Abstract
The decellularization of porcine heart tissue offers many opportunities for the production of physiologically relevant myocardial mimetic scaffolds. Earlier, we reported the successful isolation of a thin porcine cardiac extracellular matrix (pcECM) exhibiting relevant bio-mechanical properties for myocardial tissue engineering. Nevertheless, since native cardiac tissue is much thicker, such thin scaffolds may offer limited regeneration capacity. However, generation of thicker myocardial mimetic tissue constructs is hindered by diffusion limitations (∼100 μm), and the lack of a proper vascular-like network within these constructs. In our present work, we focused on optimizing the decellularization procedure for thicker tissue slabs (10–15 mm), while retaining their inherent vasculature, and on characterizing the resulting pcECM. The trypsin/Triton-based perfusion procedure that resulted in a nonimmunogenic and cell-supportive pcECM was found to be more effective in cell removal and in the preservation of fiber morphology and structural characteristics than stirring, sonication, or sodium dodecyl sulfate/Triton-based procedures. Mass spectroscopy revealed that the pcECM is mainly composed of ECM proteins with no apparent cellular protein remains. Mechanical testing indicated that the obtained pcECM is viscoelastic in nature and possesses the typical stress-strain profile of biological materials. It is stiffer than native tissue yet exhibits matched mechanical properties in terms of energy dissipation, toughness, and ultimate stress behavior. Vascular network functionality was maintained to the first three–four branches from the main coronary vessels. Taken together, these results reaffirm the efficiency of the decellularization procedure reported herein for yielding thick nonimmunogenic cell-supportive pcECM scaffolds, preserving both native tissue ultra-structural properties and an inherent vascular network. When reseeded with the appropriate progenitor cells, these scaffolds can potentially serve as ex vivo screening platforms for new therapeutics, as models for human cardiac ECM, or as biomedical constructs for patch or transmural transplantation strategies.
Introduction
Several recently reported strategies1 provide relatively thin myocardial-like constructs for use either in in vitro toxicology and drug screening studies2 or as implants showing improvements in myocardial function on transplantation in small myocardial infarction (MI) animal models3 and in a human case study of idiopathic heart dilation.4 Other studies suggested the use of natural extracellular matrix (ECM), isolated from various tissue origins, as a part of heart regeneration therapies after MI.5–13 However, since the tissue origin of the isolated acellular ECM is an issue of concern, the use of ECM obtained through the decellularization of actual heart tissue offers several advantages, including the preservation of the 3D architecture and key ECM elements of the cardiac tissue related to cell support and differentiation.1 Recent publications, including our own, reported the isolation of acellular ECM from the myocardium of rats7,12 and pigs8–11,13 as a model and platform for cardiac regeneration therapies. In these studies, the ECM was isolated using various procedures, which largely differ in terms of the detergents used (i.e., ionic/nonionic), the presence or absence of an enzymatic treatment (e.g., using trypsin), and their administration methods (i.e., perfusion, sonication, or agitation/stirring).7–9,11 Nonetheless, while these thin acellular slices might still be beneficial as patch-based therapies or for use as solubilized injectable platforms, their application in animal models has been shown to provide limited regeneration capacities.1
Myocardial mimetic tissues, with relevant physiological thickness (10–15 mm),14 may overcome some limitations associated with injection or patch-based therapies.1,7,10,12,13,15 Such thick mimetic constructs can be used for scar replacement therapy after MI or as tissue models for cellular studies and ex vivo drug screening. The thick mimetic ECMs need to be isolated from organs that share a similar physiological composition, size, and thickness with the human heart. One such organ that meets the requirements is porcine myocardium.15 The ECM isolated from the porcine heart may be a relevant clinical model for the production of ex vivo full-thickness scaffolds for scar replacement therapy.
As with thin ECM scaffolds, studies have reported isolating the entire porcine heart ECM10,13 to serve as a human model. The decellularization of the whole organ was also based on extensive use of active enzymes and highly concentrated detergents: perfusion with a combined rapid enzymatic-detergent based sequence (<10 h), including hypo/isotonic solutions, trypsin, and ionic/nonionic detergents (Triton and deoxycholic, respectively). The obtained decellularized heart can be used either for an entire organ orthotopic transplantation15 or as a source for specific tissue parts required for particular treatments (e.g., heart valves, ventricular wall slabs, etc.). However, the success of the whole heart strategy may be hampered by the limited technologies available in terms of large-scale cell expansion needed for the repopulation of the entire heart, particularly that of cardiomyocytes—the heart parenchymal cells.1,15 In the case of MI, using thick slabs originating from the whole heart may be advantageous, as this would enable scaling down the cell quantity required for repopulation while still providing proper functional support. However, such thick slabs need to be obtained while preserving the macro and micro properties of the isolated ECM, in particular its thickness, composition, and structure. Unfortunately, scaling down using the decelluarization approach just mentioned may involve uncontrolled cutting of the acellular whole heart ECM template and may damage these properties post decellularization.
Another major obstacle facing whole organ application or thick constructs, whether made using ECM, natural, or synthetic polymers, is the need for proper vascularization without which long-term construct survival is hampered. This issue of vascularization is particularly critical with construct thicknesses exceeding the diffusion barrier (i.e., ∼100 μm), where oxygen and nutrient supply is prevented and accumulated cell cytotoxic waste byproducts are retained.1,16,17 Several approaches have been suggested to overcome these limitations, including the in vitro co-cultivation of endothelial cells with pro-angiogenic cells, the embodiment of growth factors within the scaffold aimed at the in vivo attraction of angiogenic cells, and the use of micro-fabricated channels allowing nutrient flow and directing endothelial cell attachment.16 In addition, different technologies were suggested such as using scaffold-free multilayered cardiomyocyte cell sheets18,19 or femoral arterio-venous loops20,21 producing vascularized thick (1–2 mm) myocardium-like tissue. Though these technologies represent major breakthroughs, several drawbacks still limit their clinical applicability, including the repeated surgeries required for cell sheet transplantation and the morbidity at the donor site of surgical flaps proposed for the femoral arterio-venous loop system.20
We propose an alternative process involving the decellurization of scaled-down thick tissue slabs, which maintains their inherent blood vessel infrastructure for future revascularization. The ECM is acquired by cutting the whole native tissue with its inherent vasculature, before the decellularization process, and perfusing it with a combination of Triton and trypsin through its own main blood vessels. Such an approach provides a significant technical advantage by enabling the monitoring of the perfusion process and tracking of the decellurization online and in real time, facilitating the removal of the nonperfused areas during or post decellularization. Another major advantage is that by controlling this process, the inherent vascularization can be preserved for future blood vessel remodeling.
Accordingly, we extended and optimized our previously reported procedure so as to obtain a thick decellularized ECM scaffold while preserving its inherent vasculature. To improve cell removal from the slabs' inner mass, we compared the effect of perfusing the decellularization reagents through the inherent blood vessels with previously reported effects of agitation/stirring8,9 and sonication.11 The cell removal efficiency of sodium dodecyl sulfate (SDS) was also evaluated and compared with that achieved by an enzymatic procedure involving the use of trypsin. Finally, the acellular products of the optimized procedure were characterized in terms of their macro/micro 3D architecture, protein composition, biomechanical properties, in vitro immunogenicity, cell-supporting ability, and, most importantly, preservation and remodeling of their vascular network.
Methods
All experiments were carried out in accordance with the Israeli Animal Welfare (Protection and Experimentation) Law, after obtaining the permission of the Technion's Institutional Animal Care Committee. All animals received humane care in compliance with the preclinical research authority SOP-032.
Decellularization of porcine heart tissue
Hearts of healthy commercial slaughter-weight female pigs (∼6 months old) were harvested and immersed in ice-cold sterile phosphate buffer saline (PBS), supplemented with anti-bacterial and anti-mycotic antibiotics: 200 U/mL Penicillin, 0.2 mg/mL Streptomycin (Pen-Strep®, Biological Industries), and 2 μg/mL amphotericin B (Fungizone®, Invitrogen). The atria, right ventricle, and any excess fat were removed, and the left ventricle was excised in parallel to the left anterior descending (LAD) coronary artery to achieve 70 mm (w)×90 mm (l)×10 mm (t) left ventricular tissue slabs containing the LAD coronary artery and its adjacent veins.
The decellularization procedure consisted of three steps: alternating hyper/hypo tonic solutions (1.1% and 0.7% (w/v) NaCl in double distilled water (DDW), respectively); enzymatic treatment using 0.05% (w/v) trypsin (14,800 BAEE [units/mg]; Sigma) supplemented with 0.02% (w/v) EDTA (Sigma) calibrated to pH 8.2 and maintained at 37°C for optimal trypsin activity; and detergent washes with 1% (v/v) Triton-X-100 (BioLab Ltd.) in PBS supplemented with 1% (v/v) ammonium hydroxide (BioLab Ltd.). The alternating hyper/hypotonic treatment was repeated twice for each sample (2 h in each solution) at the beginning of the decellularization procedure. The enzymatic and detergent treatments were repeated for each sample in two cycles of the following sequence: trypsin digestion for 48 h followed by Triton-X-100 washes for 96 h. Decellularization solutions were replenished every 24 h. Extensive washes with DDW were conducted between the two cycles to remove any remaining Triton-X-100.
To compare the cell removal efficiency of the different methods, myocardial slabs were divided into four groups (n=4 different slabs per group) and decellularized using the following protocols: decellularization by stirring and agitation (Group 1); sonication, 30 min twice a day using a UC-02 ultrasonic cleaner (Jeio-tech, Korea, Group 2), and perfusion through the LAD coronary artery at 20 mL/min per slab using a Masterflex peristaltic pump (Cole-Parmer) of either of the complete cycles just described (Group 3) or using 1% (w/v) SDS (Sigma) in PBS, instead of the trypsin step, for a comparison (Group 4). All slabs were also immersed in the decellularization reagents throughout the process. For disinfection, the decellularized slabs were extensively washed in sterile saline, immersed overnight in 70% ethanol, and then washed in sterile DDW.
Confirmation of acellularity
Decellularized porcine cardiac ECM (pcECM) slabs of each treatment group were frozen in Tissue-Tek® OCT compound (Sakura), cross-sectioned to 10 μm, and stained using Masson tri-chrome (Bio-Optica) and oil red (Sigma), according to the manufacturer's instructions. Stained sections were visualized by inverted phase-contrast microscopy (Eclipse TE2000-E; Nikon Inc.). Image data analyses were performed on three representative cross-sections from three different sample slides using Axiovision™ software (Carl Zeiss). Staining color intensity and area were measured using the auto measurement module. Densitometric measurements were used to determine the relative percentage of red dye area in both Masson tri-chrome and oil-red stained slides (representative of cell cytoplasm and adipocyte remains, respectively). Fiber-to-fiber distance was averaged along three separate straight-line intensity profiles, measured in three distinct cross-sectional locations and determined throughout each cross-section from one side to the other. Representative images are presented.
Scanning electron microscopy
Perfusion decellularized pcECM slabs were washed and submerged in distilled water, frozen, and then lyophilized overnight. Samples were imaged using side and back scattered electrons at 0.98 Torr and 20 kV, using an FEI-Quanta 200 scanning electron microscope (OXFORD Instruments).
Multi-photon microscopy
To image pcECM ultra-structure in wet conditions, perfusion decellularized pcECM slabs were mounted onto an upright LSM510meta multi-photon microscope (Carl Zeiss) and imaged using second harmonic generation for collagen excitation, as previously reported.22 Briefly, the presented 3D image (z-stack of 110 μm obtained from 23 images of 8 bit 512×512 pixels with a 4.8 μm step size) was obtained using a Chameleon Ti-Sapphire multi-photon laser (Coherent, Inc.) at an excitation wavelength of 820 nm (4.1% of maximal laser power). Data were collected through a band pass 390–465 filter using a live-cell imaging (LCI) Plan-Neofluar 25× water immersion lens having a 0.8 numerical aperture (Carl Zeiss). Data were acquired using the LSM Image browser (Carl Zeiss) and analyzed with the ZEN 2011 lite edition (Carl Zeiss).
Proteomic analysis
A 100 mg sample pool of trypsin perfused acellular slabs (originating from three different slabs) was minced with a scalpel in PBS and then proteolysed by submerging in 8 M Urea (Sigma) with a 3 mM DTT (Sigma) reducing agent for 30 min at 60°C, followed by 10 mM iodoacetamide (Sigma) in 10 mM ammonium bicarbonate (Fluka Sigma) at room temperature for 30 min, and then trypsinization in 10 mM ammonium bicarbonate (Fluka Sigma) containing trypsin (Promega) at a 1:50 enzyme-to-substrate ratio, overnight at 37°C. The tryptic peptides were desalted using C18 stageTip (3M) and resolved by reverse-phase chromatography on 0.075×200 mm fused J&W silica capillaries (Agilent Technologies) packed with Reprosil reversed-phase material (Dr. Maisch GmbH). The peptides were eluted with linear 65 min gradients of 5% to 45% and 15 min at 95% acetonitrile (Sigma) with 0.1% formic acid (Fluka Sigma) in water at flow rates of 0.25 μL/min. Mass spectrometry was performed by an orbitrap ion-trap mass spectrometer (MS, Thermo Fisher Scientific, Inc.) in a positive mode using a repetitive full MS scan, followed by collision-induced dissociation of the seven most dominant ions selected from the first MS scan. The MS data were analyzed using Sequest 3.31 software (J. Eng and J. Yates, University of Washington and Finnigan, San Jose) searching against the vertebrate part of the Uniprot database, enabling a 5% false-positive rate (β=0.05).
Corrosion casting
Corrosion casting was performed using Batson's No. 17 Plastic Replica and Corrosion Kit (Polysciences, Inc.), according to the manufacturer's instructions. Briefly, 100 mL of base monomer solution (methyl methacrylate) were divided into two parts: The first was mixed with 6 drops of red dye (2-Naphthenecarboxylic acid-4-((5-chloro-4-methyl-2-sulfophenyl)azo)-3-hydroxy-calcium salt) and 24 mL of catalyst solution (Benzoyl peroxide); the second part was mixed with 12 drops of promoter solution (N,N-Dimethyl-4-toluidine) and stirred slowly on a magnetic stirrer. The two parts were combined, while stirring, until a homogenous solution was reached. Decellularized and native tissue samples of similar sizes (70 mm×90 mm×10 mm) containing the LAD coronary artery were clamped at most of the large exit points, submerged in 4°C DDW, and injected with the polymeric solution (∼40 mL per sample) until the specimen appeared to be fully perfused. Samples were let to cure at 4°C for 3.5 h, followed by maceration in 32% KOH (v/v) in DDW at 37°C for 96 h. Maceration solutions were gently replenished every 24 h until no tissue mass remains were visible.
Fluorescent imaging of vasculature ECM preservation
TRITC-Dextran (150 MDa; Sigma) was diluted 1:150 in 2% Alginate (Sigma) in PBS and heated to 37°C. A fluorescently labeled solution (10 mL, Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea) was manually perfused for 1 min through the decellularized pcECM slabs using a 20 mL syringe. These slabs were then imaged using the Maestro™ multispectral fluorescent imager (Cri™) and captured using Maestro 2.4 imaging software. Representative images are presented.
Mechanical evaluation of thick pcECM
Tensile testing was performed using an Instron 5567 universal testing instrument with a 500N load cell (Instron). Device control, data acquisition, and processing were performed with BlueHill® materials testing software (Instron). Three sequential uni-axial mechanical assays (cyclic strain, stress relaxation, and strain to break) were conducted with 10 min intermissions between them. Both pcECM and native specimens (n=5 slabs from different hearts per group) were cut into identical sizes (20 mm×60 mm) of similar thickness (∼10 mm) and orientation (parallel to the longitudinal axis of the heart). The specimens were hydrated with PBS (pH=7.4) under ambient temperature throughout the testing. Samples were sutured using #5-0 surgical 100% polyester sutures, passing through the whole thickness of each wide edge (1 loop/1.5 mm) of all specimens, and looped around two stainless steel rods (Ø=6.5 mm, l=30 mm), which were mounted onto the Instron apparatus (Fig. 4). To allow for reliable, consistent, repeatable results, all specimens were precycled with 10 cycles of loading at a rate of 0.05 mm/s to 15% strain, followed by unloading at the same rate, before each test.
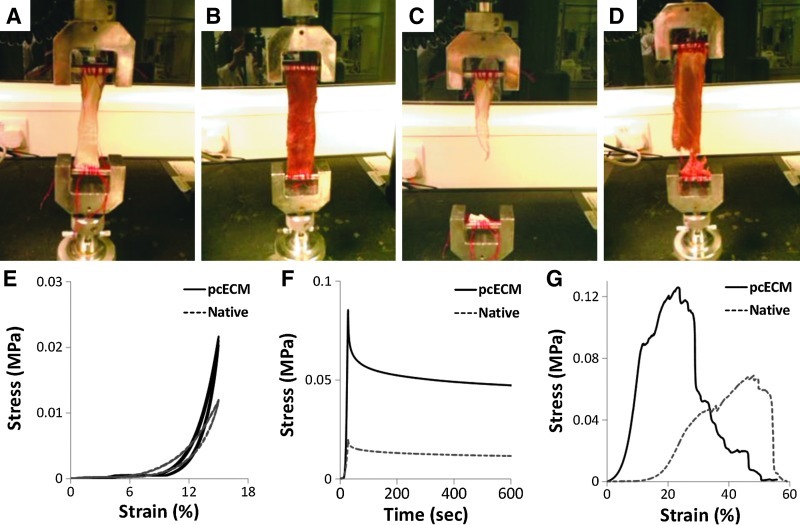
FIG. 4.
Mechanical evaluation of thick pcECM. Identical-sized samples of both pcECM (A, C) and freshly isolated native heart tissue (B, D) were mounted on an Instron 5567 machine and mechanically tested using dynamic cyclic loading (E), stress-relaxation (F), and strain-to-break (G) assays. Representative graphs and pictures are presented out of 5 repetitions per group.
Dynamic loading (cyclic strain) was carried out in a similar fashion to the precycling, and was recorded for three repetitive cycles. Energy dissipation during the dynamic loading was calculated based on the area blocked within the stress-strain “loop” curves obtained during these measurements. To evaluate the viscoelastic properties, a stress relaxation assay was performed by stretching the samples at a rate of 0.5 mm/s to 20% strain, and holding at this constant displacement for 10 min during which force decay was measured and plotted as the function of time (stress vs. time curves). Finally, samples were stretched at a constant strain rate of 0.05 mm/s until failure was defined as the complete cleavage of the specimen. True stress-strain curves were plotted for analysis, and the toughness was calculated as the total area under the curves.
Mesenchymal stem cell isolation and culture
Mesenchymal stem cells (MSCs) were isolated from the femurs and tibiae of 3 month old Sprague-Dawley rats, as previously described.23 Briefly, cells from each bone were seeded separately on culture plates and grown in low-glucose DMEM media (DMEM-LOW; Biological Industries) supplemented with 10% FCS (Biological Industries), 1% Pen-Strep, and 0.4% Fungizone in a humidified 37°C, 5% CO2 incubator. The next day, nonadherent cells were removed by several washes with a fresh medium. MSCs were allowed to grow to about 75% confluence and then harvested, re-plated (P1), and cultured for an additional week before being used in the experiments. To verify cell lineage integrity, MSCs were analyzed at passage 4 (P4) by flow cytometry for typical MSC surface markers (also described in the Supplementary data and Supplementary Fig. S2). Passage 4 was not exceeded during all cell-related experiments.
Monitoring MSC adherence and density on pcECM
Sterile pcECM scaffolds (8 mm in diameter) were immersed in 96-well plates containing DMEM-LOW, supplemented with 10% FCS, 1% Pen-Strep, and 0.4% Fungizone for 24 h in culturing conditions. To evaluate the MSCs' adherence and density on the pcECM scaffold, cell quantities were determined using AlamarBlue™ quantitative assay (AbD Serotec) according to the manufacturer's instructions and against a standard calibration curve. Cell numbers were divided by the initial seeded quantity to evaluate the adherence percentage or by the average surface area of the scaffold (0.5 cm2) to evaluate cell density. Before seeding, media were removed, and scaffolds were left to partially dry for 2 hr. MSC were harvested from tissue culture plates at 70% confluence and re-suspended in complete DMDEM-LOW, to a final concentration of 4×106 cells/mL. The cell suspension (50 μL/piece) was slowly pipetted onto the center of the round scaffolds. Seeded scaffolds were preincubated in culturing conditions for various time durations (0, 45, 90 and 270 min), washed in PBS, and transferred to new 24-well plates filled with 2 mL of DMEM-LOW supplemented with 10% (v/v) AlamarBlue. To monitor cell density over time, cell numbers were also determined on scaffolds seeded and cultured for approximately 30 days. After each AlamarBlue measurement, the scaffolds were washed to remove excess AlamarBlue and re-cultured in fresh media. To avoid deviations due to cells that had migrated from the scaffold and adhered to the plate surface, seeded scaffolds were moved to new culture plates before each measurement. To verify that the optimized perfusion decellularization procedure did not result in a less cell-supportive scaffold, cells were also seeded on our previously reported thin pcECM scaffolds,9 which served as controls, and their proliferation was measured at the same time points.
Immunogenic evaluation
RAW 264.7 cells (ATCC, TIB-71™) were seeded in six-well plates and cultured in 2 mL of high-glucose DMEM (DMEM-HIGH; Sigma) supplemented with 10% FCS, 1% Pen-Strep, and 0.4% Fungizone for 1 day, after which the medium was changed to a low-serum medium (3% serum). Once 70% confluence was reached; the cells were exposed to acellular pcECM or poly lactic-co-glycolic acid (PLGA) scaffolds prepared according to a previously reported salt-leaching protocol.17 Briefly, samples were lyophilized, minced with a scalpel, sterilized, and gently dispersed into the wells (1 mg of each sample per well, n=4 wells per group). Native cardiac tissue served as a positive control, PLGA served as a negative control, and wells with no added stimulating agents served as blank controls for basal nitric oxide (NO) secretion. After 6 h of incubation, secreted NO levels were measured and determined against a standard calibration curve (0–100 μM sodium nitrite in low-serum medium) as the free stable nitrite form (NO2−) using the Griess reagent system (Promega) and according to the manufacturer's instructions.
To determine the pcECM-induced expression of the pro-inflammatory cytokine tumor necrosis factor (TNF)-α, primary bone marrow macrophages (BMM), freshly harvested from C57 black mice, were cultured on 12-well plates at a seeding density of 150,000 cells/well and incubated with low-serum (3%) complete DMED-HIGH for 24 h. The cells were then exposed to acellular minced pcECM and PLGA scaffolds. TNF-α mRNA levels were determined using real-time reverse transcription polymerase chain reaction (real time RT-PCR). Total mRNA was extracted using Tri-reagent™ (Sigma), according to the manufacturer's instructions. RNA was used for reverse transcription (1st strand reverse-iT, ABgene) in a PCR thermo cycler (PTC-200; MJ Research). The cDNA genes were amplified with primers to TNF-α (Fw: GCC TCCCTCTCATCAGTTCT, Re: TGGTGGTTTGCTACGACGTG) and GAPDH (Fw: CACTCTTCCACCTTCGATGC, Re: AGGTCCACCACCCTGTTGC) in an Applied Biosystems real-time PCR (model 7300; Applied Biosystems) using a power SYBER® green PCR master mix (Applied Biosystems). Results were analyzed using Applied Biosystems 7300 sequence detection software version 1.3.1 and with the same threshold for all samples. To normalize the results, the TNF-α relative mRNA copy number was divided by the housekeeping gene GAPDH. Untreated wells were used to quantify basal levels of TNF-α secretion, and native tissue exposed cells served as positive controls.
Vasculature remodeling
Human umbilical vein endothelial cells (HUVECs) stably expressing green fluorescent protein (GFP) (HUVEC-GFP) were a kind gift from Prof. Gera Neufeld (Technion, Faculty of medicine) and transfected as previously published.24 PcECM slabs (20×60 mm, n=4 different slabs) containing inherent vascular infrastructure were washed with 70% ethanol, PBS, and complete EGM-2 (Lonza) medium before seeding. HUVEC-GFP cells (1×107 cells/mL) suspended in a 1 mL EGM-2 medium were injected through 1.5 french catheters (Biometrix) sutured to the vasculature inlet. The cell-seeded pcECM construct was incubated at 37°C and 5% CO2 for 2 h to allow static attachment (with gentle rotation every half hour), followed by submersion in complete growth media for 48 h static cultivation, under standard incubator conditions as earlier. To study HUVEC-pcECM interaction, the slabs were transversely cut and cryo-sectioned into 10 μm slices for histological assessment with H&E staining (Histopathology Unit, IMCB) and fluorescent imaging using a fluorescent binocular microscope (Olympus SZX16).
Statistical analysis
Data were expressed as mean values±standard deviation. analysis of variance, and two paired t-tests were used for a comparison using SAS statistical software (JMP 6.0). p<0.05 was considered significant for all comparisons.
Results
Decellularization of thick tissue slabs
Sonication (Fig. 1C, D) and perfusion, through the built-in coronary vasculature of the thick pcECM (Fig. 1E, F), were used to increase cell removal and decellularization reagent penetration. SDS, which had been previously shown as suitable for perfusion decellularization of rat hearts,7 was used as a control instead of trypsin (Fig. 1G, H). As can be seen from the Masson Trichrome and the oil red staining, and quantified using image analyses (Fig. 1I, J), both sonication and perfusion (but not agitation) yielded significant tissue decellularization (p<0.05). Thus, quantification of the relative red dye area (representative of cellular remains) in Masson tri chrome-stained slides revealed that all convection-based methodologies (sonication: 0.6%±0.1%; trypsin perfusion: 1.1%±0.5% and SDS perfusion: 1.1±0.4) resulted in significantly cleaner matrices (p<0.05) compared with the standard agitation technique (2.5%±1.1%). Furthermore, in terms of adipocyte remains, quantitative analyses performed on oil-red stained slides indicated that the trypsin-triton based procedures (agitation: 2.4%±2.1%; sonication: 1.8%±1.3% and trypsin perfusion: 0.7%±0.1%) resulted in significantly more efficient adipose tissue decellularization (p<0.05) compared with the combination of SDS and triton perfusion (15.9%±3.4%). Macroscopically, perfusion decellularized slabs appeared slightly thicker than the sonicated ones, and both were more swollen than agitation decellularized matrices (data not shown). This was further supported with a more spacious fiber morphology characterizing the perfusion-decellularized matrices (average fiber-to-fiber distance of 760±125 μm vs. 550±110 μm in sonication, 520±30 μm in the control agitation group, and 260±32 μm in the SDS-treated group, Fig. 1K). In addition, trypsin perfusion was shown to be more effective in terms of cell removal efficiency than SDS perfusion (Fig. 1I, J); thus, it was chosen as the selected methodology for the rest of this study. This procedure enabled the isolation of 14.6±1.9 mm thick acellular slabs (Supplementary Table S1). To further quantify the level of acellularity using this methodology, samples of trypsin-perfused acellular slabs were analyzed for their DNA content normalized to the dry matrix weight (Supplementary Fig. S3). The total DNA content within the acellular slab (1.5±1.3 ng DNA/mg pcECM dry weight) is significantly lower (p<0.05) than that obtained for native tissue (13.3±0.6 ng DNA/mg pcECM dry weight) which serves as a positive control.
FIG. 1.
Evaluating various procedures for thick cardiac tissue decellularization. Slabs of thick porcine cardiac tissue were decellularized using a Trypsin-Triton sequence (A–F) combined with stirring (A, B), sonication (C, D), or perfusion through the built-in coronary arteries (E, F). Trypsin was replaced with 1% SDS using perfusion (G, H). The entire process was repeated twice to allow for sufficient decellularization. Histological cross-sections of the resulting decellularized tissue are presented using Masson tri chrome staining (A, C, E, and G) blue marking extracellular matrix (ECM) fibers, red–cytoplasm, black–nuclei; and oil red staining (B, D, F, and H) - red marking adipocyte and lipid remains. Image analyses were performed to quantify the average red dye surface area ratio as a marker for cell cytoplasms (I) and adipocye remains (J) in Masson trichrome and oil red, respectively. Average fiber-to-fiber distance was also quantified through image analyses (K) Scale bars: 200 μm.
Media perfusion analyses revealed that even though the matrix is precut and leaky, outlet flow can functionally reach the exit catheter (Supplementary online movie S1,). This outlet flow corresponds to 7%–18% of the inlet flow evaluated by the relative pressures measured at the inlet and outlet connectors (Supplementary Table 1).
Preservation of ultra-structure, structural proteins, and vascular ECM network
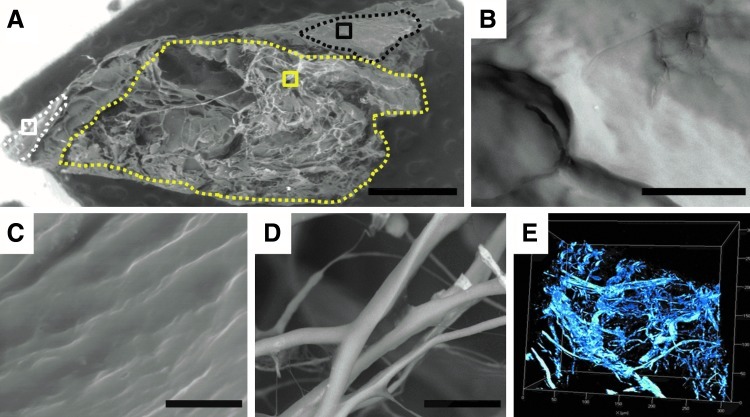
Scanning electron microscopy (SEM) and multi-photon microscopy (MPM) analyses showed that the resulting trypsin-perfused pcECMs preserve their ultra-structural characteristics and retain their nonisotropic orientation (Fig. 2). Disrupted adipose tissue remains (Fig. 2B) were found adjacent to intact epicardial layers (Fig. 2C) and to a spacious myocardial fiber mesh (Fig. 2D, E). SEM imaging did not show any cellular remains on pcECM decellularized using perfusion (Fig. 2) or sonication (data not shown). However, some cellular remains were present when pcECM was decellularized using SDS perfusion (Supplementary Fig. S4). A further indication of the effectiveness of the trypsin perfusion decellularization procedure was provided by the MS proteomic analyses, which revealed that the pcECM comprised only ECM proteins such as collagen types I, II, III, and V. No residual cellular proteins were detected (Table 1).
FIG. 2.
Scanning electron microscopy (SEM) and multiphoton imaging of porcine cardiac extracellular matrix (pcECM) ultra-structure post perfusion decellularization. SEM analysis of lyophilized pcECM (A–D) showing a scaffold overview (A) with distinct morphologies (marked by dotted region borders) attributed to a disrupted adipose tissue (B, magnification of black-squared ROI in A), intact epicardial layer (C, magnification of white squared ROI in A), and a myocardial ECM-mesh presumably supportive of cellular proliferation (D, magnification of yellow-squared ROI in A). Multiphoton microscopy revealed similar myocardial fiber orientation in wet matrices (nonlyophilized, E). Scale bars: A: 1 mm; B: 50 μm; C: 10 μm; D: 20 μm. ROI, region of interest.
Table 1.
Proteomic Analyses of Decellularized Porcine Cardiac Extracellular Matrix
| Protein | Coverage | P (pro) | Accession |
|---|---|---|---|
| Collagen I alpha-1 chain precursor | 31.78 | 8.38×10−11 | P02453 |
| Collagen I alpha-2 chain precursor | 22.21 | 7.83×10−10 | P02465 |
| Collagen III alpha-1 chain | 18.88 | 4.94×10−11 | P04258 |
| Dermatopontin precursor | 9.45 | 1.16×10−4 | P19427 |
| Collagen V alpha-2 chain precursor | 6.60 | 1.68×10−8 | P05997 |
| Collagen V alpha-1 chain precursor | 4.51 | 3.14×10−9 | Q60467 |
| Collagen II alpha-1 chain precursor | 2.69 | 1.97×10−5 | P28481 |
| Collagen II alpha-1 chain precursor | 1.61 | 6.92×10−5 | P02459 |
Mass spectrometer proteomic analyses showing various extracellular matrix proteins listed according to their coverage, from largest to smallest. Protein coverage represents the overall percentage of the respective protein positively identified by the various tryptic peptides that reached the detector. The P(pro) column represents the probability of randomly obtaining similar or better results. Accession number column refers to the protein data base accession numbers in the National Center for Biotechnology Information (NCBI) web site.
To evaluate the extent of the vasculature preservation during perfusion decellularization, we prepared corrosion casting plastic replicas of the native and perfusion-decellularized pcECM vascular trees. Representative images are shown in Figure 3 and display the preservation of the main coronary arteries up to the third and fourth branching from the primary blood vessel (Fig. 3B–E). Perfusion of fluorescently labeled dextran (150 MDa) through the remaining vascular network showed a relatively quick diffusion throughout the pcECM, suggesting that the structure of these vessels remains intact, and can, consequently, be utilized as blood vessel platforms for future tissue vascularization strategies (Fig. 4E, F).
FIG. 3.
Preservation of inherent vasculature. Macroscopic view of a 10 mm-thick acellular perfused matrix (A). Preservation of inherent vasculature was evaluated by corrosion casting of native (B, C) and acellular pcECM (D, E). Patency and functionality of the acellular vasculature ECM is demonstrated by the perfusion of fluorescently labeled Rhodamine-Dextran at consecutive time points (F and G with a 1 min interval).
Mechanical characterization
The mechanical properties of the pcECM were tested using cyclic-strain, strain-relaxation, and strain-to-break assays. Representative graphs and images were chosen from the entire data and are presented in Figure 4. The average mechanical properties across the entire data are presented in Table 2. Dynamic loading tests were performed on native and perfusion-decellularized pcECM in order to address the scaffold's mechanical compatibility with the cyclic working loads of the human heart (Fig. 4E). The similar loop-curve profiles qualitatively indicated a similar working capacity for the pcECM and the native myocardial tissue with no significant difference (p>0.05) in elastic energy dissipation (Table 2). In the stress-relaxation test, both native cardiac tissue and decellularized pcECM exhibited viscoelastic behaviors (Fig. 4F), although the pcECM showed higher ultimate stress and a relatively greater decaying rate than the native tissue. Furthermore, the thick pcECM was found to be stiffer than the native tissue as indicated by its higher elasticity modulus (p<0.05, Table 2) and much lower deformation strain (p<0.05) in the strain-to-break assay. During the strain-to-break assay, both materials were elongated gradually. Reductions of the local cross-sectional area, owing to fiber by fiber rupture (i.e., necking), were patently observed for the pcECM specimens as early as 6 min, whereas native tissue samples exhibited similar phenomena beginning only at ∼10 min of testing. For both materials, failure modes were defined based on the ASTM D3039-00 standard coding style (i.e., failure type-area-location coded by three letter codes). Both materials exhibited edge failure (Grip-At tab-Bottom, code: GAB) and mid-substance failure (Angled-Gage-Middle code: AGM). For the case of edge failure, cleavages occurred at the less adiposive edge for both specimen types. A statistical analysis of a two-tailed paired t-test performed to evaluate the mechanical parameter differences between the two groups showed that the specimens were of equal variance within their group; while the groups' means were significantly different from one another (p<0.05).
Table 2.
Summary of Mechanical Testing Results
| Assay | Parameters | pcECM | Native | p-Value |
|---|---|---|---|---|
| Cyclic strain | Max. stress (kPa) | 17.6±8.4 | 9.6±2.05 | 0.141 |
| (up to 15% strain) | Hysteresis energy dissipation (kJ/m3) | 6.8±1.9 | 7.77±1.85 | 0.212 |
| Stress relaxation | Max. stress (kPa) | 91±39 | 23.3±9.1 | 0.028 |
| (at 20% strain) | Relaxation time (sec) | 10.8±2.1 | 17.6±2.4 | 0.003 |
| Tensile failure | Young's modulus (MPa) | 2.0±1.1 | 0.37±0.15 | 0.021 |
| Ultimate stress (kPa) | 127±21 | 69±20 | 0.165 | |
| Strain at ultimate stress (%) | 25.1±3.9 | 42.9±5.4 | 0.009 | |
| Actual tearing strain (%) | 54.8±1.6 | 74.2±7.9 | 0.008 | |
| Toughness (MJ/m3) | 2.2±0.2 | 1.7±0.3 | 0.204 |
Results represented as mean±SD of the entire dataset consisting of 5 samples per tested group.
pcECM, porcine cardiac extracellular matrix.
Cell support and in vitro immunogenicity
To evaluate whether the isolated thick pcECM, obtained through our perfusion-driven procedure, still supports cell adherence and long-term survival, MSC (CD90+CD29+CD44+CD34− Supplementary Fig. S1) were seeded and cultured on the thick pcECM slabs and compared with the thin pcECM samples that had been previously shown to sustain cell attachment and survival.9 As can be seen from Figure 5A, both the thin and thick pcECM slices were shown to similarly support the adherence of MSCs, which sustained viability, at a maximal density of 100,000 cells/cm2, for approximately 30 days (Fig. 5B).
FIG. 5.
Cell support and in vitro immunogenicity of decellularized thick pcECM. Cell adherence (Mesenchymal stem cell [MSC]) to pcECM is presented for different seeding times (A). Cell (MSC) survival (density as measured by Alamar Blue™) on thin and thick pcECM seeded for 90 min and cultivated for 30 days (B). Nitric Oxide secretion by RAW 264.7 cells exposed to 1 mg/mL of minced lyophilized pcECM samples (C). Tumor necrosis factor (TNF)-α expression from C57black bone marrow macrophages exposed to pcECM as assessed by real-time reverse transcription polymerase chain reaction. The mRNA copy number of TNF-α was normalized to that of the housekeeping gene GAPDH for a comparison (D). For both immunogenicity assays (C, D), native tissue served as a positive control, and unexposed cells served as blank controls, indicating the basal expression levels of Nitric Oxide and TNF-α. Analysis of variance significant difference indicator: *(p<0.05). Histological cross-section assessment of pcECM re-seeded with human umbilical vein endothelial cells stably expressing GFP (green) and statically cultivated for 48 h. Note the monolayer of endothelial cells covering the inner lumen of a preserved blood vessel infrastructure in histological cross-sections. H&E staining present an overview of a large blood vessel. Arrows point to the endothelial monolayer location within the artery lumen (E), zoomed in magnification (F). Fluorescent imaging shows a zoomed in magnification of endothelial monolayer covering the lumens of a large vessel (G) and a smaller arteriole (H). DAPI was used for counter staining in (G, H). Scale bars: C=200 μm; F, H=100 μm, G=50 μm.
We also evaluated the immunogenicity of our decellularized pcECM. No increase was shown in the levels of NO (p>0.05), secreted by RAW macrophages, after exposure to minced pcECM compared with a commercial PLGA scaffold that served as a negative control (Fig. 5C). Furthermore, NO levels for decellularized pcECM were significantly smaller (p<0.05) than the levels induced by exposure to native tissue. Similar results were obtained with BMM expression of the pro-inflammatory cytokine TNF-α, 24 h post exposure (Fig. 5D).
Finally, we also addressed the ability of endothelial cells to repopulate the inherent vasculature infrastructure of these pcECM slabs. When reseeded, HUVEC and HUVEC-GFP cells seem to create a monolayer, surrounding the inner lumen of the inherent acellular blood vessel with no apparent signs of infiltration into the tissue bulk (Fig. 5E–H).
Discussion
To achieve clinically feasible remedies for the scar tissue formed after MI, myocardial tissue engineering has to overcome several major hurdles. These include the fabrication of physiologically relevant thick scaffolds (10–15 mm) that can be further supported by an appropriate vasculature. Such scaffolds should also possess a nonisotropic fiber orientation with a composite 3D structure-function relationship, enabling the support of reseeded cells, while withstanding the mechanical working parameters of the heart.
Aiming at achieving such thick vascularized myocardial mimetic tissue, whole heart acellular myocardial scaffolds were isolated and offered to support reseeded cells through some degree of preserved vascular structures.7,10,13 Nevertheless, in order to serve as a human model, applicability is still hindered by limited cell expansion technologies. Thus, the scaling down to smaller segments of physiologically thick transmural heart ECM may offer a step forward toward the production of such composite scaffold material. In this study, we were, thus, motivated to explore the possibility of isolating scaled down thick acellular pcECM that preserves inherent functional vascular-like structures. Such a pcECM construct will potentially be able to support reseeded cells both ex vivo and in vivo through relatively simple anastomosis to the host vasculature post transplantation.
Our previously reported decellularization protocol for thin cardiac patches9 was optimized here in two ways to fit the decellularization of much thicker myocardial tissue slabs. We employed a similar trypsin-Triton-based sequence, using perfusion, and compared it with sonication or stirring. This comparison has enabled us to assess the efficiency of perfusion-driven decellularization with these alternative methods previously reported to increase the exposure of inner tissue mass to decellularization reagents.11,25 Since SDS has already been shown to be a more effective decellularization reagent than Triton,7 we compared its performance with trypsin. However, since both SDS and trypsin (but not Triton) were reported to cause severe ECM disruption,25 and as Triton is a major component of our procedure, we replaced trypsin with SDS and monitored the acellularity level obtained after perfusion by either method.
Our results suggest that sonication and perfusion (separately) through the inherent coronary arteries lead to similar levels of acellularity, demonstrating superiority to the levels obtained using agitation and stirring. This can probably be attributed to a more directional convection of the decellularization reagents in both methods compared with the random diffusion mechanism governing agitation and stirring. Furthermore, in terms of the prevention of ECM collapse, the perfusion procedure yielded slightly better results than sonication, as indicated by the more spacious fiber morphology achieved using this approach (Fig. 1). The presence of cellular remains in the SDS-treated group causes cross-linking between the fibers and could explain the lower fiber-to-fiber distance measured in this group. Based on these results, a trypsin perfusion-driven decellularization method was selected for the rest of this study.
Another important finding of this study is that trypsin perfusion was found to be more effective and yielded better acellularity than SDS, specifically, and much to our surprise, also in the removal of lipids and adipocytes. This can be supported by the work of the Badylak group demonstrating that the combination of trypsin and Triton was highly effective in the removal of adipocytes from adipose tissue.26 In most studies, SDS has been used to replace Triton and is shown to be more effective than Triton in cell removal, although SDS is an ionic detergent and is expected to be less effective in delipidization than Triton.7,25 No study, to the best of our knowledge, has used SDS to replace trypsin or any other enzyme. Our results suggest that SDS is not only less effective than trypsin, at least when adipocytes are concerned, but also probably with other cell types as well (Supplementary Fig. S3). It is possible that trypsin is more disruptive than SDS to the adipose tissue ECM, as was also suggested by Crapo et al.,25 thus making Triton more effective in the next step of the process. Such disruption, however, was not seen in our SEM or MPM imaging—neither in the vicinity of the adipose tissue nor in other areas of the myocardium, which exhibited a well-preserved ultra-structure of several distinct morphologies. This may imply that although trypsin may disrupt ECM morphology, its disruptive effect is not extensive enough to cause severe morphology changes when used in a controlled manner.
Though any decellularization process is expected to generate some amount of ECM damage, to be clinically compatible, the extent of this damage should be determined and generally limited as much as possible.25 In order to determine the extent of ECM disruption, a proteomic analysis of the ECM protein content was performed on a representative sample pool taken from within several acellular pcECM slabs. It has already been previously reported that in native mammalian myocardium, the most abundant collagens are types I (∼85%) and III (∼11%), while the remaining 4% consist of other collagen types (IV, V, VI, and VIII).27,28 All these collagen types are interconnected to form a network of collageneous fibers that are further supported by other ECM proteins (e.g., elastin and laminin).27 From our proteomic analyses, it is evident that no traces of cellular proteins remained in the ECM, which further demonstrates the decellularization efficiency. These analyses also revealed a relatively high coverage of dermatopontin, a protein that was previously found to be associated with heart tissue at both mRNA29,30 and protein levels.31 Dermatopontin, which is also known to increase after heart infarction,30 was found to be a potent enhancer of transforming growth factor-β,32 and, as such, is expected to affect cell differentiation and proliferation in the myocardial ECM. Finally, and most importantly, all proteins identified using this analysis were ECM associated and mostly structural collageneous proteins, including collagen types I, II, III, and V, confirming the presence of known major ECM structural proteins. Nevertheless, other expected components of the ECM (e.g., collagen types IV & VIII, elastin, and laminin) were absent from this analysis, which is an indication of some ECM disruption. The absence of these proteins can probably be attributed to the fact that they are usually associated with the basal laminae of blood vessels, and were, thus, directly exposed to the perfused reagents and subjected to much harsher decellurization conditions than other components in the bulk. These phenomena were also recently reported by Akhyari et al.,12 who performed a thorough comparison between the decellularization procedure used by Ott et al.7 and that of Wainwright et al.10 Akhyari et al. showed that even short exposure to trypsin (<10 h,12) led to a dramatic decrease in collagen type IV and elastin in a perfused rat heart model. This was not observed in the SDS-based procedure reported by Ott et al.7 Akhyari et al. also claimed that neither procedure was ideal, which suggests that there is a correlation between milder and shorter decellularization procedures that produce inadequately decellularized ECM and longer and more aggressive decellularization procedures which result in “cleaner” ECM at the expense of ECM integrity loss.12
In our study, the disruptive effect of trypsin on the basement membrane is further supported by the corrosion casting images, indicating the lack of capillary beds in the acellular ECM, as would be expected from the lack of capillary constituents, that is, cells and basement membranes.33 Nevertheless, these results also indicate the preservation of a potentially functional vascular-like network up to the third or fourth branches from the main coronary artery. This preservation, therefore, implies that in the future they may possibly be used for nutrient supply in vitro, re-vascularisation in vivo, and as a possible site for anastomosis after transplantation, which would facilitate the survival of re-seeded cells on implantation. To the best of our knowledge, the extent of vascular network branching was not measured earlier with similar types of perfusion decellularized thick matrices; hence, these data could not be compared with similar reported procedures. However, as an initial assessment, the media perfusion analysis suggests that the exit catheter could be functionally used to direct the outlet flow, which was estimated to account for 7%–18% of the respective inlet flow. These values depend on the properties of the biological sample as well as on the ability to shunt large leaks along the way by suturing or cauterizing (data not shown). This functionality may prove beneficial for any future strategy, either ex vivo or in vivo, involving the use of such thick scaffolds.
An important issue regarding transmural therapy is the extent to which this obtained scaffold will withstand the physiological working conditions of the heart (i.e., ∼15%–17% longitudinal strain34). In an effort to resolve this issue, we performed mechanical analyses which revealed that the perfusion decellularized pcECM exhibits a similar cyclic response profile up to 15% strain. Energy dissipation of the decellularized pcECM, during the “working cycle”, was insignificantly different from that of the native tissue throughout the entire dataset, suggesting sufficient mechanical stability. Even though these results do not serve as an actual heart cycle mimetic, the comparison between the profiles of the native and the acellular tissue is still valid under these conditions and is indicative of the overall structural fitness of this scaffold. Surprisingly, these results were also not considerably different than those obtained for the thinner scaffolds produced using our previously reported procedure.9 Further assessment of the viscoelastic nature of this scaffold revealed a greater viscoelastic profile, having higher ultimate stress and a more rapid rate of decay (Table 2) when compared with a native tissue control. This is in agreement with the fact that the scaffold is much more polymeric in nature, lacking the damping effect of the populating cells. To better assess cell contribution to this damping effect, the repopulation of such large matrices for long time periods is required. However, given the limitations associated with static culture conditions, we found this assessment to be impractical without the use of a dynamic cultivation system, which is currently being evaluated in our lab. Furthermore, in order to theoretically calculate the viscous damping coefficients and the relaxation time of the two materials so as to evaluate their viscoelastic behaviors, an appropriate viscoelastic model algorithm, which we are currently developing, should be applied.
We further evaluated the elasticity of the scaffold (E=2.0±1.1 MPa) and found it to be stiffer than the native tissue (E=0.37±0.15 MPa). This finding correlates with the apparent lack of noncollagenous proteins (elastin in particular) in the acellular pcECM scaffold that is expected to result in reduced flexibility and increased stiffness of the pcECM compared with native tissue. The elasticity moduli measured in this study for both the native and the acellular thick slabs were comparable to those previously reported for the thin left ventricular slices.9,11 In contrast, a close examination of the deformation profile (strain-to-break assay), suggests that the isolated thick pcECM scaffold has a composite material architecture, in which some constituents serve as the “weak link,” while others are stronger and yield only at later points in time. This behavior was not observed for the thinner slices,9 further supporting the claim that the overall architecture is preserved, despite the use of trypsin. Moreover, the nonsignificant difference (p>0.05) in the ultimate stress and toughness values obtained for the pcECM and the native tissue suggests that the collagenous structural framework is well preserved.
Finally, since the thick pcECM scaffolds reported in this study were obtained through several modifications to our previously reported procedure,9 their immunogenic nature as well as their ability to support cell adherence and long-term viability were tested using several ex vivo assays. The classic macrophage stimulation pathway involves the secretion of various pro-inflammatory cytokines such as NO and TNF-α, which, in turn, recruit cells associated with classic signs of inflammation leading to allogeneic and xenogeneic transplant rejection.35 To assess whether exposed macrophages are stimulated by the resulting pcECM, macrophage cells were allowed to react with minced pcECM scaffolds, and the levels of their pro-inflammatory cytokines were determined using the Griess method (for NO) and real-time RT-PCR (for TNF-α). The thick acellular scaffolds did not elicit strong stimulation of these pro-inflammatory cytokines, suggesting their potential immunologic acceptance. This further confirms the acellularity of the pcECM scaffold comprising a mostly interspecies conserved collagenous network36 with no apparent xenogeneic cellular remains. These cellular remains are the major cause of stimulation observed within the native tissue exposed macrophages serving as positive controls in this set of experiments. This result is further supported by the recent study of Akhyari et al., who used a rat whole heart decellularization model to compare four different decellularization procedures and showed that regardless of the procedure employed, all products had similar low-to-none cytotoxicity and the ability to support de novo cellular repopulation.12 Likewise, in terms of cell adhesion and viability, the thick acellular slabs presented herein maintained cell growth, as was previously reported with thin matrices,9 demonstrating MSC survival for more than 30 days. Surprisingly, statically cultivated seeded cells did not seem to proliferate beyond their initial seeding density. This phenomenon, observed for both the thin and the thick matrices, may indicate a surface-dependent cell growth, yet merits further investigation to elucidate its governing mechanisms. Though MSC do not actually beat, it was proposed that they may serve as CM model cells.1 Despite the fact that we did not test the cultivation of actual beating cells in this work, our previous publication exhibited CM cell support by acellular myocardial ECM that would be expected to be retained with our thick pcECM as well.5 Thus, future dynamic experimentation using neonatal rat CM or MSC as model cells will be performed by multi-site injections of the cells into the myocardial cavity bulk of the isolated thick pcECM slabs. This injection methodology was already shown to be successful, giving rise to synchronous beating of reseeded thin rat whole heart ECM, accompanied by histological evidence to striated muscle fiber formation.7 However, to support this repopulation through the entire thickness of these slabs, a functional inherent vasculature has to be developed. As seen, our preliminary studies have demonstrated that HUVECs can be seeded inside the inherent vasculature infrastructure and contribute to endothelium-like monolayer formation. Moreover, a custom-made perfusion bioreactor system needs to be developed to support such thick seeded constructs. Currently, we are focusing on blood vessel remodeling aspects under static and dynamic cultivation for future in vitro vascularization strategies.
Conclusions and Future Prospects
We report the successful decellularization of a precut transmural porcine myocardial slab yielding acellular thick pcECM. The use of trypsin in the decellularization process appears to be more effective than SDS in cell removal, including coronary adipose tissue removal, yet might cause some degree of damage to the ECM proteins, particularly those associated with the basal laminae. This effect does not seem to be deleterious, as the mechanical properties, cell support, and immunogenicity exhibited by the pcECM still fit the requirements for myocardial thick-left ventricular tissue engineering purposes. The obtained scaffold preserves much of the macro to micro architecture and ultra-structures of the native tissue, as well as a functional vascular infrastructure that could be valuable for either ex vivo cultivation or future transmural transplantation therapies. Future studies will focus on the development of custom-made bioreactor systems that would mimic the physiological parameters in vitro, thus enabling the dynamic cultivation of thick tissue constructs. These constructs, repopulated with various stem and progenitor cells, will also be assessed in terms of their functional vasculature assembly and the extent of a graft nutrient supply in vitro. Further preclinical in vivo work will validate the biocompatibility and safety of these scaffolds for transplantation.
Supplementary Material
Acknowledgments
This research is most generously supported by the Israeli Science Foundation (grant no. 1563/10) and the Singapore National Research Foundation under the CREATE program: The Regenerative Medicine Initiative in Cardiac Restoration Therapy Research.
The authors would like to acknowledge the help in the preparation and analyses of the MS data presented herein extended by the Smoler Proteomics Center at the Technion – Israel Institute of Technology.
Disclosure Statement
The authors declare that they have nothing to disclose.
References
- 1.Sarig U. Machluf M. Engineering cell platforms for myocardial regeneration. Expert Opin Biol Ther. 2011;11:1055. doi: 10.1517/14712598.2011.578574. [DOI] [PubMed] [Google Scholar]
- 2.Schaaf S. Shibamiya A. Mewe M. Eder A. Stohr A. Hirt M.N., et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PloS One. 2011;6:e26397. doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann W.H. Melnychenko I. Wasmeier G. Didie M. Naito H. Nixdorff U., et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 4.Sawa Y. Miyagawa S. Sakaguchi T. Fujita T. Matsuyama A. Saito A., et al. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today. 2012;42:181. doi: 10.1007/s00595-011-0106-4. [DOI] [PubMed] [Google Scholar]
- 5.Kochupura P.V. Azeloglu E.U. Kelly D.J. Doronin S.V. Badylak S.F. Krukenkamp I.B., et al. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112:I144. doi: 10.1161/CIRCULATIONAHA.104.524355. [DOI] [PubMed] [Google Scholar]
- 6.Robinson K.A. Li J. Mathison M. Redkar A. Cui J. Chronos N.A., et al. Extracellular matrix scaffold for cardiac repair. Circulation. 2005;112:I135. doi: 10.1161/CIRCULATIONAHA.104.525436. [DOI] [PubMed] [Google Scholar]
- 7.Ott H.C. Matthiesen T.S. Goh S.K. Black L.D. Kren S.M. Netoff T.I., et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 8.Singelyn J.M. DeQuach J.A. Seif-Naraghi S.B. Littlefield R.B. Schup-Magoffin P.J. Christman K.L. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eitan Y. Sarig U. Dahan N. Machluf M. Acellular cardiac extracellular matrix as a scaffold for tissue engineering: in vitro cell support, remodeling, and biocompatibility. Tissue Eng Part C Methods. 2010;16:671. doi: 10.1089/ten.TEC.2009.0111. [DOI] [PubMed] [Google Scholar]
- 10.Wainwright J.M. Czajka C.A. Patel U.B. Freytes D.O. Tobita K. Gilbert T.W., et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16:525. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B. Borazjani A. Tahai M. Curry A.L. Simionescu D.T. Guan J., et al. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J Biomed Mater Res A. 2010;94:1100. doi: 10.1002/jbm.a.32781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akhyari P. Aubin H. Gwanmesia P. Barth M. Hoffmann S. Huelsmann J., et al. The quest for an optimized protocol for whole heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Part C Methods. 2011;17:915. doi: 10.1089/ten.TEC.2011.0210. [DOI] [PubMed] [Google Scholar]
- 13.Weymann A. Loganathan S. Takahashi H. Schies C. Claus B. Hirschberg K., et al. Development and evaluation of a perfusion decellularization porcine heart model—generation of 3-dimensional myocardial neoscaffolds. Circ J. 2011;75:852. doi: 10.1253/circj.cj-10-0717. [DOI] [PubMed] [Google Scholar]
- 14.Christman K.L. Lee R.J. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Badylak S.F. Taylor D. Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaully T. Kaufman-Francis K. Lesman A. Levenberg S. Vascularization—the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15:159. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 17.Caspi O. Lesman A. Basevitch Y. Gepstein A. Arbel G. Habib I.H., et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu T. Sekine H. Yang J. Isoi Y. Yamato M. Kikuchi A., et al. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20:708. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 19.Sekine H. Shimizu T. Hobo K. Sekiya S. Yang J. Yamato M., et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 20.Tee R. Lokmic Z. Morrison W.A. Dilley R.J. Strategies in cardiac tissue engineering. ANZ J Surg. 2010;80:683. doi: 10.1111/j.1445-2197.2010.05435.x. [DOI] [PubMed] [Google Scholar]
- 21.Morritt A.N. Bortolotto S.K. Dilley R.J. Han X. Kompa A.R. McCombe D., et al. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- 22.Chen A.C. McNeilly C. Liu A.P. Flaim C.J. Cuttle L. Kendall M., et al. Second harmonic generation and multiphoton microscopic detection of collagen without the need for species specific antibodies. Burns. 2011;37:1001. doi: 10.1016/j.burns.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Lennon D.P. Caplan A.I. Isolation of rat marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1606. doi: 10.1016/j.exphem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Varshavsky A. Kessler O. Abramovitch S. Kigel B. Zaffryar S. Akiri G., et al. Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res. 2008;68:6922. doi: 10.1158/0008-5472.CAN-07-5408. [DOI] [PubMed] [Google Scholar]
- 25.Crapo P.M. Gilbert T.W. Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown B.N. Freund J.M. Han L. Rubin J.P. Reing J.E. Jeffries E.M., et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng Part C Methods. 2010;17:411. doi: 10.1089/ten.tec.2010.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham H.K. Horn M. Trafford A.W. Extracellular matrix profiles in the progression to heart failure. European Young Physiologists Symposium Keynote Lecture-Bratislava 2007. Acta Physiol (Oxf) 2008;194:3. doi: 10.1111/j.1748-1716.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 28.Human protein databas (HPRD) John hopkins university; Jul 4, 2011. Collagen, type II, alpha 1- site of expression. [Google Scholar]
- 29.Superti-Furga A. Rocchi M. Schafer B.W. Gitzelmann R. Complementary DNA sequence and chromosomal mapping of a human proteoglycan-binding cell-adhesion protein (dermatopontin) Genomics. 1993;17:463. doi: 10.1006/geno.1993.1348. [DOI] [PubMed] [Google Scholar]
- 30.Takemoto S. Murakami T. Kusachi S. Iwabu A. Hirohata S. Nakamura K., et al. Increased expression of dermatopontin mRNA in the infarct zone of experimentally induced myocardial infarction in rats: comparison with decorin and type I collagen mRNAs. Basic Res Cardiol. 2002;97:461. doi: 10.1007/s00395-002-0371-x. [DOI] [PubMed] [Google Scholar]
- 31.Forbes E.G. Cronshaw A.D. MacBeath J.R. Hulmes D.J. Tyrosine-rich acidic matrix protein (TRAMP) is a tyrosine-sulphated and widely distributed protein of the extracellular matrix. FEBS Lett. 1994;351:433. doi: 10.1016/0014-5793(94)00907-4. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto O. Fujiwara S. Abe M. Sato Y. Dermatopontin interacts with transforming growth factor beta and enhances its biological activity. Biochem J. 1999;337:537. [PMC free article] [PubMed] [Google Scholar]
- 33.Stratman A.N. Malotte K.M. Mahan R.D. Davis M.J. Davis G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalen H. Thorstensen A. Aase S.A. Ingul C.B. Torp H. Vatten L.J., et al. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr. 2010;11:176. doi: 10.1093/ejechocard/jep194. [DOI] [PubMed] [Google Scholar]
- 35.Badylak S.F. Gilbert T.W. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert T.W. Sellaro T.L. Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.