Abstract
Human articular cartilage functions under a wide range of mechanical loads in synovial joints, where hydrostatic pressure (HP) is the prevalent actuating force. We hypothesized that the formation of engineered cartilage can be augmented by applying such physiologic stimuli to chondrogenic cells or stem cells, cultured in hydrogels, using custom-designed HP bioreactors. To test this hypothesis, we investigated the effects of distinct HP regimens on cartilage formation in vitro by either human nasal chondrocytes (HNCs) or human adipose stem cells (hASCs) encapsulated in gellan gum (GG) hydrogels. To this end, we varied the frequency of low HP, by applying pulsatile hydrostatic pressure or a steady hydrostatic pressure load to HNC-GG constructs over a period of 3 weeks, and evaluated their effects on cartilage tissue-engineering outcomes. HNCs (10×106 cells/mL) were encapsulated in GG hydrogels (1.5%) and cultured in a chondrogenic medium under three regimens for 3 weeks: (1) 0.4 MPa Pulsatile HP; (2) 0.4 MPa Steady HP; and (3) Static. Subsequently, we applied the pulsatile regimen to hASC-GG constructs and varied the amplitude of loading, by generating both low (0.4 MPa) and physiologic (5 MPa) HP levels. hASCs (10×106 cells/mL) were encapsulated in GG hydrogels (1.5%) and cultured in a chondrogenic medium under three regimens for 4 weeks: (1) 0.4 MPa Pulsatile HP; (2) 5 MPa Pulsatile HP; and (3) Static. In the HNC study, the best tissue development was achieved by the pulsatile HP regimen, whereas in the hASC study, greater chondrogenic differentiation and matrix deposition were obtained for physiologic loading, as evidenced by gene expression of aggrecan, collagen type II, and sox-9; metachromatic staining of cartilage extracellular matrix; and immunolocalization of collagens. We thus propose that both HNCs and hASCs detect and respond to physical forces, thus resembling joint loading, by enhancing cartilage tissue development in a frequency- and amplitude-dependant manner.
Introduction
Hydrostatic pressure (HP) has long been considered a variable influencing chondrocyte activity, since 1985, when Lippiello and co-workers1 first evaluated the in vitro metabolic response of articular cartilage (AC) explants (both bovine and human) to different levels of HP—75 to 375 psi (0.5–2.5 MPa). Data suggested that AC chondrocytes have the capacity to rapidly and differentially transform mechanical signals derived from application of HP into metabolic events, which are also determined by the magnitude of the applied force.1
With the emerging of tissue engineering as an independent research field, and the understanding that chondrocytes are mechanically sensitive cells,2,3 great efforts have been made to comprehend how HP, and other mechanical stimuli relevant for articular cartilage, such as compression4–7 and shear,8–12 may improve the development of cartilage tissue in vitro. In vivo, articular cartilage is exposed to a wide range of static and dynamic mechanical loads, ranging amplitudes of about 5–6 MPa for gait, and as high as 18 MPa for other movements such as running or jumping.13,14 In accordance to the biphasic model of cartilage,15 the solid components of the extracellular matrix (ECM) support shear stress, whereas the incompressible interstitial water is responsible for withstanding compressive loading, by driving out of the tissue. In view of this, 95% of the overall applied joint load is supported by interstitial fluid pressurization, so HP is the prevailing mechanical signal governing normal articular cartilage homeostasis.2,16 Most studies17–27 have focused on the effects of HP stimuli on chondrocyte-mediated synthesis and degradation of cartilage matrix macromolecules, such as proteoglycans, collagens, noncollagenous proteins, and glycoproteins. Articular chondrocytes, usually from animal source, respond positively to pulsatile (0.0125–1 Hz) HP loadings ranging 0.3–5 MPa, by increasing glycosaminoglycan (GAG) synthesis and deposition, as well as expression of healthy AC markers such as collagen type II, aggrecan, and sox-9 transcription factor.17–19,22,23 However, when high HP magnitudes are applied, in the order of 50 MPa, cell apoptosis is observed.21 The same outcome was observed when culturing human osteoarthritic chondrocytes under physiologically normal pressure magnitudes (5 MPa).20 In our study, we consider two clinically relevant cell sources as potentially responsive to HP dynamic culturing: human nasal chondrocytes (HNCs) have demonstrated to respond to physical forces resembling joint loading;28 therefore, this cell source was used as a proof of principle. Nasal cartilage tissue is characterized as hyaline cartilage, containing differentiated chondrocytes that express the ECM molecules typical of articular cartilage,29,30 and is responsive to physical forces resembling joint loading.28 Nasal septum cartilage may be obtained under local anesthesia, through a procedure considered to be less invasive as compared to localized tissue harvesting from non-load-bearing areas of the joint. This last biopsy procedure is the one normally employed when treating articular cartilage lesions using cell-based therapies such as autologous chondrocyte implantation or matrix-induced chondrocyte implantation.31,32 We aim to understand whether HNCs respond to HP loading, and consequently improve in vitro cartilage tissue development. Furthermore, and considering a more challenging approach, adipose tissue-derived stem cells (ASCs) appear to be a promising alternative cell source for cell-based therapies,33–35 as well as for cartilage tissue regeneration36–39 due to their excellent features. ASCs may be easily isolated from adipose tissue (AT) and proliferate quickly, and their chondrogenic differentiation potential has been proven.40–42 This known, we additionally hypothesize if human adipose stem cells (hASCs) respond to biomechanical stimuli, particularly HP, and if this could be employed to enhance and ameliorate cartilage tissue development in vitro, with the aim of reducing the time to therapy, and ultimately increasing graft implantation success. To achieve these outcomes, two custom-designed bioreactors were developed, allowing long-term culturing and control of loading parameters. In this study, we evaluated the effects of the loading amplitude (0.4 and 5 MPa) and the frequency (pulsatile vs. steady pressurization). Assays of the produced tissues evaluated the significance of these treatments on cartilage tissue development, as compared to a static culture condition (control condition).
Materials and Methods
Development of hydrostatic pressure bioreactors
Two original easy-to-use devices were developed to generate HP forces, which uniformly load constructs in culture. The High Hydrostatic Pressure Bioreactor (HHPB) was designed to generate physiological amplitudes of HP (up to 10 MPa), and the Low Hydrostatic Pressure Bioreactor (LHPB) was projected to load culturing constructs with shorter HP amplitudes (up to 0.5 MPa). Both devices enclose particular key characteristics, such as
(1) possibility to perform long-term culturing, up to several weeks;
(2) culture of multiple 3D constructs within a high range of dimensions;
(3) tunable loading parameters: pressure amplitudes may range 0–0.4 MPa (for LHPB) and 1.5–10 MPa (for HHPB); frequency may range 0–1 Hz;
(4) operation of devices inside standard biohazard hoods, and CO2 incubators at 37°C, in complete sterile conditions;
(5) LHPB is disposable, composed by standard, off-the-shelf components, whereas HHPB is reusable, composed by sterilizable stainless steel components.
High-HPB
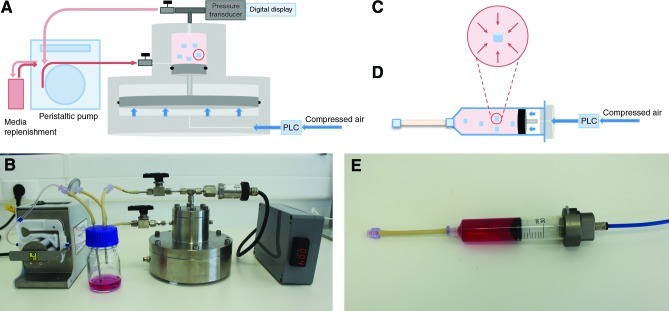
The High-HPB system (Fig.1A, B) consists of a stainless steel device (15-cm diameter×18-cm height), composed of a culture chamber (CC), an air chamber (AC), and a piston. The air pressure-driven piston creates HP in the CC, where constructs reside in the culturing medium. As filtered compressed house air enters the AC, the piston is pushed up. The ratio of the AC and CC area is 15:1, so that 15×the applied pressure is generated in the upper CC. A pressure transducer (0–16 MPa; Swagelok) with a digital display is connected to the lid of the CC, providing a real-time pressure reading. Pulsatile pressurization is controlled by a Programmable Logic Controller unit (PLC–OMRON CPM1A-30CDR-A-V1) and a solenoid valve (Camozzi A331-1C2). When nonpressurized, the culture medium is replenished through the CC by a peristaltic pump (Ismatec), from a standard 50-mL Schott bottle, with an adapted air filter.
FIG. 1.
Custom-designed hydrostatic pressure bioreactors (HPBs). (A) Schematic representation of High Hydrostatic Pressure Bioreactor (HHPB); (B) photograph of HHPB; (C) schematic representation of hydrostatic pressure (HP) forces applied to cell-encapsulated gellan gum construct; (D) schematic representation of Low Hydrostatic Pressure Bioreactor (LHPB); (E) photograph of LHPB. Color images available online at www.liebertpub.com/tea
Low-HPB
During HP loading, cells (encapsulated in gellan gum [GG] hydrogel) experience uniform normal stress, without measurable tissue strain—this is schematically represented in Figure 1C and valid within both High- and Low-HPB. The Low-HPB system (Fig.1D, E) is composed by a 30-mL luer-lok polypropylene syringe (BD Biosciences), where the rubber piston is used as a physical division between the culture medium and compressed air, used as mechanism of compression. An aluminum adapter is connected at the top of the syringe, together with a semirigid nylon tube (Legris) to conduct filtered compressed house air inside the chamber. At the bottom of the syringe, Pharmed BPT tubing (Masterflex) is connected to allow gas exchange. The PLC and solenoid valve used with HHPB allow controlled pulsatile pressurization of the culture medium. The medium is replenished through the Pharmed BPT tubing, using a syringe to avoid complex manipulation.
Isolation, expansion, and cell encapsulation in GG hydrogels
Human nasal chondrocytes
Nasal cartilage was obtained as a surgical waste from an endoscopic endonasal approach to the brain, by the neurosurgery department of our local hospital. All patients (n=10, average age of 44 years) signed an informed consent document approved by the Ethics Committee of Hospital S. Marcos (Braga). Dissected nasal cartilage (average weight of 0.67 g) was cut and digested with collagenase type II, according to a protocol described elsewhere.43 Briefly, the tissue was washed with sterile phosphate-buffered saline (PBS) (Sigma P4417), diced into 2–3-mm-thickness cubes, immersed in 20 mL trypsin-EDTA solution (Invitrogen 25300-062), and incubated for 30 min at 37°C in a rotator. The trypsin–EDTA solution was removed, and 20 mL of collagenase type II (2 mg/mL; Sigma C6885) was added and allowed to incubate for 15 h at 37°C in a rotator. The digested tissue was filtered and the cell suspension centrifuged at 1200 rpm for 8 min. The cell pellet was washed twice with PBS, and cells were counted with a hemocytometer using the viability stain trypan blue (Sigma), obtaining a cell yield of 3,500 cells/mg. Chondrocytes were expanded until passage 3 in Dulbecco's modified Eagle's medium (DMEM) high glucose (Sigma D5671); 1% nonessential aminoacids (1xMEM Invitrogen 11140-035); 20 mM L-alanyl-L-glutamine (Sigma G8541); 1% antibiotic/antimicotic 100×(15240-062 Gibco); 10 mM HEPES (Sigma H4034); and 10% Fetal Bovine Serum (Biochrom, heat-inactivated 30 min, 57°C), supplemented with basic fibroblast growth factor (bFGF) 10 ng/mL (Peprotek 100-18B). Chondrocytes were further detached from the culture flask with the trypsin–EDTA solution (Invitrogen 25300-062) and encapsulated in a GG hydrogel and cultured for 3 weeks in a medium without bFGF supplementation.
Human adipose-derived stem cells
hASCs were isolated, according to previously described methods,44 from liposuction aspirates of subcutaneous AT, donated with written consent by patients undergoing elective liposurgery. Briefly, AT was washed with PBS (1:1 v/v) and centrifuged 200 g for 5 min at room temperature (RT), and infranatants were discarded. About 1 mL of 0.2 U/mL collagenase solution (Collagenase NB 4 Standard grade; SERVA Electrophoresis, Cat. No. 17454) was added per gram of washed AT, shaked vigorously, and incubated at 37°C for 45 min in a water bath with agitation at 200 rpm. Digested AT was further centrifuged at 1200 g, 3 min, at RT, and supernatants were discarded, and the pellet (correspondent to the stromal vascular fraction—SVF) was plated in cell culture plates. Adhered cells (adipose stem cells–hASCs) were further expanded to the third passage in a high-glucose DMEM (GIBCO 11965) supplemented with 10% fetal bovine serum (FBS) (GIBCO 26140), penicillin–streptomycin (1%) (GIBCO 15140), and 1 ng/mL bFGF (Peprotech 100-18B). hASCs were further detached from the culture flask with the trypsin–EDTA solution (Invitrogen 25300-062), encapsulated in a GG hydrogel, and cultured for 4 weeks in a chondrogenic medium: DMEM supplemented with 0.1 nM dexamethasone (Sigma D2915), 50 μg/mL proline (Sigma P5607), 1 mM sodium pyruvate (Invitrogen 11360-070), 1×ITS+ (BD Bioscience), 50 μg/mL ascorbic acid 2-phosphate (Sigma A8960), penicillin–streptomycin (1%) (GIBCO 15140), and 10 ng/mL TGF -ß3 (Invitrogen PHG9302). p0 cells were examined for surface marker expression using flow cytometry. The presence of specific antigens such as CD105, CD45, CD34, CD73, and CD90 was analyzed, as previously published.44,45 hASCs were tested for their differentiation capacity into the osteogenic, chondrogenic, and adipogenic lineages.
Cell encapsulation in GG hydrogels
GG is a water-soluble gelling agent commonly used in food and pharmaceutical industry, due to its processing into transparent gels resistant to heat and acid stress. The thickness and hardness of the GG are determined by acetyl groups present: with acetyl groups, the gel is soft and elastic; without acetyl groups, firmer gels are obtained.46 Both form thermoreversible gels with different mechanical properties in the presence of metallic ions and upon temperature decrease. Once GG presents a thermosensitive behavior,47–49 with gelation close to body temperature, its application as an injectable formulation, for repair of cartilage defects, is promising.50–53
GG hydrogel was produced according to the procedure described by Oliveira JT and Reis RL et al.50–53 Briefly, powdered GG (Gelzan™ Sigma G1910) was dissolved in distilled water into a 1.5% solution and heated up to 90°C. Temperature was subsequently decreased down to 37°C–40°C, for cell encapsulation at a final concentration of 10×106 cells/mL. Cylindrical discs were made with a mold, and PBS (Sigma P4417) was used as a cross-linking agent to stabilize the hydrogel structure.
Bioreactor cultivation of tissue constructs
HNC study
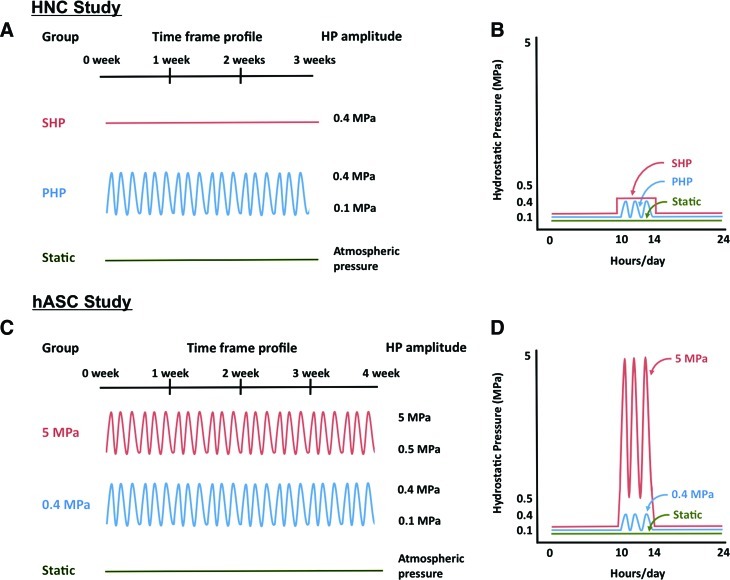
The experimental design is outlined in Figure 2. HNCs encapsulated in GG hydrogel (HNC-GG) were cultured up to 3 weeks in three conditions (Fig. 2A, B): (1) Pulsatile Hydrostatic Pressure (PHP)–culture medium pressurized between 0.1 MPa and 0.4 MPa, at a frequency of 0.1 Hz, 3 h/day, and 5 days/week; (2) Steady Hydrostatic Pressure (SHP)–culture medium pressurized at 0.4 MPa for 3 h/day, and 5 days/week; (3) Static culturing (Static)—constructs were cultured at atmospheric pressure conditions (0.1 MPa) during the total culturing period. Equal individual devices were used for each culturing regime.
FIG. 2.
HP profiles applied to constructs. Human nasal chondrocyte (HNC) study: (A) HP profile applied along 3 weeks of culture (SHP–Steady Hydrostatic Pressure; PHP–Pulsatile Hydrostatic Pressure); (B) daily HP profile applied to constructs. Human adipose stem cell (hASC) study: (C) HP profile applied along 4 weeks of culture; (D) daily HP profile applied to constructs. Color images available online at www.liebertpub.com/tea
hASC study
hASCs encapsulated in GG hydrogel (hASC-GG) were cultured up to 4 weeks in three conditions (Fig. 2C, D): (1) High HP (5 MPa)—culture medium pressurized between 0.5 MPa and 5 MPa, at a frequency of 0.5 Hz, 4 h/day, and 5 days/week; (2) Low HP (0.4 MPa)—culture medium pressurized at 0.4 MPa, at a frequency of 0.5 Hz, 4 h/day, and 5 days/week; (3) Static culturing (Static)—constructs were cultured at atmospheric pressure conditions during the total culturing period.
Cell viability and proliferation assessment
Cell viability was evaluated by a Live/Dead assay. Live cells (indicated by calcein AM; Invitrogen C3099) and dead cells (indicated by propidium iodide; Alfagene P1304MP) were imaged through a Zeiss Axioimage (RZ1M) florescence microscope. Cell metabolic activity was assessed through an MTS (3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2(4-sulfofenyl)-2H-tetrazolium) assay (VWR G3580). Constructs were washed in PBS and incubated with the MTS solution at 37°C for 3 h, after which 100 μL was transferred to a 96-well plate for optical density (OD) measurement at 490 nm. OD is directly proportional to the cellular activity, being a measure of mitochondrial activity.
Biochemical characterization
Constructs were harvested, washed in PBS, cut in half, and weighed. For DNA assay, one-half was added to 1 mL of the proteinase K digestion buffer (10 mM Tris, 1 mM EDTA, 0.1% Triton X-100, and 0.1 mg/mL proteinase K) and incubated overnight at 56°C for digestion. After centrifugation at 3000 g, for 10 min, the supernatants were removed, diluted, pippeted in duplicate into a 96-well plate, and 1:1 ratio of the picogreen solution (Quant-iT™ PicoGreen® dsDNA Kit; Invitrogen) was added. Sample fluorescence was measured with a fluorescent plate reader at excitation ∼480 nm and emission ∼520 nm. Lambda DNA was used to prepare the standard curve. For GAG quantification, one-half of constructs was added to 1 mL of the papain digestion buffer (100 mM sodium phosphate buffer, 10 mM Na2EDTA, 1.5 mg/mL L-cysteine hydrochloride, and 0.125 mg/mL papain) and incubated overnight at 60°C for digestion. After centrifugation at 10,000 g, for 5 min, 10 μL of supernatant was removed and pippeted in duplicate into a 96-well plate, and 250 μL of DMB solution (Dimethylmethylene blue) was added. Sample absorbance was measured with a microplate reader at OD 530 nm. Chondroitin 6-sulfate C sodium salt was used to prepare the standard curve.
Histology and immunohistochemistry
Ten percent formalin was used to fix samples for 1 day, and further dehydrated with a graded series of ethanol washes. Samples were embedded in paraffin, sectioned to 5 μm, and mounted on glass slides. To proceed to distinct stainings, sections were deparaffinized with Clearite and rehydrated with a graded series of ethanol washes.
Articular cartilage ECM components, such as mucopolysaccharides and GAGs, respectively, were detected by safranin O and Alcian Blue staining. Briefly, Safranin O stain was performed by staining with Weigert's iron hematoxylin, for 10 min followed by running in tap water for 10 min. Slides were then immersed in 0.02% fast green solution for 5 min and rinsed 10–15 s in 1% acetic acid. Finally, samples were stained with 0.1% Safranin O solution for 5 min. Regarding Alcian Blue staining, slides were immersed in 0.01 g/mL Alcian Blue solution for 30 min, and washed in running tap water for 2 min. A counterstain with nuclear fast red was performed for 5 min. Upon staining, sections were washed in running tap water, dehydrated with a graded series of ethanol, cleared with Clearite, and mounted with a resinous mounting medium.
For immunohistochemistry, sections were blocked with normal horse serum (NHS), stained sequentially with a primary antibody (rabbit anti-human collagen type II polyclonal antibody, abcam ab34712 and rabbit anti-human collagen type I polyclonal antibody, abcam ab292; NHS for negative control) and a secondary antibody (Vectastain Universal Elite ABC Kit; PK-6200 Vector Laboratories), and developed with the biotin–avidin system (DAB substrate kit; SK-4100 Vector Laboratories). All histological and immunohistochemical assessments were performed blindly by three independent researchers.
Quantitative real-time reverse transcriptase–polymerase chain reaction
For RNA extraction, constructs were added to 800 μL of TRIzol (Invitrogen 15596-026) and disintegrated by a mortar and pestle. Suspensions were centrifuged at 12,000 g for 10 min at 4°C to remove tissue debris and extracted with chloroform (JMGS C/4960/17). Colorless aqueous phase containing RNA was removed and mixed with an equal volume of isopropanol (Laborspirit 33539). Suspensions were again centrifuged at 12,000 g for 8 min at 4°C, and supernatant was discarded, and RNA pellet was washed with 75% ethanol. Samples were centrifuged at 7500 g for 5 min at 4°C, and supernatant was removed, and pellet was air-dried and dissolved with RNAase-free water (Invitrogen 10977035). RNA was quantified using Nanodrop® ND-1000. Approximately 1 μg of RNA was reverse-transcribed with random hexameres using the qScript™ cDNA Synthesis Kit (Quanta Biosciences 95047). Expression of collagen type II, aggrecan, Sox-9, and the housekeeping gene glyceraldehyde-3-phosphatedehydrogenase (GAPDH) was quantified using PerfeCTa™ SYBR® Green FastMix™ (Quanta Biosciences 95072) and the Mastercycler ep realplex4 (Eppendorf). Primer sequences and amplicon size are described on Table I. The expression data were normalized to GAPDH and presented as average values for each group (n=3±standard deviation [SD]).
Table 1.
Primers Used for Quantitative Real-Time Reverse Transcriptase–Polymerase Chain Reaction
| Gene | Primer forward | Primer reverse | Amplicon |
|---|---|---|---|
| Collagen type II | 5′GACAATCTGGCTCCCAAC | 5′ACAGTCTTGCCCCACTTA | 257 bp |
| Aggrecan | 5′TGAGTCCTCAAGCCTCCTGT | 5′TGGTCTGCAGCAGTTGATTC | 129 bp |
| Sox-9 | 5′TACGACTACACCGACCACCA | 5′TTAGGATCATCTCGGCCATC | 256 bp |
| GAPDH | 5′ACAGTCAGCCGCATCTTCTT | 5′ACGACCAAATCCGTTGACTC | 94 bp |
bp, base pairs; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
Data are presented as average (n=3)±SD. Statistical significance was determined using an analysis of variance followed by the Tukey's HSD (honestly significant difference) test using Prism software (Prism 4.0c; GraphPad Software Inc.).
Results
Cell proliferation and viability
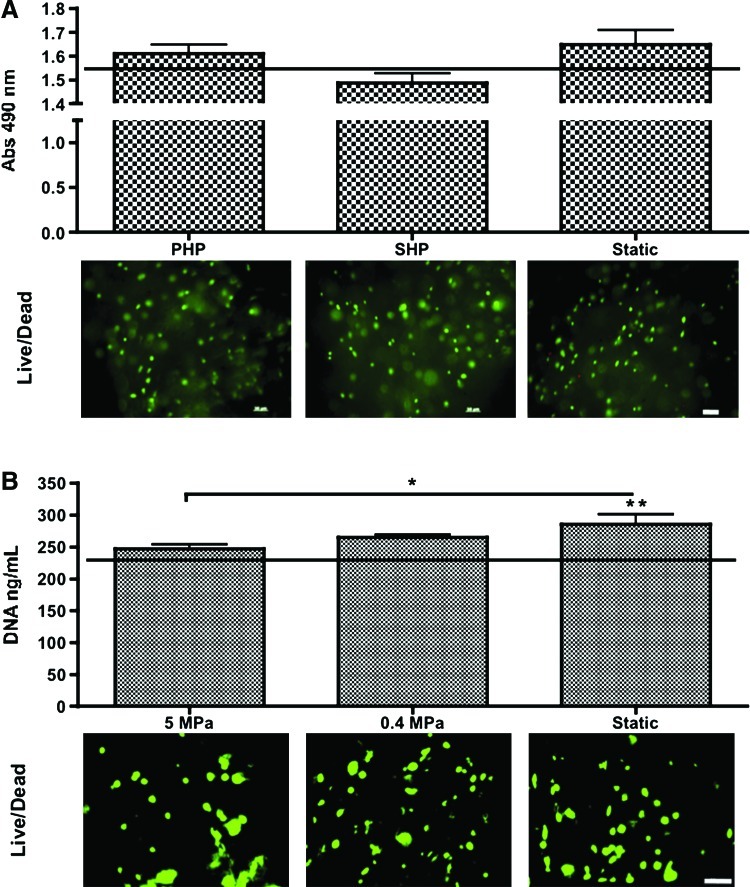
HNC metabolic activity was quantified by the reduction of the MTS reagent at the beginning and end of culture (Fig. 3A). No significant differences were obtained from day 1 to week 3 of culture, or among experimental groups. Live/Dead imaging of HNCs encapsulated in GG hydrogels corroborates with MTS data, as green viable cells are visualized by fluorescence microscopy.
FIG. 3.
Cell viability and proliferation assessment. (A) HNC study Top: Cell metabolic activity evaluated by MTS reduction. Horizontal line indicates day-1 values. n=3; no statistically significant differences between groups. Bottom: Live/Dead imaging of constructs after 3 weeks of culture. Scale bar=50 μm. (B) hASC study Top: Cell proliferation evaluated by DNA concentration. Horizontal line indicates day 1-values. n=3, *p<0.05; **p<0.01 to day 1. Bottom: Live/Dead imaging of constructs after 4 weeks of culture. Scale bar=50 μm. Color images available online at www.liebertpub.com/tea
DNA contents of hASCs encapsulated in GG hydrogels were quantified at beginning and end of culture. The group of hASCs cultured in static conditions proliferated more than those cultured in 5 MPa HP (p<0.05) and was the only group that significantly increased the cell number relatively to beginning of culture (p<0.01). The Live/Dead viability assay allowed the observation, by fluorescence microscopy, of green viable cells that enzymatically converted nonfluorescent cell-permeant calcein AM into the intensely fluorescent calcein (Fig. 3B).
Cartilage tissue development
HNC study
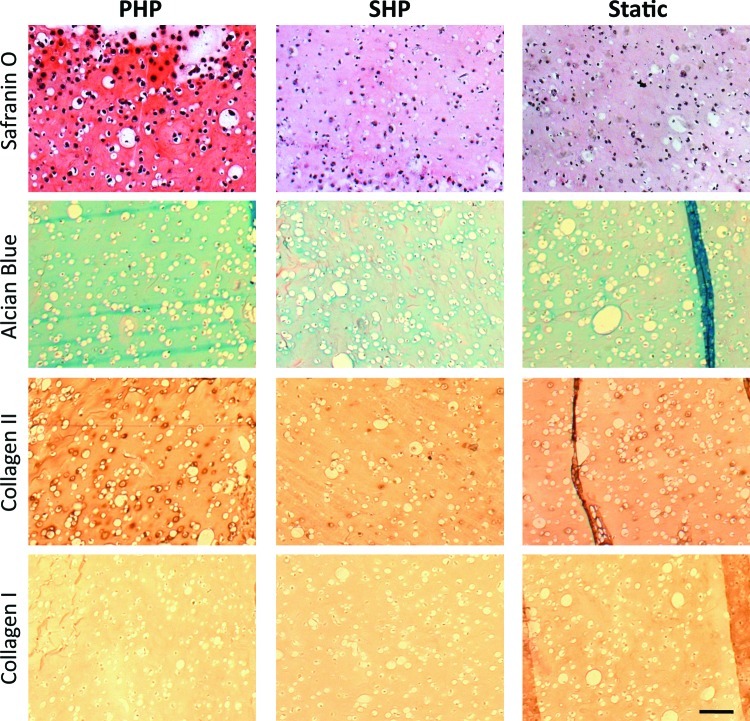
Cartilage tissue developed by HNC-GG cultured under 0.4 MPa PHP or SHP was evaluated after 3 weeks, and compared with static culturing. Cartilage ECM components, namely collagen type II (Col II) and GAGs, were localized in cross-sections of constructs (Fig. 4). Safranin O and Alcian Blue staining revealed the presence of negatively charged GAGs in cultured constructs.
FIG. 4.
HNC study: Cartilage development evaluation after 3 weeks of culture. (1st and 2nd row) Histological stainings of cartilage extracellular matrix (ECM), namely glycosaminoglycans (GAGs) (by Safranin O and Alcian Blue); (3rd and 4th row) immunohistochemical localization of collagen type II and collagen type I. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
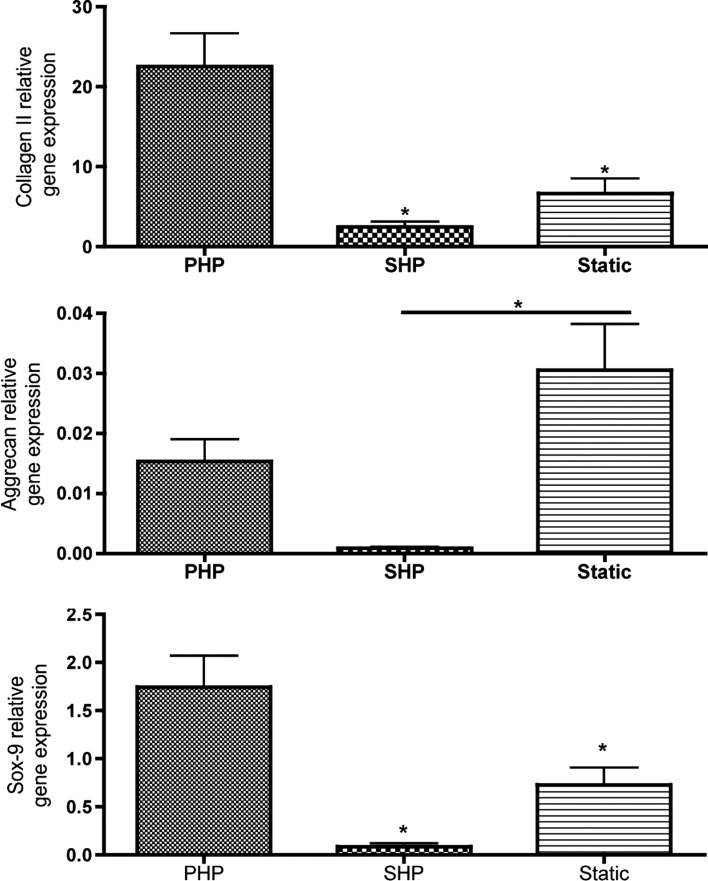
We observed that PHP induced a great increase in GAG deposition as shown by intense Safranin O staining and higher Alcian Blue staining, as compared to constructs cultured under SHP, or under static culturing conditions. Also, deposition of collagen type II, the major component of articular cartilage, was increased in the PHP group, whereas the least deposition was observed for the SHP group. Collagen type I was not detected in any of the experimental groups, which is a positive marker of development of healthy articular cartilage (Fig. 4). The gene expression profile corroborates with these observations (Fig. 5). Collagen type II-relative gene expression was overexpressed in cells cultured under PHP conditions, significantly different (p<0.05) than SHP or Static groups. Sox-9 gene expression, an important transcription factor in chondrogenesis, was also upregulated in the PHP group, statistically different than SHP and Static groups. Among these, SHP culturing did not improve either collagen type II- nor Sox-9-relative gene expression over Static culturing. Aggrecan gene expression, though, was significantly inferior (p<0.05) in cells cultured under SHP as compared to Static conditions.
FIG. 5.
HNC study: Relative gene expression obtained after culture. Collagen II, aggrecan, and sox-9 gene expression relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). n=3, *p<0.05.
hASC study
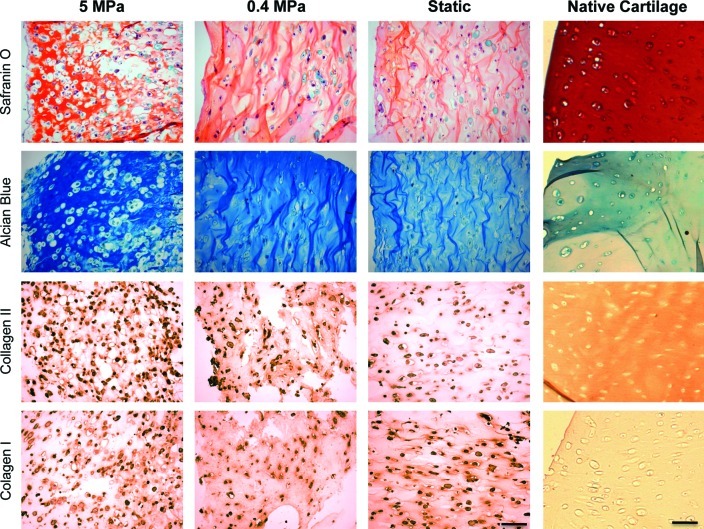
Safranin O staining and Alcian Blue staining were performed to detect GAGs in hASC-GG constructs cultured under high (5 MPa) or low (0.4 MPa) PHP, and compared to static culture conditions (Fig. 6). Both dyes stained intensely the GAGs present in constructs cultured under 5 MPa of PHP. Moreover, it was evident the presence of cells in lacunae, a characteristic of articular cartilage tissue, as observed in correspondent stainings of human native cartilage (Fig. 6, right column). Less stain intensity was observed for constructs cultured under 0.4 MPa of PHP in comparison to the 5-MPa group, yet higher than the one observed for constructs cultured under static conditions. The same trend was observed for immunolocalization of collagen type II (Fig. 6): higher deposition of this articular cartilage protein was observed in constructs of the group 5 MPa, relatively to constructs of the 0.4-MPa group, which per se, demonstrated higher collagen II deposition than in constructs that were not mechanically stimulated (Static group).
FIG. 6.
hASC study: Cartilage development evaluation after 4 weeks of culture. (1st and 2nd row) Histological stainings of cartilage ECM, namely GAGs (by Safranin O and Alcian Blue); (3rd and 4th row) immunohistochemical localization of collagen type II and collagen type I. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
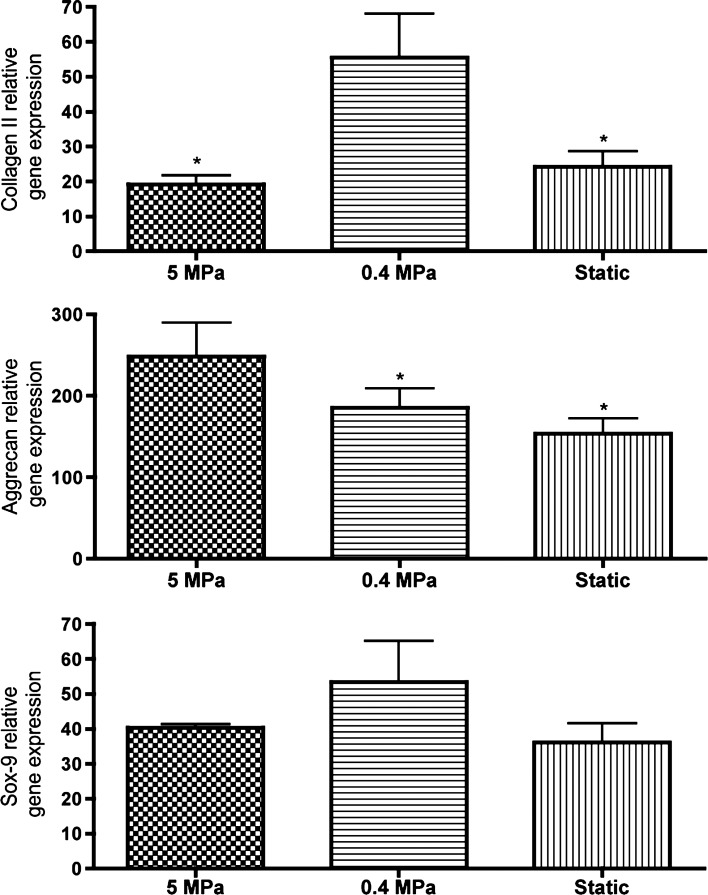
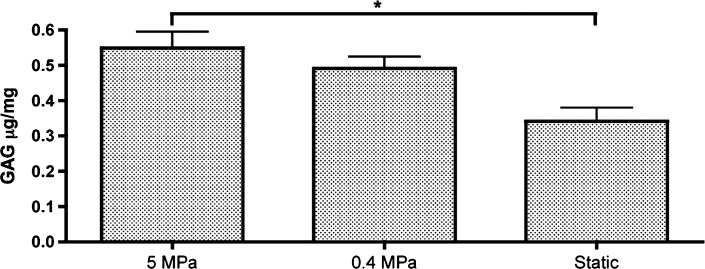
Regarding collagen type I, all groups demonstrated collagen type I deposition, with the highest intensity observed for the Static group, considerably higher than that detected in native articular cartilage (Fig. 6). Herein, the opposite trend was observed: constructs of Static group demonstrated deposition of this protein to a higher extent than constructs cultured under low PHP (0.4-MPa group), whereas constructs of the 5-MPa group demonstrated the smaller deposition of collagen type I. The increased deposition of collagen type II and low deposition of collagen type I are indicative of the development of mature and healthy articular cartilage. The relative expression of genes encoding for major articular cartilage ECM components, such as collagen II and aggrecan, was determined by quantitative real-time reverse transcriptase–polymerase chain reaction and found to be consistent with histology staining (Fig. 7). Aggrecan-relative gene expression (GE) of cells exposed to 5-MPa PHP was significantly higher (p<0.05) than GE quantified for both 0.4-MPa and Static groups. Regarding collagen type II, the highest expression was obtained for cells exposed to 0.4 MPa, which was found to be significantly different from GE quantified for both 5-MPa and Static groups. Regarding GE of Sox-9 transcription factor, no significant differences were detected between culturing groups. The ECM components were also directly quantified by biochemical assays. The amount of quantified GAGs in the constructs was superior for the 5-MPa group and found to be significantly different (p<0.05) than that observed for static culturing (Fig. 8). Although in our best condition we obtained ∼0.55 μg/mg GAG, this is yet 50 times lower the amount quantified in human articular cartilage, whose dense matrix contains about 25–30 μg/mg GAG (54).
FIG. 7.
hASC study: Relative gene expression obtained after culture. Collagen II, aggrecan and sox-9 gene expression relative to GAPDH. n=3, *p<0.05.
FIG. 8.
hASC study: GAG quantification obtained after 4 weeks of culture. n=3, *p<0.05.
Discussion
Dynamic culturing has long been adopted55–57 as a strategy to improve the development of cartilage tissue in vitro as opposed to standard static culturing techniques. We understand dynamic culturing, as the use of biomechanical stimuli that are relevant to the tissue being engineered. Articular cartilage tissue in joints such as the hip or the knee is subjected to intermittent cyclic stress that can either be compressive or shear.2 However, a major joint load is supported by interstitial fluid pressurization.3,58 HP is so essential for cartilage homeostasis such that joint immobilization or decreased loading results in cartilage thinning.59,60 HP is a fundamental mechanical stimulus governing the normal functioning of articular cartilage, which has justified its broad exploitation in our study. Two custom bioreactor systems were developed, being capable of generating and applying this mechanical stimulation to 3D tissue-engineering constructs (Fig. 1). These systems are novel and distinguish themselves from other systems described in the literature by several features, namely the possibility to long-term culture and stimulate cell-encapsulated/cell-seeded constructs in the same device up to 4 weeks (maximum evaluated). Other studies have explored HP pressurization for shorter periods, such as a few hours (1, 4, or 24 h)19–21,23,24,27 or up to 2 weeks.18,22,25,26 Carver et al. 1999,17 and lately, Gunja and coworkers 2010,61 cultured constructs up to 5 and 4 weeks, respectively, although requiring higher technical complexity. Our devices are user-friendly, and can be operated inside standard hoods and incubators during complete experimental periods, which is a feature not commonly observed in other studies.19,21,23,24,26,27,61
To validate the purpose of using HP to improve cartilage tissue development in vivo, we performed a preliminary study using healthy chondrocytes from a hyaline cartilage source (nasal septum cartilage). Nasal septum was chosen as an alternative model of healthy articular cartilage, as HNCs have proven to respond to mechanical stimulation.28 In our study, very positive outcomes were obtained, even under low levels of HP (0.4 MPa). This finding supported the subsequent study using hASCs. Our major aim was to understand if hASCs respond positively to this mechanical stimulation and develop cartilage tissue with increased properties than those obtained under standard culturing conditions, for similar culturing periods. Furthermore, we questioned if higher levels of HP within the physiological range found in human joints (5–6 MPa for gait)13,14 would improve cartilage formation. In both studies, GG hydrogel50 was used for cell encapsulation because of its proven performance on supporting cartilage tissue development in vitro50,51 and in vivo52 with chondrocytes and ASCs.52 For both studies, the cell viability was assessed. We observed that HP amplitudes of 0.4 MPa under both pulsatile and steady state regimes were not deleterious for HNCs, as no significant differences were obtained as compared to static conditions (Fig. 3A). Furthermore, for the hASC study, DNA content for all groups measured at the end of culture was superior than the initial, which demonstrated that both 0.4 and 5 MPa pulsatile-loading regimes were not harmful to cells (Fig. 3B). Although, only hASCs cultured under static conditions proliferated (p<0.01 to day-1 values), indicating that lack of HP allowed higher cell division in detriment of chondrogenic differentiation stimulation. Cell numbers used in this study compare to those quantified for native cartilage: the biopsies collected (n=10) yielded 28.0±5.8 ng DNA/mg tissue, whereas DNA quantification of cultured constructs demonstrated 20.5±2.9 ng/mg construct.
Regarding chondrogenic matrix deposition, we observed that HP culturing seemed to induce cartilage-like tissue development in a frequency- and amplitude-dependant manner. In the HNC study, more than questioning if HP would improve cartilage tissue development by these cells, we questioned whether pulsatile or steady loading would provide increased tissue formation. Therefore, HNC-GG constructs were cultured under 0.1 Hz or 0 Hz, 0.4-MPa HP loading. Higher ECM production and deposition, identified by increased GAG staining by Safranin O and Alcian Blue, as well as collagen type II immunolocalization, were obtained for pulsatile culturing (PHP group). Moreover, steady HP culturing (SHP group) seemed to suppress any chondrogenic development once very low histological detection and gene expression were obtained for this group, even lower than for static culturing (Figs. 4 and 5). To our knowledge, HNC response to HP mechanical stimulation has not been yet fully explored. In the context of mechanical stimulation, Candrian and colleagues28 evaluated HNC response to distinct compression-loading regimens and observed that these expressed collagen II, aggrecan, and hyaluronan to different extents as a response to those specific regimes. Bouchet et al.62 cultured HNCs in spinner flasks, demonstrating that this stimulation improved aggrecan and collagen type II gene expression and deposition on Cellagen™ beads. Although we have used HNCs as a cell model, we also recognize its interest as a possible heterologous approach for the treatment of cartilage focal lesions. In fact, the use of HNCs (1) avoids the need of biopsy harvesting of hyaline cartilage from nonbearing articular sites; and (2) allows good cell response to mechanical stimuli that are physiological relevant, anticipating a good adaptation of the engineered tissue to the implantation-site environment. From this preliminary study, we conclude that (1) HNCs respond to HP mechanical stimulation by increasing the cartilage formation outcome as compared to engineered constructs cultured under static conditions; (2) pulsatile HP stimulated HNCs to secrete cartilaginous ECM, whereas SHP appears to suppress cartilage development. Therefore, pulsatile HP at 0.4 MPa was chosen to stimulate hASCs in GG hydrogels toward chondrogenic differentiation and hyaline cartilage tissue formation. Herein, more than hypothesizing the ability of hASCs to sense and respond to pulsatile HP mechanical stimulation at 0.4 MPa by increasing chondrogenic differentiation and improving cartilage tissue development, we also questioned the influence of physiologic levels of HP (5 MPa) on the magnitude of these outcomes. Both hypotheses were proven. Data shown demonstrate increased aggrecan gene expression, intense GAG staining, and correspondent increased GAG quantification for the 5-MPa group, relatively to both 0.4-MPa and Static groups (Figs. 6–8). Moreover, same outputs were found greater for the 0.4-MPa group as compared to the Static group, indicating an amplitude-related response of hASCs to HP, since cartilage-related outputs were found to vary proportionally to the HP magnitude: 5 MPa > 0.4 MPa > Static. Some mechanotransduction mechanisms may play an important role in justifying these outcomes. Hydrostatic loading does not result in macroscopic deformation of the tissue once the solid matrix is incompressible, but an increase in the interstitial fluid flow and/or increased cytoskeleton changes63 promoted by a pulsatile loading might be some of the mechanisms triggering cell response over steady pressurization. Additionally, by increasing loading amplitudes, up to 5 MPa, major mechanotransduction mechanisms should respond proportionally by (1) increasing integrin-mediated responses, majorly by α5ß1 that performs as a primary bridge between the ECM and actin cytoskeleton and playing an important role on MAPK activation64; and (2) enhancing molecular conformational changes transduced to the nucleus and consequently changing accessibility of genomic DNA for transcription64; and (3) rising the direct effects on the cell membrane ion pumps and channels—with loading, the Na/K pump is inhibited, and intracellular Ca2+ wave function as one of the major signal transduction mechanism.65,66
Yet, our results are additive to those obtained by Ogawa and coworkers,39 which remain, to our knowledge, the only study that previously explored the response of hASCs to HP. In this work, hASCs were exposed to pulsatile low HP (0.5 MPa) for 1 week, followed by 3 weeks of static culturing, and evidenced increased collagen II, aggrecan, and sox-9 gene expression and ECM staining in the pressurized group, as compared to the Static group. We suggest that physiologic levels of HP (10×higher) and longer loading periods provide a more mature tissue, which is better adapted to the environment found in vivo. Given this, we conclude that (1) hASCs sense and respond to PHP stimulation at both low (0.4 MPa) and physiologic (5 MPa) pressure amplitudes; (2) physiologic HP amplitudes promoted a higher and better tissue matrix distribution, which resembles native articular cartilage.
Conclusions
By aiming the improvement of in vitro engineering of cartilage tissue, two custom-made bioreactor systems were developed being capable of generating and applying, in a controlled manner, HP as biomechanical stimuli—at high and low magnitudes—to 3D tissue-engineering constructs. In addition, HNCs and hASCs were encapsulated in GG hydrogels (ASC-GG), and cultured under several HP regimens to evaluate cartilaginous tissue formation. We observed that HNCs enhance secretion of cartilaginous ECM when cultured under pulsatile low HP, whereas same levels of HP (0.4 MPa) applied continuously inhibit ECM secretion. Moreover, hASCs cultured under same pulsatile low HP (0.4 MPa) secrete less chondrogenic ECM than under pulsatile physiologic levels of HP (5 MPa). We conclude that hASCs not only sense and respond to HP loading but also respond in different proportions in accordance to magnitude of the loading applied. Further experimental studies are needed to understand the mechanotransduction mechanisms occurring that lead to the observed cell response.
Acknowledgments
We gratefully acknowledge funding support of this work by the FCT Ph.D. grant (SFRH/BD/42316/2007 to CC). The authors thank Doctor Emílio Valls and Clínica Luso-Espanhola, Porto, for providing adipose tissue, and Professor Nuno Neves and Hospital S. Marcos, Braga, for providing cartilage tissue, both used in experiments.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lippiello L. Kaye C. Neumata T. Mankin H.J. In vitro Metabolic Response of Articular-Cartilage Segments to Low-Levels of Hydrostatic-Pressure. Connect Tissue Res. 1985;13:99. doi: 10.3109/03008208509152388. [DOI] [PubMed] [Google Scholar]
- 2.Grodzinsky A.J. Levenston M.E. Jin M. Frank E.H. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 3.Mow V. Guo X.E. Mechano-electrochemical properties of articular cartilage: Their inhomogeneities and anisotropies. Annu Rev Biomed Eng. 2002;4:175. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- 4.Seidel J.O. Pei M. Gray M.L. Langer R. Freed L.E. Vunjak-Novakovic G. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 2004;41:445. [PubMed] [Google Scholar]
- 5.Davisson T. Kunig S. Chen A. Sah R. Ratcliffe A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J Orthop Res. 2002;20:842. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang P.Y. Chow H.H. Lai J.Y. Liu H.L. Tsai W.B. Dynamic Compression Modulates Chondrocyte Proliferation and Matrix Biosynthesis in Chitosan/Gelatin Scaffolds. J Biomed Mater Res B. 2009;91B:143. doi: 10.1002/jbm.b.31384. [DOI] [PubMed] [Google Scholar]
- 7.Xie J. Han Z.Y. Matsuda T. Mechanical compressive loading stimulates the activity of proximal region of human COL2A1 gene promoter in transfected chondrocytes. Biochem Bioph Res Co. 2006;344:1192. doi: 10.1016/j.bbrc.2006.03.243. [DOI] [PubMed] [Google Scholar]
- 8.Bueno E.M. Bilgen B. Barabino G.A. Wavy-walled bioreactor supports increased cell proliferation and matrix deposition in engineered cartilage constructs. Tissue Eng. 2005;11:1699. doi: 10.1089/ten.2005.11.1699. [DOI] [PubMed] [Google Scholar]
- 9.Chen H.C. Lee H.P. Sung M.L. Liao C.J. Hu Y.C. A novel rotating-shaft bioreactor for two-phase cultivation of tissue-engineered cartilage. Biotechnol Prog. 2004;20:1802. doi: 10.1021/bp049740s. [DOI] [PubMed] [Google Scholar]
- 10.Gooch K.J. Kwon J.H. Blunk T. Langer R. Freed L.E. Vunjak-Novakovic G. Effects of mixing intensity on tissue-engineered cartilage. Biotechnol Bioeng. 2001;72:402. doi: 10.1002/1097-0290(20000220)72:4<402::aid-bit1002>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Saini S. Wick T.M. Concentric cylinder bioreactor for production of tissue engineered cartilage: effect of seeding density and hydrodynamic loading on construct development. Biotechnol Prog. 2003;19:510. doi: 10.1021/bp0256519. [DOI] [PubMed] [Google Scholar]
- 12.Alves da Silva M.L. Crawford A. Mundy J.M. Correlo V.M. Sol P. Bhattacharya M. Hatton P.V. Reis R.L. Neves N.M. Chitosan/polyester-based scaffolds for cartilage tissue engineering: assessment of extracellular matrix formation. Acta Biomater. 2010;6:1149. doi: 10.1016/j.actbio.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Hodge W.A. Fijan R.S. Carlson K.L. Burgess R.G. Harris W.H. Mann R.W. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci USA. 1986;83:2879. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rushfeldt P.D. Mann R.W. Harris W.H. Improved Techniques for Measuring In vitro the Geometry and Pressure Distribution in the Human Acetabulum .2. Instrumented Endoprosthesis Measurement of Articular Surface Pressure Distribution. J Biomech. 1981;14:315. doi: 10.1016/0021-9290(81)90041-5. [DOI] [PubMed] [Google Scholar]
- 15.Mow V.C. Ratcliffe A. Poole A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 16.Eckstein F. Reiser M. Englmeier K.H. Putz R. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging—from image to data, from data to theory. Anat Embryol. 2001;203:147. doi: 10.1007/s004290000154. [DOI] [PubMed] [Google Scholar]
- 17.Carver S.E. Heath C.A. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol Bioeng. 1999;62:166. [PubMed] [Google Scholar]
- 18.Mizuno S. Tateishi T. Ushida T. Glowacki J. Hydrostatic fluid pressure enhances matrix synthesis and accumulation by bovine chondrocytes in three-dimensional culture. J Cell Physiol. 2002;193:319. doi: 10.1002/jcp.10180. [DOI] [PubMed] [Google Scholar]
- 19.Toyoda T. Seedhom B.B. Yao J.Q. Kirkham J. Brookes S. Bonass W.A. Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis Rheum. 2003;48:2865. doi: 10.1002/art.11250. [DOI] [PubMed] [Google Scholar]
- 20.Wenger R. Hans M.G. Welter J.F. Solchaga L.A. Sheu Y.R. Malemud C.J. Hydrostatic pressure increases apoptosis in cartilage-constructs produced from human osteoarthritic chondrocytes. Front Biosci. 2006;11:1690. doi: 10.2741/1914. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura S. Arai Y. Takahashi K.A. Terauchi R. Ohashi S. Mazda O. Imanishi J. Inoue A. Tonomura H. Kubo T. Hydrostatic pressure induces apoptosis of chondrocytes cultured in alginate beads. J Orthop Res. 2006;24:733. doi: 10.1002/jor.20077. [DOI] [PubMed] [Google Scholar]
- 22.Heyland J. Wiegandt K. Goepfert C. Nagel-Heyer S. Ilinich E. Schumacher U. Portner R. Redifferentiation of chondrocytes and cartilage formation under intermittent hydrostatic pressure. Biotechnol Lett. 2006;28:1641. doi: 10.1007/s10529-006-9144-1. [DOI] [PubMed] [Google Scholar]
- 23.Sharma G. Saxena R.K. Mishra P. Differential effects of cyclic and static pressure on biochemical and morphological properties of chondrocytes from articular cartilage. Clinical Biomech. 2007;22:248. doi: 10.1016/j.clinbiomech.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Mio K. Kirkham J. Bonass W.A. Possible role of extracellular signal-regulated kinase pathway in regulation of Sox9 mRNA expression in chondrocytes under hydrostatic pressure. J Biosci Bioeng. 2007;104:506. doi: 10.1263/jbb.104.506. [DOI] [PubMed] [Google Scholar]
- 25.Gavenis K. Kremer A. Von Walter M. Hollander D.A. Schneider U. Schmidt-Rohlfing B. Effects of cyclic hydrostatic pressure on the metabolism of human osteoarthritic chondrocytes cultivated in a collagen gel. Artif Organs. 2007;31:91. doi: 10.1111/j.1525-1594.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 26.Wagner D.R. Lindsey D.P. Li K.W. Tummala P. Chandran S.E. Smith R.L. Longaker M.T. Carter D.R. Beaupre G.S. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng. 2008;36:813. doi: 10.1007/s10439-008-9448-5. [DOI] [PubMed] [Google Scholar]
- 27.Sakao K. Takahashi K.A. Arai Y. Inoue A. Tonomura H. Saito M. Yamamoto T. Kanamura N. Imanishi J. Mazda O. Kubo T. Induction of chondrogenic phenotype in synovium-derived progenitor cells by intermittent hydrostatic pressure. Osteoarthr Cartilage. 2008;16:805. doi: 10.1016/j.joca.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Candrian C. Vonwil D. Barbero A. Bonacina E. Miot S. Farhadi J. Wirz D. Dickinson S. Hollander A. Jakob M. Li Z. Alini M. Heberer M. Martin I. Engineered cartilage generated by nasal chondrocytes is responsive to physical forces resembling joint loading. Arthritis Rheum. 2008;58:197. doi: 10.1002/art.23155. [DOI] [PubMed] [Google Scholar]
- 29.Wachsmuth L. Soder S. Fan Z. Finger F. Aigner T. Immunolocalization of matrix proteins in different human cartilage subtypes. Histol Histopathol. 2006;21:477. doi: 10.14670/HH-21.477. [DOI] [PubMed] [Google Scholar]
- 30.Idrus R.B.H. Chua K.H. Shaban M. Noruddin N.A.A. Saim A.B. Tissue engineered cartilage with different human chondrocyte sources: articular, auricular and nasal septum. Med J Islam Acad Sci. 2005;15:5. [Google Scholar]
- 31.Jiang Y.Z. Zhang S.F. Qi Y.Y. Wang L.L. Ouyang H.W. Cell transplantation for articular cartilage defects: principles of past, present, and future practice. Cell transplantation for articular cartilage defects: principles of past, present, and future practice. 2011;20:593. doi: 10.3727/096368910X532738. [DOI] [PubMed] [Google Scholar]
- 32.Zheng M.H. Willers C. Kirilak L. Yates P. Xu J.K. Wood D. Shimmin A. Matrix-induced autologous chondrocyte implantation (MACI (R)): Biological and histological assessment. Tissue Eng. 2007;13:737. doi: 10.1089/ten.2006.0246. [DOI] [PubMed] [Google Scholar]
- 33.Gimble J.M. Guilak F. Bunnell B.A. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:1. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gimble J.M. Bunnell B.A. Chiu E.S. Guilak F. Concise Review: Adipose-Derived Stromal Vascular Fraction Cells and Stem Cells: Let's Not Get Lost in Translation. Stem Cells. 2011;29:749. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- 35.Schaffler A. Buchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 36.Rada T. Reis R.L. Gomes M.E. Adipose Tissue-Derived Stem Cells and Their Application in Bone and Cartilage Tissue Engineering. Tissue Eng Pt B-Rev. 2009;15 doi: 10.1089/ten.teb.2008.0423. [DOI] [PubMed] [Google Scholar]
- 37.Malafaya P.B. Pedro A.J. Peterbauer A. Gabriel C. Redl H. Reis R.L. Chitosan particles agglomerated scaffolds for cartilage and osteochondral tissue engineering approaches with adipose tissue derived stem cells. J Mater Sci Mater Med. 2005;16:1077. doi: 10.1007/s10856-005-4709-4. [DOI] [PubMed] [Google Scholar]
- 38.Mahmoudifar N. Doran P.M. Chondrogenic differentiation of human adipose-derived stem cells in polyglycolic acid mesh scaffolds under dynamic culture conditions. Biomaterials. 2010;31:3858. doi: 10.1016/j.biomaterials.2010.01.090. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa R. Mizuno S. Murphy G.F. Orgill D.P. The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2937. doi: 10.1089/ten.tea.2008.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awad H.A. Wickham M.Q. Leddy H.A. Gimble J.M. Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 41.Guilak F. Lott K.E. Awad H.A. Cao Q. Hicok K.C. Fermor B. Gimble J.M. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 42.Erickson G.R. Gimble J.M. Franklin D.M. Rice H.E. Awad H. Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 43.Crawford A. Dickson S. C. Chondrocyte isolation, expansion and culture on polymer scaffolds. In: Hollander A.P., editor; Hatton P.V., editor. Methods in Molecular Biology. Totowa, NJ: Humana Press Inc.; 2004. [DOI] [PubMed] [Google Scholar]
- 44.McIntosh K. Zvonic S. Garrett S. Mitchell J.B. Floyd Z.E. Hammill L. Kloster A. Halvorsen Y.D. Ting J.P. Storms R.W. Goh B. Kilroy G. Wu X.Y. Gimble J.M. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells. 2006;24:1246. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell J.B. McIntosh K. Zvonic S. Garretta S. Floyd Z.E. Kloster A. Di Halvorsen Y. Storms R.W. Goh B. Kilroy G. Wu X.Y. Gimble J.M. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 46.Grasdalen H. Smidsrod O. Gelation of Gellan Gum. Carbohyd Polym. 1987;7:371. [Google Scholar]
- 47.Silva N.A. Salgado A.J. Sousa R.A. Oliveira J.T. Pedro A.J. Mastronardi F. Mano J.F. Neves N.M. Sousa N. Reis R.L. Development and characterization of a novel hybrid tissue engineering based scaffold for spinal cord injury repair. Tissue Eng Part A. 2010;16:45. doi: 10.1089/ten.TEA.2008.0559. [DOI] [PubMed] [Google Scholar]
- 48.Silva-Correia J. Oliveira J.M. Caridade S.G. Oliveira J.T. Sousa R.A. Mano J.F. Reis R.L. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J Tissue Eng Regen Med. 2010;5:e97. doi: 10.1002/term.363. [DOI] [PubMed] [Google Scholar]
- 49.Silva-Correia J. Oliveira J.M. Oliveira J.T. Sousa R.A. Reis R.L. Photo-crosslinked Gellan gum-based hydrogels: methods and uses thereof. World Intellectual Property Organization (patent) WO2011/119059, Priority date: 105030 26.03.2010 PT.
- 50.Oliveira J.T. Martins L. Picciochi R. Malafaya I.B. Sousa R.A. Neves N.M. Mano J.F. Reis R.L. Gellan gum: A new biomaterial for cartilage tissue engineering applications. J Biomed Mater Res A. 2010;93A doi: 10.1002/jbm.a.32574. [DOI] [PubMed] [Google Scholar]
- 51.Oliveira J.T. Santos T.C. Martins L. Picciochi R. Marques A.P. Castro A.G. Neves N.M. Mano J.F. Reis R.L. Gellan gum injectable hydrogels for cartilage tissue engineering applications: in vitro studies and preliminary in vivo evaluation. Tissue Eng Part A. 2010;16:343. doi: 10.1089/ten.TEA.2009.0117. [DOI] [PubMed] [Google Scholar]
- 52.Oliveira J.T. Gardel L.S. Rada T. Martins L. Gomes M.E. Reis R.L. Injectable Gellan Gum Hydrogels with Autologous Cells for the Treatment of Rabbit Articular Cartilage Defects. J Orthop Res. 2010;28:1193. doi: 10.1002/jor.21114. [DOI] [PubMed] [Google Scholar]
- 53.Oliveira J.T. Sousa R.A. Reis R.L. Gellan Gum Based Hydrogels for Regenerative Medicine and Tissue Engineering Applications, Its System, and Processing Devices. World Intellectual Property Organization (patent); WO2009101518, Priority date: 103970 15.02.2008 PT. [Google Scholar]
- 54.Hoemann C.D. Molecular and biochemical assays of cartilage components. Molecular and biochemical assays of cartilage components. Methods Mol Med. 2004;101 doi: 10.1385/1-59259-821-8:127. [DOI] [PubMed] [Google Scholar]
- 55.Schulz R.M. Bader A. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur Biophys J. 2007;36:539. doi: 10.1007/s00249-007-0139-1. [DOI] [PubMed] [Google Scholar]
- 56.da Silva M.L.A. Martins A. Costa-Pinto A.R. Correlo V.M. Sol P. Bhattacharya M. Faria S. Reis R.L. Neves N.M. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J Tissue Eng Regen Med. 2011;5:722. doi: 10.1002/term.372. [DOI] [PubMed] [Google Scholar]
- 57.Goncalves A. Costa P. Rodrigues M.T. Dias I.R. Reis R.L. Gomes M.E. Effect of flow perfusion conditions in the chondrogenic differentiation of bone marrow stromal cells cultured onto starch based biodegradable scaffolds. Acta Biomater. 2011;7:1644. doi: 10.1016/j.actbio.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 58.Soltz M.A. Ateshian G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 59.Beaupre G.S. Stevens S.S. Carter D.R. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev. 2000;37:145. [PubMed] [Google Scholar]
- 60.Urban J.P. The chondrocyte: a cell under pressure. Br J Rheumatol. 1994;33:901. doi: 10.1093/rheumatology/33.10.901. [DOI] [PubMed] [Google Scholar]
- 61.Gunja N.J. Athanasiou K.A. Effects of hydrostatic pressure on leporine meniscus cell-seeded PLLA scaffolds. J Biomed Mater Res A. 2010;92A:896. doi: 10.1002/jbm.a.32451. [DOI] [PubMed] [Google Scholar]
- 62.Bouchet B.Y. Colon M. Polotsky A. Shikani A.H. Hungerford D.S. Frondoza C.G. Beta-1 integrin expression by human nasal chondrocytes in microcarrier spinner culture. J Biomed Mater Res. 2000;52:716. doi: 10.1002/1097-4636(20001215)52:4<716::aid-jbm17>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 63.Gray M.L. Pizzanelli A.M. Grodzinsky A.J. Lee R.C. Mechanical and Physicochemical Determinants of the Chondrocyte Biosynthetic Response. J Orthop Res. 1988;6 doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- 64.Shieh A.C. Athanasiou K.A. Principles of cell mechanics for cartilage tissue engineering. Ann Biomed Eng. 2003;31 doi: 10.1114/1.1535415. [DOI] [PubMed] [Google Scholar]
- 65.D'Andrea P. Calabrese A. Capozzi I. Grandolfo M. Tonon R. Vittur F. Intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. Biorheology. 2000;37 [PubMed] [Google Scholar]
- 66.Yellowley C.E. Jacobs C.R. Li Z. Zhou Z. Donahue H.J. Effects of fluid flow on intracellular calcium in bovine articular chondrocytes. Am J Physiol. 1997;273 doi: 10.1152/ajpcell.1997.273.1.C30. [DOI] [PubMed] [Google Scholar]