Background: Mitochondrial proteins are covalently modified with bioactive lipids (carbonylation) resulting in reduced metabolic function.
Results: iTRAQ-based proteomics identified the phosphate carrier and subunits of Complex I as critical carbonylation targets.
Conclusion: Oxidative stress leads to mitochondrial dysfunction through targeted lipid modification of critical proteins involved in phosphate and electron transport.
Significance: Identification of carbonylation targets enables evaluation of specific protein modification on mitochondrial function.
Keywords: Electron Transport System (ETS), Metabolic Regulation, Metabolism, Mitochondria, Oxidative Stress, Protein Carbonylation
Abstract
Carbonylation is the covalent, non-reversible modification of the side chains of cysteine, histidine, and lysine residues by lipid peroxidation end products such as 4-hydroxy- and 4-oxononenal. In adipose tissue the effects of such modifications are associated with increased oxidative stress and metabolic dysregulation centered on mitochondrial energy metabolism. To address the role of protein carbonylation in the pathogenesis of mitochondrial dysfunction, quantitative proteomics was employed to identify specific targets of carbonylation in GSTA4-silenced or overexpressing 3T3-L1 adipocytes. GSTA4-silenced adipocytes displayed elevated carbonylation of several key mitochondrial proteins including the phosphate carrier protein, NADH dehydrogenase 1α subcomplexes 2 and 3, translocase of inner mitochondrial membrane 50, and valyl-tRNA synthetase. Elevated protein carbonylation is accompanied by diminished complex I activity, impaired respiration, increased superoxide production, and a reduction in membrane potential without changes in mitochondrial number, area, or density. Silencing of the phosphate carrier or NADH dehydrogenase 1α subcomplexes 2 or 3 in 3T3-L1 cells results in decreased basal and maximal respiration. These results suggest that protein carbonylation plays a major instigating role in cytokine-dependent mitochondrial dysfunction and may be linked to the development of insulin resistance in the adipocyte.
Introduction
Obesity is a positive risk factor for the development of insulin resistance and diabetes (1). Increased adiposity is often associated with chronic inflammation, altered glucose, and lipid metabolism and oxidative stress (2). Inflammatory cytokines such as tumor necrosis factor α (TNFα) and interleukin-6 (IL-6) are produced by inflammatory macrophages that infiltrate adipose tissue and are elevated in the obese, insulin-resistant state. Increased oxidative stress and the production of reactive oxygen species (ROS)3 have been hypothesized as a mechanism linking mitochondrial dysfunction to insulin resistance (3). Although the molecular details of such signaling remain enigmatic, a number of studies have suggested that increased ROS leads to the oxidation of thioredoxin resulting in the release and activation of ASK1. Activated ASK1 initiates a cascade of phosphorylation events culminating in the activation of Jnk (4, 5) and the NF-κB pathway as well as serine phosphorylation of IRS1. Countering the concept that mitochondrial dysfunction is linked to insulin resistance is the finding that loss of mitochondrial membrane potential and ATP synthesis capacity frequently are linked to the phosphorylation of AMP-activated protein kinase, enhanced glucose and lipid oxidation, and improvement of insulin sensitivity (6).
The mitochondrion is a major source of reactive oxygen species. The transfer of electrons to molecular oxygen from the electron transport chain at sites other than Complex IV produces superoxide anion as a primary ROS species. The antioxidant enzyme manganese superoxide dismutase is abundantly expressed in mitochondria and efficiently metabolizes superoxide to hydrogen peroxide that is further reduced by enzymes such as catalase, glutathione peroxidase, and peroxiredoxin. However, in the presence of ferrous iron, hydrogen peroxide will oxidize to form hydroxide and the hydroxyl radical. The hydroxyl radical initiates peroxidation of polyunsaturated acyl chains of glycerophospholipids and triacylglycerol, leading to the production of α,β-unsaturated aldehydes such as 4-hydroxy-trans-2,3-nonenal (4-HNE) and 4-oxo-trans-2,3-nonenal that are highly reactive toward nucleophilic addition (7). These reactive lipid species can be metabolized through oxidative- or reductive enzyme-catalyzed reactions, but the predominant detoxification route is glutathionylation via glutathione S-transferase A4 (GSTA4) and subsequent cellular export of the glutathionylated lipid (8, 9). If the aldehyde escapes detoxification, it can react with the protein side chains of cysteine, histidine, and lysine residues, in a non-reversible reaction termed protein carbonylation (10).
Inflammatory cytokines down-regulate GSTA4 expression in cultured white adipocytes, and GSTA4 expression is similarly down-regulated in vivo in white adipose tissue of obese insulin-resistant C57Bl/6J mice. The down-regulation of GSTA4 is specific for white fat (visceral and subcutaneous depots) and does not occur in brown fat, liver, or muscle (11, 12). Our previous studies indicated that the level of protein carbonylation is elevated in the obese adipocyte and leads to the disruption of a number of metabolic pathways including β-oxidation, electron transport, and the citric acid cycle.
Despite the wide body of information concerning the metabolic effects of protein carbonylation in the adipocyte mitochondrion, the identification of mitochondrial targets has not been carried out. To that end, the current study was initiated to characterize mitochondrial targets of protein carbonylation and investigate how their protein function is altered. To specifically focus on protein carbonylation events linked to regulation of GSTA4, we generated GSTA4-silenced and over-expressing 3T3-L1 adipocyte cell lines and evaluated protein carbonylation via proteomic methods. We report here the identification of several differentially carbonylated mitochondrial proteins and evaluate the potential impact of such modifications on mitochondrial function.
EXPERIMENTAL PROCEDURES
Materials
The pcDNA plasmid containing aP2 promoter and intron/poly(A) was kindly provided by Dr. Ormond MacDougald (University of Michigan). Rabbit monoclonal anti-hemagglutinin (HA) antibody was obtained from Cell Signaling Technologies (Danvers, MA), anti-β-actin mouse monoclonal and PiC (SLC25A3) mouse polyclonal antibodies were obtained from Sigma, and NDUFA3 mouse polyclonal antibody was obtained from Abcam (Cambridge, MA). IRDye secondary antibodies used for immunoblotting were obtained from Odessey Imaging System (LiCor Bioscience, Lincoln, NE). Plasmids for NDUFA3 (TRCN0000041529; CCGGTGTGAGAGATGACGGGAACATCTCGAGATGTTCCCGTCATCTCTCACATTTTTG), NDUFA2 (TRCN0000041823; CCGGCGTGAGATTCGCGTTCACTTACTCGAGTAAGTGAACGCGAATCTCACGTTTTTG), SLC25A3 (TRCN0000070020; CCGGGCAACATACTTGGTGAGGAAACTCGAGTTTCCTCACCAAGTATGTTGCTTTTTG), and green fluorescent protein (RHS4459; TACAACAGCCACAACGTCTAT) used to generate lentiviruses expressing shRNA against specific targets were provided by the Biomedical Genomics Center (University of Minnesota, Minneapolis, MN). Other commercial materials were of the highest available quality.
Cell Culture
3T3-L1 cells were differentiated for 8 days using the standard methylisobutyl xanthine, dexamethasone, insulin protocol (13) supplemented with 1 μg/ml troglitazone. GSTA4-silenced (Kd) and scrambled (Scr) control adipocytes were generated as described previously (11). The extent of differentiation was identical between cell lines as evaluated by assessing lipid accumulation and the expression of adipocyte marker proteins including the adipocyte fatty acid binding protein. The aP2-HA-GSTA4 overexpressing cells were generated by ligating the 8.2-kb mouse aP2/FABP4 promoter sequence upstream of the GSTA4 coding region containing an N terminal HA tag followed by the rabbit β-globin intron/poly(A) signal. The construct in pcDNA3.1 or pcDNA3.1 alone was transfected into 3T3-L1 fibroblasts using Effectene transfection reagent (Qiagen, Valencia, CA) according to manufacturer's instructions, and pooled cells were selected for stable incorporation using 400 μg/ml Geneticin (Invitrogen) for 8 days. The culture medium was supplemented with 1 μg/ml blasticidin for GSTA4 Kd and Scr cells or 400 μg/ml Geneticin for aP2-HA-GSTA4 and pcDNA cells. NDUFA2- and NDUFA3- and SLC25A3-silenced cell lines were generated by transducing 3T3-L1 fibroblasts with lentiviral vectors as described previously (7) using 2 μg/ml puromycin for selection. A GFP plasmid was transduced in parallel for use as a transfection control.
mRNA Measurements
Adipocytes were lysed in TRIzol (Invitrogen), and RNA was isolated according to manufacturer's protocol. cDNA was prepared using iScript cDNA Synthesis kit (Bio-Rad), and relative mRNA expression was measured by real-time PCR SYBR Green Detection using the My iQ iCycler (Bio-Rad). mRNA expression was normalized to TFIIE and reported relative to control groups using the ΔΔCt method (14).
Enrichment of Mitochondrial Protein Carbonyls with Biotin Hydrazide Conjugation and Avidin Affinity Chromatography
Crude mitochondria containing endoplasmic reticulum fragments were isolated in triplicate from each of the four 3T3-L1 adipocyte cell lines (GSTA4 Kd and control, aP2-HA-GSTA4 and control) by differential centrifugation (15). Mitochondrial pellets were dissolved in biotin hydrazide coupling buffer (100 mm sodium acetate, 20 mm NaCl, 1% SDS, pH 5.5) and centrifuged at 13,000 × g for 10 min at 15 °C. Mitochondria from three independent experiments were pooled and modified with biotin hydrazide. Biotin hydrazide (5 mm, Pierce) was added to 5 mg of mitochondrial proteins for 2 h at room temperature to specifically modify aldehyde groups. Derivatized protein was diluted 1:10 in ice-cold phosphate-buffered saline (PBS) and dialyzed against the same buffer at 4 °C to remove excess biotin hydrazide. Avidin affinity columns (monomeric avidin agarose, Pierce) were washed with PBS, and 2 mm d-biotin was added to block non-reversible biotin binding sites. Excess biotin was removed with the addition of 0.1 m glycine, pH 2.8, and the column was re-equilibrated into PBS. Samples were applied, the columns were extensively washed, and the bound biotinylated proteins eluted with 3 ml of 0.1 m glycine, pH 2.8. The eluted proteins were neutralized with the addition of 750 μl of 2.5 m Tris, pH 8.5, and stored at −20 °C until further processed.
iTRAQTM Peptide Labeling and Nanoflow LC-MS/MS
100 μg of affinity-captured biotinylated protein was denatured with SDS to 0.5% and reduced with 5 mm tris(2-carboxyethyl)phosphine, and free sulfhydryls were modified with 20 mm methylmethane thiosulfonate. The solution was diluted 10-fold with buffer containing 1 mm CaCl2, and proteins were digested overnight at 37 °C with 2 μg of trypsin (Promega, Madison, WI). The resultant peptides were acidified with formic acid and purified by solid phase extraction using 3 ml MCX cartridges (Waters, Milford, MA).
For proteomic analysis, peptides were reconstituted in iTRAQTM dissolution buffer and labeled with isobaric iTRAQTM tags (Applied Biosystems, Carlsbad, CA) according to the manufacturer's protocol. After labeling, the four samples were combined, repurified with MCX cartridges, and further fractionated with strong cation exchange HPLC as described (16). Strong cation exchange fractions having an average absorbance of at least 10 milliunits at 215 nm were selected (18 total) and processed for mass spectrometry.
Reversed-phase nanoHPLC and mass spectrometry was performed as previously described (16). Briefly, fragmentation was performed in optimized PQD (pulsed Q collision-induced dissociation) mode (17) with a normalized fragmentation energy of 31 and an activation time of 100 ms. Tandem mass spectra were acquired with 2 microscans with automatic gain control settings of 30,000 charges or 100 ms. Raw spectral data were extracted and converted to mzXML format using MSconvert from ProteoWizard. Data were evaluated using SEQUEST v27.0 against a concatenated forward and reversed mouse database (derived from UniProt mouse canonical and isoform from August 2010) appended with common contaminants from the Global Proteome Machine totaling 47,932 entries. Precursor and fragment ion tolerances were set at 1 and 0.8 atomic mass units. Semi-tryptic specificity was used with up to two missed cleavage sites. A fixed modification of 144.102 atomic mass units was set for Lys and peptide N termini. Variable modifications included 15.9949 on Met and 394.2039 (4-HNE modification) on Cys, His, and Lys. Candidate identifications were organized, and peptide probabilities (18) were calculated using Scaffold v3 (Proteome software). Protein identifications were filtered to a <1% false discovery rate by using the following filter criteria: 10 ppm precursor mass tolerance, >5% peptide probability, and at least 2 unique peptides per protein identification. Relative iTRAQTM reporter ion ratios, and p values were calculated for proteins with three or more matched spectra using LTQ-iQuant software (19). For comparison of experimental samples, GSTA4 Kd and aP2-HA-GSTA4 data were normalized to respective controls. Protein subcellular localization was determined with GO terms using Uniprot and verified manually.
Cellular Respiration
Cellular respiration was analyzed in situ with the XF24 Extracellular Flux Analyzer (Seahorse Biosciences, Billerica, MA). 3T3-L1 fibroblasts were plated on V7 microplates coated with 0.2% gelatin and differentiated into adipocytes. Eight days post-differentiation, cells were washed and incubated at 37 °C without CO2 in bicarbonate-free DMEM containing 1.0 mm sodium pyruvate, 2.0 mm glutamax, and 25 mm d-glucose. SLC25A3-, NDUFA2-, and NDUFA3-silenced cells were analyzed as preadipocytes. During the assay, cell monolayers were exposed to 1 μg/ml oligomycin, 2.5 μm carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone, and 4 μm antimycin A. Respiration rates calculated as previously described (20, 21).
Electron Microscopy
Adipocytes were grown in 6-well dishes on chemically resistant Thermanox coverslips (Ted Pella, Redding, CA). Eight days post-differentiation the cells were fixed on ice with 1 ml of 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA) in 0.1 m sodium cacodylate overnight at 4 °C. Cells on thermonox coverslips were washed 3 times with 0.1 m sodium cacodylate buffer and post-fixed with 1% osmium tetroxide (Electron Microscopy Sciences). An additional set of samples was treated with 1% tannic acid, postfixed with 1% osmium tetroxide, dehydrated with ethanol, and embedded with Embed 812 resin (Electron Microscopy Sciences). Embedded samples were sectioned on a Leica UC6 Ultramicrotome (Leica Microsystems, Vienna, Austria), stained, and analyzed using a JEOL 1200 EX II transmission electron microscope (JEOL LTD, Tokyo, Japan). Images were obtained using a Veleta 2K x 2K camera with iTEM software (Olympus SIS, Munster, Germany). Cells were selected randomly, and the number of mitochondria was counted. Perimeter and area were measured for 10 randomly selected mitochondria in each cell using iTEM software (Olympus SIS).
Fluorescence Microscopy
3T3-L1 cells were differentiated on chambered 1.5 borosilicate coverglass (Lab Tek, Thermo Fisher Scientific, Rochester, NY), and adipocytes were incubated with 50 nm tetramethylrhodamine methylester perchlorate (TMRM, Molecular Probes, Invitrogen, Carlsbad, CA) at 37 °C for 30–90 min before washing and mounting in Hanks' buffered salt solution for visualization. All images were captured at 37 °C using a DeltaVision PersonalDV system (Applied Precision, Inc, Issaquah, WA) at 100× with identical settings, deconvolved, and projected with equal numbers of z-slices. For quantification of TMRM fluorescence intensity, cells were stained as described and imaged at 60× on a Nikon Biostation IM that allowed time-lapse imaging of multiple points. Equal numbers of z-slices were projected and quantified in ImageJ (NIH). Fluorescence was normalized to background non-mitochondrial fluorescence (nucleus) and relativized to controls.
Superoxide Anion Detection
Superoxide was detected in isolated mitochondria with triphenylphosphonium hydroethidine as described previously (22) but with modifications to internally control for membrane potential-dependent probe accumulation using rhodamine 123 (23).
Enzyme Assays
3T3-L1 adipocytes were lysed in 50 mm NaPO4, pH 6.5, containing 1 μm diethylene triamine pentaacetic acid, 250 μm butylated hydroxytoluene, and protease inhibitors and centrifuged at 3000 × g for 5 min at 4 °C. Each sample was diluted to a final protein concentration of 0.1 mg/ml in 50 mm sodium phosphate buffer, pH 6.5, containing 1 mm diethylenetriaminepentaacetic acid, 250 μm butylated hydroxytoluene, 1 mm glutathione, and 1 μm 4-HNE at room temperature. After 2 min, the reaction was stopped by acidification using a final concentration of 0.1% formic acid (24) and 20 μl of the reaction mixture analyzed by LC-MS/MS using an Agilent 1100 HPLC system coupled to an AB-Sciex API 2000Qtrap tandem mass spectrometer. Liquid chromatography was performed using a 2.1 × 100-mm Agilent Zorbax Eclipse Plus C18 column with a 1.8-μm particle size. Glutathionylated-HNE (GS-HNE) was detected using negative mode electrospray ionization and multiple reaction monitoring. Authentic GS-HNE was synthesized as a standard, and the transition ions at 306.1 and 272.2 atomic mass units were used for analysis as reported previously (24). GS-HNE was quantified by stable isotope dilution with GS-HNE-d3 as the internal standard (Cayman Chemical, Ann Arbor, MI).
Complex I activity was assessed as described previously but adapted to a microplate format (25). Briefly, Complex I transfers electrons from NADH to decylubiquinone that subsequently delivers them to dichlorophenylindole. Reduction of dichlorophenylindole is monitored spectrophotometrically at 600 nm. Isolated mitochondria were resuspended in 10 mm Tris-HCl, pH 7.6, and assayed in buffer containing 25 mm KH2PO4, 3.5 mg/ml fatty acid-free BSA, 60 μm dichlorophenylindole, 70 μm decylubiquinone, and 1.0 μm antimycin A. The reaction was initiated with the addition of 0.2 mm NADH, and absorbance was measured over a period of 5 min.
Statistical Analysis
All values are expressed as the mean ± S.E. Statistical significance was determined using the two-tailed Student's t test assuming unequal variances. p values ≤ 0.05 are considered significant (*) with an increased significance of p value ≤0.01 indicated (**).
RESULTS
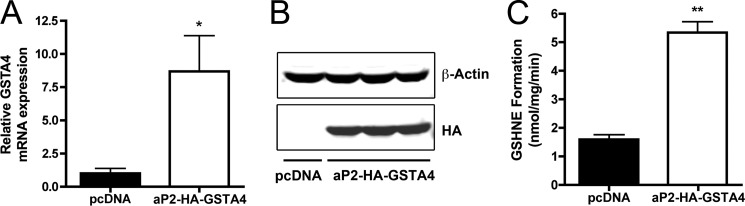
Obesity-linked insulin resistance is correlated with adipose mitochondrial dysfunction and occurs coincident with cytokine-mediated down-regulation of key antioxidant genes, particularly that encoding GSTA4. GSTA4 catalyzes the glutathionylation of reactive aldehydes such as 4-HNE and 4-oxo-trans-2,3 nonenal and functions in an antioxidant capacity reducing oxidative stress and its downstream metabolic effects due to carbonylation. GSTA4 is a cytoplasmic enzyme that translocates to the mitochondrion under conditions of oxidative stress (26). Previous results have demonstrated that selective loss of GSTA4 protein results in increased total protein carbonylation and mitochondrial dysfunction, yet the mitochondrial targets of such modification have not been identified (11). To address the role of protein carbonylation in adipocyte mitochondrial dysfunction, we utilized a strategy based on loss or gain-of-function of GSTA4. Because cytokine-treated cells exhibit a wide spectrum of effects and we wanted to focus exclusively on protein carbonylation, we developed cell lines with either reduced or augmented levels of GSTA4. We previously utilized a lentiviral-derived stable 3T3-L1 cell line harboring a shRNA targeting GSTA4 that reduces GSTA4 mRNA expression by ∼70%, similar to the extent down-regulated in murine adipose tissue of high fat fed mice. To produce an adipocyte cell line overexpressing GSTA4, we stably transfected 3T3-L1 fibroblasts with HA-tagged GSTA4 under the control of the fat-specific promoter, aP2. Several cell lines were derived, and one exhibiting stable elevated expression of HA-GSTA4 (as evaluated by immunoblotting for HA; antibodies monoselective for the murine GSTA4 isoform are not available) was selected for further study. The aP2-HA-GSTA4 cells had ∼8-fold higher mRNA expression of GSTA4 compared with control cells (Fig. 1A) and expressed HA-GSTA4 protein similarly (Fig. 1B). To verify the HA tag had no impact on the ability of GSTA4 to metabolize 4-HNE, the in vitro enzyme activity was assessed by monitoring the synthesis of GS-HNE in a glutathione and 4-HNE-dependent manner. Using cellular extracts from overexpressing (aP2-HA-GSTA4) and control adipocytes (pcDNA), GS-HNE synthesis was measured by LC-MS/MS. Overexpressing adipocytes exhibited ∼5-fold more GS-HNE production relative to controls (Fig. 1C), indicating the HA-GSTA4 retains activity in the presence of the HA epitope.
FIGURE 1.
Expression and functionality of aP2-HA-GSTA4 in 3T3-L1 adipocytes. A, relative mRNA expression of GSTA4 in control (pcDNA) and overexpressing (aP2-HA-GSTA4) adipocytes normalized to TFIIE is shown. B, expression of HA-GSTA4 (anti-HA) relative to β-actin (anti-actin) in separate overexpressing cell lines is shown. C, an in vitro GS-HNE formation assay is shown (n = 3; *, p < 0.05; **, p < 0.01).
Loss of GSTA4 in cultured 3T3-L1 adipocytes or in adipose tissue from high fat-fed C57Bl/6J mice leads to elevated mitochondrial carbonylation, diminished respiration, and altered metabolic flux through the tricarboxylic acid cycle and β-oxidation pathways (11). In addition, obesity-induced insulin resistance in humans positively correlates with protein carbonylation, indicating that increased oxidative stress leads to increased levels of carbonylated proteins (27). Because the mitochondrial electron transport chain is the major site of ROS synthesis, we utilized an unbiased proteomic approach focused on adipocyte mitochondria to define specific targets of protein carbonylation. We hypothesized that due to the reduced detoxification of reactive aldehydes in GSTA4-silenced (Kd) adipocytes and increased 4-HNE glutathionylation in aP2-HA-GSTA4 cells that we would be able to identify critical carbonylation targets in the mitochondrion that may be causal for the pathophysiology of the cells. To test this hypothesis, mitochondrial protein from GSTA4-silenced, overexpressing, and cognate control cell lines were subjected to free-carbonyl modification with biotin hydrazide, avidin enrichment chromatography, iTRAQ labeling, and mass spectrometric analysis. More than 370 proteins were identified from the proteomic analysis, and ∼52% of those proteins exhibit mitochondrial localization. This represents ∼20% of all mitochondrial proteins (28). Because the endoplasmic reticulum and mitochondrion contain sites of interaction defined by the linking proteins Mmm1/Mdm10/Mdm12/Mdm34 (29), it was not surprising that our fractionation led to identification of some non-mitochondrial proteins. Indeed, ∼13% of total carbonylated proteins were annotated as being endoplasmic reticular in origin. Other cellular locations represented include cytoplasm (12%), membrane-bound (6%), and lysosomal (5%). A complete proteomic summary of the resolved proteins can be found in supplemental Table 1. The mitochondrial pathways most represented in the dataset are those linked to oxidative phosphorylation (19%), branched chain amino acid catabolism (28%), and tricarboxylic acid cycle (33%) (Table 1). Other pathways with 10% coverage or more include fatty acid, amino acid, and ketone body metabolism (data not shown). When the carbonylation level of mitochondrial proteins in GSTA4-silenced relative to overexpressing cells was quantitated, a small number of differentially modified polypeptides was identified. Carbonylated target data in Table 2 are presented as -fold change with positive numbers indicating elevated carbonylation, and negative numbers designating decreased carbonylation relative to the compared cell type. These targets include mitochondrial phosphate carrier, two subunits of NADH dehydrogenase (Complex I of mitochondrial electron transport), translocase of the inner mitochondrial membrane 50, and valyl tRNA synthetase. Comparison of the relative carbonylation level in GSTA4-silenced versus -scrambled cells and to that in the aP2-HA-GSTA4 versus pcDNA cells suggested that the largest number of differences was measured in the aP2-HA-GSTA4 versus pcDNA cells.
TABLE 1.
Pathway analysis of carbonylated mitochondrial proteins in adipocytes
Proteins underlined are pathway-specific targets of carbonylation, whereas others are represented in two or more pathways.
| Pathway | Pathway coverage | Carbonylated proteins |
|---|---|---|
| Tricarboxylic acid cycle | 19/58 | ACO2, CS, DLD, DLST, FH, IDH1, IDH2, IDH3A, IDH3G, MDH1, MDH2, OGDH, PC, PCK2, SDHA, SDHB, SUCLA2, SUCLG1, SUCLG2 |
| Oxidative phosphorylation | 32/165 | ATP5A1, ATP5B, ATP5C1, ATP5F1, ATP5H, ATP5I, ATP5O, ATP6V1A, COX15, COX4I1, COX5A, COX5B, CYC1, MT-ATP8, MT-CO2, NDUFA2, NDUFA3, NDUFA4, NDUFA10, NDUFB4, NDUFB5, NDUFC2, NDUFS1, NDUFS3, NDUFS7, PPA2, SDHA, SDHB, UQCRB, UQCRC1, UQCRC2, UQCRFS1 |
| Branched chain amino acid catabolism | 31/111 | ACAD9, ACAD10, ACADL, ACADM, ACADS, ACADSB, ACADVL, ACAT1, ALDH2, ALDH1B1, ALDH4A1, ALDH6A1, BCAT2, BCKDHA, DBT, ECH1, ECHS1, HADH, HADHA, HADHB, HIBCH, HMGCL, HSD17B4, HSD17B10, IVD, MCCC1, MCCC2, MUT, OXCT1, PCCA, PCC |
TABLE 2.
Differentially modified targets of carbonylation in adipocyte mitochondria
Values represented as -fold change with positive numbers indicate elevated carbonylation and negative numbers designating decreased carbonylation relative to the compared cell type. Data in the GSTA4 Kd versus aP2-HA-GSTA4 column have been normalized to respective controls. p values are calculated with ratio data from three or more matched spectra.
| Accession number | Protein symbol | Protein name | GSTA4 Kd vs. Scr |
aP2-HAGSTA4 vs. pcDNA |
GSTA4 Kd vs. aP2-HAGSTA4 |
|||
|---|---|---|---|---|---|---|---|---|
| -Fold | p value | Fold | p value | Fold | p value | |||
| Q8VEM8 | MPCP | Phosphate carrier protein, mitochondrial | 2.59 | 2.5E − 06 | −2.06 | 6.9E − 05 | 5.33 | <E − 15 |
| Q9CQ91 | NDUFA3 | NADH dehydrogenase (ubiquinone) 1α subunit 3 | 1.62 | 2.9E − 01 | −2.95 | 2.8E − 06 | 4.77 | 3.3E − 06 |
| Q9D880 | TIM50 | Mitochondrial import inner membrane translocase subunit TIM50 | 1.06 | 8.8E − 01 | −2.40 | 1.7E − 03 | 2.56 | 4.0E − 02 |
| Q9CQ75 | NDUFA2 | NADH dehydrogenase (ubiquinone) 1α subunit 2 | −1.02 | 9.4E − 01 | −2.21 | 2.0E − 13 | 2.17 | 9.3E − 06 |
| Q3U2A8 | SYVM | Valyl-tRNA synthetase, mitochondrial | 1.02 | 9.3E − 01 | −1.82 | 1.6E − 10 | 1.85 | 4.7E − 02 |
| Q9DCU6 | RM04 | 39 S ribosomal protein L4, mitochondrial | −1.68 | 2.1E − 04 | −1.24 | 2.3E − 02 | −1.35 | 2.0E − 04 |
| P84091 | AP2M1 | Adaptor-related protein complex 2, μ1 subunit | −2.52 | 8.0E − 15 | 1.00 | 9.9E − 01 | −2.51 | 1.7E − 05 |
| P22315 | HEMH | Ferrochelatase, mitochondrial | −2.23 | 2.4E − 09 | 1.32 | 5.1E − 01 | −2.95 | 4.0E − 02 |
| Q8JZN7 | MIRO2 | Mitochondrial Rho GTPase 2 | −2.66 | 3.0E − 13 | 1.22 | 3.5E − 01 | −3.23 | 9.8E − 15 |
| Q8R127 | SCPDH | Saccharopine dehydrogenase (putative) | −2.36 | 1.3E − 11 | 1.52 | 1.6E − 01 | −3.58 | 1.1E − 10 |
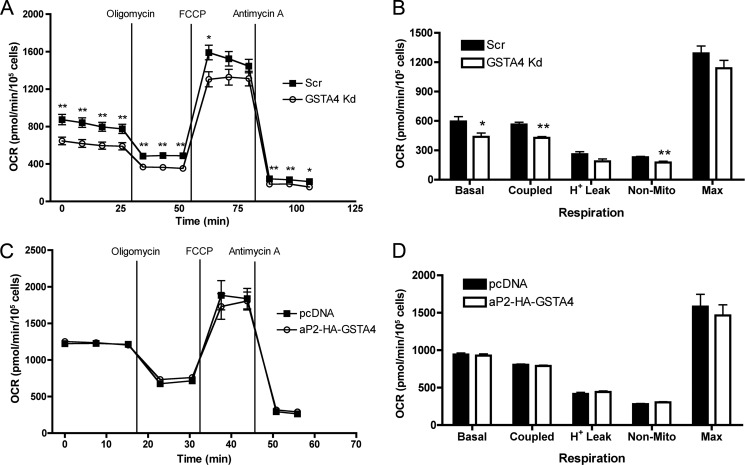
Silencing GSTA4 in 3T3-L1 adipocytes leads to loss of ADP-coupled respiration in isolated mitochondria (11). However, because these cells have compensatory changes in substrate level phosphorylation and fatty acid uptake, it was important to assess their in situ respiratory capacity. This was determined using the XF24 extracellular flux analyzer with cultured adipocytes. Similar to studies with isolated mitochondria, GSTA4-silenced cells had reduced respiratory capacity relative to control adipocytes (Fig. 2A). Respiration rates were calculated as a function of changes in the oxygen consumption rate with the indicated treatments; Fig. 2B (20, 21). The results of the respiration studies indicated that GSTA4 Kd adipocytes had ∼25% less basal and coupled respiration. Surprisingly, GSTA4 overexpression did not significantly affect oxygen consumption rate relative to controls (Fig. 2, C and D), suggesting that 3T3-L1 adipocytes have sufficient expression of endogenous GSTA4 to prevent carbonylation induced mitochondrial dysfunction.
FIGURE 2.
Oxygen consumption rates in GSTA4-silenced and -overexpressing adipocytes. A and C, shown are cellular oxygen consumption rates (OCR) for scrambled control (Scr) and GSTA4 Kd (A) and control (pcDNA) and GSTA4 overexpressing (aP2-HA-GSTA4) (C) adipocytes as measured by XF24 extracellular flux analysis. Oligomycin (50 μg/ml), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, 4 μm), and antimycin A (2.5 μm) were injected at the indicated time points. B and D, respiration rates as determined from A and C are shown. (n = 5–10; *, p < 0.05; **, p < 0.01).
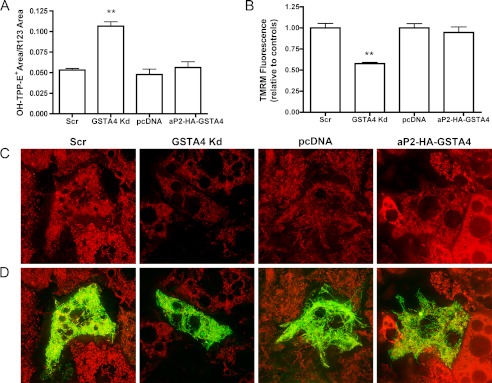
Mitochondrial respiratory efficiency is inversely proportional to superoxide anion production where inhibition of complex I leads to increased superoxide (30). As two complex I subunits were identified in our proteomic analysis of mitochondrial carbonylation and this complex is a significant site of ROS production, we estimated superoxide produced in isolated mitochondria. The accumulation of the superoxide probe, triphenylphosphonium hydroethidine, across the inner mitochondrial membrane is driven by membrane potential thus introducing a bias in product formation. To evaluate superoxide levels in a membrane potential-independent manner, we utilized rhodamine 123 that accumulates in the mitochondria based on membrane potential but has no interaction with superoxide (23) as a normalization control. Triphenylphosphonium hydroethidine-based analysis of membrane potential in the presence of rhodamine 123 demonstrated a significant increase in superoxide anion production in GSTA4 Kd adipocytes but no change with GSTA4 overexpression (Fig. 3A).
FIGURE 3.
Mitochondrial superoxide production and membrane potential. A, mitochondrial superoxide anion production normalized to rhodamine 123 membrane potential control (n = 4). B, TMRM fluorescence using ImageJ (n = 10 frames) is shown. C, TMRM staining of 3T3-L1 adipocytes is shown. D, merge of TMRM with mitochondrial targeted GFP localization control is shown is shown. (**, p < 0.01).
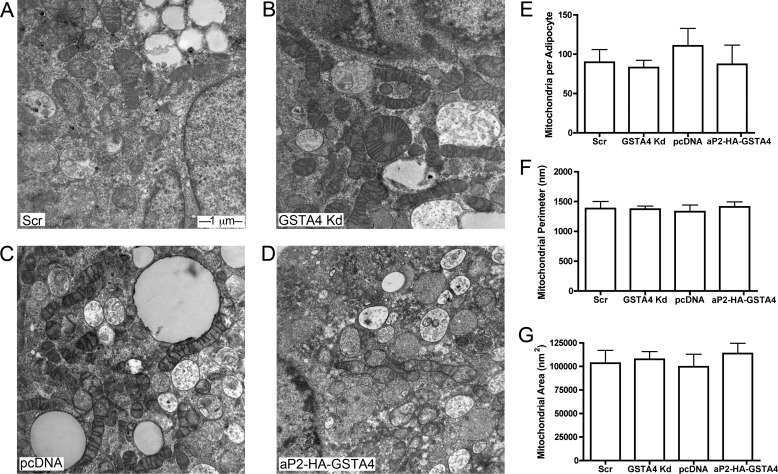
Our previous studies utilizing GSTA4-silenced adipocytes demonstrated reduced mitochondrial respiration, mitochondrial DNA (mtDNA) copy number, and expression of several key transcription factors regulating mitochondrial biogenesis (11). To determine whether mitochondrial dysfunction was a product of a decreased number of mitochondria or decreased mitochondrial activity, electron microscopy was employed. Using differentiated adipocytes from GSTA4-silenced, overexpressing, and control cell lines, mitochondrial number, area, and perimeter were evaluated (Fig. 4). Despite an ∼75% reduction in mtDNA content in GSTA4-silenced adipocytes, there was no difference in mitochondrial number, area, or density (Fig. 4, E–G). On a qualitative basis, mitochondria appeared to be irregularly shaped in GSTA4-silenced cells compared with either scrambled or overexpressing adipocytes, and the cristae were less well organized. These results suggest that decreased mitochondrial function is not linked to changes in mitochondrial number. Consistent with this, expression of autophagy markers ATG5, ATG6, ATG9, and ATG13 were not different between the cell lines nor was the phosphorylation of S6 kinase, an mTOR target (results not shown).
FIGURE 4.
Mitochondrial number and area by electron microscopy. A–D, electron micrographs at 30,000× magnification of scrambled (A), GSTA4 Kd (B), pcDNA (C), and aP2-HA-GSTA4 (D) 3T3-L1 adipocytes are shown. E and F, quantitation of mitochondrial number (E), perimeter (F), and area (G) from multiple fields (n = 70–100 each) is shown.
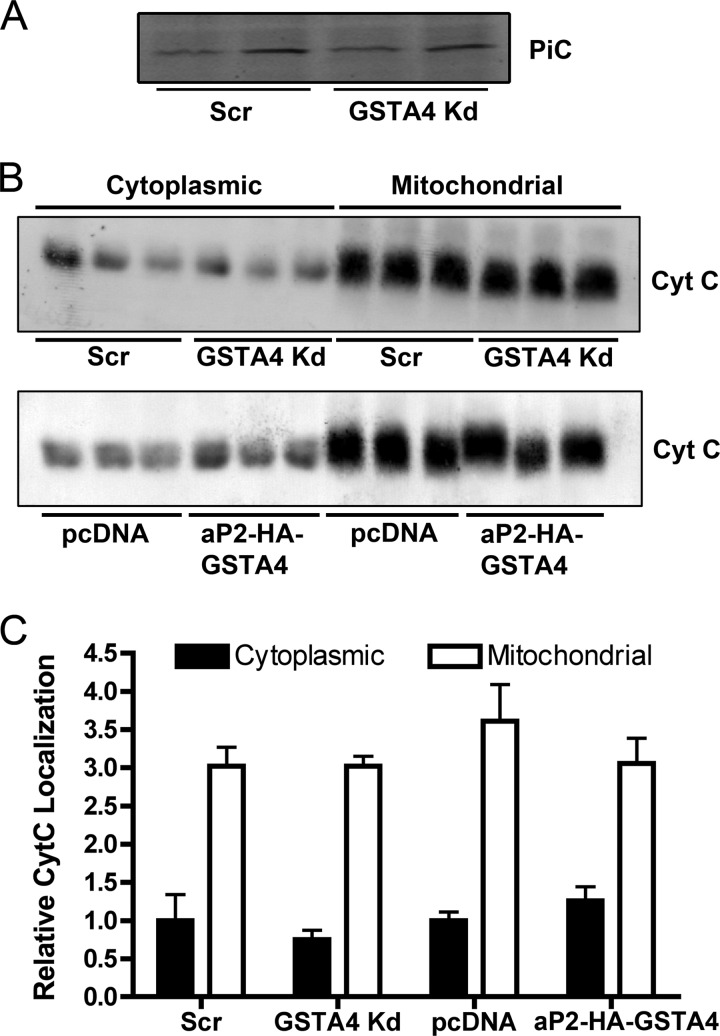
The most prominent differentially carbonylated protein identified was the mitochondrial inner membrane phosphate carrier (PiC) (Table 2). The mitochondrial phosphate carrier is a cotransporter of protons and inorganic phosphate across the inner mitochondrial membrane (31) and likely works in conjunction with the FoF1 ATPase to facilitate efficient ATP generation in response to the electrochemical gradient across the inner membrane (32). Because the PiC was a significant target of protein carbonylation (Table 2) and coupled respiration was impaired in both whole cell and isolated mitochondrial experiments (Fig. 2 (11)), the membrane potential was assayed in live cells using TMRM, a cationic probe specific for mitochondrial membrane potential. Silencing GSTA4 led to diminished membrane potential relative to control and overexpressing adipocytes under basal conditions (Fig. 3, C and D), whereas the overexpression of GSTA4 did not confer any changes in membrane potential relative to control cells. Additionally, no change in PiC protein abundance was observed (Fig. 5A). Loss of mitochondrial membrane potential is often associated with cytochrome c (Cyt C) release and the induction of the apoptotic response (33, 34); therefore, localization of Cyt C was analyzed in GSTA4-silenced and overexpressing cells. There was no increase in Cyt C release in the GSTA4-silenced cells relative to controls or decrease in aP2-HA-GSTA4 overexpressing cells (Fig. 5, B and C), indicating the apoptosis pathway had not been triggered by changes in GSTA4-dependent protein carbonylation despite the loss of activity.
FIGURE 5.
PiC protein abundance and cytochrome C localization in GSTA4-silenced and overexpressing 3T3-L1 adipocytes. A, shown is immunoblot analysis of PiC. B, immunolocalization of Cyt C protein in 3T3-L1 adipocyte cytoplasmic and mitochondrial fractions is shown. C, quantitation of immunoblots from B is shown.
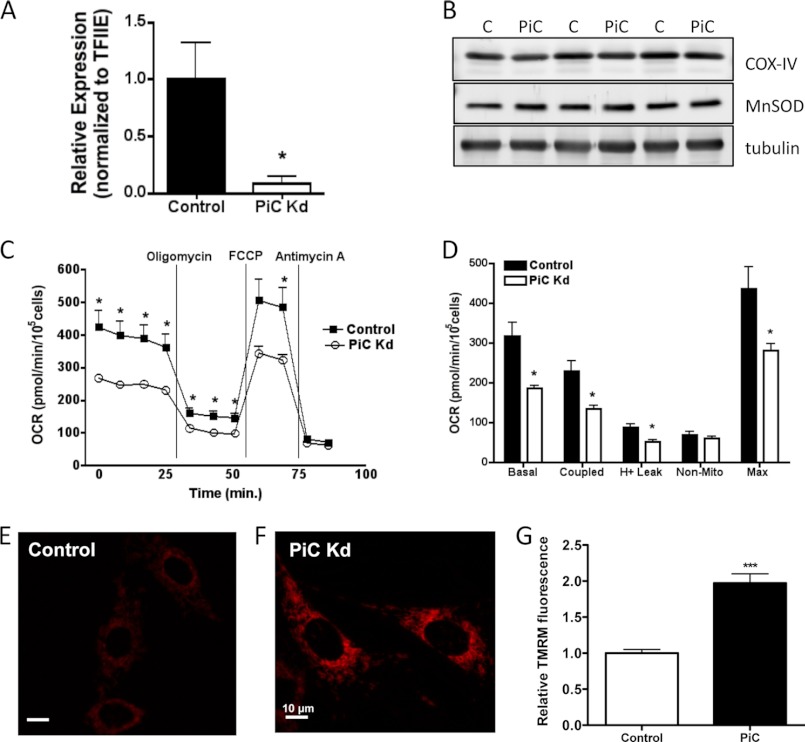
The identification of the phosphate carrier as a carbonylation target was carried out within the context of GSTA4-silenced and overexpressing cells where multiple carbonylation events give rise to the changes in respiration and mitochondrial metabolism (7). To isolate changes in mitochondrial respiration specifically linked to the phosphate carrier, we silenced the protein in 3T3-L1 cells using shRNA-directed methodologies. Fig. 6 shows that the mRNA expression of the PiC was reduced ∼90% relative to a control cell line expressing an shRNA directed to the green fluorescent protein, but the expression of mitochondrial marker proteins (cytochrome c oxidase complex IV, manganese superoxide dismutase) were not significantly affected. Respiration analysis using the Seahorse XF24 analyzer indicated that respiration was severely compromised with markedly reduced basal, coupled and maximal respiration (Fig. 6, C, D). The reduced respiration was associated with an increase in the mitochondrial membrane potential as assessed by TMRM staining (Fig. 6 E, F).
FIGURE 6.
Properties of PiC-silenced 3T3-L1 cells. A, shown is relative mRNA expression of the mitochondrial phosphate carrier in control and PiC-silenced (PiC Kd) fibroblasts normalized to TFIIE. B, expression of mitochondrial proteins cytochrome c oxidase complex IV (COX-IV) and manganese superoxide dismutase (MnSOD) in PiC-silenced 3T3-L1 cells is shown. C and D, cellular oxygen consumption rates for control and PiC-silenced cells. E and F, TMRM fluorescence (n = 30 frames, ∼ 150 cells) and G, quantitation using Image J of control and PiC-silenced cells are shown. (*, p < 0.05; ***, p < 0.001).
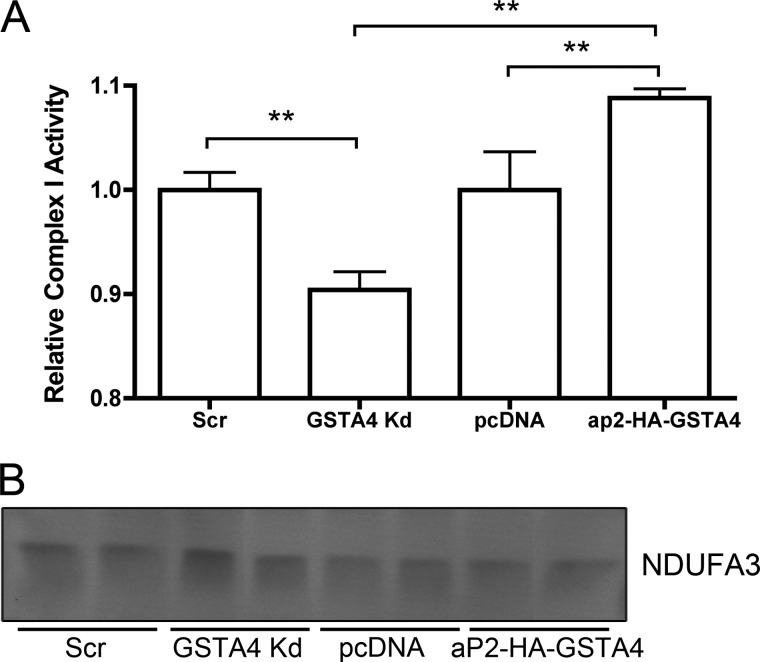
Two carbonylation targets identified are linked to Complex I (NDUFA2, NDUFA3). To investigate whether carbonylation affects Complex I activity, the transfer of electrons from NADH to dichlorophenolindophenol was evaluated spectrophotometrically (25) in mitochondria isolated from the GSTA4-silenced and overexpressing cells. As shown in Fig. 7A, the reduction of GSTA4 expression is associated with a ∼10% decrease in Complex I enzyme activity, whereas adipocytes with higher expression of GSTA4 exhibited a ∼ 10% increase in Complex I activity. No change in NDUFA3 protein expression was observed between any of the cell lines (Fig. 7B).
FIGURE 7.
Complex I activity in isolated mitochondria. A, the Complex I-dependent reduction of dichlorophenolindolephenol was monitored spectrophotometrically at 595 nm in isolated mitochondria (n = 4; ** p < 0.01). B, protein expression of NDUFA3 Complex I subunit in mitochondrial fractions (representative blot, n = 6) is shown.
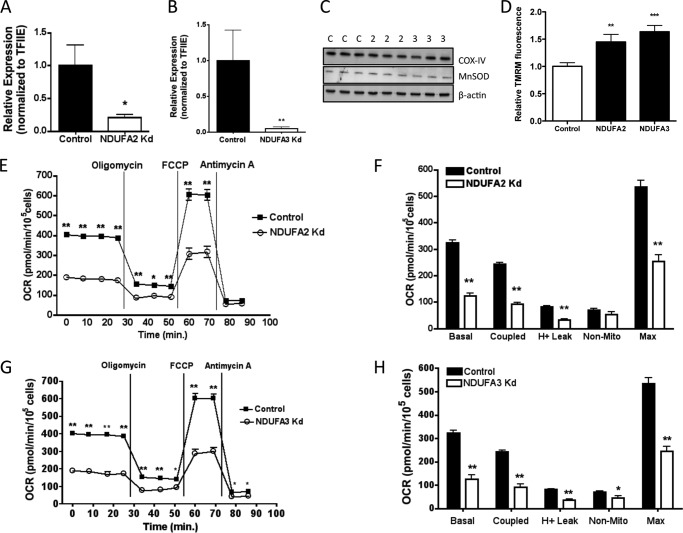
As with the phosphate carrier, to isolate specific effects due to carbonylation of NDUFA2 and NDUFA3 we utilized gene silencing as a surrogate methodology to assess loss of activity. To that end, NDUFA2 and NDUFA3 (Fig. 8) silenced cells were developed using lentiviral directed procedures and respiration assessed. The mRNA expression of NDUFA2 was reduced ∼80%, whereas that for NDUFA3 was reduced ∼90% (Fig. 8 A, B) relative to control cells expressing an shRNA directed to the green fluorescent protein. For NDUFA2 silenced cells, but not NDUFA3 silenced cells there was a corresponding decrease in the abundance of cytochrome c oxidase complex IV (COX-IV) protein (Fig. 8C). Manganese superoxide dismutase abundance was not decreased in either NDUFA2- or NDUFA3-silenced cells. Using the Seahorse XF24 analyzer, we evaluated respiratory parameters in the NDUFA2- and NDUFA3-silenced cells. Fig. 8, E–H, shows significantly decreased basal and maximal respiration in both complex I subunit-silenced cells and correspondingly decreased-coupled respiration and proton leak. Decreased proton leak in both NDUFA2- and NDUFA3-silenced cells was associated with an increase in mitochondrial membrane potential (Fig. 8D). Overall, the results indicated that decreased NDUFA2 or NDUFA3 abundance results in significantly reduced mitochondrial activity in GSTA4-silenced cells.
FIGURE 8.
Properties of NDUFA2- and NDUFA3-silenced 3T3-L1 cells. A and B, shown is relative mRNA expression of NDUFA2 (A) and NDUFA3 (B) in control and silenced (Kd) fibroblasts normalized to TFIIE. C, expression of cytochrome c oxidase complex IV (COX-IV) and manganese superoxide dismutase (MnSOD) protein in control (C) and NDUFA2 (2)- and NDUFA3 (3)- silenced cells. D, TMRM fluorescence (n = 30 frames, ∼150 cells) and quantitation using Image J of control and NDUFA2- and NDUFA3-silenced cells are shown. E–H, cellular oxygen consumption rates for control and NDUFA2- and NDUFA3-silenced cells are shown. Details of experiment as in legend to Fig. 2. (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
DISCUSSION
Oxidative stress and the production of ROS have been strongly implicated as causal in the development of obesity-linked insulin resistance, and a variety of studies have reported that anti-oxidants may have beneficial effects attenuating the characteristics of the metabolic syndrome (3, 35–37). Increased oxidative stress and production of hydroxyl radicals is linked to the hydroperoxidation of unsaturated acyl chains of membrane lipid and triglyceride. Lipid hydroperoxides undergo Hock cleavage to produce a family of α,β-unsaturated hydroxylated aldehydes that by virtue of their electron withdrawing carbonyl and hydroxyl functions are subject to Michael addition reactions centering on carbon 3 of the lipid producing a covalent protein-lipid adduct. Such alkylation, referred to as protein carbonylation, has been demonstrated in a variety of oxidative systems including inflammation, neurodegeneration, and cancer (38–40). Because the mitochondrial electron transport chain is a major site of ROS production, mitochondrial carbonylation is thought to contribute substantially to oxidative damage, although this hypothesis has not been rigorously tested. The objective of this study was to utilize a proteomic strategy to identify carbonylation targets in adipocyte mitochondria linked to down-regulation of glutathione S-transferase A4. It should be noted that the biotinylated peptides carrying the carbonyl modification are not identified. The reason for this is unclear but may be because they do not resolve during chromatography or they fragment in a manner that precludes their identification via sequence database searching. Methods are in development to isolate and analyze such alkylated peptides to determine the site(s) and stoichiometry of carbonylation. Additionally, although both amino acid side chain oxidation and carbonylation with lipid aldehydes occurs, studies indicate that side chain alkylation is ∼10-fold more prevalent than direct side chain oxidation (41).
Our previous studies have established that protein carbonylation is elevated in the adipose tissue of obese, insulin-resistant mice and in obese humans and identified soluble protein targets of carbonylation (11, 27, 42). Increased protein carbonylation has been mechanistically linked to TNFα-dependent down-regulation of the antioxidant enzyme GSTA4. In both murine and human adipose tissue the major soluble carbonylation target protein was the fatty acid-binding protein FABP4 and FABP5, and carbonylation of murine FABP4 on Cys-117 in the ligand binding cavity results in loss of fatty acid binding activity. However, biochemically, the major site of metabolic dysfunction was in mitochondrial metabolism suggesting that carbonylation of mitochondrial targets may be causative for changes in oxidative metabolism. To address this we developed adipocyte models with variable expression of GSTA4 as a means to identify and evaluate the role of protein carbonylation in the pathogenesis of obesity-linked insulin resistance. Coupling the enrichment of carbonylated mitochondrial proteins with quantitative proteomics, we identified differentially modified proteins between our cellular models of oxidative stress. Surprisingly, only a handful of proteins were differentially carbonylated, suggesting that the changes in metabolism may be focused on just a small number of metabolic loci.
Other published studies using exogenous 4-HNE treatment of cells transfected with GSTA4 have shown that localization of GSTA4 to the mitochondria (26) protects against oxidative damage (43) and resultant apoptosis (44). Proteomic analysis demonstrated that GSTA4 overexpressing adipocytes had less mitochondrial carbonylation relative to GSTA4-silenced cells (Table 2 and supplemental Table 1), but functional analysis shows this did not confer large changes to respiration, superoxide production, membrane potential, or complex I activity relative to control cells. This suggests that endogenous levels of GSTA4 are sufficient for protection against carbonylation-induced functional changes to adipocyte metabolism under normoxic conditions. Carbonylated proteins are frequently targeted for proteasome-dependent degradation (45). Proteins whose relative level of carbonylation is decreased in the GSTA4-silenced cells relative to the HA-GSTA4-overexpressing cells (Table 2) may represent targets that are selectively proteolyzed, resulting in decreased abundance. This does not pertain to the phosphate carrier or NDUFA3; their abundance did not differ between cellular states (Figs. 5 and 7, respectively).
Carbonylation of mitochondrial proteins did not change the number or size of the mitochondrial pool (Fig. 4) despite down-regulation of key mitochondrial-encoded proteins and transcription factors regulating mitochondrial biogenesis, such as peroxisome proliferator-activated receptor γ coactivator 1α and Tfam1 (11). Although highly variable, ultrastructure analysis via electron microscopy suggests disorganization of the cristae and irregular shaping in mitochondria from GSTA4-silenced adipocytes relative to those from control or GSTA4-overexpressing cells. Mitochondrial depolarization, which occurs in GSTA4-silenced adipocytes, is an important signal for mitophagic clearance (for review, see Ref. 46); however, evaluation of autophagy-signaling proteins ATG5/6/9/13 and S6K (data not shown) indicate that such cellular clearance mechanisms have not been activated.
Among the important targets of carbonylation implicated in mitochondrial dysfunction, two Complex I subunits have been identified: NDUFA2 and NDUFA3. These proteins are accessory subunits of NADH:ubiquinone oxidoreductase that transfers electrons from NADH to ubiquinol:cytochrome c oxidoreductase (complex III) (47). This large ∼1-MDa complex consists of 45 subunits, and NDUFA2 is located on the peripheral arm of complex I in the inner mitochondrial matrix where NADH binding occurs (48). Its expression in white adipose tissue is positively correlated with peroxisome proliferator-activated receptor-γ coactivator-1α expression, a potent mediator of mitochondrial biogenesis and energy expenditure (49). NDUFA3 fractionates with proteins connecting the peripheral arm to the membrane-embedded domain and is 1 of 31 subunits of complex I without an established primary function (47). Defects in complex I assembly are a common cause of mitochondrial disorders (48, 50), and mutations in complex I alone are responsible for 23% of all childhood respiratory chain defects in humans (51). Mutations in NDUFA2 affecting complex I activity frequently lead to infant mortality (52). Because proper complex I function requires intricate coordination of subunits, it is possible that post-translational modifications such as carbonylation could disrupt this process. In fact, micromolar concentrations of 4-HNE are linked to loss of mitochondrial respiration through inhibition of complex I (53). In this study carbonylation of complex I subunits is associated with impaired activity (Fig. 7) and increased membrane potential (Fig. 8D) but diminished basal and coupled respiration (Fig. 8, E–H). Increased membrane potential in the NDUFA2- and NDUFA3-silenced cells suggests that carbonylation of these targets is not likely to underlie the reduced membrane potential measured in GSTA4-silenced cells. It is interesting to note that the basal oxygen consumption rate in NDUFA2- and NDUFA3-silenced cells is considerably reduced relative to that for GSTA4-silenced cells (compare Fig. 2 to Fig. 8). This suggests that the carbonylation of NDUFA2 or NDUFA3 in GSTA4-silenced cells may have significant impact on respiration.
A second possible impact carbonylation of complex I subunits may have is on the generation of superoxide. Cellular superoxide anion is produced in part by Complex I and contributes significantly to redox-regulated cellular signaling (54–56). Carbonylation of NDUFA2 or NDUFA3, located near the NADH binding site, may interfere with the ability of complex I to efficiently transfer electrons from NADH to complex III leading to elevated superoxide generation (Fig. 3A). Consistent with this, the production rate of superoxide by Complex I is inversely proportional to the NAD+/NADH ratio (57), which is reduced in the GSTA4-silenced adipocytes (11).
One of the central functions of the mitochondrion is the generation of ATP by FoF1 ATP synthase (Complex V). This enzyme is part of a supercomplex known as the ATP synthasome that consists of the adenine nucleotide translocase, the mitochondrial phosphate carrier, and the ATP synthase (32, 58). Adenine nucleotide translocase exchanges ATP for ADP across the inner mitochondrial membrane, and PiC co-transports inorganic phosphate (Pi) and protons into the inner mitochondrial matrix. Through physical association, these complexes centralize the production of ATP by bringing the synthase together with ADP and Pi. With proper substrate availability, the FoF1 molecular motor exploits the proton gradient established by complexes I-IV of the electron transport chain to generate ATP. Both adenine nucleotide translocase and several Complex V subunits were identified as carbonylated (supplemental Table 1), but there was no significant difference in the amount of carbonylation between variable models of oxidative stress. In addition, there was no statistical difference in carbonylation of other proton-transporting polypeptides such as uncoupling proteins. However, PiC was differentially carbonylated to the greatest extent between the various cell lines (Table 2). The PiC plays a significant role in ATP synthesis as down-regulation of PiC in INS-1 β-cells leads to blunted ATP production (59). Interestingly, even with reduced respiration and mitochondrial membrane potential (Figs. 2 and 3), phosphorylation of AMP-activated kinase, a cellular indicator of the AMP:ATP ratio, is unaltered in GSTA4-silenced adipocytes4 likely due to increased basal glucose uptake (11). This suggests that GSTA4-silenced cells have reprogrammed their metabolism to rely on substrate level phosphorylation as a result of reduced mitochondrial function. As oxygen consumption is coupled to ATP production, the impaired respiration observed in GSTA4-silenced adipocytes (Fig. 2, A and B) may in part be due to carbonylation of PiC interfering with its ability to transport phosphate or protons or associate with the ATP synthasome. Consistent with this proposition, the membrane potential in PiC-silenced 3T3-L1 cells was increased suggesting an inability to efficiently transport proteins.
An alternative function for the PiC is as a component of the mitochondrial permeability transition pore (60–62), suggesting a larger role for the PiC in the regulation of mitochondrial membrane potential. Opening of the mitochondrial permeability transition pore uncouples the proton gradient and induces apoptosis by releasing Cyt C. Overexpression of PiC induces apoptosis, whereas PiC knockdown leads to reduced Cyt C release and apoptosis (61). In this study there were no changes in PiC abundance or relative cytochrome c localization between GSTA4-silenced and -overexpressing cells (Fig. 5), indicating the amount of oxidative damage observed in GSTA4-silenced adipocytes is insufficient to trigger apoptosis. Symport of protons and phosphate across the inner mitochondrial membrane by PiC uncouples the proton gradient but is an electroneutral event. The PiC contains several sulfhydryl residues that are essential for its primary function (63), and thiol reagents, such as N-ethylmaleimide, modify cysteine residues and inhibit symport activity (64). Additionally, dithiol cross-linking causes homodimerization of PiC and formation of a nonspecific anion channel (65). These observations suggest that protein carbonylation of the PiC may either affect its symport activity and/or ability to function as a component of the mitochondrial permeability transition pore.
In summary, mitochondrial protein carbonylation is regulated via the expression and activity of GSTA4 in the adipocyte. The transition from lean to obese leads to cytokine-mediated down-regulation of GSTA4 expression and concomitant modification of mitochondrial proteins. Such protein modification results in impaired mitochondrial respiration, increased superoxide production, reduced membrane potential, and decreased complex I activity. Specific targets of protein carbonylation such as complex I subunits and the PiC may contribute significantly to mitochondrial dysfunction and play an important role in regulating adipocyte metabolism. Future studies will focus on the role of specific target protein carbonylation in mitochondrial metabolism and the resultant influence on insulin signaling.
Supplementary Material
Acknowledgments
We thank the Bernlohr laboratory members who participated in helpful discussions about this work. We also thank Marissa Lee for cell culture support as well as John Chilton, Getiria Onsongo, and the Minnesota Supercomputing Institute for computational support, Todd Markowski at the University of Minnesota Center for Mass Spectrometry and Proteomics for assistance with chromatography, Duncan Clark, Andrew Lane, and Rebecca Pulver for assistance with live cell imaging, and Donsanjiv Ariyakumar for electron microscopy assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants DE017734 (to T. J. G.), 5T32DK007203 (to M. A. D.), NIH DK084669 and MN-70-043 (to D. A. B.), and DK050456 (to The Minnesota Obesity Center).

This article contains supplemental Table 1.
J. M. Curtis and D. A. Bernlohr, unpublished information.
- ROS
- reactive oxygen species
- 4-HNE
- 4-hydroxy-trans-2,3-nonenal
- GS-HNE
- glutathione-conjugated HNE
- PiC
- mitochondrial phosphate carrier
- NDUFA
- NADH dehydrogenase (ubiquinone) 1α subcomplex
- Cyt C
- cytochrome c
- Kd
- knockdown
- TMRM
- tetramethylrhodamine methylester
- Scr
- scrambled.
REFERENCES
- 1. Bonadonna R. C., Groop L., Kraemer N., Ferrannini E., Del Prato S., DeFronzo R. A. (1990) Obesity and insulin resistance in humans. A dose-response study. Metab. Clin. Exp. 39, 452–459 [DOI] [PubMed] [Google Scholar]
- 2. Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Houstis N., Rosen E. D., Lander E. (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440, 944–948 [DOI] [PubMed] [Google Scholar]
- 4. Fang J., Holmgren A. (2006) Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J. Am. Chem. Soc. 128, 1879–1885 [DOI] [PubMed] [Google Scholar]
- 5. Sharma R., Sharma A., Dwivedi S., Zimniak P., Awasthi S., Awasthi Y. C. (2008) 4-Hydroxynonenal self-limits fas-mediated DISC-independent apoptosis by promoting export of Daxx from the nucleus to the cytosol and its binding to Fas. Biochemistry 47, 143–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Senese R., Valli V., Moreno M., Lombardi A., Busiello R. A., Cioffi F., Silvestri E., Goglia F., Lanni A., de Lange P. (2011) Uncoupling protein 3 expression levels influence insulin sensitivity, fatty acid oxidation, and related signaling pathways. Pflugers Arch. 461, 153–164 [DOI] [PubMed] [Google Scholar]
- 7. Grimsrud P. A., Xie H., Griffin T. J., Bernlohr D. A. (2008) Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 283, 21837–21841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruns C. M., Hubatsch I., Ridderström M., Mannervik B., Tainer J. A. (1999) Human glutathione transferase A4-4 crystal structures and mutagenesis reveal the basis of high catalytic efficiency with toxic lipid peroxidation products. J. Mol. Biol. 288, 427–439 [DOI] [PubMed] [Google Scholar]
- 9. Engle M. R., Singh S. P., Czernik P. J., Gaddy D., Montague D. C., Ceci J. D., Yang Y., Awasthi S., Awasthi Y. C., Zimniak P. (2004) Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal. Generation and analysis of mGsta4 null mouse. Toxicol. Appl. Pharmacol. 194, 296–308 [DOI] [PubMed] [Google Scholar]
- 10. Curtis J. M., Hahn W. S., Long E. K., Burrill J. S., Arriaga E. A., Bernlohr D. A. (2012) Protein carbonylation and metabolic control systems. Trends Endocrinol. Metab. 23, 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Curtis J. M., Grimsrud P. A., Wright W. S., Xu X., Foncea R. E., Graham D. W., Brestoff J. R., Wiczer B. M., Ilkayeva O., Cianflone K., Muoio D. E., Arriaga E. A., Bernlohr D. A. (2010) Down-regulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes 59, 1132–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olefsky J. M., Glass C. K. (2010) Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 [DOI] [PubMed] [Google Scholar]
- 13. Student A. K., Hsu R. Y., Lane M. D. (1980) Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J. Biol. Chem. 255, 4745–4750 [PubMed] [Google Scholar]
- 14. Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 15. Frezza C., Cipolat S., Scorrano L. (2007) Organelle isolation. Functional mitochondria from mouse liver, muscle, and cultured fibroblasts. Nat. Protoc. 2, 287–295 [DOI] [PubMed] [Google Scholar]
- 16. Bandhakavi S., Stone M. D., Onsongo G., Van Riper S. K., Griffin T. J. (2009) A dynamic range compression and three-dimensional peptide fractionation analysis platform expands proteome coverage and the diagnostic potential of whole saliva. J. proteome Res. 8, 5590–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffin T. J., Xie H., Bandhakavi S., Popko J., Mohan A., Carlis J. V., Higgins L. (2007) iTRAQ reagent-based quantitative proteomic analysis on a linear ion trap mass spectrometer. J. Proteome Res. 6, 4200–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller A., Nesvizhskii A. I., Kolker E., Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 [DOI] [PubMed] [Google Scholar]
- 19. Onsongo G., Stone M. D., Van Riper S. K., Chilton J., Wu B., Higgins L., Lund T. C., Carlis J. V., Griffin T. J. (2010) LTQ-iQuant. A freely available software pipeline for automated and accurate protein quantification of isobaric-tagged peptide data from LTQ instruments. Proteomics 10, 3533–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brand M. D., Nicholls D. G. (2011) Assessing mitochondrial dysfunction in cells. Biochem. J. 435, 297–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jekabsons M. B., Nicholls D. G. (2004) In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. J. Biol. Chem. 279, 32989–33000 [DOI] [PubMed] [Google Scholar]
- 22. Xu X., Arriaga E. A. (2009) Qualitative determination of superoxide release at both sides of the mitochondrial inner membrane by capillary electrophoretic analysis of the oxidation products of triphenylphosphonium hydroethidine. Biochem. J. 46, 905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu X., Thompson L. V., Navratil M., Arriaga E. A. (2010) Analysis of superoxide production in single skeletal muscle fibers. Anal. Chem. 82, 4570–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Long E. K., Rosenberger T. A., Picklo M. J. (2010) Ethanol withdrawal increases glutathione adducts of 4-hydroxy-2-hexenal but not 4-hydroxyl-2-nonenal in the rat cerebral cortex. Free Radic. Biol. Med. 48, 384–390 [DOI] [PubMed] [Google Scholar]
- 25. Janssen A. J., Trijbels F. J., Sengers R. C., Smeitink J. A., van den Heuvel L. P., Wintjes L. T., Stoltenborg-Hogenkamp B. J., Rodenburg R. J. (2007) Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin. Chem. 53, 729–734 [DOI] [PubMed] [Google Scholar]
- 26. Raza H., Robin M. A., Fang J. K., Avadhani N. G. (2002) Multiple isoforms of mitochondrial glutathione S-transferases and their differential induction under oxidative stress. Biochem. J. 366, 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frohnert B. I., Sinaiko A. R., Serrot F. J., Foncea R. E., Moran A., Ikramuddin S., Choudry U., Bernlohr D. A. (2011) Increased adipose protein carbonylation in human obesity. Obesity 19, 1735–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pagliarini D. J., Calvo S. E., Chang B., Sheth S. A., Vafai S. B., Ong S. E., Walford G. A., Sugiana C., Boneh A., Chen W. K., Hill D. E., Vidal M., Evans J. G., Thorburn D. R., Carr S. A., Mootha V. K. (2008) A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kornmann B., Currie E., Collins S. R., Schuldiner M., Nunnari J., Weissman J. S., Walter P. (2009) An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325, 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Budd S. L., Castilho R. F., Nicholls D. G. (1997) Mitochondrial membrane potential and hydroethidine-monitored superoxide generation in cultured cerebellar granule cells. FEBS Lett. 415, 21–24 [DOI] [PubMed] [Google Scholar]
- 31. Stappen R., Krämer R. (1994) Kinetic mechanism of phosphate/phosphate and phosphate/OH- antiports catalyzed by reconstituted phosphate carrier from beef heart mitochondria. J. Biol. Chem. 269, 11240–11246 [PubMed] [Google Scholar]
- 32. Ko Y. H., Delannoy M., Hullihen J., Chiu W., Pedersen P. L. (2003) Mitochondrial ATP synthasome. Cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for Pi and ADP/ATP. J. Biol. Chem. 278, 12305–12309 [DOI] [PubMed] [Google Scholar]
- 33. Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. (1996) Induction of apoptotic program in cell-free extracts. Requirement for dATP and cytochrome c. Cell 86, 147–157 [DOI] [PubMed] [Google Scholar]
- 34. Bradham C. A., Qian T., Streetz K., Trautwein C., Brenner D. A., Lemasters J. J. (1998) The mitochondrial permeability transition is required for tumor necrosis factor α-mediated apoptosis and cytochrome c release. Mol. Cell. Biol. 18, 6353–6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haber C. A., Lam T. K., Yu Z., Gupta N., Goh T., Bogdanovic E., Giacca A., Fantus I. G. (2003) N-Acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo. Possible role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 285, E744–E753 [DOI] [PubMed] [Google Scholar]
- 36. Lautt W. W., Ming Z., Legare D. J. (2010) Attenuation of age- and sucrose-induced insulin resistance and syndrome X by a synergistic antioxidant cocktail. The AMIS syndrome and HISS hypothesis. Can. J. Physiol. Pharmacol. 88, 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perreault M., Marette A. (2001) Targeted disruption of inducible nitric-oxide synthase protects against obesity-linked insulin resistance in muscle. Nat. Med. 7, 1138–1143 [DOI] [PubMed] [Google Scholar]
- 38. Mark R. J., Pang Z., Geddes J. W., Uchida K., Mattson M. P. (1997) Amyloid β-peptide impairs glucose transport in hippocampal and cortical neurons. Involvement of membrane lipid peroxidation. J. Neurosci. 17, 1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagai K., Betsuyaku T., Konno S., Ito Y., Nasuhara Y., Hizawa N., Kondo T., Nishimura M. (2008) Diversity of protein carbonylation in allergic airway inflammation. Free Radic. Res. 42, 921–929 [DOI] [PubMed] [Google Scholar]
- 40. Trevisani M., Siemens J., Materazzi S., Bautista D. M., Nassini R., Campi B., Imamachi N., Andrè E., Patacchini R., Cottrell G. S., Gatti R., Basbaum A. I., Bunnett N. W., Julius D., Geppetti P. (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. U.S.A. 104, 13519–13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yuan Q., Zhu X., Sayre L. M. (2007) Chemical nature of stochastic generation of protein-based carbonyls. Metal-catalyzed oxidation versus modification by products of lipid oxidation. Chem. Res. Toxicol. 20, 129–139 [DOI] [PubMed] [Google Scholar]
- 42. Grimsrud P. A., Picklo M. J., Sr., Griffin T. J., Bernlohr D. A. (2007) Carbonylation of adipose proteins in obesity and insulin resistance. Identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol. Cell. Proteomics 6, 624–637 [DOI] [PubMed] [Google Scholar]
- 43. Yang Y., Yang Y., Xu Y., Lick S. D., Awasthi Y. C., Boor P. J. (2008) Endothelial glutathione S-transferase 4-4 protects against oxidative stress and modulates iNOS expression through NF-κB translocation. Toxicol. Appl. Pharmacol. 230, 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng J. Z., Singhal S. S., Sharma A., Saini M., Yang Y., Awasthi S., Zimniak P., Awasthi Y. C. (2001) Transfection of mGSTA4 in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis by inhibiting JNK-mediated signaling. Arch. Biochem. Biophys. 392, 197–207 [DOI] [PubMed] [Google Scholar]
- 45. Nyström T. (2005) Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 24, 1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim I., Rodriguez-Enriquez S., Lemasters J. J. (2007) Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 462, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Janssen R. J., Nijtmans L. G., van den Heuvel L. P., Smeitink J. A. (2006) Mitochondrial complex I. Structure, function, and pathology. J. Inherit. Metab. Dis. 29, 499–515 [DOI] [PubMed] [Google Scholar]
- 48. Dieteren C. E., Willems P. H., Vogel R. O., Swarts H. G., Fransen J., Roepman R., Crienen G., Smeitink J. A., Nijtmans L. G., Koopman W. J. (2008) Subunits of mitochondrial complex I exist as part of matrix- and membrane-associated subcomplexes in living cells. J. Biol. Chem. 283, 34753–34761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rutanen J., Yaluri N., Modi S., Pihlajamäki J., Vänttinen M., Itkonen P., Kainulainen S., Yamamoto H., Lagouge M., Sinclair D. A., Elliott P., Westphal C., Auwerx J., Laakso M. (2010) SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes 59, 829–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shoubridge E. A. (2001) Nuclear genetic defects of oxidative phosphorylation. Hum. Mol. Genet. 10, 2277–2284 [DOI] [PubMed] [Google Scholar]
- 51. Loeffen J. L., Smeitink J. A., Trijbels J. M., Janssen A. J., Triepels R. H., Sengers R. C., van den Heuvel L. P. (2000) Isolated complex I deficiency in children. Clinical, biochemical, and genetic aspects. Hum. Mutat. 15, 123–134 [DOI] [PubMed] [Google Scholar]
- 52. Hoefs S. J., Dieteren C. E., Distelmaier F., Janssen R. J., Epplen A., Swarts H. G., Forkink M., Rodenburg R. J., Nijtmans L. G., Willems P. H., Smeitink J. A., van den Heuvel L. P. (2008) NDUFA2 complex I mutation leads to Leigh disease. Am. J. Hum. Genet. 82, 1306–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Picklo M. J., Amarnath V., McIntyre J. O., Graham D. G., Montine T. J. (1999) 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J. Neurochem. 72, 1617–1624 [DOI] [PubMed] [Google Scholar]
- 54. Fisher-Wellman K. H., Neufer P. D. (2012) Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol. Metab. 23, 142–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Murphy M. P. (2009) How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Turrens J. F. (2003) Mitochondrial formation of reactive oxygen species. J. Physiol. 552, 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kussmaul L., Hirst J. (2006) The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. U.S.A. 103, 7607–7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen C., Ko Y., Delannoy M., Ludtke S. J., Chiu W., Pedersen P. L. (2004) Mitochondrial ATP synthasome. Three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J. Biol. Chem. 279, 31761–31768 [DOI] [PubMed] [Google Scholar]
- 59. Nishi Y., Fujimoto S., Sasaki M., Mukai E., Sato H., Sato Y., Tahara Y., Nakamura Y., Inagaki N. (2011) Role of mitochondrial phosphate carrier in metabolism-secretion coupling in rat insulinoma cell line INS-1. Biochem. J. 435, 421–430 [DOI] [PubMed] [Google Scholar]
- 60. Leung A. W., Varanyuwatana P., Halestrap A. P. (2008) The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J. Biol. Chem. 283, 26312–26323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alcalá S., Klee M., Fernández J., Fleischer A., Pimentel-Muiños F. X. (2008) A high-throughput screening for mammalian cell death effectors identifies the mitochondrial phosphate carrier as a regulator of cytochrome c release. Oncogene 27, 44–54 [DOI] [PubMed] [Google Scholar]
- 62. Basso E., Petronilli V., Forte M. A., Bernardi P. (2008) Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J. Biol. Chem. 283, 26307–26311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tyler D. D. (1969) Evidence of a phosphate-transporter system in the inner membrane of isolated mitochondria. Biochem. J. 111, 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kolbe H. V., Wohlrab H. (1985) Sequence of the N-terminal formic acid fragment and location of the N-ethylmaleimide-binding site of the phosphate transport protein from beef heart mitochondria. J. Biol. Chem. 260, 15899–15906 [PubMed] [Google Scholar]
- 65. Krämer R. (1998) Mitochondrial carrier proteins can reversibly change their transport mode. The cases of the aspartate/glutamate and the phosphate carrier. Exp. Physiol. 83, 259–265 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.