Background: Tumor-induced SERCA3 up-regulation is a major cause of death of CD4+T lymphocytes leading to immune suppression in cancer bearers.
Results: Nifetepimine down-modulates SERCA3 expression and thereby protects the lymphocytes from tumor-induced apoptosis.
Conclusion: The present finding strongly suggests nifetepimine as a potent immuno-restoring agent that protects T lymphocytes from tumor insult.
Significance: The results suggest that nifetepimine may be developed into a potent immuno-restoring agent in tumor-bearers.
Keywords: Apoptosis, Calcium ATPase, ER Stress, T Cell, Tumor Immunology
Abstract
Multiple mechanisms have been proposed by which tumors induce T cell apoptosis to circumvent tumor immune-surveillance. Although sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) have long been known to regulate intracellular Ca2+ homeostasis, few studies have examined the role of SERCA in processes of T lymphocyte survival and activation. In this context it remains largely unexplored as to how tumors jeopardize SERCA function to disable T cell-mediated anti-tumor immunity. Here, we show that human CD4+ T cells in the presence of tumor conditions manifested an up-regulation of SERCA3 expression that resulted in development of endoplasmic reticulum stress leading to CD4+ T cell apoptosis. Prostaglandin E2 produced by the tumor cell plays a critical role in up-regulating SERCA3 by enhancing the binding of its transcription factor Sp1. Gene manipulation and pharmacological approaches further established that an increase in SERCA expression also resulted in subsequent inhibition of PKCα and -θ and retention of NFκB in the cytosol; however, down-modulation of SERCA3 expression by a dihydropyrimidone derivative, ethyl-4-(3-nitro)-phenyl-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5 carboxylate (nifetepimine), protected the CD4+ T cells from tumor-induced apoptosis. In fact, nifetepimine-mediated restoration of PKC activity resulted in nuclear translocation of p65NFκB, thereby ensuring its survival. Studies further undertaken in a tumor-bearing mice model revalidated the immunoprotective role of nifetepimine. Our present study thus strongly suggests that imbalance in cellular calcium homeostasis is an important factor leading to CD4+ T cell death during cancer and holds promise that nifetepimine may have the potential to be used as an immunorestoring agent in cancer bearers.
Introduction
T lymphocytes play a crucial role in the host immune response to cancer. Accumulating evidences suggest that patients with advanced cancer show impairment in lymphocyte activation resulting in immune dysfunction (1, 2). Indeed, malignant cells often use a variety of mechanisms to evade destruction offered by the immune system (3, 4). The effect that a progressively growing tumor has on the immune response presents an important challenge to the success of T cell-based immunotherapy and cancer vaccines (5). Therefore, therapeutic approaches that can protect the immune system in cancer patients may enhance the immune competence and increase the survival.
Current evidences suggest that CD4+ T cells play a vital role in the immune attack directed against human tumors (6). An uncontrolled death of lymphocytes at the tumor site may represent a mechanism of tumor-induced immune suppression. Apoptosis of lymphocytes interacting with tumor cells may be due to the interaction between the death receptors expressed on lymphocytes with death ligands expressed on tumor cells (4). Certain tumor-derived soluble factors like prostaglandin E2 have also been identified that are also shown to alter T cell function by changing some of their signal transduction pathways (7–11). Chemnitz et al. (12) report impairment in CD4+ T cell activation in cancer patients by prostaglandin E2. Tumor-shed PGE2 have been found to make profound alteration in cytokine balance in the cancer micro-environment, which thereby contributes to T cell suppression in cancer patients (13, 14). Therefore, understanding the mechanisms of tumor-induced CD4+ T cell apoptosis as well as its alleviation by any immune-protective drug must be of great importance from the point of view of amelioration of tumor-induced immune-suppression.
Launay et al. (15) report that calcium signaling plays a significant role in T lymphocyte survival and activation. Changes in the levels of intracellular calcium (Ca2+) provide highly versatile signals that control a plethora of cellular processes, although their importance is perhaps most strikingly exemplified by their role in life-and-death decisions (16). The calcium signaling machinery promotes cell proliferation while at the same time induces apoptosis depending on the amplitude of the increase in cytosolic Ca2+, the duration of the change in cytosolic Ca2+, and the nature of the change and the location (17, 18). In fact an increase and decrease in cytosolic calcium levels has been shown to promote apoptosis (18–20). This has led to the proposal that Ca2+ pumps, which regulate Ca2+ levels in the cells, can be potential targets for different therapeutic approaches. It is known that calcium transport ATPases, associated with intracellular Ca2+ storage organelles, play a major role in controlling the subcellular distribution of Ca2+ by sequestering it from cytosol to intracellular Ca2+ pools. Because calcium accumulation into endoplasmic reticulum is accomplished by the SERCA2 pump (21), precisely regulated SERCA activity is essential for normal cell function and survival. Convincing evidences also suggest that down-modulation of some specific SERCA isoenzymes is associated with lymphocyte activation. It, therefore, becomes evident that by maneuvering SERCA pump expression status, one may effectively alter intracellular calcium homeostasis to ensure survival of CD4+ T cells, thereby ameliorating immune suppression in cancer patients.
In humans, SERCA type Ca2+ pumps are encoded by three genes (ATP2A1–3) that generate multiple isoforms of SERCA, i.e. SERCAla, b, SERCA2a-c, and SERCA3a-f by developmental or tissue-specific alternative splicing (17). In several cell types including T lymphocytes, SERCA2 is co-expressed with SERCA3 (15, 22) that finely regulates the calcium balance of the cell depending on its requirement. Ca2+ mobilization results in activation of protein kinase C (PKC) (23) that in turn stimulates transcription factors like nuclear factor-κB (NF-κB) (24). Various reports also suggest that SERCA3 up-regulation is often associated with ER stress-induced caspase activation and cell apoptosis (25). Thus modulation in the SERCA3 expression may be helpful to protect the T cells from tumor-induced apoptosis.
In humans and mice, Sp1 and Ets1 serves as two important transcription factors required for the basal transcription of the SERCA3 gene. However, mutation of the Sp1 binding sites prevents the activation of the SERCA3 gene by Ets1 (26). Thus Sp1 acts as an important transcription factor that regulates the activation of the SERCA3 gene. It has been acknowledged that PGE2 markedly enhances the phosphorylation and DNA binding capacity of Sp1 (27). Thus the tumor supernatant can alter the SERCA3 expression status of a cell by regulating its transcription factor Sp1.
On the basis of the above discussion, which highlights the importance of Ca2+ signaling in T cell survival and that the SERCA pump is instrumental in shaping the amplitude, intensity, and duration of cellular calcium signals (28), our present work was focused on exploring the possibility of overcoming tumor-induced immune evasion by maneuvering the SERCA pump. To achieve the goal, we have selected various gene manipulation and pharmacological interference approaches that involves SERCA overexpression and down-modulation using short interfering RNA and an synthetic dihydropyrimidone, ethyl-4-(3-nitrophenyl)-6-methyl-2-oxo-1,2,3,4 tetrahydropyrimidine-5 carboxylate (29–31), named as nifetepimine, and have explored its role in CD4+ T cell survival in tumor milieu. Results demonstrate that down-modulation of SERCA3 expression by nifetepimine ensured CD4+ T cell survival both in in vitro and in vivo experimental models. Underlying molecular mechanisms suggested that tumor-shed PGE2-mediated SERCA up-regulation was associated with caspase activation and T cell apoptosis. SERCA3 up-regulation also inhibited PKCα and -θ, resulting in retention of NF-κB in the cytosol, whereas down-modulation of SERCA expression by nifetepimine inhibited caspase activation and also facilitated NF-κB-dependent T cell survival. Our study thus reports for the first time the an intricate mechanism of nifetepimine-mediated immune restoration from tumor-induced immune suppression and also suggests the role of nifetepimine as a possible therapeutic agent with a strong immunmodulatory effect, which can be used to treat patients with cancer.
EXPERIMENTAL PROCEDURES
Cell Lines and Mice
The human mammary epithelial carcinoma cells (MCF-7; maintained in complete DMEM) were obtained from National Centre For Cell Science, India. Primary lesions of breast cancer tissue were obtained from Calcutta National Medical College, Kolkata, India following all ethical guidelines of the institute. Informed consent was obtained from all patients with localized disease. These tumors were exclusively primary site cancers that had not been treated with either chemotherapy or radiation. The specimens were washed with phosphate-buffered saline (PBS), cut into small pieces 5 × 5 mm in size, and immersed in 0.125% trypsin-EDTA at 4 °C overnight. Then the specimens were gently dissected with forceps into single cells, seeded on poly-L lysine-coated dishes, and cultured for 1 day at 37 °C in complete DMEM medium, keratinocyte growth medium (KGM) containing 0.1 ng/ml human recombinant epidermal growth factor, 5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 50 μg/ml gentamycin, 50 ng/ml amphotericin-B, and 15 mg/liter bovine pituitary extract. Male Swiss albino mice were obtained from Chittaranjan National Cancer Research Institute, Kolkata, India following all ethical guidelines of the institute.
Synthesis and Characterization of Nifetepimine
Nifetepimine was produced from 3-nitrobenzaldehyde, ethylacetoacetate, and urea in the presence of HCl as catalyst. The crude product was crystallized from methyl alcohol to obtain the pure, ethyl-4-(3-nitro)-phenyl-6-methyl-2-oxo-1,2,3,4 tetrahydropyrimidine-5 carboxylate (1.6 g, 40%; melting point 227–229 °C). The structure of nifetepimine was confirmed by IR (KBr palate) analysis that revealed 686.0, 693.0, 1088.4, 1224.3, 1346.5, 1525.9, 1629.8, 1688.7, 1708.6, 2821.3, 2964.2, 3100.0, 3217.7, 3329.5 cm−1. The purified compound also yielded a NMR spectra of δ 1.08 (3H, t, J = 7.1 Hz,-CH2CH3), 2.26 (3H, s,-CH3), 3.98 (2H, m, -CH2CH3), 5.29 (1H, d, J = 3.3 Hz, CH), 7.63–7.69 (2H, m, ArH), 7.87 (1H, s, NH), 8.07 (1H, t, J = 1.8 Hz, ArH), 8.11–8.13 (1H, m, ArH), 9.34 (1H, s, NH).
Isolation of CD4+ T Cells
Human venous blood from healthy adult volunteers was collected with prior consent using heparinized syringes. Whole blood (100 ml) was diluted with 150 ml of RPMI 1640 (Sigma) and then layered in centrifuge tubes onto 120 ml of Histopaque-1077 (Sigma) gradient. After centrifugation, the opaque interface containing lymphocytes was collected and washed twice in RPMI 1640, and after complete removal of the supernatant the pellet was resuspended in PBS. CD4+ T cells were purified from total leukocytes by positive selection using anti-CD4 antibody coated micro-beads (Milteny Biotech) (32). The purity of the isolated CD4+ T cells was determined by flow cytometry and found to be enriched routinely >99% CD3+ and CD4+ but was negative for CD8. Cells were cultured in RPMI 1640 (supplemented with 10 units/ml recombinant IL-2, 10% fetal bovine serum, 2 mm l-glutamine, 100 mg/ml sodium pyruvate, 100 mm nonessential amino acids, 100 mg/ml streptomycin, and 50 units/ml penicillin; Sigma) at 37 °C in humidified incubator containing 5% CO2. Viable cell numbers were determined by trypan blue exclusion test. Tumor supernatants freed from cellular components were used in a 1:1 ratio with RPMI to study the effect of tumor supernatant on CD4+ T cells. Nifetepimine (50 μm) was added along with the tumor supernatant to investigate its immune-protective effect on CD4+ T cells. To further understand the sequence of events leading to apoptosis, cells were pretreated for 2 h with 20 μm each of specific caspase 3 (z-DEVD-FMK), caspase 9(z-LEHD-FMK), and pan-caspase inhibitor (z-VAD-FMK) (Calbiochem/ED Chemicals, NJ) before treatment with the tumor supernatant.
Treatment of Animals
All experiments were performed strictly adhering to the ethical guidelines of the Institute. Male Swiss albino mice (20 g) were randomly divided into four groups of 10 animals each including (i) untreated set (non-tumor-bearing), (ii) nifetepimine-treated set (non-tumor-bearing), (iii) untreated tumor-bearing set (which were intraperitoneally injected with 1 × 106 exponentially grown Ehlrich ascites carcinoma (EAC) in 0.25 ml sterile PBS), and (iv) nifetepimine-treated tumor-bearing set. Nifetepimine (10 μg/g body weight, data for the other doses used not shown) solubilized in DMSO was injected intraperitoneally (every alternate day). Untreated mice received DMSO instead of nifetepimine. The peripheral blood was collected from the eye of the mice and followed by CD4+ T cells isolation as described above. The spleens from the mice from all the above-mentioned sets were also collected.
Flow Cytometry
For the determination of cell death, cells were stained with 7-aminoactinomycin D and annexin-V-FITC (Pharmingen) and analyzed on flow cytometer (FACSCalibur, BD Biosciences) equipped with 488-nm argon laser light source using Cell Quest Software (BD Biosciences). Electronic compensation of the instrument was done to exclude overlapping of the emission spectra. A total 10,000 events were acquired for analysis using CellQuest software. Annexin-V/7-AAD positive cells were regarded as apoptotic cells. For determination of the SERCA3 expression levels in the mice CD4+ T cells, the mice peripheral blood mononuclear cells were first incubated with FITC-tagged CD4+ antibody. The cells were then fixed, permeabilized, and then incubated with the SERCA3 antibody (Santa Cruz Biotechnology) and then with FITC-conjugated 2nd antibody followed by flow cytometric analysis.
Determination of the Cytosolic Ca2+ Concentration
Cytosolic calcium concentration was measured using a fluorometric ratio technique. Cells were centrifuged and resuspended at a density of 106 cells/ml in PBS supplemented with 1 mg/ml bovine serum albumin and incubated in the dark with Fura-2AM (final concentration, 5 μm) (Sigma) for 30 min at room temperature under slow agitation. Cells were then centrifuged and resuspended in calcium-free Hanks' buffered saline solution (135 mm NaCl, 5.9 mm KCl, 1.2 mm MgCl2, 11.6 mm Hepes, 11.5 mm glucose adjusted to pH 7.3 with NaOH) prior to measurements. After centrifugation, 0.5 to 1 × 106 cells were suspended in 3 ml of Hanks' buffered saline solution in a quartz cuvette and inserted into a Hitachi spectrofluorimeter equipped with a stirring apparatus and a thermostated (37 °C) cuvette holder and connected to a PC computer. The fluorescence was recorded at 510 nm in the spectrofluorometer using an excitation source of 340 or 380 nm. Maximum Fura-2 fluorescence (Fmax) was obtained by adding 1 μm ionomycin (Sigma) to the cell suspension in the presence of 10 mm CaCl2, and minimum fluorescence (Fmin) was determined without added calcium in the presence of 5 mm EGTA (Sigma). The cytosolic Ca2+ was calculated from the Fura-2AM fluorescence intensity as [Ca2+]cyt = Kd (F − Fmin)/(Fmax − F), where Kd = 224 nm for Fura-2, and R is the ratio of fluorescence values (F) (R = F340/F380).
Immunoblotting
Primary human CD4+ T cells were lysed in buffer (10 mm Hepes, pH 7.9, 1.5 mm MgCl2, 10 mm KCl, and 0.5 mm DTT), and nuclei were pelleted by a brief centrifugation. The supernatant was spun at 100,000 × g to get the cytosolic fraction. The nuclear extract was prepared in buffer containing 20 mm HEPES, pH 7.9, 25% (v/v) glycerol, 420 mm KCl, 1.5 mm MgCl2, 0.2 mm EDTA, 0.5 mm DTT, and 0.5 mm PMSF. All the buffers were supplemented with protease and phosphatase inhibitor cocktails. For direct Western blot analysis, cell lysates of the particular fractions containing 30 μg of protein was separated by SDS-polyacrylamide gel electrophoresis (8% for SERCA pumps and 10% for others) and transferred to nitrocellulose membrane. The protein levels of glucose-regulated protein 78 (GRP78; Sigma), SERCA3 and -2B, cleaved caspase 9 and 3, p65NF-κB, pIKBα, IL-2, and Sp1 were determined with specific antibodies (Santa Cruz Biotechnology). For PKC immunoblot analysis the cytosolic and particulate fractions were separated first by centrifuging at 1000 × g for 10 min and then at 40,000 × g for 40 min at 4 °C. The high speed pellet was designated as the membrane fraction, and the supernatant was designated as the cytosolic fraction. The proteins were then determined with anti-peptide antibodies to the PKCα and PKCθ (Santa Cruz Biotechnology). The protein of interest was visualized by chemiluminescence. Equal protein loading was confirmed by reprobing the blots with α-actin/histone H1 antibody (Santa Cruz Biotechnology).
Reverse Transcriptase PCR
SERCA3 and SERCA2b mRNA were estimated using a semiquantitative reverse transcriptase-PCR method. Briefly, total RNA from cells was extracted with TRIzol reagent (Invitrogen) and reverse-transcribed and amplified using the U. S. Biochemical Corp. RT -CR kit and TaqDNA polymerase according to the manufacturer's instructions by 30 cycles (each cycle consisting of 30 s at 94 °C, 2 min at 55 °C, and 2 min at 72 °C). The primers used to amplify SERCA2b were 5′-TCATCTTCCAGATCACACCGCT-3′/5′-GTCAAGACCAGAACATATC-3′, which cover the region from base pairs 2861 to 3132 of the human sequence (15). SERCA PLIM430 was amplified using the primers 5′-GAGTCACGCTTCCCCACCACC-3′/5′-TCAACTTCTGGCTCATTTCTT-3′, which cover the region located between base pairs 2674 and 3000 (15), and GAPDH (internal control (5′-CAGAACATCATCCCTGCCTCT-3′/5′-GCTTGACAAAGTGGTCGTTGAG-3′).
Plasmid Constructs, siRNA, and Transfection
cDNA encoding full-length SERCA3 (a generous gift from Prof. Jonathan Lytton, Department of Biochemistry and Molecular Biology, University of Calgary) were introduced into CD4+ cells using T cell nucleofactor kit (Amaxa, Koein, Germany). Isolation of stably expressing clones were done by limiting dilution and selection with IL2 (25 units/ml) and hygromycin B (800 mg/ml), and cells surviving this treatment were cloned and assessed for SERCA3 expressions by Western blot analysis. CD4+ T cells were transfected with 300 pmol of SERCA3/PKCα/PKCθ-siRNA (Santa Cruz Biotechnology) and Lipofectamine 2000 (Invitrogen) separately for 12 h. Levels of respective proteins were estimated by Western blotting.
Chromatin Immunoprecipitation Assay
A chromatin immunoprecipitation (CHIP) assay was performed using CHIP assay kit (Millipore) following the manufacturer's instructions. Isolated chromatin was precipitated with Sp1 antibody. Input DNA and rabbit IgG-pulled DNA served as controls for all the experiments. Immunoprecipitated DNA was then subjected to 40 cycles of PCR using primers for respective promoter regions as mentioned in the figures. Glyceraldehyde-3-phosphate dehydrogenase promoter was used as a nonspecific control for all the CHIP experiments.
Statistical Analysis
Values are shown as S.E. except where otherwise indicated. Data were analyzed, and when appropriate, significance of the differences between mean values was determined by Student's t test. Results were considered significant at p < 0.05.
RESULTS
Tumor-induced CD4+ T Cell Death Is Associated with SERCA3 Overexpression
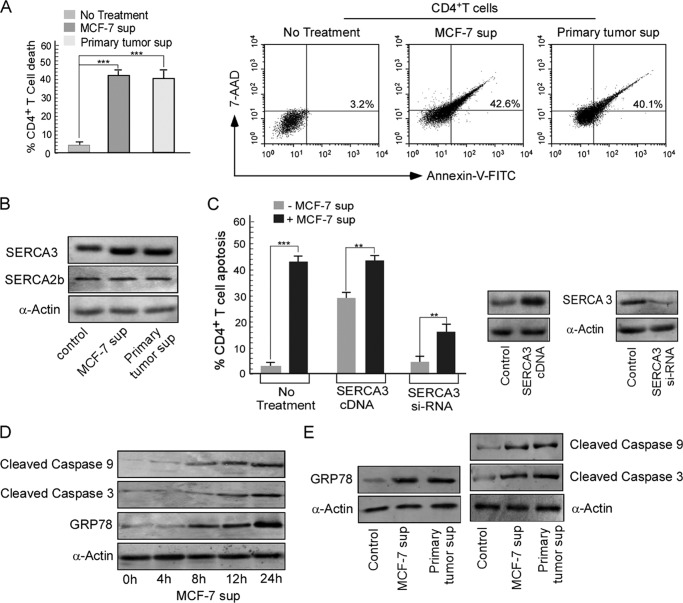
When purified CD4+ T cells were cultured in the presence of cell-free breast carcinoma cell (MCF-7) supernatant and primary tumor supernatant, a situation mimicking the tumor-bearing condition was observed. Here, we found that CD4+ T cells exhibited 42% cell death in presence of MCF-7 supernatant, whereas supernatant of primary tumor induced about 40% CD4+ T cell death as measured by trypan blue dye exclusion assay (Fig. 1A). The nature of cell death was identified as apoptosis by an annexin-V/7-AAD double-labeling assay (Fig. 1A). These results thereby prompted us to investigate the underlying mechanism behind this tumor-induced CD4+ T cell apoptosis. As modulation of ER calcium homeostasis by the SERCA pump is important in the T cell survival cascade, we raised the question as to whether tumor-induced CD4+ T cell death involved any modulation in SERCA expression.
FIGURE 1.
Tumor-induced CD4+ T cell death is associated with SERCA3 overexpression. A, graphic representation of control and tumor supernatant (sup)-treated CD4+ T cell viability was determined by trypan blue exclusion test (left panel). Dot plot analysis represents the percent of annexin V positivity in CD4+ T cells treated with MCF-7 and primary tumor supernatant (right panel). B, Western blot analysis depicts SERCA3 and SERCA2b from control, MCF-7 supernatant, and primary tumor supernatant-treated CD4+ T cells. C, control/SERCA3-cDNA/SERCA3-siRNA-transfected cells after treatment with or without MCF-7 supernatant for 24 h were scored for percent cell apoptosis by determining annexin-V/7-ADD positivity; expression levels of SERCA3 in untransfected and SERCA3-cDNA/siRNA transfected cells are shown. D, Western blot representation for GRP78, cleaved caspase 9 and 3 from control, and MCF-7 supernatant-treated CD4+ T cells were determined for different time periods. E, shown are expression levels for GRP78, cleaved caspase 9 and 3 from control, MCF-7 supernatant, and primary tumor supernatant-treated CD4+ T cells as determined by Western blotting.
Interestingly, our investigation revealed that in comparison to SERCA2b, SERCA3 expression was prominently up-regulated in CD4+ T cells treated with cell-free MCF-7 and primary tumor supernatants (Fig. 1B). To confirm the contribution of SERCA3 in tumor-induced CD4+ T cell apoptosis, first, SERCA3 gene was overexpressed in CD4+ T cell (Fig. 1C). The SERCA3-overexpressed CD4+ T cells furnished significant apoptosis (29.4%) even in the absence of the MCF-7 supernatant (Fig. 1C). In our second approach, transient silencing of SERCA3 gene (Fig. 1C) protected the CD4+ T cells from the tumor supernatant-induced apoptosis where we observed that SERCA3 knocked-out cells underwent significantly lesser apoptosis (17.5%) even up on incubation with the MCF-7 supernatant (Fig. 1C). Thus our findings so far clearly suggest the involvement of SERCA3 during CD4+ T cell apoptosis under tumor conditions.
Tumor-induced SERCA3 Up-regulation Causes ER Stress-mediated Caspase Activation Leading to CD4+ T Cell Death
Next, we further investigated how up-regulation in SERCA3 expression induced by the tumor supernatant produced death in CD4+ T cells. In accordance with previous reports, we observed that SERCA3 overexpression in CD4+ T cells was associated with the induction of ER stress. Conditions of both calcium overload and calcium depletion from the ER results in development of ER stress (25). To survive ER stress and accumulation of misfolded proteins, ER responds by triggering specific signaling pathways including an increase in chaperoning capacity (33). One of the best characterized ER chaperone proteins is the GRP78, also referred to as BiP whose expression is enhanced during ER stress. Our findings clearly illustrated that treatment of CD4+ T cells with the MCF-7 and primary tumor supernatant was associated with a rise in the GRP78 levels in a time dependent fashion (Fig. 1D), indicating ER stress development and also the activation of caspase 9 and caspase 3 leading to cell death (Fig. 1E). As our findings so far clearly highlights that up-regulation of SERCA3 expression by the tumor supernatant was responsible for CD4+ T cell apoptosis, we next sought to explore some new immunotherapeutic strategies that could effectively overcome tumor-induced immune evasion by remodeling the SERCA expression.
Nifetepimine, a Dihydropyrimidone Protects CD4+ T Cells from Tumor-induced Apoptosis
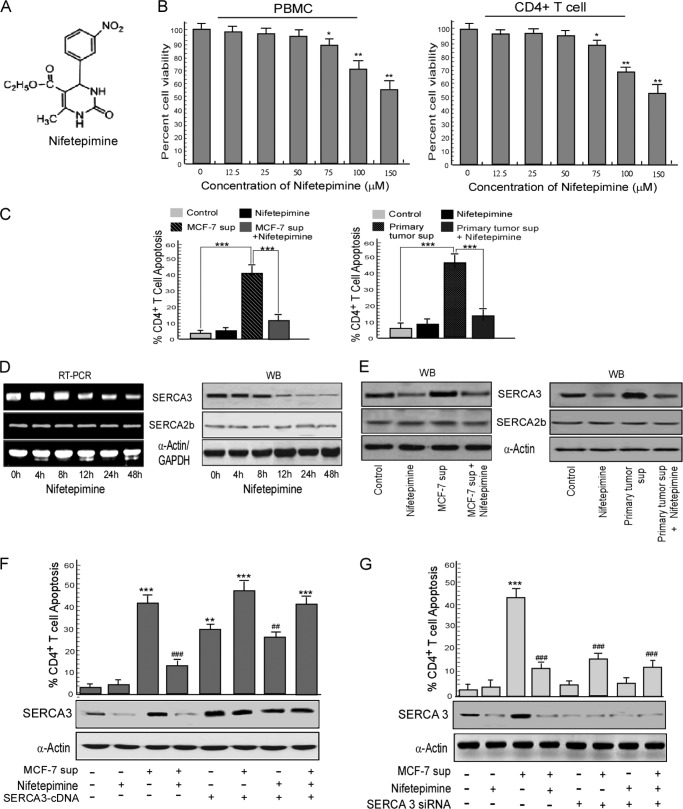
The dihydropyrimidone group of compounds are now being extensively studied worldwide for their anti-carcinogenic properties. Monastrol, a dihydropyrimidone, is already stated as an anti-cancer agent (34). However, the immune-potentiating properties of this group of compounds are yet to be investigated. Here, we have explored another dihydropyrimidone, nifetepimine (Fig. 2A) for its immune-rejuvenating characteristics. When the human peripheral blood mononuclear cells as well as the purified CD4+ T cells were cultured in the presence of nifetepimine (Fig. 2B), doses up to 75 μm produced no significant cell death. Thus to examine the effect of nifetepimine in protecting CD4+ T cell from tumor-induced apoptosis, we chose the 50 μm dose, as concentrations above 75 μm produced death in both peripheral T cells as well as purified CD4+ lymphocytes. As observed previously when purified CD4+ T cells were cultured in the presence of cell-free breast carcinoma cell (MCF-7) supernatant, a situation nearly identical to tumor-bearing condition, about 42.3% CD4+ cell death was recorded in comparison to the control (2.8% cell death) (Fig. 2C). A significant percentage of CD8+ cell death (about 45.7%) was also observed. Interestingly, nifetepimine was found to significantly restore these CD4+ T cell populations from such tumor insult (11.2% dead cells) (Fig. 2C); however, the CD8+ T cell population was not significantly recovered (36.2% dead cells) (data not shown). Similar trends were also observed by treating CD4+ T cells with primary tumor supernatant with or without nifetepimine. In that case 46.3% cell death, produced by the primary tumor supernatant, was brought down to 14.6% upon treatment with nifetepimine, thereby clearly indicating that nifetepimine could protect the CD4+ T cells from tumor-induced apoptosis (Fig. 2C). Importantly, nifetepimine itself did not change CD4+ cell numbers significantly when applied directly to control CD4+ cells. These findings thus prompted us to delineate the mechanisms underlying such an immune-protective effect of this compound on CD4+ T cells.
FIGURE 2.
Nifetepimine-induced SERCA3 down-modulation protects CD4+ T cells from tumor-induced apoptosis. A, shown is the structure of nifetepimine. B, human peripheral blood mononuclear cells (PBMC) as well as CD4+ T cells were treated with different doses of nifetepimine for 24 h and scored for percent apoptosis by annexin-V/7-AAD positivity. C, shown is a graphic representation of CD4+ T cell apoptosis in control and MCF-7/primary tumor supernatant (sup)-treated CD4+ T cells in the presence and absence of nifetepimine. D, CD4+ T cells treated with nifetepimine for different time periods were examined for SERCA3 and SERCA2b expression at both mRNA and protein levels and by RT-PCR and Western blotting (WB), respectively. E, shown is a Western blot representation for SERCA3 and SERCA2b in control and MCF-7/primary tumor supernatant-treated CD4+ T cells in the presence and absence of nifetepimine. F, shown is a graphic representation of CD4+ T cell apoptosis in control and tumor supernatant-treated SERCA3 overexpressed CD4+ T cells in the presence and absence of nifetepimine. * denotes p value with respect to the control, and # denotes p value with respect to the tumor supernatant. Under similar experimental conditions, SERCA3 levels were determined by Western blot analysis. G, a graphic representation shows annexin V positivity for CD4+ T cell from control and tumor supernatant-treated SERCA3 knocked-down sets in the presence and absence of nifetepimine. * denotes p value with respect to the control, and # denotes p value with respect to the tumor supernatant. GAPDH and α-actin were used as internal control. Values are the mean ± S.E. of three independent experiments in each case. *, p < 0.05; **, p < 0.01; ***, p < 0.001; #, p < 0.05; ##, p < 0.01; ###, p < 0.001.
CD4+ T Cell Restoration by Nifetepimine Involves Down-regulation of SERCA3 Expression
T cell activation is supported by a cascade of signaling events in which Ca2+ plays a pivotal role, and ER calcium homeostasis regulated by the SERCA pump is important in controlling this activation process (15). Because nifetepimine protected CD4+ T lymphocytes from tumor-induced apoptosis, we raised the question of whether this immune restoration involved any modulation in SERCA expression. Our Western blot analysis revealed that nifetepimine down-regulated SERCA3 expression in a time-dependent fashion both at mRNA and protein levels, whereas SERCA2b expression remained unaltered (Fig. 2D). Additionally, our investigation already revealed that SERCA3 expression was prominently up-regulated in CD4+ T cells treated with cell-free MCF-7 and primary tumor supernatants. However, nifetepimine could thwart such effects thereby ensuing in down-regulation of SERCA3 expression and bringing it close to normal (Fig. 2E) to relieve CD4+ T cells from tumor-induced apoptosis. Reflecting the results of Fig. 2E, SERCA2b expression was not prominently affected by nifetepimine in these experimental set-ups. Involvement of SERCA3 in nifetepimine-mediated CD4+ T cell recovery was further confirmed by gene manipulation approaches where overexpression of SERCA3 in CD4+ T cells significantly reduced the protective effect of nifetepimine in tumor supernatant-treated CD4+ T cells (Fig. 2F). On the other hand, transient silencing of the SERCA3 gene protected the CD4+ cells from the tumor supernatant-induced apoptosis, nearly mimicking the action of nifetepimine even in its absence (Fig. 2G). These observations so far elucidate that the changes in the SERCA3 expression patterns are important for nifetepimine-mediated protection of CD4+ T cells from tumor-induced apoptosis.
Tumor-shed PGE2 Is Responsible for CD4+ T Cell Apoptosis
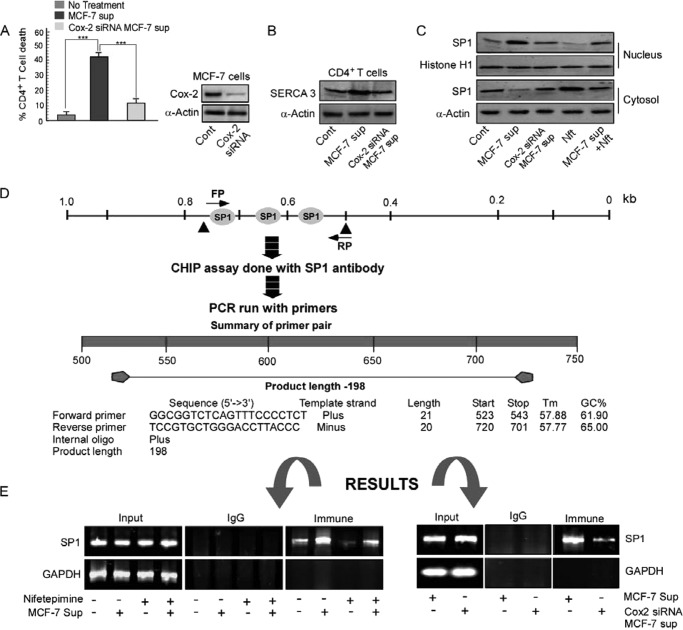
All these reactions described so far occurred independent of the direct contact of CD4+ T cells with tumor cells, thereby pointing at the possibility of the presence of tumor-shed soluble immunosuppressors in the supernatant. As previous experiments done in our laboratory (9) with MCF-7 supernatant proved that prostaglandin E2 is the major soluble immunosuppressant produced by the tumor, we checked for the involvement of PGE2 in mediating the process of T cell death. We thereby transfected the tumor cells with the Cox-2 siRNA, which blocked the release of PGE2 in cell-free tumor supernatant. Interestingly when the CD4+ T cells were treated with this supernatant, the percentage of CD4+ T cell apoptosis declined prominently (Fig. 3A). The enhancement in the SERCA3 expression level was also not observed on treating the CD4+ T cell with the supernatant obtained from the Cox-2 siRNA-transfected cells (Fig. 3B). All these findings, therefore, conclude that PGE2 liberated in the tumor supernatant is primarily responsible for CD4+ T cell killing and overexpression of SERCA3.
FIGURE 3.
Regulation of SERCA3 expression by the tumor/nifetepimine involves differential binding of Sp1 to the SERCA3 promoter. A, shown is a graphic representation of CD4+ T cell apoptosis in control, tumor supernatant (sup)-treated, and Cox-2 siRNA-transfected tumor-derived supernatant-treated CD4+ T cells; expression levels of Cox-2 in control and Cox-2 siRNA-transfected MCF-7 cells are shown. B, shown is a Western blot representation for SERCA3 in control, MCF-7 supernatant-treated, and supernatant of Cox-2 siRNA-transfected MCF-7 cell-treated CD4+ T cells. C, Western blot analysis of the cytosolic and nuclear fraction of CD4+ T cells determine the nuclear translocation of the transcription factor Sp1 from control, MCF-7 supernatant, Cox-2-knocked-down MCF-7 supernatant-, nifetepimine (Nft)-, nifetepimine- and MCF-7 supernatant-treated sets. D, a schematic representation of the SERCA3 promoter shows Sp1 binding sites and the sequence of the forward (FP) and reverse primers (RP) synthesized using Primer Blast software by NCBI against the region 523–720 bp of the SERCA3 promoter used for CHIP analysis. E, left panel, a CHIP assay in MCF-7 supernatant-treated CD4+ T cells in the presence and absence of nifetepimine demonstrates the recruitment of Sp1 onto the SERCA3 promoter; right panel, CHIP analysis in MCF-7 supernatant and Cox-2 siRNA-transfected MCF-7 supernatant-treated CD4+ T cell highlights the recruitment of Sp1 on the SERCA3 promoter; GAPDH promoter was used as control. Values are mean ± S.E. of three independent experiments in each case.
Regulation of SERCA3 Expression by Tumor Supernatant/Nifetepimine Involves the Differential Binding of Sp1 to the SERCA3 Promoter
The SERCA3 gene transcription has been known to be regulated by the binding of Sp1 to its promoter region. Therefore, we investigated the possible transcriptional regulation of SERCA3 by Sp1 on treatment with tumor supernatant and nifetepimine. Initially, we observed that the nuclear translocation of Sp1 was significantly enhanced on treatment with the tumor supernatant. On the other hand, nifetepimine significantly declined Sp1 nuclear translocation (Fig. 3C). Next, the SERCA3 promoter was screened for its Sp1 binding sites using the TRED analysis software provided by Bioinformatics and Biological Computing (BBCU). Software analysis predicted more than 2–3 potential binding sites of Sp1 within a stretch of a 200-bp region at the SERCA3 promoter. Primers were synthesized using the Primer Blast software supplied by NCBI against region 523–720 bp of the SERCA3 promoter (Fig. 3D). Thus to study the recruitment of Sp1 to the SERCA3 promoter under different conditions, we performed the chromatin immunoprecipitation assay. We observed that Sp1 was highly recruited to the SERCA3 promoter region in the MCF-7 supernatant-treated sets. On the other hand, the recruitment of Sp1 was significantly decreased in the nifetepimine-treated sets, clearly stating that nifetepimine interferes with the binding of Sp1 to the SERCA3 promoter (Fig. 3E, left panel). Our observations also highlight that in the cells treated with the supernatant from the Cox-2 siRNA-transfected tumor cells, the nuclear recruitment of Sp1 to the SERCA3 promoter was significantly declined (Fig. 3E, right panel). These observations clearly support our previous findings that nifetepimine treatment causes down-regulation of SERCA3 expression. These findings also highlight the importance of SERCA3 in the T cell survival cascade and also establishes the fact that SERCA3 is the key player that is regulated by both PGE2 and nifetepimine through its transcription factor Sp1 during T cell death and survival.
SERCA3 Down-modulation by Nifetepimine Results in a Rise in the Cytosolic Ca2+
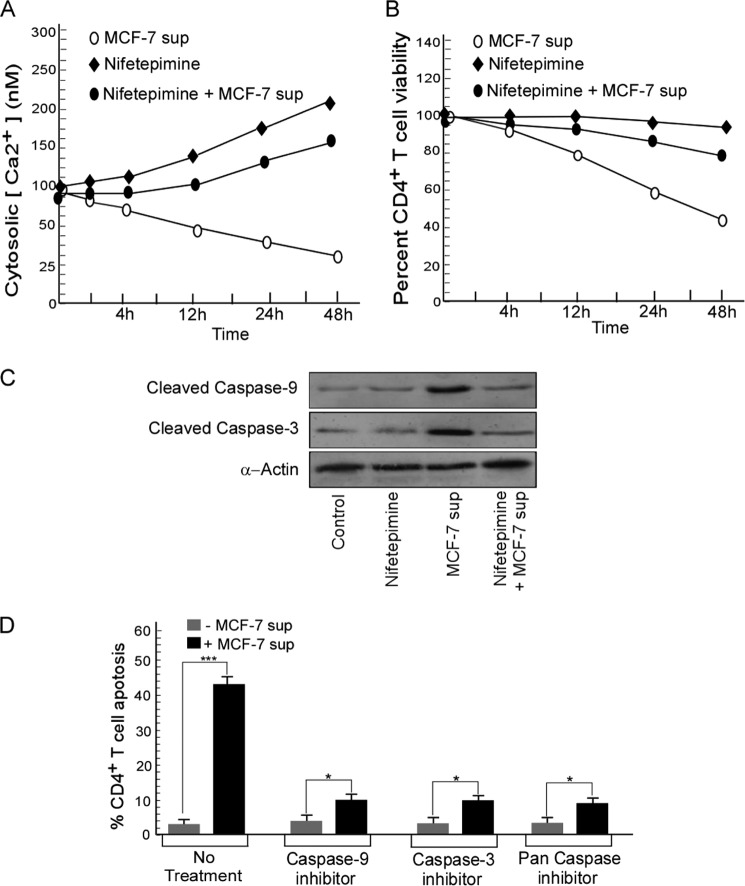
Because modulation of SERCA activity results in alterations in cytosolic Ca2+ levels, next we monitored the changes in cytosolic Ca2+ in CD4+ T cells on nifetepimine treatment. When Fura-2AM-loaded CD4+ T cells were incubated with nifetepimine, it resulted in a progressive rise in the cytosolic Ca2+ (Fig. 4A). As expected, the cell-free MCF-7 cell supernatant caused a significant reduction in the cytosolic Ca2+ of the CD4+ T cells. Interestingly, this sharp decline in intracellular Ca2+ was efficiently restored back almost to the normal level when the CD4+ T cells were treated with nifetepimine along with the tumor supernatant (Fig. 4A). As clearly illustrated in Fig. 4B, such a rise in the cytosolic Ca2+ level of CD4+ cells was associated with a greater CD4+ survival as about 41.3% cell death, associated with the tumor supernatant, was brought down to 15% up on treatment with nifetepimine for 24 h.
FIGURE 4.
Down-regulation of SERCA3 by nifetepimine causes a rise in the cytosolic Ca2+ concentration thereby blocking CD4+ T cell apoptosis. A, cytosolic Ca2+ was measured from control and tumor supernatant (sup)-treated CD4+ T cells in the presence and absence of nifetepimine. CD4+ T cells were loaded with 5 μm Fura-2AM, and the units of fluorescence, recorded at 510 nm after excitation at 340 and 380 nm, was used to calculate cytosolic Ca2+ as described above. B, in a parallel set of experiments, the percent of CD4+ T cell viability under the above-mentioned conditions was monitored with time period. C, shown is a Western blot representation for cleaved caspase 9 and 3 in control and MCF-7 supernatant-treated CD4+ T cells in the presence and absence of nifetepimine. D, shown is a graphic representation of the percentage of cell death in control and MCF-7 supernatant-treated CD4+ T cells in the presence and absence of caspase 9(z-LEHD-FMK)/caspase 3(z-DEVD-FMK)/pan-caspase(z-VAD-FMK) inhibitors. Values are the mean ± S.E. of three independent experiments in each case.
SERCA3 Down-modulation by Nifetepimine Blocks Caspase Activation and Protects T Cells from Tumor-induced Apoptosis
Our findings clearly illustrated that treatment of CD4+ T cells with the tumor supernatant was associated with a rise in the GRP78 levels, which also resulted in the activation of caspase 9 and caspase 3 leading to cell death. Conversely treatment with nifetepimine inhibited the activation of caspase 9 and caspase 3, thereby protecting the cells from tumor-induced apoptosis (Fig. 4C). The apoptosis produced by the MCF-7 supernatant was significantly abrogated in the presence of caspase 3 (z-DEVD-FMK), caspase 9(z-LEHD-FMK), and pan-caspase inhibitor (z-VAD-FMK), thereby justifying the involvement of these caspases in the apoptotic cascade (Fig. 4D).
Nifetepimine-induced SERCA3 Down Modulation Causes Membrane Translocation of PKC Isoforms
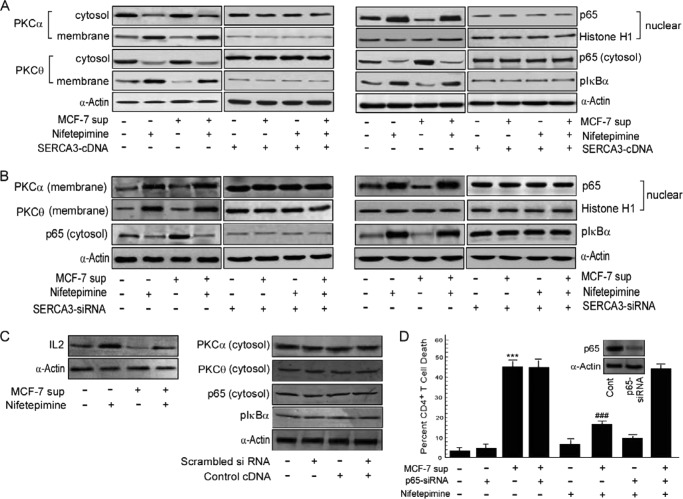
Calcium, being a ubiquitous second messenger, interacts with the growth promoting PKC, resulting in activation of the downstream signaling cascade (28). It is acknowledged that the “conventional” PKC isoform, PKCα, and the “novel” isoform PKCθ, are the major isoforms of the PKC family involved in T cell activation, with PKCα lying upstream of PKCθ in the activation process (35). PKCα binds to two Ca2+ ions when recruited to the cell membrane where it becomes activated and undergoes autophosphorylation to achieve full catalytic function (36). Because our results described increase in cytosolic Ca2+ by nifetepimine, we next checked the translocation of these PKC isoforms from cytosol to the membrane during nifetepimine-mediated restoration of CD4+ T cells from tumor-induced apoptosis. As shown in Fig. 5A, tumor supernatant significantly decreased the levels of both the PKC isoforms in CD4+ T cell membrane. In contrast, as depicted in Fig. 5A, a considerable increment in PKCα and PKCθ expression, respectively, was observed in the membrane after treatment with nifetepimine. Interestingly, it was observed that in the SERCA3-overexpressed cells, membrane translocation of both the PKC isoforms was significantly lessened (Fig. 5A), and nifetepimine failed to significantly potentiate the membrane translocation of the PKC isoforms in these transfected cells (Fig. 5A), thereby being unsuccessful in establishing its immunorestoration act. In contrast, in SERCA3 siRNA-transfected CD4+ T cells, membrane levels of both the PKC isoforms were enhanced even in the absence of nifetepimine (Fig. 5B). Moreover, in these SERCA3 knocked-out cells, the tumor supernatant failed to significantly retain PKCα and PKCθ in the cytosol (Fig. 5B). These findings strongly suggest that SERCA3 down-regulation by nifetepimine essentially not only protects T cells from tumor-induced apoptosis but also favors its survival.
FIGURE 5.
Nifetepimine-mediated SERCA3 down-modulation favors PKC activation and nuclear translocation of NF-κB. A, to determine the role of SERCA3 in regulating the downstream signaling cascade responsible for CD4+ T cell activation, SERCA3 was overexpressed in CD4+ T cells. Control and tumor supernatant (sup)-treated SERCA3-overexpressed CD4+ T cells in the presence and absence of nifetepimine were subjected to isolation of cytosolic and membrane fractions. Western blot analysis with the membrane fractions was performed to determine the changes in the membrane distribution pattern of the two PKC isoforms. The expression levels of pIκBα and p65NF-κB in the cytosol was determined with the respective antibodies. Nuclear fractions from CD4+ T cells were Western-blotted to determine nuclear translocation of p65NF-κB. B, in a parallel set of experiment, to reconfirm the involvement of SERCA3 in the lymphocyte activation process, CD4+ T cells were transfected with SERCA3-siRNA. Control and tumor supernatant-treated SERCA3-knocked-down CD4+ T cells in the presence and absence of nifetepimine were subjected to Western blot analysis with the membrane fraction to determine the membrane expression status of PKCα and PKCθ, and the cytosolic expression level of pIκBα and p65NF-κB was evaluated with the respective antibodies. The nuclear fraction isolated from the above sets of CD4+ T cells was used to detect the nuclear translocation of p65NF-κB by Western blot analysis. C, a Western blot analysis shows the changes in IL-2 expression status in control and MCF-7 supernatant-treated CD4+ T cells in the presence and absence of nifetepimine; Western blot analysis depicts the cytosolic expression status of PKCα, PKCθ, and p65NF-κB in control and scrambled siRNA/control cDNA-treated sets. D, to finally confirm the role of NF-κB in the nifetepimine-mediated protection against apoptosis, control and tumor supernatant-treated NF-κB silenced CD4+ T cells in the presence and absence of nifetepimine. Thereafter, the percent of apoptosis was determined using annexin-V/PI positivity; inset, the expression level of NF-κB in control and NF-κB-siRNA-transfected cells. *** denotes p value with respect to the control, and ### denotes p value with respect to the tumor supernatant. α-Actin/Histone H1 was used as the internal control. *, p < 0.05; **, p < 0.01; ***, p < 0.001; #, p < 0.05; ##, p < 0.01; ###, p < 0.001.
Nifetepimine-mediated SERCA3 Down Modulation Promotes NF-κB Activation
Because NF-κB plays an important role in T cell homeostasis and activation, we next evaluated the role of this transcription factor in nifetepimine-mediated CD4+ T cell restoration against tumor-induced apoptosis. Results of Fig. 5A depict that although MCF-7 supernatant retarded nuclear translocation of p65NF-κB in CD4+ T cells, nifetepimine efficiently stimulated the translocation NF-κB to the nucleus of these cells even in the presence of the tumor supernatant. It is known that the degradation of p50IκBα that retains NF-κB in the cytosol and subsequent release of p65NF-κB requires prior phosphorylation of IκBα at Ser-32 and Ser-36 residues (37). Supporting our above-mentioned results, phosphorylated IκBα level was elevated in cytosol of CD4+ T cells up on nifetepimine treatment even in the presence of tumor supernatant (Fig. 5A). In contrast, nifetepimine failed to change the pIκBα status in the cytosol and to support nuclear recruitment of p65NF-κB substantially in SERCA3-overexpressed CD4+ T cells cultured with or without MCF-7 supernatant (Fig. 5A). On the other hand, in the SERCA3-knocked down CD4+ T cells, pIκBα levels were already high in the cytosol (Fig. 5B), and thus the nuclear translocation of p65NF-κB was favored even in the absence of nifetepimine (Fig. 5B). All these results together emphasize that SERCA3 down-modulation by nifetepimine also initiates the downstream signaling that provokes CD4+ T lymphocyte reinstatement in tumor milieu.
To further establish the contribution of the transcription factor NF-κB in the immune-protective environment, we transfected CD4+ T cells with p65-siRNA. Interestingly, p65NF-κB-silencing inhibited the protective effect of nifetepimine, and 44.8% cell death was produced by the tumor supernatant even when treated with this compound (Fig. 5D). These results, therefore, signify that tumor-induced SERCA3 up-regulation inhibited p65NF-κB-dependent CD4+ T cell survival, whereas SERCA3 down-modulation caused subsequent p65NF-κB activation thereby rendering protection to the CD4+ T cells from tumor-induced apoptosis.
Nuclear Translocation of p65NF-κB Facilitates IL-2 Activation
It is well known that IL-2 is essential for T cell homeostasis and activation. Induction of IL-2 gene expression involves the activation and binding of several transcription factors to their cognate sequences of the IL-2 promoter. Of them, NF-κB, is obligatory for the activation of IL-2 (38). We, therefore, checked the changes in IL-2 expression levels induced by NF-κB activation. Our findings revealed an increment in the IL-2 protein level on treatment with nifetepimine. On the other hand, IL-2 expression that was abrogated on treatment with the MCF-7 supernatant was restored on nifetepimine treatment (Fig. 5C). This clearly highlights that nifetepimine-mediated NF-κB activation, and IL-2 expression is a prerequisite to the process of immuno restoration.
Inhibition of PKCα and PKCθ Perturbs p65NF-κB-dependent Restoration of CD4+ T Cells from Tumor-induced Apoptosis
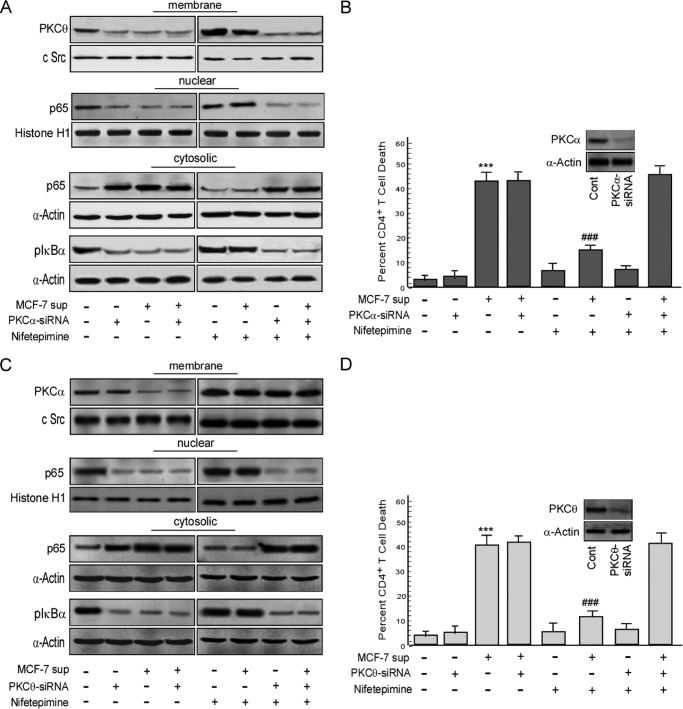
Next, in an effort to confirm the involvement of the afore-mentioned PKC isoforms in the SERCA3-mediated NF-κB regulation, we adopted gene manipulation study. To that end, inhibition of PKCα by RNA interference significantly nullified the nifetepimine-induced activation of PKCθ and retarded p65NF-κB translocation to the nucleus (Fig. 6A). These transfectants also showed a low phosphorylation status of IκBα in the cytosol even in the presence of nifetepimine (Fig. 6A). Interestingly, in these engineered cells, 45.2% cell death was produced by the tumor supernatant even in the presence of nifetepimine, thereby indicating that nifetepimine-induced protection was mediated through this conventional PKC isoforms (Fig. 6B). However, nifetepimine-induced membrane translocation of PKCα was not affected by the transient silencing of PKCθ, although the nuclear recruitment of p65NF-κB was inhibited (Fig. 6C). Confirming these results, the cytosolic pIκBα level remained low in these PKCθ-silenced cells even up on nifetepimine treatment (Fig. 6C). Nifetepimine also failed to protect PKCθ-knocked out CD4+ T cells from tumor-induced apoptosis (Fig. 6D). These results clearly defend the role of PKCα at the downstream of SERCA3 in activating PKCθ that in turn persuaded the nuclear recruitment of p65NF-κB to restore the survival pathway of CD4+ T cells from tumor insult.
FIGURE 6.
Inhibition of the PKC isoforms perturbs the restoration of CD4+ T cells from tumor-induced apoptosis. A, to justify the role of PKCα in the immunorestoration act, PKCα was inhibited in the CD4+ T cell by the gene silencing approach. Control and tumor supernatant (sup)-treated PKCα-siRNA-transfected CD4+ T cells in the presence and absence of nifetepimine were subjected to Western blot analysis with the isolated membrane fractions to determine the membrane expression status of PKCθ. Simultaneously, the cytosolic expression level of pIκBα and p65NF-κB and also the nuclear expression level of p65NF-κB were determined from the nuclear extracts in the same experimental set by Western blot analysis. B, in parallel, the above experimental set was scored for percent cell death using annexin-V/7-AAD positivity; inset, shown is the expression level of PKCα in control and PKCα-siRNA-transfected cells. *** denotes p value with respect to the control, and ### denotes p value with respect to the tumor supernatant. C, to clarify the role of PKCθ in the T cell activation cascade, the PKCθ gene was transiently silenced using PKCθ siRNA. Control and tumor supernatant-treated PKCθ-silenced CD4+ T cells in the presence and absence of nifetepimine were subjected to Western blot analysis to determine the membrane expression levels of PKCα, the cytosolic expression levels of both pIκBα and p65NF-κB, and also the nuclear expression status of p65NF-κB. D, the same experimental set was simultaneously analyzed flow cytometrically for percent cell death by labeling the cells with annexin-V/7-AAD; inset, expression level of PKCθ in control and PKCθ-siRNA-transfected cells. *** denotes p value with respect to the control, and ### denotes p value with respect to the tumor supernatant. Values are the mean ± S.E. of three independent experiments in each case. *, p < 0.05; **, p < 0.01; ***, p < 0.001; #, p < 0.05; ##, p < 0.01; ###, p < 0.001.
Validation of the Immunoprotective Mechanism of Nifetepimine in Tumor-bearing Mice Models
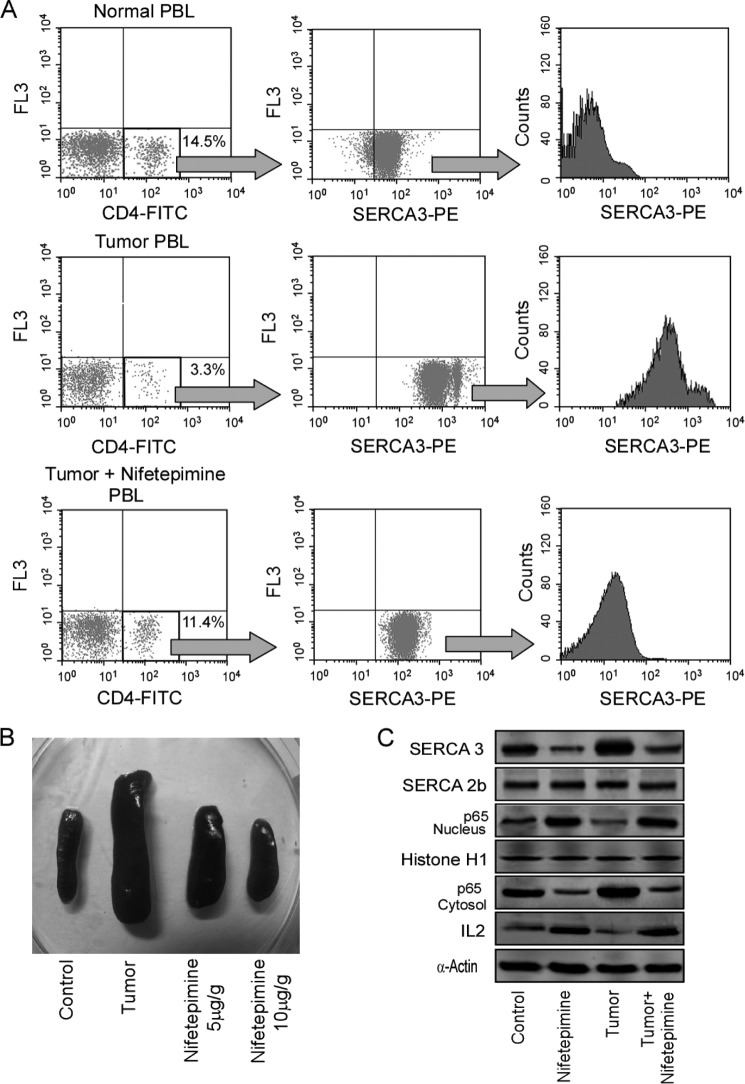
In an attempt to further validate the importance of SERCA3 down-modulation in ameliorating tumor-induced immune-suppression, the above-mentioned results were confirmed in Swiss albino mice that were injected intra-peritoneally with 1 × 106 Ehlrich ascites carcinoma cells followed by the intraperitoneal application of nifetepimine (10 μg/g of body weight) starting from 7 days after Ehlrich ascites carcinoma inoculation. As depicted from the FACS analysis in Fig. 7A, first panel, tumor burden significantly decreased CD4+ T cell number, whereas treatment with nifetepimine significantly restored the depleted cell number. These CD4+ T cell populations were further gated to determine the changes in the SERCA3 expression level. Interestingly, it was observed that the rise in the SERCA3 expression levels in the tumor-bearing mice was prominently reduced in the nifetepimine-treated sets (Fig. 7A, middle and last panel). On the other hand, it was also observed that the tumor-induced spleenomegaly was restored in the nifetepimine-treated sets (Fig. 7B). Reflecting our in vitro results, tumor burden also restricted NF-κB translocation to the nucleus of the CD4+ T cells and also IL-2 production in these tumor-bearing mice, whereas nifetepimine inhibited the same (Fig. 7C), thereby relieving these immune cells from tumor insult. These in vivo results, demonstrating the role of nifetepimine in protecting T cells from tumor-induced apoptosis by down-modulating SERCA3 expression, successfully validated the immune-protective role of this compound, thus allowing us to predict a model for nifetepimine action (Fig. 8).
FIGURE 7.
Validation of this immunoprotective mechanism by nifetepimine in tumor-bearing mice models. A, a typical flow cytometric representation shows the percentage of CD4+ T cells in the peripheral circulation from normal, tumor-bearing, and nifetepimine-treated tumor-bearing mice (left panel). Shown are dot plot analysis (middle panel) and a histogram representation for SERCA3 expression in CD4+-gated T cells of control/tumor-bearing and nifetepimine-treated tumor bearing sets (right panel). PE, phosphatidylethanolamine. B, shown is a pictorial representation of the change in spleen size isolated from control, tumor-bearing, and nifetepimine (5 and 10 μg/g body weight)-treated tumor-bearing sets. C, the expression levels of SERCA3 and SERCA2b in control and tumor-bearing mice CD4+ T cells in the presence and absence of nifetepimine treatment were determined by Western blotting. The cytosolic and the nuclear expression status of the transcription factor, p65NF-κB, and also the expression level of IL-2 was determined by Western blot analysis from the above experimental sets. α-Actin/histone H1 was used as internal control. Values are the mean ± S.E. of three independent experiments in each case. PBL, peripheral blood lymphocyte.
FIGURE 8.
Schematic diagram showing the immunoprotective effect of nifetepimine on tumor-induced CD4+ T cell apoptosis. Nifetepimine prevents tumor-induced T cell death by down-modulating SERCA3 expression, which thereby blocks the apoptotic cascade on one hand and restores the survival pathway on the other, ultimately protecting the CD4+ T cells from tumor insult. PBL, peripheral blood lymphocytes.
DISCUSSION
Despite the convincing evidence suggesting that cancer patients initiate immune reactions to their tumors, it is clear that most responses are relatively ineffective because many infiltrated tumors continue to grow progressively (5). Indeed, malignant cells use various mechanisms for evading immunity (39–41). Recent evidence from several laboratories including our own suggests that tumors produce several soluble factors that may inhibit the development of an effective antitumor immune response by inducing T cell apoptosis (1, 7–10, 42, 43). Tumor-shed PGE2 has been implicated as a potential inhibitor of T cell function in the context of malignant disease (13, 44). It is well known that PGE2 has diverse effects on CD4+ T cells leading to inhibition of T cell activation (45). Albeit the outcome of PGE2 signaling is well established, the molecular mechanisms involved are still not completely understood.
It has been well documented that calcium is involved in several mechanisms essential for normal lymphocyte proliferation and SERCA enzyme plays an important role in controlling intracellular Ca2+ homeostasis (46). In this regard, down-modulation of SERCA3 gene expression contributes to the maintenance of the activated state of T cells and helps in the alteration of the apoptotic state of the cells. Although the importance of ER Ca2+ homeostasis and SERCA pump regulation during lymphocyte activation and cancer development is clearly established (15, 16, 47), studies correlating SERCA signaling with T lymphocyte apoptosis in tumor milieu and consequently protection of T cells by potential therapeutic agents are still lacking. Our results implicate SERCA3 as being an important participant in tumor-induced apoptosis of CD4+ T cells, reflecting its role in perturbing cellular Ca2+ homeostasis that in turn leads to development of ER stress and is also inhibited PKCα/PKCθ/NF-κB survival pathway thereby leading to cell death. Our findings also highlight the mechanism by which tumor-shed PGE2 causes up-regulation of SERCA3 through activation of its transcription factor Sp1. Our work also established nifetepimine that successfully protected CD4+ T lymphocytes from tumor-induced apoptosis by diminishing the binding of Sp1 to the SERCA3 promoter, thereby blocking the apoptotic cascade and also protecting the downstream survival signaling. Thus by modulation of the cellular calcium homeostasis of the T cell in the tumor microenvironment, nifetepimine effectively rescues the cell from apoptosis.
It is known that calcium is a ubiquitous second messenger controlling a broad range of cellular functions including growth and proliferation. Therapeutic strategies based on modulating intracellular Ca2+ or targeting of Ca2+-regulated signaling factors, therefore, seems to be a promising approach to control growth and/or apoptosis. Maintenance of cellular calcium homeostasis is thus of utmost importance for regulating normal cellular function. Various protein types involved in cellular Ca2+ homeostasis function in a highly interconnected manner due to the fact that the transport activity of various SERCA and PMCA enzymes as well as the opening probability of IP3 receptor calcium channels are regulated by the Ca2+ concentration of the cytosol or of the ER lumen. This leads to generation of cellular calcium signals that selectively affect the activation of several target proteins such as PKC and calcineurin (48, 49). Because in the vicinity of the ER, variations of cytosolic calcium levels lie within the range in which SERCA3 activity is modulated by calcium, the amount of SERCA3 expressed in a cell has a major impact on determining the state of activation of the cell (46).
In this study we have found that tumor-induced CD4+ T cell apoptosis involved up-regulation of SERCA3 expression, which was associated with the development of ER stress and caspase activation. Actually the tumor-shed PGE2 causes the nuclear translocation and promoter binding of Sp1, a transcription factor essential for SERCA3 gene transcription. In contrast, nifetepimine could block the promoter binding of Sp1 and down-modulate SERCA3 expression thereby blocking the apoptotic cascade and rejuvenating CD4+ T cell activation program even in tumor microenvironment. Our gene manipulation studies have further confirmed the role of SERCA3 in deciding CD4+ T cell-fate in tumor-bearers. From all these results, one can reasonably conclude that down-regulation of SERCA3 expression by nifetepimine protects the CD4+ T lymphocytes from tumor-induced apoptosis. The rise in the cytosolic calcium levels associated with SERCA3 down-regulation induces rapid PKC activation that is critical for the T cells to enter into the proliferative phase (24). In fact, PKC plays a prominent role in the regulation of a variety of cellular functions, including Ca2+ signaling. As expected, CD4+ T lymphocyte recovery by nifetepimine involves a redistribution of the enzyme from the cytosol toward the membrane. On the other hand, the tumor supernatant renders a reduced PKC activation. These results are in line with the findings of Sun et al. (50) who reported that pharmacological down-regulation of PKCs impairs T cell activation signaling and effector functions, thereby indicating the contribution of PKCs in T cell activation. Among the PKC isoforms, PKCα and PKCθ are recruited to the inner leaflet of the plasma membrane, an event crucial to NF-κB activation (35). These results support our observation that nifetepimine-mediated T lymphocyte recovery from tumor-induced immunosuppression involves membrane translocation of both the PKC isoforms. To coalesce with the previous reports, we also found that impairment of PKCα and PKCθ activation by RNA interference blocks the activation process by interrupting the downstream NF-κB signaling that is required for the survival of the T lymphocytes (7, 51). Our in vitro findings have also been supported by our in vivo results where down-modulation of SERCA3 expression by nifetepimine in tumor-bearing mice subsequently protects the CD4+ T cells from tumor-induced apoptosis.
In conclusion, our data for the first time provide direct evidence that tumor-derived PGE2 impairs survival signals and switches on the apoptotic signals in CD4+ T cells by up-regulating SERCA3 expression. This ultimately results in impaired activation of downstream signals as demonstrated by decreased membrane translocation of PKCα and PKCθ on one hand and activation of the apoptotic cascade on the other. As a consequence, p65NF-κB translocation to nucleus is interrupted, and caspase activation is favored, thereby shifting the cellular microenvironment toward apoptosis. Based on our observations, we postulate SERCA3 as an important factor responsible for apoptosis and impaired survival signaling in CD4+ T cells in tumor environment. Modulation of the transcriptional levels of SERCA3 by both the tumor and nifetepimine play a vital role in determining the fate of the lymphocyte. In addition, our results demonstrating protection of PKCα/PKCθ/p65NF-κB signaling as well as survival of CD4+ T cells from tumor insult signify that by targeting the SERCA pump using drugs like nifetepimine may help to sustain the cell-mediated immunity of the cancer-bearer.
Acknowledgments
We gratefully acknowledge Prof. Jonathan Lytton, Department of Biochemistry and Molecular Biology, University of Calgary, Canada, for gifting us the SERCA3 clone. We also sincerely thank Dr. Gautam Das, Department of Surgery, Calcutta National Medical College, Kolkata, India for providing us with the human breast carcinoma tissues. Thanks are also due to A. Basu, R. Dutta, and K. Das for technical support.
This work was supported by the Department of Science and Technology, Council of Scientific and Industrial Research, Department of Biotechnology, Government of India.
- SERCA
- sarco(endo)plasmic reticulum calcium ATPase
- 7-ADD
- 7-amino actinomycin D
- GRP78
- glucose-regulated protein 78
- ER
- endoplasmic reticulum
- PGE2
- prostaglandin E2
- z-
- benzyloxycarbonyl
- FMK
- fluoromethyl ketone.
REFERENCES
- 1. Bhattacharyya S., Mandal D., Saha B., Sen G. S., Das T., Sa G. (2007) Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J. Biol. Chem. 282, 15954–15964 [DOI] [PubMed] [Google Scholar]
- 2. Das T., Sa G., Paszkiewicz-Kozik E., Hilston C., Molto L., Rayman P., Kudo D., Biswas K., Bukowski R. M., Finke J. H., Tannenbaum C. S. (2008) Renal cell carcinoma tumors induce T cell apoptosis through receptor-dependent and receptor-independent pathway. J. Immunol. 180, 4687–4696 [DOI] [PubMed] [Google Scholar]
- 3. Biswas K., Richmond A., Rayman P., Biswas S., Thornton M., Sa G., Das T., Zhang R., Chahlavi A., Tannenbaum C. S., Novick A., Bukowski R., Finke J. H. (2006) GM2 expression in renal cell carcinoma. Potential role in tumor-induced T-cell dysfunction. Cancer Res. 66, 6816–6825 [DOI] [PubMed] [Google Scholar]
- 4. Gastman B. R., Johnson D. E., Whiteside T. L., Rabinowich H. (2000) Tumor induced apoptosis of T lymphocytes. Elucidation of intracellular apoptotic events. Blood 95, 2015–2023 [PubMed] [Google Scholar]
- 5. Finke J., Ferrone S., Frey A., Mufson A., Ochoa A. (1999) Where have all the T cells gone. Mechanisms of immune evasion by tumors. Immunol. Today 20, 158–160 [DOI] [PubMed] [Google Scholar]
- 6. Toes R. E., Ossendorp F., Offringa R., Melief C. J. (1999) CD4+ T cells and their role in antitumor immune responses. J. Exp. Med. 189, 753–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhattacharyya S., Mandal D., Sen G. S., Pal S., Banerjee S., Lahiry L., Finke J. H., Tannenbaum C. S., Das T., Sa G. (2007) Tumor-induced oxidative stress perturbs nuclear factor κB activity-augmenting tumor necrosis factor-α-mediated T-cell death. Protection by curcumin. Cancer Res. 67, 362–370 [DOI] [PubMed] [Google Scholar]
- 8. Bhattacharyya S., Md Sakib Hossain D., Mohanty S., Sankar Sen G., Chattopadhyay S., Banerjee S., Chakraborty J., Das K., Sarkar D., Das T., Sa G. (2010) Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing host. Cell. Mol. Immunol. 7, 306–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chattopadhyay S., Bhattacharyya S., Saha B., Chakraborty J., Mohanty S., Sakib Hossain D. M., Banerjee S., Das K., Sa G., Das T. (2009) Tumor-shed PGE2 impairs IL2Rγc signaling to inhibit CD4+ T cell survival, Regulation by theaflavins. Plos One 4, e7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das T., Sa G., Hilston C., Kudo D., Rayman P., Biswas K., Molto L., Bukowski R., Rini B., Finke J. H., Tannenbaum C. (2008) GM1 and TNFa, overexpressed in renal cell carcinoma, synergize to induce T cell apoptosis. Cancer Res. 68, 2014–2023 [DOI] [PubMed] [Google Scholar]
- 11. Sa G., Das T., Moon C., Hilston C. M., Rayman P. A., Rini B. I., Tannenbaum C. S., Finke J. H. (2009) GD3, an overexpressed tumor-derived ganglioside, mediates the apoptosis of activated but not resting T cells. Cancer Res. 69, 3095–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chemnitz J. M., Driesen J., Classen S., Riley J. L., Debey S., Beyer M., Popov A., Zander T., Schultze J. L. (2006). Prostaglandin E2 impairs CD4+ T cell activation by inhibition of lck. Implications in Hodgkin's lymphoma. Cancer Res. 66, 1114–1122 [DOI] [PubMed] [Google Scholar]
- 13. Pockaj B. A., Basu G. D., Pathangey L. B., Gray R. J., Hernandez J. L., Gendler S. J., Mukherjee P. (2004) Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann. Surg. Oncol. 11, 328–339 [DOI] [PubMed] [Google Scholar]
- 14. Minakuchi R., Wacholtz M. C., Davis L. S., Lipsky P. E. (1990) Delineation of the mechanism of inhibition of human T cell activation by PGE2. J. Immunol. 145, 2616–2625 [PubMed] [Google Scholar]
- 15. Launay S., Bobe R., Lacabaratz-Porret C., Bredoux R., Kovàcs T., Enouf J., Papp B. (1997) Modulation of endoplasmic reticulum calcium pump expression during T lymphocyte activation. J. Biol. Chem. 272, 10746–10750 [DOI] [PubMed] [Google Scholar]
- 16. Roderick H. L., Cook S. J. (2008) Ca2+ signaling checkpoints in cancer. Remodeling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer 8, 361–375 [DOI] [PubMed] [Google Scholar]
- 17. Monteith G. R., McAndrew D., Faddy H. M., Roberts-Thomson S. J. (2007) Calcium and cancer. Targeting Ca2+ transport. Nat. Rev. Cancer 7, 519–530 [DOI] [PubMed] [Google Scholar]
- 18. Brini M., Carafoli E. (2009) Calcium pumps in health and disease. Physiol. Rev. 89, 1341–1378 [DOI] [PubMed] [Google Scholar]
- 19. Kluck R. M., McDougall C. A, Harmon B. V., Halliday J. W. (1994) Calcium chelators induce apoptosis. Evidence that raised intracellular ionized calcium is not essential for apoptosis. Biochim. Biophys. Acta 1223, 247–254 [DOI] [PubMed] [Google Scholar]
- 20. Mason R. P. (1999) Calcium channel blockers, apoptosis and cancer. Is there a biologic relationship? J. Am. Coll. Cardiol. 34, 1857–1866 [DOI] [PubMed] [Google Scholar]
- 21. Wuytack F., Raeymaekers L., Missiaen L. (2002) Molecular physiology of the SERCA and SPCA pumps. Cell Calcium 32, 279–305 [DOI] [PubMed] [Google Scholar]
- 22. Wuytack F., Papp B., Verboomen H., Raeymaekers L., Dode L., Bobe R., Enouf J., Bokkala S., Authi K. S., Casteels R. (1994) A sarco/endoplasmic reticulum Ca2+ ATPase 3-type Ca2+ pump is expressed in platelets, in lymphoid cells, and in mast cells. J. Biol. Chem. 269, 1410–1416 [PubMed] [Google Scholar]
- 23. Szamel M., Appel A., Schwinzer R., Resch K. (1998) Different protein kinase C Isoenzymes regulate IL-2 receptor expression or IL-2 synthesis in human lymphocytes stimulated via the TCR. J. Immunol. 160, 2207–2214 [PubMed] [Google Scholar]
- 24. Silberman D. M., Zorrilla-Zubilete M., Cremaschi G. A., Genaro A. M. (2005) Protein kinase C dependent NF-κB activation is altered in T cells by chronic stress. Cell. Mol. Life Sci. 62, 1744–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaâbane C., Corvazier E., Bredoux R., Dally S., Raïes A., Villemain A., Dupuy E., Enouf J., Bobe R. (2006) Sarco/endoplasmic reticulum Ca2+ATPase type 3 isoforms (SERCA3b and SERCA3f). Distinct roles in cell adhesion and ER stress. Biochem. Biophys. Res. Commun. 345, 1377–1385 [DOI] [PubMed] [Google Scholar]
- 26. Hadri L., Ozog A., Soncin F., Lompré A. M. (2002) Basal Transcription of the mouse sarco(endo)plasmic reticulum Ca2+-ATPase type 3 gene in endothelial cells is controlled by Ets-1 and Sp1. J. Biol. Chem. 277, 36471–36478 [DOI] [PubMed] [Google Scholar]
- 27. Kanda N., Koike S., Watanabe S. (2005) Prostaglandin E2 enhances neurotrophin-4 production via EP3 receptor in human keratinocytes. J. Pharmacol. Exp. Ther. 315, 796–804 [DOI] [PubMed] [Google Scholar]
- 28. Higgins E. R., Cannell M. B., Sneyd J. (2006) A buffering SERCA pump in models of calcium dynamics. Biophys. J. 91, 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Falsone F. S, Kappe C. O. (2001) The Biginelli dihydropyrimidone synthesis using polyphosphate ester as mild and efficient cyclocondensation/dehydration reagent. ARKIVOC 2, 122–134 [Google Scholar]
- 30. Gohain M., Prajapati D., Sandhu J. S. (2004) A novel copper-catalyzed three-component one-pot synthesis of dihydropyrimidin-2(1H)-ones using microwaves under solvent-free condition. Synlett 2, 235–238 [Google Scholar]
- 31. Kappe C. O., Kumar D., Varma R. S. (1999) Microwave-assisted high speed parallel synthesis of 4-aryl-3,4-dihydropyrimidin-2(1H)-ones using a solventless Bignelli condensation protocol. Synthesis 10, 1799–1803 [Google Scholar]
- 32. Anastassiou E. D., Paliogianni F., Balow J. P., Yamada H., Boumpas D. T. (1992) Prostaglandin E2 and other cyclic AMP-elevating agents modulate IL2 and IL2Rα gene expression at multiple levels. J. Immunol. 148, 2845–2852 [PubMed] [Google Scholar]
- 33. Kleizen B., Braakman I. (2004) Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 16, 343–349 [DOI] [PubMed] [Google Scholar]
- 34. Chin G. M., Herbst R. (2006) Induction of apoptosis by monastrol, an inhibitor of the mitotic kinesin Eg5, is independent of the spindle checkpoint. Mol. Cancer Ther. 5, 2580–2591 [DOI] [PubMed] [Google Scholar]
- 35. Trushin S. A., Pennington K. N., Carmona E. M., Asin S., Savoy D. N., Billadeau D. D., Paya C. V. (2003) Protein kinase Cα (PKCα) acts upstream of PKCθ to activate IκB kinase and NF-κB in T lymphocytes. Mol. Cell. Biol. 23, 7068–7081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Medkova M., Cho W. (1999) Interplay of C1 and C2 domains of protein kinase Cα in its membrane binding and activation. J. Biol. Chem. 274, 19852–19861 [DOI] [PubMed] [Google Scholar]
- 37. Chen Z. J., Parent L., Maniatis T. (1996) Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell 84, 853–862 [DOI] [PubMed] [Google Scholar]
- 38. Tsatsanis C., Patriotis C., Tsichlis P. N. (1998) Tpl-2 induces IL-2 expression in T-cell lines by triggering multiple signaling pathways that activate NFAT and NF-κB. Oncogene 17, 2609–2618 [DOI] [PubMed] [Google Scholar]
- 39. Nielsen M. B., Marincola F. M. (2000) Melanoma vaccines. The paradox of T cell activation without clinical response. Cancer Chemother. Pharmacol. 46, S62–S66 [DOI] [PubMed] [Google Scholar]
- 40. Real L. M., Jimenez P., Kirkin A., Serrano A., García A., Cantón J., Zeuthen J., Garrido F., Ruiz-Cabello F. (2001) Multiple mechanisms of immune evasion can coexist in melanoma tumor cell lines derived from the same patient. Cancer Immunol. Immunother. 49, 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salazar-Onfray F. (1999) Interleukin-10. A cytokine used by tumors to escape immunosurveillance. Med. Oncol. 16, 86–94 [DOI] [PubMed] [Google Scholar]
- 42. Mandal D., Lahiry L., Bhattacharyya A., Bhattacharyya S., Sa G., Das T. (2006) Tumor-induced thymic involution via inhibition of IL-7Rα and its JAK-STAT signaling pathway. Protection by black tea. Int. Immunopharmacol. 6, 433–444 [DOI] [PubMed] [Google Scholar]
- 43. Bhattacharyya A., Mandal D., Lahiry L., Sa G., Das T. (2004). Black tea protects immunocytes from tumor-induced apoptosis by changing Bcl-2/Bax ratio. Cancer Lett. 209, 147–154 [DOI] [PubMed] [Google Scholar]
- 44. Sharma S., Yang S. C., Zhu L., Reckamp K., Gardner B., Baratelli F., Huang M., Batra R. K., Dubinett S. M. (2005) Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 65, 5211–5220 [DOI] [PubMed] [Google Scholar]
- 45. Goodwin J. S, Bankhurst A. D, Messner R. P. (1977) Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J. Exp. Med. 146, 1719–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dellis O., Arbabian A., Brouland J. P., Kovàcs T., Rowe M., Chomienne C., Joab I., Papp B. (2009) Modulation of B-cell endoplasmic reticulum calcium homeostasis by Epstein-Barr virus latent membrane protein-1. Mol. Cancer 8, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gélébart P., Kovács T., Brouland J. P., van Gorp R., Grossmann J., Rivard N., Panis Y., Martin V., Bredoux R., Enouf J., Papp B. (2002) Expression of endomembrane calcium pumps in colon and gastric cancer cells. J. Biol. Chem. 277, 26310–26320 [DOI] [PubMed] [Google Scholar]
- 48. Dolmetsch R. E., Xu K., Lewis R. S. (1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 [DOI] [PubMed] [Google Scholar]
- 49. Nelson D. E., Sée V., Nelson G., White M. R. (2004) Oscillations in transcription factor dynamics. A new way to control gene expression. Biochem. Soc. Trans. 32, 1090–1092 [DOI] [PubMed] [Google Scholar]
- 50. Sun Z., Arendt C. W., Ellmeier W., Schaeffer E. M., Sunshine M. J., Gandhi L., Annes J., Petrzilka D., Kupfer A., Schwartzberg P. L., Littman D. R. (2000). PKCθ is required for TCR induced NF-κB activation in mature but not immature T lymphocytes. Nature 404, 402–407 [DOI] [PubMed] [Google Scholar]
- 51. Kane L. P., Lin J., Weiss A. (2002) It's all Rel-ative. NF-κB and CD28 costimulation of T-cell activation. Trends Immunol. 23, 413–420 [DOI] [PubMed] [Google Scholar]