Abstract
Genotype-based dosing recommendations are provided in the US FDA-approved warfarin labeling. However, data that informed these recommendations were from predominately Caucasian populations. Studies show that variants contributing to warfarin dose requirements in Caucasians provide similar contributions to dose requirements in US Hispanics, but significantly lesser contributions in African–Americans. Further data demonstrate that variants occurring commonly in individuals of African ancestry, but rarely in other racial groups, significantly influence dose requirements in African–Americans. These data suggest that it is important to consider variants specific for African–Americans when implementing genotype-guided warfarin dosing in this population.
Keywords: African–American, CYP2C9, Hispanic, polymorphism, VKORC1, warfarin
Warfarin is widely prescribed to prevent thromboembolism and while it has been in use for over 60 years, it remains a challenging drug to manage. This is predominately because of its narrow therapeutic index and the significant interpatient variability in the dose that produces therapeutic anticoagulation. Specifically, warfarin is dosed to achieve an international normalized ratio (INR) of 2–3 for most indications, with sub- or supra-therapeutic dosing increasing the risk for thrombosis or bleeding, respectively [1,2]. The warfarin dose required to achieve therapeutic levels of anticoagulation (i.e., INR of 2–3) varies as much as 20-fold between individuals [3].
According to data from the National Electronic Injury Surveillance System, warfarin is the most common drug-related cause of hospitalization for adverse events among older adults in the USA, accounting for 33% of such hospitalizations [4]. An estimated 21,010 hospitalizations from 2007 to 2009 were for warfarin-related hemorrhages. The risk for hemorrhage is particularly elevated when the INR exceeds 4, as well as during the initial months of therapy [1]. Thus, it is imperative to efficiently achieve a safe and effective level of anticoagulation for patients starting warfarin.
Current anticoagulation management guidelines recommend starting warfarin at a dose of 5 mg/day for most patients, with lower doses in the elderly or those taking medications that reduce CYP2C9-mediated warfarin metabolism [5,6]. However, the 5 mg/day dose will result in sub- or supra-therapeutic anticoagulation for approximately 60% of Caucasians and even more African–Americans [7]. The dose titration necessary in these patients prolongs the time to achieve therapeutic anticoagulation.
A number of factors influence warfarin dose requirements, including age, hepatic and renal function, concomitant use of drugs that interfere with warfarin metabolism (especially amiodarone) and vitamin K intake [5,8,9]. In addition, the genotypes for proteins involved in warfarin metabolism and pharmacodynamics provide significant contributions to warfarin dose requirements. Thus, rather than using an empiric approach to warfarin dosing, a number of investigators have proposed personalized warfarin dosing, with doses chosen based on genetic and clinical factors [7,10–13]. The goal of this individualized approach is to improve dosing accuracy and reduce the risk for sub- or supra-therapeutic anticoagulation during the initial months of therapy when the risks for adverse outcomes are highest.
African–Americans are significantly under-represented in pharmacogenetic studies with warfarin, and Hispanics are even less well represented. The performance of dosing algorithms derived from predominately Caucasian populations is questionable in under-represented patient groups. This article will discuss warfarin pharmacogenetics and its future potential, with a focus on African–American and Hispanic populations.
Genetic determinants of warfarin response
Numerous studies, predominately conducted in Caucasian and Asian populations, demonstrate that the CYP2C9 and VKORC1 genotypes contribute significantly to warfarin dose variability [3,7,14–18]. The CYP2C9 enzyme metabolizes the more active S-enantiomer of warfarin primary to an inactive 7-hydroxy metabolite, as shown in Figure 1 [5]. Warfarin inhibits VKOR to prevent generation of a reduced form of vitamin K that is necessary for γ-carboxylation and activation of clotting factors II, VII, IX and X. Thus, the CYP2C9 gene affects warfarin pharmacokinetics, while the VKORC1 gene (the gene encoding VKOR) impacts warfarin pharmacodynamics.
Figure 1. Genes affecting warfarin.
Pharmacokinetics (shown in oval boxes) and pharmacodynamics (shown in rectangular boxes).
The most extensively studied CYP2C9 variants are the CYP2C9*2 (R144C) and CYP2C9*3 (I359L) alleles, which lead to significant reductions in CYP2C9 activity. The location and frequencies of these alleles are shown in Table 1. Compared with the CYP2C9*1/*1 genotype, the CYP2C9 *1/*2, *1/*3 and *3/*3 genotypes reduce S-warfarin clearance by approximately 40, 60 and 90%, respectively [19,20]. As a result, significantly lower doses are usually needed in individuals with a CYP2C9*2 or CYP2C9*3 allele. For example, mean doses of 5.6, 3.9 and 2.9 mg/day were reported in Caucasians with the CYP2C9*1/*1, *1/*2 and *1/*3 genotypes, respectively [19]. A dose of 1 mg/day or lower may be necessary in CYP2C9*3 homozygotes. The CYP2C9*2 and *3 alleles explain 9–12% of the total variability in warfarin dose requirements in Caucasians, but significantly less in African–Americans, likely because of their lower frequency in the latter population [15,17,21]. The CYP2C9 genotype is also implicated in the risk for bleeding during warfarin therapy, especially during the warfarin initiation period [22].
Table 1.
Location and frequency of gene alleles contributing to warfarin response.
| Allele | Location | Frequency |
||
|---|---|---|---|---|
| European Caucasians | US Hispanics | African–Americans | ||
| CYP2C9*2 | Exon 3 | 0.10 | 0.07 | 0.02 |
| CYP2C9*3 | Exon 7 | 0.06 | 0.05 | 0.01 |
| CYP2C9*5 | Exon 7 | <0.01 | <0.01 | 0.01 |
| CYP2C9*6 | Exon 5 | <0.01 | <0.01 | 0.01 |
| CYP2C9*8 | Exon 3 | <0.01 | <0.01 | 0.06 |
| CYP2C9*11 | Exon 7 | <0.01 | <0.01 | 0.04 |
| CYP2C9 rs7089580 | Intronic | 0.24 | 0.11 | 0.23 |
| VKORC1 -1639A | 5-UTR | 0.40 | 0.46 | 0.11 |
| VKORC1 rs61162043 | 5-UTR | Unknown | Unknown | 0.47 |
| CALU rs339097 | Intronic | <0.01 | Unknown | 0.11–0.14 |
| CYP4F2 433M | Exon 2 | 0.23 | 0.22 | 0.09 |
| GGCX (CAA)16, 17 | Intronic | <0.01 | Unknown | 0.03 |
The VKORC1 genotype was originally recognized for causing warfarin resistance due to mutations in the gene-coding region [23]. More recently, common VKORC1 single-nucleotide polymorphisms (SNPs) occurring in gene-regulatory regions and underlying usual warfarin dose variability were discovered [14,24]. Five common VKORC1 SNPs are in strong linkage disequilibrium (i.e., almost always inherited together) in Caucasians and comprise two major haplotypes, designated as haplotypes A and B [14]. Haplotype A is associated with a twofold lower VKORC1 expression and significantly lower warfarin dose requirements [14]. Specifically, doses of 2.7, 4.9 and 6.2 mg/day were reported with the AA, AB and BB haplotype combinations, respectively.
Of the five variants comprising VKORC1 haplotypes A and B, only the -1639G>A and possibly 1173C>T SNPs appear to be functional [25]. Thus, the majority of warfarin pharmacogenetic studies have focused on one of these two SNPs. The -1639G>A and 1173C>T are in strong linkage disequilibrium across populations and are similarly predictive of warfarin dose requirements, as shown in Figure 2 [26]. Thus, only one of these SNPs needs to be taken into account for pharmacogenetic dosing of warfarin. The VKORC1 -1639G>A variant explains approximately 20–28% of the overall variability in dose requirements in Caucasians, but only 5–7% of the variability in African–Americans [15,17,21,26]. The reduced contribution of the -1639G>A genotype to dose variability in African–Americans is primarily due to the lower frequency of the -1639A (low dose) allele in this racial group (Table 1) [26]. Unlike CYP2C9, VKORC1 genotype does not appear to affect bleeding risk with warfarin [22].
Figure 2. Median warfarin dose requirements by VKORC1 -1639G>A and 1173C>T genotypes in Caucasians and African–Americans, according to data from the International Warfarin Pharmacogenetics Consortium.
Data taken from [26].
Data from two genome-wide association studies (GWAS) in Caucasians and a third in Asian individuals confirm that the VKORC1 -1639G>A, CYP2C9*2 and CYP2C9*3 polymorphisms are the primary genetic determinants of warfarin dose requirements in these populations [15–17]. The combination of VKORC1 -1639G>A, CYP2C9 (*2 and *3) and clinical factors (e.g., age, sex, weight and amiodarone use) explains approximately 55% of the total variance in warfarin maintenance dose in Caucasians, but only about 25% among African–Americans [3,26]. With the exception of the CYP4F2 genotype discussed below, no other variant met genome-wide significance for association with warfarin dose requirements in Caucasian and Asian GWAS.
Warfarin pharmacogenetic dosing tools
In response to the wealth of data supporting the CYP2C9 and VKORC1 variants as determinants of warfarin response, the US FDA-approved warfarin labeling was revised in 2007 to include pharmacogenetic information. The label was again revised in 2010 to include a dosing table based on the VKORC1 -1639G>A, CYP2C9*2, and CYP2C9*3 genotypes [27]. In addition to this table, several pharmacogenetic dosing algorithms have been published [7,10–13,28]. The two most commonly cited algorithms are the International Warfarin Pharmacogenetic Consortium (IWPC) and Gage algorithms [7,11]. Both are based on data from large, predominately Caucasian populations, and both are freely available [101]. Data from over 4000 warfarin-treated patients (55% Caucasian and 30% Asian patients) were used to derive the IWPC algorithm [7]. The Gage algorithm was derived from a population of over 1000 patients, of whom 83% were Caucasian [11].
The Gage algorithm can account for previous warfarin doses and INR measurements to refine dose prediction [29]. Thus, the Gage algorithm may be most useful for patients who have already received one or more warfarin doses at the time of algorithm use. In this regard, there are data supporting the use of pharmacogenetic algorithms even after INR results are available. Specifically, Lenzini et al. found that, even after 4–5 days of warfarin therapy, a pharmacogenetic algorithm that incorporated previous warfarin doses and INR values more accurately predicted warfarin maintenance dose than clinical factors alone [29]. In a prospective cohort included in this study, the pharmacogenetic algorithm explained 42–58% of the variance in warfarin dose at 4–5 days, whereas a clinical algorithm explained 26–43% of the variance. A more recent study by the same investigator group supports the integration of genetic data into warfarin dosing decisions even after 9 days of therapy [30]. At this time point, a pharmacogenetic algorithm explained 74% of the variability in dose compared with 65% explained by clinical factors alone (p < 0.01).
Insight into CYP2C9 genotype may also be useful with regard to decisions regarding warfarin dose titration. Specifically, reduced warfarin metabolism, secondary to CYP2C9 variant genotype prolongs the half-life of warfarin and time to achieve steady-state plasma concentration and a stable INR [31,32]. Because of the prolonged rate of dose stabilization, patients with a variant CYP2C9 allele may require slower than usual dose titration to avoid ‘over-shooting’ the target INR range.
Racial considerations in warfarin pharmacogenetics
African–American and Hispanic individuals are under-represented in warfarin pharmacogenetic studies. Specifically, only 9% of patients included in the IWPC cohort were African–American and even fewer were Hispanic [7]. Only 15% of patients included in the derivation of the Gage algorithm were African–American and 2% were Hispanic [11]. There are important differences in genetic structure and allele frequencies by race and ethnicity that may significantly impact the genotype–warfarin response relationship. Thus, it is important to confirm that associations in Caucasians extend to those of other racial and ethnic backgrounds.
Racial differences in warfarin dose requirements
Warfarin dose requirements vary significantly by race, as demonstrated in Figure 3, with higher median doses in African–Americans and lower doses in Asians compared with Caucasians [26,33]. Racial differences in genotype frequencies contribute to the observed racial differences in warfarin dose requirements [34–39,102]. In particular, the VKORC1 -1639A, CYP2C9*2 and CYP2C9*3 alleles, all of which are associated with lower warfarin doses, are significantly less common in African–Americans compared with Caucasians and Asians (Table 1). Less is known about dose requirements in Hispanics. However, the limited data available suggest that Hispanic and non-Hispanic Caucasians require similar warfarin doses [33].
Figure 3. Median warfarin dose requirements by race and ethnicity.
Racial differences in thrombotic risk
African–Americans are at greater risk for adverse outcomes as a result of subtherapeutic anticoagulation compared with Caucasians. Specifically, African–Americans have greater stroke-related disability and higher mortality rates from stroke and pulmonary embolism compared with Caucasians [40–42]. Thus, efficiently achieving therapeutic anticoagulation is especially important for African–Americans starting warfarin.
Compared with non-Hispanic Caucasians, Hispanics have a higher risk for stroke, suffer from stroke earlier in life and die at a younger age from stroke [43,44]. However, Hispanics have lower overall stroke-related mortality rates compared with non-Hispanic whites [45]. Hispanics also have a lower risk for first venous thromboembolism compared with non-Hispanic Caucasians [46]. However, Hispanic women have a higher rate of recurrent venous thromboembolism compared with non-Hispanic Caucasian women [42]. The incidence of recurrent thromboembolism is similar between Hispanic and non-Hispanic men. Thus, achieving and maintaining therapeutic anticoagulation in Hispanic women with a history of venous thromboembolism is especially important.
Racial differences in genetic ‘structure’
It is widely recognized that populations of recent African ancestry generally have more genetic variation. Furthermore, the extent of linkage disequilibrium, or the association between two SNPs on the same chromosome, can be much lower in populations of recent African ancestry than in populations of recent European or Asian ancestry [47]. In many cases, the linkage disequilibrium in the African genome extends for shorter distances and may be broken up with sections of the genome that have very low linkage disequilibrium between sections that have very high linkage disequilibrium (known as long range linkage disequilibrium) [47]. These patterns are typically absent in Caucasians.
Polymorphisms that tag for a particular haplotype, known as tag SNPs, may be associated with drug response without actually causing changes in gene function or expression. Rather, tag SNPs may be in high linkage disequilibrium with the causal variant that actually underlies the genotype–phenotype association. Until functional studies are performed, the actual causal SNP within a haplotype block is unknown. Thus, a tag SNP that is not in itself functional, but is in high linkage disequilibrium with the causal SNP, may be a good predictor of warfarin dose requirements in a European population. However, because of the lesser linkage disequilibrium in African–Americans, this SNP may not be inherited as often with the causal SNP in the African–American population. Thus, it will not be predictive of dose requirements in African–Americans. As an example, the VKORC1 1583G>C SNP was strongly associated with warfarin dose requirements in several Caucasian populations [14,48]. However, we previously observed no association between the 1583G>C SNP and dose requirements in African–Americans [49]. The 1583G>C SNP is not functional. However, in Caucasians, the 1583G>C SNP is in strong linkage disequilibrium with the functional -1639G>A SNP that decreases VKORC1 expression [14,25]. In African–Americans, the 1583G>C and -1639G>A SNPs are in much weaker linkage disequilibrium, thus explaining the lack of association with the 1583G>C SNP in this racial group. This example demonstrates the importance of validating gene–drug response associations across populations, especially when there are no functional data available for the SNP in question.
As mentioned previously, several groups have constructed warfarin dosing algorithms that include the VKORC1 -1639G>A, CYP2C9 *2 and CYP2C9*3 SNPs. These algorithms explain 50–60% of variability in warfarin dose in Caucasians and Asians, but significantly less in African–Americans [3,7,26,28,50,51]. African–American heritage was found to be a predictor of warfarin doses greater than 5 mg/day, and this association was independent of dietary and vitamin K intake [8]. On the other hand, patients from the Far East (Chinese, Japanese and Malay patients), required the lowest mean doses of warfarin at 3.1 mg/day [52–54]. Dosing algorithms that include only variants predictive in Caucasians and Asians are much less predictive in African–Americans [7,51], which may be due to the large amount of genetic diversity and decreased linkage disequilibrium in the African–American genome [55].
Warfarin pharmacogenetics in African–American individuals
CYP2C9 genotype
While African–American individuals have a lower prevalence of the CYP2C9*2 and *3 alleles compared with Caucasians, the CYP2C9*5 (D360E), *6 (10601delA) and *8 (R150H) alleles occur almost exclusively in African populations, whereas the CYP2C9*11 (R335W) allele occurs at a low frequency in both populations.The CYP2C9*8 allele is one of the most common CYP2C9 alleles in African–Americans (Table 1), occurring more often than the CYP2C9*2 and *3 alleles combined. The CYP2C9*5, *6 and *11 alleles have been associated with decreased enzyme activity, whereas functional data suggest that the effects of the CYP2C9*8 allele on drug metabolism are substrate specific [56–58]. Specifically, the CYP2C9*8 allele is reported to increase enzyme activity toward tolbutamide in vitro, decrease enzyme activity toward phenytoin in vivo and have no effect on losartan metabolism [57–59]. Consistent with the in vivo data with phenytoin, we recently found a 20% reduction in S-warfarin clearance in African–American individuals with the CYP2C9*8 allele [60].
Data from our group and others show significantly lower warfarin dose requirements in African–American individuals with a CYP2C9*5, *6, *8 or *11 allele compared with those with the CYP2C9*1/*1 genotype [21,34,61]. Specifically, we observed an 18% lower median warfarin maintenance dose in African–Americans with a CYP2C9*5, *6, *8 or *11 allele compared with those with the CYP2C9*1/*1 genotype (median dose of 5.0 mg/day vs 6.1 mg/day, p = 0.004) [21]. When taking the CYP2C9*2, *3, *5, *6, *8 and *11 alleles into account, the CYP2C9 genotype explained approximately 8% of the variability in warfarin dose requirements among African–Americans, just slightly less than that explained by the CYP2C9*2 and *3 alleles in Caucasians [15].
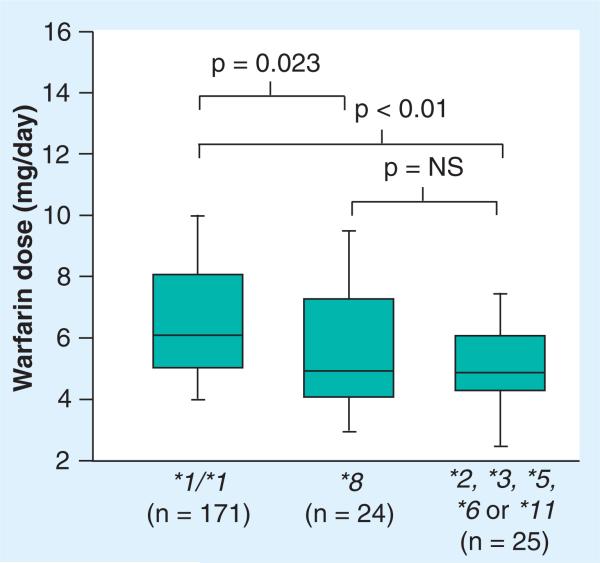
The CYP2C9*8 allele by itself is associated with significantly lower warfarin dose requirements compared with the CYP2C9 *1/*1 genotype, as shown in Figure 4 [21]. Furthermore, it predicts similar warfarin dose requirements as the other CYP2C9 alleles combined in African–Americans. Since the CYP2C9*8 allele occurs almost as often as other CYP2C9 alleles combined in African–Americans and significantly impacts warfarin dose requirements, it is especially important to consider the CYP2C9*8 allele in pharmacogenetic warfarin dosing for African–American patients [21].
Figure 4. Median (range) warfarin dose requirements in African–Americans with the CYP2C9*1/*1 genotype compared with CYP2C9*8 allele carriers and carriers of a CYP2C9*2, *3, *5, *6 or *11 allele.
Lines within boxes represent medians. Lower and upper borders of the boxes represent 25th and 75th percentiles, respectively. Whiskers below and above boxes represent 10th and 90th percentiles, respectively.
NS: Not significant.
Data taken from [21].
VKORC1 genotype
Many of the warfarin pharmacogenetic studies to date have focused on VKORC1 -1639G>A SNP, which distinguishes between haplotype groups A and B, as defined by Rieder et al. [14]. By compiling frequencies of haplotype groups found in several different studies (Table 2), we can see that, in general, African–American individuals have the highest prevalence of haplotype group B (high dose) and lowest prevalence of haplotype group A (low dose), while Asian individuals have the highest prevalence of haplotype group A [14,36]. While these haplotype groups capture 96 and 99% of the total VKORC1 haplotypes in Caucasians and Asians, respectively, they account for only 62–78% of the total VKORC1 haplotypes in African–Americans. This is due in part to the decrease in linkage disequilibrium and increase in the amount of variation found in populations of African ancestry. By focusing solely on the VKORC1 -1639G>A variant, there is a significant portion of the genetic variation in African–Americans, in terms of VKORC1 haplotype, that will not be captured.
Table 2.
Frequency of VKORC1 haplotype groups in African–American individuals compared with Caucasian and Asian individuals.
| Haplotype group | African–Americans (%) | Non-Hispanic Caucasians (%) | Asians (%) |
|---|---|---|---|
| A | 14–21 | 37–42 | 85–89 |
| B | 49–58 | 57–58 | 10–14 |
Novel CYP2C9 & VKORC1 variants
Because African–Americans were excluded from initial efforts to elucidate SNPs associated with warfarin response, our laboratory sought to identify a novel variation that affects warfarin dose in African–Americans through a targeted gene resequencing approach. Our recent findings highlighted a novel VKORC1 SNP in African–Americans that was associated with higher warfarin dose requirements in both a discovery (n = 122) and replication (n = 207) cohort [34]. This SNP (VKORC1-8191, rs61162043) was found approximately 8 kb upstream of the VKORC1 start site and was only identified via resequencing of the region in African–Americans. Furthermore, this SNP is not on the current 1 million or 2.5 million SNP arrays nor is it in strong linkage dis equilibrium with SNPs on these arrays. Therefore, conventional GWAS would not have identified this variant as associated with warfarin dose.
In addition to the VKORC1 variant, the authors also identified a novel CYP2C9 variant (rs7089580, intron 3) that was associated with higher dose requirements of warfarin [34]. The inclusion of these two SNPs in a regression model allowed us to explain 40% of the variability in warfarin dose in the combined (discovery plus replication) cohort of over 300 African–Americans. With the addition of sequence-level data, the authors will be closer to identifying novel ethnicity-specific variants GWAS and candidate gene investigations are underpowered or unable to capture.
Alternative genes associated with warfarin response
CYP4F2
The CYP4F2 enzyme is involved in the metabolism of vitamin K1. Increased vitamin K1 metabolism leads to reduced quantity of vitamin KH, available for clotting factor activation. The V433M SNP occurs in exon 2 of the CYP4F2 gene, with the 433M allele leading to lower CYP4F2 concentration and a reduced ability to metabolize vitamin K1 [62]. Consequently, individuals with the 433 M/M genotype will likely have greater vitamin K availability. The CYP4F2 V433M genotype has been associated with warfarin dose requirements in both Caucasian and Asian populations, with increased requirements with the 433 M allele [16,17,39]. The CYP4F2 genotype explains approximately 1–2% of the variability in warfarin dose requirements in these populations [17]. However, the CYP4F2 genotype was not associated with warfarin maintenance doses in African–Americans, possibly because of the low frequency of the 433M allele in this population (Table 1) [21].
CALU
Calumenin regulates γ-glutamyl carboxylation of vitamin K-dependent-clotting factors by inhibiting γ-carboxylase activity (Figure 1) [63]. As such, the CALU gene is a potential candidate for influencing warfarin pharmaco dynamics. With this in mind, Voora et al. resequenced the CALU gene and identified the rs339097 SNP, which was significantly more common in African–Americans compared with Caucasians (25% vs <1%) and was associated with warfarin dose requirements in the former population [37]. In particular, the minor G allele at this position was associated with 11% higher warfarin doses in African–Americans. A subsequent study in Egyptian individuals confirmed that the CALU variant increases warfarin dose requirements [64].
GGCX
Another potential candidate for influencing warfarin pharmacodynamics is the gene encoding γ-glutamyl carboxylase (GGCX) (Figure 1) The GGCX enzyme catalyzes the biosynthesis of vitamin K-dependent clotting factors by carboxy lating protein-bound glutamate residues. Rare GGCX mutations cause deficiencies in vitamin K-dependent clotting factors [65]. More common GGCX variants have been reported to influence warfarin dose variability; however, there are limited data with regards to this gene in African–Americans [66–68].
Our group recently found that the rs10654848 (CAA) microsatellite in intron 6 of the GGCX gene is associated with higher warfarin dose requirements in African–Americans [38]. Specifically, we identified 8–17 repeats of the CAA sequence among 338 warfarin-treated African–Americans. We found that 5% of African–Americans carried the (CAA)16 or 17 repeat, which was correlated with fourfold greater odds of needing a warfarin dose >7.5 mg/day. The GGCX (CAA)n genotype was also associated with warfarin dose variability, explaining 2% of the total variability in maintenance dose among African–Americans. In a comparative cohort of 183 Swedish warfarin-treated patients, only one patient (0.5%) had a (CAA)16 repeat, and none carried the 17 repeat. Interestingly, the Caucasian (CAA)16 repeat carrier also had the CYP2C9*1/*2 genotype and was taking amiodarone, both of which are expect to lower dose requirements. Yet, this patient required a warfarin maintenance dose of 11 mg/day. The higher prevalence of the (CAA)16 or 17 allele among African–Americans may contribute to the higher dose requirements observed in the African–American population. The mechanism underlying higher doses with the (CAA)16/17 repeat is unclear. However, one possibility is that a higher number of repeats enhances the activity of the enzyme, thus leading to greater clotting factor carboxylation and higher warfarin dose requirements.
Warfarin pharmacogenetics in Hispanic individuals
The Hispanic population is the largest and most rapidly growing minority population in the USA [103]. According to data from the US Census Bureau, there are approximately 43 million Hispanic individuals living in the USA, and this population is expected to increase more than twofold in the next 3–4 decades [103]. Despite this, most warfarin pharmacogenetic studies either excluded or enrolled a marginal percentage (<15%) of Hispanic individuals [7,11,14,69,70]. Data from the NHANES cohort demonstrate that VKORC1 alleles occur at similar frequencies in US Hispanic and non-Hispanic Caucasians [26]. However, whether VKORC1 predicts warfarin dose requirements in Hispanic individuals is not well studied.
To address this issue, our group assessed warfarin pharmacogenetics in a small inner-city US Hispanic population, mostly consisting of Mexican–Americans [33]. In addition to determining the combination of clinical and genetic factors that influence warfarin dose requirements, we tested the performance of published warfarin dosing algorithms that were derived from predominately European Caucasians, in this population. The VKORC1 -1639A, CYP2C9*2 and CYP2C9*3 alleles occurred at frequencies similar to those reported in Caucasian individuals. Compared with the VKORC1 -1639GG genotype, the median warfarin dose requirements were 30% lower with the AG genotype and 62% lower with AA genotype. Median dose requirements were 42% lower with the CYP2C9*2 or *3 allele compared with the CYP2C9*1/*1 genotype. Together with clinical factors, VKORC1 and CYP2C9 genotypes explained 56% of the interpatient variability in warfarin dose requirements in our Hispanic cohort, which is consistent with the contribution of these variables to dose requirements in non-Hispanic Caucasians [7,11,51]. Both the IWPC and Gage algorithms were predictive of warfarin dose requirements in Hispanic individuals, each explaining approximately 45% of the variance in dose. This is similar to the performance of these algorithms in non-Hispanic Caucasians [11,51,71]. These data suggest that similar factors affect warfarin dose requirements in Hispanic and non-Hispanic Caucasians and that dosing algorithms derived from non-Hispanic Caucasian cohorts are applicable to Hispanics of Mexican descent living in the USA.
Future directions in pharmacogenetic research
Novel approaches to examining gene–drug response associations
New methodology has been proposed to examine SNP association in the context of genome-wide approaches. One method that has been used in both disease association and chemotherapy response association is expression quantitative trait loci (eQTL) analysis. Such analysis involves both genome-wide SNP genotyping and gene-expression array data in the same set of individuals. This approach allows the investigator to identify a genetic variant associated with a phenotype of interest (through genome-wide SNP genotyping) as well as the gene(s) whose expression is affected by the variant (through gene-expression array data). By understanding which genes are affected by a particular variant, the investigator gains insight into the mechanism underlying the association between the variant and the outcome of interest. eQTL studies have led to novel SNP associations in chemotherapy sensitivity, which subsequently led to translatable clinical outcomes in ovarian cancer [72], as well as a publically available database to search for genotype/gene expression associations [73].
The primary drawback to these eQTL studies, particularly for applying eQTL methods to warfarin pharmacogenetic research, is that many of the studies were conducted in lympho blastic cell lines that are less than ideal for pharmacokinetic phenotypes. In particular, lymphoblastic cell lines do not express CYP450 drug metabolizing enzymes and thus, could not be used to interrogate the pharmaco genetics of warfarin metabolism. A recent publication highlighted findings in liver eQTL, which could have important implications in drug metabolism genetics [74]. There are no studies utilizing eQTL strategies for warfarin pharmacogenetics to date. Therefore, use of such would be a novel approach to examining the genetics of warfarin pharmacokinetics. However, since most of the liver eQTL studies have been conducted in predominantly Caucasian populations, the translation of these findings into other ethnicities has similar limitations as those seen in GWAS and genetic association studies discussed previously.
GWAS in African–Americans
The IWPC recently completed a GWAS in a relatively large cohort of African–Americans. Preliminary results were presented at the 2011 American Heart Association Scientific Sessions and are published in abstract form [75]. This study found novel variation in the CYP2C locus associated with warfarin dose requirements. Interestingly, this novel signal is not related to the known CYP2C9*2 and CYP2C9*3 variants that have been previously implicated as contributors to warfarin dose requirements.
Studies such as the GWAS in African–American individuals show that by studying non-Caucasian populations, one can reveal novel variation that cannot be found otherwise. However, the use of GWAS in African–American individuals also has its limitations. A recent publication by our laboratory interrogated the coverage of approximately 250 pharmacogenes (i.e., genes important in pharmacogenomics) on several different high-throughput genotyping platforms [76]. We also investigated the coverage of these pharmacogenes in the HapMap Project, which is commonly used as the reference set for SNP imputation in GWAS, compared with the 1000 Genomes Project data, which were generated through deeper sequencing and provide greater SNP coverage. The authors found that the HapMap data provided high overall coverage of pharmacogenes, with 84% of SNPs in 1000 Genomes found in the HapMap database. However, none of the genotyping platforms studied (which included the Illumina Omni 2.5 million array and the Axiom™ Genomic Database [Affymetrix, CA, USA], which contains 11 million SNPs) showed more than 85% coverage of SNPs in pharmacogenes when using 1000 Genomes data as the reference. In addition, many of the platforms studied did not reach maximum coverage of approximately 70–80% for SNPs with minor allele frequencies <20%.
These findings illustrate one of the limitations of genome-wide technologies. The implication of these findings is that SNPs with minor allele frequencies below 20% are not well represented on available platforms for genome wide analysis. When focusing on the CYP2C9 gene specifically, we found that the Axiom database showed the highest overall coverage of the platforms studied at 68%. These coverage estimates take into account linkage disequilibrium within and near the CYP2C9 gene and highlight that as much as 32% of the variation in this gene, which is important in warfarin dosing, would never be captured in a GWAS. Furthermore, the novel VKORC1 variant associated with higher warfarin dose requirements that we identified through resequencing in African–Americans (described previously) was not captured by any of the genotyping platforms evaluated, nor is it in HapMap [34]. Thus, without a sequenced-based methodology, the authors would never have uncovered this SNP.
Findings from the authors’ analysis of genotyping platforms and HapMap coverage also call into question the assumption that only very low-frequency alleles are absent in GWAS genotyping methods as they relate to pharmacogenes. While these studies were performed in a Caucasian population, we assume the coverage would be even less for populations such as African–Americans that carry greater amounts of genetic variation. Therefore, a comprehensive strategy, employing both GWAS and resequencing, may be required to fully evaluate GWAS, particularly for African–Americans.
Implementing warfarin pharmacogenetics
Genetic data are seldom used in most institutions to make warfarin dosing decisions. This is despite the substantial data supporting genetic determinants of warfarin dose requirements and the dosing tools available to assist with applying genetic data to dose prediction. Furthermore, at least five FDA-cleared platforms are available for genotype-guided dosing [77]. Guidelines from the American College of Chest Physicians and the American College of Medical Genetics actually recommend against routine use of genetic information to guide warfarin dosing [78,79]. In addition, few third party payers reimburse for genetic testing for warfarin management at this time; although the Centers for Medicare and Medicaid Services may provide coverage if testing is performed in the context of a controlled clinical study. A major reason that genotype-guided therapy is not better accepted is that many clinicians and policy makers require further evidence of the clinical utility of such testing [5,79].
Findings from two small trials and a comparative effectiveness study provide some evidence of the clinical utility of genotype-guided warfarin dosing [10,80,81]. The two trials demonstrated reduced time to reach stable warfarin dosing with a pharmacogenetic dosing approach [10,80]. In the comparative effectiveness study, patients who accepted free genotyping for the CYP2C9*2, CYP2C9*3 and VKORC1 -1639G>A variants, with results provided to their physician, had 30% fewer hospitalizations for any cause and for bleeding or thromboembolism during the 6 months following warfarin initiation compared with historical controls [81]. By contrast to these positive data, two other small trials found no benefit with pharmacogenetic dosing compared to clinical-based dosing in terms of percentage of out-of-range INR values [12,13]. However, an exploratory analysis of one trial demonstrated a significant benefit with pharmacogenetic dosing for patients with either multiple variant alleles or with no variant alleles compared with carriers of a single gene variant [12]. African–Americans were either excluded or only marginally represented in these trials. Overall, while studies provide some support with regards to the clinical utility of warfarin pharmacogenetics, the data are inconsistent and the utility of genotype-guided dosing in African–Americans in particular is unclear.
In order to more definitively address the clinical utility of pharmacogenetic warfarin dosing, several large clinical trials, including the National Heart, Lung and Blood Institute sponsored COAG trial, are addressing the issue. The COAG trial is a prospective, multicenter, double-blind trial, in which patients are randomized to a pharmacogenetic or clinical dosing strategy, with the outcome measures of percentage of time spent within the therapeutic INR range (primary outcome) and the occurrence of an INR >4 or serious event (secondary outcome) during the initial month of therapy [82] . Unlike previous trials, the COAG trial is powered to account for the potential lack of benefit with genotype-guided therapy in patients with a single variant. In addition, the COAG trial aims to enroll a significant proportion of minority patients in order to examine the utility of pharmacogenetic dosing across populations. The COAG trial is expected to be completed in March 2013.
An additional barrier to pharmacogenetic dosing for African–Americans is that African-specific variants, such as CYP2C9*8, CALU rs339097, the GGCX rs10654848 (CAA) microsatellite and the newly discovered CYP2C9 rs7089580 and VKORC1 rs61162043 alleles, are not included on FDA-cleared warfarin geno typing platforms. In addition, most pharmacogenetic algorithms do not include African-specific SNPs. An exception is the Gage algorithm, which is revised periodically as data become available. At the time of writing, the Gage algorithm includes the CYP2C9*5 and CYP2C9*6 alleles, but not the CYP2C9*8 allele.
Conclusion
African–Americans and Hispanics are significantly under-represented in pharmacogenetic studies with warfarin. There are important differences in genetic structure and genotype frequencies by race that may contribute to racial differences in warfarin pharmacogenetics. For US Hispanics, the limited data available suggest that warfarin pharmacogenetics in this population resembles that in non-Hispanic Caucasians, at least for Hispanics of Mexican descent. However, for African–Americans, the major variants influencing warfarin dose requirements in Caucasians provide lesser contribution to dose variability in African–Americans. In addition, there are variants occurring almost exclusively in persons of African descent that significantly impact warfarin dose requirements.
The most commonly used pharmacogenetic dosing algorithms were derived from predominately Caucasian populations, and while these appear to perform well in US Hispanics, their ability to predict warfarin maintenance dose is reduced in African–Americans [11,33,51]. African-specific variants that are predictive of warfarin dose requirements are not included in most dosing algorithms, the FDA-approved dosing table or on FDA-cleared genotyping platforms. Neglecting to account for these variants will likely result in lower accuracy of pharmacogenetic dosing algorithms in African–Americans. A recent model that incorporated two novel African-specific variants was able to explain 40% of the dose variability in African–Americans [34]. Refinement of this model to account for additional African-specific SNPs, such as the SNP recently identified in a GWAS, will likely further improve the utility of pharmacogenetic dosing for African–Americans [75]. Trials addressing the clinical utility of warfarin pharmacogenetics are ongoing. However, questions related to the utility of warfarin pharmacogenetics in African–Americans may remain after trial completion, given that many African-specific SNPs will not be accounted for. For pharmacogenetics to reach their full potential for persons of African ancestry, the data suggest that the African-specific variants should be accounted in dosing algorithms and genotyping efforts.
Future perspective
The Clinical Pharmacogenetics Implementation Consortium recently published guidelines on how to interpret and apply genetic test results to adjust warfarin doses. These guidelines do not address when to order a genetic tests but rather, how to dose warfarin when genetic test results are available. The guidelines strongly support the use of genetic information to guide warfarin dosing when genotype is known and recommend using either the IWPC or Gage algorithm to do so [83].
From a practical standpoint, when using genotype-guided therapy, an empiric dose of 5 mg (or lower if the patient is older or taking a CYP2C9 inhibitor) may initially be administered if genotyping results cannot be obtained prior to the first warfarin dose. Then, the Gage algorithm, which allows for integration of previous warfarin doses and INR values to refine subsequent dosing, may be used once genotype results are available. The Gage algorithm may be particularly useful for warfarin dosing in African–Americans since it is periodically updated to include variants associated with warfarin dose in various populations. The dosing table in the warfarin labeling is an alternative to the IWPC or Gage algorithm in the event that access to these algorithms is unavailable. However, data suggest that the dosing table may be less accurate at dose prediction than pharmacogenetic algorithms [84].
The ability to accurately predict dose requirements could improve time to stable warfarin dosing and potentially reduce the risks associated with sub- and supra-therapeutic anticoagulation early in the course of therapy. This would have particular implications for African–American individuals and Hispanics who are greater risk for adverse sequelae from suboptimal warfarin dosing than those of other racial or ethnic backgrounds [40–43].
Executive summary.
Genetic determinants of warfarin response
■ Substantial data show that the VKORC1 -1639G>A (or 1173C>T), CYP2C9*2 and CYP2C9*3 SNPs are the major genetic contributors to warfarin dose requirements in Caucasian and Asian individuals.
■ The combination of VKORC1 -1639G>A, CYP2C9*2, CYP2C9*3 and clinical factors (e.g., age, sex, weight and amiodarone use) explains 50–60% of the total variance in warfarin dose requirements in Caucasians, but only an estimated 25% in African–Americans.
Warfarin pharmacogenetic dosing tools
■ A US FDA-approved table and several algorithms are available to assist with genotype-guided warfarin dosing.
■ However, these dosing tools were derived largely from Caucasian populations and are less accurate in African–Americans.
Racial considerations in warfarin pharmacogenetics
■ African–American and Hispanic individuals are significantly under-represented in pharmacogenetic studies with warfarin.
■ There are important differences in genetic structure and genotype frequencies by race that may contribute to racial differences in warfarin pharmacogenetics.
Warfarin pharmacogenetics in African–American individuals
■ Variants in the CYP2C9, VKORC1, CALU and GGCX genes that occur almost exclusively in African–Americans have been significantly associated with warfarin dose requirements.
Warfarin pharmacogenetics in Hispanic individuals
■ Limited data in US Hispanics suggest that warfarin pharmacogenetics in this population resembles that in non-Hispanic Caucasians.
■ Pharmacogenetic dosing algorithms derived from predominantly Caucasian populations perform well in US Hispanics.
Future directions in pharmacogenetic research
■ The first genome-wide association study in African–Americans was recently completed and preliminary data show a novel association with warfarin response at the CYP2C locus.
■ New methodology to examine single-nucleotide polymorphisms associations with drug response in the context of genome-wide approaches, such as use of expression quantitative trait loci, could lead to novel insight into the mechanism underlying gene–warfarin response associations.
Implementing warfarin pharmacogenetics
■ The Clinical Pharmacogenetics Implementation Consortium recently provided guidelines for using genetic information to guide warfarin dosing.
■ However, barriers to clinical implementation of warfarin pharmacogenetics remain and include uncertainty as to its clinical utility.
■ An additional barrier for African–Americans is that US FDA-cleared genotyping platforms and most pharmacogenetic dosing algorithms do not include African-specific variants.
■ An ongoing clinical trial, which aims to enroll a significant number of ethnic minorities, will provide data on the clinical utility of genotype-guided warfarin dosing.
Conclusion
■ For pharmacogenetics to reach its full potential for persons of African ancestry, the data suggest that genotype-guided warfarin dosing should account for African-specific variants.
Acknowledgments
LH Cavallari has received an American Heart Association, Midwest Affiliate Grant-In-Aid (10GRNT3750024) and MA Perera has received NIH NHLBI awards (1 K23 HL089808-01A2 and HL106097). LH Cavallari is the coinvestor on the patent, CYP2C9*8 alleles correlate with decreased warfarin metabolism and increased warfarin sensitivity. US Utility Patent Application No 12/572,908. International Application No. PCT/US09/59413. Filed: 2 October 2009; Published: 27 May 2010; Pub. No. US 2010/0130599.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with a trial fibrillation. Circulation. 2007;115(21):2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 2.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N. Engl. J. Med. 2003;349(11):1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 3.Wadelius M, Chen LY, Lindh JD, et al. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009;113(4):784–792. doi: 10.1182/blood-2008-04-149070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 2011;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 5.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition). Chest. 2008;133(Suppl. 6):S160–S198. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 6.Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation. 2003;107(12):1692–1711. doi: 10.1161/01.CIR.0000063575.17904.4E. [DOI] [PubMed] [Google Scholar]
- 7.Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N. Engl. J. Med. 2009;360(8):753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Absher RK, Moore ME, Parker MH. Patient-specific factors predictive of warfarin dosage requirements. Ann. Pharmacother. 2002;36(10):1512–1517. doi: 10.1345/aph.1C025. [DOI] [PubMed] [Google Scholar]
- 9.Limdi NA, Limdi MA, Cavallari L, et al. Warfarin dosing in patients with impaired kidney function. Am. J. Kidney Dis. 2010;56(5):823–831. doi: 10.1053/j.ajkd.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang SW, Chen HS, Wang XQ, et al. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: a prospective study in Chinese patients. Pharmacogenet. Genomics. 2009;19(3):226–234. doi: 10.1097/FPC.0b013e328326e0c7. [DOI] [PubMed] [Google Scholar]
- 11.Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin. Pharmacol. Ther. 2008;84(3):326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson JL, Horne BD, Stevens SM, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116(22):2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 13.Burmester JK, Berg RL, Yale SH, et al. A randomized controlled trial of genotype-based coumadin initiation. Genet. Med. 2011;13(6):509–518. doi: 10.1097/GIM.0b013e31820ad77d. [DOI] [PubMed] [Google Scholar]
- 14.Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005;352(22):2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 15.Cooper GM, Johnson JA, Langaee TY, et al. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood. 2008;112(4):1022–1027. doi: 10.1182/blood-2008-01-134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cha PC, Mushiroda T, Takahashi A, et al. Genome-wide association study identifies genetic determinants of warfarin responsiveness for Japanese. Hum. Mol. Genet. 2011;19(23):4735–4744. doi: 10.1093/hmg/ddq389. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi F, McGinnis R, Bourgeois S, et al. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5(3):E1000433. doi: 10.1371/journal.pgen.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limdi NA, Arnett DK, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 on warfarin dose, anticoagulation attainment and maintenance among European–Americans and African–Americans. Pharmacogenomics. 2008;9(5):511–526. doi: 10.2217/14622416.9.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scordo MG, Pengo V, Spina E, et al. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin. Pharmacol. Ther. 2002;72(6):702–710. doi: 10.1067/mcp.2002.129321. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi H, Kashima T, Nomizo Y, et al. Metabolism of warfarin enantiomers in Japanese patients with heart disease having different CYP2C9 and CYP2C19 genotypes. Clin. Pharmacol. Ther. 1998;63(5):519–528. doi: 10.1016/S0009-9236(98)90103-5. [DOI] [PubMed] [Google Scholar]
- 21■.Cavallari LH, Langaee TY, Momary KM, et al. Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin. Pharmacol. Ther. 2010;87(4):459–464. doi: 10.1038/clpt.2009.223. [Demonstrates significantly lower warfarin dose requirements in African–Americans with the CYP2C9*8 allele.] [DOI] [PubMed] [Google Scholar]
- 22■.Limdi NA, McGwin G, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African–American and European–American patients on warfarin. Clin. Pharmacol. Ther. 2007;83(2):312–321. doi: 10.1038/sj.clpt.6100290. [One of the largest studies evaluating bleeding risk conferred by the CYP2C9 and VKORC1 genotypes in both African–Americans and Caucasians. The investigators found that CYP2C9, but not VKORC1, influences bleeding risk.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rost S, Fregin A, Ivaskevicius V, et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427(6974):537–541. doi: 10.1038/nature02214. [DOI] [PubMed] [Google Scholar]
- 24.D'Andrea G, D'Ambrosio RL, Di Perna P, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105(2):645–649. doi: 10.1182/blood-2004-06-2111. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Chen H, Momary KM, et al. Regulatory polymorphism in vitamin K epoxide reductase complex subunit 1 (VKORC1) affects gene expression and warfarin dose requirement. Blood. 2008;112(4):1013–1021. doi: 10.1182/blood-2008-03-144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26■■.Limdi NA, Wadelius M, Cavallari L, et al. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood. 2010;115(18):3827–3834. doi: 10.1182/blood-2009-12-255992. [Showed that the VKORC1 -1639G>A (or 1173C>T) single-nucleotide polymorphisms is predictive of warfarin dose requirements across racial groups, but provides lesser contributions to warfarin dose variability in African–Americans compared with Caucasians or Asians, largely because of its lower frequency in the former racial group.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coumadin®, package insert. Bristol-Myers Squibb; Princeton, NJ, USA: [Google Scholar]
- 28.Gage BF, Eby C, Milligan PE, et al. Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thromb. Haemost. 2004;91(1):87–94. doi: 10.1160/TH03-06-0379. [DOI] [PubMed] [Google Scholar]
- 29.Lenzini P, Wadelius M, Kimmel S, et al. Integration of genetic, clinical, and INR data to refine warfarin dosing. Clin. Pharmacol. Ther. 2010;87(5):572–578. doi: 10.1038/clpt.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horne BD, Lenzini PA, Wadelius M, et al. Pharmacogenetic warfarin dose refinements remain significantly influenced by genetic factors after one week of therapy. Thromb. Haemost. 2012;107(2):232–240. doi: 10.1160/TH11-06-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353(9154):717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 32.Lindh JD, Holm L, Andersson ML, Rane A. Influence of CYP2C9 genotype on warfarin dose requirements – a systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2009;65(4):365–375. doi: 10.1007/s00228-008-0584-5. [DOI] [PubMed] [Google Scholar]
- 33■.Cavallari LH, Momary KM, Patel SR, et al. Pharmacogenomics of warfarin dose requirements in Hispanics. Blood Cells Mol. Dis. 2011;46(2):147–150. doi: 10.1016/j.bcmd.2010.11.005. [One of the only studies to examine warfarin pharmacogenetics in US Hispanics.] [DOI] [PubMed] [Google Scholar]
- 34■.Perera MA, Gamazon E, Cavallari LH, et al. The missing association: sequencing-based discovery of novel SNPs in VKORC1 and CYP2C9 that affect warfarin dose in African Americans. Clin. Pharmacol. Ther. 2011;89(3):408–415. doi: 10.1038/clpt.2010.322. [The investigators identified novel CYP2C9 and VKORC1 variants associated with warfarin dose requirements in African–Americans through a targeted resequencing approach.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limdi NA, Beasley TM, Crowley MR, et al. VKORC1 polymorphisms, haplotypes and haplotype groups on warfarin dose among African–Americans and European–Americans. Pharmacogenomics. 2008;9(10):1445–1458. doi: 10.2217/14622416.9.10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh S, King CR, Porche-Sorbet RM, Scott-Horton TJ, Eby CS. Population variation in VKORC1 haplotype structure. J. Thromb. Haemost. 2006;4(2):473–474. doi: 10.1111/j.1538-7836.2006.01759.x. [DOI] [PubMed] [Google Scholar]
- 37.Voora D, Koboldt DC, King CR, et al. A polymorphism in the VKORC1 regulator calumenin predicts higher warfarin dose requirements in African Americans. Clin. Pharmacol. Ther. 2010;87(4):445–451. doi: 10.1038/clpt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavallari LH, Perera M, Wadelius M, et al. Association of the GGCX (CAA)16/17 repeat polymorphism with higher warfarin dose requirements in African Americans. Pharmacogenet. Genomics. 2012;22(2):152–158. doi: 10.1097/FPC.0b013e32834f288f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caldwell MD, Awad T, Johnson JA, et al. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008;111(8):4106–4112. doi: 10.1182/blood-2007-11-122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider D, Lilienfeld DE, Im W. The epidemiology of pulmonary embolism: racial contrasts in incidence and in-hospital case fatality. J. Natl Med. Assoc. 2006;98(12):1967–1972. [PMC free article] [PubMed] [Google Scholar]
- 41.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation. 2011;123(4):E18–E209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White RH, Dager WE, Zhou H, Murin S. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb. Haemost. 2006;96(3):267–273. doi: 10.1160/TH06-07-0365. [DOI] [PubMed] [Google Scholar]
- 43.Morgenstern LB, Smith MA, Lisabeth LD, et al. Excess stroke in Mexican Americans compared with non-Hispanic Whites: the brain attack surveillance in corpus christi project. Am. J. Epidemiol. 2004;160(4):376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lisabeth LD, Smith MA, Sanchez BN, Brown DL. Ethnic disparities in stroke and hypertension among women: the BASIC project. Am. J. Hypertens. 2008;21(7):778–783. doi: 10.1038/ajh.2008.161. [DOI] [PubMed] [Google Scholar]
- 45.Lisabeth LD, Risser JM, Brown DL, et al. Stroke burden in Mexican Americans: the impact of mortality following stroke. Ann. Epidemiol. 2006;16(1):33–40. doi: 10.1016/j.annepidem.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 46.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb. Res. 2009;123(Suppl. 4):S11–S17. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 47.Price AL, Weale ME, Patterson N, et al. Long-range LD can confound genome scans in admixed populations. Am. J. Hum. Genet. 83(1):132–135. doi: 10.1016/j.ajhg.2008.06.005. author reply 135–139 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caldwell MD, Berg RL, Zhang KQ, et al. Evaluation of genetic factors for warfarin dose prediction. Clin. Med. Res. 2007;5(1):8–16. doi: 10.3121/cmr.2007.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Momary KM, Shapiro NL, Viana MA, et al. Factors influencing warfarin dose requirements in African–Americans. Pharmacogenomics. 2007;8(11):1535–1544. doi: 10.2217/14622416.8.11.1535. [DOI] [PubMed] [Google Scholar]
- 50.Millican E, Jacobsen-Lenzini PA, Milligan PE, et al. Genetic-based dosing in orthopaedic patients beginning warfarin therapy. Blood. 2007;110(5):1511–1515. doi: 10.1182/blood-2007-01-069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schelleman H, Chen J, Chen Z, et al. Dosing algorithms to predict warfarin maintenance dose in Caucasians and African Americans. Clin. Pharmacol. Ther. 2008;84(3):332–339. doi: 10.1038/clpt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gan GG, Phipps ME, Lee MM, et al. Contribution of VKORC1 and CYP2C9 polymorphisms in the interethnic variability of warfarin dose in Malaysian populations. Ann. Hematol. 2011;90(6):635–641. doi: 10.1007/s00277-010-1119-6. [DOI] [PubMed] [Google Scholar]
- 53.Zhao F, Loke C, Rankin SC, et al. Novel CYP2C9 genetic variants in Asian subjects and their influence on maintenance warfarin dose. Clin. Pharmacol. Ther. 2004;76(3):210–219. doi: 10.1016/j.clpt.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Yu HC, Chan TY, Critchley JA, Woo KS. Factors determining the maintenance dose of warfarin in Chinese patients. QJM. 1996;89(2):127–135. doi: 10.1093/qjmed/89.2.127. [DOI] [PubMed] [Google Scholar]
- 55.Tishkoff SA, Dietzsch E, Speed W, et al. Global patterns of linkage disequilibrium at the CD4 locus and modern human origins. Science. 1996;271(5254):1380–1387. doi: 10.1126/science.271.5254.1380. [DOI] [PubMed] [Google Scholar]
- 56.Dickmann LJ, Rettie AE, Kneller MB, et al. Identification and functional characterization of a new CYP2C9 variant (CYP2C9*5) expressed among African Americans. Mol. Pharmacol. 2001;60(2):382–387. doi: 10.1124/mol.60.2.382. [DOI] [PubMed] [Google Scholar]
- 57.Allabi AC, Gala JL, Horsmans Y. CYP2C9, CYP2C19, ABCB1 (MDR1) genetic polymorphisms and phenytoin metabolism in a Black Beninese population. Pharmacogenet. Genomics. 2005;15(11):779–786. doi: 10.1097/01.fpc.0000174787.92861.91. [DOI] [PubMed] [Google Scholar]
- 58.Blaisdell J, Jorge-Nebert LF, Coulter S, et al. Discovery of new potentially defective alleles of human CYP2C9. Pharmacogenetics. 2004;14(8):527–537. doi: 10.1097/01.fpc.0000114759.08559.51. [DOI] [PubMed] [Google Scholar]
- 59.Allabi AC, Gala JL, Horsmans Y, et al. Functional impact of CYP2C95, CYP2C96, CYP2C98, and CYP2C911 in vivo among black Africans. Clin. Pharmacol. Ther. 2004;76(2):113–118. doi: 10.1016/j.clpt.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Lui Y, Hyun-Young J, Takahashi H, et al. Decreased warfarin clearance with the CYP2C9 R150H (*8) polymorphism. Clin. Pharmacol. Ther. 2012;91(4):660–665. doi: 10.1038/clpt.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell C, Gregersen N, Krause A. Novel CYP2C9 and VKORC1 gene variants associated with warfarin dosage variability in the South African black population. Pharmacogenomics. 2011;12(7):953–963. doi: 10.2217/pgs.11.36. [DOI] [PubMed] [Google Scholar]
- 62.McDonald MG, Rieder MJ, Nakano M, Hsia CH, Rettie AE. CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin cose in carriers of the V433M variant. Mol. Pharmacol. 2009;75(6):1337–1346. doi: 10.1124/mol.109.054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wajih N, Sane DC, Hutson SM, Wallin R. The inhibitory effect of calumenin on the vitamin K-dependent γ-carboxylation system. Characterization of the system in normal and warfarin-resistant rats. J. Biol. Chem. 2004;279(24):25276–25283. doi: 10.1074/jbc.M401645200. [DOI] [PubMed] [Google Scholar]
- 64.Shahin MH, Khalifa SI, Gong Y, et al. Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet. Genomics. 2011;21(3):130–135. doi: 10.1097/FPC.0b013e3283436b86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rost S, Fregin A, Koch D, et al. Compound heterozygous mutations in the γ-glutamyl carboxylase gene cause combined deficiency of all vitamin K-dependent blood coagulation factors. Br. J. Haematol. 2004;126(4):546–549. doi: 10.1111/j.1365-2141.2004.05071.x. [DOI] [PubMed] [Google Scholar]
- 66.Kimura R, Miyashita K, Kokubo Y, et al. Genotypes of vitamin K epoxide reductase, γ-glutamyl carboxylase, and cytochrome P450 2C9 as determinants of daily warfarin dose in Japanese patients. Thromb. Res. 2007;120(2):181–186. doi: 10.1016/j.thromres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Chen LY, Eriksson N, Gwilliam R, et al. Gamma-glutamyl carboxylase (GGCX) microsatellite and warfarin dosing. Blood. 2005;106(10):3673–3674. doi: 10.1182/blood-2005-04-1711. [DOI] [PubMed] [Google Scholar]
- 68.King CR, Deych E, Milligan P, et al. γ-glutamyl carboxylase and its influence on warfarin dose. Thromb. Haemost. 2010;104(4):750–754. doi: 10.1160/TH09-11-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwarz UI, Ritchie MD, Bradford Y, et al. Genetic determinants of response to warfarin during initial anticoagulation. N. Engl. J. Med. 2008;358(10):999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu AH, Wang P, Smith A, et al. Dosing algorithm for warfarin using CYP2C9 and VKORC1 genotyping from a multi-ethnic population: comparison with other equations. Pharmacogenomics. 2008;9(2):169–178. doi: 10.2217/14622416.9.2.169. [DOI] [PubMed] [Google Scholar]
- 71.Lubitz SA, Scott SA, Rothlauf EB, et al. Comparative performance of gene-based warfarin dosing algorithms in a multiethnic population. J. Thromb. Haemost. 2010;8(5):1018–1026. doi: 10.1111/j.1538-7836.2010.03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang RS, Johnatty SE, Gamazon ER, et al. Platinum sensitivity-related germline polymorphism discovered via a cell-based approach and analysis of its association with outcome in ovarian cancer patients. Clin. Cancer. Res. 2011;17(16):5490–5500. doi: 10.1158/1078-0432.CCR-11-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gamazon ER, Zhang W, Konkashbaev A, et al. SCAN: SNP and copy number annotation. Bioinformatics. 2010;26(2):259–262. doi: 10.1093/bioinformatics/btp644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Innocenti F, Cooper GM, Stanaway IB, et al. Identification, replication, and functional fine-mapping of expression quantitative trait loci in primary human liver tissue. PLoS Genet. 2011;7(5):E1002078. doi: 10.1371/journal.pgen.1002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perera MA, Limdi NA, Cavallari L, et al. Novel SNPs associated with warfarin dose in a large multicenter cohort of African Americans: Genome wide association study and replication results. Circulation. 2011;124(Suppl. 1):15518. [Google Scholar]
- 76.Gamazon ER, Skol AD, Perera MA. The limits of genome-wide methods for pharmacogenomics. Pharmacogenet. Genomics. 2012;22(4):261–272. doi: 10.1097/FPC.0b013e328350ca5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cavallari LH, Shin J, Perera MA. Role of pharmacogenomics in the management of traditional and novel oral anticoagulants. Pharmacotherapy. 2011;31(12):1192–1207. doi: 10.1592/phco.31.12.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schunemann HJ. Executive summary: antithrombotic therapy and prevention of thrombosis (9th Edition). American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(Suppl. 2):S7–S47. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flockhart DA, O'Kane D, Williams MS, et al. Pharmacogenetic testing of CYP2C9 and VKORC1 alleles for warfarin. Genet. Med. 2008;10(2):139–150. doi: 10.1097/GIM.0b013e318163c35f. [DOI] [PubMed] [Google Scholar]
- 80.Caraco Y, Blotnick S, Muszkat M. CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clin. Pharmacol. Ther. 2008;83(3):460–470. doi: 10.1038/sj.clpt.6100316. [DOI] [PubMed] [Google Scholar]
- 81■.Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo warfarin effectiveness study). J. Am. Coll. Cardiol. 2010;55(25):2804–2812. doi: 10.1016/j.jacc.2010.03.009. [This comparative effectiveness study demonstrates a reduction in overall hospitalization and hospitalization for bleeding or thrombosis with genotype-guided warfarin therapy.] [DOI] [PubMed] [Google Scholar]
- 82.French B, Joo J, Geller NL, et al. Statistical design of personalized medicine interventions: the Clarification of Optimal Anticoagulation through Genetics (COAG) trial. Trials. 2010;11:108. doi: 10.1186/1745-6215-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83■■.Johnson JA, Gong L, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin. Pharmacol. Ther. 2011;90(4):625–629. doi: 10.1038/clpt.2011.185. [An expert consensus panel provides recommendations for applying genetic information to guide warfarin dosing.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finkelman BS, Gage BF, Johnson JA, Brensinger CM, Kimmel SE. Genetic warfarin dosing: tables versus algorithms. J. Am. Coll. Cardiol. 2011;57(5):612–618. doi: 10.1016/j.jacc.2010.08.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Warfarin dosing. www.warfarindosing.org.
- 102.Database of single nucleotide polymorphisms. National Center for Biotechnology Information, National Library of Medicine. dbSNP accession; Bethesda (MD): www.ncbi.nlm.nih.gov/snp. ss5586420, ss52052050, ss76884483, ss105439387, ss105440151, ss13761958, ss10622649, ss2494699, ss3027906 (dbSNP Build ID:132).
- 103.US Census Bureau 1970, 1980, and 2000 decennial censuses: population projections, 1 July 2010–1 July 2050. www.census.gov.