Abstract
Autologous chondrocyte implantation is an effective treatment for damaged articular cartilage. However, this method involves surgical procedures that may cause further cartilage degeneration, and in vitro expansion of chondrocytes can result in dedifferentiation. Adipose-derived stem cells (ADSCs) may be an alternative autologous cell source for cartilage regeneration. In this study, we developed an effective method for large-scale in vitro chondrogenic differentiation, which is the procedure that would be required for clinical applications, and the subsequent in vivo cartilage formation of human ADSCs (hADSCs). The spheroid formation and chondrogenic differentiation of hADSCs were induced on a large scale by culturing hADSCs in three-dimensional suspension bioreactors (spinner flasks). In vitro chondrogenic differentiation of hADSCs was enhanced by a spheroid culture compared with a monolayer culture. The enhanced chondrogenesis was probably attributable to hypoxia-related cascades and enhanced cell–cell interactions in hADSC spheroids. On hADSCs loading in fibrin gel and transplantation into subcutaneous space of athymic mice for 4 weeks, the in vivo cartilage formation was enhanced by the transplantation of spheroid-cultured hADSCs compared with that of monolayer-cultured hADSCs. This study shows that the spheroid culture may be an effective method for large-scale in vitro chondrogenic differentiation of hADSCs and subsequent in vivo cartilage formation.
Introduction
Damaged articular cartilage has poor intrinsic regenerative capacity, due to its avascular nature. Autologous chondrocyte transplantation is a possible treatment, but this procedure requires the isolation of chondrocytes via surgery, which may cause further degeneration of the cartilage.1 An alternative approach is cartilage regeneration that utilizes mesenchymal stem cells (MSCs) isolated from adult tissues such as bone marrow2 and adipose tissue.3 Human adipose-derived stem cells (hADSCs) are relatively accessible and attractive as a cell source for cartilage regeneration, due to the simple surgical procedures, that are required to harvest the cells, the repeatable access to the subcutaneous adipose tissue, and relatively easy enzyme-based isolation procedures.4,5
Generally, in vitro chondrogenic differentiation of ADSCs is induced by culturing ADSCs in a pellet with the supplementation of transforming growth factor (TGF), dexamethasone, and ascorbate-2-phosphate.6–8 Winter et al. showed that bone marrow-derived MSCs (BMMSCs) and ADSCs showed indistinguishable chondrogenic potential in monolayer culture.9 However, in pellets or spheroids, BMMSCs showed improved chondrogenesis compared with ADSCs.6,7,9 It was reported that MSC culture in hydrogels of either collagen type II10 or hyaluronic acid11 promotes chondrogenesis. Pellet culture is inappropriate for large-scale culture to obtain adequate number of cells for clinical applications, because the pellet culture produces only one pellet in each culture tube. In contrast, cultivation using three-dimensional bioreactors (e.g., spinner flasks) can produce a large number of cell spheroids or pellets and facilitate culture in large scale.12 Earlier, we had developed a spinner flask culture system for the efficient formation of ADSC spheroids.13 In this study, the spheroid formation and chondrogenic differentiation of hADSCs were induced on a large scale by culturing hADSCs in spinner flasks (Fig. 1). The mechanisms for enhanced chondrogenic differentiation of hADSCs cultured in spheroids compared with monolayer culture were also investigated. In addition, to evaluate the in vivo cartilage forming ability of the cells, hADSCs cultured either in spheroid form or in monolayer were loaded into fibrin gels and were subcutaneously transplanted into athymic mice for 4 weeks. Cartilaginous tissue formation was evaluated with histological, immunohistochemical, reverse transcription–polymerase chain reaction (RT-PCR) and western-blot analysis.
FIG. 1.
A schematic diagram describing experimental procedure of the present study. Color images available online at www.liebertpub.com/tea
Materials and Methods
The isolation and culture of hADSCs
The hADSCs were isolated from lipoaspirates that were collected from informed and consenting patients, and subsequently cultured, as previously described.3 The hADSCs were cultured in a growth medium consisting of α-minimum essential medium (Gibco BRL, Gaithersburg, MD), 10% (v/v) fetal bovine serum (Gibco BRL), 100 U/mL of penicillin, and 100 μg/mL of streptomycin. The hADSCs at the fourth passage of cultivation were utilized for the experiments in this study.
The formation and culture of spheroids
The ADSCs were placed into a spinner flask (working volume=100 mL, Wheaton, Millville, NJ) at 1.0×106 cells/mL in differentiation medium consisting of Dulbecco's modified Eagle's medium high glucose (Gibco BRL), 50 mg/mL of ascorbic acid, and 100 nM of dexamethasone supplemented either with or without 10 ng/mL of TGF-β3 (R&D systems, Minneapolis, MN) and stirred at 40 rpm. The spinner flasks were siliconized with sigmacoat (Sigma, St. Louis, MO) to prevent cell adhesion onto the flask walls before use. The spheroids formed on day 3. For medium exchange, the spheroids were allowed to settle, and 80% of the supernatant was replaced with fresh medium every other day. As a control, hADSCs were also cultured in a monolayer with the differentiation medium.
Scanning electron microscope
For scanning electron microscope (SEM) analysis, the spheroids were washed twice with phosphate-buffered saline, prefixed with 4% (v/v) buffered glutaraldehyde (Sigma) for 1 h, and fixed with 0.1% (v/v) buffered formaldehyde (Sigma) for 24 h. The fixed specimens were dehydrated in ascending grades of ethanol, dried, and mounted on aluminum stubs. The specimens were subsequently coated with platinum using a Sputter Coater (Cressington 108; Cressington Scientific Instruments, Cranberry, PA) and examined by SEM (JSM-6330F; JEOL, Tokyo, Japan).
The transplantation of the spheroid-fibrin gel mixture
Monolayer hADSCs (3×106 cells) or hADSC spheroids (3×106 cells) in differentiation medium supplemented with or without TGF-β3 for 14 days were loaded into 60 μL fibrin gels (Greencross, Seoul, Korea) and were transplanted subcutaneously into 4 week old athymic mice (Orient Bio, Inc., Sungnam, Korea) (n=4 transplants per group). The number of monolayer hADSCs and spheroid hADSCs was determined by quantifying DNA-binding dye Hoechst 33258 dye (Molecular Probes, Eugene, OR). After 28 days, the transplants were removed. The specimens were cut in half and were subjected to analysis by histology, immunohistochemistry, RT-PCR, and western blot. The animal study was approved by the Institutional Animal Care and Use Committee at Seoul National University (#100203-4).
Histology and immunohistochemistry
The in vitro cultured spheroids were harvested on day 14. The neotissues formed by the hADSC transplantation were removed on day 28 after transplantation. The specimens were fixed in 10% (v/v) buffered formaldehyde, dehydrated in a graded ethanol series, and embedded in paraffin. The specimens were sliced into 4 μm sections. The sections were stained with hematoxylin and eosin (H&E) and alcian blue. The sections were also stained with anti-type II collagen antibodies (Abcam, Cambridge, United Kingdom) and counter-stained with 4,6 diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA) for immunohistochemical analysis. Next, the immunohistochemical images were examined with a fluorescence microscope (Olympus IX 71; Olympus, Tokyo, Japan).
RT-PCR analysis
The cell or tissue samples were lysed with TRIzol reagent (Invitrogen, Carlsbad, CA). The total RNA was extracted with chloroform (Sigma) and precipitated with 80% (v/v) isopropanol (Sigma). After the supernatant had been removed, the RNA pellet was washed with 75% (v/v) ethanol, air dried, and dissolved in 0.1% (v/v) diethyl pyrocarbonate-treated water (Sigma). The RNA concentration was determined by measuring the absorbance at 260 nm with a spectrophotometer. Reverse transcription was performed with 5 μg of pure total RNA and SuperScriptTM II reverse transcriptase (Invitrogen), and was followed by PCR amplification of the synthesized cDNA. The PCR consisted of 35 cycles of denaturing (94°C, 30 s), annealing (58°C, 45 s), and extension (72°C, 45 s), with a final extension at 72°C for 10 min. The PCR was followed by electrophoresis on a 2% (w/v) agarose gel and DNA visualization by ethidium bromide staining. The PCR products were analyzed with a gel-documentation system (Gel Doc 1000; Bio-Rad Laboratories, Hercules, CA). SOX-9 (S: 5′-CCC AAC GCC ATC TTC AAG G-3′, AS: 5′-CTG CTC AGC TCG CCG ATG T-3′), aggrecan (S: 5′-GCC TTG AGC AGT TCA CCT TC-3′, AS: 5′-CTC TTC TAC GGG GAC AGC AG-3′), hypoxia inducible factor (HIF)-1α (S: 5′-CCA GTT AGG TTC CTT CGA TCA GT-3′, AS: 5′-TTT GAG GAC TTG CGC TTT CA-3′), and TGF-β3 (S: 5′-ACA TTT CTT TCT TGC TGG-3′, AS: 5′-GGG GAA GAA CCC ATA ATG-3′) were used. β-Actin (S: 5′-CCT TCC TGG GCA TGG AGT CCT G-3′, AS: 5′-GGA GCA ATG ATC TTG ATC TTC-3′) served as the internal control. The amount of RT-PCR results was quantified with an Imaging Densitometer (Bio-Rad Laboratories).
Real-time PCR analysis
Real-time PCR analysis (n=3 per group) was performed to quantify the relative gene expression level of type II collagen (S: 5′-ATA AGG ATG TGT GGA AGC CG-3′, AS: 5′-TTT CTG TCC CTT TGG TCC TG-3′), SOX-9 (S: 5′-GGA GCT CGA AAC TGA CTG GAA-3′, AS: 5′-GAG GCG AAT TGG AGA GGA GGA-3′), aggrecan (S: 5′-GCC TTG AGC AGT TCA CCT TC-3′, AS: 5′-CTC TTC TAC GGG GAC AGC AG-3′), and TGF-β3 (S: 5′-ACA TTT CTT TCT TGC TGG-3′, AS: 5′-GGG GAA GAA CCC ATA ATG-3′). The total RNA was extracted from the hADSCs after chondrogenic differentiation treatment using 1 mL of TRIzol reagent (Invitrogen) and 200 μL of chloroform. The hADSCs and spheroid lysate samples were centrifuged at 12,000 rpm for 10 min at 4°C. The RNA pellets were washed with 75% (v/v) ethanol and dried. After the drying procedure, the samples were dissolved in RNase-free water. The iQ™ SYBR Green Supermix kit (Bio-Rad Laboratories) and the MyiQ™ single color Real-Time PCR Detection System (Bio-Rad Laboratories) were used.
Western-blot analysis
The specimens (n=3 per group) were lysed in ice-cold lysis buffer (15 mM Tris HCl (pH 8.0), 0.25 M sucrose, 15 mM NaCl, 1.5 mM MgCl2, 2.5 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol bis(2–aminoethyl ether) tetraacetic acid, 1 mM dithiothreitol, 2 mM NaPPi, 1 μg/mL pepstatin A, 2.5 μg/mL aprotinin, 5 μg/mL leupeptin, 0.5 mM phenymethylsulfonyl fluoride, 0.125 mM Na3VO4, 25 mM NaF, and 10 μM lactacystin). The protein concentration was determined with a bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL). Equal protein concentrations of each sample were mixed with Laemmli sample buffer, loaded, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% (v/v) resolving gel. The proteins that were separated by SDS-PAGE were transferred to an Immobilon-P membrane (Millipore Corp., Billerica, MA) and were probed with antibodies against collagen type II, SOX-9, aggrecan, N-cadherin, AKT, phosphorylated AKT (p-AKT), peroxisome proliferator-activated receptor gamma (PPARγ), osteocalcin (OC), osteopontin (OP), p38, and phosphorylated p38 (p-p38) (all products from Abcam). β-Actin (Abcam) served as the internal control.
Statistical analysis
Quantitative data were expressed as the mean±standard deviation. Statistical analysis was performed by analysis of variance using a Bonferroni test. A p-value of less than 0.05 was considered statistically significant.
Results
Enhanced in vitro chondrogenic differentiation by the spheroid culture of hADSCs
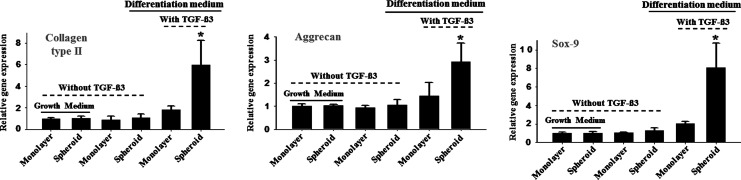
The suspension culture of hADSCs in spinner flasks induced spheroid formation (Fig. 2A). Immunohistochemical analysis for caspase-3 indicated that the cells were viable in spheroids even after 14 days of culture (Fig. 2B). hADSC spheroids cultured in spinner flasks showed significantly less caspase-3 activity compared to hADSC pellets cultured statically (Fig. 3). Qualitative analysis with RT-PCR and immunohistochemistry and quantitative analysis with real-time PCR and western blotting showed that the spheroid culture of hADSCs in differentiation medium with TGF-β3 resulted in enhanced chondrogenic differentiation, which was indicated by the enhanced expression of SOX-9, aggrecan and collagen type II compared with the monolayer culture (Figs. 4–7). TGF-β3 supplementation was required for the chondrogenic differentiation of hADSCs in vitro.
FIG. 2.
A spheroid of hADSCs at 14 days of culture. (A) A scanning electron microscopic image. (B) The staining for nuclei with DAPI (blue) and caspase-3 (red, arrows). The hADSC spheroids had a low level of apoptotic activity. The scale bars indicate 100 μm. hADSCs, human adipose-derived stem cells; DAPI, 4,6 diamidino-2-phenylindole. Color images available online at www.liebertpub.com/tea
FIG. 3.
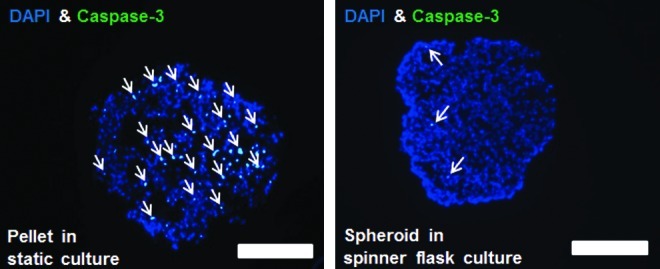
Comparison of apoptotic activity of hADSCs cultured in spheroids in spinner flasks and hADSC pellets cultured statically, as evaluated by immunohistochemistry for caspase-3. The staining for nuclei with DAPI (blue) and caspase-3 (green). The arrows indicate caspase-3-positive cells. The scale bars indicate 150 μm. Color images available online at www.liebertpub.com/tea
FIG. 4.
The expression of HIF-1α and the chondrogenic differentiation-related genes in hADSCs as evaluated by RT-PCR. The hADSCs were cultured for 14 days in either growth medium or differentiation medium with or without TGF-β3. HIF-1α, hypoxia inducible factor-1α; RT-PCR, reverse transcription–polymerase chain reaction; TGF, transforming growth factor.
FIG. 7.
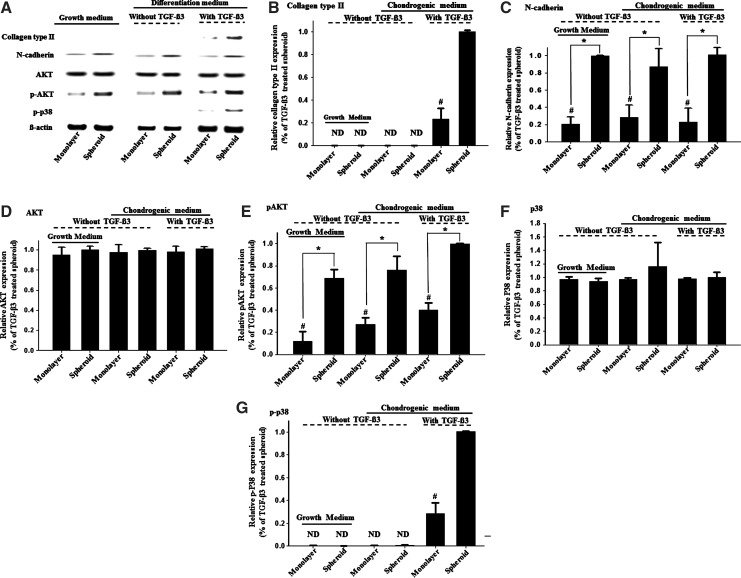
The expression of the chondrogenic differentiation- and mechanism-related genes in hADSCs as evaluated by (A) western-blot analysis and (B–G) respective quantification (n=3). The hADSCs were cultured for 14 days in either growth medium or differentiation medium with or without TGF-β3. *p<0.05. #p<0.05 compared with any group. ND, not detected.
FIG. 5.

The chondrogenic differentiation of hADSCs as evaluated by immunohistochemistry for type II collagen. Type II collagen and nuclei were stained green and blue, respectively. The hADSCs were cultured for 14 days in either growth medium or differentiation medium with or without TGF-β3. All of the scale bars indicate 100 μm. Color images available online at www.liebertpub.com/tea
The mechanisms for enhanced chondrogenic differentiation
The spheroid culture showed a dramatic increase in the expression of HIF-1α in hADSCs compared with that of the monolayer culture, regardless of the type of medium used (Fig. 4). To verify the induction of chondrogenesis by hypoxia, the expression of p-AKT and p-p38, which are known to be involved in chondrogenesis promoted by hypoxia,14 was assessed through western-blot analysis. Although AKT expression was observed in all the cultures, p-AKT expression was significantly higher in the spheroid culture (Fig. 6).The expression of p-p38 was also significantly higher in the spheroid culture than in the monolayer culture (Fig. 7). To verify the auto-induction of TGF-β3, PCR analysis was performed, and the results denoted enhanced mRNA expression of TGF-β3 in the spheroid culture with TGF-β3 supplementation (Fig. 8). The expression of N-cadherin was significantly higher in the spheroid culture than it was in the monolayer culture, which indicates the presence of enhanced cell-cell interactions in the spheroid culture (Fig. 7).
FIG. 6.
The expression of the chondrogenic differentiation-related genes in hADSCs as evaluated by real-time PCR. The hADSCs were cultured for 14 days in either growth medium or differentiation medium with or without TGF-β3. *p<0.05 compared to any group.
FIG. 8.
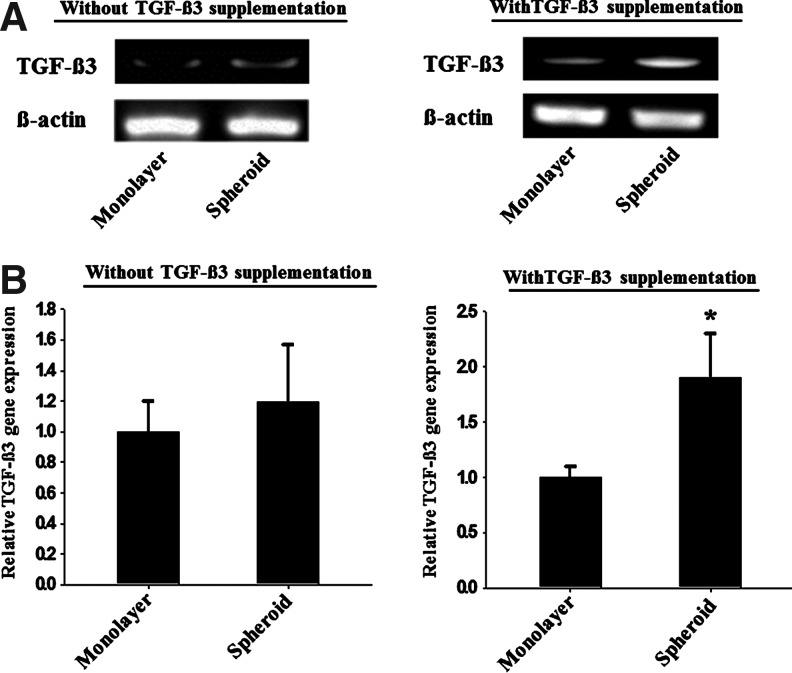
The expression of TGF-β3 mRNA in hADSCs as evaluated by (A) RT-PCR and (B) real time-PCR (n=3). The hADSCs were cultured for 14 days in either growth medium or differentiation medium with or without TGF-β3. *p<0.01 compared to other group.
Enhanced in vivo cartilage formation by the spheroid-cultured hADSCs
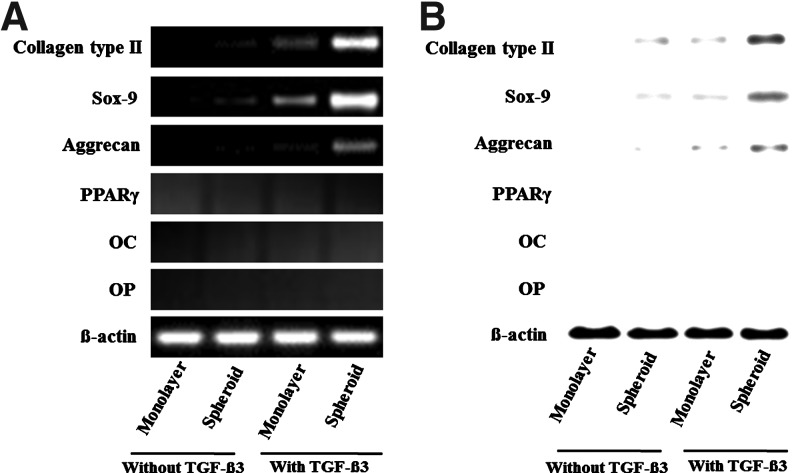
The H&E and alcian blue staining indicated the lacunae structure and enhanced deposition of glycosaminoglycan in the implants of spheroid-cultured hADSCs supplemented with TGF-β3 (Fig. 9). Extensive alcian blue staining was observed only in the spheroids with TGF-β3. Immunohistochemical analysis indicated a greater deposition of type II collagen in the implants of spheroid-cultured hADSCs supplemented with TGF-β3 than in the implants of monolayer-cultured hADSCs with TGF-β3 (Fig. 10). RT-PCR and western-blot analyses indicated the expression of type II collagen, SOX-9 and aggrecan, at both the gene and protein levels, to be the highest in the implants of spheroid-cultured hADSCs supplemented with TGF-β3 (Fig. 11). To determine whether the implanted hADSCs underwent adipogenesis or osteogenesis, the expression of PPARγ, OC, and OP were assessed by RT-PCR and western-blot analysis. Such a differentiation tendency was not observed (Fig. 11).
FIG. 9.
The in vivo cartilaginous tissue formation by the transplantation of hADSCs, cultured in a monolayer or in spheroids in differentiation medium with or without TGF-β3 and mixed with fibrin gel, into the subcutaneous space of athymic mice for 4 weeks, as evaluated by histological analysis with (A) Hematoxylin and eosin staining and (B) alcian blue staining. All of the scale bars indicate 100 μm. Color images available online at www.liebertpub.com/tea
FIG. 10.
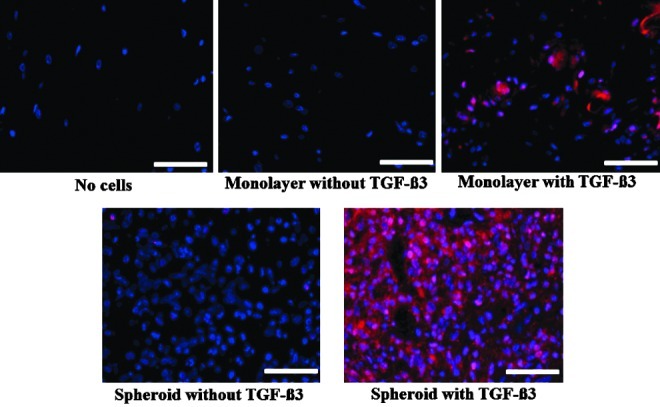
The in vivo cartilaginous tissue formation by the transplantation of hADSCs, cultured in monolayer or spheroids in differentiation medium with or without TGF-β3 into the subcutaneous space of athymic mice for 4 weeks, as evaluated by immunohistochemistry for type II collagen (red). The nuclei were stained with DAPI (blue). All the scale bars indicate 100 μm. Color images available online at www.liebertpub.com/tea
FIG. 11.
The expression of chondrogenic, adipogenic, and osteogenic differentiation markers in the tissues formed by the transplantation of hADSCs, cultured in monolayer or spheroids in differentiation medium with or without TGF-β3, into the subcutaneous space of athymic mice for 4 weeks, as evaluated by (A) RT-PCR and (B) western-blot analysis.
Discussion
The aim of this study was to evaluate the feasibility of the large-scale culture of hADSCs for chondrogenic differentiation and to elucidate the mechanisms for enhanced chondrogenesis in hADSC spheroid culture. The results demonstrated an enhanced in vitro chondrogenic differentiation of hADSCs in a spheroid culture system and the subsequent in vivo cartilage formation compared with the monolayer culture. The mechanisms for the enhanced chondrogenesis involved hypoxia-induced cascades and enhanced cell–cell interactions in the spheroids (Fig. 12).
FIG. 12.
A schematic diagram of the mechanisms for the enhanced chondrogenic differentiation of hADSCs through spheroid cultivation.
One advantage of the method of spheroid culture using suspension bioreactors compared with the conventional monolayer culture and the pellet culture is the ease of scale-up. To produce large quantities of cells that are required for clinical applications (5.2×106–7.5×107 chondrocytes per defect),15 hADSCs can be cultured as spheroids for chondrogenic differentiation in a spinner flask. In contrast, only one pellet can be formed and cultured per tube in the conventional pellet culture, which is not appropriate for large-scale culture for chondrogenic differentiation. In addition, a previous study reported extensive apoptosis of BMMSCs cultured as pellets for chondrogenic differentiation.16 Surprisingly, spheroid culture in spinner flasks reduced apoptosis of hADSCs as compared with pellet culture (Fig. 3). This could be due to improved mass transfer in spinner culture compared with static pellet culture. Therefore, the spheroid culture system has the potential to decrease the labor-intensity, enhance cell viability, and simplify the culture process to acquire a sufficient number of cells for clinical applications for cartilage tissue engineering.
Another advantage of the spheroid culture compared with the monolayer culture is the greater extent of cell–cell interactions. N-cadherin, a molecule for cell–cell adhesion, is involved in mesenchymal chondrogenesis. It was earlier shown that the inhibition of N-cadherin with neutralizing antibodies disabled the condensation of mesenchymal cells in vitro and in vivo and the subsequent chondrogenesis.17 Through N-cadherin, the spheroid enhances the cell-to-cell interactions that are analogous to the interactions that occur in precartilage condensation development.18 Therefore, a more condensed state can be obtained through spheroid culture system, which may be sufficient external stimulants for hADSCs to undergo chondrogenesis.
The cells beneath the surface of the spheroids are generally exposed to mild hypoxia, due to the diffusion limitations of oxygen.19 The hypoxic condition seems to present a more favorable microenvironment for chondrogenesis by hADSCs. This environment induces a significant expression of HIF-1α in cells in spheroids, even under normoxic culture conditions (Fig. 4). HIF-1α is a component of the intrinsic ability of cells to respond to a low oxygen environment. Given that cartilage tissue resides in a low oxygen environment, HIF-1α is thought to play a key role in maintaining the chondrocyte phenotype under such a condition.20 The activation of HIF-1α is known to stimulate the production of SOX-9, which is a chondrogenic transcription factor.21 Although the major cartilage matrix genes such as collagen type II and aggrecan are not directly up-regulated by HIF1-α, their expression is up-regulated by hypoxia through SOX-9.7,22 A previous study has also demonstrated that the ability of hypoxia to transactivate the SOX-9 promoter was almost completely abolished by the deletion of the HIF-1α consensus sites within the SOX-9 proximal promoter.23 Indeed, the expression of collagen type II and aggrecan were up-regulated in the spheroid hADSCs compared with the hADSCs in a monolayer culture (Figs. 4–7), and the monolayer cultured hADSCs, which showed no HIF-1α expression, were unable to support the enhancement of chondrogenesis.
The up-regulation of p-p38, AKT, and HIF-1α that was observed in the hADSC spheroid culture (Figs. 4 and 7) suggests that the enhanced chondrogenesis in the spheroid culture of hADSCs involves hypoxia and p38 and AKT signaling. In addition, p38, one of the major subtypes of mitogen-activated protein kinase(MAPK), and AKT, a downstream molecule of the phosphatidylinositol 3-kinase pathway, are among the most widespread signaling molecules that have been identified to be implicated in chondrocyte differentiation.23 The p38/MAPKs cascade has been established as a positive regulator in chondrogenesis by mesenchymal progenitor cells24 and was reported to regulate TGF-β-induced chondrogenesis by human mesenchymal progenitor cells.25 Inhibition of the p38/MAPKs cascade with pharmacological inhibitors suppressed cartilage matrix production.23 It was previously reported that p38 and AKT are up-regulated during hypoxic conditions, and this condition up-regulates chondrogenesis in rat MSCs.14 In this study, we showed that HIF-1α was expressed only in the spheroid-cultured hADSCs (Fig. 4) and that p-AKT was up-regulated in the spheroid-cultured hADSCs compared with monolayer-cultured hADSCs. In addition, p-p38 expression was significantly higher in the spheroid-cultured hADSCs compared with that observed in the monolayer-cultured hADSCs (Fig. 7).
The expression of TGF-β3, a chondrogenic factor, was enhanced in hADSCs that were cultured as spheroids, which is the cause of the enhanced chondrogenesis by hADSCs cultured as spheroids (Fig. 8). The mechanisms for the enhanced expression of TGF-β3 involved p38 and the autocrine action of TGF-β3 (Fig. 12). It was reported that the up-regulation of HIF-1α activates p38 by facilitating its phosphorylation.14 The activated p38, in turn, activates TGF-β3 expression.26 In addition, expressed TGF-β3 acts as an autocrine factor to induce p38 activation.26 Our data (Figs. 4, 7, and 8) suggest that the hypoxic environment in spheroids enhances TGF-β3 expression through p38 activation, to induce enhanced chondrogenesis by hADSCs cultured in spheroids.
Conclusion
The culture of hADSCs as spheroids in large-scale suspension bioreactors (i.e., spinner flasks) promoted chondrogenic differentiation. Unlike pellet culture, culture using spinner flasks is easily scalable and may be useful for the clinical application of hADSCs in cartilage regeneration, which requires a large-scale culture of cells. The mechanisms for the enhanced chondrogenesis in the hADSC spheroid cultures involve hypoxia-related cascades and enhanced cell–cell interaction.
Acknowledgments
This study was supported by grants (2009-0092213, 2009-0086518, and 2010-0020352) from the National Research Foundation of Korea.
Disclosure Statement
No competing financial interest exist.
References
- 1.Lee C.R. Grodzinsky A.J. Hsu H.P. Martin S.D. Spector M. Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. J Orthop Res. 2000;18:790. doi: 10.1002/jor.1100180517. [DOI] [PubMed] [Google Scholar]
- 2.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez A.M. Elabd C. Amri E.Z. Ailhaud G. Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:25. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Casteilla L. Planat-Benard V. Cousin B. Silvestre J.S. Laharrague P. Charrière G. Penicaud L. Plasticity of adipose tissue: a promising therapeutic avenue in the treatment of cardiovascular and blood diseases. Arch Mal Coeur Vaiss. 2005;98:922. [PubMed] [Google Scholar]
- 5.Oedayrajsingh-Varma M. van Ham S. Knippenberg M. Helder M.N. Klein-Nulend J. Schouten T.E. Ritt M.J. van Milligen F.J. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 6.Im G.I. Shin Y.W. Lee K.B. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Afizah H. Yang Z. Hui J.H. Ouyang H.W. Lee E.H. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donor. Tissue Eng. 2007;13:659. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- 8.Technau A. Froelich K. Hagen R. Kleinsasser N. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy. 2011;13:310. doi: 10.3109/14653249.2010.504769. [DOI] [PubMed] [Google Scholar]
- 9.Winter A. Breit S. Parsch D. Benz K. Steck E. Hauner H. Weber R.M. Ewerbeck V. Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 10.Bosnakovski D. Mizuno M. Kim G. Takagi S. Okumura M. Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 11.Chung C. Burdick J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu J.H. Kim M.S. Lee G.M. Choi C.Y. Kim B.S. The enhancement of recombinant protein production by polymer nanospheres in cell suspension culture. Biomaterials. 2005;26:2173. doi: 10.1016/j.biomaterials.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Bhang S.H. Cho S.W. La W.G. Lee T.J. Yang H.S. Sun A.Y. Baek S.H. Rhie J.W. Kim B.S. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Kanichai M. Ferguson D. Prendergast P.J. Campbell V.A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008;216:708. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 15.Peterson L. Minas T. Brittberg M. Lindahi A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85-A:17. doi: 10.2106/00004623-200300002-00003. [DOI] [PubMed] [Google Scholar]
- 16.Ichinose S. Tagami M. Muneta T. Sekiya I. Morphological examination during in vitro cartilage formation by human mesenchymal stem cells. Cell Tissue Res. 2005;322:217. doi: 10.1007/s00441-005-1140-6. [DOI] [PubMed] [Google Scholar]
- 17.Oberlender S.A. Tuan R.S. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120:177. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- 18.DeLise A.M. Tuan R.S. Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Dev Dyn. 2002;225:195. doi: 10.1002/dvdy.10151. [DOI] [PubMed] [Google Scholar]
- 19.Shweiki D. Neeman M. Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implication for tumor angiogenesis. Proc Natl Acad Sci U S A. 1995;92:768. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amarilio R. Viukov S.V. Sharir A. Eshkar-Oren I. Johnson R.S. Zelzer E. HIF1alpha regulation of Sox9 us necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 21.Murphy C.L. Polak J.M. Control of human articular chondrocyte differentiation by reduced oxygen tension. J Cell Physiol. 2004;199:451. doi: 10.1002/jcp.10481. [DOI] [PubMed] [Google Scholar]
- 22.Sekiya I. Tsuji K. Koopman P. Watanabe H. Yamada Y. Shinomiya K. Nifuji A. Noda M. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem. 2000;275:10738. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- 23.McMahon L.A. Prendergasi P.J. Campbell V.A. A comparison of the involvement of p38, ERK1/2 and PI3K in growth factor-induced chondrogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;368:990. doi: 10.1016/j.bbrc.2008.01.160. [DOI] [PubMed] [Google Scholar]
- 24.Oh C.D. Chang S.H. Yoon Y.M. Lee S.J. Lee Y.S. Kang S.S. Chun J.S. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem. 2000;275:5613. doi: 10.1074/jbc.275.8.5613. [DOI] [PubMed] [Google Scholar]
- 25.Robins J.C. Akeno N. Mukherjee A. Dalal R.R. Aronow B.J. Koopman P. Clemens T.L. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of SOX9. Bone. 2005;37:313. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 26.Liu G. Ding W. Neiman J. Mulder K.M. Requirement of Smad3 and CREB-1 in mediating transforming growth factor-β (TGF-β) induction of TGF-β3 secretion. J Biol Chem. 2006;281:29479. doi: 10.1074/jbc.M600579200. [DOI] [PubMed] [Google Scholar]