Summary
We report on a case of cerebral venous thrombosis (CVT) induced by oral contraception (OC) activated coagulopathy and its endovascular treatment. Deep venous system and dural sinuses thrombosis complicated with severe neurological deficit and coma due to right thalamic edema and ischemia in a young woman was treated by local thrombolysis with an administration of 0.6mg/h of the rtPA and the concomitant intravenous unfractioned heparin infusion (700 IU/h). 3D-Xra digital rotational venography performed at the beginning and after treatment confirmed thrombus resolution with rapid flow restoration. Dynamic flow imaging gives interesting information on the deep venous system and the cortical venous collectors drainage. Final NIHSS (National Institute of Health Stroke Scale) and mRS (modified Rankin Scale) confirmed an excellent clinical outcome of the interventional therapy.
Key words: cerebral venous thrombosis, 3D-Xra rotational venography, local rtPA thrombolysis, coagulopathy, oral contraception
Introduction
The risk factors associated with venous thrombosis include tissue damage and stasis, dehydratation, and congenital or aquired abnormalities of the hemostatic system. Hypercoagulable states related to the use of oral contraceptives, pregnancy, puerperium and malignancy represent a group with an increased risk for venous thrombosis 1. This case report illustrates a rare case of rapid clinical progression with a progressive loss of consciousness leading to a comatose state despite management using conventional treatment protocols in a young woman with deep venous system thrombosis treated by local thrombolytic therapy with rt-PA.
Patient Characteristics
A 24-year-old woman without any previous diseases, abortion or thrombosis history, currently on the oral contraceptive (Novynette) medication was admitted on the afternoon of May 16, 2004, to the neurological ICU of the municipal hospital with severe headache lasting one week localized behind the ears. This was accompanied by vomiting during the previous two days. Neurologically, a somnolentia of the IVth grade, apathy, dysarthria, four-fingers nuchal rigidity with a GCS of 14 points was found at the time of admission. In the evening CT scan plus conventional DSA was completed; the results showed a partial superior sagittal sinus, complete right lateral sinus, right sigmoideal sinus, vein of Galen and a straight sinus thrombotic occlusin with the thalamus and basal ganglia deep venous system drainage stagnation. The CT scan confirmed the right thalamus and basal ganglia hypodensity, the right temporooccipital area edema and a small haemorhage in the right lateral and sigmoid sinus area.
Neurological examination disclosed left sided hemiparesis, divergent strabismus due to the supranuclear lession of the IIIrd cranial nerve (thalamic ischemia and edema with brain stem compression), bilateral miosis, no papilledema, nuchal rigidity, tachycardia of the rate 120 b/min. The patient was intubated and put on artificial ventilation due to the progressive loss of consciousness leading into a comatose state. Due to this particular state the patient had to be transferred to the anaesthetic-resuscitation department. Fragmin (Dalteparin) 1000 IU/h anticoagulation and Amoksiklav (Amoxycilin + Clavulonic acid) 1,2g/8h + Ciplox (Ciprofloxacin) 100 ml/12h antibiotic therapy was administered. Ventilation with FiO2 0.3, Vt 550ml, PEEP of 5 cm of H20 was maintained. The ECG was normal showing no signs of any ischemia, 120 b/min. A follow up MRI + MR venography showed and confirmed right mesencephalon and diencephalon, right thalamic and basal ganglia ischemia plus deep veins and dural sinuses thrombosis.
Biochemical Analysis
Creat - O.72mg/dl, Glucose - 109.9 mg/dl, CRP-107.7 g/l, Coagulation: INR - l.41, Fibrinogen - 4.69 g/l, D-Dimers - 6x elevated, protein C - 45%, free protein S - 56%,APC rezistance - negative - 4,79l,AT III - 74%, Fact. II - 76% and factor VIII - 112% (percentage of standard).
On day five after admission severe symptoms with a loss of the horizontal and vertical occulocephalic reflexes progressed despite the use of the conventional anticoagulation and additional antiedematous osmotherapy protocols. The patient was referred to our hospital for CVT interventional treatment. After admission a control DSA was performed with 3D-XRA venous phase reconstruction. Incomplete internal cerebral vein, vein of Galen and straight sinus occlusion, complete right transverse sinus and sigmoid sinus thrombosis were visualised. Based on that diagnostic imaging the decision for local thrombolysis with rtPA was made.
Description of the Technique
A 5F Terumo (Radiofocus,Tokyo, Japan) sheath was introduced into the right common femoral vein for the venous approach and another 5F Terumo sheath was inserted into the right common femoral artery for right common carotid artery angiography. A positional check DSA was performed by the 4F Vertebral Aqua - Tempo catheter (Cordis - Endovascular, JJ, Miami, FL) with 3D-Xra venous phase reconstruction. The result showed complete right transverse sinus, sigmoid sinus, right internal jugular vein in the jugular foramen region and incomplete thrombosis of the straight sinus, vein of Galen, basal vein of Rosenthal (figure 1).
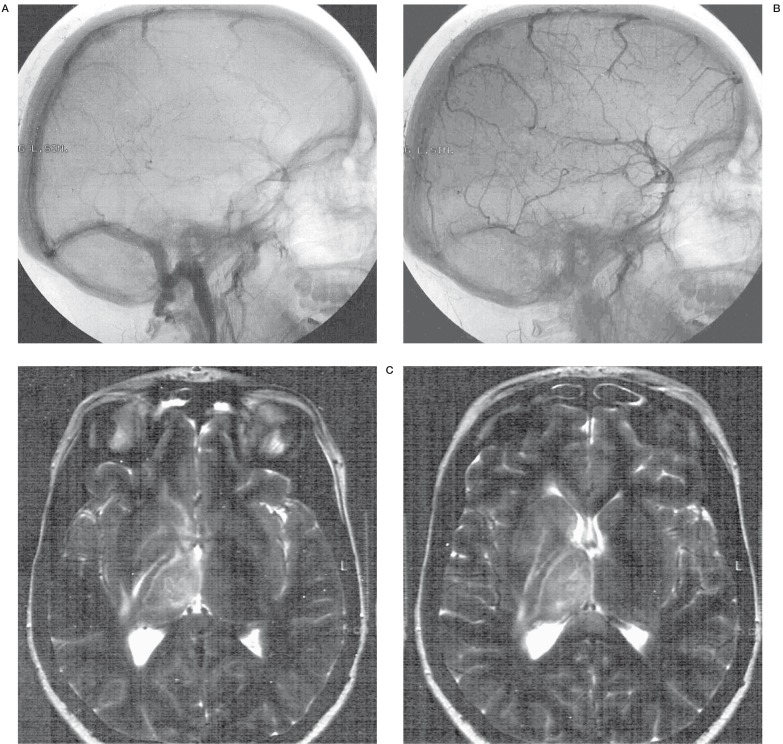
Figure 1.
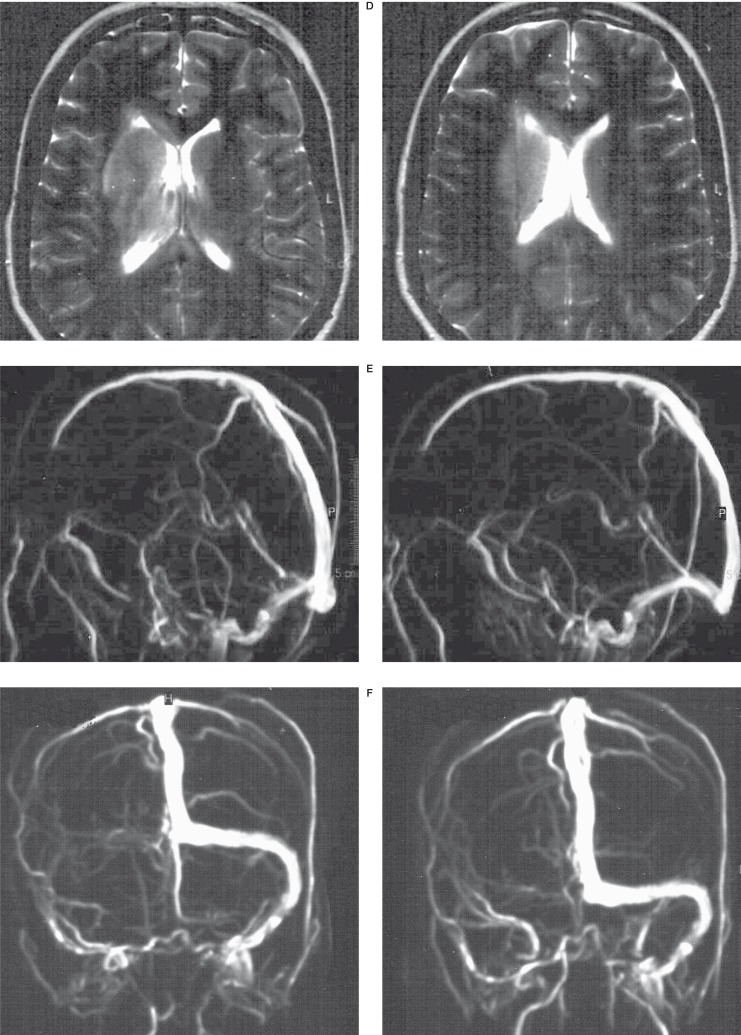
DSA,MRI + MR venography of a 24-year-old woman with CVDS thrombosis. A, B) DSA (day 1) showing no flow in the straight sinus, vein of Galen, internal cerebral veins and Basal vein of Rosenthal, cortical vein drainage was visible. C,D) T2-weighted images (day 4)- deep right thalamic and basal ganglia swelling and hyperintensity E) Lateral MR venography view (day 4) - partial superior sagittal sinus, straight sinus, complete transverse sinus thrombosis, no flow in basal vein of Rosenthal. F) Anter-posterior MR venography view - right transverse and sigmoid sinus thrombosis.
5000 units of UF Heparin were administered intraarterially. Another 4F Vertebral Aqua - Tempo catheter was introduced over the Terumo 035´/260cm (Radiofocus,Tokyo, Japan) into the right internal jugular vein and through the thrombus, it was gently navigated over the wire into the transverse sinus near the torculla (figure 2). Local thrombolysis with continuous rtPA dose of 0.6 mg/h was administered along with concomitant intravenous UF 700 IU/h Heparin infusion to achieve therapeutic anticoagulation (activated partial thromboplastin time aPTT - twice control) was started at the end of the day five. Fibrinogen, aPTT and the platelet count was checked every six hours. The duration of the thrombolytic therapy was 48 hours.
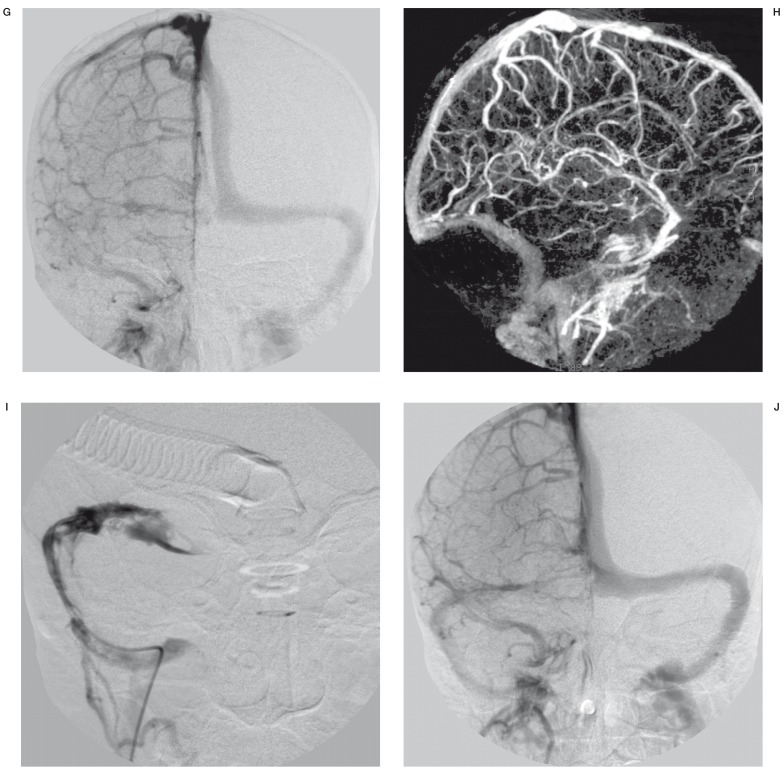
Figure 2.
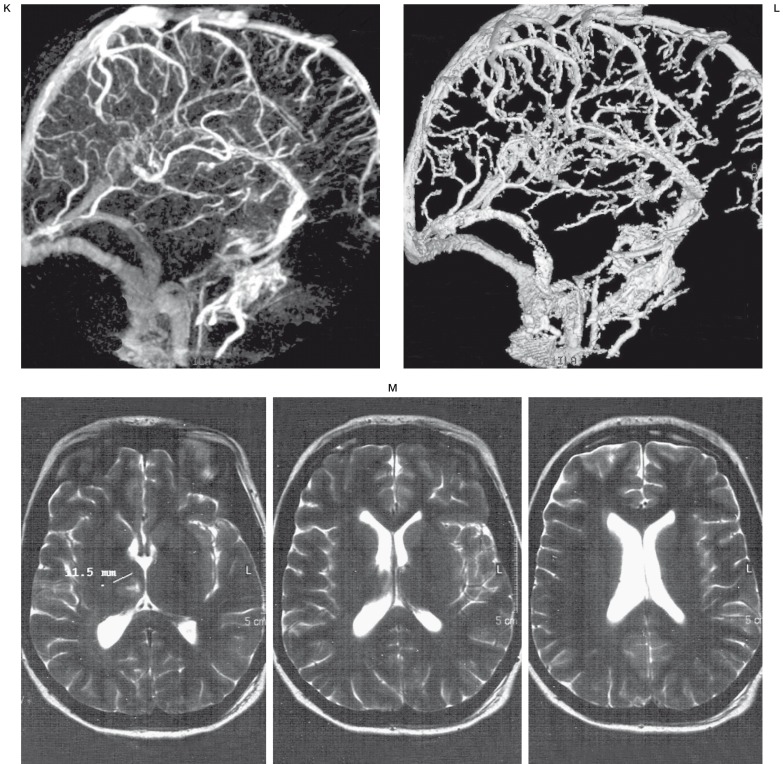
Interventional procedure + 3D-Xra control angiography and MRI after thrombolysis G) Anteroposterior DSA (day 5) - right transverse sinus and sigmoid sinus thrombosis H) 3D-Xra rotational angiography, remaining thrombosis of the straight sinus, vein of Galen and basal vein of Rosenthal. Right transverse and sigmoid sinus occlusion. I) 4F-Vertebral Tempo Cordis JJ catheter introduced into the right transverse sinus near the Torcular, through the fresh thrombus for local thrombolysis. J) Anteroposterior DSA (day 7) - 48 hours of thrombolysis - recanalisation of the right transverse and sigmoid sinus. K,L) 3D-Xra angiography with complete good straight sinus, vein of Galen, basal vein of Rosenthal and internal cerebral vein opacification. M) Follow-up MRI after one month with remaining small ischemia in the right thalamic area.
On May 22nd (day 7) the follow-up angiography with 3D-Xra venography for therapeutic effect was reassessed and the result showed deep vein system-right internal cerebral vein, basal vein of Rosenthal, vein of Galen, straight sinus and the right transverse and sigmoid sinus with internal jugulur vein bulb recanalisation and rapid cortical vein colectors drainage had been achived. Thrombolytic rtPA infusion was discontinued after flow restoration was documented. There was no bleeding on the follow-up CT scan and the follow-up MRI scan confirmed the regression of the vasogenic edema in the region of the basal ganglia and right thalamus.
After this, the patient's symptoms resolved rapidly in two days. She awoke from her coma and was extubated 24 hours after discontinuation of thrombolytic therapy. Follow-up NIHSS of 4 points on day seven after the interventional treatment confirmed excellent outcome with a modified Rankin scale (mRS) - 0p at the follow-up one month later. Coagulopathy evaluation with CVT genotyping confirmed FV -R/Q 506, FII 20210 G/Anormal genotype, MTH-FR: A/V 223 heterozygote and PAI-I 4G/5G homozygote genotype. The patient was put on 3 mg twice daily Lawarin anticoagulation treatment with INR-checked monthly.
Follow-up neuropsychological testing showed results of WAIS-R: IQ global 141, verbal 128 and nonverbal 146 with the Wechsler memory quotient test of MQ 101 - intellectual functions in the high range, good psychomotor speed, verbal expression and memory functions were apparent three months after the interventional treatment.
Discussion
The first clinical description and autopsy findings of cerebral venous thrombosis were made by Ribes in 1825 in a 45-year-old man with disseminated malignancy. In the pre-angiography era, the clinical presentation of progressive headache, papilledema, seizures, focal deficits, and coma led to a diagnosis of cerebral venous thrombosis, which was usually confirmed by the pathological findings of thrombosis of the major venous sinuses accompanied by haemorrhagic infarction.
In 2001 the International Study of Cerebral Vein Thrombosis completed the prospective enrollment of 624 patients with this disorder and has provided a wealth of clinical data 2. The deep cerebral veins, including the internal cerebral veins, and basal vein of Rosenthal, drain the deep white matter of the cerebral hemispheres and the basal ganglia. They are affected in 10% of cerebral venous thrombosis cases. Severe presentations include focal findings, such as hemipharesis or aphasia, due to bilateral or unilateral edema and haemorhagic infarction of the basal ganglia and thalami on brain imaging. Restricted water diffusion suggesting vasogenic edema is commonly found in subjects with acute CVT 3,4,5. Diagnosis of CVT in our patient was confirmed by MR imaging with MR venography and digital subtraction angiography (DSA). Liang et Al confirmed the superiority of three-dimensional contrast-enhanced MP-RAGE venography in the diagnosis of dural sinus thrombosis which was also useful in our patient 6. CT venography recently with fully automatic masking technique matched mask bone elimination (MMBE) is an alternative option for CVT diagnostic imaging 7. 3D-Xra-DSA with 3D-venography is an excellent alternative with the possibility of flow speed and direction demonstration even in the area of deep venous system.
Treating patients with intracranial venous thrombosis is dependent on the ability to clinically suspect and radiologically confirm this diagnosis. Purdon Martin and Sheehan (1941) were the first to propose anticoagulation therapy. The use of heparin is currently the first-line treatment for CVT 2. However, the effect of heparin may be too slow to help a subgroup of patients with rapidly progressive symptoms and involvement of large parts of the dural sinuses and deep cerebral veins. This subgroup should be considered for thrombolytic therapy because of the overall mortality rate of 10% 8,9,10,11. Interventional therapy with mechanical restoration of flow with clot-buster rheolytic thrombectomy was also recently demonstrated 12,13.
Deficiency of protein C, protein S, antithrombin, and factor V Leiden carrier status or resistance to activated protein C are estimated to account for 25%-35% of all occurrences of venous thrombosis 14,15.
Hormonal contraception is used by more than 100 million women throughout the world. Shortly after its introduction in the 1960s oral contraception (OC) was linked to an increased incidence of thrombovascular disease 16,17,18 This is mediated by its effects on the haemostatic system. Procoagulation activity caused by increased activity of coagulation factors VII, X and fibrinogen is a common finding in almost all preparations 19. Several studies reported that the use of oral contraceptives leads to an increased resistance to activated protein C the plasma levels of which are elevated while at the same time the plasma level of protein S decline. Fibrinolysis is stimulated in OC users. Increased fibrinolytic activity is caused by elevated plasminogen level and a higher tissue plasminogen activator (tPA) activity. Plasminogen-activator inhibitor I (PAI - I) antigen and activity decrease 20. Increased clotting activity mediated by platelet activation was also observed 21. The complex changes of the haemostatic system in OC users include stimulated procoagulation, inhibited anticoagulation and elevated fibrinolytic activity.
Novynette® is a combined low-dose oral contraception containing 20 µg of ethinyl oestradiol and 150 µg of the third generation progestogen - desogestrel. Low dose oral contraceptives with third-generation progestogens confer a higher risk of venous thrombosis than the previous generation of contraceptives. This risk is also present in women without factor V Leiden mutation or a positive family history 22. These complicated haemostatic changes combined with a homozygous form of PAI - I mutation (4G/5G) predispose OC users to a significantly higher risk of thrombotic complications as seen in our patient as homozygote genotype.
Conclusions
Direct endovascular thrombolytic therapy may dramatically improve clinical outcome in patients with deep cerebral venous system thrombosis. MRI and MR venography or 3D-XRA DSA venography can speed up the diagnostic process in a subgroup of patients with deep veins involvement and rapid clinical symptoms progression. Thrombolytic therapy can also prevent or reduce the risk of secondary complications after CVT like chronic hih intracranial preassure with visual loss and/or secondary pial or dural arteriovenous malformations 23,24.
References
- 1.Berenstein A, Lasjaunias P, Ter Brugge K.G. Venous Occlusive Disease. Surgical Neuroangiography. 2nd edition. vol 2.1 . Berlin Heidelberg: Springer-Verlag; 2004. Clinical and Endovascular Treatment Aspects in Adults; pp. 135–152. [Google Scholar]
- 2.Ferro JM, Canhao P, et al. Prognosis of cerebral vein and dural sinus thrombosis, results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 3.Forbes P.N.K, Pipe JG, Heiserman JE. Evidence for cytotoxic edema in the pathogenesis of cerebral venous infarction. Am J Neuroradiol. 2001;22:450–455. [PMC free article] [PubMed] [Google Scholar]
- 4.Peeters E, Stadnik T, et al. Diffusion-weighted MR imaging of an acute venous stroke. Am J Neuroradiol. 2001;22:1949–1952. [PMC free article] [PubMed] [Google Scholar]
- 5.Ducreux D, Oppenheim C, et al. Diffusion-weighted imaging patterns of brain damage associated with cerebral venous thrombosis. Am J Neuroradiol. 2001;22:261–268. [PMC free article] [PubMed] [Google Scholar]
- 6.Liang L, Korogi Y, et al. Evaluation of the intracranial dural sinuses with a 3D contrast-enhanced MP-RAGE sequence: prospective comparison with 2D-TOF MR venography and digital subtraction angiography. Am J Neuroradiol. 2001;22:481–492. [PMC free article] [PubMed] [Google Scholar]
- 7.Majoie CHB, van Straten M, Venema HW. Multisection CT Venography of the dural sinuses and cerebral veins by using matched mask bone elimination. Am J Neuroradiol. 2004;25:787–791. [PMC free article] [PubMed] [Google Scholar]
- 8.Rael JR, Orrison WW, Jr, et al. Direct thrombolysis of superior sagittal sinus thrombosis with coexisting intracranial haemorrhage. Am J Neuroradiol. 1997;18:1238–1242. [PMC free article] [PubMed] [Google Scholar]
- 9.Spearman MP, Jungreis ChA, et al. Endovascular thrombolysis in deep cerebral venous thrombosis. Am J Neuroradiol. 1997;18:502–506. [PMC free article] [PubMed] [Google Scholar]
- 10.Ciccone A, Canhao P, et al. Thrombolysis for cerebral vein and dural sinus thrombosis. Cochrane Corner. Stroke. 2004;35 doi: 10.1002/14651858.CD003693.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey JL, Murro GJ, et al. Cerebral venous thrombosiscombined intrathrombus rtPA and intravenous heparin. Stroke. 1999;30:489–494. doi: 10.1161/01.str.30.3.489. [DOI] [PubMed] [Google Scholar]
- 12.Opatowsky MJ, Morris PP, et al. Rapid thrombectomy of superior sagittal sinus and transverse sinus thrombosis with a rheolytic catheter device. Am J Neuroradiol. 1999;20:414–417. [PMC free article] [PubMed] [Google Scholar]
- 13.Dowd ChF, Malek AM, et al. Application of rheolytic thrombectomy device in the treatment of dural sinus thrombosis: A new technique. Am J Neuroradiol. 1999;20:568–570. [PMC free article] [PubMed] [Google Scholar]
- 14.Provenzale JM, Barboriak DP, et al. Antiphospholipid Antibodies: Findings at Arteriography. Am J Neuroradiol. 1998;19:611–616. [PMC free article] [PubMed] [Google Scholar]
- 15.Reuner KH, Ruf A, et al. Prothrombin Gene G20210. A transition is a risk factor for cerebral venous thrombosis. Stroke. 1998;29:1765–1769. doi: 10.1161/01.str.29.9.1765. [DOI] [PubMed] [Google Scholar]
- 16.Lüdemann P, Nabavi DG, et al. Factor V Leiden mutation is a risk factor for cerebral venous thrombosis. A Case - control study of 55 patients. Stroke. 1998;29:2507–2510. doi: 10.1161/01.str.29.12.2507. [DOI] [PubMed] [Google Scholar]
- 17.Cantu C, Alonso E, et al. Hyperhomocysteinemia, low folate and vitamin B12 concentrations, and methylene tetrahydrofolate reductase mutation in cerebral venous thrombosis. Stroke. 2004;35:1790–1794. doi: 10.1161/01.STR.0000132570.24618.78. [DOI] [PubMed] [Google Scholar]
- 18.Rosendaal FR, Helmerhorst FM, Vandenbroucke JP. Oral contraceptives, hormone replacement therapy and thrombosis. Thromb Haemost. 2001;86:112–123. [PubMed] [Google Scholar]
- 19.Bloemenkamp KWM, Helmerhorst FM, et al. Thrombophilias and gynaecology. Best Practice & Research Clinical Obsterics & Gynaecology. 2003;17(3):509–528. doi: 10.1016/s1521-6934(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 20.Norris LA, Bonnar J. The effect of oestrogen dose and progesteron type on haemostatic changes in women taking oral contraceptives. Br J Obstet Gynaecol. 1996;103:261–267. doi: 10.1111/j.1471-0528.1996.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Kunz F, Pechlander C, et al. Influence of oral contraceptives on coagulation tests in native blood and plasma. Am J Obstet Gynaecol. 1990;163:417–420. doi: 10.1016/0002-9378(90)90593-v. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Cardiovascular disease and steroid hormone contraception. Report of a WHO Scientific group. WHO Technical Report Series, n. 877. Geneva: World Health Organization; 1998. [PubMed] [Google Scholar]
- 23.Stolz E, Gerriets T, et al. Intracranial venous haemodynamics is a factor related to a favorable outcome in cerebral venous thrombosis. Stroke. 2002;33:1645–1650. doi: 10.1161/01.str.0000016507.94646.e6. [DOI] [PubMed] [Google Scholar]
- 24.Phatouros C, Halbach VV, et al. Acquired pial arteriovenous fistula following cerebral vein thrombosis. Stroke. 1999;30:2487–2490. doi: 10.1161/01.str.30.11.2487. [DOI] [PubMed] [Google Scholar]