Summary
Endovascular surgery has been proposed as an alternative treatment for cerebral aneurysms. However, for wide neck and large sized lesions it is very difficult to obtain complete occlusion and tissue organization.
The present study was conducted to examine the efficacy of electrical thrombosis for cerebral aneurysms and parent arterial occlusions using Interlocking Detachable Coils (IDCs), focusing on the minimum current volume and stimulation time required for stable electrical thrombosis formation.
We used ten mixed-breed adult dogs (in the study body weights 9-12 kg; males: 5, females: 5). Guiding catheter sand microcatheters were introduced into both sides of the distal external carotid artery (ECA) and placed at the same level. To prevent migration, IDCs (4 mm x 12 cm) were placed in the ECA without being detached. After confirming no vessel occlusion, we applied a positive current (2-6 mA) to the coil on one side and performed angiography every ten minutes to observe whether vessel occlusion with electrothrombosis had occurred.
It was determined that to achieve complete occlusion of the external carotid arteries in mixedbreed dogs, a minimum stimulation current of 4mA and a minimum stimulation time of ten to 20 minutes are required.
Key words: electrothrombosis, endovascular technique, interlocking detachable coil, vessel occlusion, canine, external carotid artery
Introduction
In recent years, technological advances in microcatheters and the development of Guglielmi Detachable Coils (GDCs: Boston Scientific) have allowed cerebral endovascular procedures to rapidly gain popularity in neurosurgery. As treatments for cerebral aneurysms, endovascular techniques are increasingly selected instead of traditional neurosurgical clipping. The International Subarachnoid Aneurysm Trial (ISAT) Collaboration Group reported that the outcome of ruptured intracranial aneuryms in terms of survival free of disability at one year is significantly better with endovascular coiling 1 Currently, endovascular embolization for cerebral aneurysms involves the use of a soft platinum microcoil that is inserted in a microcatheter and then placed in the aneurysm until it is filled with coils to prevent rupture. However, it is difficult to obtain complete thrombosation and organization in the long term solely with this approach, particularly with large examples with broad necks.
Additionally, although coil embolization has been widely performed for dissecting aneurysms of the vertebral artery in recent years 2, it is generally not sufficient to completely occlude parent arteries with major blood flow. In fact, we may experience treated aneurysms that angiographically appear to have dense and tight coil packing, but permit passage of injected contrast medium.
As one method to achieve successful aneurysm occlusion with endovascular coil embolization, electrothrombosis therapy has been developed. First reported by Mullan et Al 3 in the 1960's, embolization of cerebral aneurysms was performed with this therapy, along with surgery for carotid-cavernous fistulas (CCF), by Hosobuchi et Al 4. On the basis of electrothrombosis therapy carried out in the 1970's, the GDC was developed in 1991, allowing application of electrothrombosis for endovascular embolization for cerebral aneurysms 5,6.
Sadato et Al 7 performed coil embolization for artificially created cerebral aneurysms in animal experiments, applying an electric current to the coil but the outcome was poor. Part of the problem was that the wires were constructed of copper as the main material. It is anticipated that if a hard sclerotic coagulation thrombus that is not easily dissolved is added to coils, occlusion of the artery may dramatically improve and the incidence of complications associated with additional coil deployment may be also significantly reduced. In the present study, we used interlocking detachable coils (IDCs; Boston Scientific), which are employed clinically, to investigate minimum current volume and stimulation time required for stable electrical thrombosis formation.
Material and Methods
We initially used 11 mixed-breed adult dogs (body weights 9-12 kg; males; 5; females; 6) to carry out the experiments for vessel occlusion with electrothrombosis. The procedures performed under general anesthesia with halothane and sheaths were introduced into both sides of the femoral arteries. Guiding catheters (Cordis; Envoy 5 Fr) were then inserted into each common carotid artery, through which microcatheters (Cook; MicroFerret) were subsequently introduced via the inner carotid artery bifurcation into both sides of the distal external carotid arteries (ECAs). To prevent accidental migration due to blood flow, IDCs (4 mm x 12 cm) were placed in the ECAs without being detached. After confirming no vessel occlusion, we applied a positive current (2-6 mA) to the coil on one side and performed angiography every ten minutes to observe whether vessel occlusion and vasospasm had occurred and to determine the time required for occlusion. The coil on the other side was used as a control. The mobile DSA System (Siemens) was applied for angiographic analysis and a Dye Injector DPI50 (Dia Medical System) was employed to generate the electric current.
During the procedure, anticoagulant therapy was given with an intraarterial injection of heparin initial bolus of 0.1 ml/kg when inserting sheaths, followed by 20 ml/h continuous infusion of heparinized saline solution (3 ml of hepalin in 500 ml of saline) through the microcatheters placed in both ECAs. Anticoagulation time (ACT) was measured before and after the initial bolus intraarterial injection of heparin and every 30 minutes during the procedure, and then the heparin infusion rate was adjusted to maintain an ATC between two and three times the baseline. After electrical stimulation was applied for 60 minutes, both external carotid arteries were surgically removed, and histological analysis was carried out to determine alteration in vessel structures using hematoxylin-eosin (H&E) staining and in the vascular endothelium using the Elastica van Gieson stain. Of the total of 11 dogs, we excluded one whose vessel diameter was 1 mm less than the long axis of the coil (coil size impropriety). Therefore ten dogs were finally used in the present study. With the data generated, we determined the minimum stimulation load and time required to achieve stable thrombus formation.
Results
A stimulation current of 4 mA and more produced a hard sclerotic coagulation thrombus that was not affected by the pressure or stress of contrast media injection (table 1). When a stimulation current of 2 mA was applied for 60 minutes, no vessel occlusion occurred, nor was blood flow reduction due to thrombus formation observed (figure 1). When a stimulation current of 3 mA was applied for 60 minutes, complete vessel occlusion was achieved in one of three dogs (33%) (figure 2) while 4 mA and more for 60 minutes resulted in complete vessel occlusion in all the dogs (figure 3).
Table 1.
Results of vessel occlusion with electrothrombosis in ten dogs
| electric current | 2mA | 3mA | 4mA | 5mA | 6mA |
|---|---|---|---|---|---|
| vessel occlusion cases | 0 | 1 | 2 | 2 | 1 |
| non-vessel occlusion cases | 2 | 2 | 0 | 0 | 0 |
| number of cases | 2 | 3 | 2 | 2 | 1 |
| time to achieve stable | |||||
| vessel occlusion | (-) | 10 min | 10~20 min | 10~20 min | 10 min |
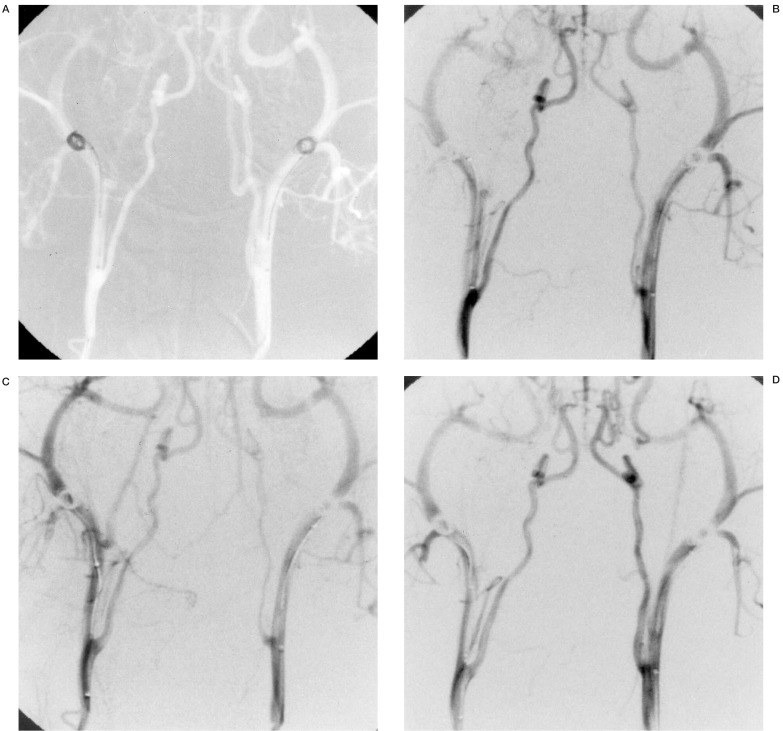
Figure 1.
Case 1: Angiography of a dog receiving a 2 mA positive current to the right coil. The figures show before (A), and 10 (B), 20 (C) and 60 (D) minutes after current application. There is no vessel occlusion.
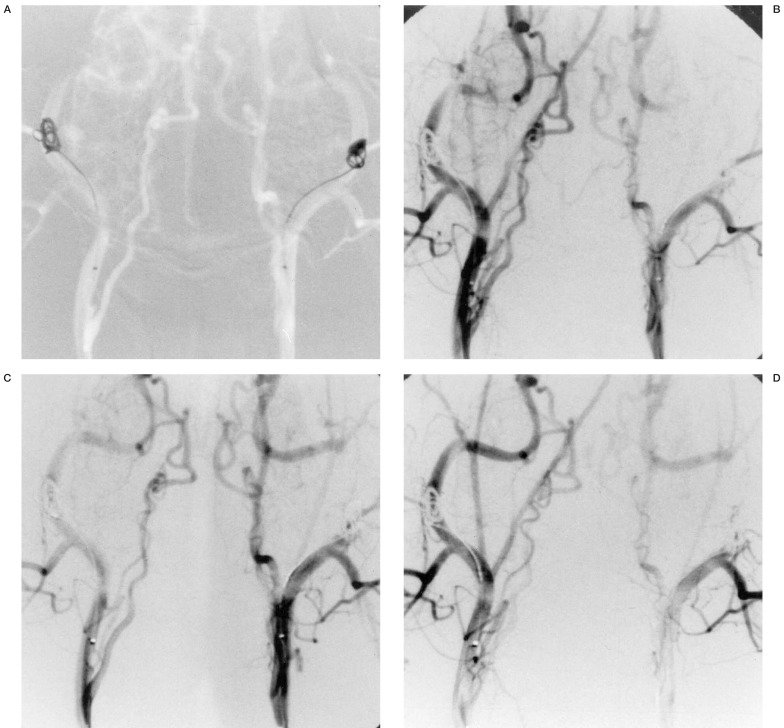
Figure 2.
Case 2: Angiography of a dog receiving a 3 mA positive current to the left coil. The figures show before (A), and 10 (B), 20 (C) and 60 (D) minutes after current application. The left ECA is occluded after 20 minutes.
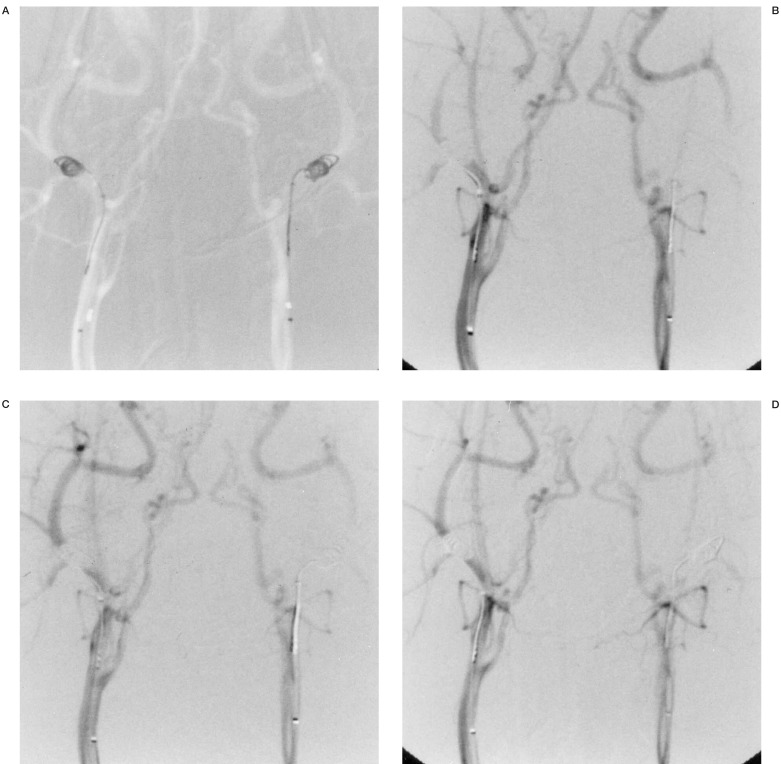
Figure 3.
Case 3: Angiography of a dog receiving a 4mA positive current to the left coil.The figures show before (A), and ten (B), 20 (C) and 60 (D) minutes after current application. The left ECA is occluded after ten minutes.
The angiographic findings provided no evidence of vasospasm in any of the vessels on the side receiving stimulation. Complete occlusion occurred as early as ten to 20 minutes after electrical stimulation and no patency of the occluded vessels was subsequently observed.
Histological examination with H&E and Elastica van Gieson staining showed neither coagulation necrosis of vascular smooth muscle cells nor traumatic injuries of vascular endothelial cells in the coil-embolized areas.
Discussion
Electrothrombosis therapy with endovascular techniques was first reported by Mullan et Al 3 and then by Hosobuchi et Al 4. This approach enhances electrothrombosis within the aneurysms. Since coils are electropositive, they attract blood elements including endothelial cells, erythrocytes, leukocytes and platelets, which are negatively charged.
Currently, endovascular embolization for cerebral aneurysms involves the use of soft platinum microcoils. To achieve satisfactory long-term success a hard sclerotic coagulation thrombus is clearly an advantage and the present results indicated that this can be effectively achieved using electrothrombosis. The clear differences between our and Sadato et Al 7 results would suggest that the material of the coils is very important and development of better liquid thrombotic substances may provide the best way to increase the occlusion rate for saccular aneurysms.
In the present study an electric current of only 2 mA applied for 60 minutes did not produce coagulation thrombi. Even using the GDCs which are now clinically applied, it takes longer for coils to become detached with such a low level of stimulation. Here a current of 3 mA caused coagulation thrombus formation, but the clots were small and fragile. In only one dog was complete vessel occlusion achieved, and in the other thrombus migration to peripheral vessels was detected. With an electric current of 4 mA or more, however, coagulation thrombi were rapidly formed and proved stable. Within the current range of 2 to 6 mA, the stimulation did not produce vasospasms, nor were vascular traumatic injuries to smooth muscle or endothelial cells histologically observed.
We must take into consideration that the blood clotting ability in dogs differs from that in humans, the fibrinolytic system being more activated than in man. There are also problems concerningwhether a thrombus might travel with the blood flow to peripheral vessels, and long-term stability needs further investigation. With regard to thrombus migration through the blood stream, a protective balloon could be inserted through the contralateral artery in the clinical case.
Conclusions
Application of an electric current directly to coils results in a coagulation thrombus and vessel occlusion. To achieve complete occlusion of external carotid arteries in mixed-breed dogs, a minimum stimulation current 4 mA for at least ten to 20 minutes is required. Problems such as possible thrombus migration to peripheral vessels and long-term stability remain to be evaluated.
Acknowledgement
This work was supported by The Marine & Fire Insurance Association of Japan, Inc.
References
- 1.Internal Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intercranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 2.Kurata A, Miyasaka Y, Fujii K, et al. Coil embolization for the treatment of ruptuted dissecting vertebral aneurysms. Am J Neuroradiol. 2001;22:11–18. [PMC free article] [PubMed] [Google Scholar]
- 3.Mullan S, Raimondi AJ, Dobben G, et al. Electrically induced thrombosis in intracranial aneurysms. J Neurosurg. 1965;22:539–547. doi: 10.3171/jns.1965.22.6.0539. [DOI] [PubMed] [Google Scholar]
- 4.Hosobuchi Y. Electrothrombosis of carotid-cavernus fistula. J Neurosurg. 1975;42:76–85. doi: 10.3171/jns.1975.42.1.0076. [DOI] [PubMed] [Google Scholar]
- 5.Guglielmi G, Vinuela F, Sepetka I. Electrothrombosis of saccular aneurysm via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75:1–7. doi: 10.3171/jns.1991.75.1.0001. [DOI] [PubMed] [Google Scholar]
- 6.Guglielmi G, Vinuela F, Dion J. Electrothrombosis of saccular aneurysm via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 7.Sadato A, Taki W, Ikeda Y, Kikuchi H. Effect of electrical thrombosis on coil embolization of experimental aneurysms. J of Neurovascular Disease. 1997;2:235–2458. [Google Scholar]
- 8.Fogelholm R, Hernesniemi J, Vapalahti M. Impact of early surgery on outcome after aneurysmal subarachnoid haemorrhage. A population-based study. Stroke. 1993;24:1649–1654. doi: 10.1161/01.str.24.11.1649. [DOI] [PubMed] [Google Scholar]