Abstract
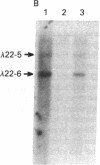
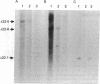
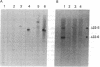
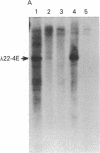
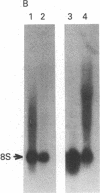
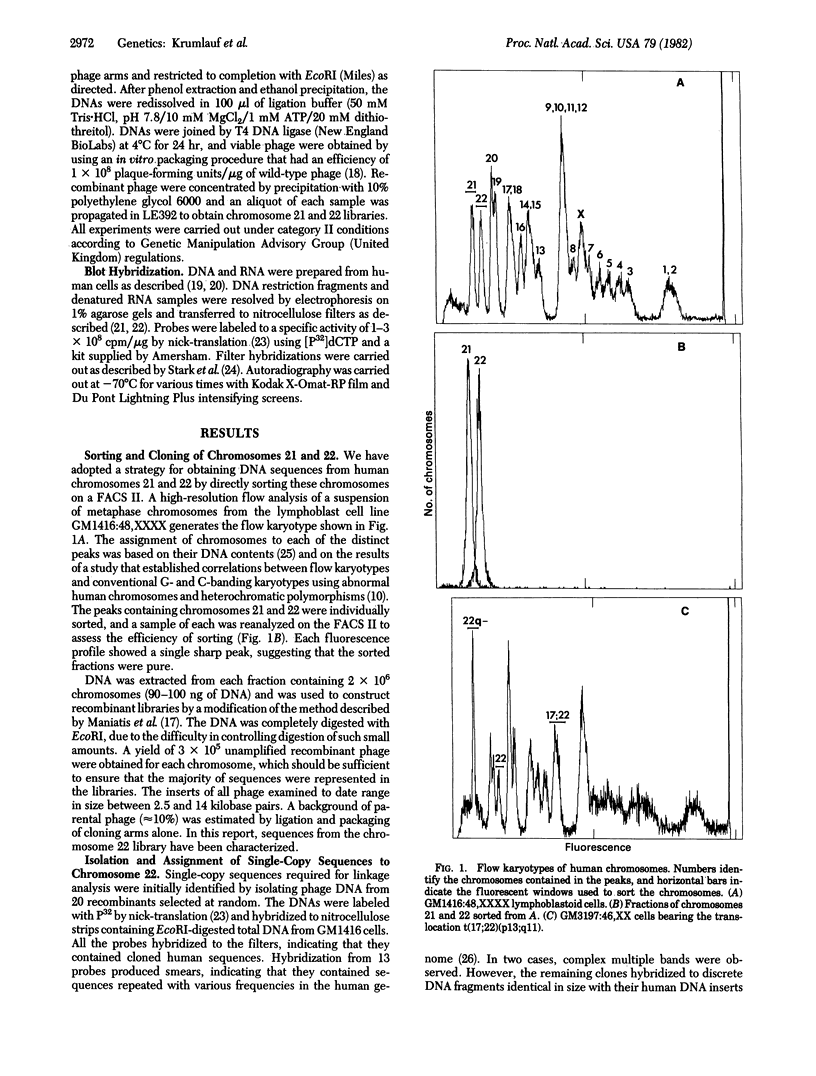
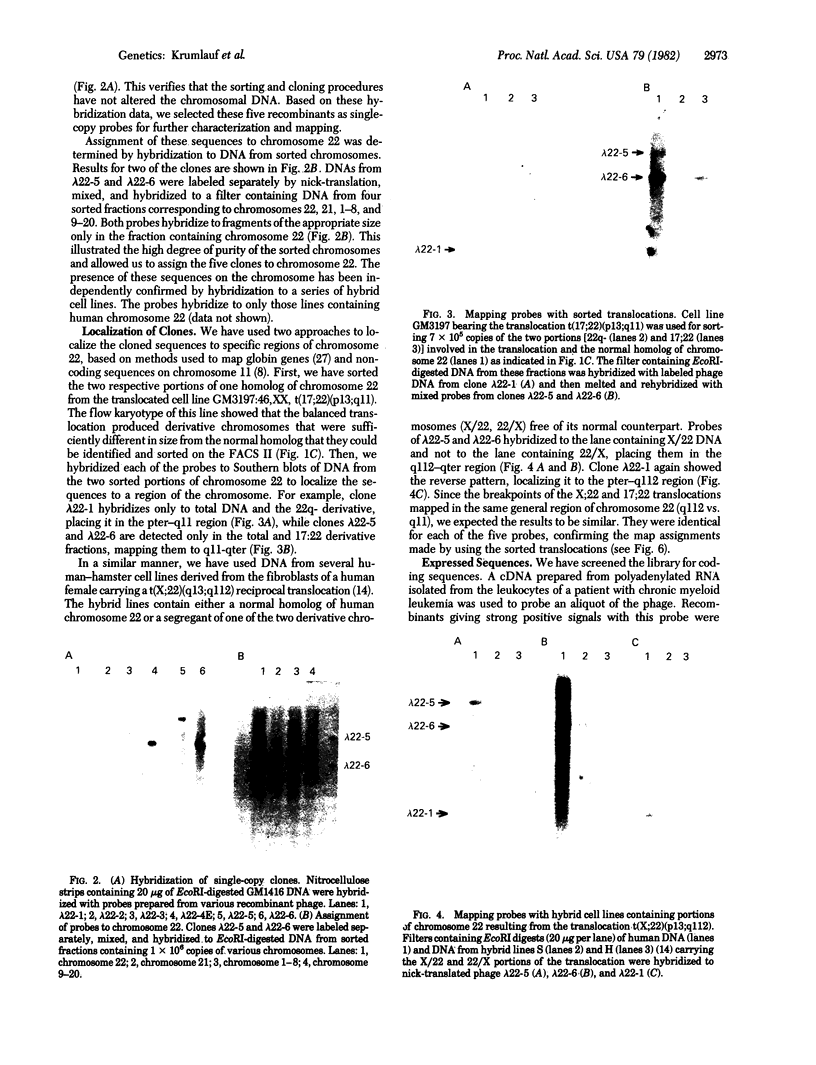
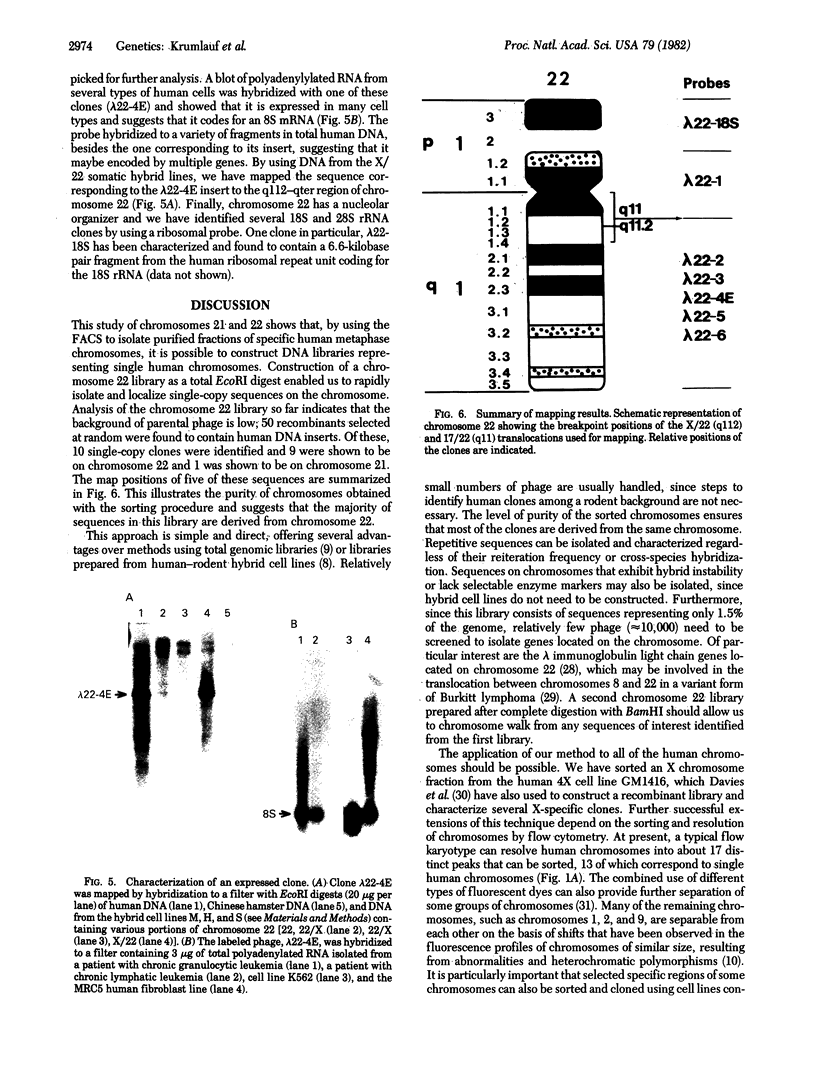
Highly purified fractions of human chromosomes 21 and 22 were isolated from a suspension of metaphase chromosomes stained with ethidium bromide by using a fluorescence-activated cell sorter (FACS II). Two recombinant DNA libraries, representing chromosomes 21 and 22, were constructed by complete digestion of DNA from these fractions with EcoRI and insertion into the vector lambda gtWES lambda B. Twenty clones selected at random from the chromosome 22 library hybridized to EcoRI-digested human DNA, and five of these clones hybridized to single bands identical in size to the phage inserts. These five single-copy sequences and a clone coding for an 8S RNA isolated by screening the chromosome 22 library for expressed sequences were characterized in detail. Hybridization of all six clones to a panel of sorted chromosomes and hybrid cell lines confirmed the assignment of the sequences to chromosome 22. The sequences were localized to regions of chromosome 22 by hybridization to translocated chromosomes sorted from a cell line having a balanced translocation t(17;22)(p13;q11) and to hybrid cell lines containing the various portions of another translocation t(X;22)(q13;q112). Five clones reside on the long arm of chromosome 22 between q112 and pter, while one clone and an 18S rRNA gene isolated from the chromosome 22 library reside pter and g112. The construction of chromosome-specific libraries by this method has the advantage of being direct and applicable to nearly all human chromosomes and will be important in molecular analysis of human genetic diseases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger R., Bernheim A., Weh H. J., Flandrin G., Daniel M. T., Brouet J. C., Colbert N. A new translocation in Burkitt's tumor cells. Hum Genet. 1979;53(1):111–112. doi: 10.1007/BF00289460. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Carrano A. V., Gray J. W., Langlois R. G., Burkhart-Schultz K. J., Van Dilla M. A. Measurement and purification of human chromosomes by flow cytometry and sorting. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1382–1384. doi: 10.1073/pnas.76.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davies K. E., Young B. D., Elles R. G., Hill M. E., Williamson R. Cloning of a representative genomic library of the human X chromosome after sorting by flow cytometry. Nature. 1981 Oct 1;293(5831):374–376. doi: 10.1038/293374a0. [DOI] [PubMed] [Google Scholar]
- Erikson J., Martinis J., Croce C. M. Assignment of the genes for human lambda immunoglobulin chains to chromosome 22. Nature. 1981 Nov 12;294(5837):173–175. doi: 10.1038/294173a0. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Keys C., VarsanyiBreiner A., Kao F. T., Jones C., Puck T. T., Housman D. Isolation and localization of DNA segments from specific human chromosomes. Proc Natl Acad Sci U S A. 1980 May;77(5):2829–2833. doi: 10.1073/pnas.77.5.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hors-Cayla M. C., Junien C., Heuertz S., Mattei J. F., Frézal J. Regional assignment of arylsulfatase A, mitochondrial aconitase and NADH-cytochrome b5 reductase by somatic cell hybridization. Hum Genet. 1981;58(2):140–143. doi: 10.1007/BF00278698. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J. DNA sequence variants in the G gamma-, A gamma-, delta- and beta-globin genes of man. Cell. 1979 Sep;18(1):1–10. doi: 10.1016/0092-8674(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Lebo R. V., Carrano A. V., Burkhart-Schultz K., Dozy A. M., Yu L. C., Kan Y. W. Assignment of human beta-, gamma-, and delta-globin genes to the short arm of chromosome 11 by chromosome sorting and DNA restriction enzyme analysis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5804–5808. doi: 10.1073/pnas.76.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Little P. F., Annison G., Darling S., Williamson R., Camba L., Modell B. Model for antenatal diagnosis of beta-thalassaemia and other monogenic disorders by molecular analysis of linked DNA polymorphisms. Nature. 1980 May 15;285(5761):144–147. doi: 10.1038/285144a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Alter B. P., Altay C., Mahoney M. J., Lazarus H., Hobbins J. C., Nathan D. G. Application of endonuclease mapping to the analysis and prenatal diagnosis of thalassemias caused by globin-gene deletion. N Engl J Med. 1978 Jul 27;299(4):166–172. doi: 10.1056/NEJM197807272990403. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Old J. M., Weatherall D. J., Nathan D. G. Partial deletion of beta-globin gene DNA in certain patients with beta 0-thalassemia. Proc Natl Acad Sci U S A. 1979 May;76(5):2400–2404. doi: 10.1073/pnas.76.5.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Deininger P. L. Sequence organization of the human genome. Cell. 1975 Nov;6(3):345–358. doi: 10.1016/0092-8674(75)90184-1. [DOI] [PubMed] [Google Scholar]
- Sillar R., Young B. D. A new method for the preparation of metaphase chromosomes for flow analysis. J Histochem Cytochem. 1981 Jan;29(1):74–78. doi: 10.1177/29.1.6162882. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Mareni C. E., Migeon B. R. Isolation and characterization of cloned DNA sequences that hybridize to the human X chromosome. Cell. 1980 Aug;21(1):95–102. doi: 10.1016/0092-8674(80)90117-8. [DOI] [PubMed] [Google Scholar]
- Wyman A. R., White R. A highly polymorphic locus in human DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6754–6758. doi: 10.1073/pnas.77.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. D., Ferguson-Smith M. A., Sillar R., Boyd E. High-resolution analysis of human peripheral lymphocyte chromosomes by flow cytometry. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7727–7731. doi: 10.1073/pnas.78.12.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]