Abstract
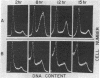
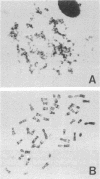
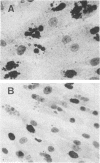
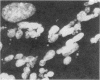
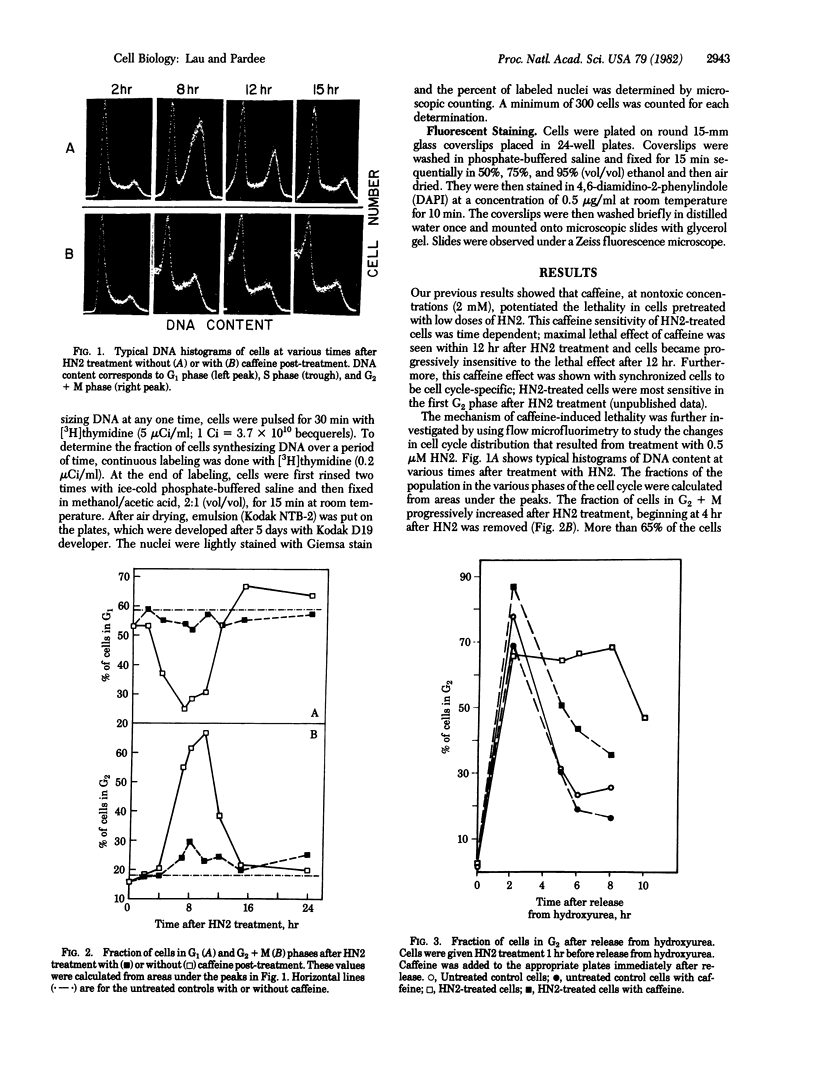
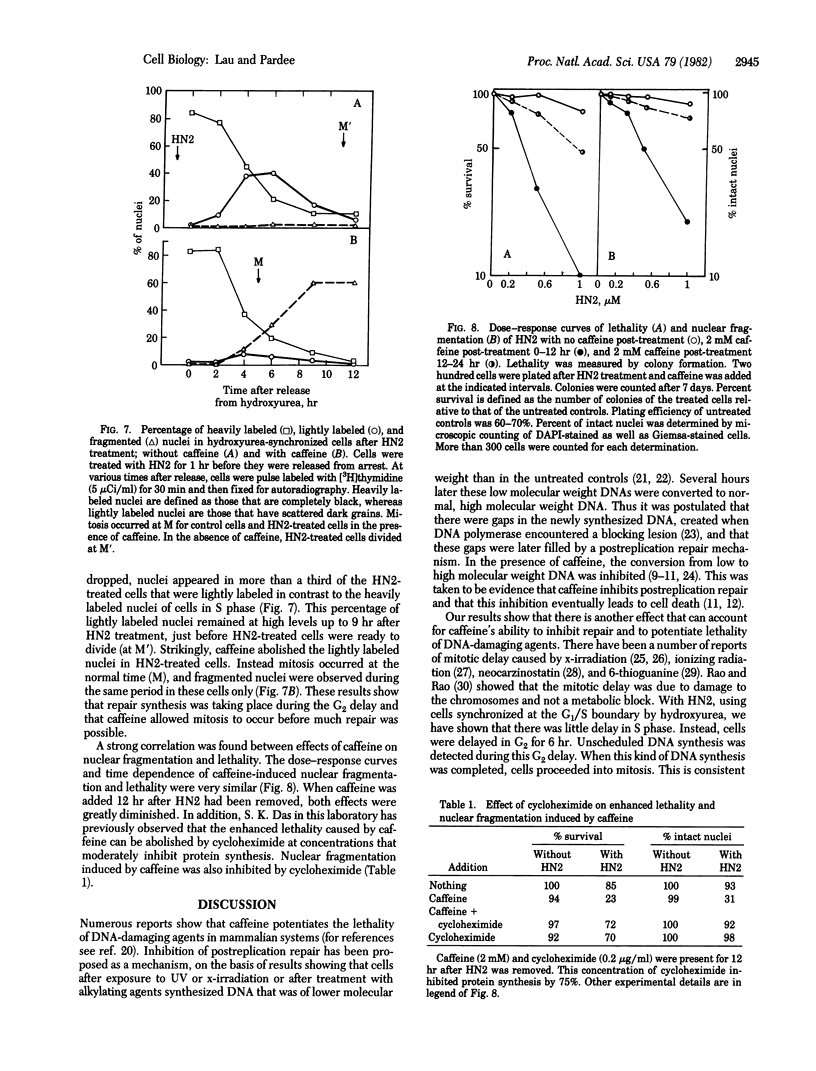
Caffeine is synergistic with many DNA-damaging agents in increasing lethality to mammalian cells. The mechanism is not well understood. Our results show that caffeine potentiates the lethality of the nitrogen mustard 2-chloro-N-(2-chloroethyl)-N-methylethanamine (HN2) by inducing damaged cells to undergo mitosis before properly repairing lesions in their DNA. Treatment with low doses of HN2 (0.5 microM for 1 hr) caused little lethality in baby hamster kidney cells (90% survival). These cells were arrested in G2 shortly after treatment with HN2 as shown by flow microfluorimetry and autoradiography. After an arrest of 6 hr, HN2-treated cells began to move into mitosis and from then on behaved like normal cells. Repair synthesis was shown to continue during the G2 arrest by using synchronized cells pulse labeled with [3H]thymidine after HN2 treatment and autoradiography. Caffeine (2mM) increased the lethality of HN2 by 5- to 10-fold. It prevented the G2 arrest. Caffeine did not prevent these HN2-treated cells from entering or completing S phase but rather allowed them to divide without finishing the repair processes and as a consequence caused nuclear fragmentation after mitosis. Caffeine-induced nuclear fragmentation and enhanced lethality were proportional, as shown with dose--response curves and time dependence. In addition, both lethality and nuclear fragmentation were abolished by low doses of cycloheximide, an inhibitor of protein synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlett C. F., Harcourt S. A., Broughton B. C. The influence of caffeine on cell survival in excision-proficient and excision-deficient xeroderma pigmentosum and normal human cell strains following ultraviolet-light irradiation. Mutat Res. 1975 Dec;33(2-3):341–346. doi: 10.1016/0027-5107(75)90209-2. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Regan J. D. Effect of caffeine on postreplication repair in human cells. Biophys J. 1974 Jul;14(7):519–527. doi: 10.1016/S0006-3495(74)85932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. Steps in DNA chain elongation and joining after ultra-violet irradiation of human cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Nov;22(5):417–424. doi: 10.1080/09553007214551301. [DOI] [PubMed] [Google Scholar]
- Busse P. M., Bose S. K., Jones R. W., Tolmach L. J. The action of caffeine on X-irradiated HeLa cells. III. Enhancement of X-ray-induced killing during G2 arrest. Radiat Res. 1978 Nov;76(2):292–307. [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair and its coupling to DNA replication in eukaryotic cells. Biochim Biophys Acta. 1978 Dec 11;516(4):489–516. doi: 10.1016/0304-419x(78)90020-3. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H. Single strand interruptions in DNA and the effects of caffeine in Chinese hamster cells irradiated with ultraviolet light. Biochem Biophys Res Commun. 1969 Jul 23;36(2):203–208. doi: 10.1016/0006-291x(69)90315-5. [DOI] [PubMed] [Google Scholar]
- Domon M., Rauth A. M. Effects of caffeine on ultraviolet-irradiated mouse L cells. Radiat Res. 1969 Jul;39(1):207–221. [PubMed] [Google Scholar]
- Fujiwara Y. Postreplication repair of alkylation damage to DNA of mammalian cells in culture. Cancer Res. 1975 Oct;35(10):2780–2789. [PubMed] [Google Scholar]
- Fujiwara Y. Postreplication repair of ultraviolet damage to DNA, DNA-chain elongation, and effects of metabolic inhibitors in mouse L cells. Biophys J. 2009 Jan 1;15(5):403–415. doi: 10.1016/S0006-3495(75)85826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Pardee A. B. S phase synchrony in monolayer CHO cultures. Exp Cell Res. 1976 Jul;100(2):265–275. doi: 10.1016/0014-4827(76)90147-6. [DOI] [PubMed] [Google Scholar]
- Iseki S., Ebina T., Ishida N. Effects of caffeine on neocarzinostatin-induced inhibition of cell cycle traverse in HeLa-S3 cells. Cancer Res. 1980 Oct;40(10):3786–3791. [PubMed] [Google Scholar]
- Kihlman B. A., Sturelid S., Hartley-Asp B., Nilsson K. The enhancement by caffeine of the frequencies of chromosomal aberrations induced in plant and animal cells by chemical and physical agents. Mutat Res. 1974 Apr;26(2):105–122. doi: 10.1016/s0027-5107(74)80041-2. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Paterson M. C., Lohman P. H., de Weerd-Kastelein E. A., Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci U S A. 1975 Jan;72(1):219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S. Effects of caffeine and theophylline on DNA synthesis in unirradiated and UV-irradiated mammalian cells. Mutat Res. 1974 Apr;26(2):73–82. doi: 10.1016/s0027-5107(74)80037-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Post-replication repair of DNA in ultraviolet-irradiated mammalian cells. No gaps in DNA synthesized late after ultraviolet irradiation. Eur J Biochem. 1972 Dec 18;31(3):438–445. doi: 10.1111/j.1432-1033.1972.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Lücke-Huhle C., Blakely E. A., Chang P. Y., Tobias C. A. Drastic G2 arrest in mammalian cells after irradiation with heavy-ion beams. Radiat Res. 1979 Jul;79(1):97–112. [PubMed] [Google Scholar]
- Murnane J. P., Byfield J. E., Ward J. F., Calabro-Jones P. Effects of methylated xanthines on mammalian cells treated with bifunctional alkylating agents. Nature. 1980 May 29;285(5763):326–329. doi: 10.1038/285326a0. [DOI] [PubMed] [Google Scholar]
- Painter R. B. DNA damage and repair in eukaryotic cells. Genetics. 1974 Sep;78(1):139–148. doi: 10.1093/genetics/78.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7315–7317. doi: 10.1073/pnas.77.12.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. P., Rao P. N. The cause of G2-arrest in Chinese hamster ovary cells treated with anticancer drugs. J Natl Cancer Inst. 1976 Nov;57(5):1139–1143. doi: 10.1093/jnci/57.5.1139. [DOI] [PubMed] [Google Scholar]
- Rauth A. M. Evidence for dark-reactivation of ultraviolet light damage in mouse L cells. Radiat Res. 1967 May;31(1):121–138. [PubMed] [Google Scholar]
- Roberts J. J., Sturrock J. E., Ward K. N. The enhancement by caffeine of alkylation-induced cell death, mutations and chromosomal aberrations in Chinese hamster cells, as a result of inhibition of post-replication DNA repair. Mutat Res. 1974 Apr;26(2):129–143. doi: 10.1016/s0027-5107(74)80043-6. [DOI] [PubMed] [Google Scholar]
- Rossow P. W., Riddle V. G., Pardee A. B. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4446–4450. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Timson J. Caffeine. Mutat Res. 1977;47(1):1–52. doi: 10.1016/0165-1110(77)90016-1. [DOI] [PubMed] [Google Scholar]
- Tobey R. A. Different drugs arrest cells at a number of distinct stages in G2. Nature. 1975 Mar 20;254(5497):245–247. doi: 10.1038/254245a0. [DOI] [PubMed] [Google Scholar]
- Waldren C. A., Rasko I. Caffeine enhancement of X-ray killing in cultured human and rodent cells. Radiat Res. 1978 Jan;73(1):95–110. [PubMed] [Google Scholar]
- Walker I. G., Reid B. D. Caffeine potentiation of the lethal action of alkylating agents on L-cells. Mutat Res. 1971 May;12(1):101–104. doi: 10.1016/0027-5107(71)90079-0. [DOI] [PubMed] [Google Scholar]
- Walters R. A., Gurley L. R., Tobey R. A. Effects of caffeine on radiation-induced phenomena associated with cell-cycle traverse of mammalian cells. Biophys J. 1974 Feb;14(2):99–118. doi: 10.1016/S0006-3495(74)70002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotring L. L., Roti Roti J. L. Thioguanine-induced S and G2 blocks and their significance to the mechanism of cytotoxicity. Cancer Res. 1980 May;40(5):1458–1462. [PubMed] [Google Scholar]
- Yen A., Pardee A. B. Arrested states produced by isoleucine deprivation and their relationship to the low serum produced arrested state in Swiss 3T3 cells. Exp Cell Res. 1978 Jul;114(2):389–395. doi: 10.1016/0014-4827(78)90497-4. [DOI] [PubMed] [Google Scholar]