Summary
A 22 year old female presented in 1987 a cardiac myxoma, removed in 1991.
In 1992 catheter angiography showed bilateral aneurysms. Conservative treatment was elected. In 2000 recurrence of the myxoma and subsequent removal, prompted new angiography. All aneurysms had decreased in size, some had spontaneously disappeared.
Key words: angiography, cerebral aneurysm, cardiac myxoma, metastasis
Introduction
A then 22-year-old women was admitted to hospital for the first time in 1985 because of recurrent leg pain. In her history she recalled cutaneous tumors. Since 1986 she has suffered from leg pain of increasing intensity. In that year episodes of pain occurred with fever leading to tentative diagnosis of bacterial endocarditis.
The immediately performed echocardiogram demonstrated a mass of 35 by 45 by 35 mm in the left ventricle. The suspected myxoma was surgically removed and confirmed histologically as papillary myxoma.
In 1987 the patient showed first symptoms of acromegaly. Because of continuing pain in both legs catheter angiography of the lower limbs was performed nine months later. Vascular occlusion could be demonstrated in both extremities. Symptoms improved after abdominal sympathectomy.
In October 1991 a recurrent tumor in the left atrium was removed.
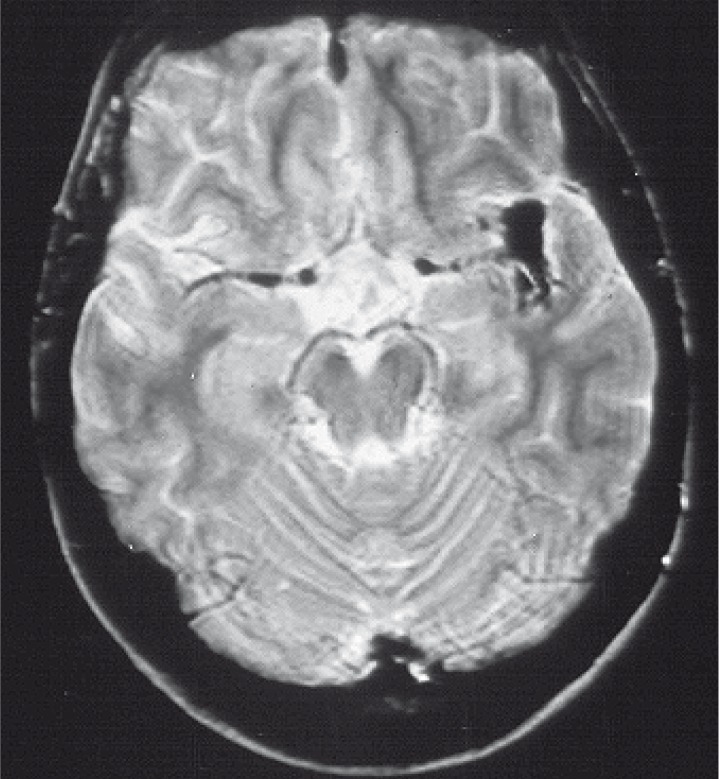
Because of acromegaly cranial magnetic resonance imaging (MRI) was performed in 1991. Images demonstrated hypophyseal adenoma. The additional finding of an unusual shape of trifurcation of left MCA led to cerebral catheter angiography (figure 2).
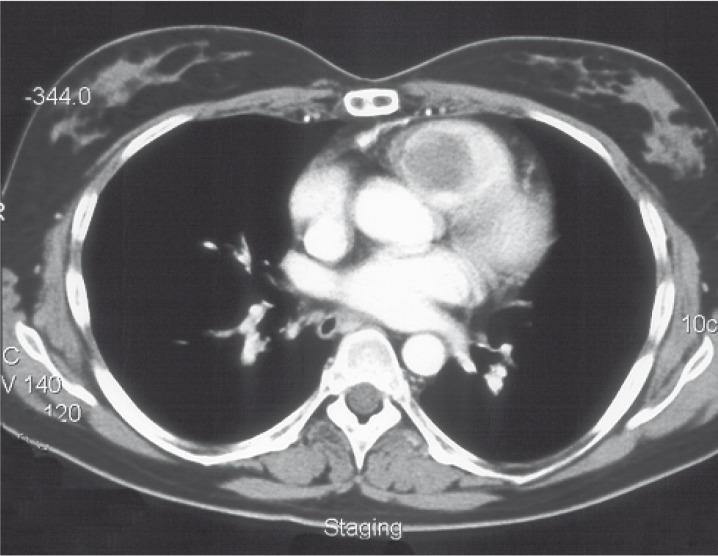
Figure 1.
CT scan demonstrates right ventricular mass (myxoma).
Figure 2.
Axial T2w image demonstrates unusual shape of trifurcation of left MCA.
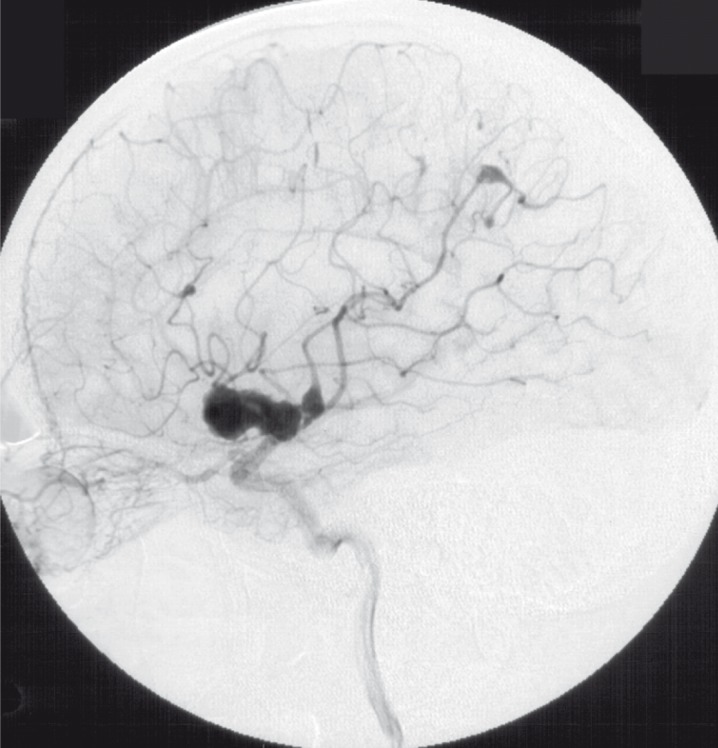
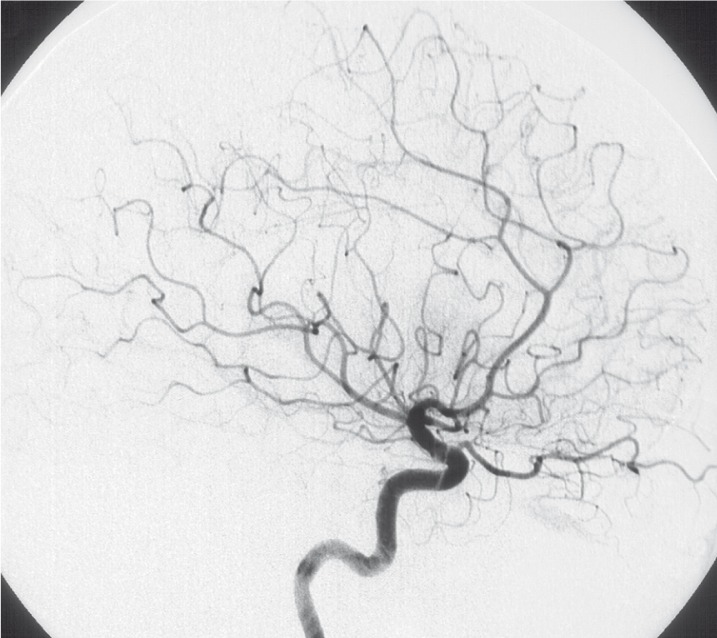
This angiography (1992) showed at least six aneurysms in both middle cerebral arteries (MCA) and right anterior cerebral artery (ACA). The largest aneurysm located at the trifurcation of the left MCA had a size of 35 x 25 x 15 mm and was slightly lobulated. All proximal branches of the trifurcation and the distal M2 segment were enclosed by the pouch of this aneurysm and could not be shown distinct in spite of multiple projections.
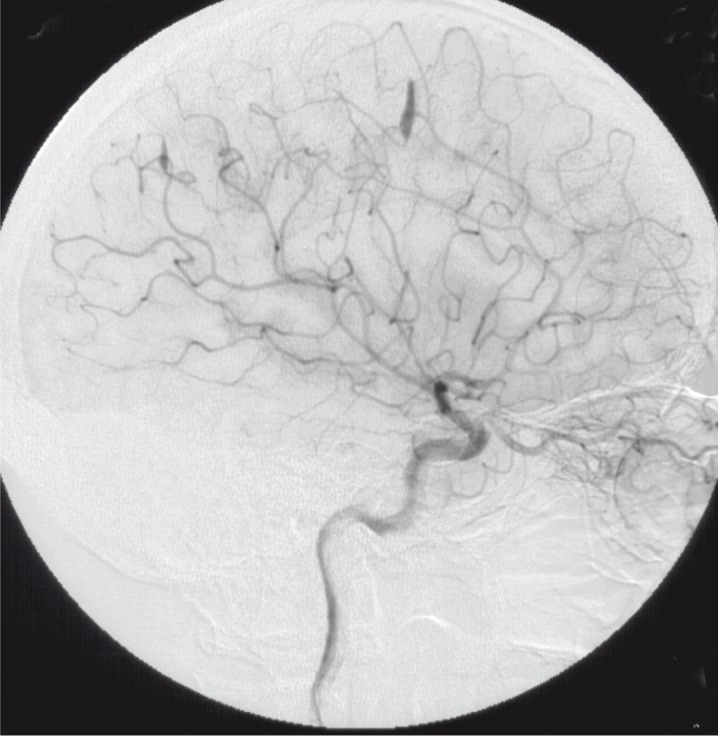
The posterior branch of the left MCA showed another two aneurysms: one straight adjacent to the large aneurysm mentioned before, and the other one in the distal occipital segments. Furthermore, another smaller aneurysm could be demonstrated at the insular branches of the left MCA (figure 3,4).
Figure 3.
Injection of left ICA lateral view (1992).
Figure 4.
Injection of left ICA frontal view (1992).
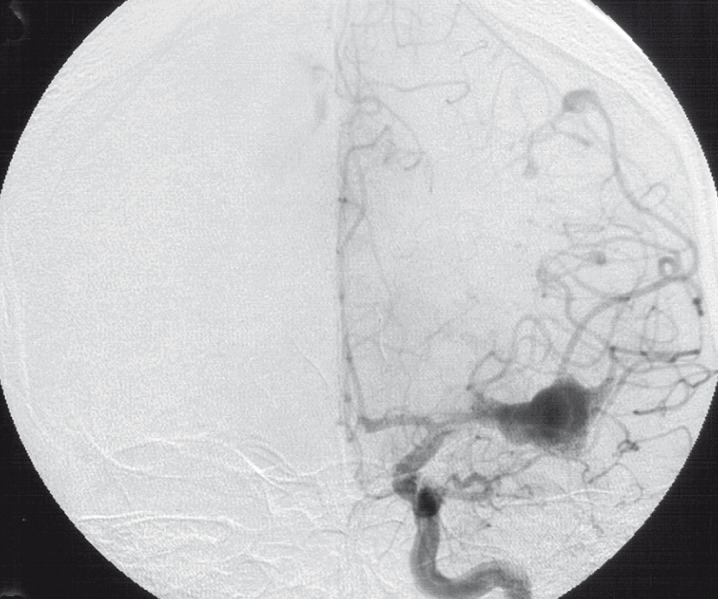
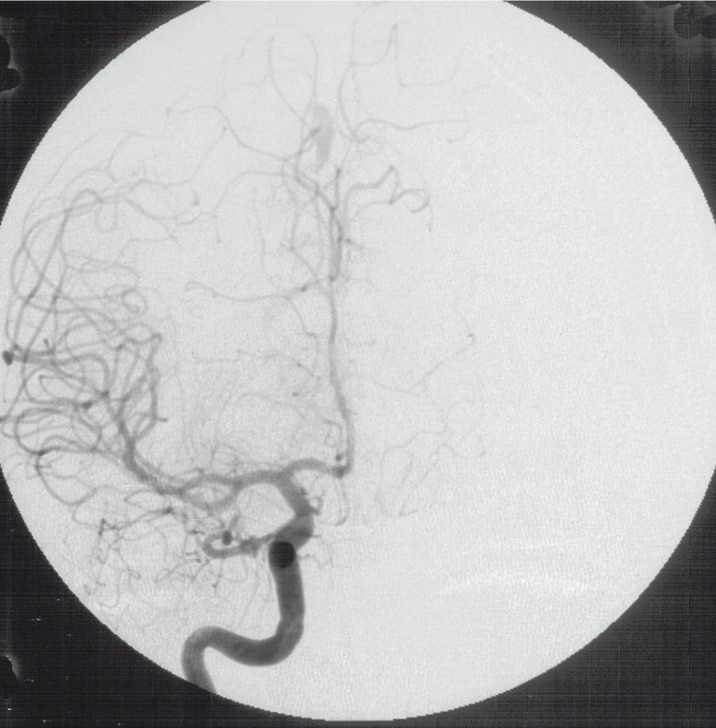
Two other aneurysms were detected at the distal occipital branches of the right MCA and at the distal segments of the right ACA. Both were of tubular and elongated shape (figure 5,6).
Figure 5.
Injection of right ICA lateral view (1992).
Figure 6.
Injection of right ICA frontal view (1992).
In the posterior circulation aneurysms could not be demonstrated.
In 2000 the patient was readmitted to the hospital because of cardiac dysfunction. A second recurrence of the tumor was detected in the right ventricle of the heart and again surgically removed again with the histological diagnosis of myxoma (figure 1).
The patient never suffered from neurological symptoms. After consultation with neuro-surgeons and with the consent of the patient the decision was made to perform repeat catheter angiography to determine therapeutic options.
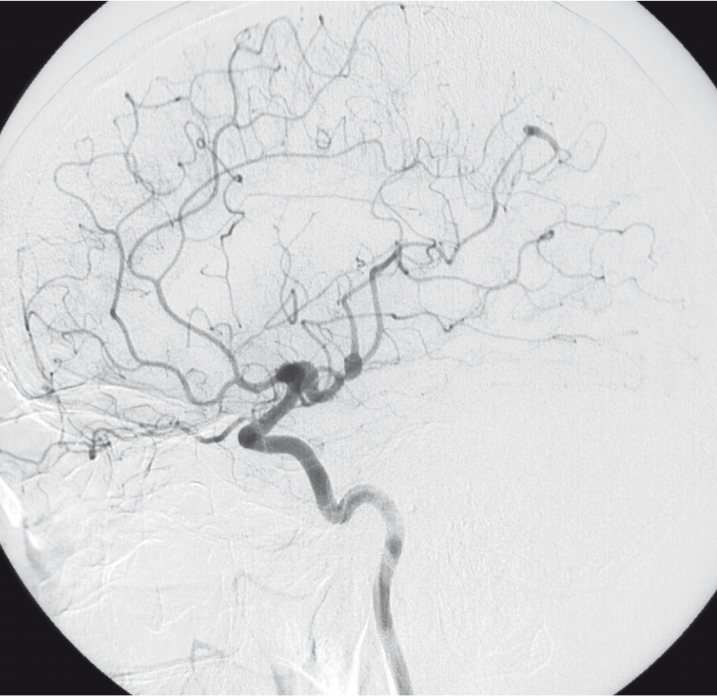
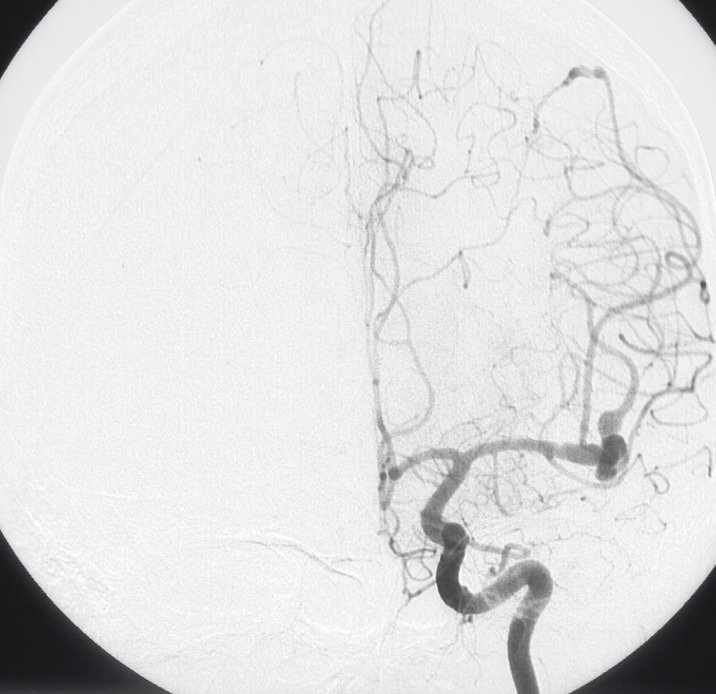
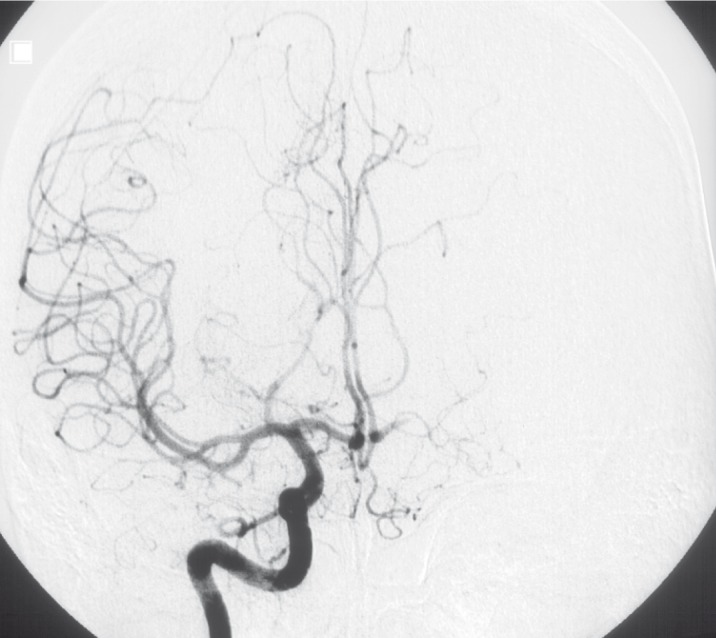
Angiography showed major changes compared with the angiogram performed 11 years before. Aneurysms on the right side were no longer present, the vessels were of normal shape and direction (figure 9,10). The left sided aneurysm of the occipital branch was somewhat more fusiform but had not grown in size. The aneurysm of the insular branch was not present. The large lobulated aneurysm of the left MCA appeared smaller and of fusiform shape. Branches of the left MCA trifurcation could be demonstrated separated from the aneurysm (figure 7,8). No new aneurysm was uncovered.
Figure 7.
Injection of left ICA lateral view (2003).
Figure 8.
Injection of left ICA frontal view (2003).
Figure 9.
Injection of right ICA lateral view (2003).
Figure 10.
Injection of right ICA frontal view (2003).
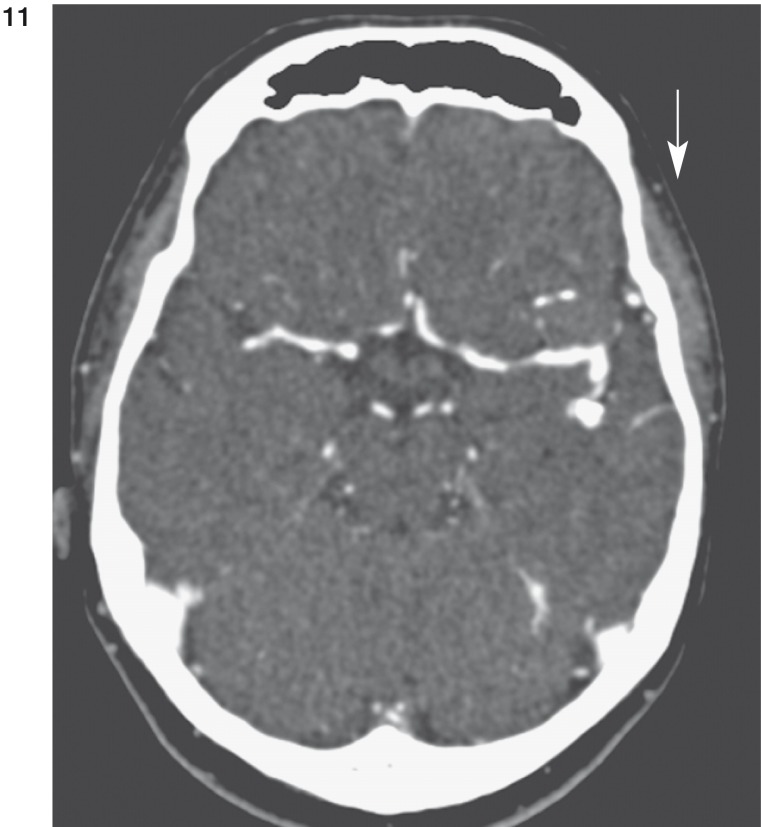
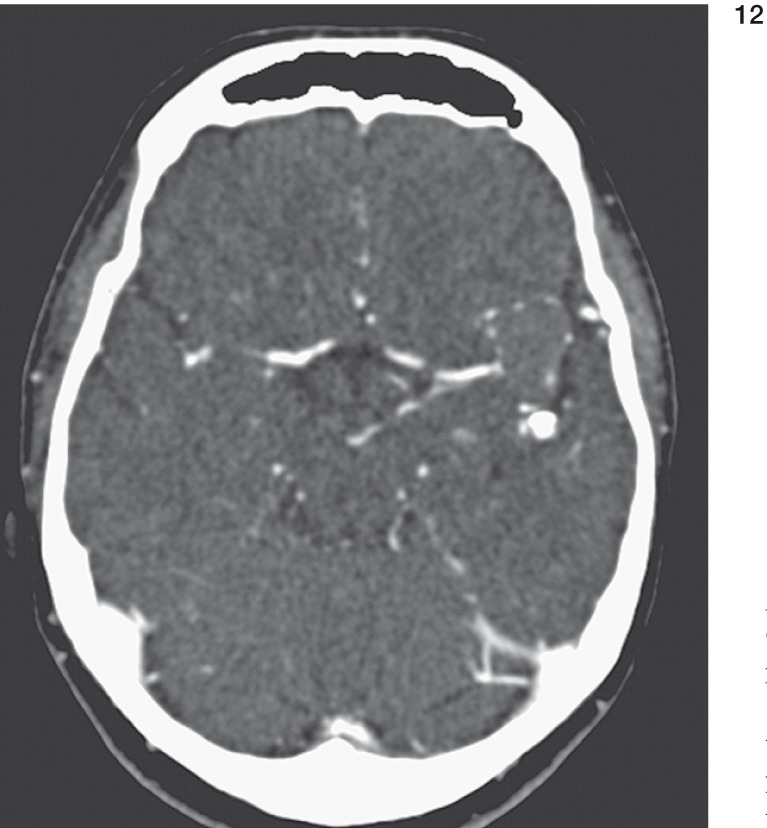
In contrast enhanced computed tomography (CT) the large aneurysm at the trifurcation of the left MCA appeared almost completely thrombosed. Marginal calcification was present (figure 11,12). The smaller branches of the posterior portion of the left MCA could be shown without thrombosis. In the insular region no aneurysm could be marked off.
Figure 11 und 12.
CT scans demonstrate marginal calcification (arrow in fig. 11) and thrombosis (arrow in fig. 12) of large aneurysm of left MCA.
The right sided aneurysms could not be found in computed tomography.
Discussion
Myxomas are the most frequent benign cardiac tumors 12. Correlation of cardiac myxomas and embolism is well known 5,13,16,18. Most often myxomas are located in the atrium of the left heart 7,10,12. Recurrence is known but rare. Tumor embolism to the brain with vessel occlusion is the most frequent complication causing neurological signs 12. Ischaemia may develop, but subarachnoid and intracerebral haemorrhage are rare 14-18. Clipping or endovascular treatment often do not represent therapeutical options because of the fusiform shape of the aneurysms 19. Technical advances in angiography and neurosurgical biopsy led to a better understanding of the pathomorphological changes of cerebral vessels in this disease. During the last 30 years different theories of aneurysm development in patients with myxomas have evolved. Complete improvement of neurological symptoms and aneurysm following surgical removal of cardiac myxoma is reported as well as progression of neurological symptoms and aneurysm growth 1,13,15,16,18.
In 1970, New et Al discussed the evolution of cerebral aneurysms in patients with cardiac myxoma following vessel wall infiltration by myxoma cells 3. This theory was validated histologically in 1995 when Furuya et Al proved active infiltration of myxoma cells into the vessel wall 1.
That fact raised the question of benignity of myxomas since infiltration is a criterion of malignant tumors or metastasis.
According to the fact of infiltration myxomatous development of aneurysms in brain vessels can show multiple pathways.
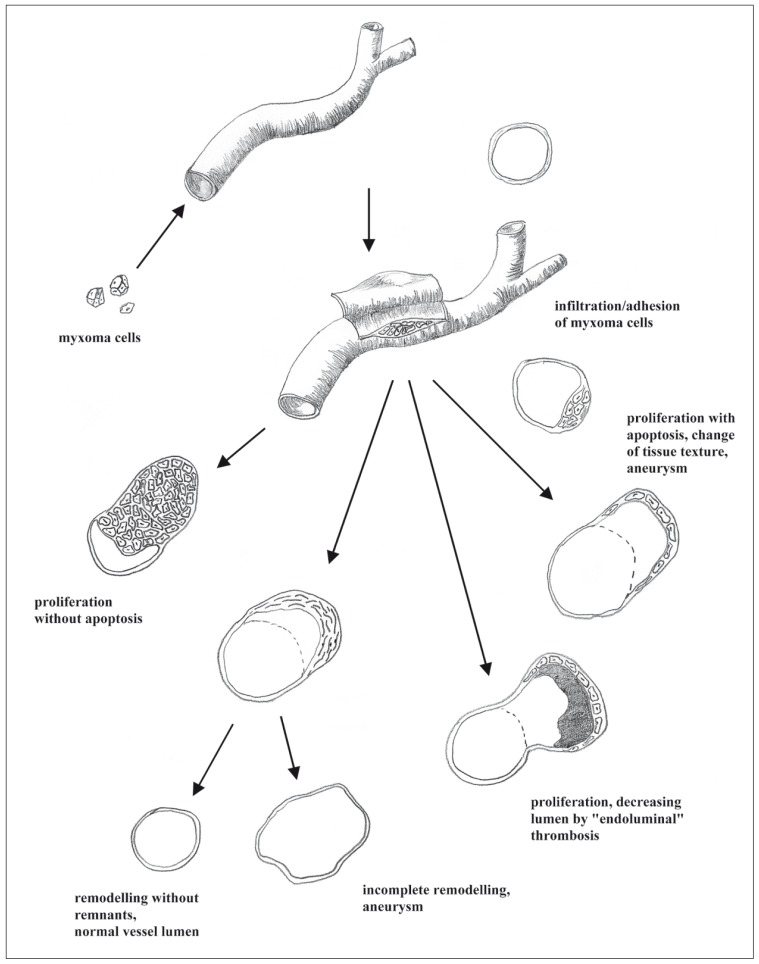
The pluripotential evolution of myxomatous aneurysms was demonstrated in our patient. Starting with detachment, tumor cells attain to intra- or extracranial vessels. Depending on the texture of the endothelium, myxoma cells adhere to the endothelium and either actively infiltrate the vessel wall or passively penetrate it through preexisting defects. Within the vessel wall the myxoma cells either proliferate with or without apoptosis. Without apoptosis the mass grows concentrically with restriction of the vessel lumen possibly resulting in brain infarction (figure 13A). Apoptosis within the myxoma results in widening of the vessel lumen caused by texture changes of the vessel wall (figure 13D). Angiographically these effects are seen either as narrowing or fusiform aneurysmatic widening.
A third pathway (figure 13B) lead to complete scarring of the myxoma lesion either followed by remodelling without any residues (figure 13E) or persisting fusiform aneurysm (figure 13F). If proliferation of myxoma cells stagnates, aneurysmatic ectasia may decrease. This fact is caused by endoluminal (mural) thrombosis resulting from turbulent blood flow inside the aneurysm (figure 13C).
Figure 13.
Pathogenesis of vessel involvement in myxoma.
In the case of the large lobulated aneurysm the lumen decreased by endoluminal thrombosis as shown in CT. Typical marginal calcification was demonstrated. Just a small remnant of aneurysm remained at the middle branch of the left MCA. On the right side in both ACA and MCA branches no aneurysm could be reproduced in follow-up angiography but nor could thrombosed residue be demonstrated in CT. This fact leads to the interpretation that scarring occurred with complete remodelling of the vessel wall. The third pathway of myxoma evolution is shown in distal left MCA branches. Angiographically these aneurysms were found partly unchanged or slightly smaller than in the first investigation. In CT minimal endoluminal thrombosis was demonstrated in some of these aneurysms. In the case of missing thrombus formation in CT we assume either incomplete remodelling with persistent aneurysm or proliferation of the myxoma cells with apoptosis.
Our patient never suffered from any neurological symptoms, which appears to be related to the fact that we never could demonstrate any narrowing or occlusion of brain vessels.
Conclusions
Besides cardiac failure and arterial embolism, development of cerebral aneurysm is a rare but important complication of cardiac myxomas. Active infiltration of myxoma cells into the vessel wall and cellular growth within the vessel wall are criteria of malignancy in this so-called "benign" tumor.
Neurological signs in patients with known myxoma or myxoma in their history must lead to cross-sectional brain imaging. Suspicious findings such as focal contrast enhancement should implicate cerebral catheter angiography to exclude or to prove formation of aneurysm.
Endovascular treatment usually is not feasible because of the fusiform shape of the aneurysms and their location in the peripheral branches of cerebral arteries. Knowledge of different pathways of myxomatous aneurysm is helpful to interpret changes in symptoms and imaging findings.
References
- 1.Kazuhide F, Sasaki T, et al. Histologically verified cerebral aneurysm formation secondary to embolism from cardiac myxoma. J Neurosurg. 1995;83:170–173. doi: 10.3171/jns.1995.83.1.0170. [DOI] [PubMed] [Google Scholar]
- 2.Stoane L, Allan JH, Jr, Collins HA. Radiologic observations in cerebral embolization from left heart myxomas. Radiology. 1966;87:262–266. doi: 10.1148/87.2.262. [DOI] [PubMed] [Google Scholar]
- 3.New PFJ, Price DL, Carter B. Cerebral angiography in cardiac myxoma. Correlation of angiographic and histopathological findings. Radiology. 1970;96:335–345. doi: 10.1148/96.2.335. [DOI] [PubMed] [Google Scholar]
- 4.Roeltgen DP, Weimer GR, Patterson LF. Delayed neurologic complications of left atrial myxoma. Neurology. 1981;31:8–13. doi: 10.1212/wnl.31.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Mattle HP, Maurer D, et al. Schroth Cardiac myxomas: a long term study. J Neurol. 1995;242(10):689–694. doi: 10.1007/BF00866921. [DOI] [PubMed] [Google Scholar]
- 6.O'Rourke F, Dean N, et al. Atrial myxoma as a cause of stroke: case report and discussion. CMAJ. 2003 Nov.196(10) [PMC free article] [PubMed] [Google Scholar]
- 7.Reyen K. Medical Progress: Cardiac Myxomas. N Engl J Med. 1995;333:1610–1617. doi: 10.1056/NEJM199512143332407. [DOI] [PubMed] [Google Scholar]
- 8.Basson CT, et al. Presentation of case: A 27-year-old woman with two intracardiac masses and a history of endocrinology. N Engl J Med. 2002:1152–1158. doi: 10.1056/NEJMcpc010057. [DOI] [PubMed] [Google Scholar]
- 9.Burke A, Virmani R. More in Cardiac Myxomas. N Engl J Med. 1996;335:1462–1464. doi: 10.1056/NEJM199611073351912. [DOI] [PubMed] [Google Scholar]
- 10.Javaheri S. Angiographic Visualization of an Atrial Myxoma. N Engl J Med. 2000;342:294–295. doi: 10.1056/NEJM200001273420417. [DOI] [PubMed] [Google Scholar]
- 11.Gopal AS, Arora NS, Messioneo FC. Angiographic Visualization of an Atrial Myxoma. N Engl J Med. 2000;342:295. doi: 10.1056/NEJM200001273420417. [DOI] [PubMed] [Google Scholar]
- 12.Kamiya H, Yasuda T, et al. Surgical Treatment of Primary Cardiac Tumors - 28 Years'Experience in Kanazawa. University Hospital Jpn Circ J. 2001;65:315–319. doi: 10.1253/jcj.65.315. [DOI] [PubMed] [Google Scholar]
- 13.Damasio H, Seabra-Gomes R, et al. Multiple cerebral aneurysms and cardiac myxoma. Arch Neurol. 1975;32(4):269–270. doi: 10.1001/archneur.1975.00490460085013. [DOI] [PubMed] [Google Scholar]
- 14.Ho KL. Neoplastic aneurysm and intracranial haemorrhage. Cancer. 1982;50(12):2935–1940. doi: 10.1002/1097-0142(19821215)50:12<2935::aid-cncr2820501238>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Gonsalves CG, Nidecker AC. Cerebral Aneurysms and cardiac myxoma. J Can Assoc Radiol. 1979;30(2):127–128. [PubMed] [Google Scholar]
- 16.Branch CL, Jr, Laster DW, Kelly DL., Jr Left atrial myxoma with cerebral emboli. Neurosurgery. 1985;16(5):675–680. doi: 10.1227/00006123-198505000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Nakata H, Yamada K, et al. A case of multiple cerebral aneurysm caused by cardiac myxoma. No Shinkei Geka. 1985;13(12):1365–1369. [PubMed] [Google Scholar]
- 18.Hung PC, Wang HS, et al. Multiple cerebral aneurysms in a child with cardiac myxoma. J Formos Med Assoc. 1992;91(8):818–821. [PubMed] [Google Scholar]
- 19.Hayashi S, Takahashi H, et al. A case of multiple cerebral aneurysm which showed rapid growth caused by left atrial myxoma. No Shinkei Geko. 1995;23(11):977–980. [PubMed] [Google Scholar]