Background: CaVβ subunits stimulate cell surface expression of CaV2.3 channels.
Results: Of 33 positions and domains tested, leucine mutants in the guanylate kinase domain of CaVβ3 decreased significantly the surface protein density of CaV2.3.
Conclusion: Leucine residues are responsible for the functional modulation by CaVβ.
Significance: A quartet of leucine residues forms the hydrophobic pocket surrounding the α-interacting domain of CaV2.3.
Keywords: Biophysics, Calcium Channels, Electrophysiology, Flow Cytometry, Membrane Trafficking, Molecular Modeling, Structural Biology
Abstract
CaVβ subunits are formed by a Src homology 3 domain and a guanylate kinase-like (GK) domain connected through a variable HOOK domain. Complete deletion of the Src homology 3 domain (75 residues) as well as deletion of the HOOK domain (47 residues) did not alter plasma membrane density of CaV2.3 nor its typical activation gating. In contrast, six-residue deletions in the GK domain disrupted cell surface trafficking and functional expression of CaV2.3. Mutations of residues known to carry nanomolar affinity binding in the GK domain of CaVβ (P175A, P179A, M195A, M196A, K198A, S295A, R302G, R307A, E339G, N340G, and A345G) did not significantly alter cell surface targeting or gating modulation of CaV2.3. Nonetheless, mutations of a quartet of leucine residues (either single or multiple mutants) in the α3, α6, β10, and α9 regions of the GK domain were found to significantly impair cell surface density of CaV2.3 channels. Furthermore, the normalized protein density of CaV2.3 was nearly abolished with the quadruple CaVβ3 Leu mutant L200G/L303G/L337G/L342G. Altogether, our observations suggest that the four leucine residues in CaVβ3 form a hydrophobic pocket surrounding key residues in the α-interacting domain of CaV2.3. This interaction appears to play an essential role in conferring CaVβ-induced modulation of the protein density of CaVα1 subunits in CaV2 channels.
Introduction
Voltage-dependent Ca2+ channels (CaV) are membrane proteins that play a key role in promoting Ca2+ influx in response to membrane depolarization in excitable cells. The total Ca2+ influx through CaV proteins is controlled by the single-channel conductance, the probability that the channel is open at a given time and voltage, what is also referred to as gating, and the number of proteins expressed at the membrane. Modulating any of these parameters could be used to alter Ca2+ influx and prevent excitable cells from Ca2+ overload.
Voltage-gated Ca2+ channels form oligomeric complexes that are classified according to the structural properties of the pore-forming CaVα1 subunit. The primary structures for 10 distinct CaVα1 subunits (1–7) are classified into three main subfamilies according to their high voltage-activated gating (HVA CaV1 and CaV2) or low voltage-activated gating (LVA CaV3). In HVA CaV1 and CaV2 channels, auxiliary subunits include a cytoplasmic CaVβ subunit, a mostly extracellular CaVα2δ subunit, and calmodulin constitutively bound to the C terminus of CaVα1 (7–12) (for review see Ref. 9).
There are four subfamilies of Cavβs, each with multiple splicing isoforms and unique modulatory functions (14, 15). Auxiliary CaVβ subunits modulate activation gating as well as inactivation kinetics of the main CaVα1 subunits of HVA CaV1 and CaV2 channels (16–23). They also promote cell surface expression in part by preventing protein ubiquitination and degradation of the protein by the ERAD complex (20, 24).
The principal CaVα1-CaVβ interaction site on the pore-forming α1 subunit is a conserved 18-residue sequence in the I-II loop called the α-interacting domain (AID)3 (25). The nanomolar affinity between the AID helix and the CaVβ protein has been thoroughly investigated (26–29). It is primarily secured by the projection of the conserved tryptophan and isoleucine (WI) pair of residues onto the CaVβ fold (27–29). Single point mutations of the C-terminal WI residues on the AID decreased by 1000-fold the affinity of the CaVβ2 for CaV1.2 (26) and decreased its cell surface density (17).
Less is known in regard to the molecular determinants in CaVβ that control protein density at the plasma membrane and modulate the activation gating of the channel. It is understood that the N terminus plays a predominant role in modulating inactivation kinetics, either directly by interacting with the CaVα1 (30, 31) and/or indirectly through palmitoylation for CaVβ2a (32). High resolution three-dimensional crystal structures have shown that CaVβ subunits consist of five distinct domains: the N terminus, a Src homology 3 (SH3) domain, a HOOK region, a guanylate kinase (GK) domain, and the C terminus (see Fig. 5A) (27–29). The GK core module appears to be sufficient to confer the activation properties (33–35). The latter includes the structural determinants of the α-binding pocket that are distributed among α3, α6, α7, and α9 helices and β9 and β10 sheets (26–29).
FIGURE 5.
A, schematic diagram of the domain organization of the CaVβ3 subunit based on the crystal structure adapted from Ref. 27 and used in (17). B, typical whole cell current traces were obtained for CaV2.3 WT in the presence of a 2 mm Ca2+ solution. CaVα2δ-1 was omitted. From left to right are shown no CaVβ3, CaVβ3 WT, and CaVβ3 fragment 58–362. C, peak current densities are plotted as a function of applied voltage. CaV2.3 WT + CaVβ3 WT and CaV2.3 WT + CaVβ3 58–362 showed a similar typical voltage-dependent activation with a >5-fold increase in peak current densities as compared with CaV2.3 WT in the absence of CaVβ3. The numerical values are shown in Table 1. D, the r50 values are shown as means ± S.E. from −15 to +5 mV for CaV2.3 WT + CaVβ3 and CaV2.3 WT + CaVβ3 58–362 (from left to right on the bar graph). The r50 values of CaV2.3 WT + CaVβ3 and CaV2.3 WT + CaVβ3 58–362 were significantly not different at p > 0.01 at all voltages.
With some notable exceptions (23, 26, 36), few studies have systematically attempted to correlate CaVβ high affinity binding with the modulation of channel gating and trafficking. Whereas interaction between CaVβ and CaVα1 is required for the trafficking of CaVα1 to the plasma membrane, it remains to be seen whether nanomolar binding of CaVβ to the AID motif of CaVα1 is required to carry both roles. Furthermore, whereas the binding of CaVβ on the AID of CaVα1 has been well characterized (17, 22, 26, 33, 37, 38), few concur on the GK residues in CaVβ that determine protein density at the plasma membrane and contribute to channel modulation.
Complications have arisen in functional studies performed with the Xenopus recombinant system (26, 27, 33, 39–41), including our own (37, 38), because endogenous CaVβs (42) are likely to boost expression of noninteracting mutants. The presence of the CaVα2δ subunit in some experiments could complicate data interpretation. In addition, the CaVβ2a subunit used in most studies is an atypical subunit with an N-terminal palmitoylation site (43) that anchors it to the membrane and could offset the disruption of the high affinity interaction site. Indeed, the palmitoylated CaVβ2a was still able to modulate the biophysical properties of CaV2.2 W391A in Xenopus oocytes, indicating that the plasma membrane anchoring afforded by its palmitoylation can substitute in some cases for high affinity interaction with the I-II linker (22).
In this work we have systematically addressed the role of the structural domains of CaVβ3 in regard to protein density and channel function after recombinant expression of HVA CaV2.3 in HEKT cells. Selective deletion of the SH3 and HOOK structural domains did not significantly alter the cell surface density or the channel functional modulation by CaVβ. More surprisingly, mutations of residues in the GK domain that were previously identified as underlying the affinity to HVA CaVα1 (26) caused little change in the cell surface density of CaV2.3 or in the channel activation gating. Nonetheless, point mutations within a quartet of leucine residues in the α3, α6, β10, and α9 regions of the GK domain significantly decreased cell surface density. Altogether our results suggest that affinity interaction in the micro- to nanomolar range is sufficient to carry the typical CaVβ-induced hyperpolarizing shift in the channel activation gating in the presence of overexpressed CaVβ subunits. Furthermore, we have identified a quartet of leucine residues in the GK domain of CaVβ3 that play a critical role in promoting surface plasma density of CaV2.3.
EXPERIMENTAL PROCEDURES
Recombinant DNA Techniques
The human CaV2.3 (GenbankTM accession number L27745) (44), the rat CaVβ3 (GenBankTM accession number M88751) (45), and the rat brain CaVα2bδ-1 (GenBankTM accession number NM_000722) (46) were used. All subunits were subcloned in commercial vectors under the control of the CMV promoter as explained earlier (17). Some electrophysiological experiments were performed with pGFP-CaVα2δ1 peGFP-C2 vector (Clontech).
The HA epitope tag (YPYDVPDYA) was inserted in the first extracytoplasmic predicted loop in Domain I at position 367 (nucleotides) for CaV2.3. The biophysical properties of the HA-tagged CaVα1 subunit of CaV2.3 expressed in HEKT cells with the auxiliary CaVβ3 subunit were found not to be significantly different from the wild-type CaV2.3 channel expressed under the same conditions (see Table 1). CaVβ3 deletion mutants were produced as described elsewhere (17). The CaVβ3 58–362 fragment was used previously in (17). The boundaries of the five domains/regions of the rat CaVβ3 are: N terminus, Met1–Pro59; SH3 domain, Val60–Ser123 and Pro170–Pro175; HOOK region, Pro120–Pro169; GK domain, Ser176–Thr360; and C terminus, His361–Tyr484 (see Fig. 5A).
TABLE 1.
Biophysical properties of CaV2.3 channels in the absence of CaVα2δ-1
CaV2.3 wild-type or HA-CaV2.3 channels were expressed in HEKT cells with CaVβ3 WT or constructs. Biophysical parameters were measured in the presence of a physiological saline containing 2 mm Ca2+ as described elsewhere (17). CaVα2δ-1 was omitted. Activation properties (E0.5,act and ΔGact) were estimated from the mean I-V relationships and fitted to a Boltzmann equation. The data are shown as the means ± S.E. of the individual experiments, and the number of experiments appears in parentheses.
| CaV2.3 WT in HEKT cells no CaVα2δ-1 | Electrophysiological properties |
|||
|---|---|---|---|---|
| E0.5,act | ΔGact | Peak density | r50 at +10 mV | |
| mV | kcal mol−1 | pA/pF | ||
| No auxiliary subunit | −5 ± 1 (18) | −0.6 ± 0.1 (18) | −6 ± 1 (18) | ND |
| + CaVβ3 | −10.7 ± 0.3 (49) | −1.32 ± 0.05 (49) | −18 ± 2 (49) | 0.38 ± 0.01 (49) |
| + CaVβ1a | −10.5 ± 0.5 (6) | −1.2 ± 0.1 (6) | −35 ± 4 (6) | 0.51 ± 0.01 (6) |
| + CaVβ1b | −12.2 ± 0.6 (12) | −1.6 ± 0.2 (12) | −68 ± 14 (12) | 0.54 ± 0.01 (12) |
| + CaVβ2a | −11.4 ± 0.6 (6) | −1.3 ± 0.1 (6) | −33 ± 4 (6) | 0.85 ± 0.02 (6) |
| + CaVβ4 | −12.1 ± 0.6 (7) | −1.5 ± 0.1 (7) | −21 ± 2 (7) | 0.64 ± 0.02 (7) |
| + CaVβ3 M196A | −7.9 ± 0.3 (22) | −0.90 ± 0.04 (22) | −14 ± 1 (22) | 0.43 ± 0.01 (22) |
| + CaVβ3 M195A | −12 ± 1 (9) | −1.5 ± 0.1 (9) | −24 ± 5 (9) | 0.53 ± 0.01 (9) |
| + CaVβ3 K198A | −11.6 ± 0.6 (10) | −1.4 ± 0.1 (10) | −13 ± 2 (10) | 0.30 ± 0.01 (10) |
| + CaVβ3 L200G | −9.8 ± 0.6 (7) | −1.16 ± 0.08 (7) | −18 ± 2 (7) | 0.44 ± 0.02 (7) |
| + CaVβ3 L303G | −7.2 ± 0.5 (7) | −0.82 ± 0.06 (7) | −17 ± 2 (7) | 0.40 ± 0.02 (7) |
| + CaVβ3 L337G | −9.3 ± 0.5 (8) | −1.2 ± 0.1 (8) | −19 ± 6 (8) | 0.46 ± 0.01 (8) |
| + CaVβ3 L342G | −10.1 ± 0.5 (8) | −1.27 ± 0.07 (8) | −15 ± 3 (8) | 0.49 ± 0.01 (8) |
| + CaVβ3 L200G/L303G | −7.8 ± 0.6 (10) | −1.0 ± 0.1 (10) | −9 ± 1 (10) | 0.40 ± 0.03 (10) |
| + CaVβ3 L200G/L303G/L337G | −7.9 ± 0.5 (12) | −1.0 ± 0.1 (12) | −6.2 ± 0.8 (12) | 0.48 ± 0.03 (12) |
| + CaVβ3 L200G/L303G/L337G/L342G | −9.1 ± 0.7 (11) | −1.1 ± 0.1 (11) | −5.7 ± 0.5 (11) | 0.45 ± 0.02 (11) |
| + CaVβ3 R307G | −15 ± 1 (10) | −2.0 ± 0.1 (10) | −66 ± 11 (10) | 0.53 ± 0.01 (10) |
| + CaVβ3 Δ57–123 | −10 ± 1 (12) | −1.2 ± 0.1 (12) | −38 ± 9 (12) | 0.36 ± 0.03 (12) |
| + CaVβ3 Δ57–180 | −9 ± 1 (6) | −1.0 ± 0.1 (6) | −7 ± 1 (6) | 0.37 ± 0.06 (6) |
| + CaVβ3 Δ170–175 (ΔPYDVVP) | −10 ± 1 (14) | −1.1 ± 0.1 (14) | −22 ± 6 (14) | 0.48 ± 0.02 (14) |
| + CaVβ3 Δ57–123 Δ170–175 (ΔSH3) | −8 ± 1 (12) | −0.9 ± 0.1 (12) | −24 ± 3 (12) | 0.40 ± 0.03 (12) |
| + CaVβ3 Δ175–180 | −7.7 ± 0.5 (12) | −1.0 ± 0.1 (12) | −7.1 ± 0.8 (12) | 0.42 ± 0.04 (12) |
| + CaVβ3 Δ122–169 | −9.5 ± 0.5 (11) | −0.95 ± 0.07 (11) | −19 ± 3 (11) | 0.35 ± 0.03 (11) |
| + CaVβ3 Δ122–175 | −7.6 ± 0.5 (11) | −0.85 ± 0.07 (11) | −19 ± 4 (11) | 0.33 ± 0.01 (11) |
| + CaVβ3 Δ58–362 | −13 ± 1 (10) | −1.4 ± 0.1 (10) | −13 ± 4 (10) | 0.31 ± 0.03 (10) |
| + CaVβ3 Δ180–364 | −8.6 ± 0.5 (9) | −1.1 ± 0.1 (9) | −7 ± 1 (9) | 0.42 ± 0.05 (9) |
| HA-CaV2.3 WT + CaVβ3 WT | −12 ± 1 (5) | −1.3 ± 0.1 (5) | −14 ± 2 (5) | 0.46 ± 0.03 (9) |
| HA-Y383F + CaVβ3 WT | −9 ± 1 (9) | −1.0 ± 0.1 (9) | −21 ± 4 (9) | 0.47 ± 0.01 (9) |
| HA-Y383A + CaVβ3 WT | −7.8 ± 0.3 (11) | −0.75 ± 0.05 (11) | −9 ± 1 (11) | 0.53 ± 0.02 (11) |
| HA-R384L + CaVβ3 WT | −12 ± 1 (5) | −1.7 ± 0.2 (4) | −13 ± 2 (4) | 0.36 ± 0.03 (5) |
| HA-R384 M + CaVβ3WT | −16 ± 2 (5) | −1.4 ± 0.1 (5) | −22 ± 2 (5) | 0.41 ± 0.04 (5) |
Cell Culture and Transfections
HEK293T or HEKT were grown in high glucose DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C under 5% CO2 atmosphere as described elsewhere (17). RT-PCR conducted in these cells failed to highlight the presence of CaVβ and CaVα2δ auxiliary subunits (47). HEKT cells (80% confluence) were transiently transfected with similar amounts of DNA (4 μg each or 12 μg total): HA-CaV2.3, CaVβ3, CaVα2δ-1, or empty vector pCMVTag5 in 10 μl of Lipofectamine 2000 (Qiagen) using a DNA:lipid ratio of 1:2.5 as described elsewhere (17). Transfection rate of the control peGFP plasmid was estimated to be 66 ± 2% (n = 8) as assessed by flow cytometry from the fluorescence of the GFP. Preliminary tests showed that CaV2.3 protein expression peaked 24–30 h after transfection.
Quantification of CaV2.3 Surface Expression with FACS
FACS experiments were conducted and analyzed as described elsewhere. Briefly, 24–28 h after transfection, the cells were harvested and stained with the anti-HA FITC conjugate (10 μg/ml) at room temperature for 45 min. A maximum of 10,000 cells resuspended in standard PBS were counted using a FACScalibur® flow cytometer (BD Biosciences) with a FITC filter (530 nm) at the flow cytometry facility located in the Department of Microbiology of the Université de Montréal. The level of background fluorescence was adjusted with cells incubated in the absence of the fluorophore. The parameters of the M1 region were set from Gaussian distribution of the fluorescence intensity obtained in the absence of the FITC antibody. The M2 region was calculated from the Gaussian distribution observed in the higher decades of the fluorescence log scale in the presence of the FITC antibody. The cell fluorescence intensity and, by extension, the cell surface expression of the HA tag were estimated from the M2 peak value (see supplemental Fig. S2 of Ref. 17). Three control conditions were always carried out for each series of experiments: (a) nontransfected HEKT cells with anti-HA FITC antibody; (b) HEKT cells transfected with HA-CaV2.3 without CaVβ3 WT; and (c) HEKT cells transfected with HA-CaV2.3 WT and CaVβ3 WT. Peak fluorescence values obtained for the control conditions were compared with previous measures for internal consistency. Each novel mutant and/or experimental condition was conducted in triplicate and sometimes repeated over 2 weeks. The experiments performed in the 24–28-h range after transfection yielded M2 values with an experimental variation lower than 10% between samples and between series of experiments. In any case, a maximum of 10% variation in the measure of fluorescence was tolerated. All control conditions were pooled and reported in Tables 3 and 4.
TABLE 3.
Fluorescent-activated cell sorting analysis of CaV2.3
FACS results obtained after the transient transfection of HA-CaV2.3 WT in either HEKT control cells or stable CaVβ3 cells. One day (24–28 h) after transfection, the cells were incubated with anti-HA FITC conjugate (10 μg/ml) at room temperature for 45 min. FACS separation of FITC positive cells was performed on a FACScalibur® flow cytometer (BD Biosciences), and fluorescence was quantified using CellQuest software (BD Biosciences).The results are reported as percentage values of cells in higher quadrant. The data were pooled from experiments carried out over a period of 12 months. The data are shown as the means ± S.E. of the individual experiments, and the number of experiments appears in parentheses. ND, not determined.
| Constructs transient expression | Fluorescence in HEKT | Fluorescence in CaVβ3 stable |
|---|---|---|
| % | % | |
| Cells no Ab | 0.22 ± 0.01 (33) | 0.21 ± 0.02 (28) |
| Cells with Ab | 0.5 ± 0.1 (35) | 0.6 ± 0.1 (30) |
| HA-CaV1.2 WT | 3.8 ± 0.5 (15) | 27 ± 1 (18) |
| HA-CaV1.2 WT + CaVβ3 | 26 ± 1 (56) | ND |
| HA-CaV2.3 WT | 1.7 ± 0.1 (31) | 20 ± 1 (30) |
| HA-CaV2.3 WT + CaVβ3 | 36.3 ± 0.08 (46) | ND |
| HA-CaV2.3 G382A | 35 ± 1 (3) | 19 ± 1 (7) |
| HA-CaV2.3 Y383A | 27 ± 1 (3) | 4.9 ± 0.6 (7) |
| HA-CaV2.3 Y383F | 37 ± 1 (3) | 13 ± 1 (7) |
| HA-CaV2.3 Y383G | 12 ± 1 (3) | 4.0 ± 0.3 (7) |
| HA-CaV2.3 R384M | 32 ± 2 (3) | 19 ± 1 (6) |
| HA-CaV2.3 R384L | 31 ± 2 (3) | 18 ± 1 (3) |
| HA-CaV2.3 W386A | 1.2 ± 0.2 (3) | 1.5 ± 0.3 (7) |
| HA-CaV2.3 WT + CaVβ1a | 45 ± 2 (3) | ND |
| HA-CaV2.3 WT + CaVβ1b | 41 ± 2 (3) | ND |
| HA-CaV2.3 WT + CaVβ2a | 9 ± 1 (5) | ND |
| HA-CaV2.3 WT + CaVβ4 | 40 ± 2 (3) | ND |
| HA-CaV2.3 WT + CaVβ3 + CaVα2δ-1 | 34 ± 2 (6) | ND |
| HA-CaV2.3 WT + CaVα2δ-1 | 1.2 ± 0.2 (7) | 21 ± 1 (5) |
| HA-CaV2.3 WT + CaM | 1.5 ± 0.2 (9) | ND |
| HA-CaV2.3 WT + CaM1234 | 1.2 ± 0.2 (9) | ND |
TABLE 4.
Fluorescent-activated cell sorting analysis of CaVβ3 constructs
FACS results obtained after the transient transfection of CaV2.3-HA WT in HEKT cells. The results are reported as percentage values of cells in the higher quadrant of fluorescence. The data were pooled from experiments carried out over a period of 12 months. The data are shown as the means ± S.E. of the individual experiments, and the number of experiments (n) appears in the last column.
| CaVβ constructs |
Fluorescence |
||
|---|---|---|---|
| Transient expression HA-CaV2.3 HEKT cells | Mean | S.E. | n |
| % | % | ||
| CaVβ3 WT | 36.3 | 0.8 | 46 |
| Δ57–123 (ΔNSH3) | 26 | 1 | 6 |
| Δ57–180 (ΔSH3) | 2.9 | 0.4 | 3 |
| Δ180–364 (ΔGK) | 1.4 | 0.4 | 5 |
| Δ54–362 (ΔSH3-GK) | 3 | 1 | 3 |
| Δ170–175 (ΔPYDVVP) | 23 | 2 | 7 |
| Δ170–175 Δ180–364 (ΔPYDVVPΔGK) | 3 | 1 | 3 |
| Δ170–175 Δ57–123 (ΔPYDVVPΔNSH3) | 25 | 2 | 8 |
| Δ195–200 (ΔMMQKAL) | 1.6 | 0.6 | 5 |
| ΔHOOK (Δ122–169) | 24 | 2 | 4 |
| ΔHOOKΔPYDVVP (Δ122–175) | 22 | 1 | 4 |
| ΔPSMRPV (Δ175–180) | 3 | 0.8 | 3 |
| P175A | 33 | 1.5 | 3 |
| P175G | 32 | 2.4 | 3 |
| P179A | 29 | 0.7 | 3 |
| P179G | 30 | 0.7 | 3 |
| M195A | 37 | 0.3 | 5 |
| M196A | 22 | 0.7 | 9 |
| K198A | 41 | 0.4 | 4 |
| L200G | 18 | 0.7 | 3 |
| S295A | 37 | 1.5 | 4 |
| R302G | 43 | 1.1 | 4 |
| L303G | 8.0 | 0.2 | 7 |
| R307A | 27 | 1.1 | 3 |
| R307G | 33 | 1.6 | 3 |
| R307K | 33 | 0.6 | 3 |
| L337G | 16 | 0.7 | 6 |
| E339G | 21 | 1.3 | 3 |
| N340G | 33 | 1.0 | 3 |
| L342G | 16 | 0.5 | 6 |
| A345G | 20.2 | 0.7 | 3 |
| L200G/L303G | 1.7 | 0.1 | 3 |
| L200G/L303G/L337G | 2.1 | 0.1 | 3 |
| L200G/L303G/L337G/L342G | 2.5 | 0.1 | 3 |
Patch Clamp Experiments in HEKT Cells
Whole cell voltage clamp recordings were performed 30 h after transfection using the methods described above in the presence of the peGFP vector (0.2 μg) as a control for transfection. Patch clamp experiments were carried out with the Axopatch 200-B amplifier (Molecular Devices, Union City, CA). Electrodes were filled with a solution containing 140 mm CsCl, 0.6 mm NaGTP, 3 mm MgATP, 10 mm EGTA, 10 mm Hepes titrated to pH 7.3 with NaOH. Pipette resistance ranged from 2 to 4 mΩ. The cells were bathed in a modified Earle's saline solution 135 mm NaCl, 20 mm TEACl, 2 mm CaCl2, 1 mm MgCl2, 10 mm Hepes titrated to pH 7.3 with KOH. PClamp software Clampex 10.2 coupled to a Digidata 1440A acquisition system (Molecular Devices) was used for on-line data acquisition and analysis. Pipette and cell capacitance cancellation and series resistance compensation were applied (up to 80%) using the cancellation feature of the amplifier. Cellular capacitance was estimated by measuring the time constant of current decay evoked by a 10-mV depolarizing pulse applied to the cell from a holding potential of −100 mV. A series of 150-ms voltage pulses were applied from a holding potential of −100 mV at a frequency of 0.2 Hz, from −80 to +60 mV at 5-mV intervals. Unless stated otherwise, the data were sampled at 5 kHz and filtered at 1 kHz. Experiments were performed at room temperature (20–22 °C). Activation parameters were estimated from the peak I-V curves obtained for each channel combination and are reported as the means of individual measurements ± S.E. as described elsewhere (48). Briefly, the I-V relationships were normalized to the maximum amplitude and were fitted to a Boltzmann equation with E0.5,act being the midpotential of activation. The free energy of activation was calculated using the midactivation potential with Equation 1,
where z is the effective charge displacement during activation, and T, F, and R have their usual meaning (49). The r50 ratio is defined as the ratio of peak whole cell currents remaining 50 ms later (I50 ms/IPeak). It has been used elsewhere to estimate inactivation kinetics of CaV2.3 (48, 50, 51).
For each novel mutant tested, the biophysical parameters of CaV2.3 WT + CaVβ3 WT were measured the same day under the same experimental conditions from the transfection protocol up to the bath solutions. Experiments performed under the same conditions yielded peak current densities ± 15% between samples and between series of experiments. All of the experiments were pooled and are reported in Tables 1 and 2.
TABLE 2.
Biophysical properties of CaV2.3 channels in the presence of CaVα2δ-1
CaV2.3 wild-type channels were expressed in HEKT cells with CaVβ3 WT or constructs with CaVα2δ-1. Biophysical parameters were measured in the presence of a physiological saline containing 2 mm Ca2+ as described elsewhere (17). Activation properties (E0.5,act and ΔGact) were estimated from the mean I-V relationships and fitted to a Boltzmann equation. The data are shown as the means ± S.E. of the individual experiments, and the number of experiments appears in parentheses.
| CaV2.3 in HEKT cells 2 mm Ca2+ | Electrophysiological properties |
|||
|---|---|---|---|---|
| E0.5,act | ΔGact | Peak density | r50 at +10 mV | |
| mV | kcal mol−1 | pA/pF | ||
| + CaVα2δ-1 | −4.0 ± 0.7 (10) | −0.44 ± 0.07 (10) | −12 ± 2 (10) | 0.43 ± 0.02 (9) |
| + CaVβ3 WT + CaVα2δ-1 | −9.1 ± 0.2 (95) | −1.3 ± 0.1 (95) | −65 ± 5 (95) | 0.25 ± 0.01 (95) |
| + CaVβ3 P175A + CaVα2δ-1 | −9.5 ± 0.9 (8) | −1.6 ± 0.3 (8) | −82 ± 21 (8) | 0.30 ± 0.01 (8) |
| + CaVβ3 M196A + CaVα2δ-1 | −8.6 ± 0.8 (9) | −1.1 ± 0.1 (9) | −41 ± 9 (9) | 0.39 ± 0.02 (9) |
| + CaVβ3 M195A + CaVα2δ-1 | −10 ± 1 (8) | −1.6 ± 0.3 (8) | −157 ± 25 (8) | 0.41 ± 0.01 (8) |
| + CaVβ3 K198A + CaVα2δ-1 | −7.7 ± 0.2 (9) | −0.97 ± 0.04 (9) | −56 ± 9 (9) | 0.26 ± 0.01 (9) |
| + CaVβ3 L200G + CaVα2δ-1 | −8.6 ± 0.5 (11) | −1.02 ± 0.07 (11) | −43 ± 6 (11) | 0.29 ± 0.02 (11) |
| + CaVβ3 S295A + CaVα2δ-1 | −8.1 ± 0.5 (10) | −1.6 ± 0.3 (10) | −71 ± 12 (10) | 0.33 ± 0.01 (10) |
| + CaVβ3 R302G + CaVα2δ-1 | −8.1 ± 0.3 (11) | −1.1 ± 0.1 (11) | −67 ± 11 (11) | 0.36 ± 0.01 (11) |
| + CaVβ3 L303G + CaVα2δ-1 | −5.3 ± 0.5 (8) | −0.60 ± 0.06 (8) | −47 ± 10 (8) | 0.34 ± 0.03 (8) |
| + CaVβ3 R307G + CaVα2δ-1 | −10.6 ± 0.6 (9) | −1.5 ± 0.1 (9) | −128 ± 20 (9) | 0.44 ± 0.01 (9) |
| + CaVβ3 R307A + CaVα2δ-1 | −13 ± 1 (10) | −1.7 ± 0.2 (10) | −98 ± 21 (10) | 0.40 ± 0.02 (10) |
| + CaVβ3 R307K + CaVα2δ-1 | −12.1 ± 0.7 (10) | −1.7 ± 0.2 (10) | −130 ± 28 (10) | 0.38 ± 0.01 (10) |
| + CaVβ3 L337G + CaVα2δ-1 | −6.5 ± 0.6 (8) | −0.80 ± 0.09 (8) | −82 ± 10 (8) | 0.33 ± 0.02 (8) |
| + CaVβ3 E339G + CaVα2δ-1 | −6.3 ± 0.6 (10) | −0.78 ± 0.08 (10) | −67 ± 12 (10) | 0.43 ± 0.01 (10) |
| + CaVβ3 L342G + CaVα2δ-1 | −7.2 ± 0.4 (8) | −0.90 ± 0.07 (8) | −78 ± 19 (8) | 0.39 ± 0.01 (8) |
| + CaVβ3 L200G/L303G + CaVα2δ-1 | −7.1 ± 0.7 (11) | −0.9 ± 0.1 (11) | −10 ± 2 (11) | 0.34 ± 0.04 (11) |
| + CaVβ3 L200G/L303G/L337G + CaVα2δ-1 | −6.6 ± 0.7 (11) | −0.81 ± 0.09 (11) | −10 ± 2 (11) | 0.41 ± 0.03 (11) |
| + CaVβ3 L200G/L303G/L337G/L342G + CaVα2δ-1 | −8.2 ± 0.5 (12) | −0.94 ± 0.07 (12) | −9 ± 1 (12) | 0.37 ± 0.04 (12) |
| + CaVβ3 Δ57–123 (ΔNSH3) + CaVα2δ−1 | −8.4 ± 0.5 (9) | −1.1 ± 0.1 (9) | −117 ± 16 (9) | 0.24 ± 0.01 (9) |
| + CaVβ3 Δ57–180 + CaVα2δ-1 | −5.3 ± 0.7 (9) | −0.6 ± 0.1 (9) | −9 ± 1 (9) | 0.43 ± 0.03 (9) |
| + CaVβ3 Δ122–169 + CaVα2δ-1 | −5 ± 1 (9) | −0.6 ± 0.1 (9) | −38 ± 7 (9) | 0.19 ± 0.01 (9) |
| + CaVβ3 Δ122–175 + CaVα2δ-1 | −5.6 ± 0.7 (10) | −0.6 ± 0.1 (10) | −32 ± 7 (10) | 0.22 ± 0.01 (10) |
| + CaVβ3 Δ175–180 + CaVα2δ-1 | −3.4 ± 0.5 (9) | −0.36 ± 0.05 (9) | −14 ± 2 (9) | 0.45 ± 0.02 (9) |
| + CaVβ3 Δ57–123 Δ170–175 (ΔSH3) + CaVα2δ-1 | −6.6 ± 0.7 (13) | −0.9 ± 0.2 (13) | −74 ± 15 (13) | 0.27 ± 0.01 (13) |
Western Blots
Protein expression of all constructs was confirmed by Western blotting in total cell lysates as previously described (17) using the following primary antibodies: anti-CaV2.3 (Alomone; 1:250), anti-HA (Covance Biotechnology, Québec, Canada) (1:500), anti-CaVβ3 (Alomone; 1:5000), and anti-GAPDH (Sigma 1:10000).
Homology Modeling
The primary sequence of the AID region from CaV2.3 was aligned with the similar regions in CaV1.2 (58% identity) by using T-Coffee (52). The computer-based molecular model of the AID from CaV2.3 in complex with CaVβ3 was obtained with Modeler 9v4 using the molecular coordinates of CaV1.2 with CaVβ3 (Protein Data Bank code 1VYT) (27). Fifty models were generated. Violations of spatial restraints were minimized using the molecular protein density function algorithm in Modeler. The model with the lowest molecular protein density function was used for this study.
RESULTS
α-Interacting Domain and Cell Surface Expression of CaV2.3
Co-expression of CaV2.3 with CaVβ3 increased whole cell currents in HEKT cells from −6 ± 1 pA/pF (n = 18) (Table 1) for the wild-type CaV2.3 channel expressed alone to a current density of −18 ± 2 pA/pF (n = 49) for CaV2.3 + CaVβ3. Similar results were obtained for the HA-tagged CaV2.3 channels (Table 1). Co-expression with CaVα2δ-1 subunit further increased whole cell currents to −65 ± 5 pA/pF (n = 95) (Fig. 1, A and B, and Table 2), confirming that CaVα2δ-1 increases whole cell current density of HVA CaV1 and CaV2 channels (16, 17). The increase in cell current density was accompanied by an apparent acceleration of the inactivation kinetics with r50 values decreasing from 0.38 ± 0.01 (n = 49) to 0.25 ± 0.01 (n = 95) at +10 mV in the presence of CaVα2δ-1 (Fig. 1C) but failed to significantly shift the midpotential of activation (E0.5,act) toward negative potentials (Tables 1 and 2), an observation that was also reported elsewhere (47).
FIGURE 1.
CaVβ3 stimulated whole cell currents and cell surface density of CaV2.3. A, whole cell current traces recorded after the transient expression of the CaV2.3 WT channel in HEKT cells. The charge carrier was 2 mm Ca2+. From left to right are shown CaV2.3 WT alone, CaV2.3 WT + CaVα2δ-1, CaV2.3 WT + CaVβ3, and CaV2.3 WT + CaVβ3 + CaVα2δ-1. A series of 150-ms voltage pulses were applied between −80 mV and +60 mV (5-mV steps) from a holding potential of −100 mV. B, peak current densities are plotted as a function of applied voltage. Channels CaV2.3 WT + CaVβ3 ± CaVα2δ-1 showed a typical voltage-dependent activation with a mean current density of −6 ± 1 pA/pF (n = 18) for CaV2.3 WT alone as compared with a current density of −18 ± 2 pA/pF (n = 49) obtained after transient overexpression of CaV2.3 WT + CaVβ3. Overexpression of CaV2.3 WT + CaVβ3 + CaVα2δ-1 increased the mean current density to −65 ± 5 pA/pF (n = 95). CaVβ3 hyperpolarized the activation potential of CaV2.3 from E0.5, act = −5 ± 1 mV (n = 18) for CaV2.3 alone to E0.5, act = −10.7 ± 0.3 mV (n = 49) for CaV2.3 + CaVβ3 channels. The numerical values can be found in Table 1. C, the r50 values (the fraction of peak whole cell currents remaining after a 50-ms pulse) are shown as the means ± S.E. from −10 to +20 mV for CaV2.3 WT + CaVβ3 and CaV2.3 WT + CaVβ3 + CaVα2δ-1 (from left to right on the bar graph). The r50 values of CaV2.3 WT + CaVβ3 ± CaVα2δ-1 were significantly different at p < 0.0001 at all voltages. D, HA-tagged CaV2.3 WT was expressed transiently either in the HEKT cell line or in the stable CaVβ3 cell line. Cell surface expression of HA-CaV2.3 WT was determined in intact cells by flow cytometry using the anti-HA FITC conjugate antibody. The histogram shows the number of fluorescent cells as a function of the experimental conditions. Cell autofluorescence (HEKT no Ab) was <1% throughout, and the addition of the FITC did not significantly increase the level of fluorescence in HEKT cells (not shown). As seen, only co-expression with CaVβ3 significantly promoted membrane expression of CaV2.3 (p < 0.001). Co-expression of CaV2.3 with CaVα2δ-1 did not alter the number of CaV2.3 at the membrane as compared with control conditions (p > 0.1). Co-expression with both auxiliary subunits did not further improve the membrane expression of CaV2.3 as compared with the CaV2.3 + CaVβ3 condition. The numerical values can be found in Tables 1–3.
Flow cytometry assays carried out with the HA-tagged CaV2.3 in the stable CaVβ3 confirmed that CaVβ is the critical auxiliary subunit in stimulating the plasma membrane density of CaV2.3 (Fig. 1D). Roughly 40% of the cells transfected with HA-CaV2.3 and CaVβ3 were fluorescent, suggesting that a substantial fraction of CaV2.3 proteins remained in cytoplasmic compartments as noted for CaV1.2 (17, 20). CaVβ1a, CaVβ1b, and CaVβ4 isoforms were as proficient as CaVβ3 in chaperoning CaV2.3 to the membrane, but the palmitoylated CaVβ2a did not appear to be as powerful as the other CaVβ subunits (Fig. 2 and Tables 1 and 3).
FIGURE 2.
CaVβ isoforms stimulated whole cell currents and cell surface density of CaV2.3. A, CaV2.3 WT was expressed transiently in the presence of CaVβ isoforms. Whole cell current traces recorded after the transient expression of the CaV2.3 WT channel in the presence of CaVβ1a, CaVβ1b, CaVβ2a, and CaVβ4 isoforms in HEKT cells. The charge carrier was 2 mm Ca2+. CaVα2δ was omitted. B, cell surface expression of HA-CaV2.3 WT was determined in intact cells by flow cytometry using the anti-HA FITC conjugate antibody. The histogram shows the number of fluorescent cells as a function of the CaVβ isoforms. C, the r50 values indicate that inactivation kinetics were significantly slower for CaV2.3 + CaVβ2a. The numerical values are shown in Tables 1–3.
There was no significant increase in cell fluorescence when overexpressing CaVα2δ-1 or calmodulin wild type alone with CaV2.3 (Table 3). Because the whole cell current density results from the NPoΔi product, where N is the number of proteins at the membrane, Po is the open channel probability, and Δi is the single channel conductance, these data suggest that CaVα2δ-1 improves the open channel probability rather than increasing the number of channels at the membrane as shown for CaV1.2 (17, 20). The strong functional modulation by CaVα2δ-1 contrasts with its milder effects on the total CaV2.3 protein density, especially when compared with the robust increase conferred by CaVβ3 (Fig. 3).
FIGURE 3.
CaVα2δ-1 mildly improves half-life of CaV2.3 upon arrest of cellular protein synthesis. HEKT cells were transiently transfected simultaneously with the indicated constructions. Exactly 24 h after transfection, subconfluent cells were incubated with cycloheximide (100 μg/ml) for up to 48 h, to block de novo protein synthesis. At the indicated time points (0 h or no cycloheximide, 6 h, 12 h, 24 h, 36 h, and 48 h), cell lysates were prepared and fractionated by SDS-PAGE (10%), followed by immunoblotting to visualize CaV2.3 (Alomone; 1:250), CaVβ3 (Invitrogen; 1:1000), CaVα2δ-1 (Alomone; 1:250), and GAPDH (Sigma; 1:10,000). The protein density of CaV2.3 in total membrane lysates was expressed relative to GAPDH and normalized to the protein density measured at time 0. The transient increase in CaV2.3 protein density measured at 6 and 12 h in the presence of CaVβ3 was not statistically significant (p > 0.01). As seen, CaVβ3 was more effective than CaVα2δ-1 in preventing the degradation of CaV2.3, but CaVα2δ-1 increased the lifetime of CaV2.3 as compared with the same protein expressed alone. The histograms were produced using values obtained from two different series of experiments and estimated with Image J. A, HA-CaV2.3 with the mock vector. B, HA-CaV2.3 with CaVβ3. C, HA-CaV2.3 with CaVα2δ-1. D, HA-CaV2.3 with CaVβ3 and CaVα2δ-1. The molecular mass of CaV2.3 is 250 kDa; that of CaVα2δ-1 is 175 kDa; that of CaVβ3 is 60 kDa; and that of GAPDH is 40 kDa.
To examine which AID residues (Fig. 4A) are required to promote cell surface expression of CaV2.3, conserved and nonconserved residues in the AID were mutated and functionally expressed in HEKT cells. Cell surface expression were maintained with CaV2.3 G382A, Y383A, and Y383F (Fig. 4, B and C), although the cell surface density decreased significantly for Y383G. The CaVβ3 modulation of activation gating and inactivation kinetics of Y383F and Y383A mutants was not significantly different from CaV2.3 WT (Fig. 4D and Table 1). Nonetheless, W386A failed to migrate to the plasma membrane. Hence, only the most severe reduction in the binding affinity as seen with W386A (26) was found to disrupt plasma membrane density.
FIGURE 4.
A, primary sequence of the AID locus in the I-II linker of CaV2.3. The residues that were mutated are underlined. B, HA-tagged CaV2.3 WT and mutants were expressed transiently with CaVβ3 WT + CaVα2δ-1. Whole cell currents were recorded in a 2 mm Ca2+ solution. From left to right are shown the typical whole cell current traces obtained for HA-CaV2.3 WT, HA-CaV2.3 Y383F, HA-CaV2.3 Y383A, and HA-CaV2.3 R384M. C, HA-tagged CaV2.3 was expressed transiently in the HEKT cell line (hatched bars) or in the stable CaVβ3 cell line (dark gray bars). Cell surface expression of CaV2.3 WT and mutants was determined in intact cells by flow cytometry using the anti-HA FITC conjugate antibody. The pattern of cell surface expression decreased in the order HA-CaV2.3 WT ≈ G382A ≈ Y383F > R384M ≈ R384L ≈ Y383A > Y383G ≫ W386A for the transient overexpressed CaVβ3. D, the r50 values for CaV2.3 mutants expressed with CaVβ3 WT and alternatively the CaVβ3 fragment 58–362 (SH3-HOOK-GK). The r50 values measured for the channel combinations displayed in B were not significantly different between 0 and +10 mV (p > 0.05).
Isothermal titration calorimetry assays (26) showed that the mutation of the nonconserved arginine residue (Arg384) located between the GY and WI in the AID helix increased the binding affinity of the CaV2.3 peptide for CaVβ2a with a Kd decreasing from 54 to 8.6 nm. CaV2.3 R384M and R384L mutations, however, did not significantly influence the number of CaV2.3 proteins at the membrane, nor did they alter the CaVβ3 modulation of channel gating (Fig. 4 and Table 1).
The SH3 and HOOK Domains of CaVβ Are Not Essential for CaVβ-mediated Modulation
We next addressed the importance of the different structural domains of CaVβ in the CaVβ-induced modulation of cell surface density and channel gating of CaV2.3. Co-expression with the SH3-HOOK-GK fragment of CaVβ3 (in amino acids 58–362) boosted cell surface expression and generated whole cell currents with biophysical parameters not significantly different from the wild-type version of CaVβ3 (Fig. 5 and Table 1), suggesting that the C-terminal does not contribute significantly to functional modulation in contrast to results obtained with CaVβ2a (19).
Dissection of the SH3-HOOK-GK fragment (amino acids 57–362) along structural boundaries yielded truncated constructs CaVβ ΔNSH3 (Δ57–123), CaVβ ΔHOOK (Δ122–169), CaVβ Δβ5 sheet (Δ170–175) (formally part of the SH3), and CaVβ ΔGK (Δ180–364) (Fig. 6). Except for the latter construct, each CaVβ construct increased surface density of CaV2.3 (Fig. 7 and Table 4) and produced channels with typical voltage-dependent activation gating (Table 1). The integrity of the CaVβ deletion constructs were confirmed by Western blots (data not shown).
FIGURE 6.
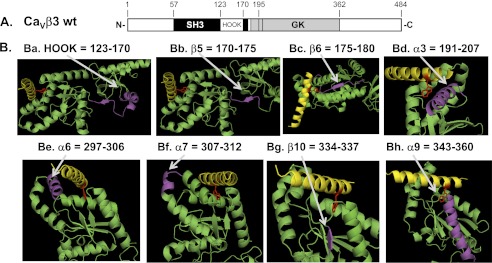
A, schematic diagram of the domain organization of the CaVβ3 subunit based on the crystal structure and adapted from (27) and used in (17). B, homology model of AID from CaV2.3 in complex with CaVβ3 highlighting structural regions in CaVβ3 and the number of amino acids marking the boundaries of the regions herein studied. The AID from CaV2.3 is shown as a yellow helix with the side chain of Trp386 protruding in red. In each panel, the region of interest is shown in violet. Panel a, CaVβ3 HOOK (123–170). Panel b, CaVβ3 β5 sheet (amino acids 170–175). Panel c, CaVβ3 β6 sheet (amino acids 175–180). Panel d, CaVβ3 α3 helix (amino acids 191–207). Panel e, CaVβ3 α6 helix (amino acids 297–306). Panel f, CaVβ3 α7 helix (amino acids 307–312). Panel g, CaVβ3 β10 sheet (amino acids 334–337). Panel h, CaVβ3 α9 helix (amino acids 343–360). The figure was produced using PyMOL (DeLano Scientific).
FIGURE 7.
A, typical whole cell current traces were recorded in a 2 mm Ca2+ solution for CaV2.3 WT in the presence of CaVβ3 deleted mutants. CaVα2δ was omitted. From left to right are shown CaVβ3 Δ57–123 (ΔNSH3), CaVβ3 Δ57–123 Δ170–175 (ΔSH3), CaVβ3 Δ122–169 (ΔHOOK), CaVβ3 Δ122–175 (ΔHOOK + Δβ5 sheet), and CaVβ3 Δ170–175 (Δβ5 sheet). B, cell surface density of HA-tagged CaV2.3 WT + CaVβ3 WT or mutants was estimated by a flow cytometry assay using the anti-HA FITC conjugate antibody. Co-expression with CaVβ3 deleted mutants Δ57–123, Δ122–169, and Δ170–175, as well as point mutations P175G and P175A in the β5 sheet of CaVβ3, promoted the cell surface expression of CaV2.3 to >70–90% of the level obtained with CaVβ3 WT. In contrast, there was no significant increase in the surface expression with CaVβ3 Δ175–180 and CaVβ3 Δ195–200 as compared with CaV2.3 in the absence of CaVβ3 (Table 4). C, mean peak current densities as a function of voltage are shown for CaV2.3 with either CaVβ3 Δ57–123 Δ170–175 (ΔSH3), CaVβ3 Δ122–169 (ΔHOOK), CaVβ3 Δ122–175 (ΔHOOK + Δβ5 sheet), or CaVβ3 Δ170–175 (Δβ5 sheet). CaVβ3 deleted constructs successfully increased peak current densities without any significant change in the activation potential as compared with CaV2.3 WT + CaVβ3 WT. D, the r50 values are shown as means ± S.E. from −5 to +15 mV for CaV2.3 WT + CaVβ3 WT and CaVβ3 Δ57–123, CaVβ3 Δ122–169, CaVβ3 Δ122–175, CaVβ3 Δ170–175, and CaVβ3 ΔSH3 (Δ57–123 Δ170–175). Inactivation kinetics were significantly slower for the Δ170–175 (p < 0.0001) at all voltages as compared with CaV2.3 WT + CaVβ3 WT. The numerical values are shown in Table 1.
The SH3 domain was shown to support channel endocytosis (53) and to promote calpain-mediated CaVβ3 proteolysis through a PEST-like motif (54). Deletion of the N-terminal of SH3 domain (Δ57–123), deletion of the six-residue β5 strand (Δ170–175 or ΔPYDVVP, formerly known as the β-interaction domain or BID (55)), or deletion of both regions (ΔSH3) yielded channels with robust peak current density and typical voltage-dependent activation gating in the absence and in the presence of CaVα2δ-1 as a background subunit (Fig. 7 and Tables 1 and 2). Deletion of the HOOK domain (Δ122–169 and Δ122–175) in CaVβ3 did not significantly alter any of the biophysical parameters (activation potential, peak current density, and inactivation kinetics) (Fig. 7 and Table 1). In contrast, small six-residue deletions in the GK domain with CaVβ3 Δβ6 or Δ175–180 and CaVβ3 Δ195–200 failed to promote cell surface density of CaV2.3 channels (Fig. 7B).
Mutations of Leucine Residues in the GK Domain Decrease Plasma Membrane Density
A mutational analysis was thus carried to seek out single residues in the GK domain that are responsible for modulation of gating and cell surface density of CaV2.3. We focused on residues of CaVβ3 that were highly likely to interact with Trp386 and Ile387 residues in CaV2.3 (38) based upon the changes in the energetics of interaction between the AID peptide from CaV1.2 and CaVβ2a (26), as well as from the predictions of the homology model (Fig. 6). Note that the GK domains of CaVβ2a and CaVβ3 are well conserved with more than 87% identity in this stretch of 188 amino acids. Residues in CaVβ3 were either substituted by glycine to minimize side chain interactions or substituted with alanine to preserve the α-helicoidal structure.
Nearly 20 point mutations were tested in the GK domain of CaVβ3, namely in the β6 sheet (P175A, P175G, P179A, and P179G); in the α3 helix (M195A, M196A, K198A, and L200G); in the β9 sheet (S295A); in the α6 helix (R302G, L303G, R307A, R307G, and R307K); in the β10 sheet (E339G, L337G, and N340G); and in the α9 helix (L342G and A345G). All CaVβ3 mutations produced functional CaV2.3 channels (Tables 1, 2, and 4). Most notably, CaVβ3 P175A (β6), CaVβ3 M196A (α3), CaVβ3 S295A (β9), CaVβ3 R302G (α6), and CaVβ3 E339G (β10) behaved like CaVβ3 WT. These CaVβ mutants stimulated cell surface density, boosted whole cell peak current density, and imparted the small but significant negative shift in the activation potential of CaV2.3 (Tables 1, 2, and 4).
Mutations at position Arg307 (R307A, R307G, and R307K) in the α6 helix significantly increased peak current densities as compared with CaVβ3 WT (Tables 1 and 2). In addition, the CaVβ3 Arg307 mutants decreased the inactivation kinetics, suggesting that the open state was stabilized by these mutants. These effects were observed despite a documented decrease in the affinity of the equivalent R356A mutation in CaVβ2a (equivalent to Arg307 in CaVβ3) with a Kd increasing from 5 to 345 nm (26).
In contrast, point mutations of four leucine residues (L200G, L303G, L337G, and L342G) significantly decreased cell surface density of CaV2.3 by ≈50–60% (Fig. 8). The three-dimensional homology model suggests that the four leucine residues in CaVβ3 could form a hydrophobic pocket surrounding CaV2.3 Trp386 and CaV2.3 Ile387 (Fig. 9). Note that the strongest effects were observed with CaVβ3 L303G, which is predicted to interact with Ile387 in CaV2.3. Multiple point mutations were constructed to test the hypothesis that the four leucine residues form a single interaction site. The double, triple, and quadruple CaVβ3 mutants L200G/L303G, L200G/L303G/L347G, and L200G/L303G/L337G/L342G CaVβ3 mutants all ablated cell surface density of CaV2.3 (Table 4). Furthermore, the peak current densities measured with these CaVβ3 mutants were not statistically different (p > 0.1) from the densities measured in the absence of CaVβ3 (Tables 1 and 2). Finally, the normalized protein density of CaV2.3 measured in membrane lysates was significantly decreased with CaVβ3 L303G as compared with CaVβ3 WT but was nearly abolished with the quadruple CaVβ3 leucine mutant L200G/L303G/L337G/L342G (Fig 10). In the context where the fluorescence level measured in our FACS assay results from the net balance between anterograde and retrograde trafficking, this observation suggests that the decreased protein density at the plasma membrane could result from a decrease in the CaV2.3 total protein density.
FIGURE 8.
A, typical whole cell current traces were recorded in a 2 mm Ca2+ solution for CaV2.3 WT in the presence of the CaVβ3 mutants. CaVα2δ-1 was omitted. From left to right are shown CaVβ3 L200G, CaVβ3 L303G, CaVβ3 L337G, and CaVβ3 L342G. B, HA-tagged CaV2.3 was expressed transiently in the HEKT cell line with CaVβ3 mutants. Cell surface expression was determined in intact cells by flow cytometry. CaVβ3 mutants L200G, L303G, L337G, and L342G curtailed membrane protein expression by 50–60%. Multiple mutations L200G/L303G (L1,2G), L200G/L303G/L337G (L1,2,3G), and L200G/L303G/L337G/L342G (L1,2,3,4G) abolished the CaVβ stimulation of cell surface density (Table 4). C, normalized peak current densities are plotted as a function of applied voltage. All of the CaVβ3 mutants produced CaV2.3 currents with similar voltage-dependent activation (Table 1). D, the r50 values are shown ± S.E. from −10 to +20 mV for CaV2.3 WT in the presence of CaVβ3 WT, CaVβ3 L200G, CaVβ3 L303G, CaVβ3 L337G, and CaVβ3 L342G (from left to right on the bar graph). Inactivation kinetics were significantly slower for CaVβ3 L342G at all voltages (p < 0.01).
FIGURE 9.
Homology model of the 18-residue-long AID region from CaV2.3 in complex with CaVβ3. The quartet of leucine residues in CaVβ3 surrounding the AID helix is shown in three different orientations. The CaVβ3 subunit is in green, and the AID helix is shown in yellow with the protruding side chains of Trp386 and Ile387 shown in red and the side chains of the four leucine residues displayed in violet. A, with the AID region shown horizontally and CaV2.3 W386A facing down. B, with the AID region shown horizontally and CaV2.3 W386A facing up. C, with the AID region shown vertically.
FIGURE 10.
CaV2.3 protein density decreased with CaVβ3 Leu mutants. A, representative Western blot analyses of HEKT cells transiently co-transfected with pCDNA3-CaV2.3-HA and pCMV-Tag5-CaVβ3 WT and mutants using anti-CaV2.3 (Alomone; 1:500), anti-CaVβ3 (Invitrogen; 1:10,000), and anti-GAPDH (Sigma; 1:10,000) antibodies. Each lane was loaded with 20 μg of protein. Lane 1, CaV2.3-HA + CaVβ3 WT. Lane 2, CaV2.3-HA + CaVβ3 L303G. Lane 3, CaV2.3-HA + CaVβ3 L200G/L303G. Lane 4, CaV2.3-HA + CaVβ3 L200G/L303G/L337G. Lane 5, CaV2.3-HA + CaVβ3 L200G/L303G/L337G/L342G. B, protein density of CaV2.3 was normalized using GAPDH as an internal loading control and estimated using Image J. Maximum density was obtained for CaV2.3 and CaVβ3. The histogram was produced using values obtained from two different gels.
DISCUSSION
There is considerable interest in identifying molecules that modulate protein-protein interactions in vivo. In this regard, specifically modulating the interaction of CaVβ with HVA CaV channels could be a strategy to design new HVA CaV agonists and antagonists. Residues of CaVβ carrying nanomolar affinity binding onto the CaVα1 of CaV1.2 have been identified from high resolution three-dimensional crystal structures and isothermal calorimetry assays (26–29). Although mutations of residues in CaVα1 abolishing the protein-protein interaction are incompatible with CaV1.2 function (17, 26), there has been some divergence regarding the functional importance of the domains in CaVβ subunits (26, 27, 33, 39–41). The range of approaches and recombinant systems could explain in part the diverse conclusions. We have thus systematically addressed the role of CaVβ in channel function based upon the structural data currently available. Our results show that protein-protein affinity measured in vitro may not be the only predictor of channel modulation especially in the context where CaVβ subunits control surface density and prevent degradation of CaVα1 subunits from CaV1 and CaV2 channels by the ERAD complex.
Site Occupancy Is Sufficient to Modulate Protein Density and Gating
It is well understood that high affinity binding of CaVβ onto the AID motif appears to be a prerequisite for both CaVβ-induced modulation of gating and CaVβ-stimulated plasma membrane trafficking of CaVα1 (17, 22, 56, 57). In our hands, mutations within the AID of CaV2.3 that were shown to moderately increase (R384M and R384L) or decrease (G382A, Y383A, and Y383F) binding affinity (26) did not alter the CaVβ3 modulation of cell surface density and hyperpolarization of gating. CaVβ mutations or CaV2.3 mutations (Y383A) that moderately reduced the number of CaV2.3 proteins at the membrane produced channels with biophysical properties (peak current density, activation gating, and inactivation kinetics) similar to CaV2.3 WT + CaVβ3 WT control channels. This result is remarkably reminiscent of the Y383S mutation in CaV2.2 that decreased the affinity of CaVβ1b binding to the I-II linker of CaV2.2 from a Kd value of 14 nm to one of 329 nm while preserving its effect on current density and cell surface expression in HEKT cells (23).
Nonetheless, complete disruption of this interaction with CaV2.3 W386A, CaVβ3 Δ175–180, or CaVβ3 Δ195–200 abrogated cell surface labeling of CaV2.3, which evidently translated in the absence of function. This is a surprising observation given that the reverse observation was reported in the Xenopus expression system. AID-deficient CaV2.3 channels migrated to the plasma membrane but were not functionally modulated by CaVβ when expressed in Xenopus oocytes (37, 38). It certainly raises questions about the transposition of data from Xenopus oocytes to mammalian cells.
Partial Deletions of the GK Domain of CaVβ3 Disrupt Plasma Membrane Density of CaV2.3
The deletion of the HOOK region and thus the absence of intramolecular coupling between the SH3 and GK in CaVβ did not impact significantly the voltage-dependent activation gating or the inactivation kinetics of CaV2.3 despite the report that the variable HOOK domain plays a role in the inactivation kinetics of CaVβ2a (40). Our observation also seems to contradict a previous report that strong SH3-GK intramolecular coupling conferred by short linkers (<3 amino acids) confers fast inactivation kinetics (19, 35). It also disagrees with the observation that the β5 sheet in the SH3 domain of CaVβ2a plays an important role in the modulation of CaV2.1 channels (35). Discrepancies can be attributed to the idiosyncrasy of the CaVβ2a subunit and/or the Xenopus expression system used in those studies. In our hands, complete deletion of the SH3 or the HOOK domains in CaVβ3 failed to abrogate either its chaperone function or its modulation of channel gating.
Leucine Residues in the GK Domain of CaVβ3 Determine Cell Surface Density of CaV2.3
Close to 20 point mutations were performed in the GK domain of CaVβ3. The majority of the mutations did not alter the CaVβ-induced stimulation of cell surface density and hyperpolarization of gating despite a documented decrease in the binding affinity (26). Among the CaVβ mutations herein tested, four mutations (M196A, L303G, R307A, and L342G) were expected to decrease significantly the binding affinity of CaVβ3 to CaV2.3 (26). CaVβ2a M245A (equivalent to CaVβ3 M196A) yielded a 200-fold decrease in the affinity for CaV1.2 (26), with the Kd increasing from 5 nm to 1.1 μm. Nonetheless, whole cell currents of CaV2.3 obtained in the presence of CaVβ3 M196A were not significantly different from those obtained with CaVβ3 WT both in terms of peak current density, voltage dependence of activation, and inactivation kinetics. In contrast, mutations of leucine residues at positions 200, 303, 337, and 342 (either individually or in combination) significantly reduced the modulation of CaV2.3 function and its cell surface density when compared with CaVβ3 WT. Remarkably, the affinities measured between the AID peptide of CaV1.2 and the CaVβ2 mutants L352A and L392A (equivalent to CaVβ3 L303 and L342) were ≈20-fold lower than for CaVβ2 WT but still 100-fold higher than with CaVβ2 M245A (26). Our functional screen thus discriminated residues in CaVβ3 that were initially believed to behave similarly based upon their binding energies.
It remains possible that the binding affinities of the CaV2.3 + CaVβ3 complex cannot be simply extrapolated from the in vitro binding energies measured with CaVβ2a bound onto the AID peptides from CaV1.2 channels (26). A recent study performed with larger peptides (≈30–40 residues) points to important structural differences between the highly conserved I-II linkers of CaV1.2 and CaV2.2 (13).
With these reservations in mind, our results altogether suggest that nanomolar protein-protein affinity may not be the sole determinant of the modulation of CaVα1 channel function by CaVβ. Given that CaV2.3 W386A was not measured at the membrane but that CaVβ3 M196A sustained surface density of CaV2.3 proteins, it can be concluded that functional modulation occurs in the nano- to micromolar range. Even with a moderate affinity, “occupancy of the AID site” could be sufficient to carry the CaVβ-induced modulation of channel function in HVA CaV1 and CaV2 channels (23). In this scheme, occupancy of the AID site by CaVβ could either unmask retention signals for protein targeting or mask ubiquitination sites on the CaVα1 subunit and prevents its degradation by the proteasome.
Conclusion
Mutations of four leucine residues (Leu200, Leu303, Leu337, and Leu342) were each expected to moderately decrease binding affinity, significantly decreasing the cell surface density of CaV2.3 protein. Simultaneous mutation of the four leucine residues completely abolished surface and total protein density of CaV2.3. We propose that these four leucine residues form a hydrophobic pocket that is required to promote van der Waals interactions with Trp386 and Ile387 (WI pair) in the AID region of CaV2.3. Hence, the four leucine residues in the GK domain of CaVβ3 and the WI pair in the AID of CaV2.3 appear to be essential determinants in the modulation of high voltage-activated calcium channel function by CaVβ auxiliary subunits.
Acknowledgments
We thank Dr. Toni Schneider (University of Cologne, Cologne, Germany) for the human CaV2.3 clone in the pcDNA3 vector; M. Serge Sénéchal and Dr. Jacques Thibodeau (Department of Microbiology and Immunology, Université de Montréal) for help with the fluorescence-activated cell sorting experiments and analysis; Guillaume Roussel for preliminary experiments; Julie Verner for cell culture; Michel Brunette for expert technical assistance; and Claude Gauthier for artwork.
This work was completed with Grant MOP13390 from the Canadian Institutes of Health Research and a grant from the Canadian Heart and Stroke Foundation (to L. P.).
- AID
- α-interacting domain
- SH3
- Src homology 3
- GK
- guanylate kinase-like.
REFERENCES
- 1. Snutch T. P., Reiner P. B. (1992) Ca2+ channels. Diversity of form and function. Curr. Opin. Neurobiol. 2, 247–253 [DOI] [PubMed] [Google Scholar]
- 2. Zhang J. F., Randall A. D., Ellinor P. T., Horne W. A., Sather W. A., Tanabe T., Schwarz T. L., Tsien R. W. (1993) Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology 32, 1075–1088 [DOI] [PubMed] [Google Scholar]
- 3. Birnbaumer L., Campbell K. P., Catterall W. A., Harpold M. M., Hofmann F., Horne W. A., Mori Y., Schwartz A., Snutch T. P., Tanabe T. (1994) The naming of voltage-gated calcium channels. Neuron 13, 505–506 [DOI] [PubMed] [Google Scholar]
- 4. Perez-Reyes E., Cribbs L. L., Daud A., Lacerda A. E., Barclay J., Williamson M. P., Fox M., Rees M., Lee J. H. (1998) Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature 391, 896–900 [DOI] [PubMed] [Google Scholar]
- 5. Cribbs L. L., Lee J. H., Yang J., Satin J., Zhang Y., Daud A., Barclay J., Williamson M. P., Fox M., Rees M., Perez-Reyes E. (1998) Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ. Res. 83, 103–109 [DOI] [PubMed] [Google Scholar]
- 6. Randall A., Benham C. D. (1999) Recent advances in the molecular understanding of voltage-gated Ca2+ channels. Mol. Cell Neurosci. 14, 255–272 [DOI] [PubMed] [Google Scholar]
- 7. Catterall W. A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 [DOI] [PubMed] [Google Scholar]
- 8. Peterson B. Z., DeMaria C. D., Adelman J. P., Yue D. T. (1999) Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron 22, 549–558 [DOI] [PubMed] [Google Scholar]
- 9. Dolphin A. C. (2009) Calcium channel diversity. Multiple roles of calcium channel subunits. Curr. Opin. Neurobiol. 19, 237–244 [DOI] [PubMed] [Google Scholar]
- 10. Dai S., Hall D. D., Hell J. W. (2009) Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol. Rev. 89, 411–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao T., Puri T. S., Gerhardstein B. L., Chien A. J., Green R. D., Hosey M. M. (1997) Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J. Biol. Chem. 272, 19401–19407 [DOI] [PubMed] [Google Scholar]
- 12. Carl S. L., Felix K., Caswell A. H., Brandt N. R., Ball W. J., Jr., Vaghy P. L., Meissner G., Ferguson D. G. (1995) Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J. Cell Biol. 129, 673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Almagor L., Chomsky-Hecht O., Ben-Mocha A., Hendin-Barak D., Dascal N., Hirsch J. A. (2012) The role of a voltage-dependent Ca2+ channel intracellular linker. A structure-function analysis. J. Neurosci. 32, 7602–7613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buraei Z., Yang J. (2010) The ß subunit of voltage-gated Ca2+ channels. Physiol. Rev. 90, 1461–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Petegem F., Minor D. L., Jr. (2006) The structural biology of voltage-gated calcium channel function and regulation. Biochem. Soc. Trans. 34, 887–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parent L., Schneider T., Moore C. P., Talwar D. (1997) Subunit regulation of the human brain α 1E calcium channel. J. Membr. Biol. 160, 127–140 [DOI] [PubMed] [Google Scholar]
- 17. Bourdin B., Marger F., Wall-Lacelle S., Schneider T., Klein H., Sauvé R., Parent L. (2010) Molecular determinants of the CaVβ-induced plasma membrane targeting of the CaV1.2 channel. J. Biol. Chem. 285, 22853–22863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao T., Chien A. J., Hosey M. M. (1999) Complexes of the α1C and β subunits generate the necessary signal for membrane targeting of class C L-type calcium channels. J. Biol. Chem. 274, 2137–2144 [DOI] [PubMed] [Google Scholar]
- 19. Takahashi S. X., Miriyala J., Tay L. H., Yue D. T., Colecraft H. M. (2005) A CaVβ SH3/guanylate kinase domain interaction regulates multiple properties of voltage-gated Ca2+ channels. J. Gen. Physiol. 126, 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Altier C., Garcia-Caballero A., Simms B., You H., Chen L., Walcher J., Tedford H. W., Hermosilla T., Zamponi G. W. (2011) The CaVβ subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat. Neurosci. 14, 173–180 [DOI] [PubMed] [Google Scholar]
- 21. Bichet D., Cornet V., Geib S., Carlier E., Volsen S., Hoshi T., Mori Y., De Waard M. (2000) The I-II loop of the Ca2+ channel α1 subunit contains an endoplasmic reticulum retention signal antagonized by the β subunit. Neuron 25, 177–190 [DOI] [PubMed] [Google Scholar]
- 22. Leroy J., Richards M. W., Butcher A. J., Nieto-Rostro M., Pratt W. S., Davies A., Dolphin A. C. (2005) Interaction via a key tryptophan in the I-II linker of N-type calcium channels is required for β1 but not for palmitoylated β2, implicating an additional binding site in the regulation of channel voltage-dependent properties. J. Neurosci. 25, 6984–6996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butcher A. J., Leroy J., Richards M. W., Pratt W. S., Dolphin A. C. (2006) The importance of occupancy rather than affinity of CaVβ subunits for the calcium channel I-II linker in relation to calcium channel function. J. Physiol. 574, 387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waithe D., Ferron L., Page K. M., Chaggar K., Dolphin A. C. (2011) β-Subunits promote the expression of CaV2.2 channels by reducing their proteasomal degradation. J. Biol. Chem. 286, 9598–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T. P., Campbell K. P. (1994) Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature 368, 67–70 [DOI] [PubMed] [Google Scholar]
- 26. Van Petegem F., Duderstadt K. E., Clark K. A., Wang M., Minor D. L., Jr. (2008) Alanine-scanning mutagenesis defines a conserved energetic hotspot in the CaVα1 AID-CaVβ interaction site that is critical for channel modulation. Structure 16, 280–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Y. H., Li M. H., Zhang Y., He L. L., Yamada Y., Fitzmaurice A., Shen Y., Zhang H., Tong L., Yang J. (2004) Structural basis of the α1-β subunit interaction of voltage-gated Ca2+ channels. Nature 429, 675–680 [DOI] [PubMed] [Google Scholar]
- 28. Opatowsky Y., Chen C. C., Campbell K. P., Hirsch J. A. (2004) Structural analysis of the voltage-dependent calcium channel β subunit functional core and its complex with the α1 interaction domain. Neuron 42, 387–399 [DOI] [PubMed] [Google Scholar]
- 29. Van Petegem F., Clark K. A., Chatelain F. C., Minor D. L., Jr. (2004) Structure of a complex between a voltage-gated calcium channel β-subunit and an α-subunit domain. Nature 429, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olcese R., Qin N., Schneider T., Neely A., Wei X., Stefani E., Birnbaumer L. (1994) The amino terminus of a calcium channel β subunit sets rates of channel inactivation independently of the subunit's effect on activation. Neuron 13, 1433–1438 [DOI] [PubMed] [Google Scholar]
- 31. Jangsangthong W., Kuzmenkina E., Khan I. F., Matthes J., Hullin R., Herzig S. (2010) Inactivation of L-type calcium channels is determined by the length of the N terminus of mutant β1 subunits. Pflugers Arch. 459, 399–411 [DOI] [PubMed] [Google Scholar]
- 32. Restituito S., Cens T., Barrere C., Geib S., Galas S., De Waard M., Charnet P. (2000) The β2a subunit is a molecular groom for the Ca2+ channel inactivation gate. J. Neurosci. 20, 9046–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gonzalez-Gutierrez G., Miranda-Laferte E., Nothmann D., Schmidt S., Neely A., Hidalgo P. (2008) The guanylate kinase domain of the beta-subunit of voltage-gated calcium channels suffices to modulate gating. Proc. Natl. Acad. Sci. U.S.A. 105, 14198–14203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dresviannikov A. V., Page K. M., Leroy J., Pratt W. S., Dolphin A. C. (2009) Determinants of the voltage dependence of G protein modulation within calcium channel β subunits. Pflugers Arch. 457, 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Y. H., He L. L., Buchanan D. R., Zhang Y., Fitzmaurice A., Yang J. (2009) Functional dissection of the intramolecular Src homology 3-guanylate kinase domain coupling in voltage-gated Ca2+ channel β-subunits. FEBS Lett. 583, 1969–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maltez J. M., Nunziato D. A., Kim J., Pitt G. S. (2005) Essential CaVβ modulatory properties are AID-independent. Nat. Struct. Mol. Biol. 12, 372–377 [DOI] [PubMed] [Google Scholar]
- 37. Berrou L., Dodier Y., Raybaud A., Tousignant A., Dafi O., Pelletier J. N., Parent L. (2005) The C-terminal residues in the α-interacting domain (AID) helix anchor CaV β subunit interaction and modulation of CaV2.3 channels. J. Biol. Chem. 280, 494–505 [DOI] [PubMed] [Google Scholar]
- 38. Berrou L., Klein H., Bernatchez G., Parent L. (2002) A specific tryptophan in the I-II linker is a key determinant of beta-subunit binding and modulation in CaV2.3 calcium channels. Biophys. J. 83, 1429–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McGee A. W., Nunziato D. A., Maltez J. M., Prehoda K. E., Pitt G. S., Bredt D. S. (2004) Calcium channel function regulated by the SH3-GK module in beta subunits. Neuron 42, 89–99 [DOI] [PubMed] [Google Scholar]
- 40. Richards M. W., Leroy J., Pratt W. S., Dolphin A. C. (2007) The HOOK-domain between the SH3 and the GK domains of CaVβ subunits contains key determinants controlling calcium channel inactivation. Channels 1, 92–101 [DOI] [PubMed] [Google Scholar]
- 41. Miranda-Laferte E., Gonzalez-Gutierrez G., Schmidt S., Zeug A., Ponimaskin E. G., Neely A., Hidalgo P. (2011) Homodimerization of the Src homology 3 domain of the calcium channel β-subunit drives dynamin-dependent endocytosis. J. Biol. Chem. 286, 22203–22210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tareilus E., Roux M., Qin N., Olcese R., Zhou J., Stefani E., Birnbaumer L. (1997) A Xenopus oocyte β subunit. Evidence for a role in the assembly/expression of voltage-gated calcium channels that is separate from its role as a regulatory subunit. Proc. Natl. Acad. Sci. U.S.A. 94, 1703–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chien A. J., Carr K. M., Shirokov R. E., Rios E., Hosey M. M. (1996) Identification of palmitoylation sites within the L-type calcium channel β2a subunit and effects on channel function. J. Biol. Chem. 271, 26465–26468 [DOI] [PubMed] [Google Scholar]
- 44. Pereverzev A., Leroy J., Krieger A., Malécot C. O., Hescheler J., Pfitzer G., Klöckner U., Schneider T. (2002) Alternate splicing in the cytosolic II-III loop and the carboxy terminus of human E-type voltage-gated Ca2+ channels. Electrophysiological characterization of isoforms. Mol. Cell Neurosci. 21, 352–365 [DOI] [PubMed] [Google Scholar]
- 45. Castellano A., Wei X., Birnbaumer L., Perez-Reyes E. (1993) Cloning and expression of a third calcium channel β subunit. J. Biol. Chem. 268, 3450–3455 [PubMed] [Google Scholar]
- 46. Williams M. E., Feldman D. H., McCue A. F., Brenner R., Velicelebi G., Ellis S. B., Harpold M. M. (1992) Structure and functional expression of α1, α2, and β subunits of a novel human neuronal calcium channel subtype. Neuron 8, 71–84 [DOI] [PubMed] [Google Scholar]
- 47. Yasuda T., Chen L., Barr W., McRory J. E., Lewis R. J., Adams D. J., Zamponi G. W. (2004) Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur. J. Neurosci. 20, 1–13 [DOI] [PubMed] [Google Scholar]
- 48. Wall-Lacelle S., Hossain M. I., Sauvé R., Blunck R., Parent L. (2011) Double mutant cycle analysis identified a critical leucine residue in the IIS4S5 linker for the activation of the CaV2.3 calcium channel. J. Biol. Chem. 286, 27197–27205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yifrach O., MacKinnon R. (2002) Energetics of pore opening in a voltage-gated K+ channel. Cell. 111, 231–239 [DOI] [PubMed] [Google Scholar]
- 50. Raybaud A., Baspinar E. E., Dionne F., Dodier Y., Sauvé R., Parent L. (2007) The role of distal S6 hydrophobic residues in the voltage-dependent gating of CaV2.3 channels. J. Biol. Chem. 282, 27944–27952 [DOI] [PubMed] [Google Scholar]
- 51. Kamp M. A., Shakeri B., Tevoufouet E. E., Krieger A., Henry M., Behnke K., Herzig S., Hescheler J., Radhakrishnan K., Parent L., Schneider T. (2012) The C-terminus of human Cav2.3 voltage-gated calcium channel interacts with alternatively spliced calmodulin-2 expressed in two human cell lines. Biochim. Biophys. Acta 1824, 1045–1057 [DOI] [PubMed] [Google Scholar]
- 52. Notredame C., Higgins D. G., Heringa J. (2000) T-Coffee. A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302, 205–217 [DOI] [PubMed] [Google Scholar]
- 53. Gonzalez-Gutierrez G., Miranda-Laferte E., Neely A., Hidalgo P. (2007) The Src homology 3 domain of the β-subunit of voltage-gated calcium channels promotes endocytosis via dynamin interaction. J. Biol. Chem. 282, 2156–2162 [DOI] [PubMed] [Google Scholar]
- 54. Sandoval A., Oviedo N., Tadmouri A., Avila T., De Waard M., Felix R. (2006) Two PEST-like motifs regulate Ca2+/calpain-mediated cleavage of the CaVβ3 subunit and provide important determinants for neuronal Ca2+ channel activity. Eur. J. Neurosci. 23, 2311–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Waard M., Scott V. E., Pragnell M., Campbell K. P. (1996) Identification of critical amino acids involved in α1-β interaction in voltage-dependent Ca2+ channels. FEBS Lett. 380, 272–276 [DOI] [PubMed] [Google Scholar]
- 56. Altier C., Dubel S. J., Barrère C., Jarvis S. E., Stotz S. C., Spaetgens R. L., Scott J. D., Cornet V., De Waard M., Zamponi G. W., Nargeot J., Bourinet E. (2002) Trafficking of L-type calcium channels mediated by the postsynaptic scaffolding protein AKAP79. J. Biol. Chem. 277, 33598–33603 [DOI] [PubMed] [Google Scholar]
- 57. Cohen R. M., Foell J. D., Balijepalli R. C., Shah V., Hell J. W., Kamp T. J. (2005) Unique modulation of L-type Ca2+ channels by short auxiliary β1d subunit present in cardiac muscle. Am. J. Physiol. Heart Circ. Physiol. 288, H2363–H2374 [DOI] [PubMed] [Google Scholar]