Abstract
Styrene oxide-cysteine adduction predominantly involves in protein covalent modification undergoing in vivo after exposed to styrene or styrene oxide. In present study, we developed an alkaline permethylation- and GC/MS-based approach to detect styrene oxide-derived protein adduction. Permethylation of the protein adducts produced two methylthiophenylethanols, namely 2-methylthio-2-phenyl-1-ethanol and 2-methylthio-1-phenyl-1-ethanol. To improve the permethylation efficiency, reaction conditions, including temperature, time, NaOH strength and molar ratio of CH3I/NaOH, were explored. Under the optimized condition, the yields of the analyte formation resulting from permethylation of authentic standard α- and β-mercapturic acids, representing α and β isomers of cysteine adducts, were 35% and 28%, respectively. Permethylation of styrene oxide-modified bovine serum albumin released the two methylthiophenylethanols with an α-/β-adduction ratio of 1.5. A concentration-dependent increase in both α- and β-adduction was observed in mouse liver microsomes incubated with styrene at various concentrations. CD-1 mice were administered intraperitoneally with styrene at doses of 0, 50 and 400 mg/kg daily for 5 days. The formation of protein adducts derived from styrene oxide in whole blood in 400 mg/kg group was observed with an α/β ratio of 4.8, suggesting the reaction of styrene oxide with cysteine residues took place more likely at α-carbon than β-carbon of styrene oxide.
Keywords: Alkaline permethylation, styrene, styrene oxide, styrene oxide-cysteine adduct, protein adduct
Introduction
Styrene is an important industrial chemical widely used in the production of plastics, synthetic rubbers, and polyester resins [1, 2]. It is also a common environmental contaminant, arising from industrial emission, engine exhaust, tobacco smoke, and natural source, and occurring in the foods and drinking water that were stored in polystyrene containers [3, 4]. Once absorbed into the body, styrene is exclusively activated via cytochrome P450 enzymes to reactive metabolite styrene-7,8-oxide (Scheme 1) [5, 6], which also is an industrial intermediate used in the production of epoxy resin and as a precursor for agrochemicals, cosmetics, and fibers. Current evidence has shown that styrene oxide can react with nucleophilic sites in DNA and proteins to form stable covalent adducts [7], possibly triggering various toxicities [5, 8, 9]. Considering the potential health risk of human exposure to styrene and styrene oxide, International Agency for Research on Cancer (IARC) has classified styrene as a possible human carcinogen (group 2B), and styrene oxide as a probable human carcinogen (group 2A).
Scheme 1.

Bioactivation of styrene and protein adduction derived from styrene oxide.
In risk assessment of occupational exposure to styrene and styrene oxide, several DNA and protein adducts have been adopted as styrene-specific biomarkers to monitor the exposure level and assess the cancer and cytogenetic damage [5, 10]. Since the protein adducts are stable, present in much larger amount than DNA, persisted over a relative long time, and are not removed by DNA repair, qualitative and quantitative determination of the protein adducts in blood and target organs can facilitate the understanding of styrene toxicity [11-13]. Previous studies have shown that styrene oxide alkylates a variety of nucleophilic sites in proteins, including cysteine sulfhydryl, histidine imidazole, lysine amino, aspartic and glutamic carboxylic groups, and the N-terminal valine [7, 14]. Among these amino acids, cysteine has been reported to have the highest reactivity towards styrene oxide when incubating styrene oxide with polyamino acids in vitro [15]. Therefore, determination of accumulated styrene oxide-cysteine protein adducts in blood provides a means for assessing time- and dose-integrated toxicity after exposure to styrene and styrene oxide.
Polyclonal antibodies to detect protein adduction derived from styrene oxide were developed in our laboratory [16, 17]. The antibody approach shares the advantage of its application in immunoblot, immuno-histologic staining and immuno-affinity chromatography, but it lacks offering quantitative assessment of protein adduction. The major approach reported for qualitative and quantitative measurement of styrene oxide-cysteine protein adducts includes Raney nickel reaction which detaches styrene oxide-cysteine adducts from protein by cleavage of the carbon-sulfur bond between the alkyl group and cysteine-S, and releases the styrene oxide moiety in the form of 1-phenylethanol and 2-phenylethanol. This method has been applied on hemoglobin and albumin samples from animals and humans exposed in vivo to styrene and styrene oxide [18-20]. However, this approach has its serious problem due to high background levels of both phenylethanols observed in control subjects [20].
Alkaline permethylation is an alternative approach to detect covalent adducts to protein sulfur nucleophiles, which is based on base-induced cleavage of the carbonsulfur bond between the alkyl group and cysteine-S. This method has been successfully employed in identification of bromobenzene-derived adducts to sulfur nucleophiles in liver proteins [21, 22]. In present study, we developed alkaline permethylation approach to detect styrene oxide-cysteine adducts from in vitro and in vivo protein samples. This permethylation approach allows us to overcome the problem of high background that Raney nickel reaction suffers.
Materials and Methods
Chemicals
Styrene (99.9%), styrene oxide (97%), 2-bromoacetophenone (98%), bromophenylacetic acid, α-bromophenylacetic acid, N-acetyl-L-cysteine, trifluoroacetic acid, sodium borohydride, sodium thiomethoxide, sodium hydrosulfide (NaHS), acetophenone-α,β-13C2 (99 atom % 13C), bromophenylacetic acid-2’,3’,4’,5’,6’-d5, sodium borohydride-d4, iodomethane, iodomethane-d3 (CD3I), bovine serum albumin (BSA), NADPH, protease XIV and heparin were purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI). N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) was from Supelco (Bellefonte, PA). Acetone, diethylether, ammonium chloride, sulfuric acid, anhydrous Na2SO4, hydrochloric acid, anhydrous methanol, anhydrous tetrahydrofuran (THF), DMSO, sodium hydroxide, silica gel, HPLC-grade acetonitrile and ethyl acetate were from EMD Biosciences (La Jolla, CA). Deionized water was prepared by Millipore MilliQ water purification system (Billerica, MA).
Chemical synthesis
2-Acetamido-3-(2-hydroxy-1-phenylethylthio)propanoic acid (α-mercapturic acid)
Bromophenylacetic acid (215 mg, 1.0 mmol) was dissolved in anhydrous methanol (15 ml), and concentrated H2SO4 (0.5 ml) was added slowly while stirring. The reaction mixture was refluxed for 2 hr and then neutralized with 2 N NaOH. After most of methanol was removed under reduced pressure, the aqueous phase was extracted with ethyl acetate for three times. The combined organic phases were washed, dried over anhydrous Na2SO4, and evaporated to dryness. The residue was purified by silica gel chromatography to give the corresponding ester, methyl bromophenylacetate (176 mg, 77%). Methyl bromophenylacetate (176 mg, 0.77 mmol) and N-acetyl-L-cysteine (130 mg, 0.80 mmol) were dissolved in methanol (10 ml). Then the reaction mixture was adjusted to pH 9-10 with 1 N NaOH and stirred at room temperature for 2 hr. After the completion of the reaction, solvent was removed under reduced pressure. The residue was acidified with 0.1 N H2SO4 to pH 2, and extracted with ethyl acetate. The combined organic phases were washed, dried over anhydrous Na2SO4, and evaporated to dryness in vacuum. The residue was chromatographed in a silica gel column to give the desired compound, 2-acetamido-3-(2-methoxy-2-oxo-1-phenylethylthio)propanoic acid (213 mg, 89%). Finely powdered sodium borohydride (115 mg, 3mmol) was suspended in anhydrous THF (10 ml) in the presence of 2-acetamido-3-(2-methoxy-2-oxo-1-phenylethylthio)propanoic acid (155 mg, 0.5 mmol) during a period of 5 min under reflux and stirring. Methanol (1.25 ml) was added dropwise in 5 min with effervescence being observed. Stirring and reflux were maintained for 1 hr, then the reaction was cooled to room temperature and quenched with saturated NH4Cl (5 ml) with stirring for 10 min. The organic layer was separated and the aqueous layer was acidified and extracted with ethyl acetate. The organic phases were combined, washed, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel chromatography to give the final compound (81 mg, 57%). H-NMR (CDCl3, 300MHz) δ 7.40-7.31 (m, 5H), 4.88-4.72 (m, 1H), 4.07-4.00 (m, 1H), 3.95-3.82 (m, 2H), 3.06-2.97 (m, 2H), 2.00 (s, 3H).
2-Acetamido-3-(2-hydroxy-2-phenylethylthio)propanoic acid (β-mercapturic acid)
2-Bromoacetophenone (199 mg, 1.0 mmol) was dissolved in methanol (10 ml), and N-acetyl-L-cysteine (180 mg, 1.1 mmol) was added while stirring. The reaction mixture was adjusted to pH 9-10 with 0.1 N NaOH, and stirred at room temperature for 3 hr. Then the mixture was diluted with water (30 ml), acidified with 1 N HCl to pH 2, and extracted with ethyl acetate. The organic phases were combined, washed, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was used in the next step without further purification. To a solution of the above crude product (225 mg, 0.8 mmol) cooled to 0 °C was added sodium borohydride (38 mg, 1.0 mmol) in several portions. The reaction mixture was stirred at room temperature for 1 hr and quenched with 1 N H2SO4. After removal of methanol, the aqueous phase was extracted with ethyl acetate. The combined organic phases were washed, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel chromatography and high performance liquid chromatography (HPLC) to give the desired compound (178 mg, 79%). H-NMR (D2O, 300MHz) δ 7.37-7.31 (m, 5H), 4.83-4.77 (m, 1H), 4.48-4.42 (m, 1H), 2.94-2.92 (m, 2H), 2.89-2.72 (m, 2H), 1.95 (s, 3H).
2-Methylthio-2-phenyl-1-ethanol (2-methylthio-2-PE)
α-Bromophenylacetic acid (430 mg, 2.0 mmol) and sodium thiomethoxide (141 mg, 2.0 mmol) were mixed in anhydrous methanol (10 ml). The mixture was stirred at room temperature for 4 hr and then acidified with 2 N HCl to pH 2. After two thirds of the solvent was removed under reduced pressure, the residue was extracted with ethyl acetate. The combined organic phases were washed, dried over anhydrous Na2SO4, and evaporated in vacuum to afford the crude product, α-(methylthio)phenylacetic acid, subjected to next step reaction without further purification. α-(Methylthio)phenylacetic acid (364 mg, 2.0 mmol) was dissolved in anhydrous methanol (10 ml), and concentrated H2SO4 (490 mg, 5.0 mmol) was added slowly while stirring. The mixture was refluxed for 3 hr and then neutralized with 2 N NaOH. After removal of methanol, the aqueous phase was extracted with ethyl acetate. The combined organic phases were washed, dried over anhydrous Na2SO4, and evaporated to dryness. The residue was purified by silica gel chromatography to give the desired compound, methyl α-(methylthio)phenylacetate (357 mg, 91%). Sodium borohydride powder (227 mg, 6.0 mmol) was suspended in anhydrous THF (20 ml) in the presence of methyl α-(methylthio)phenylacetate (196 mg, 1.0 mmol) during a period of 5 min under reflux and stirring. Methanol (2 ml) was added dropwise in 5 min with effervescence being observed. Stirring and reflux were maintained for 1 hr, and the reaction was cooled to room temperature and quenched with saturated NH4Cl (10 ml). The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The organic phases were combined, washed, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel chromatography to give the final compound (109 mg, 65%). H-NMR (CDCl3, 300MHz) δ 7.37-7.28 (m, 5H), 3.93-3.91 (m, 3H), 2.03-1.99 (s, 4H).
2-Methylthio-1-phenyl-1-ethanol (2-methylthio-1-PE)
To a solution of 2-bromoacetophenone (398 mg, 2.0 mmol) in methanol (10 ml) was added sodium thiomethoxide (140 mg, 2.0 mmol) in a hood. The reaction mixture was stirred at room temperature for 2 hr. Most of solvent was removed under reduced pressure, and the residue was extracted with ethyl acetate. The combined organic phases were washed, dried over anhydrous Na2SO4, and evaporated to dryness in vacuum. Silica gel chromatography of the residue gave pure 2-(methylthio)acetophenone (323 mg, 97.4%). 2-(Methylthio)acetophenone (166 mg, 1.0 mmol) was dissolved in methanol (10 ml) and cooled to 0-5 °C with water/ice bath. Sodium borohydride (38 mg, 1.0 mmol) was then added in several portions. The reaction mixture was stirred at 0 °C for 2 hr and quenched with 1 N H2SO4. Most of methanol was removed under reduced pressure, and the resulting aqueous residue was extracted with ethyl acetate. The combined organic phases were washed, dried over anhydrous Na2SO4, and evaporated to dryness in vacuum. The residue was purified by silica gel chromatography to offer the desired compound (153 mg, 91.1%). H-NMR (CDCl3, 300MHz) δ 7.42-7.28 (m, 5H), 4.82-4.77 (dt, J = 3Hz, J = 9Hz, 1H), 2.97 (d, J = 3Hz, 1H), 2.90 (dd, J = 3Hz, J = 14Hz, 1H), 2.75 (dd, J = 9Hz, J =14Hz), 2.15 (s, 3H).
2-Methylthio-d3-1-phenyl-1-ethanol (IS-d3)
2-Bromoacetophenone (199 mg, 1.0 mmol) was dissolved in methanol (10 ml), and finely ground NaHS (168 mg, 3.0 mmol) in water was added. The reaction mixture was adjusted to pH 9-10 with 0.1 N NaOH, and stirred at room temperature for 2 hr. After completion of the reaction, sodium borohydride (76 mg, 2.0 mmol) was added. The reduction was carried out for 1 hr while stirring, followed by addition of CD3I (750 mg, 5.0 mmol). The resulting reaction mixture was stirred at room temperature overnight. The organic solvent was removed under reduced pressure, and the residue was extracted with ethyl acetate. The organic phases were combined, washed, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel chromatography to give the final compound (41 mg, 24.0%). H-NMR (CDCl3, 300MHz) δ 7.42-7.28 (m, 5H), 4.82-4.77 (dt, J = 3Hz, J = 9Hz, 1H), 2.97 (d, J = 3Hz, 1H), 2.90 (dd, J = 3Hz, J = 14Hz, 1H), 2.75 (dd, J = 9Hz, J =14Hz).
2-Acetamido-3-(2-hydroxy-2-phenylethylthio-2-d1-1,2-13C2)propanoic acid (IS-d1 13C2)
To a solution of acetophenone-α,β-13C2 (250 mg, 2.0 mmol) and concentrated H2SO4 (20 μl) in THF (20 ml) was added N-bromosuccinimide (768 mg, 4.32 mmol) in several portions. The reaction mixture was stirred at room temperature for 6 hr, and then diluted with water (80 ml). After extraction with ethyl acetate and concentrating, the residue was purified by silica gel chromatography to give 2-bromoacetophenone-α,β-13C2 (370 mg, 92%). 2-Bromoacetophenone-α, β-13C2 (201 mg, 1.0 mmol) was dissolved in methanol (10 ml), and N-acetyl-L-cysteine (212 mg, 1.3 mmol) was added while stirring. The reaction mixture was adjusted to pH 9-10 with 1 N NaOH, and stirred at room temperature for 4 hr. Then the mixture was diluted with water (30 ml), acidified with 1 N HCl, and extracted with ethyl acetate. The organic phases were pooled, washed, dried over anhydrous Na2SO4, and concentrated under reduced pressure to give rise to the crude product that was used in the next step without further purification. To a solution of the crude compound (225 mg, 0.8 mmol) cooled to 0 °C was added sodium borohydride-d4 (38 mg, 1.0 mmol) in several portions. The reaction mixture was stirred at room temperature for 1 hr and quenched with 1 N H2SO4. After removal of methanol, the aqueous phase was extracted with ethyl acetate. The combined organic phases were washed, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel chromatography and HPLC to give the desired compound (178 mg, 79%). H-NMR (CDCl3, 300MHz) δ 7.38-7.24 (m, 5H), 4.91-4.74 (m, 1H), 3.23-3.10 (m, 1H), 3.09-2.99 (m, 2H), 2.76-2.54 (m, 1H), 2.07 (s, 3H).
(2-Phenyl-2-hydroxyethyl)mercapturic acid-2’,3’,4’,5’,6’-d5 (IS-d5)
Bromophenylacetic acid-2’,3’,4’,5’,6’-d5 (220 mg, 1.0 mmol) was dissolved in anhydrous methanol (15 ml), and concentrated H2SO4 (0.5 ml) was added slowly while stirring. The reaction mixture was refluxed for 2 hr and then neutralized with 2 N NaOH. After most of methanol was removed under reduced pressure, the aqueous phase was extracted with ethyl acetate. The combined organic phases were washed, dried over anhydrous Na2SO4, and evaporated to dryness. The residue was purified by silica gel chromatography to give compound methyl bromophenylacetate-2’,3’,4’,5’,6’-d5 (176 mg, 75%). Methyl bromophenylacetate-2’,3’,4’,5’,6’-d5 (176 mg, 0.75 mmol) and N-acetyl-L-cysteine (130 mg, 0.8 mmol) were dissolved in methanol (10 ml). Then the reaction mixture was adjusted to pH 9-10 with 1 N NaOH and stirred at room temperature for 2 hr. After completion of the reaction, the solvent was removed under reduced pressure. The residue was acidified with 0.1 N H2SO4, and extracted with ethyl acetate. The combined organic phases were washed, dried over anhydrous Na2SO4 and evaporated to dryness in vacuum. The residue was purified by silica gel chromatography to give the desired compound (203 mg, 86%). Finely powdered sodium borohydride (115 mg, 3 mmol) was suspended in anhydrous THF (10 ml) in the presence of 2-acetamido-3-(2-methoxy-2-oxo-1-phenylethylthio)propanoic acid (158 mg, 0.5 mmol) during a period of 5 min under reflux and stirring. Methanol (1.25 ml) was added dropwise in 5 min with effervescence being observed. Stirring and reflux were maintained for 1 hr, then the reaction was cooled to room temperature and quenched with saturated NH4Cl (5 ml) with stirring for 10 min. The organic layer was separated and the aqueous layer was acidified and extracted with ethyl acetate. The organic phases were combined, washed, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel chromatography to give the final compound (81 mg, 56%). H-NMR (CDCl3, 300MHz) δ 4.85-4.66 (m, 1H), 4.08-3.99 (m, 1H), 3.96-3.82 (m, 2H), 3.06-2.94 (m, 2H), 1.98 (s, 3H).
Instruments
The qualitative and quantitative analysis of alkaline permethylation products was performed on gas chromatography-mass spectrometry (GC/MS) (Agilent Technologies, Palo Alto, CA), including a HP 5890 series II gas chromatograph, HP 5971A mass selective detector, and HP 59940A MS ChemStation (HP-UX series) controller. An Agilent J&W Scientific DB-5MS 20 m × 0.18mm ID × 0.18 μm GC column was used for the analysis. The injector was in splitless mode (1 min. hold) with 10 μL injection volume at an inlet temperature of 250 °C. Helium was used as the carrier gas set at constant flow 0.49 ml/min. The oven program had an initial temperature of 60 °C, then immediately ramped at 20 °C/min to 250 °C (hold for 2 min). The mass spectrometry data were acquired in selective ion monitoring (SIM) mode. The filament delay was set at 6.0 min. This gave a total run time of 12.5 min. The peak areas were integrated in SIM chromatograms with styrene glycol: m/z 179; 2-methylthio-2-PE: m/z 137; and 2-methylthio-1-PE: m/z 179; IS-d3: m/z 179; IS-d1 13C2: m/z 181; IS-d5: m/z 142 (refer to Scheme 2 for structures of internal standards IS-d3, IS-d1 13C2, and IS-d5).
Scheme 2.
Structures of internal standards.
Some synthetic compounds were purified on a Shimadzu LC-10Avp series HPLC system (Kyoto, Japan), consisting of LC-10ADvp pump, DGU-14A degasser, SIL-10ADvp auto-injector, CTO-10Avp column oven, and SPD-M10Avp diode array UV detector. The chromatographic separation was performed on an Econosil C18 column (250 × 22.5 mm i.d., 10 μm) and Alltima C18 column (250 × 4.6 mm i.d., 5 μm, Alltech Associates Inc., Deerfield, IL) with the mobile phase composed of a mixture of water and methanol or acetonitrile.
Development and optimization of alkaline permethylation
The initial reaction mixture consisted of 1.5 μmol α–mercapturic acid, 4N NaOH, 200 μl CH3I in a total volume of 1 ml. The reactants and a small Teflon-coated magnetic stirring bar were placed in a sealed 5-ml glass ampoule (Wheaton Science Products, Millville, NJ) in order that the reaction was not influenced by volume change due to evaporation. To avoid the potential explosion of the sealed ampoule, it is advisable that the volume of the mixture is no more than 2 ml in any case, so that the upper sides of the container can act as an air-cooled reflux condenser when the reactants are heated. The ampoule was immersed up to the level of sample meniscus in an oil bath at designed temperatures, and the mixture was vigorously stirred magnetically over incubation. After the permethylation procedure ended and the reaction mixture was cooled down on ice, 10 μl of IS-d3 (Scheme 2) as internal standard was spiked into the mixture at a final concentration of 5 μg/ml, followed by extraction and derivatization procedures as described below (refer to section “Postpermethylation sample preparation”). The yield was determined by the following equation:
In order to obtain the highest yield, reaction conditions, including temperature (40, 60, 80 °C), incubation time (1, 2, 4, 6 h), NaOH concentration (1, 2, 4, 6, 8, 12 N), and volume ratio of CH3I/NaOH (0.05, 0.11, 0.25, 0.42, 0.67, 1.0), were explored systematically. Only one condition was investigated at a time. Once the optimum conditions were found, α-mercapturic acid (700 nmol) was permethylated in the presence of 10 mg or 50 mg native BSA in order to investigate the effect of the protein on the efficiency of alkaline permethylation. In addition, the yield of β-mercapturic acid under the optimum conditions was also determined. Finally, smaller scales of α- and β-mercapturic acids were permethylated to determine the lowest levels of detection.
Alkaline permethylation of styrene oxide-modified BSA
To prepare styrene oxide-modified BSA, BSA was dissolved in 0.1 M phosphate buffer (pH 7.4) to yield a final concentration of 50 mg/ml. Racemic styrene oxide was added dropwise, and the molar ratio of styrene oxide / BSA was 100:1. The mixture was left to stir gently at room temperature for 24 hr. Then, BSA was precipitated by the addition of ice-cold acetone, washed three times with cold acetone and diethylether, and then dried in vacuum at room temperature.
Before alkaline permethylation, the modified BSA was dissolved in PBS (pH 7.4) at a concentration of 20 mg/ml. A mixture of IS- d1 13C2 and IS-d5 (Scheme 2), as internal standards representing α- and β-adducts respectively, was spiked into the BSA solution (0.5 ml) to a final concentration of 10 μg/ml each. Then, CH3I and NaOH were added in order. The alkaline permethylation reaction was carried out following the optimum method described above.
Alkaline permethylation of microsomes incubated with styrene
Mouse liver microsomes were prepared from CD-1 mice (20-25 g) by differential centrifugation according to the method reported by Watt et al [23]. Microsomes (final concentration: 4.0 mg protein/ml) were mixed with 3.2 mM MgCl2, 2.0 mM NADPH, and styrene at various concentrations (0, 50, 100, 250, 500, 750, and 1000 μM) in 0.1 M potassium phosphate buffer (pH 7.4) to a total volume of 1.0 ml. The mixtures were incubated at 37 °C for 30 min, and then dialyzed (molecular weight cut off: 3,500 Da) against three changes of 1 liter of 0.1 M phosphate-buffered saline (PBS) to remove excess styrene and styrene glycol generated. Protease XIV was added (7% by weight of protein) into the dialysates, and the digestion continued at 37 °C with gentle shaking for 5 hr. After digestion, the mixture was transferred to a 5-ml glass ampoule, and the internal standard mixture, CH3I, and NaOH were added in order, followed by 2-hr alkaline permethylation at 60 °C. The experiments were performed three times in duplicate.
Animal experiments
Male CD-1 mice (27-30 g; Charles River Breeding Laboratories, Wilmington, MA) were maintained on a 12-hr light/dark cycle and allowed free access to water and food. The animals were divided into three groups of four mice each. Styrene in corn oil was administered intraperitoneally at doses of 50 mg/kg (15 mg/ml) and 400 mg/kg (120 mg/ml) for 5 days. In control group, corn oil was administered. Mice were sacrificed by carbon dioxide 24 hr after the last administration. The thoracic cavity was open to expose the heart, and blood was collected via cardiac puncture into heparin-treated tubes. Blood was thawed and frozen three times to break the red blood cells, followed by centrifugation at 3,000 rpm for 10 min. The protein contents of the supernatants were determined using bicinchoninic acid protein assay kit (Pierce Chemical, Rockford, IL). All of the samples were adjusted to 30 mg/ml protein with 0.1 M PBS buffer (pH 7.4), divided into 30 mg portions, and then stored at -80 °C until alkaline permethylation.
Alkaline permethylation of blood protein of styrene-treated mice
Before alkaline permethylation procedure, the blood samples described above were brought to room temperature, and digested with protease XIV (7% by weight of protein) at 37 °C with continuous stirring for 5 hr. Then, the digested protein solutions were transferred to 5-ml glass ampoules, and the internal standard mixture, CH3I, and NaOH were added in order. The ampoules were sealed and the alkaline permethylation procedure was carried out under the optimized condition.
Postpermethylation sample preparation
After alkaline permethylation, the reaction mixtures were cooled down on ice and then transferred to 15-ml borosilicate glass tubes, followed by extraction with ethyl acetate. The ethyl acetate extracts were pooled, dried under N2 at room temperature, and stored at -20 °C. Before GC/MS analysis, the dried residues were brought to room temperature mixed with aliquots of 100 μl of 20% BSTFA (in acetonitrile) for derivatization. After vortex and sonication each for 3 min, the reconstituted mixture was heated at 50 °C for 10 min, followed by 5-min centrifugation at 14,000 rpm. The supernatants were collected and analyzed by GC/MS.
Result and Discussion
GC/MS analysis of alkaline permethylation products
As an initial step, we permethylated styrene oxide derived α-mercapturic acid (Scheme 3) as a model compound. The permethylation reaction produced two major products, including styrene glycol and 2-methylthio-2-PE. The two products are generated by two different proposed pathways, respectively (Scheme 3). Methylation of the mercapturate (thioether) by iodomethane products the corresponding sulfonium ion as an intermediate. The sulfonium is not stable and can be decomposed via two elimination reactions in basic solution. One elimination reaction (route A of Scheme 3) involves retro-Michael addition to offer 2-methylthio-2-PE. The other (retro-SN2 reaction) produces styrene oxide, which is then spontaneously hydrated to styrene glycol (route B of Scheme 3).
Scheme 3.

Proposed mechanisms of alkaline permethylation.
Styrene oxide alkylates cysteine residues of protein at α- and β-carbon of styrene oxide, producing two positional isomers (α- and β-adducts, Scheme 1), which, upon alkaline permethylation reaction, generate three products: 2-methylthio-2-PE (from α-adducts), 2-methylthio-1-PE (from β-adducts), and styrene glycol (from both adducts). Unfortunately, electrospray mass spectrometry failed to show reasonable sensitivity to detect these products due to poor ionization. Fortunately, GC/MS (MSD) provides good detection sensitivity with excellent analyte separation. The permethylation products were silylated with BSTFA, and the resulting derivatives were analyzed by GC/MS. Figure 1 shows SIM chromatograms of GC/MS analysis of authentic standards of methylthio-PEs and styrene glycol. The parent ions of methylthio-PEs were unstable and their base peak ions in ESI full scan spectra were found to be m/z 137 (2-methylthio-2-PE, 8.9 min) and m/z 179 (2-methylthio-1-PE, 8.7 min), respectively. Styrene glycol was derivatized with BSTFA to form double silylated styrene glycol-(Si(CH3)3)2, which was also labile and the base peak ion occurred at m/z 179 (8.2 min).
Figure 1.

SIM chromatograms of 2-methytio-2-PE (A, m/z 137), 2-methytio-1-PE (B, m/z 179) and styrene diol (C, m/z 179) formed in alkaline permethylation of authentic standard α-mercapturic acid or β-mercapturic acid. The mercapturic acids were individually heated in a sealed ampoule filled with CH3I and 4N NaOH (v/v, 5:95) at 60 °C for 2 hr, followed by derivatization with BSTFA and GC/MS analysis. 2-Methytio-2-PE and 2-methytio-1-PE were only observed in permethylation of the corresponding mercapturic acids, and styrene glycol was detected in permethylated products of both meracturic acids.
More production of styrene glycol than that of methylthio-PEs was observed in permethylation of either α- or β-mercapturic acid. Naturally, styrene glycol should be preferentially chosen to monitor protein adduction derived from styrene oxide. Unfortunately, significant background of styrene glycol was observed in permethylated protein samples, such as native BSA and blank mouse liver microsomal proteins. Another problem includes potential contamination of styrene glycol from styrene metabolism. In contrast, no such background resulting from methylthio-PEs was observed in permethylation of the above protein samples. Additionally, the sulfur of methylthio-PEs comes from the moiety of adducted proteins, as opposed to that the composition of permethylation product styrene glycol has nothing to do with the adducted proteins. This made us to select methylthio-PEs as the analytes to detect styrene oxide-derived protein adduction, which substantially reduces potential false positive possibly from styrene metabolites. Quantitation of the methylthio-PEs produced was achieved by calculation based on the ratio of integrated peak areas to their corresponding internal standards, i.e. IS-d5 and IS-d1 13C2.
Optimization of reaction conditions of alkaline permethylation
In order to optimize the conditions for permethylation of styrene oxide-cysteine adducts, we were intrigued by cleaving adduct from authentic standard α-mercapturic acid which represents α-substituted protein adducts. The effects of reaction temperature, NaOH concentration, molar ratio of CH3I/NaOH, and reaction time on the yield of 2-methylthio-2-PE generated from alkaline permethylation of authentic α-mercapturic acid are shown in Figure 2. In these experiments only one parameter at a time was varied from the original conditions (80 °C, 4 N NaOH base, 1.0 mol CH3I/mol NaOH, 4-hr reaction time). Spiking with internal standard IS-d3 (Scheme 2) right after permethylation procedure allowed us to quantify the formation of methylthio-PEs. As shown in Figure 2, it appears that the reaction condition consisting of a temperature of 60 °C, a base strength of 4 N, 0.21 mol CH3I/mol NaOH, and a reaction time of 2 hr is the best for alkaline permethylation of α-mercapturic acid, which consistently gave yields of 35 ± 7.2%. In the presence of 10 mg or 50 mg native BSA, the yields of 2-methylthio-2-PE were 34 ± 8.3% and 31± 1.5 %, respectively. No statistical difference was observed, suggesting that the presence of protein at the concentrations of 10 and 50 mg/ml had no significant effect on the efficiency of alkaline permethylation. Under the optimized conditions, the yield of 2-methylthio-1-PE generated from authentic β-mercapturic acid was 28 ± 5.3%, a little lower than that of 2-methylthio-2-PE from alkaline permethylation of α-mercapturic acid. In addition, the lowest detection level of α-mercapturic acid was 70 nmol, whereas that of β-mercapturic acid only reached 200 nmol.
Figure 2.
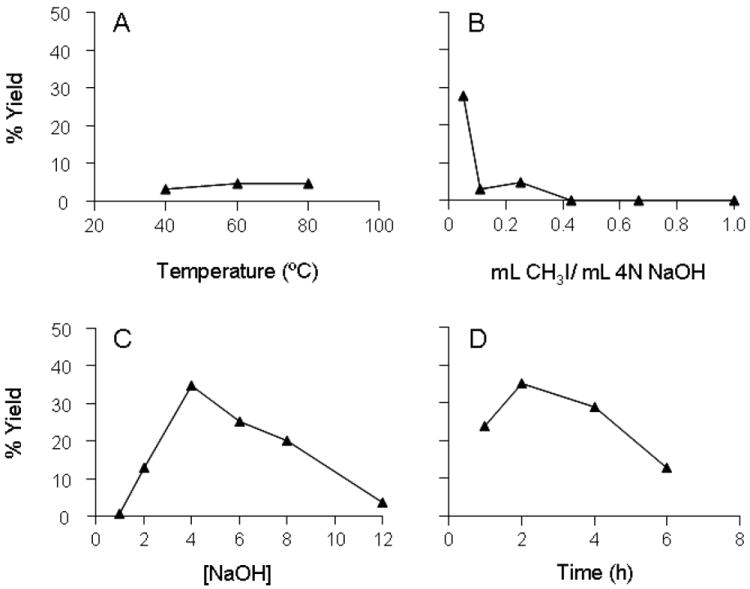
Optimization of protein-free alkaline permethylation reaction conditions. Each reaction mixture contained 1.5 μmol α-mercapturic acid, along with CH3I and NaOH. (A) Alkaline permethylation as a function of oil bath temperature. Condition: 4N NaOH, 200 μl CH3I, variable bath temperature, and 4 hr reaction time. (B) Alkaline permethylation as a function of CH3I used. Condition: 4 N NaOH, CH3I of various volumes, 60 °C bath temperature, and 4 hr reaction time. (C) Alkaline permethylation as a function of NaOH concentration. Condition: variable [NaOH], 50 μl CH3I, 60 °C bath temperature, and 4 h reaction time. (D) Alkaline permethylation as a function of reaction time. Condition: 4 N NaOH, 50 μl CH3I, 60 °C bath temperature, and variable reaction time.
Alkaline permethylation of styrene oxide-modified BSA
The optimized alkaline permethylation procedure was further applied to BSA (20 mg/ml) that has been modified with styrene oxide in vitro. Internal standards IS-d5 and IS-d1 13C2 (Scheme 2) were spiked for qualitative and quantitative analysis of styrene oxide-derived protein adduction. As expected, permethylation of the modified BSA released two methylthio-PEs along with styrene glycol (Figure 3). The amounts of 2-methylthio-2-PE and 2-methylthio-1-PE detected in 10 mg modified BSA were 53.8 nmol and 28.6 nmol, respectively. According to the yields obtained from alkaline permethylation of authentic standard α- and β-mercapturic acid, the calculated binding of α- and β- substituted adducts were 0.77 nmol/mg BSA and 0.51 nmol/mg BSA, respectively. The α-/β- binding ratio was 1.5, suggesting that styrene oxide reacts with BSA at α carbon in preference to β carbon. The same binding ratio was observed in in vitro incubation of styrene oxide with the cysteine residue of glutathione [24]. In addition, Yagen reported that the reaction of styrene oxide with N-acetylcysteine resulted in an α-/β-adduction ratio of 1.9 [25], and Hemminki published a ratio of approximately 2 generated from the reaction of styrene oxide with cysteine [26].
Figure 3.
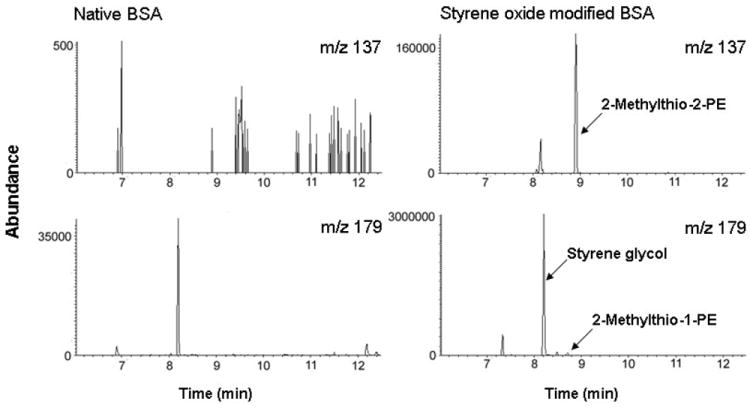
SIM chromatograms of 2-methylthio-PEs derived from native BSA (left panels) and styrene oxide-modified BSA (right panels) after alkaline permethylation procedure, followed by derivatization with BSTFA.
Alkaline permethylation of styrene-treated mouse liver microsomes
Microsomal incubation is an effective enzyme system to bioactivate styrene. Styrene oxide generated from styrene applied in the incubation system is expected to react with microsomal proteins to form protein adducts. Mouse liver microsomes were incubated with styrene at various concentrations, and the resulting incubation mixtures were permethylated and then analyzed by GC/MS. As expected, both 2-methylthio-2-PE (derivative of α-adducts) and 2-methylthio-1-PE (derivative of β-adducts) were detected in the permethylated microsomal proteins after exposure to styrene. In addition, the levels of the detected methylthio-PEs increased with the increase in the concentrations of styrene exposed (Figure 4). At the concentration of 50 μM styrene, the level of α-adducts was 0.12 ± 0.03 nmol/mg total protein, similar to that of β-adducts (0.096 ± 0.004 nmol/mg total protein). With the increase in the concentrations of styrene, the level of α-adduction became gradually higher than that of β-adduction. At 1000 μM styrene, a binding level of 0.65 ± 0.16 nmol α–adducts per mg of total protein was observed, whereas the binding via β-adduction was 0.31 ± 0.028 nmol/mg total protein, 2-fold lower than that of α-adducts (p < 0.05), which is consistent with the ratio of α- vs. β-adduction observed in the BSA experiments described above. This indicates higher reactivity of α-carbon than β-carbon of styrene oxide towards cysteine residues.
Figure 4.

Concentration-dependent increase of styrene oxide-cysteine protein adducts formed in mouse liver microsomal incubations with styrene at various concentrations (0, 50, 100, 250, 500, 750, and 1000 μM) at 37 °C for 4 hr. Protein adduction was represented by the observed 2-methylthio-2-PE (●) and 2-methylthio-1-PE (▲) generated in alkaline permethylation of the microsomal proteins. Each data represent the mean ± SD of duplicate incubations in three separate experiments (* p<0.05).
Alkaline permethylation of blood protein from styrene-treated mice
The challenges for human exposure studies include the availability of appropriate specimens and informative methods for analysis of these specimens. Blood samples are acceptable specimens in human studies. CD-1 mice were administered with styrene at doses of 0, 50, and 400 mg/kg by i.p. injection once a day for 5 days. The blood samples were harvested, followed by alkaline permethylation and GC/MS analysis. As shown in Figure 5, no methylthio-PE background was observed in the blank group. Both 2-methylthio-2-PE and 2-methylthio-1-PE were detected in the blood obtained from mice given styrene at 400 mg/kg, but they were undetectable in the group at a dose of 50 mg/kg. In the group of 400 mg/kg, the levels of α-adducts and β-adducts were 0.087 nmol and 0.018 nmol per mg of total protein, respectively. The ratio of α- over β-binding was 4.8, significantly higher than that observed in in vitro reaction of styrene oxide with BSA (a ratio of 1.5). However, the ratio of 4.8 was similar to those reported in humans after exposure to styrene (α/β = 4.7) or styrene oxide (α/β = 4.0) [20].
Figure 5.
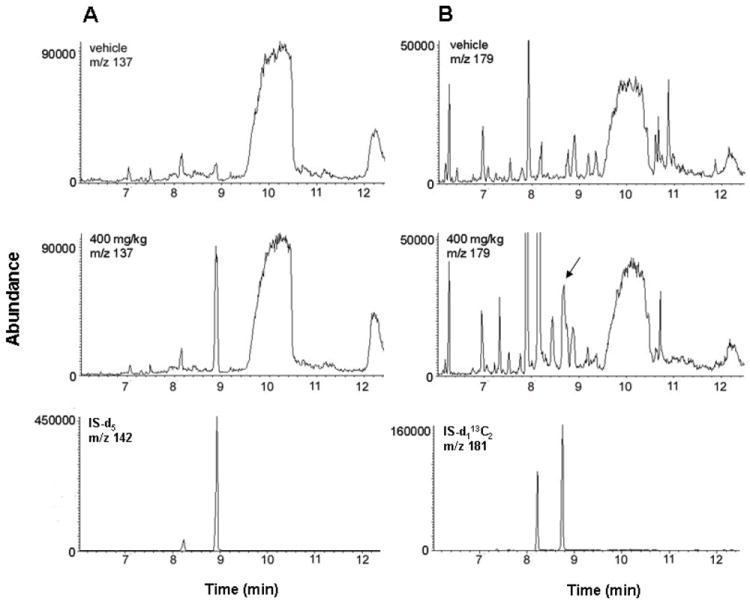
SIM chromatograms of methylthio-PEs generated by alkaline permethylation of blood samples obtained from CD-1 mice treated with vehicle or styrene (400 mg/kg dose for 5 days). (A) 2-methylthio-2-PE (m/z 137) with internal standard IS-d5 (m/z 142). (B) 2-methylthio-1-PE (m/z 179) with internal standard IS-d1 13C2 (m/z 181).
In conclusion, we developed an analytical approach to detect protein adduction derived from styrene oxide. This technique based on alkaline permethylation of protein and GC/MS analysis allows us not only to quantify the protein adduction but also to determine the chemical nature of interaction between styrene oxide and cysteine residues of protein. The developed approach provides a reliable analytical tool for quantitative assessment of styrene and styrene oxide exposure and will facilitate the mechanistic investigation of styrene toxicity.
Acknowledgments
This work was supported by NIH Grant HL080226.
Abbreviations
- BSA
bovine serum albumin
- BSTFA
N,O-bis(trimethylsilyl)trifluoroacetamide
- GC-MS
gas chromatography-mass spectrometry
- HPLC
high performance liquid chromatography
- IS-d3
2-methylthio-d3-1-phenyl-1-ethanol
- IS-d5
2-phenyl-2-hydroxyethyl mercapturic acid-2’,3’,4’,5’,6’-d5
- IS-d113C2
2-acetamido-3-(2-hydroxy-2-phenylethylthio-2-d1-1,2-13C2)propanoic acid
- 2-Methylthio-2-PE
2-methylthio-2-phenyl-1-ethanol
- 2-Methylthio-1-PE
2-methylthio-1-phenyl-1-ethanol
- α-Mercapturic acid
2-acetamido-3-(2-hydroxy-1-phenylethylthio)propanoic acid
- β-Mercapturic acid
2-acetamido-3-(2-hydroxy-2-phenylethylthio)propanoic acid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James Denis H, Castor William M. “Styrene” in Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim: 2005. [Google Scholar]
- 2.Miller RR, Newhook R, Poole A. Styrene production, use and human exposure. Crit Rev Toxicol. 1994;24:S1–S10. doi: 10.3109/10408449409020137. [DOI] [PubMed] [Google Scholar]
- 3.Fleming-Jones ME, Smith RE. Volatile organic compounds in foods: A five-year study. J Agric Food Chem. 2003;51:8120–8217. doi: 10.1021/jf0303159. [DOI] [PubMed] [Google Scholar]
- 4.Tang W, Hemm I, Eisenbrand G. Estimation of human exposure to styrene and ethylbenzene. Toxicology. 2000;144:39–50. doi: 10.1016/s0300-483x(99)00188-2. [DOI] [PubMed] [Google Scholar]
- 5.Vodicka P, Koshinen M, Naccarati A, Oesch-Bartlomowicz B, Vodickova L, Hemminki K, Oesch F. Styrene metabolism, genotoxicity, and potential carcinogenicity. Drug Metab Rev. 2006;38:805–853. doi: 10.1080/03602530600952222. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima T, Elovaara E, Gelboin FJ, Vainio H, Aoyama T. Characterization of the human cytochrome P450 isozymes responsible for styrene metabolism. In: Sorsa M, Peltonen K, Vainio H, Hemminki K, editors. Butadiene and styrene: Assessment of Health Hazards, IARC Scientific Publication No. 127. Lyon: IARC; 1993. pp. 101–108. [PubMed] [Google Scholar]
- 7.Phillillps DH, Farmer PB. Evidence for DNA and protein binding by styrene and styrene oxide. Crit Rev Toxicol. 1994;24:S35–S46. doi: 10.3109/10408449409020139. [DOI] [PubMed] [Google Scholar]
- 8.Bond JA. Review of the toxicology of styrene. Crit Rev Toxicol. 1989;19:227–249. doi: 10.3109/10408448909037472. [DOI] [PubMed] [Google Scholar]
- 9.Hall TA, Rebert CS. Review of neurotoxicity of styrene in humans. Vet Hum Toxicol. 1994;36:249. [PubMed] [Google Scholar]
- 10.Rappaport SM, Yeowell-O’Connell K, Bodell W, Yager JW, Symanski E. An investigation of multiple biomarkers among workers exposed to styrene and styrene-7,8-oxide. Cancer Res. 1996;56:5410–5416. [PubMed] [Google Scholar]
- 11.Skipper PL, Tannenbaum SR. The role of protein adducts in the study of chemical carcinogenesis. Prog Clin Biol Res. 1990;340C:301–310. [PubMed] [Google Scholar]
- 12.van Welie RTH, van Djick RGJM, Vermeulen NPE, van Sittert NJ. Mercapturic acids, protein adducts, and DNA adducts as biomarkers of electrophilic chemicals. Crit Rev Toxicol. 1992;22:271–306. doi: 10.3109/10408449209146310. [DOI] [PubMed] [Google Scholar]
- 13.Törnqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adduct: quantitative and qualitative aspects of their formation, analysis and applications. J Chromtogra B. 2002;778:279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 14.Jagr M, Mrza J, Linhart I, Stransky V, Pospisil M. Synthesis and characterization of styrene oxide adducts with cysteine, histidine, and lysine in human globin. Chem Res Toxicol. 2007;20:1442–1452. doi: 10.1021/tx700057t. [DOI] [PubMed] [Google Scholar]
- 15.Hemminki K. Reactions of methylnitrosourea, epichlorhydrin, styrene oxide and acetoxyacetylaminofluorene with polyamino acids. Carcinogenesis. 1983;4:1–3. doi: 10.1093/carcin/4.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Yuan W, Chung J-K, Zheng J. Development of polyclonal antibodies for detection of styrene oxide modified proteins. Chem Res Toxicol. 2007;20:316–321. doi: 10.1021/tx600340c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen S, Zhang F, Zeng S, Tian Y, Chai X, Gee S, Hammock BD, Zheng J. Development of enantioselective polyclonal antibodies to detect styrene oxide protein adducts. Anal Chem. 2009;81:2668–2676. doi: 10.1021/ac8023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ting D, Smith MT, Doane-Setzer P, Rappaport SM. Analysis of styrene oxide-globin adducts based upon reaction with Raney nickel. Carcinogenesis. 1990;11:755–760. doi: 10.1093/carcin/11.5.755. [DOI] [PubMed] [Google Scholar]
- 19.Rappaport SM, Ting D, Jin Z, Yeowell-O’connell K, Waidyanatha S, McDonald T. Application of Raney nickel to measure adducts of styrene oxide with hemoglobin and albumin. Chem Res Toxicol. 1993;6:238–244. doi: 10.1021/tx00032a014. [DOI] [PubMed] [Google Scholar]
- 20.Yeowell-O’Connell K, Jin Z, Rappaport SM. Determination of albumin and hemoglobin adducts in workers exposed to styrene and styrene oxide, Cancer Epidemiol. Biomarkers Prev. 1995;5:205–215. [PubMed] [Google Scholar]
- 21.Slaughter DE, Hanzlik RP. Identification of epoxide- and quinine- derived bromobenzene adducts to protein sulfur nucleophiles. Chem Res Toxicol. 1991;4:349–359. doi: 10.1021/tx00021a015. [DOI] [PubMed] [Google Scholar]
- 22.Slaughter DE, Zheng J, Harriman S, Hanzlik RP. Identification of covalent adducts to protein sulfur nucleophiles by alkaline permethylation. Anal Biochem. 1993;208:288–295. doi: 10.1006/abio.1993.1048. [DOI] [PubMed] [Google Scholar]
- 23.Watt KC, Morin DM, Kurth MJ, Mercer RS, Plopper CG, Buckpitt AR. Glutathione conjugation of electrophilic metabolites of 1-nitronaphthalene in rat tracheobronchial airways and liver: identification by mass spectrometry and proton nuclear magnetic resonance spectroscopy. Chem Res Toxicol. 1999;12:831–839. doi: 10.1021/tx990023v. [DOI] [PubMed] [Google Scholar]
- 24.Pacheka J, Gariboldi P, Cantoni L, Belvedere G, Mussini E, Salmona M. Isolation and structure determination of enzymatically formed styrene oxide glutathione conjugates. Chem Biol Interact. 1979;27:313–322. doi: 10.1016/0009-2797(79)90134-0. [DOI] [PubMed] [Google Scholar]
- 25.Yagen B, Hernandez O, Bend JR, Cox RH. Synthesis and relative stereochemistry of the four mercapturic acids derived from styrene oxide and N-acetylcysteine. Chem Biol Interact. 1981;34:57–67. doi: 10.1016/0009-2797(81)90090-9. [DOI] [PubMed] [Google Scholar]
- 26.Hemminki K. Binding of styrene oxide to amino acids, human serum proteins, and hemoglobin. Arch Toxicol. 1986;9(suppl):286–290. doi: 10.1007/978-3-642-71248-7_46. [DOI] [PubMed] [Google Scholar]


