Abstract
In bone tissue engineering, growth factors are widely used. Bone morphogenetic proteins (BMPs) and vascular endothelial growth factor (VEGF) are the most well-known regulators of osteogenesis and angiogenesis. We investigated whether the timing of dual release of VEGF and BMP-2 influences the amount of bone formation in a large-animal model. Poly(lactic-co-glycolic acid) (PLGA) microparticles (MPs) were loaded with BMP-2 or VEGF to create sustained-release profiles, and rapidly degrading gelatin was loaded with either growth factor for fast-release profiles. To study in vivo osteogenicity, the two delivery vehicles were combined with biphasic calcium phosphate (BCP) scaffolds and implanted in 10 Beagle dogs for 9 weeks, at both ectopic (paraspinal muscles) and orthotopic sites (critical-size ulnar defect). The 9 ectopic groups contained combined or single BMP/VEGF dosage, in sustained- or fast-release profiles. In the ulnae of 8 dogs, fast VEGF and sustained BMP-2 were applied to one leg, and the other received the opposite release profiles. The two remaining dogs received bilateral control scaffolds. Bone growth dynamics was analyzed by fluorochrome injection at weeks 3, 5, and 7. Postoperative and posteuthanization X-rays of the ulnar implants were taken. After 9 weeks of implantation, bone quantity and bone growth dynamics were studied by histology, histomorphometry, and fluorescence microscopy. The release of the growth factors resulted in both enhanced orthotopic and ectopic bone formation. Bone formation started before 3 weeks and continued beyond 7 weeks. The ectopic BMP-2 fast groups showed significantly more bone compared to sustained release, independent of the VEGF profile. The ulna implants revealed no significant differences in the amount of bone formed. This study shows that timing of BMP-2 release largely determines speed and amount of ectopic bone formation independent of VEGF release. Furthermore, at the orthotopic site, no significant effect on bone formation was found from a timed release of growth factors, implicating that timed-release effects are location dependent.
Introduction
Osteoinduction, that is, the induction of osteoprogenitors toward the osteogenic lineage, is an important aspect of bone healing, which occurs during bone-grafting procedures and fracture healing, and is normally a part of the successful process of tissue regeneration. This process is active when autologous bone-grafting procedures are used in orthopedic surgery, for example, in spinal fusion and nonunions. Unfortunately, several disadvantages are associated with graft harvesting, such as limited availability of grafts and donor-site morbidity.1,2 Therefore, alternative strategies have been developed for the regeneration of bone, for example, the use of bioactive molecules known to be involved in the process of osteoblast differentiation.
Bone morphogenetic proteins (BMPs), bioactive molecules first found in demineralized bone, are widely studied in developmental and skeletal biology; they belong to the transforming growth factor β superfamily, which also includes other growth factors such as activins and inhibins. Members of the BMP family, comprising over 30 members, half of whom in mammals, are divided into different subgroups based on their amino acid sequences.3,4 Not all of the members of the BMP family possess osteogenic properties: BMP 2, 4, 6, 7, and 9 are osteogenic.5–9 Several of these BMPs have initially proven their inductive capacity by inducing bone formation at ectopic sites.10,11 Later, they have been described to initiate both cartilage and bone progenitor cell differentiation and to control the formation of new bone through both endochondral and intramembranous ossification.12,13 Recombinant DNA technologies have allowed the optimization of BMP-2 and BMP-7 protein preparation, which has already resulted in clinical successes.14,15 A disadvantage however is that the current clinical available delivery vehicles of these BMPs, which still need to be optimized, and furthermore that supraphysiologic dosages are used. The collagen powder or sponges used show large initial burst release, while during fracture repair, BMP expression increases until day 21, suggesting that it is needed for a longer period of time.16–18
Apart from induction by BMPs, bone formation is an angiogenesis-dependent process. This vessel formation is important for transport of nutrients, oxygen, and cells toward and removal of waste products from the newly formed bone. Many studies focus on the use of the bioactive molecule vascular endothelial growth factor (VEGF), the key mediator in angiogenesis. As a result of being important in angiogenesis, VEGF has shown to be important during early fracture repair and endochondral and intramembranous ossification.19–21 In line with this, experimental models have shown that normal fracture healing is disturbed if VEGF is inhibited.22,23
Communication between osteoprogenitor and endothelial cells is mediated by several mechanisms: the direct process of gap–junctional communication and the indirect process involving the soluble growth factors VEGF and BMP-2.24,25 VEGF has been described to interact in a synergistic manner with BMP-2 and BMP-4 in both bone formation and bone healing by increasing cell survival, angiogenesis, and enhancing cell recruitment.26 Furthermore, VEGF can stimulate chemotaxis and differentiation of osteoblasts,27 osteoclasts,28 and mesenchymal progenitor cells,29 and furthermore, VEGF may also directly contribute to bone formation by enhancing BMP-2 mRNA and protein expression.18 Moreover, osteoblasts are known to be able to synthesize VEGF when oxygen levels are low, and in this way actively enhance the process of angiogenesis.30 Given the importance of the angiogenesis–osteogenesis coupling, combining the most important bioactive molecules involved in these processes can be beneficial for bone regeneration.31
One of the major problems considering release of growth factors from delivery vehicles is the loss of biological activity. Since these growth factors have short biological half-lives, keeping the concentration of growth factor in the therapeutic window for a sufficient period of time is suggested to be important in mimicking natural bone regeneration.32 Additionally, the timing of this release could be important to simulate the natural expression pattern of these growth factors. There are several studies that use a dual release system of VEGF and BMP-2 for the enhancement of bone healing, especially in small-animal models, but they do not consider the different timing of this release.33–37 One study does investigate timing of this release in a small-animal model, but only uses fast delivery of VEGF and sustained delivery of BMP-2, based on their expression pattern in normal bone repair.38
Therefore, we investigated whether the timing of sequentially released BMP-2 and VEGF is of importance in bone formation in a large-animal model. We made use of fast- and sustained-release systems for proteins that were earlier described and validated for both BMP-2 and VEGF sequentially.38,39 The effects of VEGF and BMP-2 were studied in ectopic and orthotopic implantation models in dogs, and after 9 weeks of implantation, bone quantity and bone growth dynamics were studied.
Materials and Methods
Experimental design
A total of 10 dogs were used for the experiment after approval of the local Ethics Committee for Animal Experimentation and in compliance with the Institutional Guidelines on the Use of Laboratory Animals. A rapidly degrading hydrogel (gelatin) served as a delivery vehicle for fast (f) release, whereas poly(lactic-co-glycolic acid) (PLGA) microparticles (MPs) served as a sustained (s)-release vehicle. The bone-forming capacity of constructs containing these growth factors was studied in vivo at both ectopic (intramuscular) and orthotopic (critical-size ulnar defect) locations. Eight animals received 9 ectopic implants and 2 ulnar constructs: (1) BMP-2 sustained/VEGF fast and (2) BMP-2 fast/VEGF sustained (Table 1). Two animals received scaffolds without growth factors in the ulnar defect, serving as controls for scaffold-mediated bone regeneration. Fluorochrome labels were administered after 3, 5, and 7 weeks to analyze bone growth dynamics. Directly after implantation and after euthanization, X-rays were taken again to analyze bone formation around the implants. Furthermore, the bone formation in and around the implants was analyzed in more detail by histology, histomorphometry, and fluorescence microscopy.
Table 1.
Implantation Groups
| Condition | MPs | Gelatin | Location (n) |
|---|---|---|---|
| BMPf/VEGFs | VEGF | BMP | i.m (8)/ulna(8) |
| BMPf/VEGFf | BMP+VEGF | i.m. (8) | |
| BMPs/VEGFf | BMP | VEGF | i.m (8)/ulna(8) |
| BMPs/VEGFs | BMP+VEGF | i.m. (8) | |
| BMPs | BMP | i.m. (8) | |
| VEGFs | VEGF | i.m. (8) | |
| BMPf | BMP | i.m. (8) | |
| VEGFf | VEGF | i.m. (8) | |
| Control | i.m (8)/ulna (4) |
The total amount of bone morphogenetic protein-2 was 12 μg/intramuscular, and 120 μg/ulnar implant.
The amount of VEGF was 0.4 μg/i.m. and 4 μg/ulnar implant.
f, fast; s, sustained; i.m., intramuscular; VEGF, vascular endothelial growth factor; BMP, bone morphogenetic protein.
Construct design and preparation
The constructs consisted of three major components. PLGA MPs (Medisorb®, Lakeshore Biomaterials) for sustained release, loaded with either rhBMP-2 (INFUSE®; Medtronic) or rhVEGF-165 (R&D systems). A rapidly degrading hydrogel (gelatin from porcine skin; Sigma-Aldrich) for fast release loaded with either rhBMP-2 or rhVEGF and biphasic calcium phosphate (BCP) (Progentix) was used as a scaffold and combined with these delivery vehicles.
The BCP consisted of 80%±5% (mean±SD) (w/v) hydroxyapatite and 20%±5% (w/v) β-tricalcium phosphate, and total porosity was 70%±5%, macroporosity 55%±5%, and microporosity 20%±5%. For ectopic implantation, particles of ø 2–3 mm were used, and for ulnar implantation, cylinders of 22-mm height, 10-mm diameter, and a 4-mm-diameter central canal. Finally, they were cleaned in an ultrasonic bath and sterilized by autoclave.
Based on earlier literature, we assumed that entrapment efficiencies and in vivo release profiles were similar as described before. PLGA MPs (Medisorb; Lakeshore Biomaterials) for sustained release were loaded with rhBMP-2 (Medtronic) or rhVEGF-165 (R&D systems) and prepared using a previously described water-in-oil-in-water (W1-O-W2) double-emulsion solvent extraction technique38–42 up to 1 month before implantation. Four different groups of MPs were produced: BMP high, BMP low, VEGF high, and VEGF low. For this, 100 μL of a BMP-2 stock (8 mg/mL, or 0.8 mg/mL) or 135 μL of a VEGF stock (0.2 mg/mL, or 0.02 mg/mL) or a control solution (BMP-2 buffer containing 5 mM glutamic acid, 2.5 (w/v) glycine, 0.5% (w/v) sucrose, and 0.01% (v/v) Tween 80, pH4.5; INFUSE, Medtronic) was emulsified in a solution of 500 mg of PLGA (L:G ratio of 50:50, Mw 23 kDa) and in 1.25 mL dichloromethane. These growth factor amounts were based on the entrapment efficiency in earlier studies39,41 combined with a dosage 100–4000-times above the ED50 described for both growth factors. This mixture was re-emulsified for 30 s in 2 mL of 1% (w/v) aqueous poly(vinyl alcohol) (PVA, 87%–89% mole hydrolyzed; Sigma-Aldrich) solution to create the double emulsion. This double emulsion was then added to 100 mL of a 0.3% (w/v) aqueous PVA solution and 100 mL of a 2% (v/v) aqueous isopropanol solution and stirred for 1 h. The MPs were centrifuged, collected, washed twice with ddH2O, vacuum-dried overnight into a free flowing powder system (Savant Speedvac systems), and stored at −20°C.
The hydrogel for fast release was made of a 1 mL aqueous gelatin solution (10% w/v) crosslinked with 40 μL aqueous glutaraldehyde (10% v/v) solution as described before.38 For the orthotopic implants, hydrogel (1 mL) was pipetted in the central canal of the BCP scaffold and served as a plug when it was polymerized. The MPs (for sustained release) were premixed with the gelatin and pipetted in the central canal of the scaffold on top of the plug (1 mL). After 1 h of crosslinking at 4°C, scaffolds were put in 100 mM aqueous glycine solution for 1 h to block the residual aldehyde groups of glutaraldehyde (Fig. 1A).
FIG. 1.
Samples before implantation. (A) BCP-cylinder h 22 mm, Ø 10 mm, core 4 mm, the scheme on the right side of Figure A indicates the gelatin (containing the growth factor for fast release) combined with the PLGA MPs (containing the growth factor for sustained release). (B) BCP-particles Ø2–3 mm, molded in an 8×8×8-mm chamber slide, the scheme above this indicates the gelatin (containing the growth factor for fast release) combined with the PLGA MPs (containing the growth factor for sustained release). BCP, biphasic calcium phosphate; PLGA, poly(lactic-co-glycolic acid); MPs, microparticles. Color images available online at www.liebertpub.com/tea
For the ectopic implants, chamber slides (Sigma-Aldrich) were used as a mold to make them equally sized (8×8×8 mm, Fig. 1B). The chamber slides were filled with BCP particles, and the hydrogel mixed with MPs containing VEGF/BMP-2 was pipetted into the chamber slides (0.5 mL). They underwent the same crosslinking procedure as the orthotopic implants, and all implants were stored at −20°C before implantation the day before surgery. The fast-released growth factor was injected into the hydrogel (cylinder and blocks) after thawing and just before the operation and was allowed to diffuse in the material. Based on previous studies, the entrapment efficiency was estimated to be 85% or 1.36 μg BMP-2 per mg PLGA that resulted in a total BMP-2 loading of 12 μg per ectopic implant and 120 μg per ulnar implant (both 88 mg PLGA). The same was done for the VEGF implants, resulting in a total loading of 0.4 μg per ectopic implant and 4 μg for ulnar implants (both 108 mg PLGA).38 The calculated concentrations of growth factors in these implants are much higher (100–4000×) than the in vitro effective dosage known for the growth factors (ED50 of VEGF is 2–6 ng/mL; ED50 of BMP-2 is 20–50 ng/mL, as stated in the product sheets) to make sure enough that BMP-2/VEGF was available in vivo during the 9-week implantation period. The ectopic control groups consisted of BCP mixed with gelatin/control MPs without growth factor, and orthotopic control implants consisted of an empty BCP scaffold with gelatin/control MPs. All scaffolds were stored at −20°C until use. The presence of calcium phosphate ceramics was not expected to influence the fast- and sustained-release profiles.43
Animals and surgical procedure
Ten beagle dogs were used for the in vivo experiment. The age of the dogs was 1.5±0.27 years and weight 13.7±2.1 kg. The procedures were performed under general anesthesia. Anesthesia was initiated using intravenously administered medetomidine (Domitor; Pfizer Animal Health B.B., Capelle a/d Ijssel) and propofol (Rapinovet; Schering-Plough Animal Health N.V.). After intubation, it was maintained by inhalation anesthesia with isoflurane, nitrous oxide, and oxygen. Amoxicillin with clavulanic acid (Augmentin; SmithKline Beecham Farma B.V.) were administered intravenously (20 mg/kg body weight) as prophylactic antibiotics before the start of the operation. Both front limbs were shaved, disinfected, and draped in a sterile fashion. The ulna was exposed through a craniolateral approach using a longitudinal skin incision ending 4-cm proximal of the ulnar styloid process. The periosteum was preserved by making a longitudinal incision and careful periosteal elevation. A 22.5-mm bone segment was removed using an oscillating saw and ample lavage. In this way, a critical-sized bone defect was created.44 The prepared implants were placed in the defects according to a randomized design. The periosteal cylinder was approximated with an absorbable suture material. The subcutaneous tissues and skin were closed in a routine fashion. A protective bandage was applied for 3 days. Next, a midline incision was made to expose the paraspinal muscles. For ectopic implantation, intramuscular pockets (in the paraspinal muscle) were created through separate fascia incisions, and the constructs were placed in these pockets according to a randomized block schedule (Table 1), after which they were closed by nonresorbable sutures, for localization purposes. The muscle fascia, subcutaneous tissues, and skin were closed subsequently in layers. Direct postoperative X-rays were taken. The dogs received analgesics (Temgesic) for 3 days postoperatively. Full loading of the legs was permitted immediately after surgery. The dogs were nursed until complete recovery from their surgery. After this, they went back to their kennel and fed a standard commercial dog food twice a day with water available ad libitum.
Fluorochromes were administered at week 3 (Calcein Green, 10 mg/kg, i.v.; Sigma), week 5 (Oxytetracyclin, Engemycine 32 mg/kg, i.m.; Mycofarm), and week 7 (Xylenol Orange, 80 mg/kg, i.v.; Sigma) to analyze onset of bone formation. After 9 weeks, all animals were euthanized with an overdose of pentobarbital (Organon), and radiographs were taken in anterior-posterior and medial-lateral direction. Samples were retrieved for histological and histomorphometric analysis.
Histology
The orthotopic and subcutaneous implants were explanted after 9 weeks and fixed in 4% formalin, dehydrated by ethanol series, and embedded in polymethylmethacrylate (MMA). Two 10-μm-thick longitudinal sections were cut through the middle of each implant using a sawing microtome (Leica). The first section remained unstained for fluorescence microscopy, and the other section was stained with methylene blue and basic fuchsin. High-resolution digital photos were made from the stained sections for histomorphometric analysis. Bone and scaffold were pseudocolored yellow and green, respectively, using Adobe Photoshop CS3. KS400 software (version 3; Zeiss) was used to perform histomorphometry. A custom macro was used to determine the area of interest, the area of scaffold, the area of bone, the scaffold outline available for bone apposition, and the contact length of bone and scaffold. This allowed the calculation of the percentage bone in available space, called bone area%=[bone area/(total area–scaffold area)] ×100%, and the percentage bone apposition against the scaffold, contact%=(bone-to-scaffold contact length/scaffold outline)×100%. Fluorochrome labels were analyzed using a light/fluorescence microscope (X-Cite; EXFO) equipped with a quadruple filter block (Chroma 104158; Chroma Technology). The presence or absence of each of the three fluorochrome labels was scored for all implants.45
The 10 Beagle dogs underwent surgery without any complications (OR time 111.6±16.0 min.), and they recovered well. After surgery, the dogs received pain relief and were almost immediately able to stand on both their legs. No dogs were lost during the 9-week follow-up, and no complications in wound healing were observed. Seventy out of 72 implants were retrieved successfully after 9 weeks of implantation, one VEGF sustained (VEGFs) and one BMP-2 fast (BMPf) implant were not found.
Statistical analysis
Sample size was determined by performing a power analysis based on histomophometry from earlier studies. Power of 0.9 was used, and α 0.05 and minimal difference in bone contact% and area% between the implanted groups were expected to be 40% (with a variance of 30%). For this study, at least eight animals were needed. For statistical evaluation of the bone quantification of ectopic implants, statistical differences between the implanted groups were analyzed by a repeated measures analysis. For the orthotopic implants, statistical differences in bone formation between the two implanted groups were analyzed by a Student's t-test. Results are reported as means±standard deviation and were considered statistical significant at p<0.05.
Results
Results of the ectopic implants
Histology of the ectopic implants
Explanted ectopic samples were analyzed using basic fuchsin/methylene blue staining of MMA-embedded sections and showed various amounts of bone in all groups. Strikingly, we found extensive bone bridging between different particles, especially when BMPf was implanted (Fig. 2A). When BMP-2 sustained (BMPs) was implanted, newly formed bone was present mostly against the borders of the scaffolds (Fig. 2B). No clear signs of degradation of the BCP scaffolds could be observed. No signs of remnants of the MPs or gelatin were found, suggesting that they fully degraded. Scaffolds were surrounded by newly formed bone with osteoblasts lining the surface and osteocytes lying in lacunas (Fig. 2C). Foreign-body multinucleated giants cells were often lining the surface of the scaffold at sites where bone was not present (Fig. 2C), and in rare cases, they were also found on the bone (Fig. 2D). In addition, tissue resembling bone marrow could be seen in the implants (Fig. 2D). The newly formed tissue within the implants was well vascularized.
FIG. 2.
Bone formation in the ectopic implants. (A) BMPf/VEGFf group: note the bone bridging between the particles; (B) BMPs/VEGFs group: less to no bridging, white arrow pointing to a blood vessel; (C) BMPf/VEGFs group (these cells/structures were seen in all groups): bone-lining cells/OB, MNGCs, OC with a black arrow pointing at it, white arrow pointing to a blood vessel; (D) most-pronounced in BMPf groups: bone marrow, and note the MNGC lying against the bone, suggesting it is an osteoclast. (A–D) Methylene blue/basic fuchsin stainings. (E and F) fluorochrome detection, green=3-week label; orange=5-week label; red=7-week label; white arrow pointing at the 5-week label; and the black and white arrows pointing at the 7-week label. B, bone; MNGC, multinucleated giant cell; OB, osteoblast; OC, osteocyte; V, vessel; BM, bone marrow; S, scaffold; BMP, bone morphogenetic proteins; VEGF, vascular endothelial growth factor; f, fast; s, sustained. Color images available online at www.liebertpub.com/tea
Onset and growth dynamics of newly formed bone
Analysis of bone growth dynamics by incorporation of fluorochrome labels in the ectopic samples (Table 2) revealed that bone formation almost always started within the first 3 weeks after implantation (in 7/8 or 8/8 implants), except in the VEGFs and control groups, where the 3-week label (calcein green) showed less presence. Bone formation had started in the outer rim of the scaffolds, as seen by the green label that was usually present in the periphery of the implants. The yellow (tetracycline) and red labels (xylenol orange) were present all over the scaffolds (Fig. 2E). A remarkable difference in onset of bone formation was found in the VEGFs and control group. While bone formation started within the first 3 weeks after implantation in some scaffolds, it did not start before 7 weeks in the others as indicated by the absence of all 3 fluorochromes.
Table 2.
Onset of Bone Formation in Ectopic Constructs
| Condition | Calcein green week-3 label | Tetracycline week-5 label | Xylenol Orange week-7 label |
|---|---|---|---|
| BMPf/VEGFs | 8/8 | 8/8 | 8/8 |
| BMPf/VEGFf | 8/8 | 8/8 | 8/8 |
| BMPs/VEGFf | 7/8 | 8/8 | 8/8 |
| BMPs/VEGFs | 8/8 | 8/8 | 8/8 |
| BMPs | 8/8 | 8/8 | 8/8 |
| VEGFs | 4/7 | 4/7 | 4/7 |
| BMPf | 7/7 | 7/7 | 7/7 |
| VEGFf | 7/8 | 7/8 | 7/8 |
| Control | 3/8 | 3/8 | 3/8 |
Incorporation of fluorochrome labels at weeks 3, 5, and 7, presence scored per implant.
Bone formation analysis
The amount of bone formed in each group was determined by histomorphometric analysis (Fig. 3). Bone contact% and bone area% showed approximately similar results. Largest bone contact% was seen in BMPf-release groups, ranging from 15.2%±8.1% to 25.8%±16.1%. BMPf groups showed significantly higher bone contact% than BMPs-release groups (p<0.01) (area%, 22.2%±10.9% to 34.0%±8.4%, p<0.01). The addition of VEGF had no statistically significant effect on bone contact% (p=0.239), nor on area% (p=0.132) (Fig. 3). Overall, BMPf groups induced more bone than BMPs groups. BMPs groups showed bone contact% of 7.0%±4.7% to 9.9%±7.5% and bone area% of 4.8%±4.5% to 10.6%±7.8%, which was higher than VEGFs and VEGF fast (VEGFf) groups. Furthermore, no significant differences in bone area% and bone contact% were seen between the VEGFs and VEGFf groups. Control scaffolds without growth factors showed little bone formation in only 3/8 samples. Bone formation in these controls was variable, 0.5%±0.27% bone area% and 1.17%±0.65% bone contact%.
FIG. 3.
Bone quantification in ectopic implants: bone contact% and bone area%, both after 9 weeks of ectopic implantation in the dog. The results are represented as mean±standard deviation. BMPf groups show significant higher bone contact% and area% compared to the BMPs groups (*p<0.01), without an effect of VEGF.
Results of the ulnar implantation
Radiographs of the ulnar implants, postoperative and after euthanization
The postoperative radiographs showed similar placement of all ulnar implants ∼2 cm above the styloid process of the ulna (Fig. 4A). Radiographs after termination showed bone formation in the direction of the implants (Fig. 4B, C). Some of the implants had been broken (two in every group) as was seen in the proximal part of the scaffold in Figure 4C. A radiolucent line (Fig. 4C, arrow) was seen in all radiographs mainly at the proximal part of the scaffolds, which might indicate pseudoarthrosis.
FIG. 4.
Mediolateral X-rays: (A) BMPf/VEGFs, postoperatively, (B) BMPs/VEGFf posteuthanization, intact scaffold with obvious bone formation, (C) BMPf/VEGFs posteuthanization, broken scaffold with obvious bone formation toward the scaffold (arrow), and (D) control scaffold, posteuthanization.
Histology of the ulnar implants
Although some of the implants had broken edges, no clear signs of scaffold degradation were observed. Bone formation could be seen in all growth factor-loaded implants. Most of it was found in the scaffold material and less in the central canal of the cylinders (Fig. 5A). Overall, the aspect of the newly formed bone was comparable to the ectopic implants and contained bone lining cells, multinucleated giant cells (MNGCs), and blood vessels (Fig. 5B). In contrast to the ectopic implants, cartilage had formed outside the proximal border between the scaffold and ulna (Fig. 5C). This could indicate endochondral ossification taking place. The controls showed only small amounts of newly formed bone in 3 out of 4 implants.
FIG. 5.
Histology shown in orthotopic (ulnar) implants. (A) BMPf/VEGFs group: complete scaffold, (B) extensive bone formation with bone-lining cells/osteoblast, (C) cartilage formation seen in especially the proximal parts of several scaffolds. (D) Fluorochrome detection. (A–C) Methylene blue/basic fuchsin stainings; (D) fluorescence microscopy, green=3-week label, orange=5-week label, and red=7-week label. Color images available online at www.liebertpub.com/tea
Onset and growth dynamics of newly formed bone
For analysis of bone growth dynamics, the ulnar implants were divided into three regions (Table 3): 1) the scaffold material itself, 2) the central core of the scaffold, and 3) outside the scaffold. In 6 out of 8 dogs, bone formation started in the BMPs/VEGFf groups before 3 weeks (calcein green label) in the pores of the scaffold (Fig. 5D). In the BMPf/VEGFs groups, it always started before 3 weeks. Outside the scaffolds, bone was always present independent of which group it was. In the central canal of the scaffold, bone formation started almost always between 3 and 5 weeks. Only in the control groups, bone formation started later than 7 weeks in 50% of the cases.
Table 3.
Onset of Bone Formation in Orthotopic (Ulnar) Constructs
| Condition | Calcein Green week-3 label | Tetracycline week-5 label | Xylenol Orange week-7 label |
|---|---|---|---|
| Within scaffold | |||
| BMPs/VEGFf | 6/8 | 8/8 | 8/8 |
| BMPf/VEGFs | 8/8 | 8/8 | 8/8 |
| Control | 2/4 | 3/4 | 3/4 |
| Central canal | |||
| BMPs/VEGFf | 1/8 | 7/8 | 7/8 |
| BMPf/VEGFs | 1/8 | 8/8 | 8/8 |
| Control | 1/4 | 2/4 | 2/4 |
| Outside scaffold | |||
| BMPs/VEGFf | 8/8 | 8/8 | 8/8 |
| BMPf/VEGFs | 8/8 | 8/8 | 8/8 |
| Control | 4/4 | 4/4 | 4/4 |
Incorporation of fluorochrome labels at weeks 3, 5, and 7, presence scored per implant.
Bone formation analysis
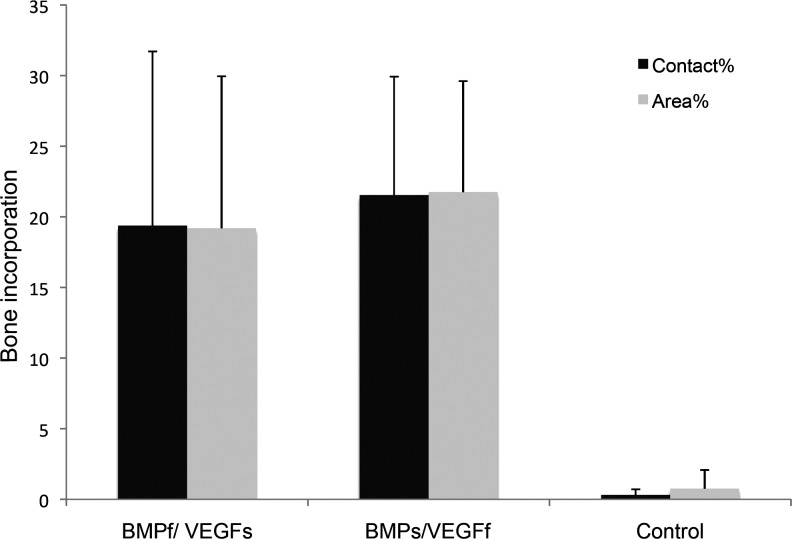
Bone was quantified within the pores of the implant. Bone growth in the central canal and outside the scaffold was excluded for the calculation of bone contact% and bone area%. Comparable to the ectopic implants, results of bone contact% and area% are quite similar. No significant differences in the bone quantity between BMPf/VEGFs and BMPs/VEGFf groups could be observed (p=0.506). Bone contact% for BMPs/VEGFf was 21.5%±8.4% and 19.4%±12.3% for BMPf/VEGFs. Both growth factor-containing groups did show significant differences compared to the control groups (p<0.01) (Fig. 6).
FIG. 6.
Bone quantification in orthotopic (ulnar) implants: bone contact% and bone area%, both after 9 weeks of orthotopic implantation in the dog. The results are represented as mean±standard deviation. BMPf/VEGFs and BMPs/VEGFf groups both show significant higher contact% and area% compared to the control groups (p<0.01), but no significant effect compared to each other (p=0.506).
Discussion
The aim of this study was to assess whether the timing of a dual release of BMP-2 and VEGF is important in enhancing bone formation in an ectopic and orthotopic (ulnar) implantation model in dogs, since they are the most important regulators in osteogenesis and angiogenesis. To obtain a dual-release profile, BCP scaffolds were combined with PLGA microspheres and a gelatin hydrogel as controlled delivery vehicles. MPs were used for the sustained release, and gelatin for a more rapid release. These constructs were implanted at an ectopic as well as an orthotopic (ulnar) location in dogs. The amount of growth factor incorporated in the MPs was based on the entrapment efficiency measured in an earlier study by radiolabeling the BMP-2 and the fact that they showed an almost complete release without retention.39 We have presumed a complete release of growth factors in this larger-animal model, because no signs of the MPs or gelatin were found in the histological samples. Previously, it has been shown that incorporation of growth factors in gelatin results in a rapid release in a 2-week period,38,46,47 and PLGA has been widely used for sustained release profiles of VEGF and BMP-2, capable of releasing growth factors for an 8-week period in vivo.38,47,48
The early osteogenic effect of both growth factors, analyzed by calcein green fluorescence, was clearly present at both the ectopic and orthotopic location (within the scaffold). Although there was no significant influence of the VEGF on bone area and contact percentage after 12 weeks, fluorochrome analysis suggested an effect of timing of VEGF in the absence of BMP-2. While only 4 out of 7 VEGFs scaffolds showed the presence of the 3-week label, calcein green was present in 7 out of 8 scaffolds in the VEGFf group. The difference in early bone formation in both ectopic VEGF groups might be attributed to both the vasculogenic and the chemotactic properties of VEGF on osteoblasts, osteoclasts, and endothelial cells.27,49 These properties are important during the first weeks of the implantation period, and the gelatin has shown a high initial burst release during the first few days in our previous study. Therefore, the release from the MPs, which had a more sustained-release profile over 56 days in this same study, might be too slow to exhibit the chemotactic effect.38 In the orthotopic implants, bone formation within the growth factor-containing scaffolds also started earlier compared to the control group. However, also in these control groups, 50% of implants showed some early bone formation, especially at the periphery, probably resulting from an osteogenic effect of the periost covering these implants. The later onset of bone formation observed in the cavity of the orthotopic implants is most likely due to the lack of cells and blood vessels in that location.
The role of the dual delivery of VEGF and BMP-2 on the total amounts of in vivo bone formation ectopically, analyzed by histomorphometry, is diverse. A very clear effect of timing of BMP was seen. Significantly, more bone was found in the BMPf groups compared to the BMPs groups without an influence of VEGF in either form. Since there is a little difference between the 5- and 7-week incorporation of fluorochromes, initiation of bone formation appears to be the determining factor in these experiments. Therefore, the lower BMP-2 concentrations at early time points in the sustained delivery vehicles are less effective to initiate bone formation. Since previous studies showed that a similar sustained BMP-2-release profile from a microsphere/polymer composite was more efficient than the rapid BMP-2 release from the gelatin hydrogel, this effect cannot be exclusively attributed to the pharmacokinetics.38,41 The prolonged local BMP-2 retention in the microsphere-containing gelatin hydrogel might make it more susceptible to spontaneous and/or enzymatic degradation and might result in lower total dose of BMP-2 available from sustained delivery vehicles. As a result, the microsphere–gelatin composite is less attractive as a sustained delivery vehicle for tissue engineering.
As mentioned previously, no significant additional effect of VEGF was seen on the amount of ectopic bone formation, which is similar to results of earlier studies on bone formation in a rat critical-size cranial defect model,33,50 where even higher amounts of VEGF were used. Nevertheless, VEGF groups without BMP-2 showed some bone formation in this study, which might be attributed to the earlier-described chemotactic properties of VEGF.27,49 This only accounts for the VEGFf group, and was not significant. Other studies did see an additional effect of VEGF ectopically.34,38,51 The discrepancy might be that VEGF was not a restrictive factor in our study, and furthermore the use of a different kind of scaffold material. In this study, we used the osteoconductive ceramic BCP, as opposed to polymer scaffolds in other studies. This ceramic induced differentiation of multipotent stromal cells (MSCs) toward osteoblasts, which efficiently synthesize VEGF and thereby render the addition of extra-VEGF ineffective.51,52 Furthermore, the surgical procedures resulted in local bleeding and hematoma formation in the muscle of the dogs, which could have served as a local source for other chemotactic and angiogenic growth factors contributing to early bone regeneration both at the orthotopic and ectopic location.53,54
In the orthotopic location, no statistical significant differences were seen between the BMPf/VEGFs and BMPs/VEGFf groups, indicating that timing of release of BMP-2 and VEGF using these compositions has no influence on orthotopic bone regeneration in this setting. The different effects of growth factor release in the ectopic and orthotopic site might be explained by the location. Overall, the orthotopic defect is less challenging with regard to MSC recruitment. The periost covering the ulnar implants and the exposed marrow cavity contain MSCs, which could have contributed to this bone formation making it not a very challenging model. The osteoinductive properties of the scaffold material allow these cells to differentiate into osteoblasts to form bone within the pores of the scaffold.55 It is possible that the BMP-2 and VEGF were both needed to initiate the bone-healing cascade, but that the timing of this release was not important, since other (growth) factors and MSCs had a more prolonged effect on this bone formation. Although bone quantification was only performed within the pores of the implant, bone formation was also observed along the borders and the central cavity of the implant. However, radiological and histomorphometric analysis showed no large differences between these groups.
In conclusion, this study showed differential orthotopic and ectopic bone formation induced by timed release of BMP-2. No significant additional effect of VEGF was seen on the amount of ectopic bone formation, and no significant influence of timing of dual VEGF and BMP-2 delivery was seen on orthotopic bone regeneration, whereas at the ectopic site timing of BMP-2 release significantly influences bone formation.
Acknowledgments
The authors acknowledge the Dutch Program for Tissue Engineering (grant UGT.6743), and the Smart Mix Program of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science for financial support. The authors also thank H. Yuan (Progentix, The Netherlands) for kindly providing the BCP scaffold materials and P. Westers for advice on the statistical analysis.
Disclosure Statement
No competing financial interests exist.
References
- 1.Silber J.S. Anderson D.G. Daffner S.D. Brislin B.T. Leland J.M. Hilibrand A.S. Vaccaro A.R. Albert T.J. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Finkemeier C.G. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A:454. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Reddi A.H. Haudenschild D.R. BMP Family. Maryland Heights, MO: Elsevier Academic Press; 2000. p. 747. [Google Scholar]
- 4.Wozney J.M. Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998;346:26. [PubMed] [Google Scholar]
- 5.Rengachary S.S. Bone morphogenetic proteins: basic concepts. Neurosurg Focus. 2002;13:e2. doi: 10.3171/foc.2002.13.6.3. [DOI] [PubMed] [Google Scholar]
- 6.Wozney J.M. Overview of bone morphogenetic proteins. Spine (Phila Pa 1976) 2002;27:S2. doi: 10.1097/00007632-200208151-00002. [DOI] [PubMed] [Google Scholar]
- 7.Bessa P.C. Casal M. Reis R.L. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 8.Bessa P.C. Casal M. Reis R.L. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 9.Kang Q. Sun M.H. Cheng H. Peng Y. Montag A.G. Deyrup A.T. Jiang W. Luu H.H. Luo J. Szatkowski J.P. Vanichakarn P. Park J.Y. Li Y. Haydon R.C. He T.C. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson O.S. Urist M.R. Dawson E.G. Schmalzried T.P. Finerman G.A. Bone repair induced by bone morphogenetic protein in ulnar defects in dogs. J Bone Joint Surg Br. 1986;68:635. doi: 10.1302/0301-620X.68B4.3733844. [DOI] [PubMed] [Google Scholar]
- 11.Wang E.A. Rosen V. D'Alessandro J.S. Bauduy M. Cordes P. Harada T. Israel D.I. Hewick R.M. Kerns K.M. LaPan P., et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87:2220. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanada K. Solchaga L.A. Caplan A.I. Hering T.M. Goldberg V.M. Yoo J.U. Johnstone B. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem. 2001;81:284. doi: 10.1002/1097-4644(20010501)81:2<284::aid-jcb1043>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Bostrom M.P. Lane J.M. Berberian W.S. Missri A.A. Tomin E. Weiland A. Doty S.B. Glaser D. Rosen V.M. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res. 1995;13:357. doi: 10.1002/jor.1100130309. [DOI] [PubMed] [Google Scholar]
- 14.Govender S. Csimma C. Genant H.K. Valentin-Opran A. Amit Y. Arbel R. Aro H. Atar D. Bishay M. Borner M.G. Chiron P. Choong P. Cinats J. Courtenay B. Feibel R. Geulette B. Gravel C. Haas N. Raschke M. Hammacher E. van der Velde D. Hardy P. Holt M. Josten C. Ketterl R.L. Lindeque B. Lob G. Mathevon H. McCoy G. Marsh D. Miller R. Munting E. Oevre S. Nordsletten L. Patel A. Pohl A. Rennie W. Reynders P. Rommens P.M. Rondia J. Rossouw W.C. Daneel P.J. Ruff S. Ruter A. Santavirta S. Schildhauer T.A. Gekle C. Schnettler R. Segal D. Seiler H. Snowdowne R.B. Stapert J. Taglang G. Verdonk R. Vogels L. Weckbach A. Wentzensen A. Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Friedlaender G.E. Perry C.R. Cole J.D. Cook S.D. Cierny G. Muschler G.F. Zych G.A. Calhoun J.H. LaForte A.J. Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S151. [PMC free article] [PubMed] [Google Scholar]
- 16.Uludag H. D'Augusta D. Palmer R. Timony G. Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46:193. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Cho T.J. Gerstenfeld L.C. Einhorn T.A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 18.Bouletreau P.J. Warren S.M. Spector J.A. Peled Z.M. Gerrets R.P. Greenwald J.A. Longaker M.T. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg. 2002;109:2384. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu D.E. Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34:680. doi: 10.1016/j.bone.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Maes C. Stockmans I. Moermans K. Van Looveren R. Smets N. Carmeliet P. Bouillon R. Carmeliet G. Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J Clin Invest. 2004;113:188. doi: 10.1172/JCI19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Street J. Bao M. deGuzman L. Bunting S. Peale F.V., Jr. Ferrara N. Steinmetz H. Hoeffel J. Cleland J.L. Daugherty A. van Bruggen N. Redmond H.P. Carano R.A. Filvaroff E.H. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaigler D. Wang Z. Horger K. Mooney D.J. Krebsbach P.H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21:735. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 23.Eckardt H. Ding M. Lind M. Hansen E.S. Christensen K.S. Hvid I. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J Bone Joint Surg Br. 2005;87:1434. doi: 10.1302/0301-620X.87B10.16226. [DOI] [PubMed] [Google Scholar]
- 24.Villars F. Guillotin B. Amedee T. Dutoya S. Bordenave L. Bareille R. Amedee J. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Cell Physiol. 2002;282:C775. doi: 10.1152/ajpcell.00310.2001. [DOI] [PubMed] [Google Scholar]
- 25.Gerber H.P. Vu T.H. Ryan A.M. Kowalski J. Werb Z. Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 26.Peng H. Wright V. Usas A. Gearhart B. Shen H.C. Cummins J. Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayr-Wohlfart U. Waltenberger J. Hausser H. Kessler S. Gunther K.P. Dehio C. Puhl W. Brenner R.E. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 28.Engsig M.T. Chen Q.J. Vu T.H. Pedersen A.C. Therkidsen B. Lund L.R. Henriksen K. Lenhard T. Foged N.T. Werb Z. Delaisse J.M. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151:879. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiedler J. Leucht F. Waltenberger J. Dehio C. Brenner R.E. VEGF-A and PlGF-1 stimulate chemotactic migration of human mesenchymal progenitor cells. Biochem Biophys Res Commun. 2005;334:561. doi: 10.1016/j.bbrc.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 30.Akeno N. Czyzyk-Krzeska M.F. Gross T.S. Clemens T.L. Hypoxia induces vascular endothelial growth factor gene transcription in human osteoblast-like cells through the hypoxia-inducible factor-2alpha. Endocrinology. 2001;142:959. doi: 10.1210/endo.142.2.8112. [DOI] [PubMed] [Google Scholar]
- 31.Kempen D.H. Creemers L.B. Alblas J. Lu L. Verbout A.J. Yaszemski M.J. Dhert W.J. Growth factor interactions in bone regeneration. Tissue Eng Part B Rev. 2010;16:551. doi: 10.1089/ten.teb.2010.0176. [DOI] [PubMed] [Google Scholar]
- 32.Seeherman H. Wozney J. Li R. Bone morphogenetic protein delivery systems. Spine (Phila Pa 1976) 2002;27:S16. doi: 10.1097/00007632-200208151-00005. [DOI] [PubMed] [Google Scholar]
- 33.Patel Z.S. Young S. Tabata Y. Jansen J.A. Wong M.E. Mikos A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng H. Usas A. Olshanski A. Ho A.M. Gearhart B. Cooper G.M. Huard J. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20:2017. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 35.Roldan J.C. Detsch R. Schaefer S. Chang E. Kelantan M. Waiss W. Reichert T.E. Gurtner G.C. Deisinger U. Bone formation and degradation of a highly porous biphasic calcium phosphate ceramic in presence of BMP-7, VEGF and mesenchymal stem cells in an ectopic mouse model. J Craniomaxillofac Surg. 2010;38:423. doi: 10.1016/j.jcms.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Kanczler J.M. Ginty P.J. White L. Clarke N.M. Howdle S.M. Shakesheff K.M. Oreffo R.O. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2009;31:1242. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W. Wang X. Wang S. Zhao J. Xu L. Zhu C. Zeng D. Chen J. Zhang Z. Kaplan D.L. Jiang X. The use of injectable sonication-induced silk hydrogel for VEGF(165) and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials. 2011;32:9415. doi: 10.1016/j.biomaterials.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kempen D.H. Lu L. Heijink A. Hefferan T.E. Creemers L.B. Maran A. Yaszemski M.J. Dhert W.J. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 39.Kempen D.H. Lu L. Classic K.L. Hefferan T.E. Creemers L.B. Maran A. Dhert W.J. Yaszemski M.J. Non-invasive screening method for simultaneous evaluation of in vivo growth factor release profiles from multiple ectopic bone tissue engineering implants. J Control Release. 2008;130:15. doi: 10.1016/j.jconrel.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oldham J.B. Lu L. Zhu X. Porter B.D. Hefferan T.E. Larson D.R. Currier B.L. Mikos A.G. Yaszemski M.J. Biological activity of rhBMP-2 released from PLGA microspheres. J Biomech Eng. 2000;122:289. doi: 10.1115/1.429662. [DOI] [PubMed] [Google Scholar]
- 41.Kempen D.H. Lu L. Hefferan T.E. Creemers L.B. Maran A. Classic K.L. Dhert W.J. Yaszemski M.J. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rui J. Dadsetan M. Runge M.B. Spinner R.J. Yaszemski M.J. Windebank A.J. Wang H. Controlled release of vascular endothelial growth factor using poly-lactic-co-glycolic acid microspheres: in vitro characterization and application in polycaprolactone fumarate nerve conduits. Acta Biomater. 2012;8:511. doi: 10.1016/j.actbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi Y. Yamamoto M. Tabata Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials. 2005;26:4856. doi: 10.1016/j.biomaterials.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Theyse L.F. Oosterlaken-Dijksterhuis M.A. van Doorn J. Dhert W.J. Hazewinkel H.A. Growth hormone stimulates bone healing in a critical-sized bone defect model. Clin Orthop Relat Res. 2006;446:259. doi: 10.1097/01.blo.0000203490.21206.7f. [DOI] [PubMed] [Google Scholar]
- 45.van Gaalen S.M. Kruyt M.C. Geuze R.E. de Bruijn J.D. Alblas J. Dhert W.J. Use of fluorochrome labels in in vivo bone tissue engineering research. Tissue Eng Part B Rev. 2010;16:209. doi: 10.1089/ten.TEB.2009.0503. [DOI] [PubMed] [Google Scholar]
- 46.Kim T.K. Burgess D.J. Pharmacokinetic characterization of 14C-vascular endothelial growth factor controlled release microspheres using a rat model. J Pharm Pharmacol. 2002;54:897. doi: 10.1211/002235702760089009. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto M. Takahashi Y. Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24:4375. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto M. Ikada Y. Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12:77. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 49.Deckers M.M. Karperien M. van der Bent C. Yamashita T. Papapoulos S.E. Lowik C.W. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- 50.Young S. Patel Z.S. Kretlow J.D. Murphy M.B. Mountziaris P.M. Baggett L.S. Ueda H. Tabata Y. Jansen J.A. Wong M. Mikos A.G. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kakudo N. Kusumoto K. Wang Y.B. Iguchi Y. Ogawa Y. Immunolocalization of vascular endothelial growth factor on intramuscular ectopic osteoinduction by bone morphogenetic protein-2. Life Sci. 2006;79:1847. doi: 10.1016/j.lfs.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 52.Wang D.S. Yamazaki K. Nohtomi K. Shizume K. Ohsumi K. Shibuya M. Demura H. Sato K. Increase of vascular endothelial growth factor mRNA expression by 1,25-dihydroxyvitamin D3 in human osteoblast-like cells. J Bone Miner Res. 1996;11:472. doi: 10.1002/jbmr.5650110408. [DOI] [PubMed] [Google Scholar]
- 53.Pintucci G. Moscatelli D. Saponara F. Biernacki P.R. Baumann F.G. Bizekis C. Galloway A.C. Basilico C. Mignatti P. Lack of ERK activation and cell migration in FGF-2-deficient endothelial cells. FASEB J. 2002;16:598. doi: 10.1096/fj.01-0815fje. [DOI] [PubMed] [Google Scholar]
- 54.Anitua E. Andia I. Ardanza B. Nurden P. Nurden A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 55.Kruyt M.C. de Bruijn J.D. Wilson C.E. Oner F.C. van Blitterswijk C.A. Verbout A.J. Dhert W.J. Viable osteogenic cells are obligatory for tissue-engineered ectopic bone formation in goats. Tissue Eng. 2003;9:327. doi: 10.1089/107632703764664792. [DOI] [PubMed] [Google Scholar]