Background: Caspase-14 activation is associated with keratinocyte terminal differentiation.
Results: Kallikrein-related peptidase-7 (KLK7) generates an activation intermediate comprising 20- and 8-kDa subunits by cleavage after Tyr178 of procaspase-14. This intermediate cleaves procaspase-14 at Asp146, generating mature, active caspase-14.
Conclusion: Caspase-14 maturation is mediated by KLK7 and an intermediate form of caspase-14.
Significance: Caspase-14 has a unique activation mechanism.
Keywords: Caspase, Enzyme Processing, Epidermis, Kallidrein, Keratinocytes
Abstract
The maturation and activation mechanisms of caspases are generally well understood, except for those of caspase-14, which is activated at the onset of keratinocyte terminal differentiation. We investigated the possible involvement of epidermal proteases expressed in the late stage of differentiation, and found that the chymotrypsin-like serine protease kallikrein-related peptidase-7 (KLK7) cleaved procaspase-14 at Tyr178, generating an intermediate form that consists of a large (20 kDa) and a small subunit (8 kDa). We prepared an antibody directed to this cleavage site (h14Y178 Ab), and confirmed that it recognized a 20-kDa band formed when procaspase-14 was incubated with chymotrypsin or KLK7. We then constructed a constitutively active form of the intermediate, revC14-Y178. The substrate specificity of revC14-Y178 was completely different from that of caspase-14, showing broad specificity for various caspase substrates except WEHD-7-amino-4-trifluoromethylcoumarin (AFC), the preferred substrate of active, mature caspase-14. Km values for VEID-AFC, DEVD-AFC, LEVD-AFC, and LEHD-AFC were 0.172, 0.261, 0.504, and 0.847 μm, respectively. We confirmed that the mature form of caspase-14 was generated when procaspase-14 was incubated with KLK7 or revC14-Y178. Expression of constitutively active KLK7 in cultured keratinocytes resulted in generation of both the intermediate form and the mature form of caspase-14. Immunohistochemical analysis demonstrated that the intermediate form was localized at the granular layer. Our results indicate that regulation of procaspase-14 maturation during terminal differentiation is a unique two-step process involving KLK7 and an activation intermediate of caspase-14.
Introduction
Caspases are members of the cysteine protease superfamily and cleave proteins specifically after Asp residues (1). Caspases are divided into two groups, initiator caspases (caspase-1, -4, -5, -8, -9, and -10) and executioner caspases (caspase-3 and -7) (2). Initiator caspases are activated in three ways, involving various oligomeric activation platforms, i.e. apoptosome, death-inducing signaling complex, and inflammasome (3, 4). Apoptosome activates caspase-9, and death-inducing signaling complex induces activation of caspase-8 and -10 (4). Inflammatory caspases, caspase-1 and -5, are similarly activated via inflammasome (5). Subsequently these initiator caspases activate executioner caspase-3 and caspase-7. In some cases, a maturation process involving cleavage of propeptides and removal of the linker region follows (6). Among the caspases, caspase-14 shows restricted expression, being found only in epidermis and its appendages and thymic Hassall's bodies (7–9). Although it is structurally similar to caspase-1, it is thought to function in keratinocyte terminal differentiation (10, 11).
We have recently purified active caspase-14 from extract of human cornified cells (12), and found that it consists of a large subunit (p17) and a small subunit (p11) generated by the removal of a 6-amino acid linker peptide. The C-terminal of the large subunit was identified as Asp-146. This is identical with the granzyme B-cleaved activation site, although granzyme B is not the physiological activation enzyme of caspase-14 (12, 13). A cleavage site-directed antibody, h14D146, was prepared and found to react only with active caspase-14. This form is present in the stratum corneum (SC),2 indicating that maturation of caspase-14 occurs during the transition from stratum granulosum (SG) to SC.
To maintain epidermal barrier function and homeostasis, old and nonfunctional corneocytes should be removed promptly by shedding. This process, desquamation, is accomplished via degradation of corneodesmosomes by a proteolytic cascade of human kallikrein-related peptidases (KLKs) (14). It is thought that the cascade is initiated by activation of trypsin-like pro-KLK5, either by autoactivation or by matriptase in Netherton syndrome (15). Subsequently KLK5 activates other trypsin-like KLKs and chymotrypsin-like KLK7 by proteolytic release of the amino-terminal propeptide. Although most of the 15 kallikreins are expressed in skin (16), KLK5 and KLK7 are highly expressed in differentiated keratinocytes (17).
On the other hand, the activation and maturation mechanisms of caspase-14 remain to be fully established. Cleavage of procaspase-14 occurs only at the transition stage from SG to SC. Many proteolytic enzymes could be activated at this stage. Interestingly activation of caspase-14 requires a high concentration of kosmotropic salt in vitro (13), although it is not clear whether this condition is also required for maturation, in other words, cleavage of caspase-14 at Asp146. In the present study, we investigated the mechanism of caspase-14 maturation. We show that it is completely different from those of other caspases, being regulated by KLK7 and an intermediate form of caspase-14.
EXPERIMENTAL PROCEDURES
Cell Culture
Human keratinocytes derived from normal foreskin (Kurabo, Osaka, Japan) were cultured in Humedia KG2 supplemented with epidermal growth factor (0.1 ng/ml), insulin (10 μg/ml), hydrocortisone (0.5 μg/ml), bovine pituitary extract (0.4%), gentamicin (50 μg/ml), and amphotericin B (50 ng/ml). All experiments were carried out on cells at the third or fourth passages.
Tissue Specimens
Human skin specimens were obtained, with prior informed consent, from patients who underwent plastic surgery. The study was approved by the Ethical Committee of Tokyo Medical University and the Shiseido Committee on Human Ethics. Tissue samples were fixed with acetone and embedded in paraffin.
Preparation of Pro-KLK7 and Its Activation
Pro-KLK7 cDNA was PCR cloned into the BamHI and SalI sites of the pQE-100 vector (Qiagen Inc., Valencia, CA) using forward primer 5′-CGGGATCCATGGCAAGATCCCTTCTCCTGCCCC-3′ and reverse primer 5′-ACGCGTCGACTTAGCGATGCTTTTTCATGGTGTCA-3′. Recombinant proteins were purified by Ni-NTA-agarose (Qiagen) and ion-exchange Mono Q chromatography with an increasing gradient of NaCl concentration up to 0.5 m. Activation of pro-KLK7 was accomplished by incubation with tosylphenylalanyl chloromethyl ketone-treated trypsin-agarose for 1 h at room temperature. Activity of KLK7 is measured using chromogenic substrate, MeO-Suc-Arg-Pro-Tyr-p-nitroanilide (S-2586) (Pharmacia Biotech Inc.), and read at 405 nm.
Procaspase-14 Activation Study
Procaspase-14 cDNA was PCR cloned into the pQE 30 vector (Qiagen), using forward primer 5′-ATGAGCAATCCGCGGTCTTTGGAA-3′ and reverse primer 5′-CTAATCTCCACCTACTGTTTCACCGGGGT-3′. Recombinant proteins were purified by Ni-NTA-agarose and Mono Q chromatography. Recombinant procaspase-14 was incubated with active caspase-1 to -10, trypsin (tosylphenylalanyl chloromethyl ketone-treated, Sigma), chymotrypsin (tosyllysine chloromethyl ketone-treated, Sigma), or active KLK7.
Determination of the C-terminal End of the Intermediate Form
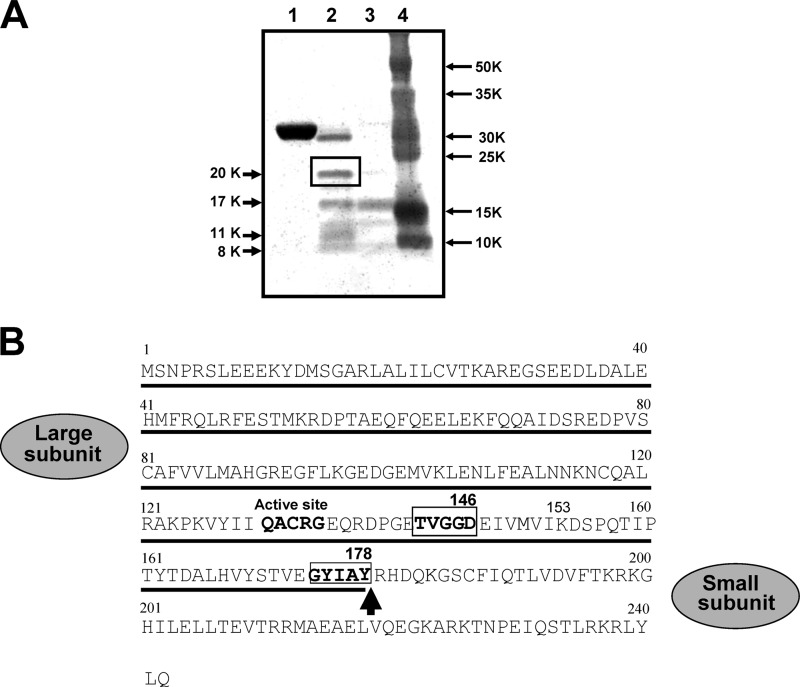
Procaspase-14 was incubated with 50 or 250 ng of KLK7 for 1 h. After SDS-PAGE, protein bands were visualized by staining with Coomassie Brilliant Blue (Bio-Rad). The 20-kDa band was excised from the gel, minced into pieces of ∼1 mm3 and digested with lysyl-endopeptidase. In-gel digestion was carried out in the presence of 50% (v/v) 18 O water to incorporate differentially labeled peptides. Peptides were extracted from the gel, and MALDI-TOF/MS analysis was carried out using a BIFLEX III (Bruker Daltonics, Inc., Billerica, MA).
Preparation of Cleavage Site-directed Antibody
Cleavage site-directed antibody (h14Y178 Ab) was prepared by immunizing rabbits with synthetic hexapeptide GYIAY, which corresponds to the C-terminal end of p20, according to the method of Kouroku et al. (36). h14Y178 Ab was purified by means of affinity chromatography on GYIAY-coupled agarose.
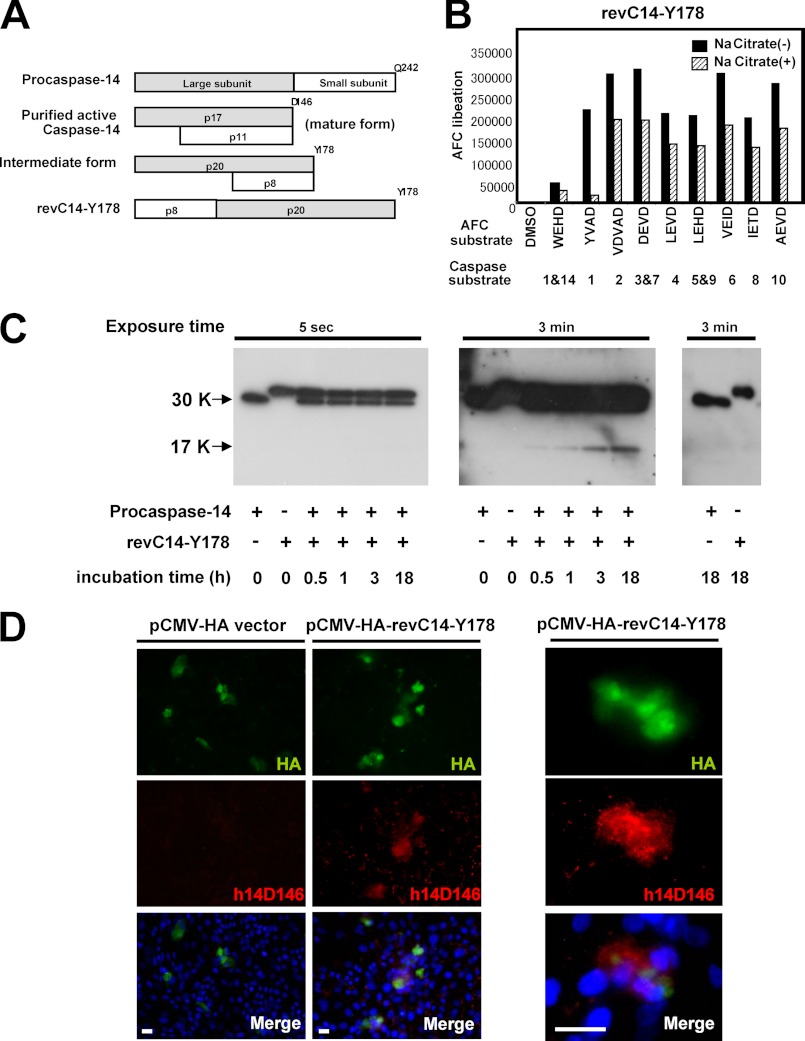
Construction of Constitutively Active Intermediate Form (revC14-Y178)
Human caspase-14 was purified from cornified cells according to our previous work, using successive chromatographies (12). Based on the sequence information, a constitutively active intermediate form (revC14-Y178) was prepared by reversing the large and small subunits, ending at Tyr178 (see Fig. 3A). revC14-Y178 cDNA was inserted into the pET 15b vector at the BamHI/XhoI sites. Recombinant protein was purified by Ni-NTA-agarose (Qiagen) and Mono Q chromatography.
FIGURE 3.
Constitutively active intermediate form showed a characteristic feature in substrate specificity. A, schematic illustration of various forms of caspase-14. A constitutively active intermediate form (revC14-Y178) was constructed by reversing p20 and p8. B, substrate specificity of revC14-Y178. Enzymatic properties of revC14-Y178 were examined using various caspase substrates in the presence or absence of 1.2 m sodium citrate. C, generation of p17 by revC14-Y178. Results from different exposure times were shown. Left panel (5 s exposure) indicated that a decrease of procaspase-14 was observed in the presence of revC14-Y178 with prolonged incubation. revC14-Y178 has a higher molecular mass (1.2 kDa larger) than procaspase-14. Middle panel (3 min exposure) showed that generation of p17 increased during incubation. Procaspase-14 or revC14-Y178 by itself did not show any changes for 18 h incubation (right panel). D, immunocytochemical analysis of revC14-Y178-transfected keratinocytes. revC14-Y178-expressing cells were also positive for h14D146 staining. Enlarged figures were also shown. Scale bars, 100 μm.
Measurement of Caspase Activity and Determination of Km Values
Caspase-14 activity was measured using Ac-WEHD-7-amino-4-trifluoromethylcoumarin (AFC) as a substrate. Fluorometric substrates were purchased from BioVision. Active caspase-1–10 were purchased from Biosciences. The assay buffer consisted of 0.1 m HEPES buffer (pH 7.5), 0.06 m NaCl, 0.01% CHAPS, 5 mm DTT, and 1.2 m sodium citrate (final concentration). revC14-Y178 (5 μg/ml, 10 μl) was preincubated in the assay buffer (80 μl) for 15 min at room temperature. After addition of 20 μl of 100 μm Ac-WEHD-AFC, the mixture was incubated for 30 min. Ac-YVAD-AFC, Ac-VDVAD-AFC, Ac-DEVD-AFC, Ac-LEVD-AFC, Ac-VEID-AFC, Ac-IETD-AFC, Ac-LEHD-AFC, and Ac-AEVD-AFC were also used to examine the enzymatic properties of the intermediate form. The activity of revC14-Y178 was measured using a 2030 Multilabel Reader ARVO X3 (Perkin Elmar, Woltham, MA) with excitation at 355 nm and emission at 460 nm. Km values for hydrolysis of these chromogenic substrates were determined from Lineweaver-Burk plots.
Western Blot Analysis
SDS-PAGE was carried out using a 5–20% gradient gel. After electrophoresis, proteins were transferred onto a polyvinylidine difluoride membrane (Immobilon-P, Millipore, Bedford, MA) and incubated with appropriate antibodies. Peroxidase-labeled anti-rabbit IgG (GE Healthcare, UK) or anti-mouse IgG was used as a secondary antibody, and immunoreactive proteins were visualized by chemiluminescence using ECL Plus (GE Healthcare).
Construction of Pro-KLK7, Active KLK7, and RevC14-Y178 Expression Vectors
Pro-KLK7 was PCR cloned using the primers, 5′-GGAATCCGGATGGCAAGATCCCTTCTCCTGCC-3′ and 5′-CCGCTCGAGTTAGCGATGCTTTTTCATGGTGTC-3′ and inserted into pCMV-HA vector at the EcoRI/XhoI site. Active KLK7 expression vectors were constructed using pH3700-pL2 vector, kindly provided by Dr. L. B. Taichman (37), driven by the involucrin promoter. cDNA of the active form of KLK7 was amplified by PCR using primers 5′-GGAATTCTGATTATTGATGGCGCCCCATGTGCAA-3′ and 5′-ATAGTTTAGCGGCCGCTTAGCGATGCTTTTTCATGGTGTC-3′. The PCR product was cloned at the EcoRI/NotI sites (pInv-HA-active KLK7). cDNA of revC14-Y178 was first cloned into pCMV-HA vector at the EcoRI/KpnI site, using primers 5′-GGAATTCCATGAGCAATCCGCGGTCTTTGGAA-3′ and 5′-GGGGTACCCTAGTAGGCGATGTATCCCTCTAC-3′(pCMV-HA-revC14-Y178). This was re-cloned into the pCMV vector at the XhoI/NotI site, using primers 5′-CCGCTCGAGATGAGCAATCCGCGGTCTTTGGAA-3′ and 5′-ATAGTTTAGCGGCCGCCTAGTAGGCGATGTATCCCTCTAC-3′. These constructs were introduced into keratinocytes during the growth phase using FuGENE (Roche). As controls, pInv-HA vector was similarly transfected.
Immunostaining
Dual stainings were carried out 2 days after stimulation of differentiation in prolonged culture. Expression of the mature form and the intermediate form was detected by immunostaining with anti-HA, h14D146, and h14Y178 antibodies. To visualize nuclei, sections were immersed in 10 ng/ml of DAPI (4′,6′-diamidino-2-phenylindole; Molecular Probes) for 5 min and washed 3 times with PBS. Normal skin specimens were stained similarly. In addition, tetramethylbenzidine was used for color development of some tissue sections.
Proximity Ligation Assay (PLA)
PLA reaction was performed using pairs of antibodies, anti-caspase-14 Ab (H99) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and monoclonal anti-KLK7 Ab (Santa Cruz Biotechnology, Inc.), and h14Y178 Ab and anti-KLK7 Ab, on sections of normal human skin, according to the manufacturer's instructions. Antibody concentrations used were 0.5 μg/ml (H99), 1 μg/ml (h14Y178 Ab) and 1 μg/ml (anti-KLK7 Ab).
RESULTS
Chymotrypsin-like Serine Protease Generated a Large Subunit from Procaspase-14
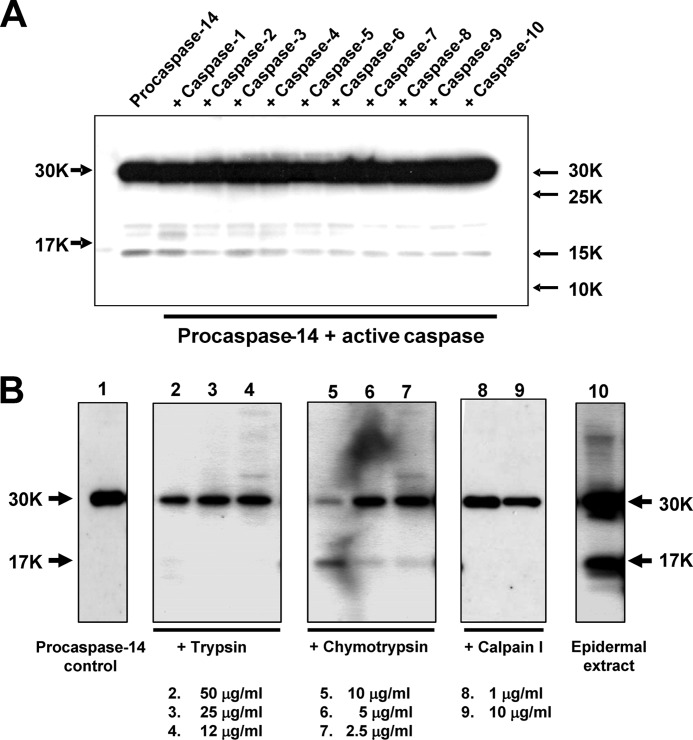
We first examined the effects of other caspase family members (caspase-1 through caspase-10). None of these caspases showed any processing activity on procaspase-14 (Fig. 1A). Because maturation of caspase-14 occurs in the boundary area between SG and SC, we then tested three nonspecific proteases, i.e. trypsin, chymotrypsin (serine protease), and calpain I (cysteine protease), as representatives of those expressed in this region. Many of the KLKs are trypsin-like serine proteases, although some are chymotrypsin-like (KLK3, KLK7, and KLK9) or mixed type (KLK11 and KLK14) (18). Calpain I was chosen as a representative of cysteine proteases and an interactant with caspase-14 in the natural moisturizing factor (NMF) generation pathway (19). Aspartic proteases and metalloproteases were not included, because processing at acidic pH is unlikely and the latter would not be expressed in SG. Interestingly, Western blot analysis using an antibody to the large subunit demonstrated that only chymotrypsin generated an immunoreactive band of 17 kDa that is identical with the large subunit of caspase-14 and with the band obtained with granzyme B (Fig. 1B). The large subunit was confirmed to be immunoreactive with h14D146 Ab. In contrast, trypsin and calpain degraded procaspase-14 and did not yield any immunoreactive bands.
FIGURE 1.
Generation of a large subunit (p17) by proteolytic processing. A, Western blot analysis of procaspase-14 processing by other caspase members. Procaspase-14 was incubated with each active caspase. None of the active caspases generated a p17 band. B, effects of trypsin, chymotrypsin, and calpain I. Procaspase-14 was incubated with these proteases for 60 min and the products were analyzed by Western blotting. Each lane contained different concentrations of proteases as indicated in the figure. Chymotrypsin liberated a p17 band that was positive for H99 antibody (directed to the caspase-14 large subunit).
KLK7 Generated an Intermediate Form from Procaspase-14
We next examined whether the chymotryptic enzyme in epidermis, KLK7, is able to generate the 17-kDa band. When procaspase-14 was incubated with a relatively low concentration of KLK7, we observed five protein bands of 20, 17, 13, 11, and 8 kDa (Fig. 2A). The 17- and 11-kDa bands may correspond to the large and small subunits of the active form of caspase-14, respectively. When a higher concentration of KLK7 was used, the 17-kDa band (p17) was predominantly produced. We were interested in the 20-kDa band (p20), because the size of the caspase-14-related fragment has not previously been observed. This protein band was digested and subjected to MALDI-TOF/MS analysis. An isolated peptide containing the C-terminal end had a molecular mass of 2804.33, which is almost identical with the theoretical mass of DSPQTIPTYTDALHVYSTVEGYIAY, 2804.99. Because KLK7 is a chymotryptic enzyme that cleaves after aromatic amino acids, we concluded that Tyr178 is the cleavage site of procaspase-14 by KLK7. Because the 8-kDa band was also produced during incubation with KLK7, this could be the corresponding small subunit of the 20-kDa band formed after the cleavage at Tyr178. The primary structure of the intermediate form is proposed to be as shown in Fig. 2B. KLK7 cleaves procaspase-14 at Tyr178 and generates an intermediate form with p20 (Met1–Tyr178) and p8 (Arg179–Gln242) subunits. Because KLK7 is a chymotryptic enzyme and its activity is restricted to cleavage after aromatic amino acids, it is unlikely that KLK7 itself can generate the mature form, because this would require cleavage at Asp146. A possible explanation is that the intermediate form may itself have caspase-like enzymatic properties.
FIGURE 2.
Chymotrypsin-like KLK7 generated p17 and p20 bands. A, Coomassie Brilliant Blue staining of procaspase-14 processing by KLK7. Procaspase-14 was incubated with different concentrations of active KLK7 for 15 min. The newly generated p20 band (boxed) was subjected to C-terminal analysis. Lane 1, procaspase-14; lane 2, procaspase-14 + active KLK7 (50 ng); lane 3, procaspase-14 + active KLK7 (250 ng); lane 4, molecular weight markers. B, comparison of p17 and p20. C-terminal sequence analysis of p20 identified Tyr178 as the cleavage site by KLK7.
Constitutively Active Intermediate Form Showed Distinct Substrate Specificity
To examine this possibility, we next prepared a constitutively active intermediate form by reversing p20 (Met1–Tyr178) and p8 (Arg179–Gln242) (Fig. 3A). We named this revC14-Y178 and examined its substrate specificity using various caspase substrates. Interestingly, WEHD-AFC, the preferred substrate for caspase-14 and -1, was least well hydrolyzed by revC14-Y178 among the substrates tested (Fig. 3B). In contrast, revC14-Y178 possessed strong hydrolytic activities toward other caspase substrates including YVAD-AFC, another caspase-1 substrate, without showing any marked preference. Furthermore, high concentrations of kosmotropic ions such as citrate ions, which are essential for mature caspase-14 activity, were not required, but even suppressed revC14-Y178 activity. We determined the Km values for representative caspase substrates, VEID-AFC, DEVD-AFC, LEVD-AFC, and LEHD-AFC, as 0.172, 0.261, 0.504, and 0.847 μm, respectively. Next we examined whether the intermediate form can generate the large subunit (p17) of mature caspase-14. When procaspase-14 was incubated with revC14-Y178, we found that p17 was produced in a time-dependent manner (Fig. 3C). Procaspase-14 or revC14-Y178 by itself did not generate the mature form for 18 h incubation. We also examined the effect of overexpression of this intermediate form. cDNA of revC14-Y178 was inserted to the pCMV-HA vector and expressed in cultured human keratinocytes. revC14-Y178-expressing cells with HA positivity were stained with h14D146 Ab, indicating generation of mature caspase-14 (Fig. 3D).
Cleavage Site-directed Antibody Confirmed Generation of the Intermediate Form and Subsequently the Mature Form
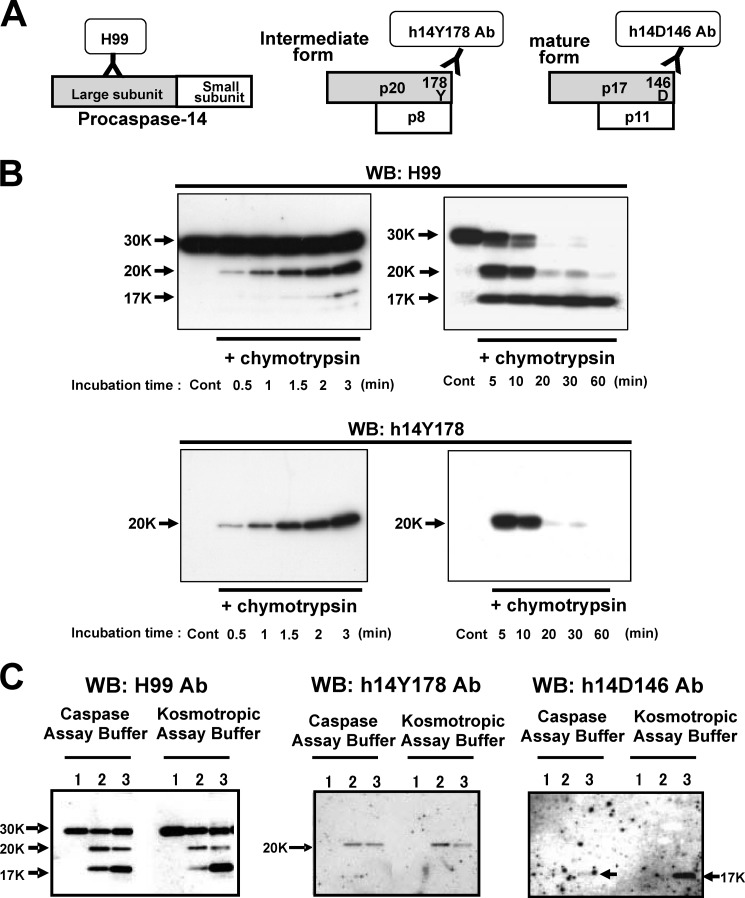
We developed an antibody directed to the cleavage site Tyr178 (h14Y178 Ab). h14Y178 Ab recognized only the p20 subunit but showed no reactivity with procaspase-14. Using various antibodies that recognize procaspase-14 and large subunit (H99), the active (mature) form (h14D146), and the intermediate form (h14Y178), we examined the activation cascade of procaspase-14 (Fig. 4A). In this experiment, we used agarose-immobilized chymotrypsin because of its easy availability and ability to remove activated enzyme from the assay mixture. Time course analysis clearly demonstrated that generation of the 20-kDa fragment occurred first at the very early time points (detected with h14Y178 Ab), and that the 17-kDa band subsequently increased during prolonged incubation (Fig. 4B). These results indicate that the intermediate form is generated at the early stage of procaspase-14 activation. Because activation of caspase-14 requires high concentrations of kosmotropic ions, we also tested the effect of kosmotropic ion on the maturation process. Chymotrypsin-treated procaspase-14 was isolated and further incubated with procaspase-14 with or without 1.2 m sodium citrate. The p20 and p17 bands were produced under both conditions (Fig. 4C). We also confirmed that p17 is recognized by h14D146 Ab, although the band was less clear in normal assay buffer than in the kosmotropic buffer, probably because mature caspase-14 is more stable in the kosmotropic buffer.
FIGURE 4.
Western blot analysis with cleavage site-specific antibodies. A, schematic illustration of antibodies used in this study. H99 is directed to the large subunit, and thus also recognizes procaspase-14. h14Y178 Ab is directed to the KLK7 cleavage site, Tyr178, and detects only p20, the intermediate form. h14D146 Ab is a cleavage site-directed antibody that recognized only p17, the mature form of the large subunit. B, Western blot (WB) analysis to detect generation of the intermediate form by chymotrypsin. Procaspase-14 was incubated with chymotrypsin for, 30 s to 3 min (left panels) and 5 to 60 min (right panels). The upper panels show H99-stained degradation products. Lower panels indicate generation of the intermediate form (p20) detected with h14Y178 Ab. The proform (p30) decreases rapidly after 5 min upon incubation with chymotrypsin. p20 is generated at 30 s, gradually increased up to 5 min and decreased thereafter. In contrast, p17, the mature form of large subunit, increased with time. C, effect of kosmotropic ion on procaspase-14 processing. Procaspase-14 was incubated with chymotrypsin with or without 1.3 m sodium citrate, a prerequisite ion for caspase-14 activation. Procaspase-14 was incubated with chymotrypsin-agarose and supernatant was added to the assay mixture containing procaspase-14 with or without 1.3 m sodium citrate. Processed fragments were verified with H99, h14Y178, and h14D146 antibodies. Lane 1, procaspase-14; lane 2, procaspase-14 + chymotrypsin-treated procaspase-14 (×1/10); lane 3, procaspase-14 + chymotrypsin-treated procaspase-14 (×1).
Immunohistochemical Study Showed That KLK7 Generated the Intermediate Form and the Mature Form in Cultured Keratinocytes
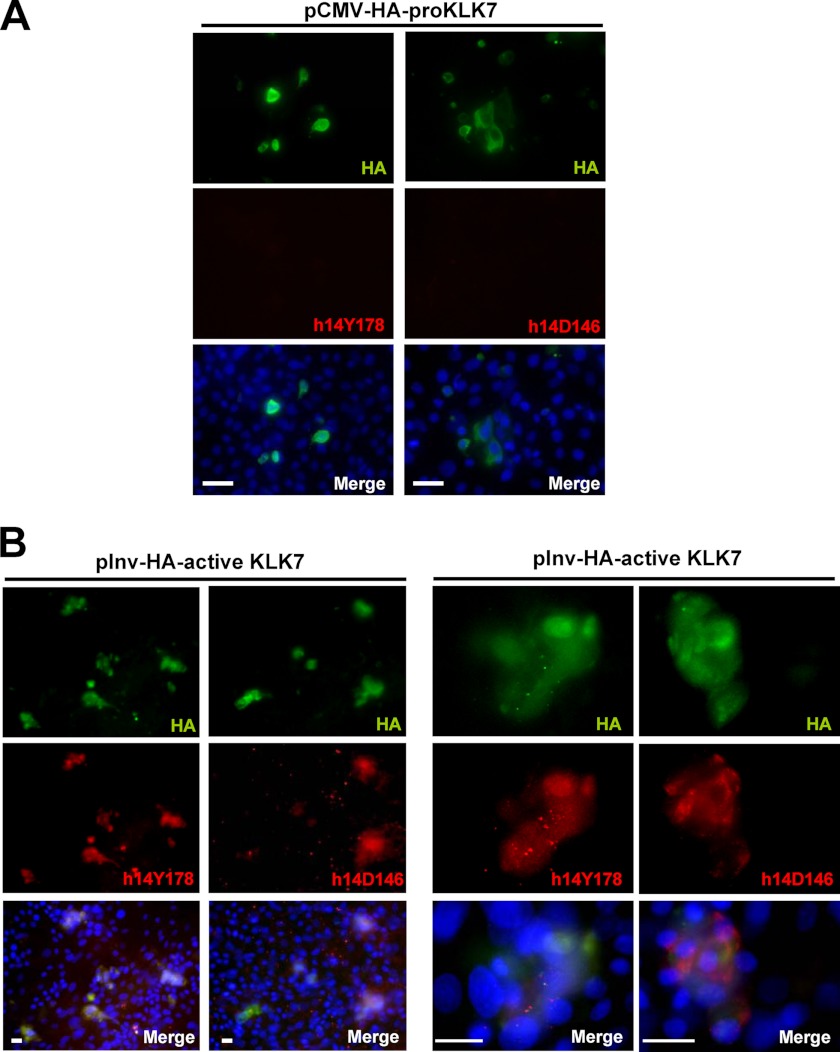
We prepared an expression vector for constitutively active KLK7 and expressed it in human keratinocytes. Because caspase-14 is strongly up-regulated after confluence, we examined the processing of these forms of caspase-14 under this condition. The proform or active form of the KLK7 construct with an HA tag was introduced into keratinocytes and 2 days after confluence, cells were immunostained with anti-HA, h14Y178 Ab, or h14D146 Ab (Fig. 5). Overexpression of pro-KLK7 did not induce production of either the intermediate form or the mature form as judged with h14Y178 Ab or h14D146 Ab. In contrast, active KLK7-transfected cells revealed that HA-positive cells were h14Y178-positive, and most of the HA-positive cells also showed positive staining for h14D146. These results indicate that KLK7 generates the intermediate form and it proceeds to the mature form production in the cell level, as well.
FIGURE 5.
KLK7 generates the intermediate form, followed by appearance of the mature form of caspase-14 in keratinocytes. A, normal human keratinocytes transfected with pCMV-HA-pro-KLK7 were stained with h14Y178 and h14D146 antibodies. B, keratinocytes transfected with pInv-HA-active KLK7 were stained with anti-HA, h14Y178, and h14D146 antibodies. Enlarged figures were also shown. Scale bars, 100 μm.
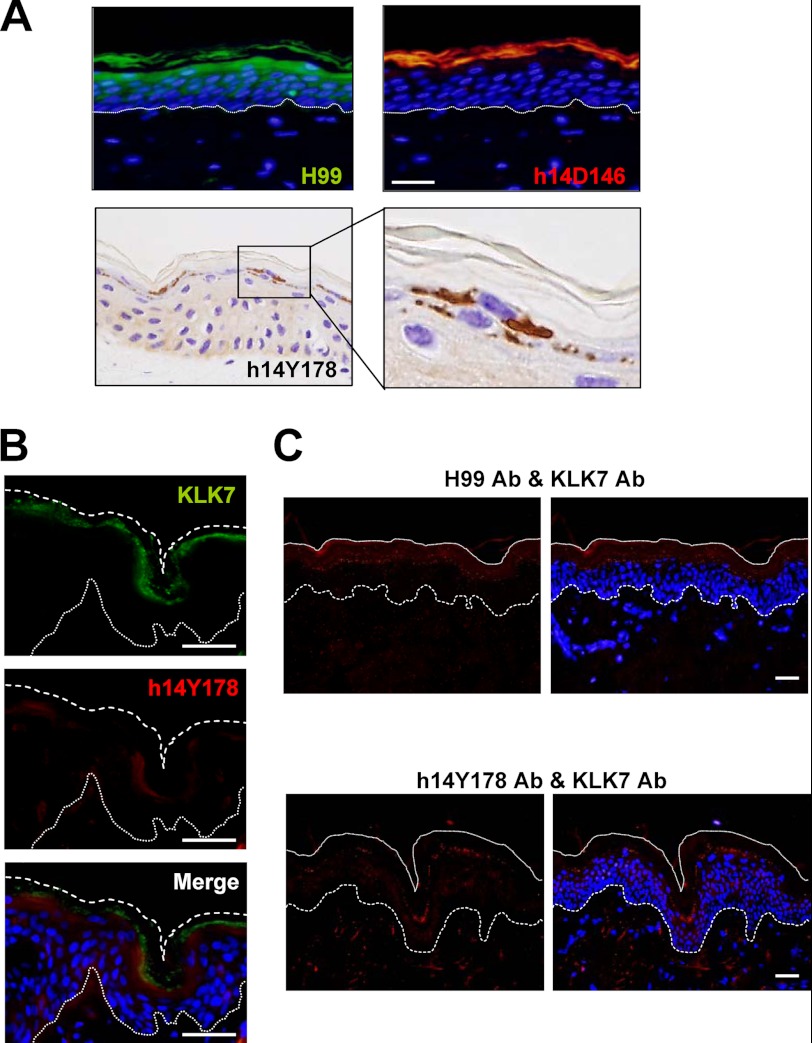
The Intermediate Form Is Present at the Boundary Area and Interacts with KLK7
Last we investigated the localization of the intermediate form in normal human epidermis, compared with that of the proform and the mature form (Fig. 6A). h14Y178 Ab staining was confined to the cytoplasm of granular cells just below the SC. The mature form stained with h14D146 Ab was localized in the SC, whereas the staining of the proform started from the upper spinous cells, as previously reported (12). Transient appearance of the intermediate form just prior to corneocyte formation strongly supports the involvement of the proposed activation cascade of procaspase-14 in vivo. We also investigated dual staining for KLK7 and the intermediate form. KLK7 was co-localized with the intermediate form at the granular layer, although it showed a broad distribution in the cornified layer as well (Fig. 6B). Furthermore, PLA demonstrated a close association between procaspase-14 and KLK7, as well as between the intermediate form and KLK7 in vivo (Fig. 6C). Strongly confined and punctate signals were detected for these pairs at the boundary area between the SG and SC, where the maturation of procaspase-14 took place.
FIGURE 6.
The intermediate form is localized in the upper granular cells and is associated with KLK7 in normal human epidermis. A, localization of procaspase-14 (green fluorescence), mature caspase-14 (red fluorescence) and the intermediate form (brown color) in normal human epidermis. B, dual staining for KLK7 and the intermediate form. They are colocalized in the granular layer. The merged figure is also shown. C, association of KLK7 and the intermediate form. The PLA method indicated that KLK7 and procaspase-14, as well as KLK7 and the intermediate form, are associated together in the granular to cornified layer in normal human epidermis. Scale bars, 50 μm.
DISCUSSION
Our hypothesis that differentiation-related proteases could be involved in the activation of caspase-14 prompted us to investigate the activity toward procaspase-14 of representative serine and cysteine proteases expressed in the upper epidermis. Our results indicate the occurrence of a unique maturation cascade of caspase-14, in which chymotrypsin-like enzyme KLK7 generates an intermediate form, which in turn generates the mature form.
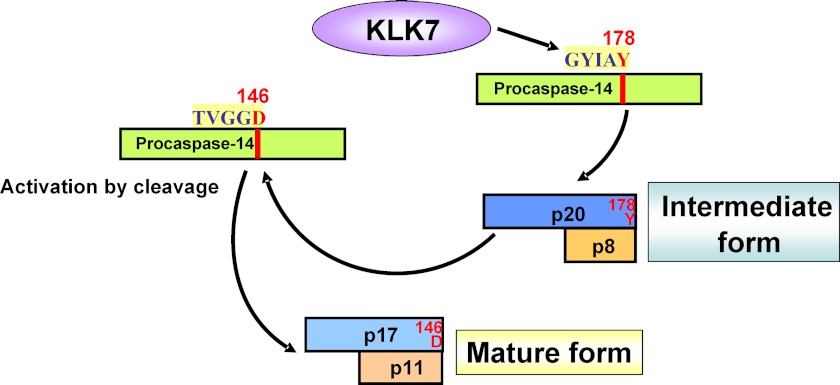
This kind of mechanism is unique among caspases, for which the activation process is usually initiated by apoptosome, death-inducing signaling complex, or inflammasome (3, 4). We confirmed the formation of the intermediate form by raising a cleavage site-directed antibody, h14Y178 Ab. This antibody was prepared in the same manner as h14D146, an antibody specific to the active, mature form of caspase-14 (12). h14Y178 Ab specifically recognizes p20, the large subunit of the intermediate form, and does not react with procaspase-14. Using h14Y178 and h14D146 antibodies, we successfully followed the generation of the intermediate and mature forms from procaspase-14 after incubation with KLK7 and chymotrypsin. Furthermore, in normal human epidermis, staining for the intermediate form was clearly confined to the transitional area from SG to SC, just where localization of the mature form begins. Thus the life cycle of caspase-14 can be interpreted as follows. Synthesis of procaspase-14 is initiated at the stratum spinosum and peaks at the SG. Prepro-KLK7 is synthesized similarly, and just prior to the terminal differentiation, activated KLK7 cleaves procaspase-14 at Tyr178, transiently generating the intermediate form (p20/p8). This form in turn has the ability to generate the mature form, p17/p11 from procaspase-14 (Fig. 7).
FIGURE 7.
Summary of proposed scheme of caspase-14 maturation. During the maturation cascade of caspase-14, chymotrypsin-like KLK7 plays a critical role. KLK7 cleaves procaspase-14 after Tyr178 and produces an intermediate form (p20/p8). This intermediate form acts on procaspase-14 to generate the mature form of caspase-14, p17/p11.
We also developed a constitutively active intermediate form, revC14-Y178, based on the strategy used in our previous study. Interestingly, revC14-Y178 showed a different substrate specificity from active caspase-14, and this seems consistent with the idea that the physiological role of the intermediate form is distinct from that of mature caspase-14 (p17/p11).
It was recently reported that caspase-14 knock-out mice showed defects in filaggrin degradation and impaired barrier function as judged from higher values of trans-epidermal water loss (9). Filaggrin is a source of NMFs, which are essential for skin hydration and barrier function (19, 20). Genetic studies have shown that loss-of-function mutations in FLG, the human gene encoding profilaggrin and filaggrin, are a major risk factor for the development of atopic dermatitis (AD) (21, 22). It was proposed that caspase-14 is involved in the first step of the filaggrin degradation pathway (16), and this is supported by the fact that filaggrin has two sites susceptible to cleavage by caspase-14 (23). Generation of NMFs could be accomplished by further digestion with calpain I, followed by digestion to free amino acids by a neutral cysteine protease, bleomycin hydrolase (19). Thus, defects in the procaspase-14 maturation pathway are likely to result in down-regulation of NMF production.
Many KLKs are expressed in human epidermis and some have been suggested to be involved in desquamation (24–26). However, the physiological roles of KLKs are not likely to be limited to degradation of corneodesmosomes in the epidermis, as various roles have been suggested in other tissues and in cancerous conditions (18, 27). KLKs are synthesized as prepro forms and processed to still inactive pro forms. Although pro-KLKs are secreted into lamellar granules and further into the intercellular space of the SC (26), this might not be the case for all the KLKs. Indeed, presence of a PI-6·KLK8 complex has been reported in the cytoplasm of differentiated keratinocyte (28, 29). Furthermore, pro-KLK8 is activated by the cleavage at Lys32 (28). They also suggested Lys-specific enterokinase could be involved in this process, because it is expressed at the SG/SC boundary area (30). This may indicate that activation of pro-KLK8 occurs in the cytoplasm and its activity needs to be regulated inside the cell. To examine the site for procaspase-14 processing, we tried DAB staining, fluorescence staining, and PLA. DAB staining with higher magnification showed more precisely that the intermediate form localized in the cytoplasm of granular cells, although KLK7 showed broader distribution up to the cornified layer. The PLA method further supported that strong positive signals between the intermediate form and KLK7 were evident at the granular layer. All of these data suggest that the intermediate form is generated at the granular layer by interaction with KLK7. Because caspase-14 is found ultrastructurally in nuclei and keratohyalin granules of the granular cells with increasing intensity toward the transitional layer (31), our results strongly indicate at least partial cytoplasmic localization of KLK7 in SG. In addition, we prepared cultured keratinocytes constitutively expressing active KLK7 after confluence. Expression of KLK7 in the cytoplasm was confirmed, and these cells became h14Y178-positive and h14D146-positive, indicating generation of both the intermediate form and the mature form of caspase-14, respectively. These lines of evidence suggest that KLK7 is a physiological activator of caspase-14.
In some skin diseases such as AD, Netherton syndrome, and psoriasis (32–34), KLKs including KLK7 are up-regulated in the lesional epidermis. On the other hand, quantification of total caspase-14 and active caspase-14 in AD skin in our previous work clearly showed marked decreases of procaspase-14 and active caspase-14 in extracts from both nonlesional and lesional skin (35). Active caspase-14 levels were down-regulated to less than 1/10 of the levels in the lesional extracts. Overproduction of active KLK7 is likely to induce excessive synthesis of the intermediate form. This may result in shortage of procaspase-14 and a decrease of mature caspase-14. Indeed, our preliminary study showed a broad distribution of the intermediate form from stratum spinosum to SG and disappearance of the mature form in SC of lesional skin with AD and psoriasis. We still do not know whether the intermediate form possesses other physiological functions in addition to caspase-14 processing. Whatever the case, impairment of the caspase-14 processing pathway is expected to be associated with abnormal differentiation. Thus, in summary, we have shown here that the activation of procaspase-14 to caspase-14, which is associated with terminal differentiation of keratinocytes, is mediated by KLK7 and an intermediate form of caspase-14, and is different from the activation mechanisms of all other known members of the caspase family.
Footnotes
- SC
- stratum corneum
- SG
- stratum granulosum
- AD
- atopic dermatitis
- AFC
- 7-amino-4-trifluoromethylcoumarin
- KLK5
- kallikrein-related peptidase-5
- KLK7
- kallikrein-related peptidase-7
- NMF
- natural moisturizing factor
- PLA
- proximity ligation assay
- Ni-NTA
- nickel-nitrilotriacetic acid.
REFERENCES
- 1. Miller D. K. (1997) The role of the caspase family of cysteine proteases in apoptosis. Semin. Immunol. 9, 35–49 [DOI] [PubMed] [Google Scholar]
- 2. Kumar S. (2007) Caspase function in programmed cell death. Cell Death Differ. 14, 32–43 [DOI] [PubMed] [Google Scholar]
- 3. Lamkanfi M., Festjens N., Declercq W., Vanden Berghe T., Vandenabeele P. (2007) Caspases in cell survival, proliferation, and differentiation. Cell Death Differ. 14, 44–55 [DOI] [PubMed] [Google Scholar]
- 4. Xu G., Shi Y. (2007) Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res. 17, 759–771 [DOI] [PubMed] [Google Scholar]
- 5. Franchi L., Eigenbrod T., Muñoz-Planillo R., Nuñez G. (2009) The inflammasome. A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10, 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pop C., Salvesen G. S. (2009) Human caspases. Activation, specificity, and regulation. J. Biol. Chem. 284, 21777–21781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van de Craen M., Van Loo G., Pype S., Van Criekinge W., Van den brande I., Molemans F., Fiers W., Declercq W., Vandenabeele P. (1998) Identification of a new caspase homologue. Caspase-14. Cell Death Differ. 10, 838–846 [DOI] [PubMed] [Google Scholar]
- 8. Eckhart L., Ban J., Fischer H., Tschachler E. (2000) Caspase-14. Analysis of gene structure and mRNA expression during keratinocyte differentiation. Biochem. Biophys. Res. Commun. 277, 655–659 [DOI] [PubMed] [Google Scholar]
- 9. Denecker G., Hoste E., Gilbert B., Hochepied T., Ovaere P., Lippens S., Van den Broecke C., Van Damme P., D'Herde K., Hachem J. P., Borgonie G., Presland R. B., Schoonjans L., Libert C., Vandekerckhove J., Gevaert K., Vandenabeele P., Declercq W. (2007) Caspase-14 protects against epidermal UVB photodamage and water loss. Nat. Cell Biol. 9, 666–674 [DOI] [PubMed] [Google Scholar]
- 10. Eckhart L., Declercq W., Ban J., Rendl M., Lengauer B., Mayer C., Lippens S., Vandenabeele P., Tschachler E. (2000) Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J. Invest. Dermatol. 115, 1148–1151 [DOI] [PubMed] [Google Scholar]
- 11. Nicotera P., Melino G. (2007) Caspase-14 and epidermis maturation. Nat. Cell Biol. 9, 621–622 [DOI] [PubMed] [Google Scholar]
- 12. Hibino T., Fujita E., Tsuji Y., Nakanishi J., Iwaki H., Katagiri C., Momoi T. (2010) Purification and characterization of active caspase-14 from human epidermis and development of the cleavage site-directed antibody. J. Cell. Biochem. 109, 487–497 [DOI] [PubMed] [Google Scholar]
- 13. Mikolajczyk J., Scott F. L., Krajewski S., Sutherlin D. P., Salvesen G. S. (2004) Activation and substrate specificity of caspase-14. Biochemistry 43, 10560–10569 [DOI] [PubMed] [Google Scholar]
- 14. Brattsand M., Stefansson K., Lundh C., Haasum Y., Egelrud T. (2005) A proteolytic cascade of kallikreins in the stratum corneum. J. Invest. Dermatol. 124, 198–203 [DOI] [PubMed] [Google Scholar]
- 15. Sales K. U., Masedunskas A., Bey A. L., Rasmussen A. L., Weigert R., List K., Szabo R., Overbeek P. A., Bugge T. H. (2010) Matriptase initiates activation of epidermal pro-kallikrein and disease onset in a mouse model of Netherton syndrome. Nat. Genet. 42, 676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Komatsu N., Takata M., Otsuki N., Toyama T., Ohka R., Takehara K., Saijoh K. (2003) Expression and localization of tissue kallikrein mRNAs in human epidermis and appendages. J. Invest. Dermatol. 121, 542–549 [DOI] [PubMed] [Google Scholar]
- 17. Egelrud T., Brattsand M., Kreutzmann P., Walden M., Vitzithum K., Marx U. C., Forssmann W. G., Mägert H. J. (2005) hK5 and hK7, two serine proteinases abundant in human skin, are inhibited by LEKTI domain 6. Br. J. Dermatol. 153, 1200–1203 [DOI] [PubMed] [Google Scholar]
- 18. Sotiropoulou G., Pampalakis G., Diamandis E. P. (2009) Functional roles of human kallikrein-related peptidases. J. Biol. Chem. 284, 32989–32994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamata Y., Taniguchi A., Yamamoto M., Nomura J., Ishihara K., Takahara H., Hibino T., Takeda A. (2009) Neutral cysteine protease bleomycin hydrolase is essential for the breakdown of deiminated filaggrin into amino acids. J. Biol. Chem. 284, 12829–12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horii I., Nakayama Y., Obata M., Tagami H. (1989) Stratum corneum hydration and amino acid content in xerotic skin. Br. J. Dermatol. 121, 587–592 [DOI] [PubMed] [Google Scholar]
- 21. O'Regan G. M., Sandilands A., McLean W. H., Irvine A. D. (2008) Filaggrin in atopic dermatitis. J. Allergy Clin. Immunol. 122, 689–693 [DOI] [PubMed] [Google Scholar]
- 22. Palmer C. N., Irvine A. D., Terron-Kwiatkowski A., Zhao Y., Liao H., Lee S. P., Goudie D. R., Sandilands A., Campbell L. E., Smith F. J., O'Regan G. M., Watson R. M., Cecil J. E., Bale S. J., Compton J. G., DiGiovanna J. J., Fleckman P., Lewis-Jones S., Arseculeratne G., Sergeant A., Munro C. S., El Houate B., McElreavey K., Halkjaer L. B., Bisgaard H., Mukhopadhyay S., McLean W. H. (2006) Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 38, 441–446 [DOI] [PubMed] [Google Scholar]
- 23. Hoste E., Kemperman P., Devos M., Denecker G., Kezic S., Yau N., Gilbert B., Lippens S., De Groote P., Roelandt R., Van Damme P., Gevaert K., Presland R. B., Takahara H., Puppels G., Caspers P., Vandenabeele P., Declercq W. (2011) Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J. Invest. Dermatol. 131, 2233–2241 [DOI] [PubMed] [Google Scholar]
- 24. Jiang R., Shi Z., Johnson J. J., Liu Y., Stack M. S. (2011) Kallikrein-5 promotes cleavage of desmoglein-1 and loss of cell-cell cohesion in oral squamous cell carcinoma. J. Biol. Chem. 286, 9127–9135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borgoño C. A., Michael I. P., Komatsu N., Jayakumar A., Kapadia R., Clayman G. L., Sotiropoulou G., Diamandis E. P. (2007) A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J. Biol. Chem. 282, 3640–3652 [DOI] [PubMed] [Google Scholar]
- 26. Ishida-Yamamoto A., Kishibe M. (2011) Involvement of corneodesmosome degradation and lamellar granule transportation in the desquamation process. Med. Mol. Morphol. 44, 1–6 [DOI] [PubMed] [Google Scholar]
- 27. Sotiropoulou G., Pampalakis G. (2010) Kallikrein-related peptidases. Bridges between immune functions and extracellular matrix degradation. Biol. Chem. 391, 321–331 [DOI] [PubMed] [Google Scholar]
- 28. Eissa A., Amodeo V., Smith C. R., Diamandis E. P. (2011) Kallikrein-related peptidase-8 (KLK8) is an active serine protease in human epidermis and sweat and is involved in a skin barrier proteolytic cascade. J. Biol. Chem. 286, 687–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scott F. L., Sun J., Whisstock J. C., Kato K., Bird P. I. (2007) SerpinB6 is an inhibitor of kallikrein-8 in keratinocytes. J. Biochem. 142, 435–442 [DOI] [PubMed] [Google Scholar]
- 30. Nakanishi J., Yamamoto M., Koyama J., Sato J., Hibino T. (2010) Keratinocytes synthesize enteropeptidase and multiple forms of trypsinogen during terminal differentiation. J. Invest. Dermatol. 130, 944–952 [DOI] [PubMed] [Google Scholar]
- 31. Alibardi L., Dockal M., Reinisch C., Tschachler E., Eckhart L. (2004) Ultrastructural localization of caspase-14 in human epidermis. J. Histochem. Cytochem. 52, 1561–1574 [DOI] [PubMed] [Google Scholar]
- 32. Voegeli R., Rawlings A. V., Breternitz M., Doppler S., Schreier T., Fluhr J. W. (2009) Increased stratum corneum serine protease activity in acute eczematous atopic skin. Br. J. Dermatol. 161, 70–77 [DOI] [PubMed] [Google Scholar]
- 33. Simon M., Tazi-Ahnini R., Jonca N., Caubet C., Cork M. J., Serre G. (2008) Alterations in the desquamation-related proteolytic cleavage of corneodesmosin and other corneodesmosomal proteins in psoriatic lesional epidermis. Br. J. Dermatol. 159, 77–85 [DOI] [PubMed] [Google Scholar]
- 34. Komatsu N., Saijoh K., Kuk C., Liu A. C., Khan S., Shirasaki F., Takehara K., Diamandis E. P. (2007) Human tissue kallikrein expression in the stratum corneum and serum of atopic dermatitis patients. Exp. Dermatol. 16, 513–519 [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto M., Kamata Y., Iida T., Fukushima H., Nomura J., Saito M., Tajima M., Okubo Y., Momoi T., Tsuboi R., Hibino T. (2011) Quantification of activated and total caspase-14 with newly developed ELISA systems in normal and atopic skin. J. Dermatol. Sci. 61, 110–117 [DOI] [PubMed] [Google Scholar]
- 36. Kourku Y., Urase K., Fujita E., Isahara K., Ohsawa Y., Uchiyama Y., Momoi M. Y., Momoi T. (1998) Detection of activated caspase-3 by a cleavage site-directed antiserum during naturally occurring DRG neurons apoptosis. Biochem. Biophys. Res. Commun. 247, 780–784 [DOI] [PubMed] [Google Scholar]
- 37. Carroll J. M., Albers K. M., Garlick J. A., Harrington R., Taichman L. B. (1993) Tissue- and stratum-specific expression of the human involucrin promoter in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 90, 10270–10274 [DOI] [PMC free article] [PubMed] [Google Scholar]