Abstract
Vanillin is one of the major components of vanilla, a commonly used flavoring agent and preservative and is known to exert potent antioxidant and anti-inflammatory activities. In this work, vanillin-incorporated poly(lactic-co-glycolic acid) (PLGA) films and scaffolds were fabricated to evaluate the effects of vanillin on the inflammatory responses and extracellular matrix (ECM) formation in vitro and in vivo. The incorporation of vanillin to PLGA films induced hydrophilic nature, resulting in the higher cell attachment and proliferation than the pure PLGA film. Vanillin also reduced the generation of reactive oxygen species (ROS) in cells cultured on the pure PLGA film and significantly inhibited the PLGA-induced inflammatory responses in vivo, evidenced by the reduced accumulation of inflammatory cells and thinner fibrous capsules. The effects of vanillin on the ECM formation were evaluated using annulus fibrous (AF) cell-seeded porous PLGA/vanillin scaffolds. PLGA/vanillin scaffolds elicited the more production of glycosaminoglycan and collagen than the pure PLGA scaffold, in a concentration-dependent manner. Based on the low level of inflammatory responses and enhanced ECM formation, vanillin-incorporated PLGA constructs make them promising candidates in the future biomedical applications.
Introduction
Tissue engineering is a discipline of regenerative medicine that seeks to repair and replace the functions of damaged tissues or organs through exogenous delivery of cells or the use of other biological materials.1 It has been rapidly growing as a promising strategy in regenerative medicine by employing principles and methods of engineering and life science.2–4 The ultimate goal of tissue engineering is to provide a temporary matrix to replace extracellular matrix (ECM) upon which cells can be seeded to synthesize new ECM as the temporary matrix degrades.5 Therefore, tissue engineering requires scaffolds that provide a suitable surface for cell attachment, proliferation, differentiation, and migration. In addition, the scaffolds deliver biologically active molecules, leading to the repair or replacement of damaged tissues.6 In this regard, biodegradable scaffolds are essential components in tissue engineering, and it is very critical to select the proper scaffold materials that effectively promote cell growth, proliferation, and facilitate the ECM regeneration.
Various biodegradable polymers have been extensively used in tissue engineering, which have suitable physicochemical, biological, and mechanical properties. Polymers used as tissue engineering scaffolds can be grouped into natural polymers and synthetic polymers.6 Natural polymers include collagen, hyaluronic acid, alginate, gelatin, gum, keratin, bladder submucosa, and small intestinal submucosa. They have advantages such as excellent biocompatibility and minimal inflammatory responses following implantation. However, they have insufficient mechanical properties when used individually.7 Synthetic polymers are mainly hydrophobic polyesters, including poly(lactic acid), poly(glycolic acid), poly(lactic-co-glycolic acid) (PLGA), and poly(ɛ-caprolactone). Among aliphatic polyesters, PLGA has been most widely used for biomedical applications, in particular, tissue engineering scaffolds and drug delivery systems due to excellent physicochemical properties, biocompatibility and biodegradability.8,9 PLGA has been utilized as scaffolds in various formations, including fibers, sheets, porous beads, and tubes. PLGA degrades by nonspecific hydrolytic scission of ester bonds in its backbone. The degradation period of PLGA can be controlled from weeks to over a year by varying the composition ratio and processing conditions.10 However, its applications have been hampered mainly by immunologic responses. PLGA degrades into its original monomers, lactic acid and glycolic acid, which decreases the pH in the surrounding tissues, leading to local inflammatory reaction and potential poor tissue regeneration.6,7,11,12 Moreover, implanted biomaterials induce a series of immunological reactions in response to injury caused by implantation procedures and lead to acute inflammation marked by dense infiltration of inflammatory cells at the tissue–biomaterial interface.13,14 Therefore, there have been considerable efforts to reduce the inflammatory responses of PLGA by using various biological molecules and anti-inflammatory drugs.6,10,11,15 For example, dexamethansone, a potent anti-inflammatory drug was incorporated into PLGA scaffolds to reduce the inflammatory responses and control foreign body reactions.16,17 However, dexamethasone also inhibits or downregulates endogenous vascular endothelial growth factors in a variety of cells, leading to the retardation of wound healing and tissue regeneration.13,15
Vanillin (4-hydroxy-3-methoxybenzylaldehyde) is a phenolic aldehyde compound with a low water solubility (log P of 1.21).18 It is one of the major constitutes of natural vanilla and is also one of the major therapeutic components of Gastrodia elata that has been a commonly used herbal agent for the treatment of headache and various inflammatory diseases.19 Vanillin has been widely used as a flavoring agent and preservative in food and cosmetics. Recent studies demonstrated that vanillin plays a protective role against protein oxidation and lipid peroxidation in hepatic mitochondria and also scavenges superoxide and hydroxyl radicals, indicating its potential to prevent oxidative damages in tissues.20 Vanillin has also been shown to inhibit the lipopolysaccharide (LPS)-stimulated nuclear factor kappa-B activation and cyclooxygenase-2 gene expression in murine macrophages and reduce the expression of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, interferon-γ, and tumor necrosis factor-alpha (TNF-α).18,20 Based on its potent antioxidant and anti-inflammatory activities, we hypothesized that vanillin reduces the inflammatory responses caused by PLGA-based tissue engineering scaffolds under physiological conditions.
The major objectives of this work are the evaluation of the beneficial effects of vanillin on the PLGA-induced inflammatory responses. We prepared PLGA films and porous PLGA scaffolds containing various contents of vanillin and investigated their antioxidant and anti-inflammatory activities in vitro and in vivo. In addition, annulus fibrous (AF) cell-seeded PLGA scaffolds were implanted into the back of mice and the beneficial effects of vanillin on the ECM formation were investigated.
Materials and Experiments
Preparation of PLGA/vanillin films
PLGA (molecular weight 90 kDa, 75/25 Resomor® RG755) was obtained from Boehringer Ingelheim Pharma KG. Vanillin was obtained from Sigma-Aldrich and was used without further purification. PLGA/vanillin films were prepared using a solvent evaporation method. PLGA was dissolved in dichloromethane (Samchun Pure Chemicals), to which a various amount of vanillin was added. The solution was thoroughly mixed by mechanical stirring and slowly poured into a glass dish (30 mm in diameter), followed by drying at room temperature to cast a film. Films were further dried in a vacuum oven at room temperature for 1 week and sterilized using an aqueous solution of ethanol (70%) for 2 h.
Preparation of porous PLGA/vanillin scaffolds
Porous scaffolds were prepared by a conventional salt-reaching method.21 In brief, 1 g of a mixture of PLGA and vanillin with various vanillin content (1, 5, and 10 wt%) was dissolved in 4 mL of dichloromethane. NaCl (9 g) was added to the solution and the mixture was mixed thoroughly by mechanical stirring. The mixture of PLGA, vanillin, and salt was poured in a mold (7-mm diameter, 3-mm thickness) and allowed to dry overnight at room temperature. The resulting cast of dried mixture was removed and placed into a vacuum oven for 24 h to remove remaining dichloromethane. The mixture was soaked in deionized water for 2 days to allow NaCl to leach out and then freeze-dried overnight at −80°C. The prepared PLGA/vanillin scaffolds were sterilized with 70% ethanol.
Cell culture
RAW 264.7 (the mouse leukemic monocyte macrophage cell line) was obtained from the Korean Cell Line Bank (KCLB). The cells were cultured in the Dulbecco's modified Eagle's medium (DMEM, high glucose, Gibco®; Invitrogen) containing 10% (v/v) fetal bovine serum (FBS, Gibco), and 1% antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin (Invitrogen). AF cells were obtained from 4-week-old New Zealand White rabbits and were cultured in the DMEM including 10% (v/v) FBS and 1% (v/v) antibiotics. These cells were grown in an incubator with 5% CO2 at 37°C. The medium was replaced with a fresh medium every 3 days throughout the experiment.
Contact angle measurement
To test the wetting properties of PLGA/vanillin films (10×10 mm), the water contact angle was measured. A droplet of deionized water (10 μL) was deposited on the surface of films and the angle formed between the liquid/solid interface and the liquid/vapor interface was measured using a contact angle goniometer (CAM-PLUS micro).
Scanning electron microscopy
PLGA/vanillin films were frozen in liquid nitrogen and fractured by mechanical force. The fractured surface was mounted on a metal stub and sputter-coated with Au-Pd. The morphology of the fractured surface of PLGA/vanillin films was observed using a scanning electron microscope (SEM; S-2250N, Hitachi) at 15 kV.
Release kinetics of vanillin from the PLGA/vanillin films
To investigate the release behaviors of vanillin from the PLGA/vanillin (5%) films, the prepared films (100 mg) were placed in a test tube containing 5 mL of phosphate-buffered saline (PBS, Takara Bio, Inc.) at 37°C. At appropriate time intervals (5, 10, 15, 20, 25, and 30 min), 500 μL of the supernatant was removed and replaced with the same amount of fresh PBS. Vanillin released from PLGA/vanillin films was analyzed using a high performance liquid chromatography system (Futecs), equipped with a P1000 solvent pump unit and an UV1000 UV–visible detector operating at 254 nm.
Cell viability assay
PLGA/vanillin films were cut into a circle with a diameter of 1.5 cm and placed on the bottom of wells of 24-well culture plates. The fibroblasts (NIH 3T3) were seeded on the films at a density of 1×105 cells/well (n=3). After cell seeding on the film in a well, cells were cultured for 1 and 3 days. Cell viability was analyzed using a modified 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium-bromide (MTT; Sigma-Aldrich) assay. Cells were treated with 100 μL of MTT solution (5 mg/mL stock in PBS) and incubated at 37°C. After 4 h, intense violet-colored formular was observed in the wells. The cells-seed filmsamples were then transferred into a test tube and dissolved in 1 mL of dimethyl sulfoxide. The absorbance at 570 nm was measured using a microplate reader (E-Max, Molecular Device Co.). The cell viability was determined by comparing the absorbance of cells cultured on PLGA/vanillin films and cells on culture plates.
Attachment of cells on PLGA/vanillin films
To study the cell attachment and morphology of fibroblasts on PLGA/vanillin films, cells were observed using a scanning electron microscope. The PLGA/vanillin films were cut into a circle with a diameter of 1.5 cm and placed on the bottom of the 24-well plate. Fibroblasts were seeded on the films at a density of 1×105 and cultured for 3 days. The cell-seeded films in 24-well plates (Falcon®) were removed and washed with PBS. The cells attached to the film were fixed 2.5% glutaraldehyde (Sigma-Aldrich) in PBS at room temperature for 24 h and these cells were dehydrated in ethanol-graded series (50%, 60%, 70%, 80%, 90%, and 100%) for 30 min each and allowed to dry on a clean bench. The films containing cells were mounted on a conductive metal stuband coated with platinum using a plasma sputter (Emscope SC500K) under argon gas and examined using SEM.
Detection of reactive oxygen species produced in cells
The level of intracellular reactive oxygen species (ROS) generated in cells was determined by measuring dichlorofluorescein (DCF) fluorescence (Ex. 450 nm, Ex. 520 nm). Briefly, the RAW 264.7 cells were seeded on the PLGA/vanillin films in a culture plate and cultured for 2 days. Cells cultured without PLGA/vanillin films and cells treated with LPS (Sigma-Aldrich) were served as controls. Dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma-Aldrich) was used to monitor the level of intracellular ROS production. DCFH-DA (2 μL of 20 μM) was added to each well. After 30 min of incubation at 37°C with 5% CO2, cells were harvested and analyzed with 10,000 events per sample by using a flow cytometry (FACS caliber; Benton-Dickson).
Inflammatory responses of macrophages to PLGA/vanillin films
TNF-α released from the cells was measured using a TNF-α ELISA set (BD optELA™) according to the manufacturer's protocol. RAW 264.7 cells were cultured on the PLGA/vanillin films in 24-well plates and cultured. Untreated cells and LPS-treated cells were served as controls. The culture medium was collected on days 1, 2, and 3 and centrifuged at 9,800 g for 15 min. The supernatants were obtained and their absorbance was measured at 450 nm using a microplate reader (E-max, Molecular Device). The content of TNF-α released was measured using a standard curve from the standard kit.
In vivo host tissue responses
Wister rats (∼200 g, Orient Animal) were anesthetized by an intramuscular injection of a mixture (150 μL) of Zoletil 50 and Domitor (the ratio of 2:1). PLGA/vanillin films (6×6 mm) sterilized with 70% of ethanol were implanted into the subcutaneous dorsum of rats. For histological examination, animals were euthanized and implanted films were extracted from the tissue at 1, 3, or 6 weeks postimplantation. The extracted tissues surrounding films were fixed in 10% formalin (Sigma-Aldrich) and fabricated in paraffin blocks. The specimens were embedded in paraffin and sectioned a microtome (Thermo Scientific) to a 4 μm thickness. The sections were stained using protocols for staining with hematoxylin and eosin (H&E) and ED-1. All experiment procedures were performed with the approval of the Chonbuk National University Animal Care Committee, Jeonju, Korea.
Animal study and immunohistological and biochemical assays
A volume of 15 μL of AF cell suspensions containing 1.5×105 cells were seeded on the top surface of PLGA/vanillin scaffolds in the DMEM containing 10% (v/v) FBS and then incubated for 10 days in 24-well plates. The cell culture medium was changed with a fresh medium every 2 days. The cell-scaffold constructs were implanted in subcutaneous tissues on the dorsal site of eight nude mice (Orient Animal). Mice were sacrificed at 1, 3, or 6 weeks after implantation, and the cell-scaffold constructs were harvested. The constructs were fixed in 4% paraformaldehyde for 12 h, paraffin embedded, sectioned, and prepared for H&E staining and immunohistochemistry (Safranin-O and collagen type I), as previously reported.22 For biochemical assays, the constructs were lyophilized and enzymatically digested with papain solution (125 μg/mL. 10 mM L-cystein, 100 mM Na2HPO4, 5 mM EDTA at pH 6.8) for 16 h at 60°C as previously reported.23 The glycosaminoglycan (GAG) content was determined using a dimethylmethylene blue (DMMB) spectrometric assay (Sigma-Aldrich) at 490 nm. The collagen content was determined by the spectroscopic assay at 570 nm after the incubation for 30 min with chloramines-T and Enrlich's aldehyde solution.
Statistical analysis
All descriptive statistical parameters such as mean values, medians, and standard deviations for the calculated parameters were generated with MS Excel 2007. The student's t-test was used to test for significant difference between two test groups. All data are given as mean value±standard error, if not otherwise indicated.
Results
Physicochemical properties of PLGA/vanillin films
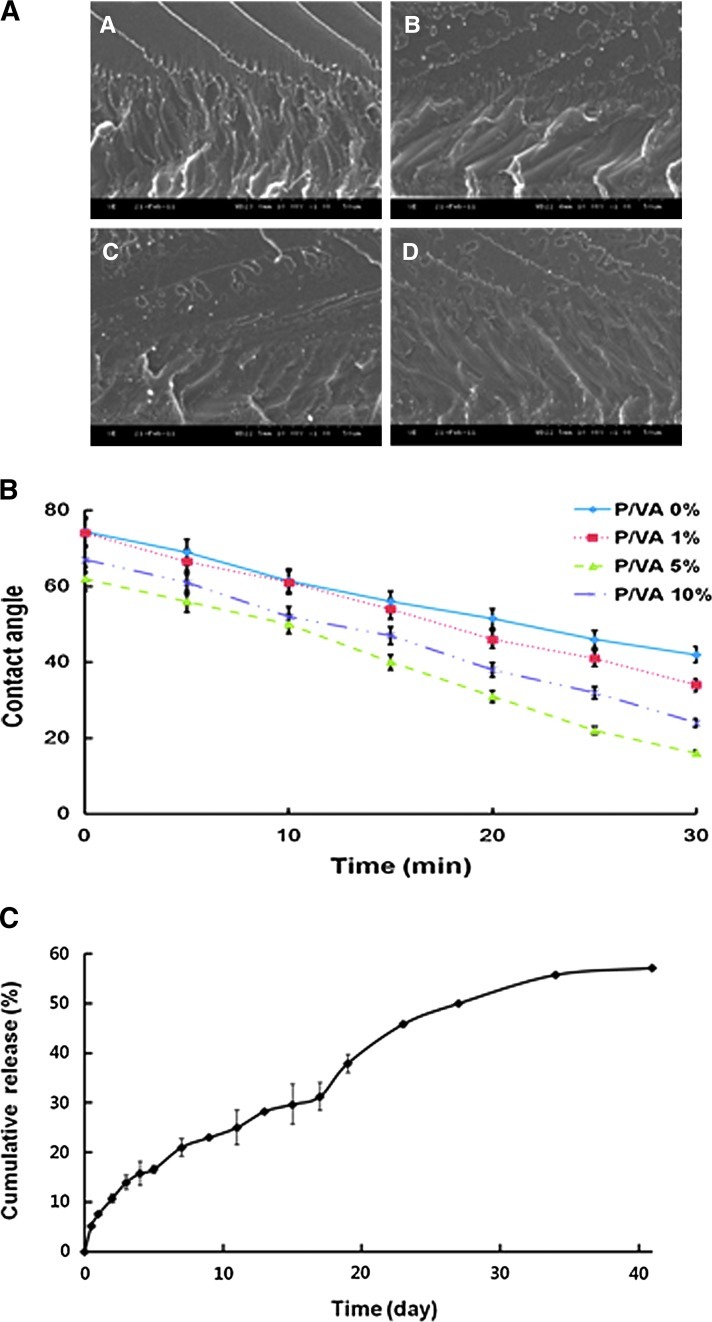
The morphology of PLGA/vanillin films was observed to investigate the miscibility of the blend films. As shown in Figure 1A, vanillin was evenly distributed in the matrix of PLGA, with less or no phase separation. The high miscibility of vanillin with PLGA may be attributed to the presence of aldehyde and phenol groups that can form hydrogen bonds with ester bonds in the backbone of PLGA.24,25 We next examined the wetting properties of PLGA/vanillin films by monitoring the change of water contact angles on their surface. Figure 1B shows that the contact angle of water on the film surface decreased with time. The contact angle decreased with increasing the content of vanillin, indicating that vanillin possessing aldehyde and phenol groups provided the films with hydrophilic nature. Vanillin is admixed with PLGA in the films and could be released during the hydrolytic degradation of PLGA. To investigate the release kinetics of vanillin from the PLGA/vanillin films, the films were incubated in phosphate buffer solution (pH 7.4) at 37°C. Figure 1C shows the release kinetics of vanillin from the PLGA/vanillin (5 wt%). Vanillin was released continuously from the film over the experimental period of 40 days as PLGA undergoes hydrolytic degradation. Half of the vanillin incorporated in the film was released at ∼24 days after incubation.
FIG. 1.
Characterization of poly(lactic-co-glycolic acid) (PLGA)/vanillin films with various concentrations of vanillin. (A) Representative scanning electron microscope (SEM) images of the fractured surface of PLGA/vanillin films (A: 0 wt%; B: 1 wt%; C: 5 wt%; D: 10 wt%). (B) The contact angle of water deposited on the surface of PLGA/vanillin (P/VA) films as a function of time. (C) The release kinetics of vanillin from the PLGA/vanillin (5 wt%) film at 37°C. Data presented are means±SD from three different experiments. Color images available online at www.liebertpub.com/tea
Viability and proliferation of cells on the PLGA/vanillin films
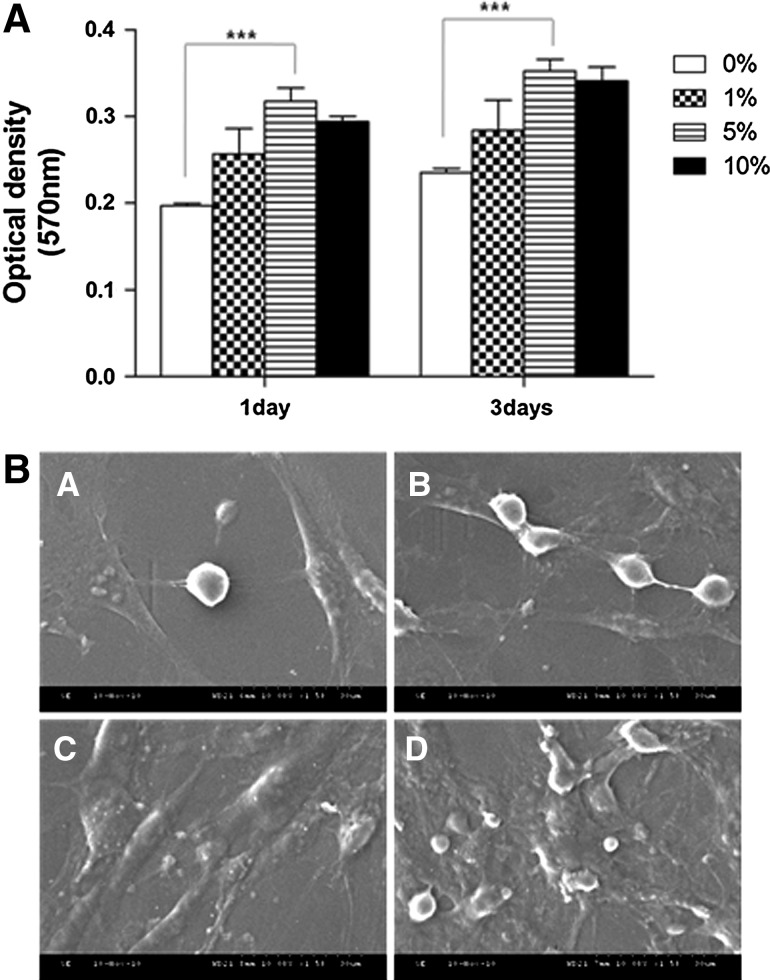
As shown in Figure 2A, the viability was dependent on the vanillin content and increased with time. PLGA/vanillin (5 wt%) films showed significantly higher cell viability than the PLGA film (0 wt%). However, further increase in the vanillin content (10 wt%) did not enhance the cell viability. Scanning electron microscopy was performed to observe the morphology of cells cultured on PLGA/vanillin films for 3 days. Cells cultured on PLGA films with or without vanillin had spread out and a flat appearance with dendritic extensions, which is typical morphology of fibroblast.26 However, PLGA/vanillin films showed more cell attachment than the pure PLGA film (Fig. 2B), as expected from the result of the MTT assay. We also found that films with higher vanillin contents exhibited the more cell-spreading patterns with more cell–biomaterial interactions and cell–cell adhesion. The results suggest that vanillin-incorporated PLGA films provide better environments for cell proliferation and growth.
FIG. 2.
Cell growth and proliferation on PLGA/vanillin films. (A) The cell viability assay at days 1 and 3. Data presented are means±SD from three different experiments. ***P<0.001. (B) Representative SEM images of cells cultured on PLGA/vanillin films (A: 0 wt%; B: 1 wt%; C: 5 wt%; D: 10 wt%).
Antioxidant and anti-inflammatory activities of PLGA/vanillin films
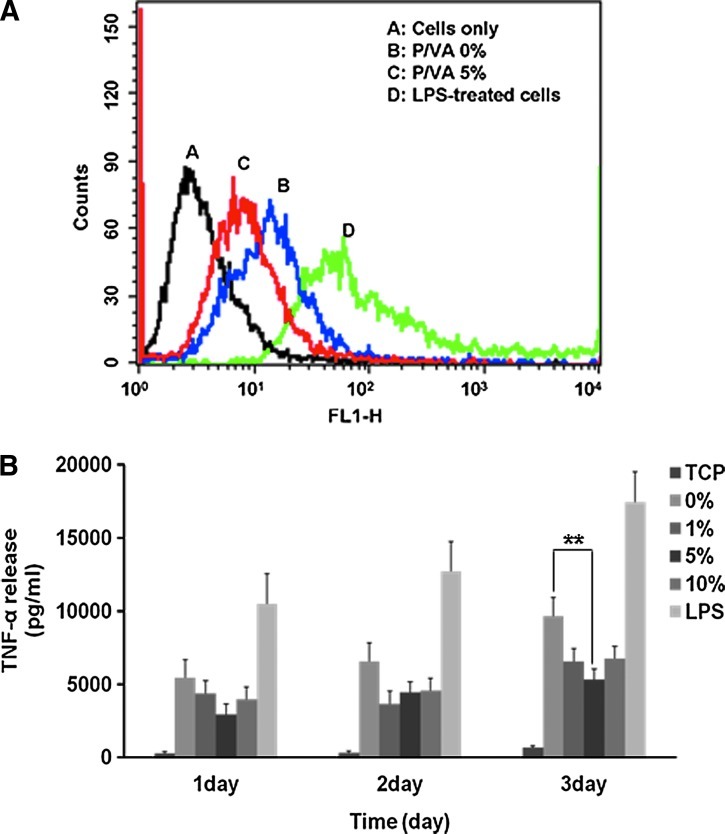
Cells cultured on pure PLGA and PLGA/vanillin films were treated with DCFH-DA and the relative extent of ROS generation was measured by flow cytometry (Fig. 3A). The flow cytometry result in Figure 3A showed that the fluorescence of cells cultured on the PLGA/vanillin (5 wt%) film is less than that of the PLGA/vanillin (0 wt%) film. Cells grown on the tissue culture plate (TCP) and LPS-treated cells on TCP served as controls. Cells grown on TCP showed a minimal ROS generation, evidenced by a weak DCF-fluorescence (line A in Fig. 3A). Cells cultured on the pure PLGA film (line B, P/VA 0 wt%) exhibited a remarkable shift of DCF fluorescence to the right direction, demonstrating that they were stimulated to generate ROS such as hydrogen peroxide. They generated ROS remarkably less than the LPS-treated group, but remarkably more than those cultured on the PLGA/vanillin (5 wt%) film, suggesting that vanillin incorporated in the film exerted a potent antioxidant activity.
FIG. 3.
Antioxidant and anti-inflammatory activity of PLGA/vanillin films. (A) The representative flow cytometry analysis showing the effects of PLGA/vanillin (P/VA) films on the generation of reactive oxygen species. (B) The effects of vanillin on the production of tumor necrosis factor-alpha (TNF-α) in RAW 264.7 cells. **P<0.01. Values shown are means±SD for quadruplicate cultures. Color images available online at www.liebertpub.com/tea
We also measured the level of TNF-α production to investigate the effects of vanillin on inflammatory responses of macrophages to PLGA films. As shown in Figure 3B, cells cultured on pure PLGA films generated a significantly higher level of TNF-α than those cultured on TCP, but remarkably lower than the LPS-treated group in a short-term culture for 3 days. However, cells grown on PLGA/vanillin (5 wt%) films showed a lower TNF-α generation than those on the pure PLGA film. PLGA/vanillin (5 wt%) films elicited significantly less TNF-α generation than those on pure PLGA films at a time point of 3 days.
Host tissue responses to PLGA/vanillin films
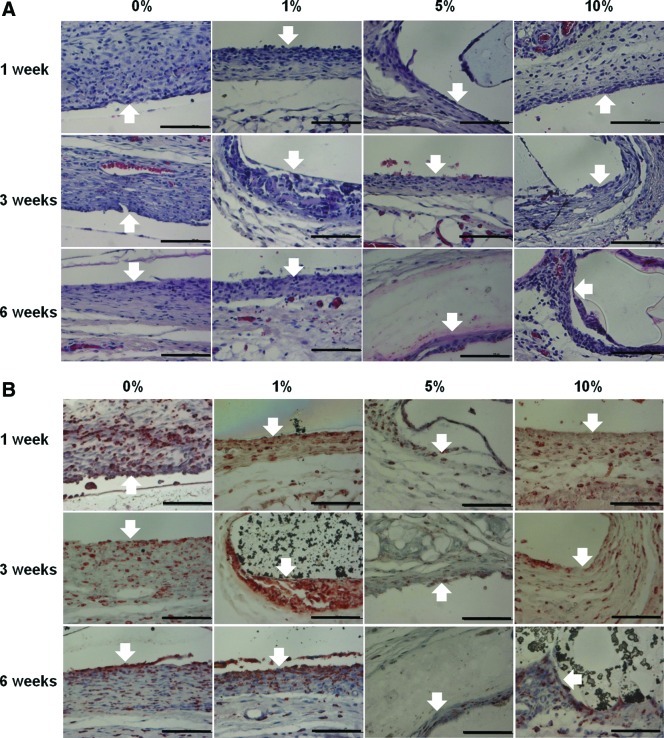
As shown in Figure 4A, severe granulomatous inflammatory responses were observed on the surface of pure PLGA films, evidenced by the accumulation of macrophages and multinucleated giant cells. There was a dense accumulation of inflammatory cells at the interface of tissues and PLGA films, with a thick wall of granulomatous infiltration and fibrosis after 1 week of implantation. Although, the numbers of inflammatory cells and the thickness of the wall of granulomatous infiltration were reduced with time, there was still a dense accumulation of inflammatory cells at the interface at 6 week postimplantation. However, the incorporation of vanillin appeared to coincide with reduction of the severity of the inflammatory responses, as supported by the less accumulation of inflammatory cells and the thinner wall of the granulomatous response. In particular, the severity of infiltration of inflammatory cells and the thickness of the granulomatous wall were the most reduced at the interface with the PLGA/vanillin (5 wt%) film after 6 weeks of implantation.
FIG. 4.
Histological studies of tissues neighboring the PLGA/vanillin films with a various content of vanillin after 1, 3, and 6 weeks of implantation. The arrows indicate the interface between the film and tissue. (A) Hematoxylin and eosin (H&E) stained section of tissues. (B) ED-1 stained section of tissues. All images are ×400 magnification (scale bars=100 μm). Color images available online at www.liebertpub.com/tea
Figure 4B shows the histological sections of tissues stained with a macrophage marker ED-127 Marked and dense infiltration of ED-1 positive cells was observed in the wall of granulomatous reaction at the interface with the PLGA film at 1 week and the numbers of ED-1 positive cells were reduced at 3 and even more at 6 weeks postimplant. PLGA/vanillin films were surrounded by a thinner wall with reduced numbers of ED-1 positive cells than the PLGA film. In particular, the numbers of ED-1 positive cells were markedly reduced at the interface with the PLGA/vanillin (5 wt%) film at 3 and 6 weeks of implantation. The aforementioned observations provide support to the notion that vanillin plays an important role in reducing PLGA-induced acute inflammatory responses in the subcutaneous tissue of the rat.
Culture of AF cells on porous PLGA/vanillin scaffolds
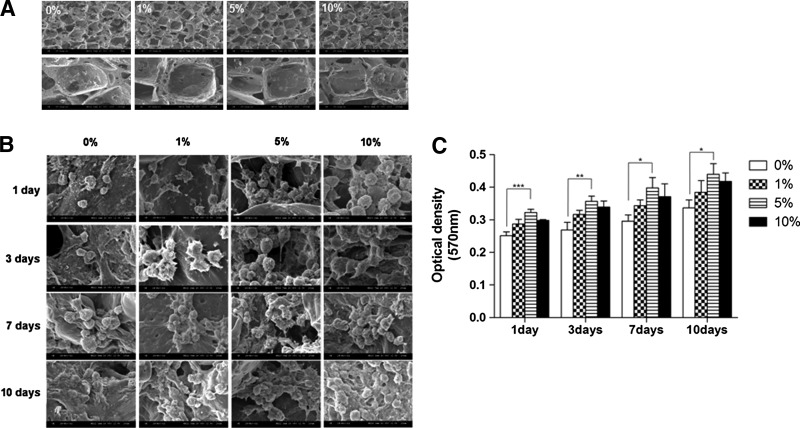
Porous PLGA/vanillin scaffolds were prepared by a salt leaching method. The morphology of PLGA/vanillin scaffolds are shown in Figure 5A. The incorporation of vanillin shows no influence on the fabrication and morphology of scaffolds. Cells were observed not only on the surface of scaffolds, but also inside of the scaffolds (Fig. 5B). The number of cells attached to and proliferated in scaffolds increased with time and there appeared to be more cells with vanillin-incorporated PLGA scaffolds. Cells spread and stretched into the inter-connected pores of scaffolds, indicating the cells were viable and functioning normally.
FIG. 5.
Cell culture in porous PLGA/vanillin scaffolds. (A) Representative SEM images of porous PLGA and PLGA/vanillin scaffolds (top panel: ×50 magnification, bottom panel: ×200 magnification). (B) Representative SEM images of annulus fibrous (AF) cell-seeded PLGA and PLGA/vanillin scaffolds. (C) The cell viability assay of cell-seeded scaffolds. *P<0.05, **P<0.01, ***P<0.001. Values shown are means±S.D (n=3).
The cell proliferation in the scaffolds was assessed by measuring the cell viability by the MTT assay. As shown in Figure 5C, in general, the cell viability in the pure PLGA scaffold increased with time for a period of 1, 3, 7, and 10 days. Vanillin-incorporated PLGA scaffolds showed a higher cell proliferation than the pure PLGA scaffold. In comparison with the pure PLGA scaffold, a significantly higher cell proliferation was observed with the PLGA/vanillin (5 wt%) scaffold for 3 days of culture.
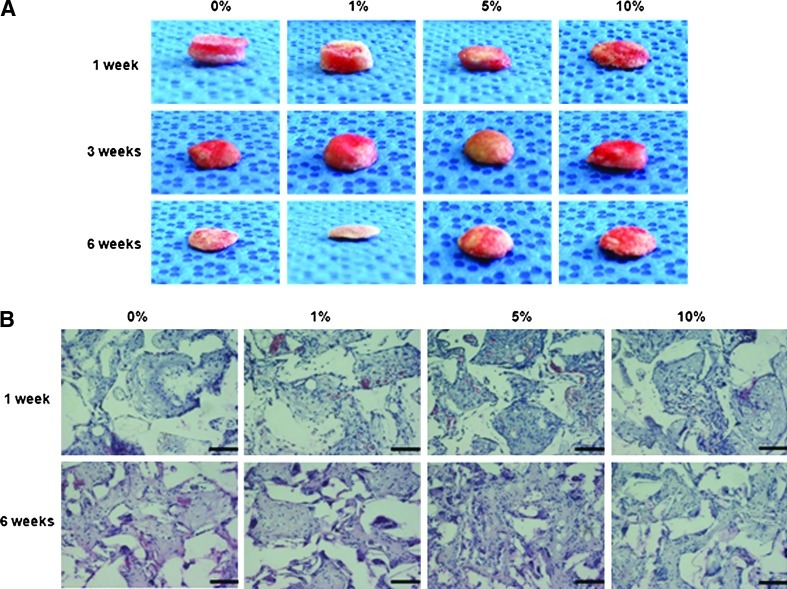
To further investigate the cell proliferation in PLGA/vanillin scaffolds in vivo, AF cell-seeded PLGA/vanillin scaffolds were implanted into a mouse. After 1 and 6 weeks of implantation, the cell-scaffold constructs were harvested and their histology was evaluated microscopically. Figure 6A shows the gross photographs of AF cell-scaffold constructs after 1, 3, and 6 weeks of implantation. After 1 week of implantation, cell-scaffold constructs with pure PLGA and PLGA/vanillin (1 wt%) scaffolds were thicker than scaffolds with 5 and 10 wt% vanillin. However, the cell-scaffold constructs with pure PLGA and PLGA/vanillin (1 wt%) showed a higher reduction in thickness than those containing more than PLGA/vanillin (5 wt%) by 6 weeks time point. PLGA/vanillin (5 wt%) showed the highest thickness after 6 weeks of implantation. As shown in Figure 6B, H&E staining of the cell-scaffold constructs after week 1 and 6 clearly indicated cell proliferation, confirming the data obtained from SEMimages. PLGA/vanillin scaffolds showed a higher cell proliferation than the pure PLGA scaffold, with the highest cell density on scaffolds containing 5 wt% vanillin at 6 weeks.
FIG. 6.
Images of AF cell-seeded PLGA/vanillin scaffolds implanted in a mouse. (A) Gross photographs and (B) H&E staining of cell-scaffold constructs. All images are ×200 magnification (scale bars=50 μm). Color images available online at www.liebertpub.com/tea
ECM formation in AF cell-seeded PLGA/vanillin scaffolds in vivo
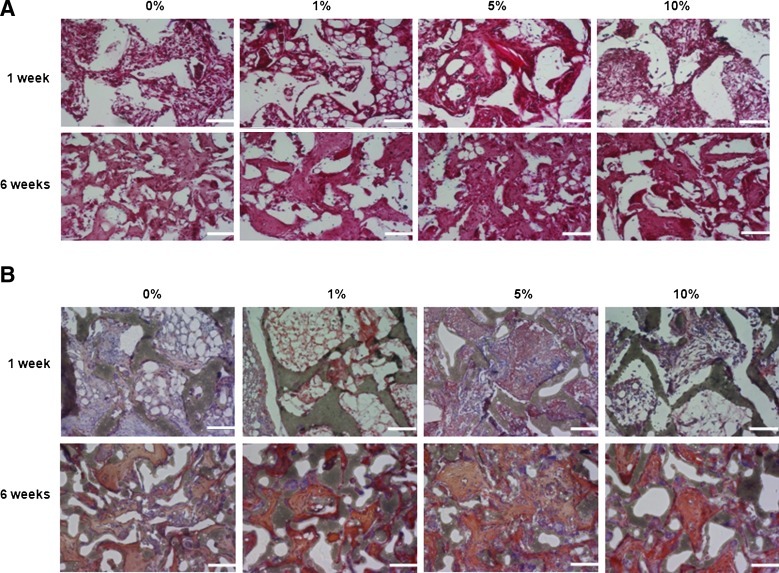
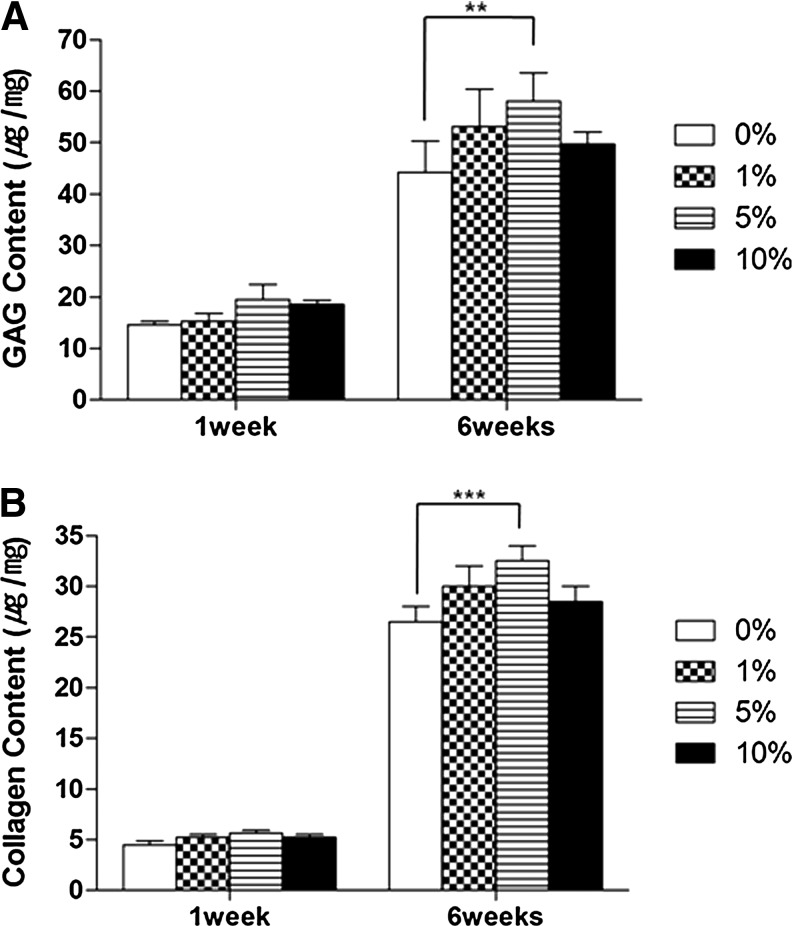
To determine if AF cells grown in PLGA/vanillin scaffolds form tissues, the generation of ECM was assessed by measuring the production of GAG and type I collagen (Fig. 7A, B, respectively). In Figure 7A, Safranin-O staining showed the matrix production by AF cells within the inter-connected pores of scaffolds. Areas of larger and more intense Safranin-O staining were observed with scaffolds containing vanillin. In addition, more intense Safranin-O staining was observed after 6 weeks compared to 1 week of implantation. A similar trend was observed in Figure 7B for collagen type I production for AF-cell-seeded scaffolds. The amounts of GAG and collagen type I accumulated in scaffolds were determined to further confirm the ECM formation. As expected from Figure 7, a marked accumulation of cartilage-like matrix with strong Safranin-O and type I collagen staining was observed inside and scaffolds as shown in Figure 8. The production of both GAG and type I collagen production increased with time and their more production was observed with vanillin-incorporated PLGA scaffolds. PLGA/vanillin (5 wt%) scaffolds allowed the most GAG and collagen I production.
FIG. 7.
Histological studies of AF cell-seeded PLGA/vanillin scaffolds after 1 and 6 weeks of implantation. Safranin-O (A) and collagen I (B) stained section of tissues. All images are ×200 magnification (scale bars=100 μm). Color images available online at www.liebertpub.com/tea
FIG. 8.
GAG and collagen type I production in AF cell-seeded PLGA/vanillin scaffolds. (A) GAG production as determined by a dimethylmethylene blue assay, (B) Collagen type I production determined by using chloramines-T and Enrlich's aldehyde solution. **P<0.05, ***P<0.001. Values shown are means±SD (n=3).
Discussion
Vanillin is considered generally regarded as safe and has excellent biocompatibility with oral LD50 of 1.58∼2.8 g/kg.28,29 A large body of evidence suggests that vanillin exhibits potent antioxidant, anti-inflammatory, antimutagenic, and antifungal properties.28,30 However, to our best knowledge, there have been no studies to explore the anti-inflammatory activity of vanillin in tissue engineering applications. Therefore, this study was aimed at assessing the effects of vanillin on the PLGA-induced inflammatory responses and ECM formation in vivo as well as in vitro. We prepared PLGA/vanillin films with various compositions to determine the conditions for optimal physical and biological properties as well as processability. After careful consideration on the miscibility and compressive strength, we developed PLGA/vanillin films that contained up to 10 wt% of vanillin. A solvent evaporation method generated strong and flexible PLGA/vanillin films and the film formability of PLGA was not altered by the incorporation of vanillin <10 wt%. After admixing with PLGA, vanillin was evenly distributed in PLGA films with no or less phase separation due to the hydrogen bonds between its aldehyde or alcohol groups and ester groups of PLGA. Vanillin incorporated in the films is responsible for the increased hydrophilicity of films, which was evidenced by the change of the water contact angle with time. The larger reduction of the contact angle with PLGA/vanillin films than the pure film suggests that the vanillin facilitates wetting and water adsorption on the film. It has been reported that the hydrophilic surface of substances has negative free energy of adhesion and show high potential to support cell attachment and proliferation.7 Therefore, the increased hydrophilic surface of the PLGA/vanillin films accounts for their more cell–biomaterials interactions and higher cell attachment than the PLGA film. The more favorable environment for cell growth and proliferation induced by vanillin can be further supported by the higher cell proliferation in the PLGA/vanillin scaffolds (Fig. 5B).
ROS are known to play essential roles in the expression of genes associated with inflammatory responses.31,32 The immune system is known to determine the course of potential inflammatory responses to foreign substances in a host tissue and their potential activation of the immune system is one of critical issues that dictate the successful application of biomaterials.33,34 A large amount of ROS produced by activated macrophages is a mediator of tissue damages and inflammatory responses. In addition, TNF-α is one of proinflammatory cytokines involving the systemic inflammation and is known to be produced by stimulated macrophages.11,34,35 Therefore, ROS and TNF-α may serve as an excellent indicator of macrophage activation during inflammatory responses.7 In this regard, we investigated the level of ROS and TNF-α on cells cultured on PLGA/vanillin films to explore their potential for biomedical applications. The effects of vanillin on the generation of ROS in macrophages were studied using dichlorofluorescien-diacetate (DCFH-DA) as an ROS probe. DCFH-DA has been widely used to probe intracellular ROS including hydrogen peroxide, because it reacts with ROS to become an oxidized DCF, which can emit strong fluorescence.36–38 As shown in Figure 3, the pure PLGA film generated a large amount of ROS and TNF-α due to transitory stimulation of mitochondrial activity and oxidative stress.30,32 However, the incorporation of vanillin significantly reduced the PLGA-induced oxidative stress and inflammatory responses. The observations suggest that antioxidant vanillin reduces the inflammatory responses of macrophages to PLGA films and may play beneficial roles in tissue engineering scaffolds.
We also performed the histological studies to investigate the effects of vanillin on host tissue responses to PLGA films for 1, 3, and 6 weeks (Fig. 4). The tissues surrounding the pure PLGA film were separated from the implant by a wall of granulomatous reaction that in time became a fibrous capsule, suggesting prominent host tissue responses. PLGA/vanillin films, however, were surrounded by a wall of granulomatous reaction that was markedly reduced in thickness and had fewer inflammatory cells at all time points studied. As expected from the slow and sustained release profiles (Fig. 1C), the reduced inflammatory responses of host tissues to PLGA/vanillin films may be attributed to vanillin, which was expected to be continuously released from films to inhibit the generation of proinflammatory mediators such as TNF-α and ROS. The apparent potent antioxidant and anti-inflammatory activity of vanillin is in good agreement with the previous studies.39,40
AF is a fibrous ring forming the outer layer of the intervertebral disc and consists of ECM composed of both type I and type II collagen.41,42 In this study, AF cells were used as a model cell to investigate the effects of vanillin on the cell attachment, proliferation, and ECM production in porous PLGA/vanillin scaffolds. AF cells were proliferating on the initial seeding site on the top of the surface of the scaffold as well as penetrating into the internal pore structures. AF cells proliferated much faster on the vanillin-incorporated PLGA scaffolds over 10 days of culture, suggesting that PLGA/vanillin scaffolds provide better environment for the growth and proliferation of AF cells than pure PLGA scaffolds and allow a high-density three-dimensional culture of cells. In particular, PLGA/vanillin (5 wt%) scaffolds induced the largest cell proliferation. To further study the effects of vanillin on ECM formation by AF cells, we examined the level of newly synthesized GAG and type I collagen after 1 and 6 weeks of implantation. As reported in Figures 7 and 8, AF cells seeded in PLGA/vanillin scaffolds (5 wt%) produced 5-fold more GAG and collagen than pure PLGA scaffolds. The results also demonstrate that AF cells growing in the inter-connected pores of PLGA/vanillin scaffolds maintained their phenotype in the constructs. The largest ECM production was observed with 5 wt% vanillin-incorporated PLGA scaffolds, which is in a good agreement with Figure 5B. The finding is also in good agreement with the previous studies reporting that the number of cells is known to play an essential role in the production of ECM.43 The highest level of cell proliferation and largest collagen and GAG production with PLGA/vanillin (5 wt%) scaffolds could be also supported by the thickness of the cell-seeded scaffolds. As shown in Figure 6A, PLGA/vanillin (5 wt%) scaffolds exhibited the highest thickness after 6 weeks of implantation. Overall, our results suggest that vanillin significantly reduces the PLGA-induced oxidative stress and inflammatory responses and promotes the ECM production.
Conclusions
We developed PLGA films and porous scaffolds containing various contents of vanillin to investigate the effects of vanillin on the PLGA-induced inflammatory responses and ECM production. Vanillin was evenly distributed in the PLGA matrix and increased the hydrophilicity of the films, leading to the enhanced cell attachment, growth, and proliferation. Vanillin incorporated in the films was released in a slow and continuous manner and exerted potent antioxidant and anti-inflammatory activities, evidenced by the reduced generation of ROS, reduced inflammatory cell recruitment and thin fibrous capsules. AF cells seeded in the porous PLGA/vanillin scaffolds showed more growth and proliferation than the pure PLGA scaffold. In addition, PLGA/vanillin scaffolds facilities the more production of GAG and collagen than the pure PLGA scaffold, in a concentration-dependent manner. Given their highly potent antioxidant and anti-inflammatory activities and enhanced ECM production, we anticipate that PLGA/vanillin hybrids are a promising candidate as a tissue engineering scaffold.
Acknowledgments
This study was supported by the grant from the National Research Foundation (2010-0021903), the Stem Cell Research Center of the 21st Century Frontier Research Program (SC4140), the World Class University program (R31-20029) funded by the Ministry of Education, Science and Technology and the Musculoskeletal Bio-organ Center from the Korea Ministry of Health and Welfare (A040003), Republic of Korea.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lee J.H. Park S.J. Chun H.J. Kim C.H. Differentiation regulation by microenvironmental interaction control between stem cell and extracellular matrix. Int J Tissue Regen. 2010;1:1. [Google Scholar]
- 2.Zhang X.H. Reagan M.R. Kaplan D.L. Electrospun silk biomaterial scaffolds for regenerative medicine. Adv Drug Deliv Rev. 2009;61:988. doi: 10.1016/j.addr.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim C.H. Khil M.S. Kim H.Y. Lee H.U. Jahng K.Y. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J Biomed Mater Res Part B Appl Biomater. 2006;78B:283. doi: 10.1002/jbm.b.30484. [DOI] [PubMed] [Google Scholar]
- 4.Cao W.L. Wang A.J. Jing D.H. Gong Y.D. Zhao N.M. Zhang X.F. Novel biodegradable films and scaffolds of chitosan blended with poly(3-hydroxybutyrate) J Biomater Sci Polym Ed. 2005;16:1379. doi: 10.1163/156856205774472308. [DOI] [PubMed] [Google Scholar]
- 5.Muschler G.E. Nakamoto C. Griffith L.G. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am Vol. 2004;86A:1541. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Kim M.S. Ahn H.H. Shin Y.N. Cho M.H. Khang G. Lee H.B. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials. 2007;28:5137. doi: 10.1016/j.biomaterials.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Song Y. Kwon J. Kim B. Jeon Y. Khang G. Lee D. Physicobiological properties and biocompatibility of biodegradable poly(oxalate-co-oxamide) J Biomed Mater Res Part A. 2011;98A:517. doi: 10.1002/jbm.a.33135. [DOI] [PubMed] [Google Scholar]
- 8.Kim S. Seong K. Kim O. Seo H. Lee M. Khang G. Lee D. Polyoxalate nanoparticles as a biodegradable and biocompatible drug delivery vehicle. Biomacromolecules. 2010;11:555. doi: 10.1021/bm901409k. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W. Shi Y.A. Chen Y.Z. Ye J.A. Sha X.Y. Fang X.L. Multifunctional Pluronic P123/F127 mixed polymeric micelles loaded with paclitaxel for the treatment of multidrug resistant tumors. Biomaterials. 2011;32:2894. doi: 10.1016/j.biomaterials.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki Y. Sawada S. Ishihara K. Khang G. Lee H.B. Reduction of surface-induced inflammatory reaction on PLGA/MPC polymer blend. Biomaterials. 2002;23:3897. doi: 10.1016/s0142-9612(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 11.Yoon S.J. Kim S.H. Ha H.J. Ko Y.K. So J.W. Kim M.S. Il Yang Y. Khang G. Rhee J.M. Lee H.B. Reduction of inflammatory reaction of poly(D,L-lactic-Co-glycolic acid) using demineralized bone particles. Tissue Eng Part A. 2008;14:539. doi: 10.1089/tea.2007.0129. [DOI] [PubMed] [Google Scholar]
- 12.Ho M.L. Fu Y.C. Wang G.J. Chen H.T. Chang J.K. Tsai T.H. Wang C.K. Controlled release carrier of BSA made by W/O/W emulsion method containing PLGA and hydroxyapatite. J Control Release. 2008;128:142. doi: 10.1016/j.jconrel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Patil S.D. Papadmitrakopoulos F. Burgess D.J. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117:68. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Kose G.T. Korkusuz F. Korkusuz P. Hasirci V. In vivo tissue engineering of bone using poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) and collagen scaffolds. Tissue Eng. 2004;10:1234. doi: 10.1089/ten.2004.10.1234. [DOI] [PubMed] [Google Scholar]
- 15.Thevenot P.T. Nair A.M. Shen J.H. Lotfi P. Ko C.Y. Tang L.P. The effect of incorporation of SDF-1 alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 2010;31:3997. doi: 10.1016/j.biomaterials.2010.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morais J.M. Papadimitrakopoulos F. Burgess D.J. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. Aaps J. 2010;12:188. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon J.J. Kim J.H. Park T.G. Dexamethasone-releasing biodegradable polymer scaffolds fabricated by a gas-foaming/salt-leaching method. Biomaterials. 2003;24:2323. doi: 10.1016/s0142-9612(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 18.Murakami Y. Hirata A. Ito S. Shoji M. Tanaka S. Yasui T. Machino M. Fujisawa S. Re-evaluation of cyclooxygenase-2-inhibiting activity of vanillin and guaiacol in macrophages stimulated with lipopolysaccharide. Anticancer Res. 2007;27:801. [PubMed] [Google Scholar]
- 19.Liu J.K. Mori A. Antioxidant and prooxidant activities of P-hydroxybenzyl alcohol and vanilline-Effects on free radicals, brain peroxidation and degradation of benzoate, deoxyribose, amino-acids and DNA. Neuropharmacology. 1993;32:659. doi: 10.1016/0028-3908(93)90079-i. [DOI] [PubMed] [Google Scholar]
- 20.Lim E.J. Kang H.J. Jung H.J. Song S. Lim C.J. Park E.H. Anti-angiogenic, anti-inflammatory and anti-nociceptive activities of vanillin in ICR mice. Biomolecules Ther. 2008;16:132. [Google Scholar]
- 21.Pattison M.A. Wurster S. Webster T.J. Haberstroh K.M. Three-dimensional, nano-structured PLGA scaffolds for bladder tissue replacement applications. Biomaterials. 2005;26:2491. doi: 10.1016/j.biomaterials.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Hwang Y.S. Sangaj N. Varghese S. Interconnected Macroporous Poly(Ethylene Glycol) Cryogels as a Cell Scaffold for Cartilage Tissue Engineering. Tissue Eng Part A. 2010;16:3033. doi: 10.1089/ten.TEA.2010.0045. [DOI] [PubMed] [Google Scholar]
- 23.Munirah S. Kim S.H. Ruszymah B.H.I. Khang G. The use of fibrin and poly(lactic-co-glycolic acid) hybrid scaffold for articular cartilage tissue engineering: an in vivo analysis. Eur Cells Mater. 2008;15:41. doi: 10.22203/ecm.v015a04. [DOI] [PubMed] [Google Scholar]
- 24.Besseau F. Lucon M. Laurence C. Berthelot M. Hydrogen-bond basicity pK(HB) scale of aldehydes and ketones. J Chem Soc [Perkin 2] 1998;101 [Google Scholar]
- 25.Kumar S.S. Priyadarsini K.I. Sainis K.B. Free radical scavenging activity of vanillin and o-vanillin using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical. Redox Rep. 2002;7:35. doi: 10.1179/135100002125000163. [DOI] [PubMed] [Google Scholar]
- 26.Yuen F.L.Y. Zak G. Waldman S.D. Docoslis A. Morphology of fibroblasts grown on substrates formed by dielectrophoretically aligned carbon nanotubes. Cytotechnology. 2008;56:9. doi: 10.1007/s10616-007-9113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meinel L. Hofmann S. Karageorgiou V. Kirker-Head C. McCool J. Gronowicz G. Zichner L. Langer R. Vunjak-Novakovic G. Kaplan D.L. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26:147. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 28.Abraham D.J. Mehanna A.S. Wireko F.C. Whitney J. Thomas R.P. Orringer E.P. Vanillin, A potential agent for the treatment of sickle-cell-anemia. Blood. 1991;77:1334. [PubMed] [Google Scholar]
- 29.Fitzgerald D.J. Stratford M. Gasson M.J. Narbad A. Structure-function analysis of the vanillin molecule and its antifungal properties. J Agric Food Chem. 2005;53:1769. doi: 10.1021/jf048575t. [DOI] [PubMed] [Google Scholar]
- 30.Kim S. Lee Y. Park H. Hong D. Khang G. Lee D. Reduced inflammatory responses to Poly(lactic-co-glycolic acid) by the incorporation of hydroxybenzyl alcohol releasing polyoxalate. Macromolecular Res. 2011;19:1242. [Google Scholar]
- 31.Yang Y.W. Hsu P.Y.J. The effect of poly(D,L-lactide-co-glycolide) microparticles with polyelectrolyte self-assembled multilayer surfaces on the cross-presentation of exogenous antigens. Biomaterials. 2008;29:2516. doi: 10.1016/j.biomaterials.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Serrano M.C. Pagani R. Manzano M. Comas J.V. Portoles M.T. Mitochondrial membrane potential and reactive oxygen species content of endothelial and smooth muscle cells cultured on poly(epsilon-caprolactone) films. Biomaterials. 2006;27:4706. doi: 10.1016/j.biomaterials.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Yim E.S. Zhao B. Myung D. Kourtis L.C. Frank C.W. Carter D. Smith R.L. Goodman S.B. Biocompatibility of poly(ethylene glycol)/poly(acrylic acid) interpenetrating polymer network hydrogel particles in RAW 264.7 macrophage and MG-63 osteoblast cell lines. J Biomed Mater Res Part A. 2009;91A:894. doi: 10.1002/jbm.a.32311. [DOI] [PubMed] [Google Scholar]
- 34.Panilaitis B. Altman G.H. Chen J.S. Jin H.J. Karageorgiou V. Kaplan D.L. Macrophage responses to silk. Biomaterials. 2003;24:3079. doi: 10.1016/s0142-9612(03)00158-3. [DOI] [PubMed] [Google Scholar]
- 35.Wu Z. Zhang J. Nakanishi H. Leptomeningeal cells activate microglia and astrocytes to induce IL-10 production by releasing pro-inflammatory cytokines during systemic inflammation. J Neuroimmunol. 2005;167:90. doi: 10.1016/j.jneuroim.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Kim S. Park H. Song Y. Hong D. Kim O. Jo E. Khang G. Lee D. Reduction of oxidative stress by p-hydroxybenzyl alcohol-containing biodegradable polyoxalate nanoparticulate antioxidant. Biomaterials. 2011;32:3021. doi: 10.1016/j.biomaterials.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 37.Lim E.J. Kang H.J. Jung H.J. Park E.H. Anti-angiogenic, anti-inflammatory and anti-nociceptive activity of 4-hydroxybenzyl alcohol. J Pharm Pharmacol. 2007;59:1235. doi: 10.1211/jpp.59.9.0007. [DOI] [PubMed] [Google Scholar]
- 38.Robinson J.P. Bruner L.H. Bassoe C.-F. Hudson J.L. Ward P.A. Phan S.H. Measurement of intracellular fluorescence of human monocytes relative to oxidative metabolism. J Leukoc Biology. 1988;43:304. doi: 10.1002/jlb.43.4.304. [DOI] [PubMed] [Google Scholar]
- 39.Lirdprapamongkol K. Kramb J.P. Suthiphongchai T. Surarit R. Srisomsap C. Dannhardt G. Svasti J. Vanillin Suppresses Metastatic Potential of Human Cancer Cells through PI3K Inhibition and Decreases Angiogenesis in Vivo. J Agric Food Chem. 2009;57:3055. doi: 10.1021/jf803366f. [DOI] [PubMed] [Google Scholar]
- 40.Wu S.L. Chen J.C. Li C.C. Lo H.Y. Ho T.Y. Hsiang C.Y. Vanillin improves and prevents trinitrobenzene sulfonic acid-induced colitis in Mice. J Pharm Exp Ther. 2009;330:370. doi: 10.1124/jpet.109.152835. [DOI] [PubMed] [Google Scholar]
- 41.Sato M. Kikuchi M. Ishihara M. Asazuma T. Kikuchi T. Masuoka K. Hattori H. Fujikawa K. Tissue engineering of the intervertebral disc with cultured annulus fibrosus cells using atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS scaffold) Med Biol Eng Comput. 2003;41:365. doi: 10.1007/BF02348444. [DOI] [PubMed] [Google Scholar]
- 42.Wan Y.Q. Feng G. Shen F.H. Laurencin C.T. Li X.D. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials. 2008;29:643. doi: 10.1016/j.biomaterials.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 43.Helen W. Gough J.E. Cell viability, proliferation and extracellular matrix production of human annulus fibrosus cells cultured within PDLLA/Bioglass (R) composite foam scaffolds in vitro. Acta Biomaterialia. 2008;4:230. doi: 10.1016/j.actbio.2007.09.010. [DOI] [PubMed] [Google Scholar]