Abstract
Floxuridine (5-fluorodeoxyuridine, FdUrd), a U.S. Food and Drug Administration-approved drug and metabolite of 5-fluorouracil, causes DNA damage that is repaired by base excision repair (BER). Thus, poly(ADP-ribose) polymerase (PARP) inhibitors, which disrupt BER, markedly sensitize ovarian cancer cells to FdUrd, suggesting that this combination may have activity in this disease. It remains unclear, however, which DNA repair and checkpoint signaling pathways affect killing by these agents individually and in combination. Here we show that depleting ATR, BRCA1, BRCA2, or RAD51 sensitized to ABT-888 (veliparib) alone, FdUrd alone, and FdUrd + ABT-888 (F+A), suggesting that homologous recombination (HR) repair protects cells exposed to these agents. In contrast, disabling the mismatch, nucleotide excision, Fanconi anemia, nonhomologous end joining, or translesion synthesis repair pathways did not sensitize to these agents alone (including ABT-888) or in combination. Further studies demonstrated that in BRCA1-depleted cells, F+A was more effective than other chemotherapy+ABT-888 combinations. Taken together, these studies 1) identify DNA repair and checkpoint pathways that are important in ovarian cancer cells treated with FdUrd, ABT-888, and F+A, 2) show that disabling HR at the level of ATR, BRCA1, BRCA2, or RAD51, but not Chk1, ATM, PTEN, or FANCD2, sensitizes cells to ABT-888, and 3) demonstrate that even though ABT-888 sensitizes ovarian tumor cells with functional HR to FdUrd, the effects of this drug combination are more profound in tumors with HR defects, even compared with other chemotherapy + ABT-888 combinations, including cisplatin + ABT-888.
Introduction
FdUrd, a metabolite of 5-fluorouracil, is a U.S. Food and Drug Administration-approved therapy for hepatic metastases of colorectal and other tumors of the gastrointestinal tract (Power and Kemeny, 2009). Although FdUrd is a metabolite of 5-fluorouracil, multiple studies have demonstrated that these agents have disparate mechanisms of action in human tumor cells (Wyatt and Wilson, 2009). After uptake, 5-fluorouracil is converted to metabolites that disrupt RNA and DNA metabolism (Longley et al., 2003), but recent studies have found that its ability to disrupt DNA metabolism has minimal effects on cytotoxicity in some cell lines, suggesting that toxicity is caused by disruption of RNA metabolism (Gmeiner et al., 2010; Geng et al., 2011; Huehls et al., 2011; Pettersen et al., 2011). In contrast, FdUrd primarily kills cells by disrupting DNA metabolism after its conversion to two active metabolites, 5′-fluoro-2′-deoxyuridine monophosphate and 5′-fluoro-2′-deoxyuridine triphosphate (Wyatt and Wilson, 2009). 5′-Fluoro-2′-deoxyuridine monophosphate inhibits thymidylate synthase, thereby disrupting dNTP ratios and causing massive accumulation of dUTP. This dUTP, along with 5′-fluoro-2′-deoxyuridine triphosphate, is directly incorporated by replicative DNA polymerases, leading to the accumulation of uracil and 5-fluorouracil in the genome.
Collectively, the disruption of dNTPs and accumulation of genomic uracil and 5-fluorouracil activate the ATR and ATM checkpoint signaling pathways (Parsels et al., 2004; Wilsker and Bunz, 2007; Liu et al., 2008; Jardim et al., 2009; Geng et al., 2011; Huehls et al., 2011). Uracil and 5-fluorouracil substitutions are also targeted by DNA repair pathways (Wyatt and Wilson, 2009). 5-Fluorouracil mispairs may be recognized by the mismatch repair pathway, an event thought to reduce survival of FdUrd-exposed cells (Meyers et al., 2001; Liu et al., 2008; Jardim et al., 2009). Alternatively, both uracil and 5-fluorouracil are targets of base excision repair (BER), which is initiated by uracil glycosylases that remove these lesions, leaving an abasic site. The abasic site is processed by an apurinic/apyridinic endonuclease (APE1), creating a nick that attracts poly(ADP-ribose) polymerase (PARP) and XRCC1, a scaffold protein that recruits additional repair proteins. Consistent with the idea that BER productively repairs these lesions, disabling the repair proteins APE1, XRCC1, or PARP increases cell killing by FdUrd (McNeill et al., 2009; Geng et al., 2011; Huehls et al., 2011). Likewise, small-molecule PARP inhibitors sensitize ovarian and colon cancer cell lines to FdUrd but not to 5-fluorouracil (Geng et al., 2011; Huehls et al., 2011).
PARP inhibitors have garnered significant attention as antitumor agents, especially since the demonstration that the PARP inhibitor olaparib (AZD2281) has single-agent activity in ovarian tumors with mutations in BRCA1 and BRCA2 (Kummar et al., 2012). Likewise, PARP inhibitors may be useful in tumors that lack mutations in BRCA1 or BRCA2 but that have defects in HR repair, a feature known as “BRCAness” (Turner et al., 2004). Although it remains unclear what causes BRCAness, it has been reported that reduced BRCA1/2 expression, defects in signaling pathways that influence HR repair (e.g., ATM, ATR, and Chk1), or defects in other proteins that regulate or participate in HR (e.g., Fanconi anemia pathway members, RAD51, and PTEN) sensitize to PARP inhibitors, thus suggesting that these defects may contribute to BRCAness (McCabe et al., 2006).
In addition to using PARP inhibitors as monotherapies, there is interest in combining these agents with conventional chemotherapy agents. Indeed, we recently reported that PARP inhibitors synergize remarkably with FdUrd, with toxicities that exceed those seen with other chemotherapy agents used to treat ovarian cancer. Accordingly, such results suggest that combining FdUrd with PARP inhibitors may be worthy of clinical studies, especially in ovarian cancer in which both FdUrd and PARP inhibitors have activity as single agents (Muggia et al., 1996; Kummar et al., 2012).
Before launching such trials, it is important to understand how the individual drugs and the drug combination affect tumor cells. This is especially important because combining a DNA-damaging agent, such as FdUrd, with an agent [veliparib (ABT-888)] that inhibits the repair of those lesions may create DNA damage that differs from the damage caused by FdUrd alone. Thus, different DNA repair and/or checkpoint pathways may assume importance in cells exposed to F+A. We therefore undertook a systematic analysis of the major DNA repair and checkpoint signaling pathways to determine which of these pathways affect the survival of ovarian cancer cells treated with FdUrd, ABT-888, and the F+A combination. Our studies reveal novel insights into the DNA repair pathways that affect FdUrd, ABT-888, and F+A tumor cell killing.
Materials and Methods
Cell Lines and Culture.
OVCAR-8, a gift from Dominic Scudierio (National Cancer Institute, Bethesda, MD), and SKOV-3 (American Type Culture Collection, Manassas, VA) were cultured at 37°C in 5% CO2 with 8 or 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA) in RPMI 1640 medium (Mediatech, Herndon, VA). For clonogenic assays, media were supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (Mediatech). Cell lines were reinitiated every 3 months from cryopreserved stocks prepared upon receipt from the indicated sources.
Materials.
Reagents were from the following suppliers: FdUrd, Bedford Laboratories (Bedford, OH); ABT-888 [Selleck Chemicals (Houston, TX) and ChemieTek (Indianapolis, IN)]; cisplatin, Teva Pharmaceuticals (Irvine, CA) gemcitabine, Eli Lilly & Co. (Indianapolis, IN); doxorubicin, melphalan, and topotecan, Sigma-Aldrich (St. Louis, MO); and SuperSignal Pico West, Thermo Fisher Scientific, Waltham, MA). All other materials were from Sigma-Aldrich.
Antibodies to the following antigens were obtained as follows: phospho-Ser317-Chk1, R&D Systems (Minneapolis, MN); phospho-Thr68-Chk2, ATR, AKT, phospho-Ser473-AKT, BRCA1, BRCA2, KU80, PTEN, horseradish peroxidase-linked rabbit IgG, and horseradish peroxidase-linked mouse IgG, Cell Signaling Technology, Inc. (Danvers, MA); Chk1, Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); Chk2, MSH2, RAD51, and ATM, Epitomics, (Burlingame, CA); phospho-Ser139-H2AX, Millipore Corporation (Billerica, MA); XRCC1, Bethyl Laboratories (Montgomery, TX); FANCD2, GeneTex (Irvine, CA); RAD18, Novus Biologicals, Inc. (Littleton, CA); XPA, Neomarkers (Fremont, CA); fluorescein-conjugated goat anti-mouse IgG, Invitrogen (Carlsbad, CA); and HSP90 D. Toft (Mayo Clinic, Rochester, MN).
Cell Transfections and siRNAs.
Cells were transfected as described previously (Huehls et al., 2011) and cultured 48 h before use. Sequences of siRNAs used were as follows: ATM-1, 5′-AAGCACCAGTCCAGTATTGGC-3′ (Wang and Qin, 2003); ATR-2, 5′-CCTCCGTGATGTTGCTTGA-3′ (Casper et al., 2004); Chk1-1, 5′-AAGCGTGCCGTAGACTGTCCA-3′ (Zhao et al., 2002); BRCA1-1, 5′-GUGGGUGUUGGACAGUGUA-3′ (Bartz et al., 2006); BRCA2-1, 5′-GACUCUAGGUCAAGAUUUA-3′ (Bartz et al., 2006); FANCD2-1, 5′-GGUCAGAGCUGUAUUAUUC-3′ (Wagner and Karnitz, 2009); Ku80-1, 5′-GCGAGUAACCAGCUCAUAA-3′ (Nimura et al., 2007); MSH2-1, 5′-CTGAAGTAATAGCAAAGAA-3′ (Geng et al., 2011); PTEN-1, 5′-AAGAGGAUGGAUUCGACUUAGAC-3′ (Hamada et al., 2005); RAD18-1, 5′-GCTCTCTGATCGTGATTTA-3′ (Geng et al., 2011); RAD51, ON-TARGETplus SMARTpool-human RAD51 (Dharmacon RNA Technologies, Lafayette, CO); XPA-1, 5′-GTCAAGAAGCATTAGAAGA-3′ (Biard et al., 2005); XRCC1-2, 5′-CUCGACUCACUGUGCAGAAUU-3′ (Luo et al., 2004); and luciferase, 5′-CTTACGCUGAGUACUUCGA-3′ (Elbashir et al., 2001).
Clonogenic Assays, Cell Lysis, Immunoblotting, Phospho-H2AX Staining, and Cell Irradiation.
Clonogenic assays, cell lysis, and immunoblotting were performed as described previously (Wagner and Karnitz, 2009). For clonogenic assays, percentage survival at each drug concentration was normalized to the vehicle-treated control for the given siRNA. For phospho-H2AX analysis, cells were stained as described but with 2 μg/ml anti-phospho-H2AX antibody (Lansiaux et al., 2007). Cells were exposed to ionizing and ultraviolet radiation using a RS-2000 Biological Irradiator (Rad Source, Suwanee, GA) and a UVC-515 Ultraviolet Multilinker (Ultra-Lum, Carson, CA), respectively, 4 to 6 h after cell plating.
Results
PARP Inhibition Enhances FdUrd-Induced Chk1 and Chk2 Activation.
We have shown previously that FdUrd activates the ATR and ATM checkpoint signaling pathways and that ATR, but not ATM, promotes survival of ovarian and colon cancer cells treated with FdUrd (Geng et al., 2011; Huehls et al., 2011). However, these studies did not assess whether these checkpoint pathways are important in cells treated with the drug combination, which could induce DNA damage that differs from that induced by FdUrd alone. Moreover, they did not address the role of Chk1, an ATR substrate that is activated by other nucleoside analogs and antimetabolites and that protects tumor cells from the toxic effects of these agents. Correspondingly, there is intense interest in combining small-molecule Chk1 inhibitors, which are currently in clinical trials, with various chemotherapy agents. To examine the roles of these pathways in ovarian cancer cells exposed to FdUrd + ABT-888 (F+A), we assessed whether they were activated by ABT-888 (veliparib) alone, FdUrd alone, or the combination F+A. For these studies, we used mismatch repair-proficient OVCAR-8 and mismatch repair-deficient SKOV-3 cells (Roschke et al., 2002), which are derived from serous epithelial ovarian cancers. These cell lines have wild-type BRCA1, BRCA2, and PTEN (Ikediobi et al., 2006; Garnett et al., 2012) and very limited sensitivity to PARP inhibitors, indicating that they have functional HR repair (Huehls et al., 2011).
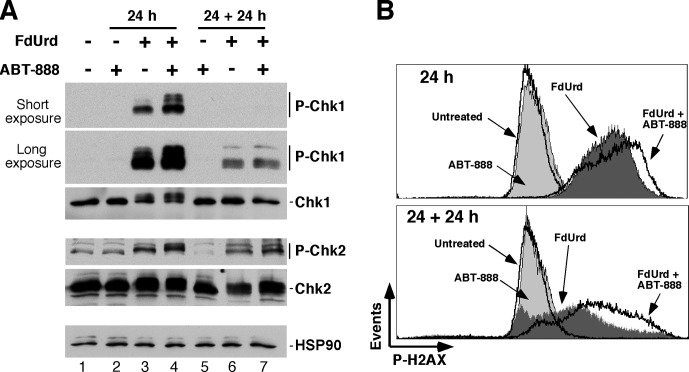
On the basis of our previous finding that continuous ABT-888 treatment after a 24-h exposure to F+A markedly increased toxicity (Huehls et al., 2011), two exposure paradigms were used for these studies. In the first, cells were exposed to FdUrd alone, ABT-888 alone, or F+A for 24 h and then analyzed for phosphorylation of Chk1 and Chk2, which are markers for ATR and ATM activation, respectively. In the second, cells were exposed to the same agents for 24 h and washed, and ABT-888 was readded to the samples that originally contained ABT-888. The cells were then analyzed after culturing for an additional 24 h (indicated as 24 + 24 h). As shown in Fig. 1A, ABT-888 alone did not provoke Chk1 or Chk2 phosphorylation under either exposure paradigm. When ABT-888 was added with FdUrd, the PARP inhibitor increased FdUrd-induced Chk1 and Chk2 phosphorylation (Fig. 1A, cf. lanes 3 and 4) at the 24-h time point. After removal of FdUrd [with the continued presence of ABT-888 (24 + 24 h)], Chk1 phosphorylation was markedly reduced compared with that seen after the 24-h exposure, and ABT-888 did not increase Chk1 phosphorylation. In contrast, FdUrd-induced Chk2 phosphorylation persisted in the 24 + 24-h samples, again with increased levels in the cells cotreated with ABT-888. Taken together, these results show that ABT-888 increases Chk1 and Chk2 activation, suggesting that PARP inhibition blocks the repair of lesions inflicted by FdUrd.
Fig. 1.
PARP inhibition increases FdUrd-induced Chk1 and Chk2 activation and causes persistent DNA damage. OVCAR-8 cells were treated with 2 μM FdUrd and 3 μM ABT-888. After 24 h of incubation, one set of cells (labeled 24 h, lanes 2–4) was collected. The second set of cells (labeled 24 + 24 h, lanes 5–7) was washed after 24 h to remove FdUrd, and 3 μM ABT-888 was readded to samples that initially contained ABT-888. These cells were then cultured an additional 24 h. A, cell lysates were immunoblotted for the indicated antigens. B, cells were stained to detect phospho-Ser139-H2AX (P-H2AX) and analyzed by flow cytometry. P, phospho; HSP90, heat shock protein 90.
ABT-888 Blocks the Repair of FdUrd-Induced DNA Damage.
To assess DNA damage caused by these agents, we examined histone H2AX phosphorylation, which serves as a surrogate marker for DNA damage such as double-stranded DNA breaks, replication stress, and other types of DNA damage. ABT-888 alone did not induce H2AX phosphorylation (Fig. 1B). In contrast, FdUrd triggered robust H2AX phosphorylation at 24 h, which was enhanced when ABT-888 was added with FdUrd. Analysis of the 24 + 24-h samples showed that after the removal of FdUrd, the level of H2AX phosphorylation was decreased in the cells treated with FdUrd alone, suggesting that the DNA damage was being repaired. Interestingly, however, in the FdUrd-treated cells that were cultured in the continued presence of ABT-888, phospho-H2AX levels remained high, indicating that ABT-888 slowed the repair of lesions induced by FdUrd and/or produced new lesions that are repaired more slowly. Taken together, these results suggest that PARP inhibition increases FdUrd-induced activation of the Chk1 and Chk2 signaling and causes persistence of FdUrd-induced DNA damage.
ATR, but Not Chk1 or ATM, Plays a Critical Role in Ovarian Cancer Cells Treated with FdUrd, ABT-888, or F+A.
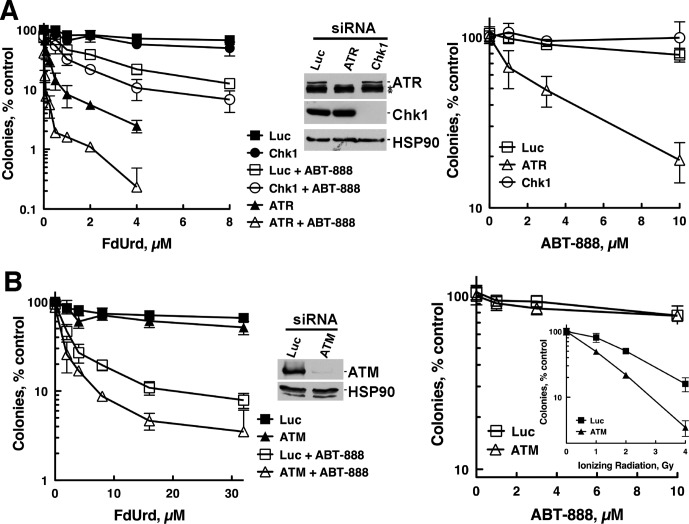
Given that the ATR and ATM pathways are hyperactivated when PARP is inhibited, we asked whether depletion of ATM, ATR, or Chk1 affected proliferation after exposure to FdUrd, ABT-888, or F+A. Consistent with previous reports (Geng et al., 2011; Huehls et al., 2011), ATR depletion sensitized OVCAR-8 cells to ABT-888 (Fig. 2A, right). ATR-depleted cells were also markedly sensitized to FdUrd alone, and these cells were even more sensitive to the F+A combination (Fig. 2A, left), indicating that ATR protects these cells from damage inflicted by each agent individually and in combination. Analyses of cells depleted of ATM revealed a very different result. Although ATM-depleted OVCAR-8 cells were more sensitive to ionizing radiation (Fig. 2B, right, inset), they were not more sensitive to FdUrd alone or F+A (Fig. 2B, left). Likewise, these ATM-depleted OVCAR-8 (Fig. 2B) and SKOV-3 cells [which were also sensitized to ionizing radiation (Supplemental Fig. 1, A and G)] were not more sensitive to ABT-888 alone (Supplemental Fig. 1F). Taken together, these results demonstrate that the ATR checkpoint pathway, but not the ATM pathway, protects ovarian cancer cells from the antiproliferative effects of FdUrd alone, ABT-888 alone, and F+A.
Fig. 2.
ATR depletion, but not ATM or Chk1 depletion, sensitizes OVCAR-8 cells to FdUrd alone, ABT-888 alone, and the F+A combination. OVCAR-8 cells were transfected with control luciferase (Luc), Chk1, ATR, or ATM siRNAs, and 48 h later, cells were processed for clonogenic assays and immunoblotting to assess siRNA efficacy. For clonogenic assays, cells were treated with indicated concentrations of FdUrd and 3 μM ABT-888 for 24 h and washed. Then 3 μM ABT-888 was readded to the cultures that initially contained ABT-888, and the plates were cultured for 7 to 8 days until colonies formed. For cells exposed only to ABT-888 (right), the exposure was continuous. *, nonspecific bands.
The finding that ATR is important prompted us to examine Chk1, an ATR substrate that protects cancer cells from replication stress, especially the stress induced by other nucleoside analogs (i.e., gemcitabine and cytarabine) and antimetabolites (Zhou and Bartek, 2004). Transfection of OVCAR-8 cells with Chk1 siRNA effectively depleted Chk1 (Fig. 2A) and robustly sensitized to the nucleoside analog gemcitabine (Supplemental Fig. 2), a result in accord with results of previous studies (Karnitz et al., 2005; Matthews et al., 2007). Despite this profound Chk1 depletion, OVCAR-8 cells were not sensitized to FdUrd alone, F+A (Fig. 2A, left), or ABT-888 (Fig. 2A, right). Likewise, Chk1-depleted SKOV-3 cells (Supplemental Fig. 1B) were not sensitized to FdUrd (Supplemental Fig. 1F) but were sensitized to gemcitabine (Supplemental Fig. 1H). These results demonstrate that even though Chk1 is activated by FdUrd, a nucleoside analog and antimetabolite, and hyperactivated by F+A, this kinase plays a limited, if any, role in facilitating the survival of cells exposed to these agents, including ABT-888.
BER Promotes the Survival of Cells Treated with FdUrd Alone but Not with ABT-888.
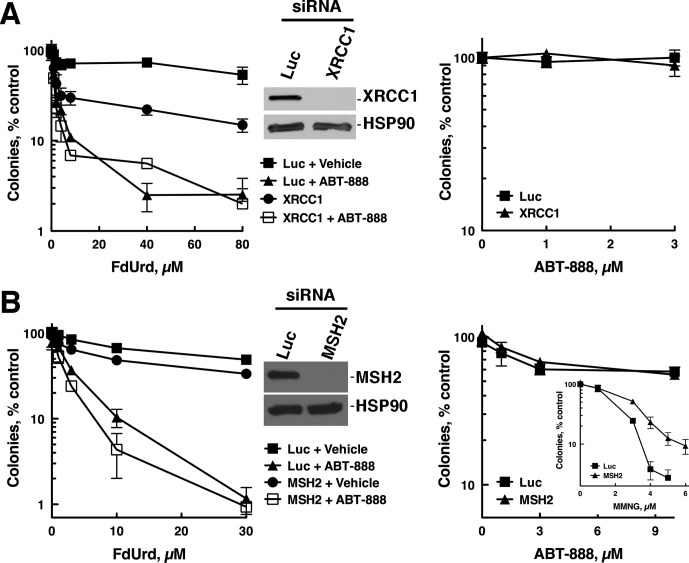
We next asked which DNA repair pathways were important in cells treated with FdUrd alone and F+A by first focusing on BER, a pathway that repairs FdUrd-induced lesions (Huehls et al., 2011; Pettersen et al., 2011) and that requires functional PARP (Horton and Wilson, 2007). Consistent with previous results (Huehls et al., 2011), depletion of XRCC1, a central scaffolding component of the BER pathway, sensitized OVCAR-8 cells to FdUrd alone (Fig. 3A, left). It is noteworthy, however, that 1) XRCC1 depletion did not sensitize to FdUrd as effectively as did ABT-888, 2) XRCC1 depletion did not sensitize to ABT-888 (Fig. 3A, right), and 3) addition of ABT-888 to XRCC1-depleted cells further sensitized to FdUrd. These results demonstrate that PARP inhibition sensitizes cells even when the key BER protein, XRCC1, is depleted. This suggests that XRCC1 depletion may not completely disable BER, in which case PARP inhibition must further suppress BER. Alternatively, drug-inhibited PARP may exert dominant-negative effects, as has been seen when it is combined with other agents (Patel et al., 2012), or PARP may participate in other cellular functions that promote the survival of cells exposed to FdUrd.
Fig. 3.
The BER pathway, but not the mismatch repair pathway, protects cells from FdUrd and F+A. OVCAR-8 cells were transfected with control luciferase (Luc) and XRCC1 (A) or MSH2 (B) siRNAs and plated as single cells, allowed to adhere, exposed to the indicated concentrations of FdUrd with or without 3 μM ABT-888 for 24 h, and washed. Then 3 μM ABT-888 was readded to the cultures that initially contained ABT-888, and the plates were cultured until colonies formed. For cells exposed only to ABT-888 (right), the exposure was continuous.
Disabling Mismatch, Translesion Synthesis, Fanconi Anemia, Nucleotide Excision, or Nonhomologous End-Joining Repair Pathways Does Not Affect Sensitivity to FdUrd, ABT-888, or F+A.
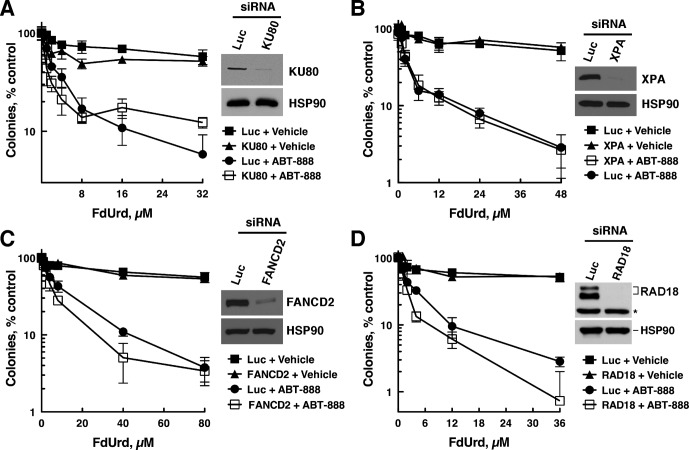
In addition to BER, we assessed the roles of five other DNA repair pathways: 1) mismatch repair, which requires MSH2 (Kunkel and Erie, 2005); 2) translesion synthesis, which requires RAD18-mediated ubiquitylation of proliferating cell nuclear antigen to facilitate postreplication repair (Ciccia and Elledge, 2010), 3) Fanconi anemia interstrand cross-link repair, which requires FANCD2 (Ciccia and Elledge, 2010), 4) nucleotide excision repair, which corrects bulky DNA lesions and requires XPA, needed for both global genome and transcription-coupled nucleotide excision repair (de Boer and Hoeijmakers, 2000), and 5) nonhomologous end-joining, which requires KU80 (Ciccia and Elledge, 2010).
To examine these pathways, we depleted OVCAR-8 cells of a key repair pathway component (MSH2, KU80, FANCD2, RAD18, or XPA) and treated the cells with FdUrd, ABT-888, or F+A. It is noteworthy that sensitivity to FdUrd alone or F+A was not affected by depletion of MSH2 (Fig. 3B), KU80 (Fig. 4A), XPA (Fig. 4B), FANCD2 (Fig. 4C), or RAD18 (Fig. 4D), even though depletion of each protein was sufficient to affect the sensitivity of these cells to control DNA-damaging agents [N-methyl-N′-nitro-N-nitrosoguanidine for MSH2 (Fig. 3B, right, inset), ionizing radiation for KU80 (Supplemental Fig. 3A), ultraviolet light for XPA (Supplemental Fig. 3B), cisplatin for RAD18, and FANCD2 (Supplemental Fig. 3, C and D)]. Similarly, the sensitivity of OVCAR-8 cells to ABT-888 was not affected by depleting MSH2, KU80, FANCD2, or RAD18 (Fig. 3B, right; Supplemental Fig. 4, A–C), whereas depletion of BRCA1 markedly sensitized to this agent (Supplemental Fig. 4D). The observation that FANCD2 depletion did not sensitize to ABT-888 was unexpected, because mouse fibroblasts lacking FANCD2 are sensitive to PARP inhibitors (McCabe et al., 2006). We therefore determined the impact of depleting FANCD2 in SKOV-3 cells (Supplemental Fig. 1D), which also were not sensitized to ABT-888 (Supplemental Fig. 1F). Taken together, these results indicate that the DNA damage inflicted by F+A is probably not acted on productively by these DNA repair pathways. Furthermore, they demonstrate that depleting FANCD2 does not affect sensitivity to ABT-888 in OVCAR-8 and SKOV-3 cells.
Fig. 4.
Nonhomologous end-joining, Fanconi anemia, translesion synthesis, and nucleotide excision repair pathways do not protect cells from FdUrd, ABT-888, or F+A. OVCAR-8 cells were transfected with control luciferase (Luc), KU80 (A), XPA (B), FANCD2 (C), or RAD18 (D) siRNAs, and 48 h after transfections cells were exposed to the indicated concentrations of FdUrd with or without 3 μM ABT-888 for 24 h and washed. Then 3 μM ABT-888 was readded to the cultures that initially contained ABT-888, and the plates were cultured until colonies formed. *, nonspecific band. RAD18 migrates as two bands, with the slowest migrating as a result of monoubiquitylation.
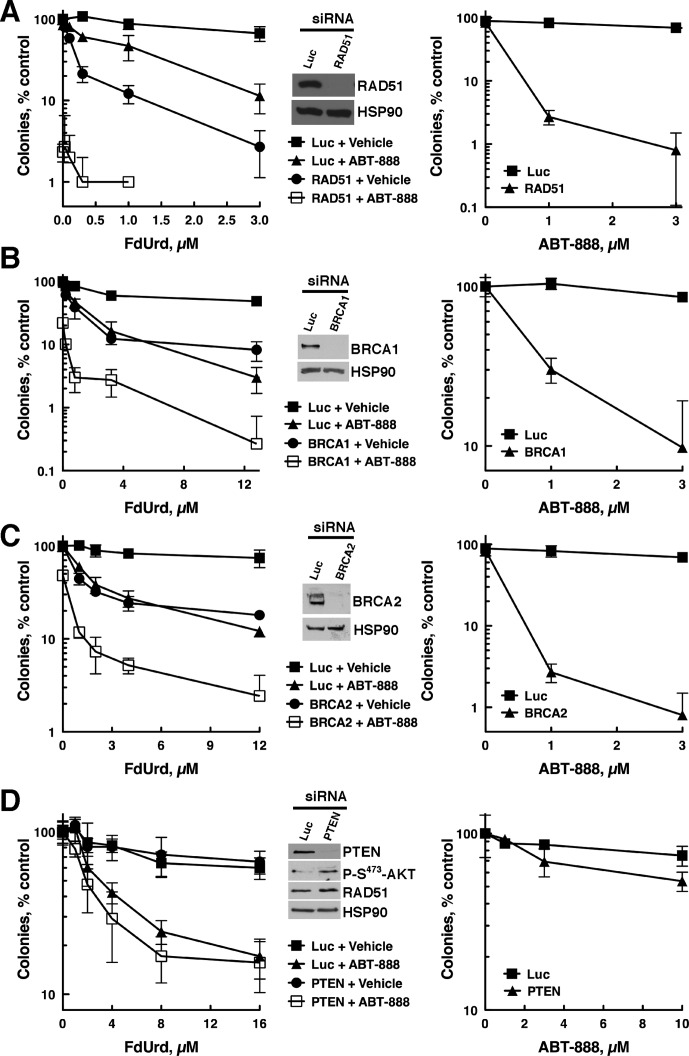
Depletion of HR Repair Proteins BRCA1, BRCA2, and RAD51 Sensitizes to FdUrd, ABT-888, and F+A.
Our results so far indicate that inhibition of PARP in cells treated with FdUrd causes increased DNA damage (Fig. 1B) that is not repaired by translesion synthesis, nucleotide excision, Fanconi anemia, or nonhomologous end-joining repair pathways. Moreover, our results also demonstrate that ATR, but not the ATR substrate Chk1, plays a critical role in cells treated with F+A (Fig. 2A). The observation that Chk1 depletion did not affect sensitivity of the cells to FdUrd, ABT-888, or F+A suggests that the function of ATR in cells treated with these agents may be channeled through other substrates. Although hundreds of ATR substrates have been identified, we turned our attention to HR repair proteins because 1) several HR proteins are ATR substrates, including BRCA1 and BRCA2 (Tibbetts et al., 1999; Gatei et al., 2001; Ciccia and Elledge, 2010), 2) BRCA1, BRCA2, and RAD51 play important roles at stalled replication forks and at DSBs, two genotoxic events that occur when cells are treated with FdUrd, and 3) defects in HR sensitize to ABT-888 alone (Martin et al., 2008).
For these studies, we depleted RAD51, which is required for HR (Ciccia and Elledge, 2010), and BRCA1 or BRCA2, two genes that are frequently mutated in ovarian cancer (Cancer Genome Atlas Research Network, 2011) and that play pivotal roles in HR (Ciccia and Elledge, 2010). OVCAR-8 cells depleted of RAD51, BRCA1, or BRCA2 (Fig. 5, A–C, insets) were sensitive to ABT-888 alone (Fig. 5, A–C, right), as expected for cells with defective HR. Surprisingly, depletion of these three HR proteins also markedly sensitized cells to FdUrd alone (Fig. 5, A–C, left), indicating that FdUrd causes damage that is repaired by the HR pathway. Most importantly, OVCAR-8 cells depleted of RAD51, BRCA1, or BRCA2 (Fig. 5, A–C, left), as well as SKOV-3 cells depleted of BRCA1 (Supplemental Fig. 1C), were exquisitely sensitive to F+A (Supplemental Fig. 1J). Taken together these results suggest that this combination of chemotherapy agents may be especially toxic in ovarian cancers with defects in HR.
Fig. 5.
Disruption of HR repair sensitizes ovarian cancer cells to FdUrd, ABT-888, and F+A. OVCAR-8 cells were transfected with control luciferase (Luc), RAD51 (A), BRCA1 (B), BRCA2 (C), or PTEN (D) siRNAs, and 48 h after transfections cells were exposed to the indicated concentrations of FdUrd with or without 3 μM or ABT-888 for 24 h and washed. Then 3 μM ABT-888 was readded to the cultures that initially contained ABT-888, and the plates were cultured until colonies formed. For cells exposed only to ABT-888 (right), the exposure was continuous.
PTEN Depletion Does Not Affect Sensitivity to FdUrd, ABT-888, or F+A.
Cells with disabled PTEN have been reported to be sensitive to PARP inhibition and to have reduced RAD51 and HR repair (Shen et al., 2007; Mendes-Pereira et al., 2009; Dedes et al., 2010; McEllin et al., 2010). Given that PTEN may regulate RAD51 and is frequently mutated in ovarian cancer (Cancer Genome Atlas Research Network, 2011), we depleted PTEN from OVCAR-8 and SKOV-3 cells, which have wild-type PTEN (Ikediobi et al., 2006). Immunoblotting demonstrated that PTEN levels were reduced in OVCAR-8 (Fig. 5D, inset) and SKOV-3 cells (Supplemental Fig. 1E). Accordingly, activating AKT phosphorylation on Ser473 was increased, indicating that PTEN depletion caused accumulation of its substrate (phosphatidylinositol 3,4,5-trisphosphate), the second messenger that activates AKT. Despite this profound PTEN depletion, RAD51 levels were unaltered in OVCAR-8 (Fig. 5D) or SKOV-3 cells (Supplemental Fig. 1E). Consistent with the lack of an effect on RAD51 levels, PTEN-depleted OVCAR-8 cells were not sensitized to FdUrd or F+A (Fig. 5D, left). OVCAR-8 (Fig. 5D, right) and SKOV-3 (Supplemental Fig. 1F) cells depleted of PTEN were also not sensitized to ABT-888.
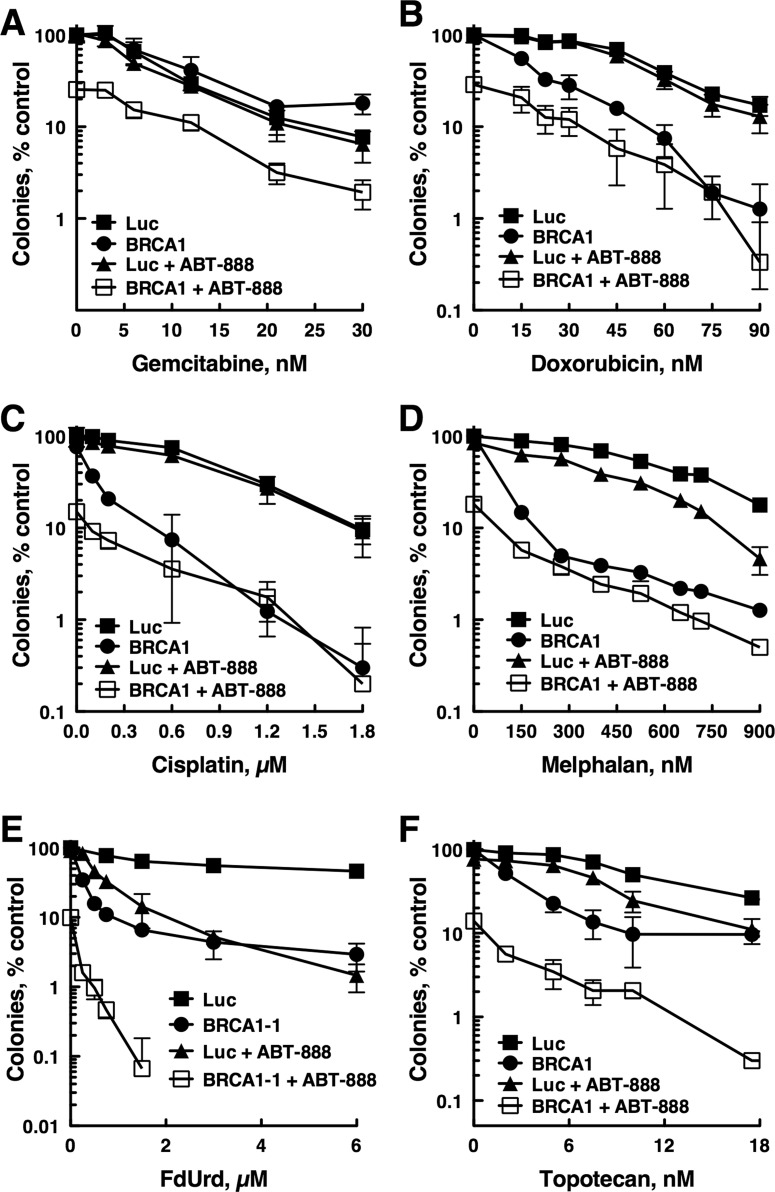
F+A Is More Cytotoxic Than Other Chemotherapy + ABT-888 Combinations in BRCA1-Depleted Ovarian Cancer Cells.
The results in Fig. 5 (and Supplemental Figs. 1 and 4) show that cells with disabled RAD51, BRCA1, or BRCA2 are very sensitive to F+A. However, this sensitivity may be no more than would be seen when ABT-888 is combined with other chemotherapy agents. To address this, we compared the antiproliferative activities of multiple chemotherapy agents used to treat ovarian cancer alone and combined with ABT-888 in BRCA1-depleted OVCAR-8 cells (Fig. 6). These results demonstrated several points. First, BRCA1 depletion sensitized to ABT-888, demonstrating that the BRCA1 depletions were sufficient to affect sensitivity to a PARP inhibitor. Second, BRCA1 depletion sensitized to all genotoxic agents except gemcitabine. Third, in cells with functional HR (luciferase siRNA-transfected cells), PARP inhibition did not sensitize to cisplatin or doxorubicin. Fourth, PARP inhibition did not further sensitize BRCA1-depleted cells to doxorubicin, cisplatin, or melphalan. Fifth, and in sharp contrast to what was observed with cisplatin, ABT-888 further sensitized BRCA1-depleted cells to FdUrd and topotecan, with the sensitization to FdUrd being the most profound. Additional experiments demonstrated that BRCA1 depletion in SKOV-3 also sensitized to FdUrd and ABT-888 and that these depleted cells were exceptionally sensitive to the F+A combination (Supplemental Fig. 1J).
Fig. 6.
The combination F+A is highly cytotoxic in BRCA1-deficient ovarian cancer cells compared with other ABT-888+chemotherapy combinations. OVCAR-8 cells transfected with control luciferase (Luc) or BRCA1 siRNA were exposed to the indicated concentrations of gemcitabine (A), doxorubicin (B), cisplatin (C), melphalan (D), FdUrd (E), or topotecan (F) and 3 μM ABT-888 for 24 h and washed. Then 3 μM ABT-888 was readded to the cultures that initially contained ABT-888, and the plates were cultured until colonies formed.
Discussion
We initiated these studies to determine whether defects in DNA repair or checkpoint signaling pathways influence the toxicity of F+A. We reasoned that if this drug combination moves into clinical trials, it will be useful to determine what DNA repair and/or checkpoint pathways affect tumor cell survival, because such information may help identify patients most likely to respond to the drug combination. Given that FdUrd and PARP inhibitors have activity in ovarian cancer, our studies focused on this tumor type.
FdUrd disrupts dNTP ratios and causes misaccumulation of uracil and 5-fluorouracil in the genome. These misincorporated bases are recognized by the BER pathway. However, it is not known whether these lesions are substrates for other repair pathways that could productively repair them. Nor is it known whether attempted repair and/or replication of these lesions creates DNA damage that is more toxic, a possibility, considering that FdUrd induces double-strand DNA breaks (Yoshioka et al., 1987; Tang et al., 1996; Meyers et al., 2001). Finally, it is not clear whether PARP inhibition, which disrupts DNA repair, alters the lesions that are ultimately induced by FdUrd. Thus, rather than directly assessing the types of damage produced by each agent alone and in combination, an approach that requires the development of many assays, we instead focused on identifying which DNA repair pathways affect cell killing. To that end, we systematically inactivated the major signaling and repair pathways and examined the effects on cells exposed to FdUrd, ABT-888, and F+A.
Our initial studies focused on the mismatch repair pathway, because defective mismatch repair has been reported to increase resistance to FdUrd-induced death, probably by preventing the attempted (but ultimately futile) mismatch repair of fluorouracil-guanine mispairs (Meyers et al., 2001; Liu et al., 2008). In contrast to these previous reports, we showed that effectively depleting MSH2 did not affect sensitivity to FdUrd or F+A but did reduce sensitivity to N-methyl-N′-nitro-N-nitrosoguanidine, an agent that produces lesions that are substrates for mismatch repair. It is noteworthy that similar results, showing that defects in mismatch repair in colon cancer cells do not (or only minimally) affect sensitivity to FdUrd have been recently reported (Pettersen et al., 2011). Taken together, these results suggest that defective mismatch repair is not universally linked to altered FdUrd sensitivity.
We also found that disabling mismatch, nucleotide excision, Fanconi anemia, nonhomologous end-joining, or translesion synthesis repair pathways did not sensitize cells to FdUrd or F+A, indicating that these pathways do not productively repair lesions induced directly or indirectly by FdUrd or even by F+A. Our results also demonstrate that these repair pathways do not make lesions induced by FdUrd alone or F+A more toxic. Given that FdUrd activates ATM and causes DSBs (Yoshioka et al., 1987; Tang et al., 1996; Meyers et al., 2001; Parsels et al., 2004; Wilsker and Bunz, 2007; Liu et al., 2008; Jardim et al., 2009; Geng et al., 2011; Huehls et al., 2011) and given that F+A induces more H2AX phosphorylation than FdUrd alone (see Fig. 1B), we were surprised that depletion of ATM or KU80, two proteins that participate in signaling from and repair of DSBs, did not affect the survival of cells treated with these agents. In contrast, disabling ATR, which responds to replication stress, sensitized cells to FdUrd alone and F+A, suggesting that these treatments are creating damage that causes replication stress rather than double-stranded DNA breaks.
It is noteworthy that, despite the critical role of ATR, depletion of Chk1, a key ATR substrate, did not affect sensitivity to FdUrd or F+A (but did sensitize to gemcitabine). These findings indicate that the replication stress caused by FdUrd must differ substantially from the stress created by other nucleoside analogs and suggest that other ATR substrates play critical roles in cells treated with this agent. Indeed, several proteins that participate in HR are phosphorylated and regulated by ATR. Therefore, we found that depletion of BRCA1, BRCA2, or RAD51 markedly sensitized to FdUrd. Although these HR proteins could participate in the repair of FdUrd-induced DSBs, they may also participate in HR-dependent resolution of stalled replication forks and/or stabilize the forks, an emerging role for these proteins (Schlacher et al., 2011; Feng and Zhang, 2012).
Although the major goal of these studies was to identify the checkpoint and DNA repair pathways important in cells treated with FdUrd and F+A, the present findings also shed light on the role of these pathways in ovarian cancer cells treated with ABT-888. In particular, we found that depletion of ATR, BRCA1, BRCA2, and RAD51 sensitized to ABT-888. In contrast, depletion of ATM, Chk1, FANCD2, RAD18, KU80, and XPA did not sensitize to ABT-888. These findings indicate several important points related to ovarian cancer cells exposed to a PARP inhibitor. They are consistent with previous results showing that disruption of BRCA1, BRCA2, or RAD51 markedly sensitizes cells to PARP inhibitors. They also agree with observations that disabling ATR, which regulates BRCA1, BRCA2, RAD51, and HR, sensitizes tumor cells to PARP inhibitors.
It is noteworthy, however, that the present findings differ in several important ways from those of other studies that have examined which DNA repair and checkpoint pathways affect sensitivity to PARP inhibitors. First, depletion of PTEN, which was shown previously to regulate RAD51 levels (Shen et al., 2007), did not sensitize these ovarian cell lines to PARP inhibitors or decrease RAD51 levels. Although this result differs from results of studies in which disabling PTEN sensitized to PARP inhibitors (Mendes-Pereira et al., 2009; Dedes et al., 2010; McEllin et al., 2010), our studies agree with recent reports showing that genetic disruption of PTEN or depletion of PTEN did not affect RAD51 levels, HR, or sensitivity to PARP inhibitors (Gupta et al., 2009; Fraser et al., 2012). Second, we found that depleting ATM did not sensitize to ABT-888, a result that contrasts with others, in which disruption of this kinase sensitized to PARP inhibitors (McCabe et al., 2006; Weston et al., 2010; Williamson et al., 2010, 2012; Golla et al., 2011). Third, our studies showed that depletion of FANCD2, a central participant in the Fanconi anemia repair pathway, did not sensitize to ABT-888. This finding differs from published findings, which showed that Fancd2(−/−) [as well as Fanca(−/−) and Fancc(−/−)] mouse fibroblasts were sensitive to PARP inhibitors (McCabe et al., 2006). Fourth, we found that profound Chk1 depletion did not affect ABT-888 sensitivity. This result contrasts with studies showing that Chk1 depletion or inhibition sensitizes tumor cells to PARP inhibition in other experimental systems (McCabe et al., 2006; Mitchell et al., 2010; Tang et al., 2012), thus suggesting that the requirement for Chk1 in PARP-inhibited cells may vary significantly among tumor cell types. Finally, the observation that Chk1 depletion did not influence FdUrd toxicity was unexpected given that Chk1 has been implicated in the regulation of RAD51 and HR (Sørensen et al., 2005), which we showed markedly protect cells from FdUrd. The apparent lack of need for Chk1 suggests that ATR may regulate BRCA1/BRCA2/RAD51 independently of Chk1.
There are several possible explanations for why our results differ from those of previous studies. On the one hand, these siRNA-mediated depletions may not be sufficiently effective to uncover potential roles in ABT-888-exposed cells. This seems unlikely given that the depletions profoundly reduced protein expression and sensitized to appropriate DNA-damaging agents (or, in the case of PTEN, increased activating phosphorylation of AKT). On the other hand, serous epithelial ovarian cancer cells may differ with respect to the experimental models used in the previous studies. This consideration is particularly relevant because 1) the genes encoding ATM, Fanconi anemia pathway members, and PTEN are mutated in some ovarian cancers (Cancer Genome Atlas Research Network, 2011), and 2) defects in these pathways have been linked to BRCAness, which predicts sensitivity to PARP inhibitors. If disabling these pathways does not sensitize to PARP inhibitors in all settings, our results suggest that caution is warranted when evaluating the effectiveness of PARP inhibitors against ovarian tumors with mutations in these genes.
In summary, our studies have furthered our understanding of the DNA repair pathways that affect the survival of ovarian cancer cells treated with FdUrd and F+A, specifically demonstrating that disabling HR increases ovarian cancer cell killing by FdUrd alone and ABT-888 alone. More impressive, our studies demonstrate that even though ABT-888 sensitizes cells with functional HR to FdUrd, a combination of F+A may be most effective in tumors with defects in HR and that this combination may be more effective than other chemotherapy + ABT-888 combinations.
Supplementary Material
Acknowledgments
We thank D. Scuderio for OVCAR-8 cells, S. Kaufmann and P. Haluska for insightful discussion, D. Toft for antibodies, P. Becker for manuscript preparation; and the Mayo Flow Cytometry/Optical Morphology Core facility.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Cancer Institute [Grants R01-CA084321, P50-CA136393]; the National Institutes of Health National Institute for General Medical Sciences [Grant T32-GM072474]; a Mayo Clinic Eagles Pilot Project Award; and the Mayo Clinic.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- FdUrd
- 5-fluorodeoxyuridine, floxuridine
- BER
- base excision repair
- PARP
- poly(ADP-ribose) polymerase
- AZD2281
- olaparib
- PTEN
- phosphatase and tensin homolog
- ABT-888
- veliparib
- F+A
- FdUrd + ABT-888
- siRNA
- small interfering RNA
- DSB
- DNA double-strand break.
Authorship Contributions
Participated in research design: Huehls, Wagner, Huntoon, and Karnitz.
Conducted experiments: Huehls, Wagner, Huntoon, and Karnitz.
Contributed new reagents or analytic tools: Huehls, Wagner, Huntoon, and Karnitz.
Performed data analysis: Huehls and Karnitz.
Wrote or contributed to the writing of the manuscript: Huehls and Karnitz.
References
- Bartz SR, Zhang Z, Burchard J, Imakura M, Martin M, Palmieri A, Needham R, Guo J, Gordon M, Chung N, et al. (2006) Small interfering RNA screens reveal enhanced cisplatin cytotoxicity in tumor cells having both BRCA network and TP53 disruptions. Mol Cell Biol 26:9377–9386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biard DS, Despras E, Sarasin A, Angulo JF. (2005) Development of new EBV-based vectors for stable expression of small interfering RNA to mimick human syndromes: application to NER gene silencing. Mol Cancer Res 3:519–529 [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474:609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper AM, Durkin SG, Arlt MF, Glover TW. (2004) Chromosomal instability at common fragile sites in Seckel syndrome. Am J Hum Genet 75:654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. (2010) The DNA damage response: making it safe to play with knives. Mol Cell 40:179–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Hoeijmakers JH. (2000) Nucleotide excision repair and human syndromes. Carcinogenesis 21:453–460 [DOI] [PubMed] [Google Scholar]
- Dedes KJ, Wetterskog D, Mendes-Pereira AM, Natrajan R, Lambros MB, Geyer FC, Vatcheva R, Savage K, Mackay A, Lord CJ, et al. (2010) PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med 2:53ra75. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494–498 [DOI] [PubMed] [Google Scholar]
- Feng Z, Zhang J. (2012) A dual role of BRCA1 in two distinct homologous recombination mediated repair in response to replication arrest. Nucleic Acids Res 40:726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M, Zhao H, Luoto KR, Lundin C, Coackley C, Chan N, Joshua AM, Bismar TA, Evans A, Helleday T, et al. (2012) PTEN deletion in prostate cancer cells does not associate with loss of RAD51 function: implications for radiotherapy and chemotherapy. Clin Cancer Res 18:1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, Greninger P, Thompson IR, Luo X, Soares J, et al. (2012) Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483:570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatei M, Zhou BB, Hobson K, Scott S, Young D, Khanna KK. (2001) Ataxia telangiectasia mutated (ATM) kinase and ATM and Rad3 related kinase mediate phosphorylation of Brca1 at distinct and overlapping sites. In vivo assessment using phospho-specific antibodies. J Biol Chem 276:17276–17280 [DOI] [PubMed] [Google Scholar]
- Geng L, Huehls AM, Wagner JM, Huntoon CJ, Karnitz LM. (2011) Checkpoint signaling, base excision repair, and PARP promote survival of colon cancer cells treated with 5-fluorodeoxyuridine but not 5-fluorouracil. PLoS ONE 6:e28862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner WH, Reinhold WC, Pommier Y. (2010) Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol Cancer Ther 9:3105–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golla RM, Li M, Shen Y, Ji M, Yan Y, Fu K, Greiner TC, McKeithan TW, Chan WC. (2011) Inhibition of poly(ADP-ribose) polymerase (PARP) and ataxia telangiectasia mutated (ATM) on the chemosensitivity of mantle cell lymphoma to agents that induce DNA strand breaks. Hematol Oncol http://dx.doi.org/10.1002/hon.1020 [DOI] [PubMed]
- Gupta A, Yang Q, Pandita RK, Hunt CR, Xiang T, Misri S, Zeng S, Pagan J, Jeffery J, Puc J, et al. (2009) Cell cycle checkpoint defects contribute to genomic instability in PTEN deficient cells independent of DNA DSB repair. Cell Cycle 8:2198–2210 [DOI] [PubMed] [Google Scholar]
- Hamada K, Sasaki T, Koni PA, Natsui M, Kishimoto H, Sasaki J, Yajima N, Horie Y, Hasegawa G, Naito M, et al. (2005) The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev 19:2054–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JK, Wilson SH. (2007) Hypersensitivity phenotypes associated with genetic and synthetic inhibitor-induced base excision repair deficiency. DNA Repair (Amst) 6:530–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehls AM, Wagner JM, Huntoon CJ, Geng L, Erlichman C, Patel AG, Kaufmann SH, Karnitz LM. (2011) Poly(ADP-ribose) polymerase inhibition synergizes with 5-fluorodeoxyuridine but not 5-fluorouracil in ovarian cancer cells. Cancer Res 71:4944–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikediobi ON, Davies H, Bignell G, Edkins S, Stevens C, O'Meara S, Santarius T, Avis T, Barthorpe S, Brackenbury L, et al. (2006) Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther 5:2606–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardim MJ, Wang Q, Furumai R, Wakeman T, Goodman BK, Wang XF. (2009) Reduced ATR or Chk1 expression leads to chromosome instability and chemosensitization of mismatch repair-deficient colorectal cancer cells. Mol Biol Cell 20:3801–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnitz LM, Flatten KS, Wagner JM, Loegering D, Hackbarth JS, Arlander SJ, Vroman BT, Thomas MB, Baek YU, Hopkins KM, et al. (2005) Gemcitabine-induced activation of checkpoint signaling pathways that affect tumor cell survival. Mol Pharmacol 68:1636–1644 [DOI] [PubMed] [Google Scholar]
- Kummar S, Chen A, Parchment RE, Kinders RJ, Ji J, Tomaszewski JE, Doroshow JH. (2012) Advances in using PARP inhibitors to treat cancer. BMC Med 10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Erie DA. (2005) DNA mismatch repair. Annu Rev Biochem 74:681–710 [DOI] [PubMed] [Google Scholar]
- Lansiaux A, Léonce S, Kraus-Berthier L, Bal-Mahieu C, Mazinghien R, Didier S, David-Cordonnier MH, Hautefaye P, Lavielle G, Bailly C, et al. (2007) Novel stable camptothecin derivatives replacing the E-ring lactone by a ketone function are potent inhibitors of topoisomerase I and promising antitumor drugs. Mol Pharmacol 72:311–319 [DOI] [PubMed] [Google Scholar]
- Liu A, Yoshioka K, Salerno V, Hsieh P. (2008) The mismatch repair-mediated cell cycle checkpoint response to fluorodeoxyuridine. J Cell Biochem 105:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley DB, Harkin DP, Johnston PG. (2003) 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3:330–338 [DOI] [PubMed] [Google Scholar]
- Luo H, Chan DW, Yang T, Rodriguez M, Chen BP, Leng M, Mu JJ, Chen D, Songyang Z, Wang Y, et al. (2004) A new XRCC1-containing complex and its role in cellular survival of methyl methanesulfonate treatment. Mol Cell Biol 24:8356–8365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SA, Lord CJ, Ashworth A. (2008) DNA repair deficiency as a therapeutic target in cancer. Curr Opin Genet Dev 18:80–86 [DOI] [PubMed] [Google Scholar]
- Matthews DJ, Yakes FM, Chen J, Tadano M, Bornheim L, Clary DO, Tai A, Wagner JM, Miller N, Kim YD, et al. (2007) Pharmacological abrogation of S-phase checkpoint enhances the anti-tumor activity of gemcitabine in vivo. Cell Cycle 6:104–110 [DOI] [PubMed] [Google Scholar]
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, Giavara S, O'Connor MJ, Tutt AN, Zdzienicka MZ, et al. (2006) Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 66:8109–8115 [DOI] [PubMed] [Google Scholar]
- McEllin B, Camacho CV, Mukherjee B, Hahm B, Tomimatsu N, Bachoo RM, Burma S. (2010) PTEN loss compromises homologous recombination repair in astrocytes: implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res 70:5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill DR, Lam W, DeWeese TL, Cheng YC, Wilson DM., 3rd (2009) Impairment of APE1 function enhances cellular sensitivity to clinically relevant alkylators and antimetabolites. Mol Cancer Res 7:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, Waldman T, Lord CJ, Ashworth A. (2009) Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med 1:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. (2001) Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res 61:5193–5201 [PubMed] [Google Scholar]
- Mitchell C, Park M, Eulitt P, Yang C, Yacoub A, Dent P. (2010) Poly(ADP-ribose) polymerase 1 modulates the lethality of CHK1 inhibitors in carcinoma cells. Mol Pharmacol 78:909–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggia FM, Liu PY, Alberts DS, Wallace DL, O'Toole RV, Terada KY, Franklin EW, Herrer GW, Goldberg DA, Hannigan EV. (1996) Intraperitoneal mitoxantrone or floxuridine: effects on time-to-failure and survival in patients with minimal residual ovarian cancer after second-look laparotomy–a randomized phase II study by the Southwest Oncology Group. Gynecol Oncol 61:395–402 [DOI] [PubMed] [Google Scholar]
- Nimura Y, Kawata T, Uzawa K, Okamura J, Liu C, Saito M, Shimada H, Seki N, Nakagawara A, Ito H, et al. (2007) Silencing Ku80 using small interfering RNA enhanced radiation sensitivity in vitro and in vivo. Int J Oncol 30:1477–1484 [PubMed] [Google Scholar]
- Parsels LA, Parsels JD, Tai DC, Coughlin DJ, Maybaum J. (2004) 5-Fluoro-2′-deoxyuridine-induced cdc25A accumulation correlates with premature mitotic entry and clonogenic death in human colon cancer cells. Cancer Res 64:6588–6594 [DOI] [PubMed] [Google Scholar]
- Patel AG, Flatten KS, Schneider PA, Dai NT, McDonald JS, Poirier GG, Kaufmann SH. (2012) Enhanced killing of cancer cells by poly(ADP-ribose) polymerase inhibitors and topoisomerase I inhibitors reflects poisoning of both enzymes. J Biol Chem 287:4198–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen HS, Visnes T, Vågbø CB, Svaasand EK, Doseth B, Slupphaug G, Kavli B, Krokan HE. (2011) UNG-initiated base excision repair is the major repair route for 5-fluorouracil in DNA, but 5-fluorouracil cytotoxicity depends mainly on RNA incorporation. Nucleic Acids Res 39:8430–8444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power DG, Kemeny NE. (2009) The role of floxuridine in metastatic liver disease. Mol Cancer Ther 8:1015–1025 [DOI] [PubMed] [Google Scholar]
- Roschke AV, Stover K, Tonon G, Schäffer AA, Kirsch IR. (2002) Stable karyotypes in epithelial cancer cell lines despite high rates of ongoing structural and numerical chromosomal instability. Neoplasia 4:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlacher K, Christ N, Siaud N, Egashira A, Wu H, Jasin M. (2011) Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 145:529–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. (2007) Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128:157–170 [DOI] [PubMed] [Google Scholar]
- Sørensen CS, Hansen LT, Dziegielewski J, Syljuåsen RG, Lundin C, Bartek J, Helleday T. (2005) The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol 7:195–201 [DOI] [PubMed] [Google Scholar]
- Tang HY, Weber KL, Lawrence TS, Merchant AK, Maybaum J. (1996) Dependence of fluorodeoxyuridine-induced cytotoxicity and megabase DNA fragment formation on S phase progression in HT29 cells. Cancer Chemother Pharmacol 37:486–490 [DOI] [PubMed] [Google Scholar]
- Tang Y, Hamed HA, Poklepovic A, Dai Y, Grant S, Dent P. (2012) Poly(ADP-ribose) polymerase 1 modulates the lethality of CHK1 inhibitors in mammary tumors. Mol Pharmacol 82:322–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh SY, Taya Y, Prives C, Abraham RT. (1999) A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev 13:152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Tutt A, Ashworth A. (2004) Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer 4:814–819 [DOI] [PubMed] [Google Scholar]
- Wagner JM, Karnitz LM. (2009) Cisplatin-induced DNA damage activates replication checkpoint signaling components that differentially affect tumor cell survival. Mol Pharmacol 76:208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Qin J. (2003) MSH2 and ATR form a signaling module and regulate two branches of the damage response to DNA methylation. Proc Natl Acad Sci USA 100:15387–15392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston VJ, Oldreive CE, Skowronska A, Oscier DG, Pratt G, Dyer MJ, Smith G, Powell JE, Rudzki Z, Kearns P, et al. (2010) The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood 116:4578–4587 [DOI] [PubMed] [Google Scholar]
- Williamson CT, Kubota E, Hamill JD, Klimowicz A, Ye R, Muzik H, Dean M, Tu L, Gilley D, Magliocco AM, et al. (2012) Enhanced cytotoxicity of PARP inhibition in mantle cell lymphoma harbouring mutations in both ATM and p53. EMBO Mol Med 4:515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CT, Muzik H, Turhan AG, Zamò A, O'Connor MJ, Bebb DG, Lees-Miller SP. (2010) ATM deficiency sensitizes mantle cell lymphoma cells to poly(ADP-ribose) polymerase-1 inhibitors. Mol Cancer Ther 9:347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsker D, Bunz F. (2007) Loss of ataxia telangiectasia mutated- and Rad3-related function potentiates the effects of chemotherapeutic drugs on cancer cell survival. Mol Cancer Ther 6:1406–1413 [DOI] [PubMed] [Google Scholar]
- Wyatt MD, Wilson DM., 3rd (2009) Participation of DNA repair in the response to 5-fluorouracil. Cell Mol Life Sci 66:788–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka A, Tanaka S, Hiraoka O, Koyama Y, Hirota Y, Ayusawa D, Seno T, Garrett C, Wataya Y. (1987) Deoxyribonucleoside triphosphate imbalance. 5-Fluorodeoxyuridine-induced DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. J Biol Chem 262:8235–8241 [PubMed] [Google Scholar]
- Zhao H, Watkins JL, Piwnica-Worms H. (2002) Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci USA 99:14795–14800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Bartek J. (2004) Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer 4:216–225 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.