Abstract
The ideally engineered bone should have similar structural and functional properties to the native tissue. Although structural integrity is critical for functional bone regeneration, we know less about modulating the structural properties of the engineered bone elicited by bone morphogenetic protein (BMP) than efficacy and safety. Erythropoietin (Epo), a primary erythropoietic hormone, has been used to augment blood transfusion in orthopedic surgery. However, the effects of Epo on bone regeneration are not well known. Here, we determined the role of Epo in BMP2-induced bone regeneration using a cranial defect model. Epo administration improved the quality of BMP2-induced bone and more closely resembled natural cranial bone with a higher bone volume (BV) fraction and lower marrow fraction when compared with BMP2 treatment alone. Epo increased red blood cells (RBCs) in peripheral blood and also increased hematopoietic and mesenchymal stem cell (MSC) populations in bone marrow. Consistent with our previous work, Epo increased osteoclastogenesis both in vitro and in vivo. Results from a metatarsal organ culture assay suggested that Epo-promoted osteoclastogenesis contributed to angiogenesis because angiogenesis was blunted when osteoclastogenesis was blocked by alendronate (ALN) or osteoprotegerin (OPG). Earlier calcification of BMP2-induced temporary chondroid tissue was observed in the Epo+BMP group compared to BMP2 alone. We conclude that Epo significantly enhanced the outcomes of BMP2-induced cranial bone regeneration in part through its actions on osteoclastogenesis and angiogenesis.
Introduction
The repair of large bone defects due to trauma, infection, or tumor resection remains a significant clinical challenge. As a promising alternative strategy to autografts, bone morphogenetic protein (BMP) is often used for clinical bone repair because of its unique osteogenic capability and relatively straightforward handling properties. The dose of BMP-2 or -7 required for spinal fusion or tibial fracture nonunion is reported to be as high as 14 mg1–3 because of the lower efficacy in humans compared to preclinical animal studies.4 Considering the risks resulting from such high doses of BMP,5,6 alternative technologies, such as gene therapy,7–10 polymer-based drug delivery,11,12 and multiple factor release,13–15 have been developed to increase the osteogenic capacity of BMP. Unfortunately, translating these intriguing technologies into effective clinical therapeutics has been slow because of their complexity and lingering safety concerns. Therefore, it is desirable to develop a clinically applicable approach to improve bone regeneration. In addition to efficacy and safety, little attention has been paid to the quality of the regenerated bone, including structure, biomechanics, and the relationship to other marrow components.16 The quality of the engineered bone is critical because it not only affects biomechanical function, but it is also a key determinant for its physiological roles and for adequate protection and support of the underlying tissues.16
Bone formation induced by BMPs develops through an endochondral ossification process that includes mesenchymal progenitor cell recruitment, condensation, and differentiation into chondrocytes.17 This tissue subsequently undergoes hypertrophy and is invaded by blood vessels, osteoblasts, osteoclasts, and hematopoietic cells, and finally bone and mature hematopoietic tissues are formed.18,19 The newly formed bone is continuously modified by the natural remodeling process that depends on resorption and formation20 and this native remodeling process can also be modified by physiological factors such as parathyroid hormone.21 These findings support the development of new strategies to modulate BMP2-engineered bone by administration of other physiological factors such as erythropoietin (Epo).
Epo is the principal hormone that stimulates red blood cell (RBC) production in the marrow and has been widely used for treatment of anemias caused by chronic kidney disease and chemotherapy in cancer patients.22,23 Epo has also been approved for use in patients undergoing orthopedic surgery in the United States as a therapeutic alternative to blood transfusions.24 However, the effects of Epo on bone regeneration are not well established.
It has long been suggested that there is a synergistic link between bone formation and hematopoiesis. In support of this concept is the observation that osteoblasts are required for hematopoiesis and function along with endothelial sinusoids as a primary component of the hematopoietic stem cell (HSC) niche.25,26 Conversely, it has been demonstrated that HSCs directly support mesenchymal stem cell (MSC) differentiation to osteoblasts.27 Further studies indicate that Epo may couple bone formation and hematopoiesis by regulating the cell behavior of osteoblasts, osteoclasts, and MSCs.28 Likewise, a dual role for osteoclasts in bone homeostasis has recently been emphasized. Osteoclasts can not only recruit MSCs to the site of bone remodeling29,30 but also can stimulate bone formation29 while Epo can function to stimulate osteoclastogenesis in vitro.28,31 Consequently, this interdependent relationship between blood and bone lead us to hypothesize that Epo may also function as a novel physiological modulator of bone regeneration. Based on the emerging roles of Epo in bone formation, we hypothesized that Epo can serve as a physiological regulator to enhance the outcome of BMP2-induced bone regeneration. We therefore aimed to determine the role of Epo in bone tissue engineering and the potential mechanisms by which Epo regulates bone regeneration. Our ultimate goal is to develop a clinically applicable approach to enhance the outcomes of tissue-engineering-based bone regeneration.
Materials and Methods
Critical-sized cranial bone defect model
Care and use of the laboratory animals followed the guidelines established by the University of Michigan Committee for the Use and Care of Animals. C57BL/6 mice (4–6 weeks; Harlan Laboratories) were used in all the animal studies. Animals were anesthetized by intraperitoneal injection of ketamine (Hospira, Inc.) and xylazine, and buprenorphine (Reckitt Benckiser Pharmaceuticals, Inc.) was given before and after the surgery as an analgesic. The skin over the skull was shaved and prepared with a povidone-iodine (Rite Aid, Corporation) scrub. A ∼0.7-cm full-thickness incision was made and one 5-mm defect was created within the parietal bone with a trephine bur. The periosteum was completely cleared from the surface of the cranium by scraping while the underlying dura mater was kept intact. One microgram of recombinant human BMP2 (rhBMP2; ProSpec-Tany Technogene Ltd.) was resuspended in 10 μL of collagen I (BD Biosciences) and then incorporated into a 3.5×3.5×3.5 mm gelatin scaffold (Gelfoam; Pharmacia and Upjohn). The BMP2/collagen/scaffolds or collagen/scaffolds were allowed to gel for 30 min at 37°C before transplantation into the defects. Sterile grafts were then placed directly in the defects and the overlying tissue was closed with surgical staples. Staples were removed 10 days after surgery. Saline (0.9% sodium chloride; Hospira, Inc.) or 1000 U/mL Epo (EPOGEN; Amgen) was subcutaneously injected into the defect area after implantation every other day for up to 2 weeks. A total of 78 mice were used in our animal experiments, and they were divided into four groups and treated as follows. (1) Control group: the mice were implanted with a collagen/scaffold and injected with saline; four, four, five, and eight animals of which were euthanized at 5, 7, 14, and 42 days after surgery, respectively. (2) Epo group: the mice were implanted with a collagen/scaffold and injected with Epo; four, five, four, and eight animals of which were euthanized at 5, 7, 14, and 42 days after surgery, respectively. (3) BMP2 group: the mice were implanted with a BMP2/collagen/scaffold and injected with saline; four, six, four, and eight animals of which were euthanized at 5, 7, 14, and 42 days after surgery, respectively. (4) BMP2+Epo group: the mice were implanted with a BMP2/collagen/scaffold and injected with Epo; four, six, four, and eight animals of which were euthanized at 5, 7, 14, and 42 days after surgery, respectively.
Microcomputed tomography three-dimensional reconstruction and bone morphometry
The calvaria at 6 weeks were fixed for 2 days with Z-fix (Anatech Ltd.) and then moved to 70% ethanol and scanned at a voxel size of 18 μm using a microcomputed tomography (μCT) scanner (GE Healthcare Pre-Clinical Imaging). Micro View software (GE Healthcare Pre-Clinical Imaging) was used to generate a three-dimensional (3D) reconstruction from the set of scans. A user-defined 3D contour was drawn to include only the bone and inside marrow cavity within the defined cylinder space. User-defined contours were drawn every five images and interpolated for all images in between. A fixed threshold (1000) was used to extract the mineralized bone phase and the bone morphometry was calculated.
Histological analysis
The calvaria were fixed with Z-fix before histologic processing at the University of Michigan School of Dentistry histology core facility. Ten-micrometer sections were cut from the middle of the scaffolds and stained with hematoxylin and eosin or Safranin O, for light microscopic observation. For immunohistochemistry detection, antigens in rehydrated sections were retrieved in a pressure cooker for 15 min, incubated with anti-CD31 antibody (Abcam) at 1:100 dilutions at 37°C for 2 h, and detected by an ImmPACT DAB substrate kit (Vector Laboratories) using manufacturer's instructions.
Peripheral blood analysis and flow cytometry
Peripheral blood was analyzed at the Unit for Laboratory Animal Medicine pathology core facility of the University of Michigan. Sca-1+Lin-CD45− and Sca-1+Lin-CD45− cells in bone marrow were analyzed by a BD FACSAria™ flow cytometer (BD Biosciences) as previously described.32 Briefly, mononuclear cells were resuspended in Dulbecco's phosphate-buffered saline (Invitrogen) with 2% fetal bovine serum (FBS; Invitrogen). The following murine antibodies (BD Biosciences) were used to stain these cells: biotin-conjugated rat anti-mouse Ly-6A/E (Sca-1) (clone E13-161.7), anti-CD45R/B220-PE (clone RA3-6B2), anti-Gr-1-PE (clone RB6-8C5), anti-TCRαβ PE (clone H57-597), anti-TCRγζ PE (clone GL3), anti-CD11b PE (clone M1/70), and anti-Ter-119 PE (clone TER-119), and streptavidin-PE-Cy5 conjugate, anti-CD45-APC-Cy7 (clone 30-F11).
Osteoclastogenesis in vitro and in vivo
Serum was collected from peripheral blood when the mice were euthanized at 1 week (n=4) and 2 weeks (n=4). The levels of tartrate-resistant acid phosphatase form 5b (TRAP-5b) in serum were determined by a Mouse TRAP kit according to the manufacturer's instructions (Immunodiagnostic Systems, Inc.). In vitro osteoclastogenesis was performed as previously described.31 Briefly, whole bone marrow cells from one mouse were seeded onto one 24-well plate. Cells were treated for 1 week with 20 U/mL recombinant human Epo and/or 10 ng/mL rhBMP2 with medium containing 25 ng/mL recombinant mouse receptor activator of nuclear factor ligand (RANKL; PeproTech) and 25 ng/mL macrophage-colony stimulating factor (PeproTech). Osteoclasts were evaluated by TRAP staining (Sigma-Aldrich) according to the manufacturer's instructions and the numbers of TRAP-positive multinucleated cells (three or more nuclei per cell) were counted.
In vitro angiogenesis assay
The fetal mouse metatarsal angiogenesis assay was conducted as described with modifications.33 Briefly, metatarsals were dissected from ∼17-day embryos (C57BL/6; Harlan) and cultured in 24-well plates containing 150 μL α-minimum essential medium (Invitrogen) and 10% FBS (Invitrogen) for the first 3 days. The medium was replaced by 250 μL fresh medium containing vascular endothelial growth factor (VEGF, 10 ng/mL; PeproTech), Epo (10 U/mL; Amgen), BMP2 (10 ng/mL; PeproTech), alendronate (10 nM, ALN; Calbiochem), or osteoprotegerin (1 μg/mL OPG; R&D Systems, Inc.) on fourth day. Explants were cultured for another 7 days and then fixed and stained for CD31. The CD31-positive area was normalized to the bone area (n=4–6). Human umbilical vein endothelial cells (HUVECs; Invitrogen) were used to study the direct effects of Epo on endothelial cells. For proliferation assays, 5000 cells per well were plated in 24-well plates and cultured in low serum growth supplement (Invitrogen) containing the medium 200PRF (Invitrogen). The cells were stimulated with VEGF (10 ng/mL; PeproTech) or Epo (10 or 20 U/mL; Amgen) for 2 days. The relative cell number was measured by a colorimetric method using a 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl) 2-(4-sulfonphenyl)-2H-tetrazolium compound (Cell-Titer 96 Aqueous One Solution Cell Proliferation Assay; Promega). The endothelial tube formation assay was performed according to the manufacturer's instructions. Briefly, the HUVECs were plated on Geltrex™-coated 96-well plate at 5×104 cells per well. The cells were cultured with Epo or VEGF overnight and then fixed and stained with CellTracker™ Green 5-chloromethylfluorescein diacetate (Invitrogen).
Statistics and image editing
To test the significance of observed differences between the study groups, a two-tailed homoscedastic t-test was applied. A value of p<0.05 was considered to be statistically significant. Values are reported as the mean±standard deviation. Brightness and contrast were adjusted equally across all images for improved visibility.
Results
Epo improved BMP2-induced cranial bone regeneration
Several important parameters of BMP2-induced bone regeneration were significantly modulated by Epo administration in a mouse critical-sized cranial defect model. Three-dimensional images reconstructed from μCT data indicated no significant bone formation in control and Epo-treated groups, although a few small isolated bony islands were observed in the middle of the defect area. In contrast, 1 μg of BMP2 suspended in collagen/gelatin scaffolds resulted in nearly complete healing of the 5-mm critical cranial defects in both the BMP2 and BMP2+Epo groups (Fig. 1A). The regenerated tissue in the BMP2-treated group exhibited a structure with a larger total volume (TV) and marrow cavity compared with the BMP2+Epo group (Fig. 1B). These visualized differences were quantified by μCT analysis where the native calvarium from equivalent regions served as the reference for the analysis (Table 1). The bone volume (BV) fraction (BV/TV, 0.53±0.09%) of the BMP2-regenerated tissue was significantly lower than the native calvarium (0.92±0.05%), while the TV and BV were significantly higher. The administration of Epo generated a significantly higher BV fraction, lower TV, and similar BV compared to the BMP2-treated group. Further, the trabecular bone thickness in the combination group was also higher than the BMP2 group, but lower than native bone. Histological examination indicated significantly more bone marrow in the BMP2 group than in the BMP2+Epo group (right panel of Fig. 1B, C). The native calvarium had a typical lamellar structure surrounding a sparsely populated marrow cavity. No new bone or marrow tissue was observed in the defects of the control and Epo-treated groups and were instead filled with only fibrous connective tissue and residual collagen scaffold (Fig. 1C). Significant bone and marrow tissue was generated in both the BMP2- and BMP2+Epo-treated groups. Consistent with the μCT observation, the ratio of bone area was significantly increased by Epo while the ratio of marrow area was decreased by histological analyses (data not shown). The total area of the bone and marrow was decreased by Epo, although it was higher than the native calvarial bone. Taken together, the in vivo data indicated that Epo strongly augmented the structural properties and nature of the marrow of the BMP2-generated bone.
FIG. 1.
Epo improved BMP2-induced cranial bone regeneration. Different structures of regenerated bone were observed in the BMP2 and BMP2+Epo groups at 6 weeks. (A) Three-dimensionally reconstructed microcomputed tomography images of cranial bone regeneration. (B) Low-magnification histological analysis of the whole cranial bone section. A larger total volume and marrow cavity was observed in the BMP2 group compared with the BMP2+Epo group. The black arrows indicate the edges of the surgical defects. The scale bar represents 500 μm. (C) High-magnification H&E-stained section from the middle of the implants. The natural calvarium served as a reference. Improved bone fraction and decreased marrow fraction of regenerated tissues were observed in the BMP2+Epo group compared with the BMP2 group. The scale bar in (C) represents 100 μm. Representative data are shown (n=8). Epo, erythropoietin; BMP, bone morphogenetic protein; H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/tea
Table 1.
Erythropoietin Increases Bone Volume/Total Volume and Bone Mineral Density (Microcomputed Tomography Analysis)
| Natural crania | BMP2 | BMP2+Epo | p-Value | |
|---|---|---|---|---|
| Total volume of ROI (mm3) | 2.21±0.27 | 11.15±2.55 | 7.05±2.26 | 0.004 |
| Volume of bone (mm3) | 2.03±0.18 | 5.82±1.38 | 4.71±1.44 | 0.139 |
| BMCs (mg) | 1.47±0.28 | 3.37±0.86 | 3.07±1.13 | 0.556 |
| BMD (mg/cc) | 725.97±129.73 | 579.10±49.13 | 644.66±77.77 | 0.063 |
| BV/TV | 0.92±0.05 | 0.53±0.10 | 0.68±0.14 | 0.021 |
| BS/BV | 16.22±2.77 | 30.30±5.98 | 22.34±7.94 | 0.040 |
| Tb.Th. | 0.13±0.03 | 0.07±0.01 | 0.10±0.04 | 0.036 |
| Tb.N. | 7.43±1.00 | 7.75±0.93 | 7.23±1.52 | 0.424 |
| Tb.Sp. | 0.01±0.01 | 0.06±0.01 | 0.04±0.02 | 0.093 |
Data are mean±SD.
p-Value indicates BMP2 versus BMP2+Epo group.
BMP, bone morphogenetic protein; EPO, erythropoietin; ROI, region of interest; BMCs, bone mineral contents; BMD, bone mineral density; BV/TV, bone volume/total volume; BS/BV, bone surface/bone volume; Tb.Th., trabecular thickness; Tb.N., trabecular number; Tb.Sp., trabecular spacing.
Epo increased the stem cell pools in bone marrow
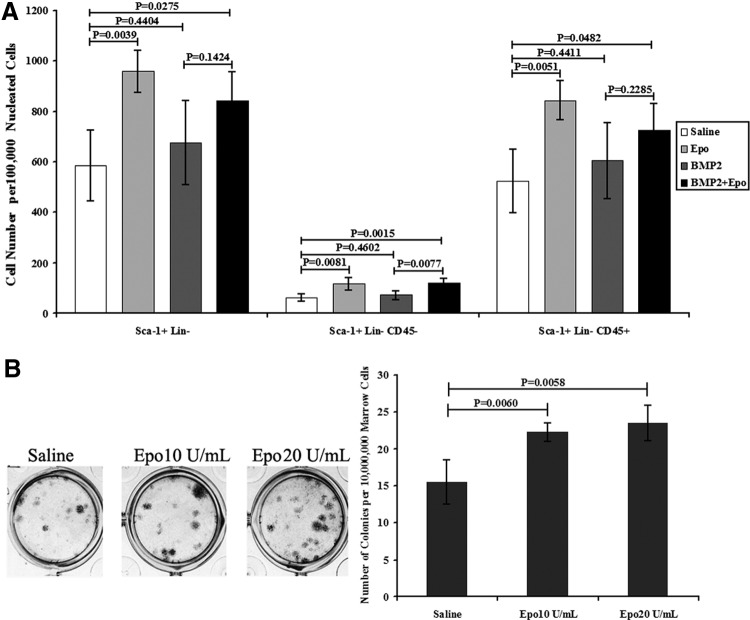
Epo administration increased both the hematopoietic and mesenchymal progenitor cells in bone marrow. As expected, erythropoiesis in peripheral blood was enhanced as early as 5 days post–Epo treatment. The erythropoietic response, including hematocrit, RBC, and hemoglobin levels, was significantly increased in the BMP2+Epo group compared with Epo group (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). In line with the expanded RBCs in peripheral blood, the size and weight of spleens were increased by Epo administration (data not shown). In addition to increased RBC numbers in peripheral blood, fluorescence-activated cell sorting analysis of multilineage progenitor cells (Sca-1+Lin−)34 in bone marrow was also increased after Epo treatment (Fig. 2A). The majority of the Sca-1+Lin− cells were hematopoiesis-related cells that were positive for CD45 and that were significantly increased by Epo. Notably, Epo significantly increased the non-HSCs (Sca-1+Lin-CD45−). Further, the capacity of bone marrow stromal cells to form colony forming units (CFU-F) was increased by Epo treatment (Fig. 2B). Therefore, Epo administration was able to increase RBCs in peripheral blood and also increase the hematopoietic and mesenchymal progenitor cell populations in the bone marrow.
FIG. 2.
Epo increased stem cell populations in vivo and in vitro. (A) Stem cell populations in bone marrow were altered by Epo administration. Hematopoietic progenitor cell (Sca-1+Lin−CD45+) and non-hematopoietic progenitor cell (Sca-1+Lin-CD45−) numbers were both elevated in bone marrow by Epo at 2 weeks posttreatment. (B) CFU-F of whole bone marrow cells in vitro. Whole bone marrow cells were treated with Epo (5 and 10 U/mL) for 1 week and then fixed and stained with H&E. Epo significantly increased the capability of the CFU-F of the bone marrow cells in vitro. Data are presented as the mean±SD (n=4). CFU-F, colony forming units; SD, standard deviation.
Epo increased osteoclastogenesis in vitro and in vivo
Considering the important role of osteoclasts in bone remodeling and regeneration, evidence for osteoclastogenesis in all groups was examined by the serum TRAP-5b assay (Fig. 3A). At 1 week, TRAP-5b was increased by both Epo and BMP2. The highest level of TRAP-5b was observed in the combined treatment group of BMP2+Epo. After 2 weeks, only the combined group demonstrated significantly greater TRAP-5b levels compared to the control (scaffold only) group, suggesting that Epo and BMP2 had synergistic effects on ostoeclastogenesis (Fig. 3A). RANKL-induced osteoclastogenesis was elevated by Epo treatment in vitro (Fig. 3B). However, BMP2 had limited effects on osteoclastogenesis in vitro. That is, no synergistic effect was observed in vitro. RANKL was essential for in vitro osteoclastogenesis as no osteoclasts were induced in all the groups without RANKL (data not shown). Therefore, the data demonstrated that Epo treatment increased osteoclastogenesis in vitro and in vivo.
FIG. 3.
Epo increased osteoclastogenesis in vivo and in vitro. (A) TRAP-5b levels in serum at 1 and 2 weeks posttreatment. TRAP-5b in serum was elevated by both BMP2 and Epo at 1 week. The combination of Epo and BMP2 showed the highest osteoclastogenesis at 1 and 2 weeks. Data are presented as the mean±SD (n=4). (B) Osteoclastogenesis of whole bone marrow cells in vitro. Osteoclasts were stained with TRAP after 1 week of culture. RANKL-induced osteoclast numbers were significantly increased by Epo treatment. BMP2 had limited effects on osteoclastogenesis even at high dosage (50 ng/mL). Data are presented as the mean±SD (n=4). TRAP-5b, tartrate-resistant acid phosphatase form 5b; RANKL, receptor activator of nuclear factor ligand. Color images available online at www.liebertpub.com/tea
Epo increased angiogenesis
Angiogenesis was evaluated in vivo by counting the number of CD31+ blood vessels in the newly regenerated tissues. More blood vessels were observed in regenerated tissues of BMP2+Epo-treated groups compared with the BMP2 group at 1 week (Fig. 4A). No noticeable differences in blood vessel number were observed between the BMP2 and BMP2+Epo groups at 2 weeks postsurgery (data not shown). The ability of Epo to increase angiogenesis was confirmed using the fetal mouse metatarsal assay in vitro (Fig. 4B). In contrast to Epo treatment, or the combination of Epo and BMP2, BMP2 and saline had no detectable effects on angiogenesis after 1 week of treatment. However, to determine whether Epo could stimulate angiogenesis by directly targeting endothelial cells, the proliferation and endothelial tube formation of HUVECs was investigated. Epo and VEGF induced similar proliferation rates of HUVECs (Fig. 4C, left panel), and also increased the HUVEC tube formation by VEGF in vitro (Fig. 4C, right panel). Interestingly, Epo-induced angiogenesis was largely blunted when osteoclastogenesis was blocked by ALN or OPG in the metatarsal organ culture assay (Fig. 4D). These data suggested that osteoclastogenesis is essential for Epo-mediated angiogenesis.
FIG. 4.
Epo stimulated angiogenesis in vivo and in vitro. (A) CD31+ blood vessels in regenerated tissues. Blood vessel numbers were significantly higher in the BMP2+Epo group than in the BMP2 group at 1 week. Representative data are shown (n=4). The scale bar represents 50 μm. (B) Angiogenesis using fetal mouse metatarsal assay. Epo-stimulated angiogenesis was detected using CD31 staining in vitro. The CD31-positive area was normalized to the bone area. Data are presented as the mean±SD (n=4–6). (C) The proliferation of HUVECs was strongly increased by Epo treatment. Data are presented as the mean±SD (n=4). Epo exhibited a less potent effect on tube formation of HUVECs compared with VEGF. Representative data are shown (n=4). (D) Epo-stimulated angiogenesis was blunted by ALN or OPG. Data are presented as the mean±SD (n=4–6). HUVECs, human umbilical vein endothelial cells; VEGF, vascular endothelial growth factor; ALN, alendronate; OPG, osteoprotegerin. Color images available online at www.liebertpub.com/tea
Epo promoted BMP2-induced endochondral bone formation
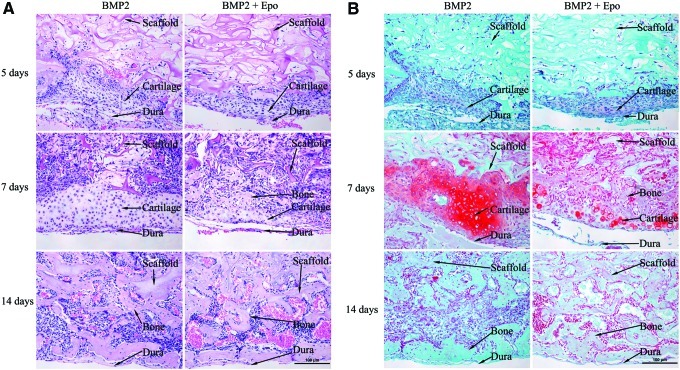
To examine the role of Epo during early osteogenesis in vivo, the cranial grafts were retrieved at 5, 7, and 14 days after implantation (Fig. 5). By 5 days, the surrounding tissues started migrating into the collagen scaffolds. Condensed mesenchymal cells were found between the scaffold and dura mater in the BMP2- and BMP2+Epo-treated groups (Fig. 5A). These cells stained positive with Safranin O (red color) (Fig. 5B), a stain that identifies proteoglycans in cartilaginous matrix. The chondroid tissues were only observed in the BMP2- and BMP2+Epo-treated groups. This indicated that BMP2 released from the scaffold was likely responsible for progenitor cell recruitment and differentiation at an early time point. At day 7, more tissue invaded the scaffolds in all groups compared to earlier points. A thick chondroid-like area between the scaffold and dura was observed in both the BMP2- and BMP2+Epo-treated groups. This chondroid tissue was located exclusively on the dural side of the scaffold, which suggested that this tissue was mainly derived from cells originating from the dura mater. Different features of chondroid tissue were noticed between the BMP2- and BMP2+Epo-treated groups. Compared to the BMP2 group, the BMP2+Epo-treated groups exhibited more calcified areas in the chondroid tissue as well as more blood vessel invasion in the hypertrophic cartilage at the time points studied. Moreover, the total Safranin O staining–positive area in each sample was significantly greater in the BMP2 group than in the BMP2+Epo group. By 14 days, the chondroid tissues in both BMP2 and BMP2+Epo groups were completely replaced by immature bone tissue (Fig. 5A, B).
FIG. 5.
Epo stimulated BMP2-induced endochondral bone formation. (A) H&E-stained sections at 5, 7, and 14 days. Chondroid-like tissues were found between the scaffold and dura mater in the BMP2- and BMP2+Epo-treated groups as early as 5 days. At day 7, more tissue invaded into the scaffolds in all groups compared to earlier points. Greater cartilage area was observed in the BMP2 group compared with the BMP2+Epo-treated groups. Epo treatment induced more calcified areas in the chondroid tissue. At day 14, the chondroid tissues in both BMP2 and BMP2+Epo groups were completely replaced by immature bone tissues. (B) Safranin O–stained sections at 5, 7, and 14 days. The cartilage was confirmed by Safranin O staining (red color). Similar pattern of the tissues in the regenerated area was observed. The scaffold, cartilage, and bone tissues were pointed out with the arrows. The representative data were shown (n=4–6). The scale bar represented 100 μm. Color images available online at www.liebertpub.com/tea
Discussion
Despite understanding the function of Epo as a hematopoiesis-inducing hormone and our recognition of the close functional relationship between blood and bone, the roles of Epo in bone regeneration are not well appreciated or understood.35 Our current data indicate that Epo increases both RBCs and their progenitor cells (Sca-1+Lin−CD45+), and the non-HSCs (Sca-1+Lin−CD45−) (Fig. 2) that have been characterized to be multipotent stem cell population in adult tissues.32 Interestingly, this adult stem cell can be mobilized and recruited to injured tissues.36,37 These observations, coupled with previous reports, suggest that Epo increases the stem cell pool in bone marrow and contributes to local bone regeneration induced by BMPs.38,39
In line with the increase in hematopoietic-related progenitor cells, Epo treatment increased the number of osteoclasts that are derived from the hematopoietic lineage (Fig. 3).35,40 This is a potentially important observation because the mechanical integrity of the skeleton is maintained by continuous bone remodeling, including bone formation by osteoblasts and bone resorption by osteoclasts.20 Although osteoclasts play an essential role in bone remodeling, their contributions to bone tissue engineering have long been overlooked.41 To mimic natural bone remodeling and repair, the activity of osteoclasts would ideally be involved in the engineering of bone. This participation by osteoclasts would therefore potentiate the newly generated bone to be recognized and integrated, and thus become a functional component of the host bone.42,43
BMP2 has been shown to enhance osteoclastogenesis44,45 and serum TRAP levels were increased by BMP and Epo in our in vivo studies (Fig. 3A). However, the effects of BMP2 on osteoclastogenesis were only observed at 1 week and not at 2 weeks. This short-term effect is likely due to the rapid diffusion and degradation of BMP2 after implantation. Our in vitro data also suggest that a high dosage (>50 ng/mL) is required for BMP2-enhanced osteoclastogenesis (Fig. 3B). The repeated administration of Epo can therefore sustain osteoclastogenesis followed by osteogenesis in BMP2-induced generated tissues, which potentially contributes to accelerating bone remodeling and improved structural quality of the engineered bone.
The anabolic effects of Epo on vertebral bone formation have also been observed in newborn and young mice (<6 weeks).28 However, catabolic effects were recently reported in long bones using 9-week-old mice.46 These findings suggest that the effects of Epo may depend on the rates of native bone formation. In developing bone, or BMP-induced regenerating bone, Epo increases bone formation by increasing bone remodeling. In mature or old bone, Epo potentially increases more bone resorption than bone formation. The dose of Epo in our animal experiments was 1000 U/kg, which was based on our preliminary data. In our previous work, Epo administration at 6000 U/kg via intraperitoneal injection for 4 weeks significantly increased vertebral bone formation.28 To decrease the potential side effects of the high dose of Epo, we used a relatively low dose (1000 U/kg) and short duration (2 weeks) in our current work. Similarly, we injected Epo directly into the defect site to increase the local effects of Epo while reducing its systemic hematopoietic response. As our data indicated, the cranial bone regeneration was significantly improved while the RBC numbers were moderately elevated. It was reported that Epo at a dose of 500 U/kg significantly increased the fracture healing in a mouse femoral osteotomy model.47 However, 300 U/kg and 10-day intraperitoneal injection of Epo was found to decrease the trabecular BV in mouse tibia.46 Therefore, the dosage, duration, and route for Epo administration, and the anatomic site of bone to be regenerated may also be important considerations for the application of Epo for bone tissue engineering.
Consistent with angiogenesis being essential for bone formation and regeneration,48–52 angiogenic factors, such as VEGF, have been shown to improve BMP-induced bone regeneration, and have proven to be an effective strategy in preclinical bone tissue engineering studies.13–15,53 In view of its direct stimulation of endothelial cells in vitro, Epo has been recognized as an angiogenic factor for over 20 years.54,55 Much later, Epo was reported to exhibit a nearly equivalent angiogenic potential as VEGF in vitro.56 Moreover, the role of Epo in angiogenesis was confirmed using gene knockout strategies in mice.57 Angiogenesis was also demonstrated to be the primary reason for the tissue-protective effect of Epo observed in neurological and cardiovascular tissues.23 Recently, one group reported Epo to improve long bone healing, possibly due to its angiogenic effects.47,58,59 Despite these findings, the role of Epo in bone repair remains largely unknown.
In this study, the Epo-induced increase in blood vessel formation was also noticed in BMP2-induced cranial bone regeneration as early as 1 week (Fig. 4A). It was noted that the Epo-stimulated angiogenesis was blunted when osteoclastogenesis was inhibited by ALN or OPG (Fig. 4D). This finding suggests that osteoclastogenesis is also important for the Epo-mediated effects on angiogenesis. These data may also explain why the more potent angiogenic effects of Epo were observed in the metatarsal bone model (organ culture including osteoclastogenesis) rather than in the endothelial tube formation model (HUVECs without osteoclasts) and is supported by the recent report on the novel role of osteoclasts for bone angiogenesis.33
Although Epo increased osteoclastogenesis and angiogenesis and thus improved BMP2-induced bone regeneration, the mechanism by which Epo changed the ratio of BMP2-generated bone and marrow is not clear. Cranial bone is normally repaired through the intramembranous bone formation pathway, as it is in normal development. However, endochondral bone formation was observed in the healing of critical-sized cranial defects elicited by exogenous BMPs.48,60 The transient cartilagenous phase was replaced by bone tissue and was mediated by angiogenesis.61 Cartilage formation was induced by BMP2 at 1 week in our experiments (Fig. 5). The increased blood vessel invasion and accompanied cartilage turnover were possibly induced by Epo-mediated angiogenesis. Therefore, the duration of BMP2-induced endochondral ossification was shortened by Epo-elevated blood vessel invasion. Although the total BMP2-regenerated BV was not changed by Epo, the volume of marrow was significantly decreased by Epo because the endochondral ossification was regarded as essential for bone marrow formation.62
Our working model for the roles of Epo in BMP2-induced bone regeneration is summarized in Figure 6. In summary, Epo can significantly improve BMP2-induced bone regeneration in critical-sized cranial bone defects. Increased osteoclastogenesis and angiogenesis were stimulated by Epo both in vitro and in vivo. Considering the important roles of osteoclasts in bone remodeling, hematopoiesis, and angiogenesis, we hypothesize that osteoclastogenesis is the primary means for Epo-improved bone regeneration. As a pleiotropic physiological factor, Epo administration may be a clinically applicable approach to promote bone regeneration.
FIG. 6.
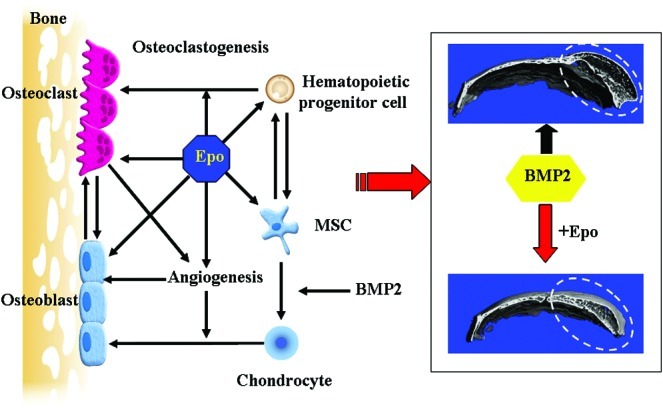
Model of Epo's action on BMP2-induced bone regeneration. Hematopoietic progenitor cell and MSC populations were increased in bone marrow by Epo administration. The increased hematopoietic progenitor cells may be responsible for Epo-stimulated osteoclastogenesis. MSCs are expected to contribute to the local bone regeneration. Osteoclasts are not only essential for coupling bone formation and bone resorption, but also important for Epo-stimulated angiogenesis. Angiogenesis is required for the transition of BMP2-generated cartilage to bone. Therefore, the structural properties of the BMP2-generated bone are strongly augmented by Epo treatment. Epo is a pleiotropic physiological factor in bone regeneration. The dashed area is the region of bone regeneration. MSCs, mesenchymal stem cells. Color images available online at www.liebertpub.com/tea
Supplementary Material
Acknowledgments
This study was supported by NIH grants DK082481 and DE020721. The authors also thank Dr. Bill King for helpful discussions and comments on the article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Schliephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31:469. doi: 10.1054/ijom.2002.0244. [DOI] [PubMed] [Google Scholar]
- 2.Giannoudis P.V. Tzioupis C. Clinical applications of BMP-7—the UK perspective. Injury. 2005;36:47. doi: 10.1016/j.injury.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Luhmann S.J. Bridwell K.H. Cheng I. Imamura T. Lenke L.G. Schootman M. Use of bone morphogenetic protein-2 for adult spinal deformity. Spine. 2005;30:S110. doi: 10.1097/01.brs.0000175184.27407.6a. [DOI] [PubMed] [Google Scholar]
- 4.Einhorn T.A. Clinical applications of recombinant human BMPs: early experience and future development. J Bone Joint Surg Am. 2003;85A:82. doi: 10.2106/00004623-200300003-00014. [DOI] [PubMed] [Google Scholar]
- 5.Cahill K.S. Chi J.H. Day A. Claus E.B. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302:58. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 6.Gautschi O.P. Frey S.P. Zellweger R. Bone morphogenetic proteins in clinical applications. Anz J Surg. 2007;77:626. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 7.Scheller E.L. Krebsbach P.H. Gene therapy: design and prospects for craniofacial regeneration. J Dent Res. 2009;88:585. doi: 10.1177/0022034509337480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu W.K. Sugiyama O. Park S.H. Conduah A. Feeley B.T. Liu N.Q., et al. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931. doi: 10.1016/j.bone.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Nussenbaum B. Rutherford R.B. Teknos T.N. Dornfeld K.J. Krebsbach P.H. Ex vivo gene therapy for skeletal regeneration in cranial defects compromised by postoperative radiotherapy. Hum Gene Ther. 2003;14:1107. doi: 10.1089/104303403322124819. [DOI] [PubMed] [Google Scholar]
- 10.Krebsbach P.H. Gu K. Franceschi R.T. Rutherford R.B. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11:1201. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 11.Richardson T.P. Peters M.C. Ennett A.B. Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 12.Wei G.B. Jin Q.M. Giannobile W.V. Ma P.X. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G.H. Corsi-Payne K. Zheng B. Usas A. Peng H.R. Huard J. The dose of growth factors influences the synergistic effect of vascular endothelial growth factor on bone morphogenetic protein 4-induced ectopic bone formation. Tissue Eng Part A. 2009;15:2123. doi: 10.1089/ten.tea.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kempen D.H.R. Lu L.C. Heijink A. Hefferan T.E. Creemers L.B. Maran A., et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y.C. Kaigler D. Rice K.G. Krebsbach P.H. Mooney D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 16.Smith D.M. Cooper G.M. Mooney M.P. Marra K.G. Losee J.E. Bone morphogenetic protein 2 therapy for craniofacial surgery. J Craniofac Surg. 2008;19:1244. doi: 10.1097/SCS.0b013e3181843312. [DOI] [PubMed] [Google Scholar]
- 17.Reddi A.H. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 18.Elizabeth A.W. Bone morphogenetic proteins (BMPs): therapeutic potential in healing bony defects. Trends Biotechnol. 1993;11:379. doi: 10.1016/0167-7799(93)90096-R. [DOI] [PubMed] [Google Scholar]
- 19.Tsumaki N. Yoshikawa H. The role of bone morphogenetic proteins in endochondral bone formation. Cytokine Growth Factor Rev. 2005;16:279. doi: 10.1016/j.cytogfr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Hadjidakis D.J. Androulakis I.I. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 21.Song J.H. Kiel M.J. Wang Z. Wang J.C. Taichman R.S. Morrison S.J., et al. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood. 2010;115:2592. doi: 10.1182/blood-2009-01-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007;78:183. doi: 10.1111/j.1600-0609.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 23.Arcasoy M.O. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 24.Goodnough L.T. Monk T.G. Andriole G.L. Erythropoietin therapy. N Engl J Med. 1997;336:933. doi: 10.1056/NEJM199703273361307. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J.W. Niu C. Ye L. Huang H.Y. He X. Tong W.G., et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 26.Calvi L.M. Adams G.B. Weibrecht K.W. Weber J.M. Olson D.P. Knight M.C., et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 27.Jung Y. Song J. Shiozawa Y. Wang J. Wang Z. Williams B., et al. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008;26:2042. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiozawa Y. Jung Y. Ziegler A.M. Pedersen E.A. Wang J. Wang Z., et al. Erythropoietin couples hematopoiesis with bone formation. PLoS One. 2010;5:e10853. doi: 10.1371/journal.pone.0010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pederson L. Ruan M. Westendorf J.J. Khosla S. Oursler M.J. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105:20764. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Y. Wu X.W. Lei W.Q. Pang L.J. Wan C. Shi Z.Q., et al. TGF-beta 1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J. Jung Y. Sun H. Joseph J. Mishra A. Shiozawa Y., et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113:220. doi: 10.1002/jcb.23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kucia M. Reca R. Campbell F.R. Zuba-Surma E. Majka M. Ratajczak J., et al. A population of very small embryonic-like (VSEL) CXCR4+SSEA-1+Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 33.Cackowski F.C. Anderson J.L. Patrene K.D. Choksi R.J. Shapiro S.D. Windle J.J., et al. Osteoclasts are important for bone angiogenesis. Blood. 2010;115:140. doi: 10.1182/blood-2009-08-237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobsen S.E.W. Borge O.J. Ramsfjell V. Cui L. Cardier J.E. Veiby O.P., et al. Thrombopoietin, a direct stimulator of viability and multilineage growth of primitive bone marrow progenitor cells. Stem Cells. 1996;14:173. doi: 10.1002/stem.5530140722. [DOI] [PubMed] [Google Scholar]
- 35.Taichman R.S. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 36.Zuba-Surma E.K. Kucia M. Dawn B. Guo Y. Ratajczak M.Z. Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kucia M. Zhang Y.P. Reca R. Wysoczynski M. Machalinski B. Majka M., et al. Cells enriched in markers of neural tissue-committed stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia. 2005;20:18. doi: 10.1038/sj.leu.2404011. [DOI] [PubMed] [Google Scholar]
- 38.Otsuru S. Tamai K. Yamazaki T. Yoshikawa H. Kaneda Y. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun. 2007;354:453. doi: 10.1016/j.bbrc.2006.12.226. [DOI] [PubMed] [Google Scholar]
- 39.Otsuru S. Tamai K. Yamazaki T. Yoshikawa H. Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 40.Roodman G.D. Cell biology of the osteoclast. Exp Hematol. 1999;27:1229. doi: 10.1016/s0301-472x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 41.Han D. Zhang Q. An essential requirement for osteoclasts in refined bone-like tissue reconstruction in vitro. Med Hypotheses. 2006;67:75. doi: 10.1016/j.mehy.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Nakagawa K. Abukawa H. Shin M.Y. Terai H. Troulis M.J. Vacanti J.P. Osteoclastogenesis on tissue-engineered bone. Tissue Eng. 2004;10:93. doi: 10.1089/107632704322791736. [DOI] [PubMed] [Google Scholar]
- 43.Ingber D.E. Mow V.C. Butler D. Niklason L. Huard J. Mao J., et al. Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 2006;12:3265. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 44.Jensen E.D. Pham L. Billington C.J. Espe K. Carlson A.E. Westendorf J.J., et al. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J Cell Biochem. 2010;109:672. doi: 10.1002/jcb.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamiya N. Mishina Y. New insights on the roles of BMP signaling in bone—a review of recent mouse genetic studies. Biofactors. 2011;37:75. doi: 10.1002/biof.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singbrant S. Russell M.R. Jovic T. Liddicoat B. Izon D.J. Purton L.E., et al. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood. 2011;117:5631. doi: 10.1182/blood-2010-11-320564. [DOI] [PubMed] [Google Scholar]
- 47.Garcia P. Speidel V. Scheuer C. Laschke M.W. Holstein J.H. Histing T., et al. Low dose erythropoietin stimulates bone healing in mice. J Orthop Res. 2011;29:165. doi: 10.1002/jor.21219. [DOI] [PubMed] [Google Scholar]
- 48.Peng H. Wright V. Usas A. Gearhart B. Shen H.-C. Cummins J., et al. Synergistic enhancement of bone formation and healing by stem cell–expressed VEGF and bone morphogenetic protein-4. J Clin Investig. 2002;110:751. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y. Wan C. Deng L. Liu X. Cao X. Gilbert S.R., et al. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Investig. 2007;117:1616. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanczler J.M. Oreffo R.O.C. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cells Mater. 2008;15:100. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 51.Wan C. Gilbert S.R. Wang Y. Cao X. Shen X. Ramaswamy G., et al. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc Natl Acad Sci U S A. 2008;105:686. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schipani E. Maes C. Carmeliet G. Semenza G.L. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009;24:1347. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kent Leach J. Kaigler D. Wang Z. Krebsbach P.H. Mooney D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 54.Anagnostou A. Lee E.S. Kessimian N. Levinson R. Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial-cells. Proc Natl Acad Sci U S A. 1990;87:5978. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anagnostou A. Liu Z.Y. Steiner M. Chin K. Lee E.S. Kessimian N., et al. Erythropoietin receptor messenger-RNA expression in human endothelial-cells. Proc Natl Acad Sci U S A. 1994;91:3974. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaquet K. Krause K. Tawakol-Khodai M. Geidel S. Kuck K.H. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64:326. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- 57.Kertesz N. Wu J. Chen T.H.P. Sucov H.M. Wu H. The role of erythropoietin in regulating angiogenesis. Develop Biol. 2004;276:101. doi: 10.1016/j.ydbio.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 58.Holstein J.H. Menger M.D. Scheuer C. Meier C. Culemann U. Wirbel R.J., et al. Erythropoietin (EPO)—EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sci. 2007;80:893. doi: 10.1016/j.lfs.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 59.Holstein J.H. Orth M. Scheuer C. Tami A. Becker S.C. Garcia P., et al. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone. 2011;49:1037. doi: 10.1016/j.bone.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Nussenbaum B. Rutherford R.B. Krebsbach P.H. Bone regeneration in cranial defects previously treated with radiation. Laryngoscope. 2005;115:1170. doi: 10.1097/01.MLG.0000166513.74247.CC. [DOI] [PubMed] [Google Scholar]
- 61.Street J. Bao M. deGuzman L. Bunting S. Peale F.V. Ferrara N., et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan C.K.F. Chen C.-C. Luppen C.A. Kim J.-B. DeBoer A.T. Wei K., et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.