Abstract
The biomechanical function of the vocal folds (VFs) depends on their viscoelastic properties. Many conditions can lead to VF scarring that compromises voice function and quality. To identify candidate replacement materials, the structure, composition, and mechanical properties of native tissues need to be understood at phonation frequencies. Previously, the authors developed the torsional wave experiment (TWE), a stress-wave-based experiment to determine the linear viscoelastic shear properties of small, soft samples. Here, the viscoelastic properties of porcine and human VFs were measured over a frequency range of 10–200 Hz. The TWE utilizes resonance phenomena to determine viscoelastic properties; therefore, the specimen test frequency is determined by the sample size and material properties. Viscoelastic moduli are reported at resonance frequencies. Structure and composition of the tissues were determined by histology and immunochemistry. Porcine data from the TWE are separated into two groups: a young group, consisting of fetal and newborn pigs, and an adult group, consisting of 6–9-month olds and 2+-year olds. Adult tissues had an average storage modulus of 2309±1394 Pa and a loss tangent of 0.38±0.10 at frequencies of 36–200 Hz. The VFs of young pigs were significantly more compliant, with a storage modulus of 394±142 Pa and a loss tangent of 0.40±0.14 between 14 and 30 Hz. No gender dependence was observed. Histological staining showed that adult porcine tissues had a more organized, layered structure than the fetal tissues, with a thicker epithelium and a more structured lamina propria. Elastin fibers in fetal VF tissues were immature compared to those in adult tissues. Together, these structural changes in the tissues most likely contributed to the change in viscoelastic properties. Adult human VF tissues, recovered postmortem from adult patients with a history of smoking or disease, had an average storage modulus of 756±439 Pa and a loss tangent of 0.42±0.10. Contrary to the results of some other investigators, no significant frequency dependence was observed. This lack of observable frequency dependence may be due to the modest frequency range of the experiments and the wide range of stiffnesses observed within nominally similar sample types.
Introduction
During phonation, the vocal folds (VFs) are driven into a shear-dominated wave-like motion at frequencies between 100 and 1000 Hz, experiencing strains of up to 30%.1 The unique biomechanical function and response of the VFs to aerodynamic forces are determined by the viscoelastic properties of the tissue, which are themselves primarily determined by the structure and composition of the extracellular matrix (ECM).2–6 VFs are laminated structures that consist of three layers: the epithelium, the lamina propria (LP), and the vocalis muscle.7,8 The human LP is further divided into three layers: superficial, intermediate, and deep LP. While all three layers are necessary for phonation, the epithelium and the superficial LP are the most important components of the VF vibrating structure.9 Damage to the VFs due to voice abuse, trauma, or surgery leads to scar tissue formation throughout the LP, though primarily to the superficial layer.10,11 Compared to normal tissue, the scarred VFs are significantly less pliable, inhibiting voice production and compromising voice quality. Additionally, cancer may require a complete removal of the VFs. As a result, patients are socially isolated due to the inability to produce sound. An estimated 3%–9% of the population has some type of voice dysfunction.12
Given the lack of satisfactory methods for the restoration of VF function, we are interested in creating autologous, injectable constructs that will regenerate the VFs and allow normal vibratory function in patients suffering from various voice-related disorders. We aim at long-term tissue regeneration rather than temporary amelioration of the symptoms. Specifically, the authors aim to return voice function or improve voice quality by creating new materials—specifically synthetic hydrogels—that mimic the structure, composition, and viscoelastic properties of the VF tissues.13–19 Other groups have utilized animal-derived collagen sponges seeded with autologous mesenchymal stromal cells for the treatment of VF scarring, with some success in animal models.20
Understanding viscoelastic properties of VF tissues is important for modeling phonation and treating voice disorders, as well as for developing materials for VF tissue engineering. In particular, shear properties of VF tissues—and potential replacements—are most critical due to the propagation of shear waves during phonation.10 Over the past decades, several studies have focused on measuring the viscoelastic properties of VF tissues in shear using commercial or custom-built rheometers.21–25 While the upper frequency limit has been increased to ∼250 Hz by reducing machine inertia, these techniques are still based on the assumption that the stress is uniform through the sample thickness; consequently, interpretation of the results of rheometric tests does not take into account wave propagation through the sample. The authors have previously shown that above ∼10 Hz, omitting sample inertia in interpreting data from a torsional rheometer can induce large errors in determining the viscoelastic properties of very soft tissues.26
To address this issue, the torsional wave experiment (TWE) was developed.26,27 Briefly, in a TWE, a right-circular cylindrical sample is sandwiched between two hexagonal acrylic plates and aligned so that the acrylic plates and the sample share a common axis. The driven lower plate oscillates, inducing the upper plate to oscillate as well. Rotation of the upper plate is measured via an optical lever, and the linear viscoelastic shear properties of the specimen are calculated from the frequency dependence of the response. Previous studies have shown the TWE's ability to provide high-frequency viscoelastic shear properties on synthetic gels13,14,19,26,28 and natural tissues.29 Additionally, it is important to correlate the viscoelastic properties of the VFs with the histological structure and composition of the tissue, so the structure–property relationship can be understood, and suitable replacement materials can be developed.
The current work focuses on determining the linear viscoelastic shear properties of VFs at physiologically relevant frequencies. Porcine VFs are the focus of this work owing to their structural similarity to human VFs30–35 and the availability of tissue samples of varying age and gender. The LP of 38 pigs, varying in age from fetal to 2+-year olds, were tested using the TWE.26 To enable comparisons of TWE results with traditional rheometric measurements, three adult porcine tissues were subjected to rheological measurements at low frequencies. Parallel histological analyses were conducted to gain an understanding of the structure–property relationship. Additionally, 22 human VF tissues, recovered postmortem from patients aged 39–93 years old with a history of smoking or disease, were tested by the TWE. Owing to the uncertainty of the state of the human tissues, no histological data are presented.
Materials and Methods
Tissue procurement
Fetal (11 specimens), 6–9-month-old (5 specimens) and 2+-year-old (15 specimens) porcine larynges were procured from Animal Technologies, Inc. (Tyler, TX) or a local butcher house (Salem Packing). Additionally, larynges from newborn (<5-day old, two specimens) and 3–4-month-old (five specimens) pigs were obtained from the University of Pennsylvania School of Veterinary Medicine and the Brown University, respectively. With the exception of 3–4-month-old specimens, all tissue samples were either snap-frozen and stored in liquid nitrogen for subsequent mechanical testing or dissected and processed for paraffin or OCT embedding immediately upon reception. The 3–4-month-old VFs were removed within 20 minutes postmortem, wrapped in gauze, and soaked in phosphate-buffered saline 7.4 at room temperature until testing. All fresh specimens were tested within 10 h postmortem, as guided by a prior report36 that storing VF samples in saline at room temperature for 24 h did not affect their viscoelastic properties. Human larynges were procured from the National Disease Research Interchange. Larynges were dissected postmortem from 39–93- year-old individuals (Table 1) and were shipped fresh on ice and snap-frozen in liquid nitrogen once received. Table 1 summarizes the gender, age, race, and relevant health history data for all human specimens.
Table 1.
Summary of Human Specimens
| Sex | Age | Race | Smoking | Surgeries | Intubation | Chemotherapy |
|---|---|---|---|---|---|---|
| M | 39 | Caucasian | x | |||
| M | 58 | Caucasian | x | x | ||
| M | 59 | Caucasian | x | x | ||
| M | 61 | Caucasian | x | x | ||
| M | 61 | Caucasian | x | |||
| M | 62 | Caucasian | x | x | x | |
| M | 63 | Caucasian | x | x | ||
| M | 64 | Caucasian | x | x | ||
| M | 65 | Caucasian | x | x | ||
| M | 65 | Caucasian | x | x | ||
| M | 67 | Caucasian | x | |||
| M | 67 | Caucasian | x | |||
| M | 78 | Caucasian | x | x | ||
| M | 84 | Caucasian | x | |||
| M | 93 | Caucasian | ||||
| F | 42 | Caucasian | x | x | ||
| F | 55 | Caucasian | x | x | ||
| F | 59 | Caucasian | x | |||
| F | 61 | Caucasian | x | |||
| F | 69 | Caucasian | x | |||
| F | 70 | Caucasian | x | |||
| F | 91 | Caucasian | x |
Information provided by the National Disease Research Interchange.
Mechanical testing
Except for five fresh 3–4-month-old pig VFs, the remaining tissues in the current study were cryopreserved via snap-freezing in liquid nitrogen. Snap-freezing is not expected to have a major effect on mechanical properties, as it has been shown that long storage of canine VFs at −80°C after snap-freezing does not alter the tissue's viscoelastic shear modulus significantly.36 Before testing, all frozen specimens were thawed in a warm water bath (∼37°C) until defrosted. The larynges were then cut sagittally, only cutting through the anterior side, and spread open to reveal the VFs. The VFs were then separately excised from the larynx and kept hydrated while not in use. A circular sample was then removed with a dermal punch, and the muscle layer was dissected with a scalpel and scissors. While the adult VFs were removed with a 5-mm dermal punch, fetal VFs were removed with a 3-mm dermal punch. Excluding fetal pig specimens, which allowed for only one sample, a larynx produced one testable specimen from each VF (left and right) though all 2+-year-old and some 6–9-month-old pigs allowed for two samples per VF (four samples per larynx). All samples were taken from the mid-portion of the VFs and consisted of all layers of the LP and the epithelium.
Torsional wave experiment
In the TWE, the sample is sandwiched between two acrylic plates that share a common vertical axis. These plates are considered rigid with respect to the sample. The sample was maintained at physiologically relevant conditions at a temperature of ∼37°C and a relative humidity >95% using an environmental chamber. The lower plate is driven by a galvanometer (model G112; Cambridge Technology) to oscillate sinusoidally about the vertical axis at angles less than ±0.2° to ensure that the strain is in the small strain range where linear viscoelasticity is expected to apply. During rotation, a torsional stress wave propagates through the sample, exciting the top plate. The top plate then rotates with the angular motion described by26
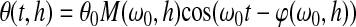 |
(1) |
where θ is the angular rotation, h is the height of the sample, θ0 is the amplitude of rotation of the bottom plate, M(ω0, h) is the amplification factor, defined as the ratio of the magnitude of rotation of the top plate divided by that of the bottom plate, ω0 is the angular frequency of rotation of the galvanometer, and  is the phase shift between the top and bottom plate. The amplification factor is26
is the phase shift between the top and bottom plate. The amplification factor is26
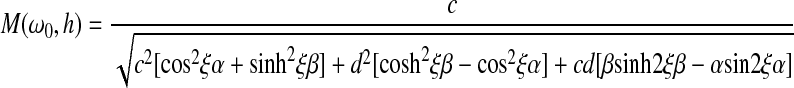 |
(2) |
where26
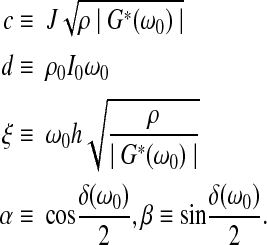 |
(3) |
The complex viscoelastic shear modulus G*(ω0) is expressed in terms of its magnitude |G*(ω0)| and loss angle δ(ω0); J is the sample's polar moment of inertia per unit length, and ρ the density; I0 and ρ0 are the top plate's polar moment of inertia and density, respectively. Sample height and diameter were measured via in situ images taken before and after the experiment; the sample density was assumed to be 1000 kg/m3. During sample preparation, specimens were trimmed to ensure that the sample was a right-circular cylinder (i.e., flat). After the experiment, if the image taken showed the acrylic plates to be nonparallel, the test data were considered invalid.
During a test, the galvanometer is driven by a computer-controlled function generator, which steps through a series of frequencies that includes the resonance frequency of the sample. The rotations of the top and bottom plate are monitored via an optical lever technique, utilizing a photodiode detector that is masked so that the voltage change is proportional to the angular rotation. To calculate the viscoelastic shear properties, the experimental amplification factor M(ω0, h) is fit, in the least-squares sense, to Equation (2) over a range of frequencies that includes the resonance frequency. The data fit assumes that any frequency dependence of the complex shear modulus is weak enough that the modulus can be assumed to be constant over the small frequency range for which the data are fit. The complex modulus obtained from the best fit is interpreted as its value at the resonance frequency, ωf. The TWE yields the magnitude of the complex modulus (|G*(ωf)|) and the loss angle (δ(ωf)) at the resonance frequency, from which the storage modulus 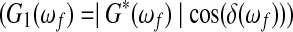 and loss tangent (tan(δ(ωf))) can be computed. The storage modulus indicates the elastic stiffness of the material, while the loss tangent indicates the ratio of the loss modulus
and loss tangent (tan(δ(ωf))) can be computed. The storage modulus indicates the elastic stiffness of the material, while the loss tangent indicates the ratio of the loss modulus  to the storage modulus. For brevity, the argument (ωf) will be dropped in the remainder of the article. The resonance frequency of the sample depends on sample properties and size, as well as the mass density and dimensions of the top plate. Increasing sample radius or decreasing sample thickness increases the resonance frequency. Decreasing the thickness or the mass moment of inertia of the top plate increases the resonance frequency. The authors have found that the fit of the frequency dependence of the amplification factor to that obtained from the experiments is very good in the current study and in many others.13,14,19,26,28,29 Three consecutive repeats of the TWE were performed on each sample.
to the storage modulus. For brevity, the argument (ωf) will be dropped in the remainder of the article. The resonance frequency of the sample depends on sample properties and size, as well as the mass density and dimensions of the top plate. Increasing sample radius or decreasing sample thickness increases the resonance frequency. Decreasing the thickness or the mass moment of inertia of the top plate increases the resonance frequency. The authors have found that the fit of the frequency dependence of the amplification factor to that obtained from the experiments is very good in the current study and in many others.13,14,19,26,28,29 Three consecutive repeats of the TWE were performed on each sample.
Rheometric measurements
The viscoelastic shear properties of three VF specimens were measured at low frequencies using a TA Instruments ARG2 rheometer with a parallel-plate geometry (8-mm-diameter plates). A circular cylinder (5-mm in diameter) of excised tissue was placed between the plates, and the gap between the two plates was adjusted so that they were in contact with the sample with a minimum normal force. The sample was subjected to a strain-controlled oscillatory deformation at a frequency of 0.1 to 100 rad/s (∼0.016 to 16 Hz), with a strain amplitude of ∼0.15%. All measurements were performed at a temperature of 37°C using a heated bottom plate. Samples were kept hydrated by sealing the edges with mineral oil. When possible, two VF biopsies were performed on each VF, resulting in a maximum of four samples per specimen.
Histology
Upon completion of the TWE, samples were embedded in OCT (Tissue-Tek), snap-frozen on dry ice, and stored at −80°C. Samples were pre-equilibrated at −20°C overnight, and 16-μm sections were cut using a Leica CM3050S Cryostat, and mounted on ColorFrost microscope slides (Fisher). Histological staining by hematoxylin and eosin (H&E), Alcian blue (pH 2.5), and Movat's pentachrome was performed following standard protocols,37 visualized on a Nikon TS100-F phase-contrast microscope, and imaged with a Nikon Coolpix camera. H&E stained cell nuclei purple and the surrounding matrix pink. Alcian blue stained glycosaminoglycans (GAGs) blue and cells pink, whereas Movat's pentachrome stained the cell nuclei, cytoplasm, collagen, elastin fibers, and GAGs black, red, yellow, purple/black, and green/blue, respectively.
Statistical methods
Quantitative data from the TWE for one sample are the result of an average of at least three consecutive repeats. Except for one data point, left and right VF samples of the same larynx are averaged if tested at resonance frequencies within 30 Hz of each other, and error bars represent the standard deviation of these means. One adult porcine data point, with a mean frequency at 140 Hz, consisted of tests at 100, 140, and 180 Hz. Statistical significance was determined using the nonparametric Mann–Whitney U-test (Wilcoxon rank-sum test) or the Kruskal–Wallis test. With the small sample sizes reported here, the difference between the U-test and Student's t-test were negligible; the U-test was used for its robustness. Values were considered to be statistically different for p<0.05.
Results
Porcine samples
Figure 1 shows a representative plot of the amplification factor versus frequency for a porcine VF. The data points are the average of three repeats on the same sample, and the error bars are one standard deviation of the average. The inset in the figure shows the sample as a right-circular cylinder between the hexagonal acrylic plates. This experiment shows a strong correlation of the measured amplification factor with predictions of the linear viscoelastic model of Equation (2) (R2=0.997).
FIG. 1.

Amplification factor (M) versus frequency for a representative porcine vocal fold sample averaged over three repeats. Symbols are the experimental data points, and the line is the least-squares linear viscoelastic model fit. Error bars are the standard deviation from three repeats on one sample. Inset shows the sample sandwiched between the two hexagonal acrylic plates. Color images available online at www.liebertpub.com/tea
Storage moduli and loss tangents for all porcine samples are plotted against the resonance frequencies in Figure 2A. Porcine samples from different age groups are plotted separately. The specimen gender is not differentiated. Due to the small specimen size and difficulty associated with tissue dissection, only one sample was collected from each fetal larynx. Consequently, no error bars are presented for the fetal data points. Adult pigs are stiffer than young pigs, though the loss tangents for the two groups are similar. The resonance frequencies of the young pig samples are grouped at the lower end of the plot range due to the small sample size and lower shear modulus. Likewise, the 3–4-month-old fresh pig sample resonance frequencies are lower than the 6–9-month-old and 2+-year-old pig samples. It is not apparent from these data that the storage modulus is frequency dependent; a large specimen-to-specimen variation is obtained across the entire frequency range tested.
FIG. 2.
Storage modulus and loss tangent for all porcine samples tested as a function of (A) frequency and (B) maximum strain. Data points are an average of all samples from one larynx, and the error bars are one standard deviation of these samples, with only one side shown for clarity. Filled symbols represent the storage modulus (G1, left axis), whereas the open symbols represent the loss tangent [tan(δ), right axis]. The abbreviations in the figure indicate the age of the tissues: 2+ YO: 2+ years old; 6–9 MO: 6–9 months old; 3–4 MO: 3–4 months old. Color images available online at www.liebertpub.com/tea
Figure 2B shows the storage modulus and loss tangent plotted as a function of strain. The strain is the engineering shear strain for small deformations, defined as 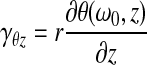 where r is the radial distance; θ(ω0,
z) is the angular rotation given by Equation (1); z is the coordinate along the axis of the TWE; and ω0 is the angular frequency. The calculated strain is the maximum strain in the entire sample over one cyclic oscillation; explicitly, this is the strain experienced by a material point on the outer radius of the cylindrical sample. Different samples experience different strains during the TWE due to the nature of the test; the amplification factor for each experiment is dependent on the viscoelastic properties and the sample size. For two samples of the same geometry, the sample with a lower loss tangent would have a higher amplification factor, and therefore experiences larger strains. The maximum strain experienced by any sample is 5.4%; all other samples experienced strains <5%. As seen in Figure 2B, no dependence on strain is observed; there is no discernible trend of the storage modulus or loss tangent with strain. The maximum or minimum storage modulus is not from a sample tested at either the highest or lowest strain. If the shear strain in the sample was no longer in the small strain regime, it is expected that the modulus and loss tangent would change with strain. As the values do not change, the assumption of linear viscoelasticity appears to be appropriate.
where r is the radial distance; θ(ω0,
z) is the angular rotation given by Equation (1); z is the coordinate along the axis of the TWE; and ω0 is the angular frequency. The calculated strain is the maximum strain in the entire sample over one cyclic oscillation; explicitly, this is the strain experienced by a material point on the outer radius of the cylindrical sample. Different samples experience different strains during the TWE due to the nature of the test; the amplification factor for each experiment is dependent on the viscoelastic properties and the sample size. For two samples of the same geometry, the sample with a lower loss tangent would have a higher amplification factor, and therefore experiences larger strains. The maximum strain experienced by any sample is 5.4%; all other samples experienced strains <5%. As seen in Figure 2B, no dependence on strain is observed; there is no discernible trend of the storage modulus or loss tangent with strain. The maximum or minimum storage modulus is not from a sample tested at either the highest or lowest strain. If the shear strain in the sample was no longer in the small strain regime, it is expected that the modulus and loss tangent would change with strain. As the values do not change, the assumption of linear viscoelasticity appears to be appropriate.
Porcine VF specimens were also tested in a commercial rheometer. Data from these tests are shown in Figure 3A. Each series is the result from one specimen, with three to four samples from each larynx averaged to produce the data. A maximum frequency of ∼16 Hz was chosen according to the results of Jiao et al.,26 as this is the limiting frequency at which inertial effects of the sample become important, and measured values no longer accurately reflect material properties. Figure 3A shows that for all three porcine samples tested, as expected, the storage modulus increases with the frequency, from 220–1036 Pa at 0.016 Hz to 5976–8214 Pa at 10 Hz. A sharp increase in the storage modulus can be seen at the high end of the frequency range; this increase is most likely due to the limitations of the rheometer. One specimen (moduli plotted as green squares) begins to show a sharp increase in modulus at ∼3 Hz, while the other two samples do not exhibit this upturn until ∼10 Hz. Similar to the porcine samples tested with the TWE, there is a large specimen-to-specimen variation.
FIG. 3.
Storage modulus and loss tangent for all adult porcine specimens tested with the rheometer (A) and the averaged rheometer data presented with the torsional wave experiment (TWE) data (B). Symbols in (A) denote three different pig tissues, and the error bars are one standard deviation of all samples from one larynx. In (B), data at low frequencies (below 20 Hz) are from the commercial rheometer, and data in gray with the point-down triangle (▾) are deemed inaccurate, as per the Discussion section. Error bars on the rheometer data are one standard deviation of all data, whereas error bars for the TWE data are for all samples from one larynx. Filled symbols represent the storage modulus (G1, left axis), whereas the open symbols represent the loss tangent [tan(δ), right axis]. Color images available online at www.liebertpub.com/tea
Figure 3B combines the storage moduli and loss tangent data from the commercial rheometer and the TWE for 6–9 month-old and 2+year-old porcine VFs. The storage modulus curve is an average of rheometer data reported in Figure 3A, ranging from 696 Pa at 0.016 Hz to 6980 Pa at 16 Hz. TWE storage moduli, ranging from 545 Pa at 35.5 Hz to 5250 Pa at 200 Hz as shown previously in Figure 2A, appear to show continuous extension of rheometer data at low frequencies (0.016–0.3 Hz). Most of the storage moduli from samples tested with the rheometer had a lower storage modulus than those tested with the TWE. Similarly, loss tangents of the samples tested with the rheometer is lower than for samples tested with the TWE, although the frequency ranges differed.
Upon completion of the TWE, the intact samples were collected for standard histological staining. Figures 4 and 5 show H&E, Alcian blue, and Movat's pentachrome staining results after OCT embedding and cryosectioning. All three types of staining revealed that while the VFs of 6–9-month-old and 2+-year-old pigs are structurally similar, they are distinctly different from those of the fetal pigs. In particular, fetal VFs appear to be globally loose, and generally disorganized, with abundant spaces devoid of any staining, while tissues from 6–9-month-old and 2+-year-old pigs (referred to as adult) appear dense and highly organized, with scarce spaces devoid of staining. In the fetal tissues, the elastin fibers, stained purple/black in Movat's pentachrome staining, appear short and aggregated, while collagen, stained yellow in Movat's, does not appear to be abundant. No specific fiber orientation was observed. Conversely, the adult tissues display a fibrous network of elastin and collagen, where the elastin fibers are long, aligned, and connected to one another. Collagen is present throughout the thickness of the adult tissues. In the fetal tissues, some small, sparse pockets of GAGs stained blue in both Alcian blue and Movat's staining are present; they appear to be laryngeal glands.8,38,39 With age, those laryngeal glands become more prominent; located just before the vocalis muscle, they are essentially composed of GAGs and cells.8,38,39 Also evident is the thickening of the epithelium with age; in the fetal VF, the epithelium layer is thin, ∼10 μm, in the 6–9-month-old tissue its thickness is 10–40 μm, while the 2+-year-old tissues show a 30–50-μm-thick epithelium layer.
FIG. 4.
Cryosectioned tissues samples stained with hematoxylin and eosin (H&E), Alcian blue, and Movat's pentachrome. Images show fetal, 6–9-month-old, and 2+-year-old porcine VFs after the TWE. All cryosections for each age group are from the same specimen. Cell pockets are circled in the Alcian blue image of the fetal tissue. Scale bar for all images: 200 μm. Color images available online at www.liebertpub.com/tea
FIG. 5.
H&E, Alcian blue, and Movat's pentachrome staining of fetal (A), 6–9-month-old (B), and 2+-year-old (C) porcine VF cryosections. (A) The epithelium, the lamina propria, and the vocalis muscle are labeled as 1, 2, and 3, respectively. Circled regions indicate intense glycosaminoglycan staining. (B) The last row shows laryngeal glands further down in the VF. Tissue protrusion at the edge is circled, and laryngeal glands are boxed. (C) Tissue protrusion at the edge is circled. Scale bar: 500 μm. Color images available online at www.liebertpub.com/tea
The immunohistochemically stained tissues, shown in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/tea), highlight elastin and type I collagen fibers and confirmed the histological observations described just above. Histologically stained sections of the entire VF specimens are shown for all age groups in Figure 5. After opening up the larynx, the midsections of the VFs were carved out and embedded in OCT. The embedded tissues were then cryosectioned longitudinally, which corresponds to a coronal section of the larynx. From left to right, in Figure 5, one can see the vocalis muscle, the LP, and the epithelium layer, labeled as 1, 2, and 3, respectively. In addition to the features seen in Figure 4, a pocket of GAGs can be seen in the fetal VF that is not present in other VF age groups. One particular pocket (circled in Fig. 5A) is located deep in the LP, adjacent to the vocalis muscle in the upper section of the tissue. Figure 5B shows the sections for tissues of 6–9-months old. A tissue protrusion appeared in the upper edge of the VF. The laryngeal glands are clearly present in the lower section of the VF. At 2+ years of age, the tissue protrusion is prominent and highly hydrated as indicated by the empty space in Figure 5C. Below this protrusion, the VFs have become longer and more dense.
Most histological studies on VFs have used paraffin as an embedding material4,8,34,35,40–44 due to paraffin's superior preservation of tissue structure when compared to OCT. However, OCT embedding is easier to perform and allows for more antibodies to be used. In this study, OCT was chosen over paraffin as the embedding material, although some porcine samples have been embedded in both media, and the results are compared in Supplementary Figure S2. Since paraffin-embedded samples showed essentially the same results as those shown by the OCT-embedded specimens, OCT embedding was confirmed as a viable alternative to paraffin embedding for histological observation of VFs.
Human samples
Storage moduli and loss tangents for all human samples are plotted against resonance frequencies of the samples in Figure 6A. It should be noted that all samples came from elderly or diseased patients, so samples may not be representative of healthy VF tissues. Male human VF samples had resonance frequencies between 40 and 175 Hz; samples had an average storage modulus of 843 Pa, with values between 165 and 1636 Pa. Females were slightly softer with an average modulus of 617 Pa, and values ranging from 246 to 1268 Pa for a range of resonance frequencies between 37.5 and 110 Hz. Loss tangent values for male and female human VF were similar. Average values were 0.41 and 0.43 for males and females, respectively. For males, the loss tangents ranged from 0.23 to 0.57; for females, the range was from 0.31 to 0.66. Male and female populations have been shown separately, but the storage moduli and loss tangents of the populations can be considered the same (p=0.20 and p=0.57, respectively). A large specimen-to-specimen variation was found due in part to the large age range covered and the pathological conditions of the specimens. This variation is believed to be due to inherent variability in tissues, not due to experimental error, as repeats of each experiment were consistent.
FIG. 6.
Storage modulus and loss tangent for all human samples tested as a function of frequency (A) and the maximum strain (B). Data points are an average of all samples from one larynx, and the error bars are one standard deviation of these samples, with only one side shown for clarity. Filled symbols represent the storage modulus (G1, left axis), whereas the open symbols represent the loss tangent [tan(δ), right axis]. Color images available online at www.liebertpub.com/tea
Figure 6B shows the storage moduli and loss tangents for all human VFs plotted against the maximum strain in the samples. The maximum strain experienced is 7.7%, with all, but two, samples experiencing maximum strains <4%. The two specimens that experienced the largest strain also had the lowest modulus of all samples tested. No reliable data are available on the linear range of VFs. Similar to the porcine data presented in Figure 2B, results for these samples are still considered valid.
Statistical analysis
Table 2 shows, for all species and groups tested, the frequency range of the resonance frequencies and the average values and standard deviations of the storage moduli and loss tangents; results from the Mann–Whitney U-test and Kruskal–Wallis tests are also presented. For example, Table 2 shows the following results for 2+-year-old porcine samples: the average storage modulus was 2207 Pa with a standard deviation of 1440 Pa; the mean loss tangent was 0.38 with a standard deviation of 0.10; resonance frequencies of the samples ranged from 36 to 195 Hz.
Table 2.
Means and Standard Deviations of All Species and Age Groups Tested with p-Values from the Mann–Whitney U-Test or Kruskal–Wallis Test
| Group | Frequency range (Hz) | G1Mean±SD (Pa) | tan(δ)Mean±SD |
|---|---|---|---|
| 2+-year-old | 36–195 | 2207±1440 | 0.38±0.10 |
| 6–9-month-old | 45–200 | 2548±1329 | 0.37±0.10 |
| p-value | 0.4417 | 0.6838 | |
| 2+-year-old | 36–195 | 2207±1440 | 0.38±0.10 |
| 3–4-month-old—fresh | 50–65 | 987±259 | 0.32±0.03 |
| p-value | 0.0790 | 0.2416 | |
| 6–9-month-old | 45–200 | 2548±1329 | 0.37±0.10 |
| 3–4-month-old—fresh | 50–65 | 987±259 | 0.32±0.03 |
| p-value | 0.0190 | 0.5185 | |
| 2+-year-old | 36–195 | 2207±1440 | 0.38±0.10 |
| 6–9-month-old | 45–200 | 2548±1329 | 0.37±0.10 |
| 3–4-month-old—fresh | 50–65 | 987±259 | 0.32±0.03 |
| p-value | 0.0743 | 0.4796 | |
| Newborn | 24, 30 | 282±22 | 0.27±0.02 |
| Fetal | 14–28 | 416±146 | 0.42±0.14 |
| p-value | 0.2727 | 0.1212 | |
| 2+-year-old | 36–195 | 2207±1440 | 0.38±0.10 |
| 6–9-month-old | 45–200 | 2548±1329 | 0.37±0.10 |
| 3–4-month-old—fresh | 50–65 | 987±259 | 0.32±0.03 |
| Newborn | 24, 30 | 282±22 | 0.27±0.02 |
| Fetal | 14–28 | 416±146 | 0.42±0.14 |
| p-value | 8.69E-06 | 0.1648 | |
| Human male | 40–175 | 843±480 | 0.41±0.09 |
| Human female | 38–110 | 617±337 | 0.43±0.10 |
| 2+-year-old | 36–195 | 2207±1440 | 0.38±0.10 |
| 6–9-month-old | 45–200 | 2548±1329 | 0.37±0.10 |
| 3–4-month-old—fresh | 50–65 | 987±259 | 0.32±0.03 |
| p-value | 1.09E-05 | 0.0986 | |
| Human male | 40–175 | 843±480 | 0.41±0.09 |
| Human female | 38–110 | 617±337 | 0.43±0.10 |
| p-value | 0.2002 | 0.5742 |
The Mann–Whitney U-test was performed on entries with only two groups, whereas the Kruskal–Wallis test was performed on entries with more than two groups. A p-value<0.05 indicates that the medians of the populations are statistically different.
Table 2 also shows that the viscoelastic properties of 2+-year-olds and 6–9-month-olds can be considered statistically the same (G1, p=0.44; tan(δ), p=0.69). Additionally, Table 2 shows that porcine data can be separated into two groups: young and adult. The young group consists of newborn and fetal pigs (G1, p=0.27; tan(δ), p=0.12), whereas the adult group contains the 2+-year-olds, 6–9-month-olds, and the fresh 3–4-month-olds (G1, p=0.07; tan(δ), p=0.48). The conclusion that the newborn and fetal pigs' viscoelastic properties are the same may not be statistically meaningful due to the low number of samples (2 newborn pigs and 10 fetal pigs). The resonance frequencies of the fetal and newborn pig VF samples are lower than those of the adults. These low resonance frequencies are due to both the low modulus of the samples and their small diameter. Lastly, Table 2 shows results from the Kruskal–Wallis test on all porcine VF data tested. Statistically, storage moduli for all porcine groups are different, whereas the loss tangents are the same (G1, p=8.7E-06; tan(δ), p=0.16). This difference demonstrates that VF storage moduli increase with age. Finally, the statistical analysis shows that human and porcine adult VFs are statistically different with the porcine storage modulus being greater than that of humans (G1, p=1.9E-05; tan(δ), p=0.10), and that male and female human VFs are statistically the same (G1, p=0.20; tan(δ), p=0.57).
Discussion
The unique vibratory function of VFs results from their viscoelastic properties, which in turn are determined by the structure and composition of the ECM. In the current work, we aimed not only to determine the linear viscoelastic shear properties of VF tissues at physiologically relevant frequencies but also to correlate the properties with the structure and organization of the tissues.
Porcine data
Several groups have shown that in humans, age and gender affect the VF ECM composition and organization, which in turn affect the viscoelastic properties of the tissue; direct correlation between age, gender, and tissue viscoelastic properties was rarely addressed.4,5,8,22,40–42,45,46 In this study, we report that an age dependency in the viscoelastic shear properties of porcine VFs correlated to an age dependency in the histological structure of the same tissues. A gender dependency in pig samples could not be examined, as most of the tissues were from males.
Porcine VF LP is a bilayered tissue with three major components: cells, most of which are fibroblasts; fibrous proteins, such as collagen and elastin, which form a complex network; and interstitial fillers, including hyaluronic acid (HA) and sulfated GAGs.2,7,34,35,38,47 While collagen and elastin contribute to the tensile strength and elasticity of the tissue, respectively, HA is the primary component that determines the viscous response of the VF,6 though HA also contributes to the tissues' stiffness. Consequently, differences in the LP ECM composition and organization result in altered viscoelastic properties. A primary goal of the current investigation was to understand the viscoelastic properties of VF tissues at frequencies close to human phonation. A second goal of this study was to correlate the tissue mechanics with the evaluation of ECM composition and microstructure.
As stated in the results, porcine VF TWE data are separated into two groups: adult and young pigs. Figures 4, 5, and Supplementary Figure S1 show that the VFs of 6–9-month-old and 2+-year-old pigs are compositionally and structurally similar, but differ significantly from the fetal tissues. Further examination of the stained tissues shows that fetal porcine VFs were generally loose, with abundant space devoid of staining, and that the elastin fibers appeared immature. Conversely, the older VF specimens appear denser, with the elastic fibers being longer and forming a network. The epithelium also becomes thicker with age. Similar to human VF, elastin content in pig VF increases with age, and the elastic fiber diameter becomes larger4; this aging effect can be seen in Figures 4 and 5. In older tissues, elastin forms a more complex network; as aging occurs, elastin turnover decreases, crosslinking increases, and the network stiffens.4,47 Generally, increases in collagen and elastin content lead to an increase in tissue stiffness. For example, scarring is associated with fibrosis and significant matrix stiffening.6 Additionally, disorganization of the ECM increases the stiffness of the tissue.43,44 The increase in thickness of the epithelium with age also contributes to increased stiffness.
To further understand limitations on experiments performed with the conventional rheometer, results presented in Figure 3 can be analyzed to determine effects of experimental equipment. The raw phase output from the rheometer gives a measure of the torque required to rotate the head compared to that being used to rotate the sample, that is, the machine inertia. As this value approaches 180 degrees (completely out of phase with the motion), the majority of the torque is applied to the rotating head. For 2+-year-old pigs of the size tested with this instrument, this crossover occurs at a conservative value of ∼0.3 Hz, which is the frequency at which the raw phase begins to drastically increase to 180 degrees. This value of 0.3 Hz is close to the 0.14 Hz value obtained in the derivation in the Supplementary Data. For typical 6–9-month-old and 2+-year-old porcine VF samples, the rheometer allows for accurate measurements below 0.3 Hz. Data above 0.3 Hz are discarded as inaccurate. Similarly, data from human VF would have a lower maximum testable frequency in a commercial rheometer due to the lower modulus expected at low frequencies.
Although the range of frequencies at which TWEs are applicable, and have been validated by Jiao et al.,26 are higher than that for which the commercial rheometer tests are valid, it is of interest to compare the results obtained by the two methods as a way of looking for trends in the dependence of viscoelastic properties of porcine VF on frequency. Figure 3B shows a comparison of the averaged rheometer data for the 2+-year-old and 6–9-month-old cryopreserved porcine VFs with averaged data from TWEs. Data from the rheometer considered inaccurate are shown in the figure by labeling these points with a downward-pointing gray triangle (▾). At ∼3 Hz, the rheometer data begin to rise past the linear trend seen at lower frequencies. This trend is most likely due to sample inertia. The machine inertia error, which is expected at ∼0.3 Hz, is indistinguishable in these data. Figure 3B shows that it may be difficult to determine the limiting frequency at which rheometer data are reliable simply by looking at the frequency dependence of output for the viscoelastic property that one is trying to measure. Additionally, Figure 3B shows that data extrapolated from the rheometer measurements at low frequencies (i.e., below ≈2 Hz) agree quite well with the TWE data collected at a high frequency. Although overcoming machine inertia is feasible with careful instrument design, the inherent limitation of the rheometer is reached when sample inertia begins to dominate. The TWE removes this limitation, and allows for accurate high-frequency testing in a manner that leaves samples available for histological analysis. Unlike some tests performed with a rheometer, the TWE is enclosed in an environmental chamber kept at relevant conditions, so no sealing agent (e.g., mineral oil) is needed to keep the sample hydrated. Additionally, experiments take significantly less time than those performed with a rheometer. A typical TWE performed by an experienced operator may take only 15 minutes for three repeats of one sample, thus rendering the alteration of the tissue structure unlikely. Overall, it appears that TWE data may be most reliable for higher frequencies, whereas rheometric data reported previously may provide a reliable indication of frequency dependence at lower frequencies if the instrumentation and experiment are properly designed and interpreted.
Typically, VFs are considered to have frequency-dependent material properties (e.g., Chan and Titze,22 Chan and Rodriguez,25 and Titze et al.48). Least-squares data fits with a power law are often used to characterize the frequency dependence. An attempt was made to fit data in the current study in the same way:
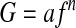 |
(4) |
where G is the modulus (G1 or G2); f is the frequency (in Hz); and a and n are the least-squares fit parameters. Table 3 shows the fits for the data in the current work, with the fit parameters a and n as well as the coefficient of correlation (R2) for the moduli of adult pigs, fetal pigs, and human specimens. In contrast to other data in the literature, the data presented here does not show statistically significant frequency dependence (R2<0.17). This does not imply that VFs are not frequency dependent, which would be contrary to the results obtained in different studies using different methodologies. For a single sample, the TWE yields one complex modulus at one frequency, whereas a rheometer gives moduli at successive frequencies. It is hypothesized that the specimen-to-specimen variability is greater than the increase in modulus due to increasing frequency. Moreover, the range of resonance frequencies in the TWE measurements is less than a decade and is probably insufficient to reveal the frequency dependence of the viscoelastic properties. Others39 have not been able to observe frequency dependence of human VFs when measuring the nonlinear elastic properties of the VF cover in tension at 1 Hz or 10 Hz. This lack of observed frequency dependence does not imply that measurements of high-frequency viscoelastic properties are not needed, but that the limited frequency range covered here may not show the frequency dependence. One potentially surprising result is that the loss tangent of all groups tested is statistically the same; it is not apparent that they should be. If artificial damping dominated the TWE, the loss tangents determined from these experiments could be high and similar. However, the authors have previously performed experiments on materials that were nearly elastic (tan(δ)≈0),14,27,49 indicating that the system is essentially free from artificial damping, so the statistical equivalence of loss tangents across all groups is believed to be valid.
Table 3.
Fit Parameters (a and n) for Porcine and Human Vocal Folds Based on Equation 4
| |
G1 |
G2 |
||||
|---|---|---|---|---|---|---|
| Tissue | a | n | R2 | a | n | R2 |
| Human | 382.102 | 0.087 | 0.064 | 92.611 | 0.210 | 0.066 |
| Porcine—adult | 371.319 | 0.367 | 0.073 | 75.126 | 0.498 | 0.110 |
| Porcine—fetal | 31.433 | 0.873 | 0.162 | 25.861 | 0.627 | 0.108 |
G1, storage modulus; G2, loss modulus; R2, correlation coefficient.
Human data
Although human specimens in this study were not ideal, a few trends were evident. Unlike porcine VF specimens, human VF tissues studied did not show any age dependency, in either the TWE data (Fig. 6A and Table 2), or in the histological stainings (data not shown). The lack of age dependence is most likely due to the small developmental range covered, 39–93 years old, which places all specimens in mid-to-late adulthood. In humans, the newborn VFs are monolayered and uniform, containing more HA, but less fibrous proteins than their adult counterpart.41,42 The trilayered structure, characterized by the layers' different ECM compositions, will not show until 11–12 years of age. The differentiation of the VFs after birth is thought to be driven by phonation.42,50 In fact, adult VFs that have not been subjected to phonation lack this trilayered structure.51 Conversely, phonation is not the only factor responsible for the VF development; hormones also seem to influence development and differentiation.42,52,53 Therefore, the most critical viscoelastic property changes in human VFs take place before 20 years of age, which may explain why no age dependency was found in the human data. These factors also explain the division of the pig mechanical and histological results described above into two groups: fetal/newborn and 6–9-month old/2+-year old. Additionally, viscoelastic shear properties of male and female human VFs tested in this study were statistically the same, even though female human VFs appear slightly softer than male human VFs.
Although human VFs are more complex than porcine VFs, basic comparisons between the two can be made. Examining the storage modulus data, adult pig VFs are stiffer than human VFs. Several factors could explain this difference. Hahn et al. have reported that pig VFs have slightly more collagen than human VFs, 52% dry weight versus 42%,35 and that porcine VFs contain much more HA than human VFs (2.2% versus 0.7%).34 Interestingly, Chan and Titze found that removal of HA from human VF LP decreased its stiffness by 35%.6 Elastin content in pig (7.5% dry weight) and human (8.5% dry weight) VF is similar, although the elastin in porcine VF is distributed more evenly throughout the thickness of the tissue and is more oriented than in human VF.34,38 ECM quantification of VF tissues has not been studied comprehensively, so the observations reported above have to be used carefully. Nevertheless, those observations indicate that porcine VF would be expected to be stiffer than human VF, thus being in agreement with the observations of this study.
Finally, the presence of laryngeal glands higher in the porcine VF than in the human VF could also contribute to the higher stiffness of porcine VF.8,38,39 Using a 5-mm dermal punch to dissect the VF, it is likely that the porcine samples would contain some laryngeal glands, while the human samples might not. This hypothesis is confirmed by the histological observations. Additionally, human tissues with the highest shear moduli showed more prominent glandular regions (data not shown).
Conclusion
Porcine VFs ranging from fetal to 2+-year old and adult human tissues were tested using the TWE in combination with histological analyses to correlate the structure and composition of the tissues with their viscoelastic properties. Storage moduli of porcine VFs were found to be age dependent, with the adult tissues being stiffer than newborn and fetal tissues. The lower storage modulus of fetal VFs was shown to be related to the presence of immature elastin and collagen fibers and the absence of an interconnected network structure in the tissue. Additionally, the young VF tissues were looser and had a thinner epithelium. Rheometric and TWE data for adult porcine VF samples showed that while rheometric data may be reliable at low frequencies, the TWE is more reliable at high frequencies. Finally, unlike porcine VFs, viscoelastic properties of human VFs were not found to be gender or age dependent, although only adult pathological tissues were tested. Generally, storage moduli of adult pigs were stiffer than those of humans, most likely due to their different ECM composition. The results from this study constitute a benchmark for material properties for the VF tissue-engineering field. Reliable high-frequency properties are needed to aid in quantitatively evaluating novel replacement materials. This study provides a foundation for such efforts.
Supplementary Material
Acknowledgments
We thank Drs. Ina Dobrinski and James Harper for providing some of the porcine specimens. This work was funded by the National Institute on Deafness and Other Communication Disorders (NIDCD, R01 DC008965).
Disclosure Statement
No competing financial interests exist.
References
- 1.Titze I.R. Principles of Voice Production. New Jersey: Prentice Hall; 1994. [Google Scholar]
- 2.Gray S.D. Titze I.R. Alipour F. Hammond T.H. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109:77. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- 3.Gray S.D. Titze I.R. Chan R.W. Hammond T.H. Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope. 1999;109:845. doi: 10.1097/00005537-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hammond T.H. Gray S.D. Butler J. Zhou R. Hammond E. Age- and gender-related elastin distribution changes in human vocal folds. J Otolaryngol Head Neck Surg. 1998;119:314. doi: 10.1016/S0194-5998(98)70071-3. [DOI] [PubMed] [Google Scholar]
- 5.Hammond T.H. Gray S.D. Butler J.E. Age- and gender-related collagen distribution in human vocal folds. Ann Otol Rhinol Laryngol. 2000;109:913. doi: 10.1177/000348940010901004. [DOI] [PubMed] [Google Scholar]
- 6.Chan R.W. Gray S.D. Titze I.R. The importance of hyaluronic acid in vocal fold biomechanics. J Otolaryngol Head Neck Surg. 2001;124:607. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- 7.Gray S.D. Cellular physiology of the vocal folds. Otolaryngol Clin North Am. 2000;33:679. doi: 10.1016/s0030-6665(05)70237-1. [DOI] [PubMed] [Google Scholar]
- 8.Hirano M. Structure of the vocal fold in normal and disease states: anatomical and physical studies. ASHA Rep. 1981;11:11. [Google Scholar]
- 9.Hirano M. Kurita S. Nakashima T. Vocal Fold Physiology Conference. San Diego, CA: College-Hill Press; 1981. Vocal fold physiology: contemporary research and clinical issues; pp. 23–43. [Google Scholar]
- 10.Hirano M. Phonosurgery: basic and clinic investigations. Otologia (Fukuoka) 1975;21:239. [Google Scholar]
- 11.Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg. 2005;13:143. doi: 10.1097/01.moo.0000162261.49739.b7. [DOI] [PubMed] [Google Scholar]
- 12.Williams N.R. Occupational groups at risk of voice disorders: a review of the literature. Occup Med (Lond) 2003;53:456. doi: 10.1093/occmed/kqg113. [DOI] [PubMed] [Google Scholar]
- 13.Farran A.J.E. Teller S.S. Jha A.K. Jiao T. Hule R.A. Clifton R.J. Pochan D.P. Duncan R.L. Jia X. Effects of matrix composition, microstructure, and viscoelasticity on the behaviors of vocal fold fibroblasts cultured in three-dimensional hydrogel networks. Tissue Eng Part A. 2010;16:1247. doi: 10.1089/ten.tea.2009.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L. Teller S.S. Clifton R.J. Jia X. Kiick K.L. Tunable mechanical stability and deformation response of a resilin-based elastomer. Biomacromolecules. 2011;12:2302. doi: 10.1021/bm200373p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grieshaber S.E. Farran A.J.E. Lin-Gibson S. Kiick K.L. Jia X. Synthesis and characterization of elastin-mimetic hybrid polymers with multiblock, alternating molecular architecture and elastomeric properties. Macromolecules. 2009;42:2532. doi: 10.1021/ma802791z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahiner N. Jha A.K. Nguyen D. Jia X. Fabrication and characterization of cross-linkable hydrogel particles based on hyaluronic acid: potential application in vocal fold regeneration. J Biomater Sci Polym Ed. 2008;19:223. doi: 10.1163/156856208783432462. [DOI] [PubMed] [Google Scholar]
- 17.Jha A.K. Hule R.A. Jiao T. Teller S.S. Clifton R.J. Duncan R.L. Pochan D.J. Jia X. Structural analysis and mechanical characterization of hyaluronic acid-based doubly cross-linked networks. Macromolecules. 2009;42:537. doi: 10.1021/ma8019442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jha A.K. Malik M.S. Farach-Carson M.C. Duncan R.L. Jia X. Hierarchically structured, hyaluronic acid-based hydrogel matrices via the covalent integration of microgels into macroscopic networks. Soft Matter. 2010;6:5045. doi: 10.1039/C0SM00101E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia X. Yeo Y. Clifton R.J. Jiao T. Kohane D.S. Kobler J.B. Zeitels S.M. Langer R. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 20.Ohno S. Hirano S. Kanemaru S.-I. Kitani Y. Kojima T. Tateya I. Nakamura T. Ito J. Implantation of an atelocollagen sponge with autologous bone marrow-derived mesenchymal stromal cells for treatment of vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2011;120:401. doi: 10.1177/000348941112000610. [DOI] [PubMed] [Google Scholar]
- 21.Chan R.W. University of Iowa; 1998. Shear properties of vocal fold mucosal tissues and their effect on vocal fold oscillations [Ph.D. thesis] [Google Scholar]
- 22.Chan R.W. Titze I.R. Viscoelastic shear properties of human vocal fold mucosa: measurement methodology and empirical results. J Acoust Soc Am. 1999;106:2008. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 23.Chan R.W. Estimation of viscoelastic shear properties of vocal-fold tissues based on time–temperature superposition. J Acoust Soc Am. 2001;110:1548. doi: 10.1121/1.1387094. [DOI] [PubMed] [Google Scholar]
- 24.Chan R.W. Measurements of vocal fold tissue viscoelasticity: approaching the male phonatory frequency range. J Acoust Soc Am. 2004;115:3161. doi: 10.1121/1.1736272. [DOI] [PubMed] [Google Scholar]
- 25.Chan R.W. Rodriguez M.L. A simple-shear rheometer for linear viscoelastic characterization of vocal fold tissues at phonatory frequencies. J Acoust Soc Am. 2008;124:1207. doi: 10.1121/1.2946715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao T. Farran A.J.E. Jia X. Clifton R.J. High frequency measurements of viscoelastic properties of hydrogels for vocal fold regeneration. Exp Mech. 2009;49:235. doi: 10.1007/s11340-008-9126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clifton R.J. Jia X. Jiao T. Bull C. Haln M.S. Viscoelastic response of vocal fold tissues and scaffolds at high frequencies. In: Holzapfel G.A., editor; Ogden R.W., editor. Mechanics of Biological Tissue. New York: Springer; 2006. pp. 445–455. [Google Scholar]
- 28.Grieshaber S.E. Nie T. Yan C. Zhong S. Teller S.S. Clifton R.J. Pochan D.J. Kiick K.L. Jia X. Assembly properties of an alanine-rich, lysine-containing peptide and the formation of peptide/polymer hybrid hydrogels. Macro Chem Phys. 2011;212:229. doi: 10.1002/macp.201000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiao T. Clifton R.J. Converse G.L. Hopkins R.A. Measurements of the effects of decellularization on viscoelastic properties of tissues in ovine, baboon, and human heart valves. Tissue Eng Part A. 2012;18:423. doi: 10.1089/ten.tea.2010.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alipour F. Jaiswal S. Phonatory characteristics of excised pig, sheep, and cow larynges. J Acoust Soc Am. 2008;123:4572. doi: 10.1121/1.2908289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alipour F. Jaiswal S. Vigmostad S. Vocal fold elasticity in the pig, sheep, and cow larynges. J Voice. 2011;25:130. doi: 10.1016/j.jvoice.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang J.J. Raviv J.R. Hanson D.G. Comparison of the phonation-related structures among pig, dog, white-tailed deer, and human larynges. Ann Otol Rhinol Laryngol. 2001;110:1120. doi: 10.1177/000348940111001207. [DOI] [PubMed] [Google Scholar]
- 33.Regner M.F. Robitaille M.J. Jiang J.J. Interspecies comparison of mucosal wave properties using high-speed digital imaging. Laryngoscope. 2010;120:1188. doi: 10.1002/lary.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn M.S. Kobler J.B. Starcher B.C. Zeitels S.M. Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix I: elastic fibers and hyaluronic acid. Ann Otol Rhinol Laryngol. 2006;115:156. doi: 10.1177/000348940611500213. [DOI] [PubMed] [Google Scholar]
- 35.Hahn M.S. Kobler J.B. Zeitels S.M. Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix II: collagen. Ann Otol Rhinol Laryngol. 2006;115:225. doi: 10.1177/000348940611500311. [DOI] [PubMed] [Google Scholar]
- 36.Chan R.W. Titze I.R. Effect of postmortem changes and freezing on the viscoelastic properties of vocal fold tissues. Ann Biomed Eng. 2003;31:482. doi: 10.1114/1.1561287. [DOI] [PubMed] [Google Scholar]
- 37.Bancroft J.D. Gamble M. London: Harcourt Publishers Limited; 2002. Theory and Practice of Histological Techniques. [Google Scholar]
- 38.Garrett C.G. Coleman J.R. Reinisch L. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope. 2000;110(5 Pt 1):814. doi: 10.1097/00005537-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Chan R.W. Fu M. Young L. Tirunagari N. Relative contributions of collagen and elastin to elasticity of the vocal fold under tension. Ann Biomed Eng. 2007;35:1471. doi: 10.1007/s10439-007-9314-x. [DOI] [PubMed] [Google Scholar]
- 40.Prades J.-M. Dumollard J.M. Duband S. Timoshenko A. Richard C. Dubois M.D. Martin C. Peoc'h M. Lamina propria of the human vocal fold: histomorphometric study of collagen fibers. Surg Radiol Anat. 2010;32:377. doi: 10.1007/s00276-009-0577-9. [DOI] [PubMed] [Google Scholar]
- 41.Sato K. Hirano M. Nakashima T. Fine structure of the human newborn and infant vocal fold mucosae. Ann Otol Rhinol Laryngol. 2001;110:417. doi: 10.1177/000348940111000505. [DOI] [PubMed] [Google Scholar]
- 42.Hartnick C.J. Rehbar R. Prasad V. Development and maturation of the pediatric human vocal fold lamina propria. Laryngoscope. 2005;115:4. doi: 10.1097/01.mlg.0000150685.54893.e9. [DOI] [PubMed] [Google Scholar]
- 43.Hirano S. Minamiguchi S. Yamashita M. Ohno T. Kanemaru S. Kitamura M. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399. doi: 10.1016/j.jvoice.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Thibeault S.L. Gray S.D. Bless D.M. Chan R.W. Ford C.N. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 45.Hammond T.H. Zhou R. Hammond E. Pawlak A. Gray S.D. The intermediate layer: a morphologic study of the elastin and hyaluronic acid constituents of normal human vocal folds. J Voice. 1997;11:59. doi: 10.1016/s0892-1997(97)80024-0. [DOI] [PubMed] [Google Scholar]
- 46.Sato K. Hirano M. Age-related changes of elastic fibers in the superficial layer of the lamina propria of vocal folds. Ann Otol Rhinol Laryngol. 1997;106:44. doi: 10.1177/000348949710600109. [DOI] [PubMed] [Google Scholar]
- 47.Kurita S. Nagata K. Hirano H. A comparative study of the layer structure of the vocal fold. In: Bless D.M., editor; Abbs J.H., editor. Vocal Fold Physiology: Contemporary Research and Clinical Issues. San Diego, CA: College Hill Press; 1983. pp. 3–21. [Google Scholar]
- 48.Titze I.R. Klemuk S.A. Gray S.D. Methodology for rheological testing of engineered biomaterials at low audio frequencies. J Acoust Soc Am. 2004;115:392. doi: 10.1121/1.1631941. [DOI] [PubMed] [Google Scholar]
- 49.Jia X. Burdick J.A. Kobler J. Clifton R.J. Rosowski J.J. Zeitels S.M. Langer R. Synthesis and characterization of in situ cross-linkable hyaluronic acid-based hydrogels with potential application for vocal fold regeneration. Macromolecules. 2004;37:3239. [Google Scholar]
- 50.Titze I.R. Hitchcock R.W. Broadhead K. Webb K. Li W. Gray S.D. Tresco P.A. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37:1521. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Sato K. Nakashima T. Nonaka S. Harabuchi Y. Histopathologic investigations of the unphonated human vocal fold mucosa. Acta Otolaryngol. 2008;128:694. doi: 10.1080/00016480701675643. [DOI] [PubMed] [Google Scholar]
- 52.Newman S.R. Butler J. Hammond E. Gray S.D. Preliminary report on hormone receptors in the human vocal fold. J Voice. 2000;14:72. doi: 10.1016/s0892-1997(00)80096-x. [DOI] [PubMed] [Google Scholar]
- 53.Rios O.A.B. Duprat A.D.C. Santos A.R.D. Immunohistochemical searching for estrogen and progesterone receptors in women vocal fold epithelia. Braz J Otorhinolaryngol. 2008;74:487. doi: 10.1016/S1808-8694(15)30593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







