Summary
We describe a 44 year-old woman with dissection of the distal third of the basilar artery presenting with subarachnoid haemorrhage. She was treated with a Neuroform stent (Boston Scientific), the first stent designed for intracranial navigation and recently approved by the FDA to treat wide-necked cerebral aneurysms and allow reconstruction of the internal lumen. In our patient, the dissection involved the origin of the anterosuperior cerebellar artery and the origin of the right posterior cerebral artery. The stent was positioned without complications during or after the procedure. Intra and periprocedural thrombolytic therapy was given followed by an antiaggregant (100 mg Aspirin) for a year after treatment. Subsequent angio-MR and angiographic monitoring disclosed resolution of the dissection and normalization of the basilar artery lumen. She currently lives a controlled but normal life.
Key words: basilar artery, dissection, intracranial stent, MRI, MRA, subarachnoid haemorrhage
Case Report
A 44-year-old woman was taken to the emergency room for loss of consciousness (lasting about a minute) following violent headache. Alert, disorientated, poorly cooperative and in pain, she presented right-side weakness. The patient gradually regained consciousness and right motor strength. Brain CT scan showed diffuse subarachnoid haemorrhage mainly in the interpeducular region with mild dilation of the ventricular system. History-taking disclosed hepatitis C virus and untreated arterial hypertension. Seven to eight hours after the onset of symptoms, angiography revealed mild ectasia of the distal portion of the basilar artery confined to the left lateral part of the vessel with a right side hyperintense signal in SE T1 and T2 weighted images ascribed to an intramural thrombus. Follow-up angiography one week later confirmed the right lateral arterial dissection with formation of a pseudo-aneurysm involving the origin of the superior cerebellar artery and right posterior cerebral artery. The site and extension of the dissection were studied in detail together with the anatomy of the vessels involved and the compensatory circulation. A second minor dissection of the extracranial vertebral artery (intratransverse stretch) was also diagnosed in the C2 vertebral body. Endovascular treatment by stent placement was planned.
Endovascular Treatment
Angiography under general anaesthesia via the right femoral artery confirmed the dissection was unchanged. Under full heparinization a Neuroform stent 4.5 mm in diameter x 20 mm in length (Boston Scientific) was positioned in the distal stretch of the basilar artery. The final angiographic sequences showed a substantially regular arterial lumen and a proportional reduction of the false lumen of the dissection. Anticoagulant therapy with Enoxheparin (Clexane) was administered for 48h followed by antiaggregant. Cardioaspirin (100 mg) was subsequently given for a year. The patient was discharged ten days later after angio-MR follow-up disclosed a good flow image in the basilar artery and no evidence of the pseudo-aneurysm. The patient received a strictly planned monitoring schedule with MR and angio-MR scans repeated at one, three, eight and 15 months after the procedure. Monitoring findings showed a normalization and the patient currently leads a normal life under treatment for arterial hypertension.
Figure 1.
CT demonstrates diffuse subarachnoid haemorrhage mainly in prepontine and interpeduncular cisterns.
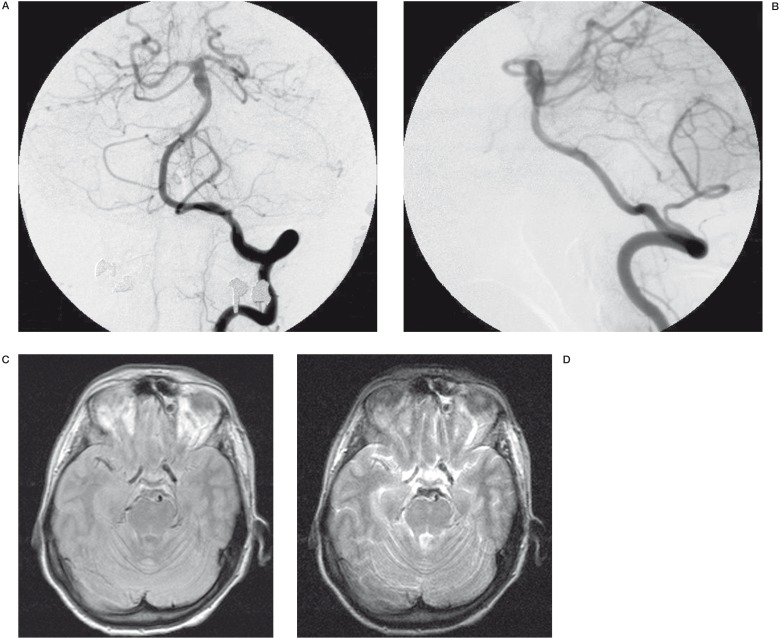
Figure 2.
A,B) Angiography A-P and L-L: mild ectasia of the distal porzion of the basilar artery confined to the lateral part of the vessel with formation of a pseudo-aneurysm involving the origin of the superior cerebellar artery and right posterior cerebral artery; MR Axial DP image C) and MR Axial T2-weighted image D) reveal hyperintense signal in the same part of the vessel ascribed to an intramural thrombus.
Discussion
The clinical signs of certebrobasilar dissection remain unsettled 1. Onset with subarachnoid haemorrhage or brain stem ischaemia required prompt treatment due to the high incidence of rebleeding 2,3. Rabinov 4 links the common onset with subarachnoid haemorrhage to the presence of a thinner tunica media in the intradural stretch of the vertebral artery and an adventiitia with fewer elastic fibres than the extracranial vertebral artery. This entails a detailed diagnostic work-up based on angiography MR and angio-MR scans. Hosoya et Al 5 claim that angiography is required to establish a final diagnosis of dissection whereas it is not essential in cases of intramural haematoma which is displayed in MR and angio-MR scans. In our patient, onset with suubarachnoid haemorrhage, negative angiographic findings in the anterior circulation and ectasia of the distal third of the basilar artery led to a suspicion of vessel dissection. MR and angio-MR scans were required for diagnostic confirmation in the acute phase. Only the combined analysis of both investigations disclosed a haematoma in the arterial wall leading to a suspicion of dissection. Angiographic follow-up better displayed the morphology of the dissection, clearly showing the dissected intimal flap and the associated dissecting aneurysm, demonstrating the dynamics of the lesion and its possible progression. The hyperintense signal encountered in the vessel in T1 weighted sequences suggests intramural haematoma. The diagnostic difficulty may be due to the fact that the signal hyperintensity may be more evident in subacute and chronic stages and masked by hyperintense cisternal material caused by bleeding. Biplanar angiography (ideally in 3D with the possibility of rotation) is essential for accurate diagnosis as it will help to outline the linear defect caused by the intimal flap thereby distinguishing between the true and false lumen. In addition, three-dimensional images may define the circumferential involvement of the dissecting aneurysm.
The choice of treatment has been based on the generally well established experience of the endovascular treatment of extracranial arterial dissection by deployment of the stent 6,7,8. The same approach in the treatment of intracranial arteries was made possible by the recent introduction of the first self-expanding stent devised for intracranial navigation, the Neuroform stent (Boston Scientific) known to be highly flexible and adaptable. The aim of treatment in cases of basilar artery dissection is to afford long-lasting protection, guarantee redistribution of arterial flow only within the vessel lumen and to offer physical support to endothelial regrowth 9. According to Fiorella 9, dissecting fusiform aneurysms are suitable for stenting as they lack a defined neck and often involve the whole circumference of the vessel wall. In addition, the elasticity of the intimal flap adapts it to the pressure exerted by the stent itself. As the device is self-expanding, the vessel wall can be remodelled without the risks linked to the placement of a balloon expandable stent.
Figure 3.
Endovascular treatment: Neuroform Stent was positioned in the distal portion of the basilar artery. Plain film shows the radiopaque distal markers of the stent.
Although only preliminary findings are available on the use of the Neuroform stent to treat arterial dissections, having been designed for wide-necked aneurysms, the device has long been used in stenting vertebrobasilar and isolated basilar artery dissection 11,3. To date, only three cases of vertebrobasilar stenting have been described 9 treated with three Neuroform stents in one, two in one and only one in the third. The problems encountered are linked to locating the site for stent release, the risks of handling the microguide and microcatheter during insertion into the dissection site and subsequently during stent release. In our case, the vertebral artery did not present significant tortuosity during navigation and the microguide was inserted beyond the left P1 without difficulty. A delicate anatomical point was seen in the extracranial portion of the vertebral artery which had a small parietal dissection with an intimal flap of a few millimetres. This dissection was not treated as some resistance was felt on delicately probing the flap with the microguide, as in a stable situation. The Neuroform stent was released with its distal tips positioned exactly in the apex of the vessel to enclose the origin of the vessels involved in the dissection, the posterior cerebral artery and the superior cerebellar artery which remained patent. In our case, the area to be treated was pinpointed exactly without a too proximal release. Subarachnoid haemorrhage was a controindication to antiaggreganting therapy prior to the procedure. Stent thrombogencity subsequently required anti-aggregant treatment for a year after the procedure.
Figure 4.
MRA two weeks after treatment demonstrates the normalization of the distal basilar artery lumen (A and B), with good signal of flow at P1 bilaterally.
Figure 5.
MRA 1 year after treatment shows a good signal of flow at the level of the distal portion of the basilar artery and at P1 bilaterally with a right diameter of these vessel. MRA also shows the stent like an abcense of flow around the hyperintense signal (A and B).
Conclusions
Our patient was successfully treated for dissection of the distal third of the basilar artery using a Neuroform stent, new titanium device for intracranial navigation. Although this stent was designed for wide-necked aneurysms, it can also be used for the endovascular treatment of other conditions. Stent placement in cases of basilar artery dissection offers a solution to a condition otherwise difficult to treat.
References
- 1.Hosoya Stroke. 2003;30:1083. [Google Scholar]
- 2.Mizutani T, Aruga T, Kirino T. Recurrent Subarachnoid Haemorrhage from Untreated Ruptured Vertebrebobasilar Dissecting Aneurysms. Neurosurgery. 1995;36:905–911. doi: 10.1227/00006123-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Willing SJ, Skidmore F, et al. Treatment of Acute Intracranial Vertebrobasilar Dissection with Angioplasty and Stent Placement: Report of Two Cases. Am J Neuroradiol. 2003;24:985–989. [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinov JD, Frank RH, et al. Endovascular Management of vertebrobasilar Dissecting Aneurysms. Am J Neuroradiol. 2003;24:1421–1428. [PMC free article] [PubMed] [Google Scholar]
- 5.Hosoya T, Adachi M, et al. Clinical and Neuroradiological Featurs of Intracranial Vertebrobasilar Artery Dissection. Stroke. 1999;30:1083–1090. doi: 10.1161/01.str.30.5.1083. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg R, Khwaja J, et al. Aortic dissection: new prospective and treatment paradigms. European Journal of Vascular and Endovascular Surgery, 2003;26:570–586. doi: 10.1016/s1078-5884(03)00415-5. [DOI] [PubMed] [Google Scholar]
- 7.Biggs KL, Chiou AC, et al. Endovascular repair of a spontaneous artery dissection with carotid stent and coils. J Vasc Surg. 2004;40:170–173. doi: 10.1016/j.jvs.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Schievink WI. The treatment of spontaneous carotid and vertebral artery dissections. Curr Opin Cardiol 2000. 2000;15:316–321. doi: 10.1097/00001573-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Fiorella D, Albuquerque FC, et al. Preliminary experience using the Neuroform Stent for the Treatment of Cerebral Aneurysms. Neurosurgery. 2004;54:6–17. doi: 10.1227/01.neu.0000097194.35781.ea. [DOI] [PubMed] [Google Scholar]
- 10.Howington JU, Hanel RA, et al. The Neuroform Stent, the first Microcathether-delivered stent for use in intracranial circulation. Neurosurgery. 2004;54:2–5. doi: 10.1227/01.neu.0000099370.05758.4d. [DOI] [PubMed] [Google Scholar]
- 11.Price R, Sellar R, et al. Traumatic Vertebral Artery Dissection and Vertebrobasilar Arterial Thrombosis Successfully Treated with Endovascular Thrombolysis and Stentig. Am J Neuroradiol. 1998;19:1677–1680. [PMC free article] [PubMed] [Google Scholar]
- 12.Broadbent LP, Moran CJ, et al. Management of Neuroform Stent Dislodgement and Misplacemen. Am J Neuroradiol. 2003;24:1819–1822. [PMC free article] [PubMed] [Google Scholar]
- 13.Horowitz MB, Purdy PD. The use of stents in the management of neurovascular disease: a review of historical and present status. Neurosurgery. 2000;46:1335–1342. doi: 10.1097/00006123-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Malek AM, Higashida RT, et al. Tandem intracranial stent deployment for treatment of an iatrogenic, flow-limiting, basilar artery dissection: technical case report. Neurosurgery. 1999;45:919–924. doi: 10.1097/00006123-199910000-00042. [DOI] [PubMed] [Google Scholar]