Abstract
Although DNA is most widely known to store and pass along genetic information, the discovery of G-quadruplex structures has illuminated a new role of DNA in biology. DNA G-quadruplexes are four-stranded globular nucleic acid secondary structures formed in specific G-rich sequences with biological significance, such as human telomeres and oncogene promoters. This review focuses on the unimolecular DNA G-quadruplexes, which can readily form in solution under physiological conditions and are considered to be most biologically relevant. Available structural data show a great conformational diversity of unimolecular G-quadruplexes, amenable to small molecule drug targeting. The relationship of sequence, structure, and stability of unimolecular DNA G-quadruplexes, as well as the recent progress on interactions with small molecule compounds and insights into rational design of G-quadruplex-interactive molecules, will be discussed.
Keywords: G-quadruplexes, oncogene promoters, human telomeres, small molecule interactions, rational drug design
INTRODUCTION
Although DNA is most widely known to store and pass along genetic information, the discovery of G-quadruplex structures has illuminated a new role of DNA in biology (Yang and Okamoto 2010). G-quadruplexes are four-stranded globular nucleic acid secondary structures, formed by one or more DNA molecules (Figure 1). Deviating from Watson-Crick hydrogen bonding found in B-form duplex DNA, the planar, stacked tetrads of G-quadruplexes are formed by Hoogsteen hydrogen bonding of guanine bases (Sen and Gilbert 1988) (Figure 1 A–B). G-quadruplexes can readily form in solution under physiological conditions. The formation and stabilization of G-quadruplexes are dependent on monovalent cations, specifically K+ and Na+ (Sen and Gilbert 1990) (Figure 1 C–F), where K+ is considered to be more physiologically relevant due to its high intracellular concentration (~140 mM) versus that of Na+ (~10 mM). The stabilization is due to the coordination of the positively charged cations with the electronegative O6 atoms in the center channel of the adjacent stacked G-tetrads (van Mourik and Dingley 2005) (Figure 1A–B).
Figure 1.
(A) Schematic illustration of a G-tetrad, a square planar alignment of four guanines connected by cyclic Hoogsteen hydrogen bonding between the N1, N2 and O6, N7 of guanine bases. The H1–H1 and H1–H8 connectivity patterns detectable in NOESY experiments is also shown. (B) An example of a G-tetrad structure. The guanines in a G-tetrad can adopt either syn or anti glycosidic conformation. The guanines from the parallel G-strands adopt the same glycosidic conformation and the guanines from the antiparallel G-strands adopt the opposite glycosidic conformations. (C) A schematic tetrameric and dimeric G-quadruplex composed of three G-tetrads. Cations (K+ or Na+), shown as blue balls, are needed to stabilize G-quadruplexes by coordinating with the eight electronegative carbonyl oxygen O6 atoms of the adjacent G-tetrads. And examples of monomeric (unimolecular) G-quadruplexes with parallel-stranded (D), mixed parallel/antiparallel (E), and antiparallel folding (F).
The G-tetrad structure was first characterized in a gelatinous substance formed by guanosines (Gellert et al. 1962). Biologically relevant G-quadruplexes were first discovered in eukaryotic chromosomal telomeric DNA (Henderson et al. 1987; Sundquist and Klug 1989). Human telomeres contain a 5–10 kb d(TTAGGG)n tandem sequence with a 50–600 nt single-stranded 3’ overhang at the chromosome ends, which can form DNA G-quadruplexes (Moyzis et al. 1988; Wright et al. 1997; Colgin and Reddel 1999; de Lange 2005; Sfeir et al. 2005). The formation of DNA G-quadruplexes in the human telomere has been shown to inhibit the activity of telomerase, which is activated in 80–85% of cancer cells, and is therefore considered to be a potential anticancer drug target (Hurley et al. 2000; Neidle and Parkinson 2002; De Cian et al. 2007; Punchihewa and Yang 2009).
More recently, DNA G-quadruplexes have been found in the proximal location of promoters, which are mostly TATA-less, in a number of human genes involved in growth and proliferation, as potential transcriptional regulators (Rustighi et al. 2002; Qin and Hurley 2008; Brooks and Hurley 2009), such as insulin (Hammond-Kosack et al. 1992), c-MYC (Simonsson et al. 1998; Siddiqui-Jain et al. 2002), VEGF (Sun et al. 2005), HIF-1α (De Armond et al. 2005), sMtCK (Yafe et al. 2005), BCL-2 (Dai et al. 2006; Dexheimer et al. 2006), KRAS(Cogoi and Xodo 2006), Rb (Xu and Sugiyama 2006), c-KIT (Rankin et al. 2005; Fernando et al. 2006), RET (Guo et al. 2007), PDGF-A (Qin et al. 2007), c-MYB(Palumbo et al. 2008), hTERT (Palumbo et al. 2009), and PDGFR-β (Qin et al. 2010). c-MYC, one of the most commonly deregulated genes in human cancers, has a DNA G-quadruplex motif in the promoter Nuclease Hypersensitive Element (NHE) III1 which regulates 75–85% of its total transcription (Berberich and Postel 1995; Simonsson et al. 1998), and is the most extensively studied system for the promoter G-quadruplexes (Brooks and Hurley 2009). The G-quadruplex structure formed in the c-MYC promoter functions as a transcriptional silencer (Siddiqui-Jain et al. 2002); G-quadruplex-interactive drugs lower c-MYC transcription in vivo (Grand et al. 2002; Ou et al. 2007). A special requirement for promoter sequences is that the G-quadruplex must be generated in a region of duplex rather than single-stranded DNA as in the case of telomeres. Recent studies from the Levens lab at NCI (Kouzine et al. 2008; Lavelle 2008) elegantly demonstrated the effect of transcriptionally induced supercoiling in vivo in the c-MYC promoter. While polyG/polyC regions have a propensity to form single-stranded DNA, it is the negative superhelicity, generated as a result of the active transcriptional machinery, that results in the intermediate single-stranded forms which spontaneously form the more stable G-quadruplex structures (Brooks and Hurley 2009). Additionally, two proteins have been demonstrated to interact with the c-MYC promoter G-quadruplex and are involved in gene transcription: nucleolin specifically binds and stabilizes the c-MYC promoter G-quadruplex and functions as a transcription repressor (Gonzalez et al. 2009), whereas NM23-H2 destabilizes the c-Myc promoter G-quadruplex and functions as a transcription activator (Dexheimer et al. 2009; Thakur et al. 2009).
In addition, G-quadruplexes have been found in other regions of the genome, such as immunoglobulin switch regions (Maizels 2006), ribosomal DNA (Drygin et al. 2009), and RNA (Kostadinov et al. 2006; Kumari et al. 2007; Wieland and Hartig 2007). This review will focus on the unimolecular (monomeric or intramolecular) DNA G-quadruplexes (referred herein as G-quadruplexes) (Figure 1 D–F), which are considered to be most biologically relevant and to be potential drug targets.
DNA G-QUADRUPLEX FOLDS
Unimolecular G-quadruplexes, formed by a single G-rich DNA molecule, are globularly folded nucleic acid structures and can readily form under physiological conditions (Figure 1 D–F). Most unimolecular G-quadruplexes have three tetrads, with two or four also reported. Available structural data show a great conformational diversity of unimolecular G-quadruplexes, including the number of G-tetrads, folding topologies and loop structures (Figure 1 D–F). The structural diversity makes G-quadruplexes attractive targets for small molecule drug targeting (Yang and Okamoto 2010). Generally, intramolecular quadruplex-forming sequences have four or more G tracts involved in tetrad formation with various numbers of interspersing residues that form loops connecting each tetrad plane. A unimolecular G-quadruplex contains four G-tracts (continuous or discontinuous), three (or more) loops, and two flanking segments (Figure 1 D–F). The four G-tracts of a unimolecular G-quadruplex can be parallel or antiparallel, and the loops can be of three conformations: strand-reversal (or propeller, connecting adjacent parallel strands), lateral (or edgewise, connecting adjacent antiparallel strands), and diagonal (connecting diagonal antiparallel strands on opposite sides of the tetrad) (Figure 1 D–F). The sugar glycosidic conformation can be anti or syn ; on a G-tetrad, guanines from parallel G-tracts have the same glycosidic conformation, whereas those from antiparallel G-tracts have a different conformation (Figure 1B).
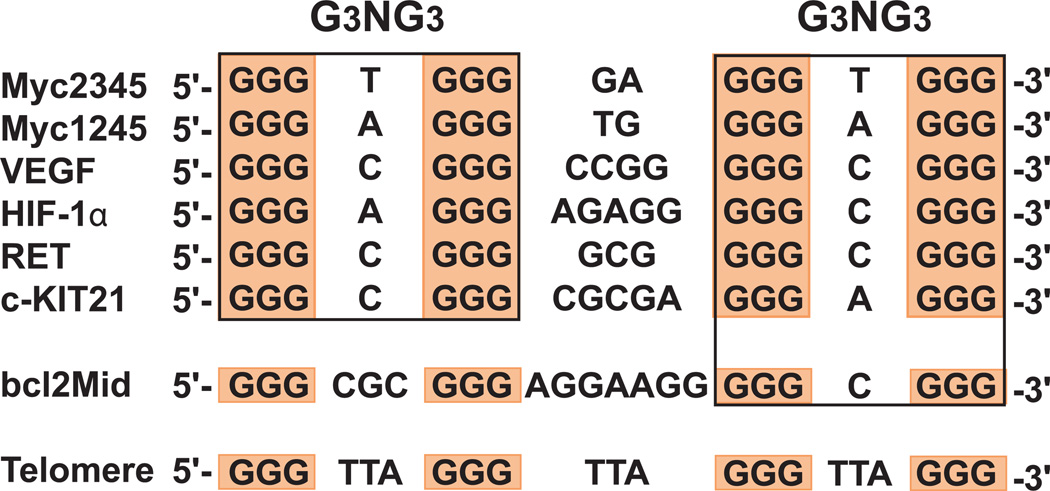
G-quadruplexes structures are uniquely determined by the primary nucleotide sequences, in a manner analogous to protein folding from a primary amino acid sequence. However, a certain G-rich sequence may adopt different G-quadruplex structures in the presence of different cations, as in the case of the human telomeric DNA; and a sequence may fold into more than one conformation. Nature appears to be very elegant in choosing the different sequences: the highly conserved human telomeric DNA sequence consists of tandem repeats of d(TTAGGG)n (Figure 2) and therefore does not have sequence diversity. However, it is able to form various G-quadruplex structures with low energy difference and thus impart intrinsic structure polymorphism. The human oncogene promoter sequences, which are each unique and thus have sequence diversity (Figure 2), appear to form different structures determined by each specific sequence.
Figure 2.
Comparison of G-quadruplex-forming sequences in selected gene promoters and human telomeres. The promoter sequences that form parallel-stranded G-quadruplexes are boxed and all contain two G3NG3 motifs.
Human Telomeric G-quadruplexes
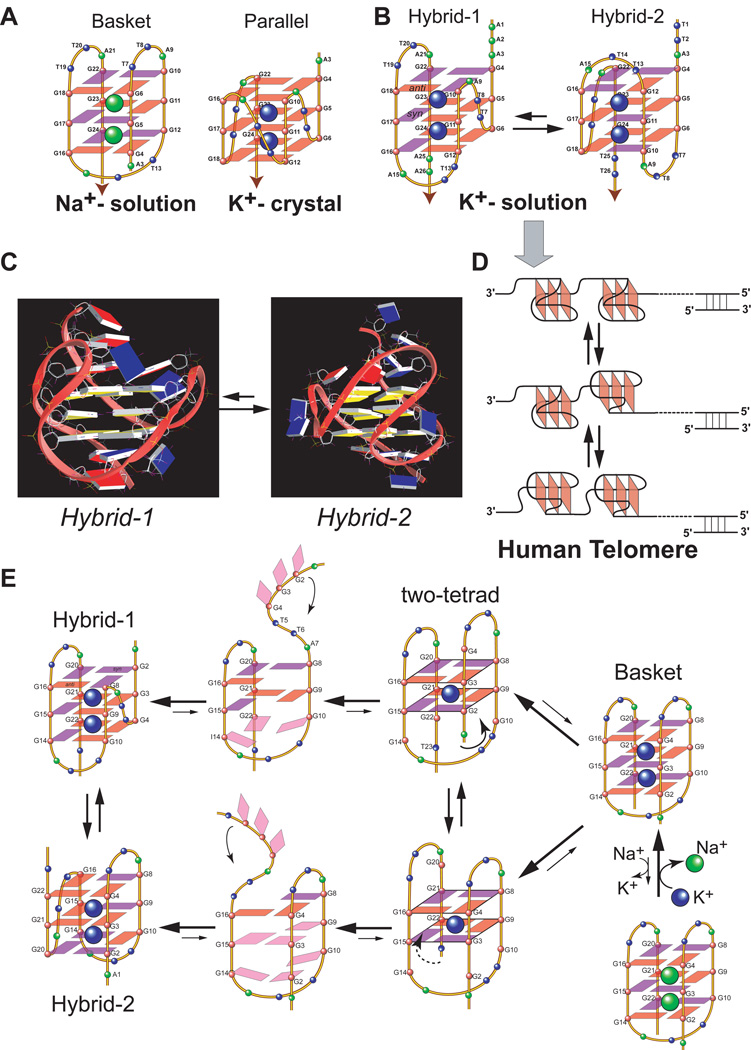
The human telomeric DNA was first found to form a basket-type G-quadruplex conformation in Na+ solution (Wang and Patel 1993) (Figure 3A left). Later it was found to form a parallel-stranded G-quadruplex conformation in the presence of K+ in the crystalline state (Parkinson et al. 2002) (Figure 3A right). More recently, the human telomeric DNA was shown to predominantly form hybrid-type G-quadruplexes in K+ solution (Ambrus et al. 2006; Luu et al. 2006; Phan et al. 2006; Xu et al. 2006; Dai et al. 2007; Dai et al. 2007; Phan et al. 2007) (Figure 3B). Two hybrid structures were found to coexist in equilibrium in human telomeric sequences in K+ solution and appear to have similar stability with a low energy difference, with the hybrid-2 form being the major conformation in extended sequences (Dai et al. 2007). The two hybrid forms have distinct yet related folding topologies which are stabilized by the specific capping structures formed by the flanking and loop residues (Figure 3C). While the energy difference of the two hybrid forms appears to be sufficiently small that they coexist in human telomeric sequences, the kinetics of the interconversion between the two forms appears to be rather slow (Dai et al. 2007), indicating high-energy barrier intermediate(s) for interconversion. A two-G-tetrad basket-type structure was more recently found to form in human telomeric sequences with no 5’-flanking segment (Lim et al. 2009; Zhang et al. 2010), which is likely to be an intermediate of the interconversion of different telomeric forms (Figure 3E).
Figure 3.
(A) Folding structures of the basket-type human telomeric G-quadruplex formed in Na+ solution (left) and the parallel-stranded human telomeric G-quadruplex formed in the presence of K+ in crystalline state (right). (B) Folding structures of hybrid-1 (left) and hybrid-2 (right) human telomeric G-quadruplexes present in equilibrium in K+ solution. red box = (anti) guanine, magenta box = (syn) guanine. (C) The representative NMR structures of hybrid-1 and hybrid-2 telomeric G-quadruplexes that are in equilibrium in K+ solution (guanine: yellow; adenine: red; thymine: blue). (D) A model showing a DNA secondary structure composed of compact-stacking multimers of hybrid-type G-quadruplexes in human telomeres, with an equilibrium between hybrid-1 and hybrid-2 forms. (E) A model of the interconversion between different forms of human telomeric G-quadruplexes through a strand-reorientation mechanism. A two-tetrad form is likely to be a transition intermediate.
Importantly, the hybrid structures suggest a straightforward means for multimer formation within the 3′ overhang of the human telomeres (Figure 3D) (Petraccone et al. 2008). The polymorphism of telomeric DNA may be related to the presence of a 3-nt strand-reversal TTA loop in both hybrid structures. A strand-reversal loop usually favors short loop sizes of 1 and 2 nt in K+ solution (Ambrus et al. 2005; Dai et al. 2006). This may also explain why the parallel form is disfavored in K+ solution, as it would contain three 3-nt TTA loops in strand-reversal loop conformation. However, the presence of the strand-reversal TTA loop positions the 3’ and 5’ ends of away from each other, thereby making the hybrid forms a straightforward platform for efficient multimer packing with possible end-to-end stacking in human telomeres (Figure 3D).
It is interesting to note that the structure polymorphism appears to be intrinsic to the highly conserved human telomeric sequence (Dai et al. 2008). It is the asymmetry of the TTA loop that determines the possibility of the formation of two closely related but distinct asymmetric hybrid structures (Figure 3C). The presence of adenine in the TTA loop of the human telomeric sequence, which was missing in the telomeres of lower organisms, provides a more selective basis for distinct capping structures, such the T:A:T triple in the hybrid-2 structure and the adenine triple in the hybrid-1 structure, to selectively stabilize different forms (Dai et al. 2007; Dai et al. 2007) (Figure 3C). Unlike the G-rich sequences in gene promoter regions, the telomeric DNA sequence contains the same tandem repeats d(TTAGGG). Nature may have chosen this specific sequence with its asymmetry and the structural polymorphism, which may provide a means to control the telomere biology such as protein recognition. The structural polymorphism of human telomeric G-quadruplexes may present an attractive means for targeting by small molecules, which may readily surpass the small energy barrier between various forms and subsequently change the dynamics as well as protein recognition of human telomeres.
Promoter G-quadruplexes
In contrast to the human telomeric sequence with the same d(TTAGGG) repeat, the G-quadruplex-forming gene proximal promoter regions are more diverse in sequence with varying numbers and lengths of G-tracts and intervening bases(Qin and Hurley 2008). Each promoter region often contains more than four G-tracts, thus it can potentially form multiple G-quadruplexes using different combinations of four G-tracts, with each G-quadruplex a potential mixture of loop isomers due to the unequal numbers of guanines on each G-tract. Unlike telomeric G-quadruplexes, which contain invariant TTA loops and appear to be similar in energy, the potential G-quadruplexes formed in a promoter region may differ greatly in energy, with one (or more) major stable structure(s) and other minor unstable structures. Thus it is important to determine the most stable conformation, which is most likely to be biologically relevant, in a promoter sequence. The combination of mutational analysis, DMS footprinting, and NMR spectroscopy has been shown to be a powerful method to identify the major G-quadruplex formed under physiological conditions and to determine the appropriately mutated sequence which forms only the major and most biologically relevant G-quadruplex for structure determination and its drug interactions. It is noteworthy that a small molecule compound may selectively bind a minor conformation and thus shift the equilibrium.
c-MYC is the first and most extensively studied system for the promoter G-quadruplex formation. The major G-quadruplex formed in the c-MYC promoter is a parallel-stranded structure with two G3NG3 single-nt strand-reversal loops (Ambrus et al. 2005) (Figure 4). Since then, there has been a growing list of molecular structures reported for G-quadruplexes formed in gene promoters (Yang and Okamoto 2010). Parallel-stranded structures are found to be more common in the promoter G-quadruplexes, and a robust parallel-stranded structure motif with a 1-nt loop (as in the G3NG3 sequence where N is a loop residue) has been found to be highly prevalent in the promoter sequences (Figure 2). Again, we focus on the unimolecular structures formed in gene promoters.
Figure 4.
(A) The promoter structure of the human c-MYC gene. The G-rich NHE III1 is shown, with guanine runs underlined. (B) The c-MYC promoter sequence and its modifications. (C) The folding structure of the major G-quadruplex formed in the c-MYC promoter, MycG4, a parallel-stranded structure with (1:2:1) loop-size arrangement (left); and the representative NMR structure of MycG4, with two potassium ions coordinated between the G-tetrads shown as green spheres (right). Guanine = yellow, adenine = red, thymine = blue.
The c-MYC promoter G-quadruplex, a prototype for the unimolecular parallel G-quadruplex
The G-quadruplex-forming region in the c-MYC promoter, a 27-nt c-MYC NHE III1 sequence (mycPu27), is comprised of five consecutive runs of guanines (Figure 4A). By mutational analysis in conjunction with DMS footprinting and luciferase reporter assays, the major G-quadruplex formed in the c-MYC NHE III1 in K+ solution has been shown to involve the 3′ II, III, IV, and V-runs of guanines (myc2345, Figure 4B) (Siddiqui-Jain et al. 2002; Seenisamy et al. 2004). Myc2345 can form a mixture of four loop isomers which has been shown to adopt a parallel-stranded folding topology in K+ solution (Phan et al. 2004; Seenisamy et al. 2004). We have determined the K+-solution structure of the major loop isomer of myc2345, which has a 1:2:1 loop-size arrangement, using mycPu22 (MycG4, Figure 4C) (Ambrus et al. 2005). The thermodynamic and kinetic study of the four c-MYC loop isomers in K+ solution show that the difference in stabilities of the four loop isomers, and hence between the parallel G-quadruplexes with similar loop lengths, is more significant than previously recognized (Hatzakis et al. 2010). More recently, a G-quadruplex involving the 5' I, II, III, and IV-runs of guanines (myc1234, Figure 4B) is found to form in the c-MYC NHE III1 in a supercoiled plasmid system (Sun and Hurley 2009). The NMR and thermodynamic study in K+ solution show that the major G-quadruplex formed in myc1234, although sharing the same parallel folding with 1:2:1 loop-size arrangement, is markedly less stable than that formed in myc2345 (ΔTm~12 °C in 20 mM K+), likely due to different base conformations of the single-nt loops (Mathad et al. 2011) (mycPu19, Figure 4B).
The major c-MYC promoter G-quadruplex (MycG4, Figure 4C) is a parallel-stranded structure with three tetrads, connected by two 1-nt loops (G3NG3 motif) and one 2-nt loop (Ambrus et al. 2005). This is the first molecular structure that contains the G3NG3 parallel-stranded motif with a 1-nt strand-reversal loop, which appears to be highly stable. The stability of the parallel-stranded G3NG3 motif is shown by the NMR structure to be highly favored due to the proximity of the 3’-end of one strand to the 5’-end of the adjacent strand caused by the right-handed twist of the two adjacent G-strands (Figure 4C right). Both of the 5′- and the 3′-flanking segments form well-defined structures capping the ends of the MycG4 G-quadruplex (Figure 4C right).
Other gene promoter sequences that form standard parallel G-quadruplexes
In addition to c-MYC, parallel-stranded G-quadruplexes have been found to form in a number of other human oncogene promoter regions, such as VEGF (Guo et al. 2008), HIF-1α (De Armond et al. 2005), c-KIT (Fernando et al. 2006), RET (Guo et al. 2007), and PDGF-A (Qin et al. 2007). All of these parallel-stranded promoter G-quadruplexes contain two G3NG3 motifs at their 1st and 3rd loops, with variable lengths of the middle loop (Figure 2), except for PDGF-A promoter which forms a four G-tetrad parallel-stranded G-quadruplex(Qin et al. 2007). We have recently determined the NMR K+-solution structure of the major G-quadruplex formed in the human VEGF promoter (Agrawal in submission), which has previously been shown to adopt a parallel folding with a 1:4:1 loop-size arrangement (Guo et al. 2008) (Figure 5A). Our NMR data show that, while the VEGF promoter G-quadruplex is also a parallel structure, unlike the 2-nt middle loop of the MycG4 that stays in the groove, the 4-nt middle loop of the VEGF-G4 appears to stretch over the terminal tetrad to form specific capping structures with the flanking segments. In addition, the VEGF-G4 is less stable than MycG4 in K+ solution. Therefore, while parallel folding is common to the promoter G-quadruplexes, each structure is likely to have unique capping and loop structures, formed by its specific flanking sequences and variable middle loop, which together also determine the stability of the overall G-quadruplex structure.
Figure 5.
(A) The folding structure of the major G-quadruplex formed in the VEGF promoter region. (B) The sequence and folding topology of one G-rich region (c-KIT87up) of the human c-KIT gene promoter. (C) The sequence of the G-rich region of the human c-Myb gene promoter.
Variant parallel-stranded G-quadruplexes formed in gene promoter sequences
Interestingly, several variant parallel structures have been shown to form in the gene promoter sequences.
Parallel-stranded G-quadruplex with a broken-strand
The G-quadruplex formed in the human c-KIT promoter sequence c-KIT87up (Rankin et al. 2005) (Figure 5B) has been shown to form a unique snapback parallel-stranded G-quadruplex with a broken strand (Phan et al. 2007) (Figure 5B). Despite the presence of four three-guanine tracts in this sequence, the backbone of one G-strand of the G-tetrad core is interrupted (Figure 5B), so that this three G-tetrad-structure can have two 1-nt strand-reversal loops (G3NG3 and G3NG…GG) connecting the two adjacent parallel-stranded motifs (Figure 5B). Therefore, in addition to three double-chain-reversal loops, this snapback parallel-stranded structure contains a fourth lateral loop.
Parallel-stranded G-quadruplex with a heptad plane
The G-rich proximal promoter of human c-MYB gene is unique in its sequence, which contains three imperfect repeats of (GGA)4 (Figure 5C). The major structure formed in this sequences appears to be a dimer composed of two parallel-stranded unimolecular heptad:tetrad (H:T) structures (Figure 1D middle) involving two consecutive 5’ end (GGAGGAGGAGG) repeats (Palumbo et al. 2008). The unimolecular T:H:H:T structure has been previously shown by NMR to form in a d(GGA)8 sequence (Matsugami et al. 2003).
Non-parallel-stranded G-quadruplex formed in the BCL-2 promoter – also has a G3NG3
The 39-mer G-rich region of the human BCL-2 promoter (Figure 6A) contains six G-tracts, and the G-quadruplex formed in the middle four G-tracts (bcl2MidG4) has been shown to be one of the most stable unimolecular structures. We have determined the folding and molecular structure of bcl2MidG4 using the bcl2Mid sequence in K+ solution, which is a hybrid-type G-quadruplex of three tetrads connected by two 3-nt and 7-nt lateral loops and a 1-nt strand-reversal loop (Dai et al. 2006; Dai et al. 2006) (Figure 6B). Interestingly, the bcl2Mid sequence exhibits some similarity to those promoter sequences that form the parallel-stranded structures (Figure 2), which all contain an extended middle loop. However, the 1st loop of bcl2Mid is 3 nt, versus 1 nt in the other sequences, and adopts a lateral versus strand-reversal conformation which appears to determine the overall folding of the bcl2MidG4 structure, namely the hybrid-type mixed parallel/antiparallel-stranded, as opposed to the parallel-stranded, G-quadruplex. This indicates that the strand-reversal loop of a parallel-stranded motif clearly favors a short loop-size, particularly 1 nt as seen in the G3NG3 motif. Notably, bcl2Mid also contains a 1-nt loop which forms a strand-reversal conformation, again stressing the robustness of the G3NG3 parallel-stranded motif (Figure 6A).
Figure 6.
(A) The promoter structure of the human BCL-2 gene. The G/C-rich region of the promoter is shown, with guanine runs underlined. The bcl2Mid sequence of the middle four G-tracts is also shown. (B) The folding structure of the major G-quadruplex formed in the BCL-2 promoter, bcl2MidG4 (left), and its NMR K+-solution structure (right).
More complex G-quadruplexes formed in the gene promoter sequences
More complex G-quadruplexes have been suggested in several gene promoter sequences. For example, two separate G-quadruplex-forming sequences (c-KIT21 and c-KIT87up), which are about 30 bases apart, have been discovered within a nuclease hypersensitive region of the human c-KIT promoter (Rankin et al. 2005; Fernando et al. 2006). While the snapback parallel-stranded G-quadruplex has been shown to form in c-KIT87up (Phan et al. 2007) (Figure 5B), a standard unimolecular parallel-stranded G-quadruplex appears to form in c-KIT21 in K+ solution (Hsu et al. 2009). The human c-MYB promoter appears to form a dimer of two heptad:tetrad structures involving two (GGAGGAGGAGG) repeats (Palumbo et al. 2008) (Figure 5C). More recently, two stacked G-quadruplexes separated by 7 bases have been found in the human hTERT promoter, with one being the standard parallel G-quadruplex and another being a unique hybrid-type structure with a 26-nt middle loop which likely forms a secondary stem-loop structure (Palumbo et al. 2009). We anticipate that the horizon of the promoter G-quadruplexes will expand, as more G-quadruplexes formed in the promoter sequences are being discovered or characterized.
GiNGj sequence motif forms robust parallel-stranded structure motif with a 1-nt loop and is highly prevalent in the promoter sequences
Significantly, despite the manifold variations of promoter quadruplexes, there is a wide prevalence of the G3NG3 (or more generally GiNGj) sequence motif, which forms a robust parallel-stranded structure motif with a 1-nt loop (Figure 2). Actually, this robust motif has been found in all types of the promoter G-quadruplexes, such as standard parallel structures (e.g., c-MYC, VEGF), variant parallel structures (e.g., c-KIT snapback), and hybrid-type mixed parallel/antiparallel structures (e.g., BCL-2, hTERT). In fact, the G3NG3 motif is so common in the quadruplex-forming gene promoter sequences that it could be inferred that the robust parallel-stranded motif with a 1-nt loop may be evolutionarily selected to serve as a stable foundation for the promoter G-quadruplexes to build upon.
INTERACTIONS OF G-QUADRUPLEXES WITH SMALL MOLECULES
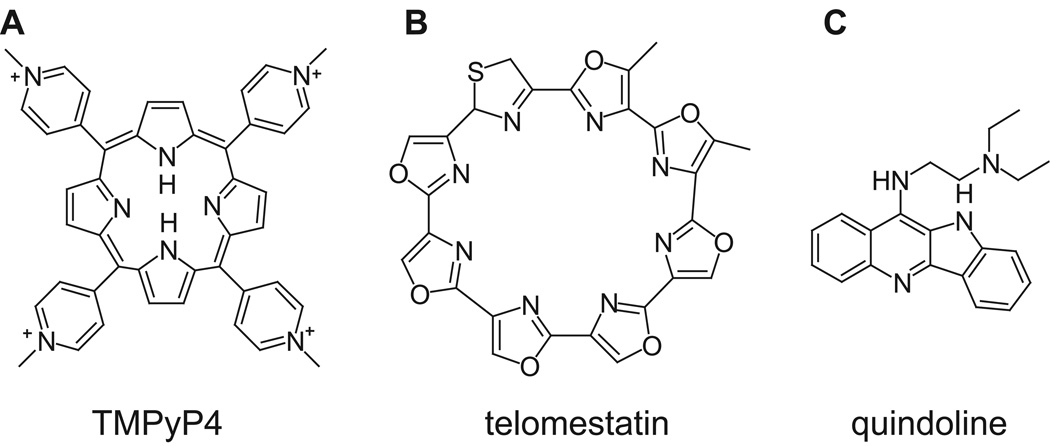
As the biological significance of the DNA G-quadruplex has been recognized, the development of G-quadruplex-interactive compounds has intensified. Some G-quadruplex-interactive compounds have become prospective anticancer agents that display relatively low cytotoxicity (Yang and Okamoto 2010). In particular, Quarfloxin, a first-in-class G-quadruplex-interactive drug reached phase 2 clinical trials (Drygin et al. 2009). However, finding small molecule compounds that bind specifically to a unimolecular G-quadruplex has not been easy. Although there is a growing list of molecular structures reported for G-quadruplexes formed in gene promoters, the structures of their drug complexes have been more difficult to obtain. The previous dogma on the optimal G-quadruplex-interactive compounds has focused on those with symmetric cyclic fused rings, such as TMPyP4 (Han et al. 1999) (Figure 7A) and telomestatin (Shin-ya et al. 2001) (Figure 7B) as they provide maximized stacking interactions with the external G-tetrad. However, we have examined the binding of such G-quadruplex-interactive compounds to various unimolecular DNA G-quadruplexes, and found that those compounds with large symmetric cyclic fused rings in general do not bind unimolecular G-quadruplexes specifically as shown by poorly-resolved NMR spectrum of the complex.
Figure 7.
Formulas of TMPYP4 (A), telomestatin (B), and quindoline (C).
We have very recently determined the NMR solution structure of a 2:1 complex of a small molecule quindoline (Figure 7C) with the major G-quadruplex formed in the c-MYC promoter (Dai et al. 2011), which represents the first ligand complex structure of a biologically relevant unimolecular promoter quadruplex (Figure 8). As the c-MYC promoter G-quadruplex is a prototype of this family, this complex structure provides important new insights into the structure-based rational design of drugs that bind to unimolecular parallel G-quadruplexes commonly found in promoter elements. Most of the previous ligand–G-quadruplex complex structures have been determined by X-ray crystallography and are derived from telomeric sequences that form bimolecular and tetramolecular species (Neidle 2009). The only known unimolecular ligand complex is a TMPyP4 complex with a quadruplex formed in a modified c-MYC promoter sequence (Phan et al. 2005). However, this G-quadruplex is not observed in the wild-type c-MYC promoter sequence and thus does not appear to be biologically relevant, as it contains a guanine-to-inosine substitution at the guanine position shown to be critical for the G-quadruplex formation in the wild-type mycPu27 sequence (Figure 3) under physiologically relevant conditions by DMS footprinting and luciferase reporter data (Siddiqui-Jain et al. 2002). In addition, this guanine-to-inosine substitution induces a G-strand discontinuity in the tetrad core, which needs to be fulfilled by a downstream guanine that is clearly shown not to be involved in the G-tetrad formation in the wild-type mycPu27 sequence by DMS footprinting (Siddiqui-Jain et al. 2002). In this complex the TMPyP4 stacks over the 5’-end but the orientation of TMPyP4 was not resolved by NMR data and a well-defined binding pocket was not observed. In this review we will focus on our recent quindoline complex structure of the major c-MYC promoter G-quadruplex MycG4 (Dai et al. 2011).
Figure 8.
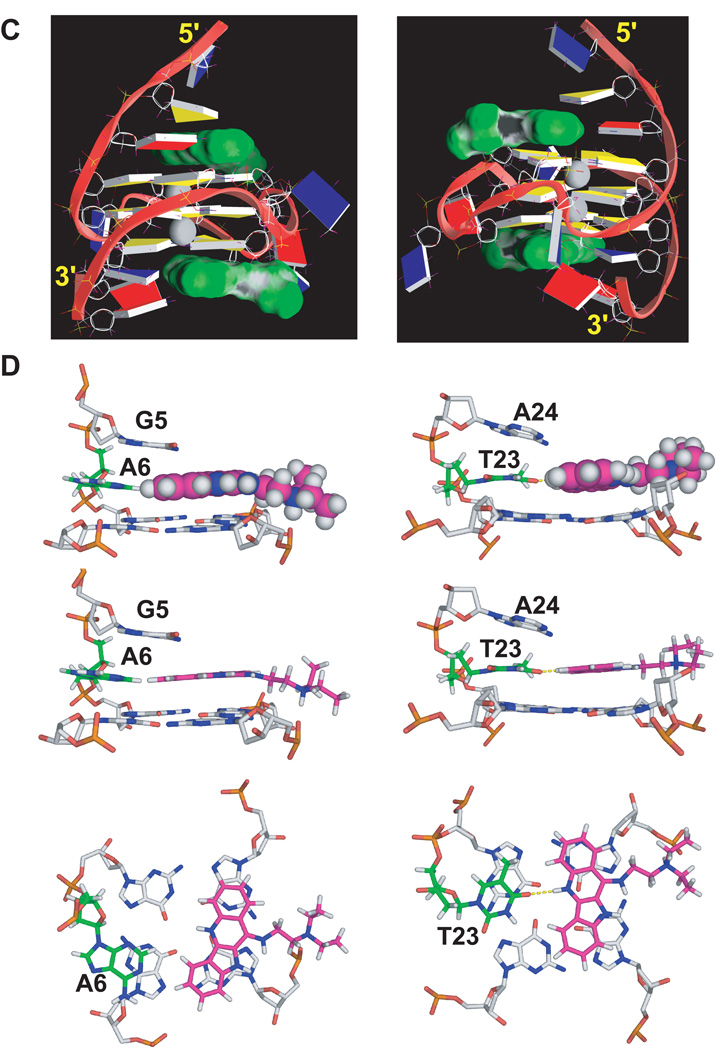
(A) Imino proton regions of the 1D 1H NMR titration spectra of mycPu22 with quindoline in K+ solution. The assignments of imino protons of the free DNA and 2:1 quindoline:DNA complex are shown above the spectra. The imino protons from the 5’ G-tetrad are colored in red, the middle G-tetrad in blue, and the 3’ G-tetrad in green. (B) The imino regions of 1D 1H NMR spectra of MycG4 (left) and the 2:1 quindoline:MycG4 complex (right) at various temperatures in pH6 10 mM K+ solution. (C) A representative model of the NMR-refined 2:1 quindoline:MycG4 complex structure from two different views. The quindoline molecules are shown in space-filling model in green. The two potassium ions are shown as white balls. guanine = yellow, adenine = red, thymine = blue. (D) Different views of the drug-induced binding pockets at the 5’-end (left) and at the 3’-end (right).
Quindoline binds the MycG4 to form a well-defined 2:1 drug-DNA complex
The quindoline molecule (Figure 7C) is a derivative of the natural product cryptolepine (Guyen et al. 2004; Zhou et al. 2005), and has been shown to stabilize the G-quadruplex formed in the c-MYC promoter and thus inhibit the expression of c-MYC in the hepatocellular carcinoma cell line H2p G2 (Ou et al. 2007). As described previously, the major c-MYC promoter G-quadruplex formed in mycPu22 (MycG4, Figure 4C) is a 3-tetrad parallel-stranded G-quadruplex with three strand-reversal loops of T, TA, and T. NMR spectroscopy is a very useful method to study G-quadruplexes and their drug interactions. MycG4 exhibits twelve well-resolved imino proton peaks for the twelve tetrad-guanines of three G-tetrads by NMR, which are isolated from other groups of protons. Upon quindoline binding, NMR titration data show a new set of imino proton signals, which become twelve well-resolved peaks at the drug equivalence of 2N, indicating a stable 2:1 quindoline-DNA complex is formed with the MycG4 (Figure 8A). The same 2:1 complex was shown to form for the wild-type sequence. The NMR variable temperature data showed that binding of quindoline increased the stability of MycG4 by more than 15°C in its melting temperature (Figure 8B), indicating the same complex structure formed at both conditions. It is interesting to note that quindoline binds the two ends of MycG4 with different affinities: it binds the 5’-end with high affinity at the physiological salt condition but binds the 3’-end with high affinity only at the low salt condition.
Unexpected drug-induced reorientation of the two flanking sequences: “induced intercalated triad pocket”
While the parallel folding of MycG4 stays the same, the NMR solution structure of the 2:1 quindoline MycG4 complex structure shows that both flanking two-base sequences undergo unexpected large conformational changes to assemble new drug binding pockets, in a manner somewhat analogous to a reorganized ligand-induced fit observed in riboswitches (Mandal and Breaker 2004). This unexpected drug-induced rearrangement is distinct from the previously proposed model wherein the planar G-quadruplex-interactive compounds would intercalate between the external G-tetrad and the flanking residues of the pre-formed “drug binding pockets”. Instead of binding within a preformed pocket provided by the flanking bases, both quindoline molecules bind to the MYC quadruplex in an “induced-fit” manner such that, upon drug binding, the conformation of flanking segments is changed dramatically so that a new drug binding pocket is formed (Figure 8C). Together with the −1 or +1 flanking residue, each quindoline molecule forms an additional plane, stacking over a total of three of the four guanines in the external G-tetrads. The +2 and −2 flanking residues then wrap over the newly formed quindoline-base planes at each end, respectively. We name this an “induced intercalated triad pocket” to describe the coplanar arrangements of the adenine (−1) or thymine (+1) with the quindoline (which overlay two external tetrad-guanines) and their combined intercalation between the external tetrad and −2 or +2 base (Figure 8D).
Differences between the 5’- and 3’ quindoline-complex: stacking and electronic interactions
The structure of the 2:1 quindoline–MycG4 complex is unique in that it forms new drug binding pockets at both ends of the unimolecular c-MYC G-quadruplex. While both 3’ and 5’ quindoline complexes show large reorientation of the flanking sequences and overall similar features, there are identifiable differences which emphasize the importance of both stacking and electronic interactions. The origin of these differences is due to inherent structural features that are associated with the 3’ and 5’ faces as well as the flanking sequences. The binding of quindoline at the 5’-end is more favored at physiologically relevant K+ concentration, because the 5’-face is more hydrophobic and more accessible for ligand stacking (Figure 8C). Binding of the 5’-end quindoline is more dependent on the stacking interactions that are inherent in the induced binding pocket created by the 5’ tetrad and the two flanking bases at −1 and −2 and is K+ concentration-independent. In contrast, the 3’-end is more hydrophilic and less accessible for ligand stacking. Indeed, the overlay of the 3’-quindoline with the adjacent G-tetrad is clearly less extensive as compared to that of the 5’-quindoline (Figure 8D). Consequently, the increased stability of the 3’-end quindoline complex at lower ionic strength is likely to be related to the specific H-bond interaction between QuiN1H and T23O4 (Figure 8D right) and relies less on capping interactions. These types of subtle but important differences related to the effect of ionic strength in ligand recognition between the 3’ and 5’ ends can only be monitored in solution and thus are amenable to NMR studies.
Insights into structure-based design of molecules that interact with unimolecular parallel G-quadruplex structures
An important implication from the 2:1 quindoline-MycG4 complex structure is that compounds containing a smaller asymmetric stacking moiety, in particular the crescent shape moiety, with appropriate functional groups, are more likely to bind in a defined manner to a unimolecular G-quadruplex, unlike the previously recognized paradigm that the preferred G-quadruplex-interactive compounds are those with symmetric cyclic fused rings, typified by TMPyP4, to maximize stacking interactions with the external G-tetrad (Figure 7). Our structure shows that specific binding and selectivity of the quindoline and other similar crescent-shaped molecules are determined by both the identity of the binding end (3’ or 5’) and the flanking two bases (Figure 8C). The crescent-shaped quindoline, importantly, provides optimal overlay of two guanines of the external G-tetrad (Figure 8D). Additionally, the electrostatic interaction between the diethylamino group in the side chain of quindoline and the DNA phosphate backbone could help orient and stabilize the quindoline nucleus, which in turn specifically pinpoints the potential location of substituents that can interact in the grooves and with the loops. It is anticipated that small changes in both the shape and electronic structure of the ligand, as well as the identity of the flanking bases making up the intercalated triad pockets, will affect the precise positioning of the ligand relative to the adjacent G-tetrad. Thus our structure for the first time describes the importance of the two flanking bases as well as the shape of the ligand in determining drug binding specificity.
The 2:1 quindoline–c-MYC G-quadruplex complex described here provides an important case study for the biologically important parallel intramolecular G-quadruplexes in promoter elements. For this “induced intercalated triad pocket” to form, a suitable single-stranded flanking segment containing at least two bases must exist, which has been shown in the c-MYC promoter under negative supercoiled conditions (Sun and Hurley 2009). In addition, there are two dimeric telomeric G-quadruplexes in which a thymine residue is recruited into a similar in-line triad plane (Haider et al. 2003; Campbell et al. 2008). Although the multimeric nature of both structures make them less relevant to the unimolecular structures, the hijacking of a base into the ligand plane reinforces the principle described here for both quindoline G-quadruplex complexes. Therefore, this reorientation occurs at both ends of the MycG4 and the recruitment of a flanking base into the plane of the ligand suggests a significant feature of drug complexes with G-quadruplexes. This divides the molecular mechanism for G-quadruplex recognition by small molecules of this type into two clear sequential steps, involving first the newly recognized induced intercalated triad pocket recognition and second the groove/loop interactions. The first step provides new opportunities for selective recognition by small molecules that bind to unimolecular parallel structures commonly found in gene promoters.
CONCLUSIONS AND FUTURE DIRECTION
DNA G-quadruplexes are four-stranded globular nucleic acid secondary structures formed in specific G-rich sequences. DNA G-quadruplexes can readily form under physiological conditions; the stable formation of G-quadruplexes requires monovalent cations, in particular K+ and Na+. Unimolecular DNA G-quadruplexes have recently been demonstrated to form as a regulatory element in regions of biological significance, specifically the human telomeres and the proximal promoter regions of oncogenes. Unimolecular DNA G-quadruplexes exhibit great conformational diversity, which comes not only from different folding patterns, but also from specific loop conformations and capping structures. Different sequences can adopt distinct topologies; but a given sequence can also fold into a variety of different conformations. The highly conserved human telomeric DNA sequence with the tandem repeats of d(TTAGGG) is able to form different G-quadruplex structures with small energy difference. This intrinsic structure polymorphism of the human telomeric sequence appears to be closely related to the invariable TTA loops of the human telomeric G-quadruplexes. In contrast, the G-quadruplex-forming promoter regions are more diverse in sequence and can potentially form multiple G-quadruplexes and loop isomers, thus it is important to determine the most stable and thus the biologically relevant conformation in a promoter sequence. The parallel-stranded structures are found to be more common in the promoter G-quadruplexes, while the strand-reversal loop of a parallel-stranded motif clearly favors a short loop-size, particularly the 1-nt loop. Intriguingly, the robust parallel-stranded structure motif G3NG3 with a 1-nt loop is found to be highly prevalent in the promoter sequences. Not only does each parallel-stranded structure contain unique capping and loop structures determined by the specific flanking and loop sequences, but the parallel-stranded G-quadruplexes also come in variant forms, such as the heptad-tetrad or with a broken-strand.
Unimolecular DNA G-quadruplexes formed in the human telomeres and the oncogene proximal promoter regions have been shown to be amenable to small molecule drug targeting and thus are considered as a new class of molecular targets for cancer therapeutics. Unlike the previously recognized paradigm that the preferred G-quadruplex-interactive compounds are those with symmetric cyclic fused rings, typified by TMPyP4, to maximize stacking interactions with the external G-tetrad, our recent NMR solution structure of a 2:1 complex of quindoline with the major c-MYC promoter G-quadruplex suggested that compounds containing a smaller asymmetric stacking moiety with appropriate functional groups are more likely to bind in a defined manner to a unimolecular G-quadruplex. The crescent shape moiety of quindoline provides optimal overlay of two guanines of the external G-tetrad, while the specific binding and selectivity of such molecules are determined by both the binding site (3’ or 5’) and the flanking bases, in addition to the specific interaction between the side chain(s) and the DNA phosphate backbone in the grooves or of the loops. While this complex structure provides important insights into the specific molecular binding to unimolecular parallel G-quadruplexes commonly found in promoter elements, it is noted that different paradigms likely exist for antiparallel or mixed parallel/antiparallel structures. The conformational diversity indicates that in principle DNA G-quadruplexes can be specifically targeted by small molecules; however, complete specificity may not be necessary, as in the case of kinase inhibitors. In contrast, selectivity towards G-quadruplex structure over duplex DNA is critical for quadruplex-targeting small molecule drugs. We anticipate this new paradigm of specific G-quadruplex binding by molecules with smaller asymmetric stacking moiety with appropriate functional groups will be seen in more small molecule-quadruplex complexes and will be taken into serious consideration in structure-based rational design of G-quadruplex-interactive small molecule drugs. While recent progress in the structural studies suggested a number of rules governing G-quadruplex folding, complete prediction of a G-quadruplex conformation has not yet been made possible. Thus structure characterization remains essential to understand G-quadruplex folding, structures, and small molecule binding interactions, as well as biological functions such as protein recognition.
Acknowledgements
This research was supported by the National Institutes of Health (1S10RR16659, CA122952, GM083117, CA153821). We thank Dr. Megan Carver for proofreading the paper.
References
- Agrawal P, Hatzakis E, Guo K, Yang D. Solution Structure of the G-Quadruplex formed in the proximal promoter region of the human VEGF gene. doi: 10.1093/nar/gkt784. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrus A, Chen D, Dai J, Bialis T, Jones RA, Yang DZ. Human telomeric sequence forms a hybrid-type intramolecular G-quadruplex structure with mixed parallel/antiparallel strands in potassium solution. Nucleic Acids Research. 2006;34(9):2723–2735. doi: 10.1093/nar/gkl348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrus A, Chen D, Dai J, Jones RA, Yang DZ. Solution structure of the biologically relevant G-quadruplex element in the human c-MYC promoter. Implications for G-quadruplex stabilization. Biochemistry. 2005;44(6):2048–2058. doi: 10.1021/bi048242p. [DOI] [PubMed] [Google Scholar]
- Berberich SJ, Postel EH. Puf/Nm23-H2/Ndpk-B Transactivates a Human C-Myc Promoter-Cat Gene Via a Functional Nuclease Hypersensitive Element. Oncogene. 1995;10(12):2343–2347. [PubMed] [Google Scholar]
- Brooks TA, Hurley LH. The role of supercoiling in transcriptional control of MYC and its importance in molecular therapeutics. Nature Reviews Cancer. 2009;9(12):849–861. doi: 10.1038/nrc2733. [DOI] [PubMed] [Google Scholar]
- Campbell NH, Parkinson GN, Reszka AP, Neidle S. Structural basis of DNA quadruplex recognition by an acridine drug. Journal of the American Chemical Society. 2008;130(21):6722–6724. doi: 10.1021/ja8016973. [DOI] [PubMed] [Google Scholar]
- Cogoi S, Xodo LE. G-quadruplex formation within the promoter of the KRAS proto-oncogene and its effect on transcription. Nucleic Acids Research. 2006;34(9):2536–2549. doi: 10.1093/nar/gkl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LM, Reddel RR. Telomere maintenance mechanisms and cellular immortalization. Current Opinion in Genetics & Development. 1999;9(1):97–103. doi: 10.1016/s0959-437x(99)80014-8. [DOI] [PubMed] [Google Scholar]
- Dai J, Carver M, Punchihewa C, Jones RA, Yang DZ. Structure of the Hybrid-2 type intramolecular human telomeric G-quadruplex in K+ solution: insights into structure polymorphism of the human telomeric sequence. Nucleic Acids Research. 2007;35(15):4927–4940. doi: 10.1093/nar/gkm522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Carver M, Yang DZ. Polymorphism of human telomeric quadruplex structures. Biochimie. 2008;90(8):1172–1183. doi: 10.1016/j.biochi.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Chen D, Jones RA, Hurley LH, Yang DZ. NMR solution structure of the major G-quadruplex structure formed in the human BCL2 promoter region. Nucleic Acids Research. 2006;34(18):5133–5144. doi: 10.1093/nar/gkl610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Dexheimer TS, Chen D, Carver M, Ambrus A, Jones RA, Yang DZ. An Intramolecular G-Quadruplex Structure with Mixed Parallel/Antiparallel G-strands Formed in the Human BCL-2 Promoter Region in Solution. Journal of the American Chemical Society. 2006;128(4):1096–1098. doi: 10.1021/ja055636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Punchihewa C, Ambrus A, Chen D, Jones RA, Yang DZ. Structure of the intramolecular human telomeric G-quadruplex in potassium solution: a novel adenine triple formation. Nucleic Acids Research. 2007;35(7):2440–2450. doi: 10.1093/nar/gkm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JX, Carver M, Hurley LH, Yang DZ. Solution Structure of a 2:1 Quindoline-c-MYC G-Quadruplex: Insights into G-Quadruplex-Interactive Small Molecule Drug Design. Journal of the American Chemical Society. 2011;133(44):17673–17680. doi: 10.1021/ja205646q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Armond R, Wood S, Sun DY, Hurley LH, Ebbinghaus SW. Evidence for the presence of a guanine quadruplex forming region within a polypurine tract of the hypoxia inducible factor 1 alpha promoter. Biochemistry. 2005;44(49):16341–16350. doi: 10.1021/bi051618u. [DOI] [PubMed] [Google Scholar]
- De Cian A, Lacroix L, Douarre C, Temime-Smaali N, Trentesaux C, Riou JF, Mergny JL. Targeting telomeres and telomerase. Biochimie. 2007;90(1):131–155. doi: 10.1016/j.biochi.2007.07.011. [DOI] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes & Development. 2005;19(18):2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Dexheimer TS, Carey SS, Zuohe S, Gokhale VM, Hu X, Murata LB, Maes EM, Weichsel A, Sun D, Meuillet EJ, Montfort WR, Hurley LH. NM23-H2 may play an indirect role in transcriptional activation of c-myc gene expression but does not cleave the nuclease hypersensitive element III1. Molecular Cancer Therapeutics. 2009;8(5):1363–1377. doi: 10.1158/1535-7163.MCT-08-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexheimer TS, Sun D, Hurley LH. Deconvoluting the structural and drug-recognition complexity of the G-quadruplex-forming region upstream of the bcl-2 P1 promoter. Journal of the American Chemical Society. 2006;128(16):5404–5415. doi: 10.1021/ja0563861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drygin D, Siddiqui-Jain A, O'Brien S, Schwaebe M, Lin A, Bliesath J, Ho CB, Proffitt C, Trent K, Whitten JP, Lim JKC, Von Hoff D, Anderes K, Rice WG. Anticancer Activity of CX-3543: A Direct Inhibitor of rRNA Biogenesis. Cancer Research. 2009;69(19):7653–7661. doi: 10.1158/0008-5472.CAN-09-1304. [DOI] [PubMed] [Google Scholar]
- Fernando H, Reszka AP, Huppert J, Ladame S, Rankin S, Venkitaraman AR, Neidle S, Balasubramanian S. A Conserved Quadruplex Motif Located in a Transcription Activation Site of the Human c-kit Oncogene. Biochemistry. 2006;45(25):7854–7860. doi: 10.1021/bi0601510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M, Lipsett MN, Davies DR. Helix formation by guanylic acid. Proceedings of the National Academy of Sciences of the United States of America. 1962;48:2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez V, Guo K, Hurley L, Sun D. Identification and Characterization of Nucleolin as a c-myc G-quadruplex-binding Protein. Journal of Biological Chemistry. 2009;284(35):23622–23635. doi: 10.1074/jbc.M109.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand CL, Han H, Munoz RM, Weitman S, Von Hoff DD, Hurley LH, Bearss DJ. The Cationic Porphyrin TMPyP4 Down-Regulates c-MYC and Human Telomerase Reverse Transcriptase Expression and Inhibits Tumor Growth in Vivo. Molecular Cancer Therapeutics. 2002;1(8):565–573. [PubMed] [Google Scholar]
- Guo K, Gokhale V, Hurley LH, Sun D. Intramolecularly folded G-quadruplex and i-motif structures in the proximal promoter of the vascular endothelial growth factor gene. Nucleic Acids Research. 2008;36(14):4598–4608. doi: 10.1093/nar/gkn380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K, Pourpak A, Beetz-Rogers K, Gokhale V, Sun D, Hurley LH. Formation of pseudosymmetrical G-quadruplex and i-motif structures in the proximal promoter region of the RET oncogene. Journal of the American Chemical Society. 2007;129(33):10220–10228. doi: 10.1021/ja072185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyen B, Schultes CM, Hazel P, Mann J, Neidle S. Synthesis and evaluation of analogues of 10H-indolo[3,2-b]-quinoline as G-quadruplex stabilising ligands and potential inhibitors of the enzyme telomerase. Organic & Biomolecular Chemistry. 2004;2(7):981–988. doi: 10.1039/b316055f. [DOI] [PubMed] [Google Scholar]
- Haider SM, Parkinson GN, Neidle S. Structure of a G-quadruplex-ligand complex. Journal of Molecular Biology. 2003;326(1):117–125. doi: 10.1016/s0022-2836(02)01354-2. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack MC, Dobrinski B, Lurz R, Docherty K, Kilpatrick MW. The human insulin gene linked polymorphic region exhibits an altered DNA structure. Nucleic Acids Research. 1992;20(2):231–236. doi: 10.1093/nar/20.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han FXG, Wheelhouse RT, Hurley LH. Interactions of TMPyP4 and TMPyP2 with quadruplex DNA. Structural basis for the differential effects on telomerase inhibition. Journal of the American Chemical Society. 1999;121(15):3561–3570. [Google Scholar]
- Hatzakis E, Okamoto K, Yang DZ. Thermodynamic Stability and Folding Kinetics of the Major G-Quadruplex and Its Loop Isomers Formed in the Nuclease Hypersensitive Element in the Human c-Myc Promoter: Effect of Loops and Flanking Segments on the Stability of Parallel-Stranded Intramolecular G-Quadruplexes. Biochemistry. 2010;49(43):9152–9160. doi: 10.1021/bi100946g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E, Hardin CC, Walk SK, Tinoco I, Jr, Blackburn EH. Telomeric DNA oligonucleotides form novel intramolecular structures containing guanine-guanine base pairs. Cell. 1987;51(6):899–908. doi: 10.1016/0092-8674(87)90577-0. [DOI] [PubMed] [Google Scholar]
- Hsu STD, Varnai P, Bugaut A, Reszka AP, Neidle S, Balasubramanian S. A G-Rich Sequence within the c-kit Oncogene Promoter Forms a Parallel G-Quadruplex Having Asymmetric G-Tetrad Dynamics. Journal of the American Chemical Society. 2009;131(37):13399–13409. doi: 10.1021/ja904007p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LH, Wheelhouse RT, Sun D, Kerwin SM, Salazar M, Fedoroff OY, Han FX, Han H, Izbicka E, Von Hoff DD. G-quadruplexes as targets for drug design. Pharmacology & Therapeutics. 2000;85(3):141–158. doi: 10.1016/s0163-7258(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Kostadinov R, Malhotra N, Viotti M, Shine R, D'Antonio L, Bagga P. GRSDB: a database of quadruplex forming G-rich sequences in alternatively processed mammalian pre-mRNA sequences. Nucleic Acids Research. 2006;34:D119–D124. doi: 10.1093/nar/gkj073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzine F, Sanford S, Elisha-Feil Z, Levens D. The functional response of upstream DNA to dynamic supercoiling in vivo. Nature Structural & Molecular Biology. 2008;15(2):146–154. doi: 10.1038/nsmb.1372. [DOI] [PubMed] [Google Scholar]
- Kumari S, Bugaut A, Huppert JL, Balasubramanian S. An RNA G-quadruplex in the 5 ' UTR of the NRAS proto-oncogene modulates translation. Nature Chemical Biology. 2007;3(4):218–221. doi: 10.1038/nchembio864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle C. DNA torsional stress propagates through chromatin fiber and participates in transcriptional regulation. Nature Structural & Molecular Biology. 2008;15(2):123–125. doi: 10.1038/nsmb0208-123. [DOI] [PubMed] [Google Scholar]
- Lim KW, Amrane S, Bouaziz S, Xu WX, Mu YG, Patel DJ, Luu KN, Phan AT. Structure of the Human Telomere in K+ Solution: A Stable Basket-Type G-Quadruplex with Only Two G-Tetrad Layers. Journal of the American Chemical Society. 2009;131(12):4301–4309. doi: 10.1021/ja807503g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu KN, Phan AT, Kuryavyi V, Lacroix L, Patel DJ. Structure of the human telomere in K+ solution: An intramolecular (3+1) G-quadruplex scaffold. Journal of the American Chemical Society. 2006;128(30):9963–9970. doi: 10.1021/ja062791w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels N. Dynamic roles for G4 DNA in the biology of eukaryotic cells. Nature Structural & Molecular Biology. 2006;13(12):1055–1059. doi: 10.1038/nsmb1171. [DOI] [PubMed] [Google Scholar]
- Mandal M, Breaker RR. Gene regulation by riboswitches. Nature Reviews Molecular Cell Biology. 2004;5(6):463. doi: 10.1038/nrm1403. [DOI] [PubMed] [Google Scholar]
- Mathad RI, Hatzakis E, Dai J, Yang D. c-MYC promoter G-quadruplex formed at the 5'-end of NHE III1 element: insights into biological relevance and parallel-stranded G-quadruplex stability. Nucleic Acids Research. 2011;39(20):9023–9033. doi: 10.1093/nar/gkr612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsugami A, Okuizumi T, Uesugi S, Katahira M. Intramolecular Higher Order Packing of Parallel Quadruplexes Comprising a G:G:G:G Tetrad and a G(:A):G(:A):G(:A):G Heptad of GGA Triplet Repeat DNA. Journal of Biological Chemistry. 2003;278(30):28147–28153. doi: 10.1074/jbc.M303694200. [DOI] [PubMed] [Google Scholar]
- Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle S. The structures of quadruplex nucleic acids and their drug complexes. Current Opinion in Structural Biology. 2009;19(3):239–250. doi: 10.1016/j.sbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Neidle S, Parkinson G. Telomere maintenance as a target for anticancer drug discovery. Nature Reviews Drug Discovery. 2002;1(5):383–393. doi: 10.1038/nrd793. [DOI] [PubMed] [Google Scholar]
- Ou TM, Lu YJ, Zhang C, Huang ZS, Wang XD, Tan JH, Chen Y, Ma DL, Wong KY, Tang JCO, Chan ASC, Gu LQ. Stabilization of G-quadruplex DNA and down-regulation of oncogene c-myc by quindoline derivatives. Journal of Medicinal Chemistry. 2007;50(7):1465–1474. doi: 10.1021/jm0610088. [DOI] [PubMed] [Google Scholar]
- Palumbo SL, Ebbinghaus SW, Hurley LH. Formation of a Unique End-to-End Stacked Pair of G-Quadruplexes in the hTERT Core Promoter with Implications for Inhibition of Telomerase by G-Quadruplex-Interactive Ligands. Journal of the American Chemical Society. 2009;131(31):10878–10891. doi: 10.1021/ja902281d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo SL, Memmott RM, Uribe DJ, Krotova-Khan Y, Hurley LH, Ebbinghaus SW. A novel G-quadruplex-forming GGA repeat region in the c-myb promoter is a critical regulator of promoter activity. Nucleic Acids Research. 2008;36(6):1755–1769. doi: 10.1093/nar/gkm1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson GN, Lee MPH, Neidle S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature. 2002;6891;417:876–880. doi: 10.1038/nature755. [DOI] [PubMed] [Google Scholar]
- Petraccone L, Trent JO, Chaires JB. The Tail of the Telomere. Journal of the American Chemical Society. 2008;130(49):16530–16532. doi: 10.1021/ja8075567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan AT, Kuryavyi V, Burge S, Neidle S, Patel DJ. Structure of an unprecedented G-quadruplex scaffold in the human c-kit promoter. Journal of the American Chemical Society. 2007;129(14):4386–4392. doi: 10.1021/ja068739h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan AT, Kuryavyi V, Gaw HY, Patel DJ. Small-molecule interaction with a five-guanine-tract G-quadruplex structure from the human MYC promoter. Nature Chemical Biology. 2005;1(3):167–173. doi: 10.1038/nchembio723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan AT, Kuryavyi V, Luu KN, Patel DJ. Structure of two intramolecular G-quadruplexes formed by natural human telomere sequences in K+ solution. Nucleic Acids Research. 2007;35(19):6517–6525. doi: 10.1093/nar/gkm706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan AT, Luu KN, Patel DJ. Different loop arrangements of intramolecular human telomeric (3+1) G-quadruplexes in K+ solution. Nucleic Acids Research. 2006;34(19):5715–5719. doi: 10.1093/nar/gkl726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan AT, Modi YS, Patel DJ. Propeller-type parallel-stranded G-quadruplexes in the human c-myc promoter. Journal of the American Chemical Society. 2004;126(28):8710–8716. doi: 10.1021/ja048805k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punchihewa C, Yang DZ. Therapeutic Targets and Drugs: G-Quadruplex and G-Quadruplex Inhibitors. In: Hiyama K, editor. Cancer Drug Discovery and Development: Telomeres and Telomerase in Cancer. Totowa, NJ: Humana Press; 2009. pp. 251–280. [Google Scholar]
- Qin Y, Fortin JS, Tye D, Gleason-Guzman M, Brooks TA, Hurley LH. Molecular Cloning of the Human Platelet-Derived Growth Factor Receptor beta (PDGFR-beta) Promoter and Drug Targeting of the G-Quadruplex-Forming Region To Repress PDGFR-beta Expression. Biochemistry. 2010;49(19):4208–4219. doi: 10.1021/bi100330w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Hurley LH. Structures, folding patterns, and functions of intramolecular DNA G-quadruplexes found in eukaryotic promoter regions. Biochimie. 2008;90(8):1149–1171. doi: 10.1016/j.biochi.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Rezler EM, Gokhale V, Sun D, Hurley LH. Characterization of the G-quadruplexes in the duplex nuclease hypersensitive element of the PDGF-A promoter and modulation of PDGF-A promoter activity by TMPyP4. Nucleic Acids Research. 2007;35(22):7698–7713. doi: 10.1093/nar/gkm538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S, Reszka AP, Huppert J, Zloh M, Parkinson GN, Todd AK, Ladame S, Balasubramanian S, Neidle S. Putative DNA quadruplex formation within the human c-kit oncogene. Journal of the American Chemical Society. 2005;127(30):10584–10589. doi: 10.1021/ja050823u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustighi A, Tessari MA, Vascotto F, Sgarra R, Giancotti V, Manfioletti G. A polypyrimidine/polypurine tract within the Hmga2 minimal promoter: A common feature of many growth-related genes. Biochemistry. 2002;41(4):1229–1240. doi: 10.1021/bi011666o. [DOI] [PubMed] [Google Scholar]
- Seenisamy J, Rezler EM, Powell TJ, Tye D, Gokhale V, Joshi CS, Siddiqui-Jain A, Hurley LH. The dynamic character of the G-quadruplex element in the c-MYC promoter and modification by TMPyP4. Journal of the American Chemical Society. 2004;126(28):8702–8709. doi: 10.1021/ja040022b. [DOI] [PubMed] [Google Scholar]
- Sen D, Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- Sen D, Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature. 1990;344(6265):410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- Sfeir AJ, Chai WH, Shay JW, Wright WE. Telomere-end processing: The terminal nucleotides of human chromosomes. Molecular Cell. 2005;18(1):131–138. doi: 10.1016/j.molcel.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Shin-ya K, Wierzba K, Matsuo K, Ohtani T, Yamada Y, Furihata K, Hayakawa Y, Seto H. Telomestatin, a novel telomerase inhibitor from Streptomyces anulatus. Journal of the American Chemical Society. 2001;123(6):1262–1263. doi: 10.1021/ja005780q. [DOI] [PubMed] [Google Scholar]
- Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsson T, Pecinka P, Kubista M. DNA tetraplex formation in the control region of c-myc. Nucleic Acids Research. 1998;26(5):1167–1172. doi: 10.1093/nar/26.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Hurley LH. The importance of negative superhelicity in inducing the formation of G-quadruplex and i-motif structures in the c-Myc promoter: implications for drug targeting and control of gene expression. Journal of Medicinal Chemistry. 2009;52(9):2863–2874. doi: 10.1021/jm900055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun DY, Guo KX, Rusche JJ, Hurley LH. Facilitation of a structural transition in the polypurine/polypyrimidine tract within the proximal promoter region of the human VEGF gene by the presence of potassium and G-quadruplex-interactive agents. Nucleic Acids Research. 2005;33(18):6070–6080. doi: 10.1093/nar/gki917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist WI, Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989;342(6251):825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- Thakur RK, Kumar P, Halder K, Verma A, Kar A, Parent J-L, Basundra R, Kumar A, Chowdhury S. Metastases suppressor NM23-H2 interaction with G-quadruplex DNA within c-MYC promoter nuclease hypersensitive element induces c-MYC expression. Nucleic Acids Research. 2009;37(1):172–183. doi: 10.1093/nar/gkn919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mourik T, Dingley AJ. Characterization of the monovalent ion position and hydrogen-bond network in guanine quartets by DFT calculations of NMR parameters. Chemistry-a European Journal. 2005;11(20):6064–6079. doi: 10.1002/chem.200500198. [DOI] [PubMed] [Google Scholar]
- Wang Y, Patel DJ. Solution structure of the human telomeric repeat d[AG3(T2AG3)3] G-tetraplex. Structure. 1993;1(4):263–282. doi: 10.1016/0969-2126(93)90015-9. [DOI] [PubMed] [Google Scholar]
- Wieland M, Hartig JS. RNA quadruplex-based modulation of gene expression. Chemistry & Biology. 2007;14(7):757–763. doi: 10.1016/j.chembiol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes & Development. 1997;11(21):2801–2809. doi: 10.1101/gad.11.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Noguchi Y, Sugiyama H. The new models of the human telomere d[AGGG(TTAGGG)3] in K+ solution. Bioorganic & Medicinal Chemistry. 2006;14(16):5584–5591. doi: 10.1016/j.bmc.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sugiyama H. Formation of the G-quadruplex and i-motif structures in retinoblastoma susceptibility genes (Rb) Nucleic Acids Research. 2006;34(3):949–954. doi: 10.1093/nar/gkj485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yafe A, Etzioni S, Weisman-Shomer P, Fry M. Formation and properties of hairpin and tetraplex structures of guanine-rich regulatory sequences of muscle-specific genes. Nucleic Acids Research. 2005;33(9):2887–2900. doi: 10.1093/nar/gki606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Okamoto K. Structural insights into G-quadruplexes: towards new anticancer drugs. Future Medicinal Chemistry. 2010;2(4):619–646. doi: 10.4155/fmc.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Dai J, Veliath E, Jones RA, Yang DZ. Structure of a two-G-tetrad intramolecular G-quadruplex formed by a variant human telomeric sequence in K+ solution: insights into the interconversion of human telomeric G-quadruplex structures. Nucleic Acids Research. 2010;38(3):1009–1021. doi: 10.1093/nar/gkp1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J-L, Lu Y-J, Ou T-M, Zhou J-M, Huang Z-S, Zhu X-F, Du C-J, Bu X-Z, Ma L, Gu L-Q, Li Y-M, Chan AS-C. Synthesis and Evaluation of Quindoline Derivatives as G-Quadruplex Inducing and Stabilizing Ligands and Potential Inhibitors of Telomerase. Journal of Medicinal Chemistry. 2005;48(23):7315–7321. doi: 10.1021/jm050041b. [DOI] [PubMed] [Google Scholar]