Background: Gangliosides are receptors for bacterial toxins.
Results: Alpha-toxin from Clostridium perfringens specifically interacts with GM1a.
Conclusion: Trp-84 and Tyr-85 of alpha-toxin are the residues that interact with GM1a, leading to activation of TrkA in A549 cells.
Significance: These results define the role of GM1a-TrkA as a receptor for alpha-toxin.
Keywords: Bacterial Signal Transduction, Cell Biology, Ganglioside, Interleukin, Tetanus Toxin, A549 Cell, Alpha-toxin, GM1a, IL-8, TrkA
Abstract
Clostridium perfringens alpha-toxin is the major virulence factor in the pathogenesis of gas gangrene. Alpha-toxin is a 43-kDa protein with two structural domains; the N-domain contains the catalytic site and coordinates the divalent metal ions, and the C-domain is a membrane-binding site. The role of the exposed loop region (72–93 residues) in the N-domain, however, has been unclear. Here we show that this loop contains a ganglioside binding motif (H … SXWY … G) that is the same motif seen in botulinum neurotoxin and directly binds to a specific conformation of the ganglioside Neu5Acα2-3(Galβ1-3GalNAcβ1-4)Galβ1-4Glcβ1Cer (GM1a) through a carbohydrate moiety. Confocal microscopy analysis using fluorescently labeled BODIPY-GM1a revealed that the toxin colocalized with GM1a and induced clustering of GM1a on the cell membranes. Alpha-toxin was only slightly toxic in β1,4-N-acetylgalactosaminyltransferase knock-out mice, which lack the a-series gangliosides that contain GM1a, but was highly toxic in α2,8-sialyltransferase knock-out mice, which lack both b-series and c-series gangliosides, similar to the control mice. Moreover, experiments with site-directed mutants indicated that Trp-84 and Tyr-85 in the exposed alpha-toxin loop play an important role in the interaction with GM1a and subsequent activation of TrkA. These results suggest that binding of alpha-toxin to GM1a facilitates the activation of the TrkA receptor and induces a signal transduction cascade that promotes the release of chemokines. Therefore, we conclude that GM1a is the primary cellular receptor for alpha-toxin, which can be a potential target for drug developed against this pathogen.
Introduction
Bacterial phospholipase Cs (PLCs)2 are secreted proteins that have preferences for different phospholipids (1, 2). Bacterial PLCs that hydrolyze phosphatidylcholine and sphingomyelin are important virulence factors in the pathogenesis of diseases caused by Listeria monocytogenes, Pseudomonas aeruginosa, and several Clostridium spp. (3–5). Clostridium perfringens, the pathogenic bacterium most widely distributed in nature (6), produces one PLC alpha-toxin that is highly cytotoxic and myotoxic and can lead to hemolysis and the release of superoxide radicals and inflammatory cytokines (7–13). The toxin has been associated with enteritis in domestic animals, and Crohn disease and gas gangrene in humans (14–17).
We previously reported that alpha-toxin-induced activation of endogenous PLC and sphingomyelinase via pertussis toxin-sensitive GTP-binding protein plays an important role in hemolysis of rabbit and sheep erythrocytes (8, 11, 18). We also reported that alpha-toxin simultaneously induced the formation of diacylglycerol through the activation of endogenous PLC and phosphorylation of ERK1/2, NFκB, and p38MAPK via activation of tyrosine kinase A (TrkA) in human lung adenocarcinoma epithelial cell line (A549) cells and that these events induced the release of interleukin-8 (IL-8) (19). In addition, the toxin-induced release of TNF-α was found to be dependent on the activation of ERK1/2 signal transduction via the phosphorylation of TrkA in neutrophils and macrophages (13). Inhibition of the toxin-induced phosphorylation of TrkA by erythromycin led to the arrest of the toxin-induced events (13), thus supporting the hypothesis that TrkA is a key signaling molecule in the action induced by alpha-toxin.
Gangliosides may have either 1 (a-series), 2 (b-series), or 3 (c-series) sialic acid residues linked to the 3-position of the inner galactose moiety or may lack sialic acid altogether (0-series) (20). Gangliosides are present in the plasma membrane of vertebrate cells with their oligosaccharide chains exposed to the external environment, and they have been implicated as cell surface receptors for several bacterial toxins. Purified tetanus neurotoxin (TeNT) (21) and botulinum neurotoxin (BoNT) (22) mainly interact with the b-series gangliosides GD3, GD1b, GT1b, and GQ1b. Cholera toxin binds with high affinity and specificity to the ganglioside GM1 (23). Mutoh et al. (24–26) report that GM1 can bind to the TrkA protein, where it regulates the receptor and enhances activation by the direct interaction of GM1 with TrkA in the lipid rafts on the plasma membrane. Ichikawa et al. (27) reported that clustering of GM1 promotes the enrichment of TrkA in the lipid rafts. In this study, we have investigated the role of gangliosides in the pathogenicity of alpha-toxin, and analyzed the binding region with which the toxin associates with gangliosides.
EXPERIMENTAL PROCEDURES
Cell Culture and Drugs
A549 cells were cultured at 37 °C in growth medium (DMEM with 10% horse serum). To prepare ganglioside-depleted A549 cells, the seeded cells were grown in the presence of medium containing 1, 5, or 10 μm d-threo-1-phenyl-2-hexadecanoylamino-3-morpholino-1-propanol (PPMP) (Matreya) for 4 days. The cells were used for immunocytochemistry and cytokine release measurements 4 days after seeding. GM1a was obtained commercially (Calbiochem). Neuraminidase from C. perfringens was obtained from Sigma. Alexa488-conjugated cholera toxin B subunit was purchased from Molecular Probes. All other chemicals were of analytical grade.
Purification of Alpha-toxin
Alpha-toxin was overexpressed in Bacillus subtilis ISW1214 that had been transformed with the plasmid vector, pHY300PLK, carrying the cDNA for alpha-toxin. The expression and purification of recombinant alpha-toxin was performed as described previously (28).
Cy3-coupled Toxin
Alpha-toxin (3 mg/ml) was labeled with the Cy3-labeling kit (GE healthcare) following the manufacturer's protocol. The release of IL-8 from A549 cells treated with Cy3-coupled alpha-toxin (Cy3-alpha-toxin) was the same as that of cells treated with non-labeled toxin.
Glycoarray Assay
The glycoarray plates (Sumitomo Bakelite) were incubated with 100 μg of Cy3-alpha-toxin in PBS containing 1 mm CaCl2 for 15 min; this was followed by washing the plate with PBS. Bound Cy3-alpha-toxin was measured with an Affymetrix 428 (Affymetrix).
Binding of Alpha-toxin to A549 Cells
A549 cells were seeded on a poly-l-lysine glass bottom dish (MatTek). Cy3-alpha-toxin fixed with 2% paraformaldehyde in PBS was added at room temperature for 15 min; the cells were then washed 3 times with PBS. The fluorescence level of Cy3-alpha-toxin in each cell was analyzed by fluorescent microscopy (BIOREVO BZ-9000; Keyence) and the associated analysis software package (BZ-H2A).
IL-8 ELISA
Immunoreactive IL-8 was quantified in cell culture supernatants by a double-Ab ELISA kit using rIL-8 as a standard (R&D Systems) following the manufacturer's protocol.
Confocal Detection of GM1a
A549 cells were seeded on a poly-l-lysine glass bottom dish (MatTek), and 500 nm BODIPY-GM1a (Molecular Probes) was added to the cells for 5 min at 25 °C. The cells were then treated with alpha-toxin or Cy3-alpha-toxin (1.0 μg/ml) at 37 °C, fixed with 4% paraformaldehyde in PBS at room temperature for 15 min, and washed 3 times with PBS. The nuclei were stained with Hoechst33342. Stained cells were visualized using confocal microscopy (Nikon, A1R).
Mice
Twenty-five-week-old male wild-type mice (C57BL/6 strain; Nihon SLC) were used. β1,4-N-Acetylgalactosaminyltransferase knock-out (GalNAcT−/−) mice and α2,8-sialyltransferase-knock-out (ST−/−) mice were constructed previously (29–32). Experimental protocols were approved by the Institute Animal Care and Use Committee at Tokushima Bunri University. The mice were housed in plastic cages under controlled environmental conditions (temperature, 22 °C; humidity, 55%). Food and water were freely available.
Culture of Macrophages
Mouse macrophages were isolated from the cell contents of peritoneal exudates with 2 ml of phenol red-free RPMI1640 medium (Wako Pure Chemical Industries) supplemented with 5% fetal bovine serum (FBS) (Biowest). After centrifugation at 170 g for 10 min at 4 °C, the cell pellet was resuspended in phenol red-free RPMI1640 medium supplemented with 5% FBS. Adherent macrophage monolayers were obtained by plating the cells in 96- or 48-well plastic trays (Falcon).
Site-directed Mutagenesis
The Transformer Site-directed Mutagenesis kit (Takara) was used with the following primers to prepare the modified plasmids: D81A, 5′-AATTTCTCAAAGGCCAATAGTTGGTAT-3′; N82A, 5′-TTCTCAAAGGACGCGTCTTGGTATTTA-3′; S83A, 5′-TCAAAGGATAATGCCTGGTATTTAGCT-3′; W84A, 5′-AAGGATAATAGCGCTTATTTAGCTTAT-3′; Y85A, 5′-GATAATAGTTGGGCCCTAGCTTATTCT-3′; Y88A, 5′-TGGTATTTAGCTGCCTCTATACCTGAC-3′. The genetic sequence of alpha-toxin in each plasmid was confirmed with an ABI310 PRISMTM genetic analyzer (Life Technologies).
Preparation of GM1a Liposomes
Phosphatidylcholine from egg yolk (Nacalai), sphingomyelin from bovine brain (Nacalai), GM1a (Calbiochem), and cholesterol (Nacalai) in a 1:1:1:2 molar ratio were dried from chloroform/methanol (1:2 v/v) under nitrogen gas, resuspended in HBS-N (0.01 m HEPES, pH 7.4, 0.15 m NaCl) (GE Healthcare), and sonicated in a water bath for 5 min to ensure that the lipid was fully hydrated. The liposome suspension was passed 21 times (0.5 ml at a time) through a Liposofast (Avestin) liposome extruder with 100-nm pore size polycarbonate membranes.
Alpha-toxin Membrane Binding Assay
The membrane-binding assay was performed by surface plasmon resonance analysis using a Biacore3000 system (GE Healthcare) and the associated analysis software package (BIAevaluation) (GE Healthcare). Alpha-toxin and variants at concentrations of 5.0, 2.5, 1.0, 0.5, and 0 nm were applied to GM1a liposome-coated L1sensor chips at a flow rate of 5.0 μl/min in a running buffer (0.01 m HEPES, pH 7.4, 0.15 m NaCl, 1 mm CaCl2) at 25 °C. Dissociation was monitored for at least 150 s in a constant flow of enzyme-free running buffer.
In Silico Docking Simulation Analysis of GM1a with Alpha-toxin
All molecular modeling studies were performed using the Molecular Operating Environment software (MOE2011; Chemical Computing Group). The x-ray crystallographic structure of alpha-toxin was obtained from the Protein Data Bank (PDB ID 1CA1) (33). This toxin was prepared for docking studies by adding hydrogen atoms with standard geometries, placing the carbohydrate moiety of GM1a in the vicinity of alpha-toxin residues Trp-84 and Tyr-85, and coordinating the sialic acid of GM1a with Trp-84. The structure was minimized using the MMFF94s force-field.
Statistical Analysis
All data presented are expressed as the mean ± S.E. Mean values among experimental groups were compared using Student's t test, and p < 0.01 was considered statistically significant.
RESULTS
Gangliosides Mediate the Release of IL-8 Induced by Alpha-toxin
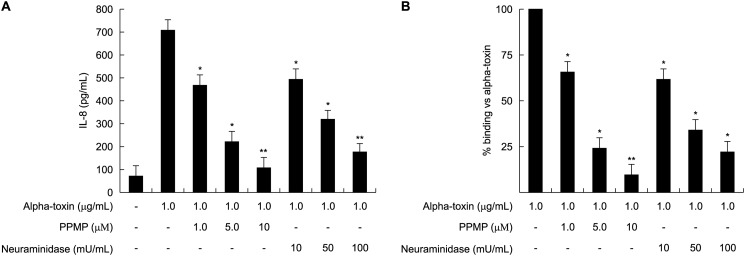
Alpha-toxin induces the release of IL-8 from A549 cells (19). To investigate the effect of gangliosides and sialic acid on the binding of alpha-toxin to A549 cells and on the release of IL-8, the cells were treated with various concentrations of PPMP, which is a ganglioside synthase inhibitor, or neuraminidase from C. perfringens, which catalyzes the hydrolysis of terminal sialic acid residues, at 37 °C for 4 days or 60 min, respectively. The inhibition of ganglioside synthesis and depletion of sialic acid in A549 cells were confirmed with Alexa488-conjugated cholera toxin B subunit that specifically binds to GM1a and possesses weaker affinity to GD1b (34). PPMP- or neuraminidase-treated A549 cells lost their ability to bind to cholera toxin B subunit (supplemental Fig. S1). After this pretreatment, the cells were incubated with 1.0 μg/ml alpha-toxin. The treatment with both PPMP and neuraminidase inhibited the release of IL-8 from A549 cells treated with alpha-toxin in a dose-dependent manner (Fig. 1A). Furthermore, the binding of Cy3-alpha-toxin to the cells was also inhibited by both PPMP and neuraminidase in a dose-dependent manner (Fig. 1B). In addition, treatment of HepG2 and HEK293 cells with these inhibitors resulted in reduction of binding of alpha-toxin to the same level as in A549 cells (data not shown). This suggests, therefore, that gangliosides containing sialic acid play an important role in the binding of the toxin.
FIGURE 1.
Relationship between gangliosides and release of IL-8 induced by alpha-toxin. A549 cells (1.0 × 107 cells/ml) were treated with various concentrations of PPMP or neuraminidase at 37 °C for 4 days or 60 min, respectively, and then the cells were incubated with 1.0 μg/ml alpha-toxin. A, IL-8 in the culture supernatants 3 h after the addition of the toxin was determined by ELISA. Values represent the mean ± S.E.; n = 3; *, p < 0.01; **, p < 0.005, compared with the release of IL-8 in the cells treated with alpha-toxin. B, the pretreated cells with these inhibitors were incubated with Cy3-alpha-toxin for 15 min. The cells were fixed in 4% paraformaldehyde and analyzed using fluorescence microscopy. The fluorescence intensity in the visual fields was measured as described under “Experimental Procedures.” The binding of alpha-toxin to the intact cells was set as the maximal response (100%) against which all other results were compared. Values represent the mean ± S.E.; n = 3; *, p < 0.01; **, p < 0.005, compared with the binding of alpha-toxin in the untreated cells.
Alpha-toxin Binds Directly to GM1a
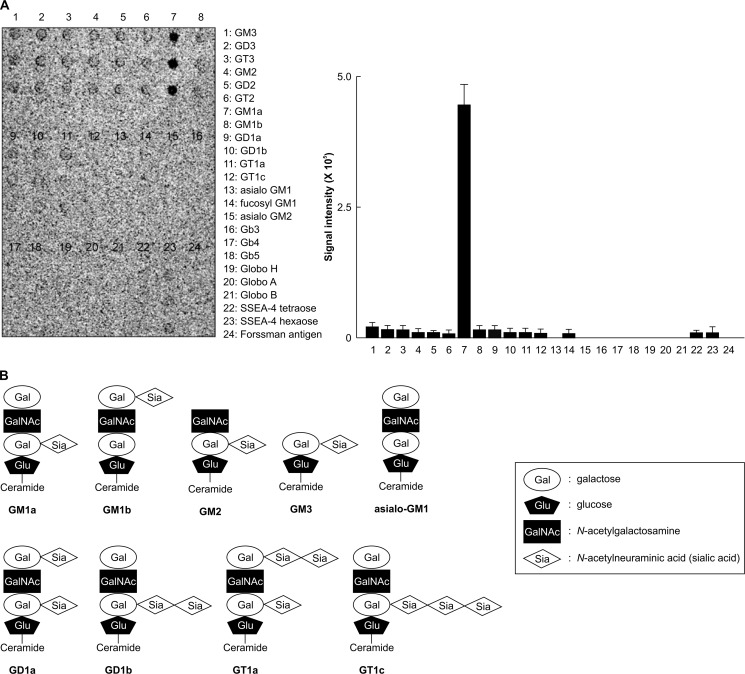
We investigated the interaction of alpha-toxin with various gangliosides using an in vitro glycoarray system (Fig. 2A). The array plate was incubated with 100 μg/ml Cy3-alpha-toxin at 37 °C for 60 min, and the fluorescence signal of alpha-toxin was strongly localized at the spot corresponding to the carbohydrate chain of GM1a (Fig. 2A). The toxin was slightly bound to gangliosides that contained sialic acid but not to gangliosides such as asialo-GM1, which lack sialic acid (Fig. 2A). Binding of alpha-toxin to GM1b, GM2, and GM3 was significantly weaker than that to GM1a (Fig. 2A). Because GM1b, GM2, and GM3 contain sialic acid (Fig. 2B), our results indicate that sialic acid is not sufficient for the specific binding of alpha-toxin to gangliosides. Furthermore, the binding of alpha-toxin to GD1a, GD1b, GT1a, and GT1c, which have the same carbohydrate chain as GM1a but contain different numbers of sialic acid residues (Fig. 2B), was substantially less than the binding to GM1a (Fig. 2A). Therefore, it appears that the structural conformation of GM1a is important for its binding to alpha-toxin.
FIGURE 2.
Glycoarray analysis. A, the glycoarray plate was incubated with 100 μg/ml Cy3-alpha-toxin for 15 min. Fluorescence intensity of the toxin on the plate was measured with a fluorescence scanner. Values represent the mean ± S.D. *, p < 0.01 (n = 3). B, schematic figures of representative gangliosides described in the study are shown. SSEA-4 tetraose, Neu5Acα2-3Galβ1-3GalNAcβ1-3Gal; SSEA-4 hexaose, Neu5Acα2-3Galβ1-3GalNAcβ1-3Galα1-4Galβ1-4Glc.
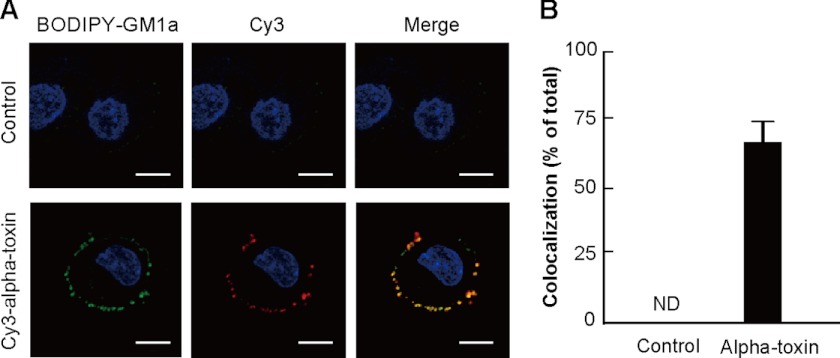
Colocalization of GM1a and Alpha-toxin
We examined whether alpha-toxin colocalized with GM1a on the cell membrane by incubating BODIPY-GM1a-labeled cells with Cy3-alpha-toxin at 37 °C for 15 min. The BODIPY-GM1a in the cells treated with alpha-toxin aggregated on the cell membranes, but aggregation in control cells was undetectable in our experimental conditions (Fig. 3A). Cy3-alpha-toxin was colocalized at sites of GM1a aggregation (Fig. 3A), and the percentage of colocalization was ∼70% (Fig. 3B). Therefore, it appears that alpha-toxin specifically binds to GM1a on biological membranes.
FIGURE 3.
Localization of alpha-toxin and GM1a. A, A549 cells stained with BODIPY-GM1a (green) were incubated with 1.0 μg/ml Cy3-alpha-toxin (red) at 37 °C for 15 min. The cells were fixed in 4% paraformaldehyde and stained with Hoechst 33342. Alpha-toxin, GM1a, and nuclei were visualized using confocal laser microscopy. The data represent the means for three independent experiments. Scale bar, 10 μm. B, the percentage of colocalization was calculated for each combination of fluorescence markers by analysis of each confocal plane containing more than 30 cells. Values represent the mean ± S.E.; n = 3; *, p < 0.003, compared with the colocalization of alpha-toxin with GM1a in untreated cells. ND, not determined.
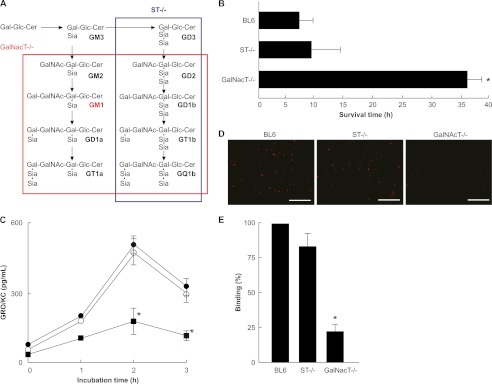
Sensitivity of Wild-type, GalNAcT−/−, and ST−/− Mice to Alpha-toxin
Next, we examined the response of GalNAcT−/− mice (lacking GM1a, GM2, GD2, GD1a, GD1b, GT1a, GT1b, GQ1b, etc.) and ST−/− mice (lacking b- and c-series gangliosides) to alpha-toxin (Fig. 4A). We injected 200 ng of alpha-toxin intraperitoneally into wild-type, GalNAcT−/−, and ST−/− mice. The wild-type and ST−/− mice succumbed to the toxic effects of the toxin within about 10 h, whereas the average survival time for GalNAcT−/− mice was 36 h (Fig. 4B). Macrophages isolated from wild-type, GalNAcT−/−, and ST−/− mice were incubated with 1.0 μg/ml alpha-toxin at 37 °C for various periods of time. As shown in Fig. 4C, the release of GRO/KC (a member of the CXC chemokine family with homology to human IL-8) from macrophages isolated from GalNAcT−/− mice was significantly lower than that from macrophages isolated from wild-type or ST−/− mice. Furthermore, the binding of Cy3-alpha-toxin to the macrophages from GalNAcT−/− mice was weaker than that to macrophages from wild-type and ST−/− mice (Fig. 4D).
FIGURE 4.
Sensitivity of wild-type and knock-out mice to alpha-toxin. A, the red or blue frame indicates the ganglioside biosynthesis pathway that is blocked in the GalNacT−/− or ST−/− mice, respectively. Cer, ceramide; Sia, sialic acid; LacCer, lactosylceramide. B, 25-week-old male mice weighing 25–28 g were injected intraperitoneally with 200 ng of alpha-toxin. The average survival time of 4 mice is shown. C, the macrophages from various mice were incubated with alpha-toxin for the indicated periods. GRO/KC in the serum was assayed with ELISA. Values represent the mean ± S.E.; n = 3; *, p < 0.01, compared with the release of GRO/KC from wild-type mouse macrophages. D, the macrophages from various mice were incubated with Cy3-alpha-toxin (1.0 μg/ml) for 15 min are shown. The cells were fixed in 4% paraformaldehyde and analyzed using fluorescence microscopy. The data represent the mean for three independent experiments. Scale bar, 100 μm. E, the fluorescence intensity in the visual fields was measured as described under “Experimental Procedures.” The binding of alpha-toxin to the cells from wild-type mice was set as the maximal response (100%), against which all other results were compared. Values represent the mean ± S.E.; n = 3; *, p < 0.01, compared with the binding of alpha-toxin to macrophages from wild-type mice.
Role of Amino Acids in the Loop Region of Alpha-toxin
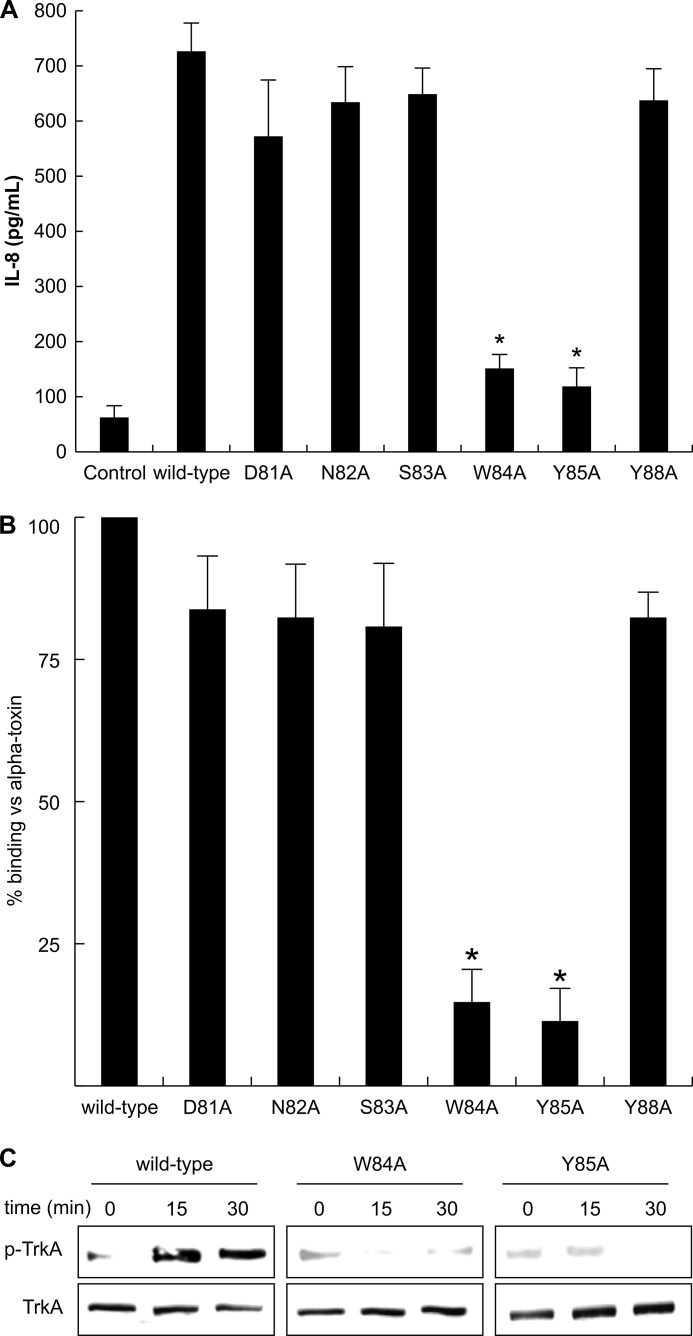
Clark et al. (35) previously suggested that the 72–93-residue loop in alpha-toxin participates in membrane binding. Previous experiments with TeNT and BoNT found that the lactose- and ganglioside-GT1b-binding site is characterized by the presence of the peptide motif H … SXWY … G, with the tryptophan and tyrosine residues being especially important (34, 37). This motif is also found in alpha-toxin (Fig. 5). We focused on Trp-84, Tyr-85, and their surrounding residues and constructed the following alpha-toxin variants with single amino acid changes in this loop: D81A, N82A, S83A, W84A, Y85A, and Y88A. To determine whether these variants induce the release of IL-8 from A549 cells, the cells were incubated with each of the variants for 3 h at 37 °C. As shown in Fig. 6A, the release of IL-8 by D81A, N82A, S83A, and Y88A was the same as that for the wild-type toxin, but very little release was seen with the W84A and Y85A variants. In addition, the binding of Cy3-W84A and Cy3-Y85A to A549 cells was about 20% that of the Cy3-wild-type toxin (Fig. 6B). Moreover, the W84A and Y85A variants failed to induce phosphorylation of TrkA in A549 cells (Fig. 6C).
FIGURE 5.
Amino acid sequence alignment. The amino acid residues forming the ganglioside-binding pocket in BoNT/A, BoNT/B, TeNT, and alpha-toxin are presented as white letters on a black background. Positions of amino acids of alpha-toxin selected for mutational analyses are highlighted by asterisks above the alpha-toxin sequence.
FIGURE 6.
The role of amino acids coordinated at the loop region of the alpha-toxin. A, A549 cells (1 × 107 cells/ml) were incubated with various toxins (1.0 μg/ml) at 37 °C for 3 h. IL-8 in the culture supernatants was assayed with an ELISA kit. Values represent the mean ± S.E.; n = 3; *, p < 0.005, compared with the release of IL-8 in the cells treated with wild-type toxin. B, A549 cells (1 × 107 cells/ml) were incubated with Cy3-variant toxin (1.0 μg/ml) for 15 min. The cells were fixed in 4% paraformaldehyde and analyzed using fluorescence microscopy. The fluorescent intensity in the visual fields was measured as described under “Experimental Procedures.” The binding of wild-type toxin to the cells was set as the maximal response (100%), against which all other results were compared. Values represent the mean ± S.E.; n = 3; *, p < 0.01, compared with the binding of the wild-type toxin in A549 cells. C, A549 cells (1 × 107 cells/ml) were incubated with wild-type toxin or the W84A and Y85A variants at 37 °C for 15 and 30 min. The lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies against phosphorylated TrkA and TrkA. The data represent the mean for three independent experiments.
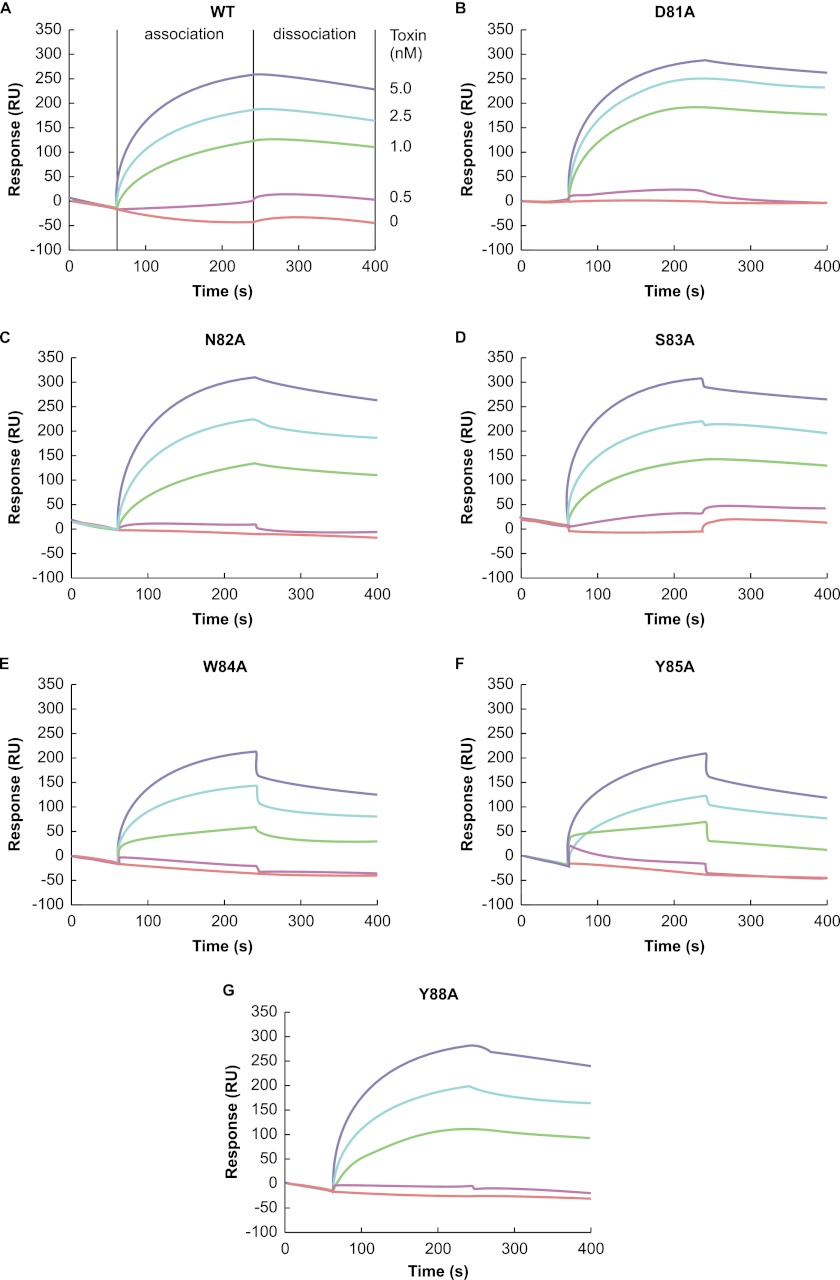
Surface Plasmon Resonance Analysis
We analyzed the affinity of these variants for GM1a-liposomes by surface plasmon resonance. Fig. 7 shows both the liposome association, represented by an increase in resonance units, and dissociation, represented by a decrease in resonance units. Wild-type toxin rapidly associated with GM1a-liposomes and slowly dissociated from them, and the resonance units of wild-type toxin binding to GM1a-liposomes increased dose-dependently (red for vehicle, pink for 0.2 nm, green for 1.0 nm, blue for 2.5 nm, and navy for 5.0 nm) (Fig. 7A). The steady-state binding parameters were determined with the BIAevaluation software, and the KD value for the wild-type toxin was estimated to be 23 nm. The binding curves for the alpha-toxin variants (Fig. 7, B–G) showed estimated KD values of 34, 54, 68, 520, 448, and 48 nm for D81A, N82A, S83A, W84A, Y85A, and Y88A, respectively. The KD values of W84A and Y85A were about 20-fold higher than that of the wild-type toxin, suggesting that the affinity of W84A and Y85A to GM1a-liposomes is markedly reduced compared with that of the wild-type toxin.
FIGURE 7.
Surface plasmon resonance analysis of binding of variants to GM1a-liposomes. Binding of the wild-type toxin (A) and the D81A (B), N82A (C), S83A (D), W84A (E), Y85A (F), and Y88A (G) variants to GM1a-liposomes on L1 sensor chips was measured on a Biacore 3000. Blue, light blue, green, pink, and red lines indicate the concentrations of the variants with 5.0, 2.5, 1.0, 0.5, and 0 nm alpha-toxin, respectively. Association and dissociation represent the buffer in the presence or absence of the input toxins, respectively. A representative result from one of three experiments is shown.
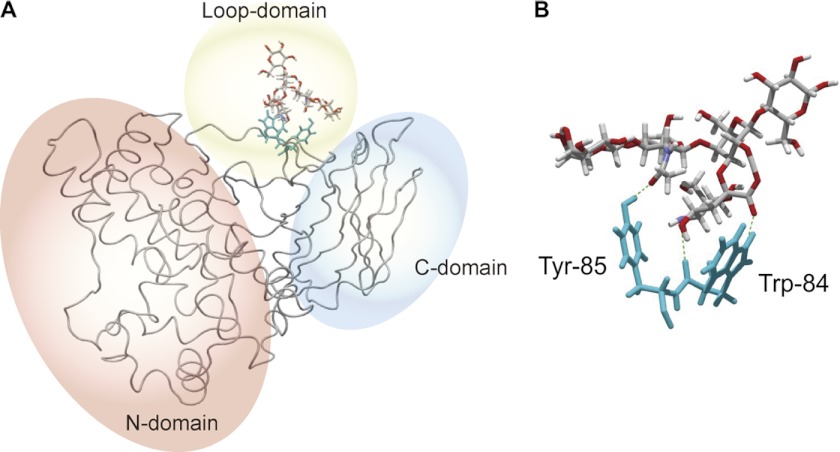
Model for Binding of Alpha-toxin to the Carbohydrate Moiety of GM1a
Next, we investigated the mode of binding between alpha-toxin and GM1a, especially as it relates to residues Trp-84 and Tyr-85, by in silico docking simulation analyses of this interaction at the tertiary structure level using the MOE software. The analysis was based on the structure of the complex between BoNT/A and GT1b (PDB ID 2VU9). Fig. 8A shows the entire structure that resulted from the docking simulation analysis between alpha-toxin and GM1a. Trp-84 interacted with the sialic acid of GM1a through a hydrophobic ring stacking mechanism and a hydrogen bond, and Tyr-85 interacted with the galactosamine through a hydrogen bond (Fig. 8B).
FIGURE 8.
Binding model of alpha-toxin and the carbohydrate moiety of GM1a. A, simulation analysis of the docking of the carbohydrate moiety of GM1a onto alpha-toxin is shown. B, shown is a close-up view of the modeled interactions between the carbohydrate moiety of GM1a and Trp-84 and Tyr-85 of alpha-toxin. Hydrogen bonds are represented by dashed lines.
DISCUSSION
Alpha-toxin is the major virulence factor in the pathogenesis of gas gangrene, a devastating disease associated with traumatic injuries or surgical wounds (14). Despite the recent identification of the residues critical for the toxicity of alpha-toxin (36–38), the binding mechanism of this toxin is still not completely understood. In this study we have shown that the pretreatment of A549 cells with PPMP resulted in attenuation of alpha-toxin binding. Alpha-toxin specifically bound to the ganglioside GM1a and induced the activation of TrkA, and we found that GM1a plays an important role in the lethality of alpha-toxin in mice. Our mutagenesis study has now identified two specific amino acid residues, Trp-84 and Tyr-85, that play a critical role in the binding of alpha-toxin to GM1a.
We measured the binding capacity of alpha-toxin to various gangliosides and found that alpha-toxin specifically binds to GM1a. Sialic acid is required for the binding because asialo-GM1 (which lacks sialic acid) did not bind to alpha-toxin. In addition, neuraminidase dose-dependently inhibited the binding of alpha-toxin to A549 cells and the subsequent release of IL-8. These results support those reported by Flores-Díaz et al. (1) that GM1a protected artificial and cellular membranes from the disruption caused by alpha-toxin, whereas asialo-GM1a did not. GT1b, GQ1b, and GD1b, which contain the same sugar chains as GM1a but have different numbers of sialic acids, bound to alpha-toxin less tightly than GM1a, suggesting that the conformation of GM1a for alpha-toxin binding is important. Alpha-toxin bound to GM2 through the same carbohydrate moiety as GM1a, but its binding was less extensive than to GM1a. This suggests that the additional Galβ1 residue at the 5′ end of the carbohydrate moiety of GM1a is required for effective binding to alpha-toxin. Flores-Díaz et al. (1) reported that alpha-toxin has higher affinity for GT1b and GD1a (b-series) than GM1a and GD1a (a-series) galactosides. This difference is probably due to the difference in assay systems. In our binding assays we used a plate coated with sugar chains from various gangliosides that minimized the conformational possibilities of the glycolipid oligosaccharides.
The response of GalNAcT−/− mice, which lack all complex gangliosides including GM1a, to alpha-toxin was lower than that of wild-type mice. On the other hand, the response of ST−/− mice, which lack b- and c-series gangliosides, to the toxin was the same as that of wild-type mice. These results show that the toxic effect of alpha-toxin in mice is mediated primarily by a-series gangliosides and by GM1a in particular. The b- and c-series gangliosides may not be essential for alpha-toxin at the initial step during the intoxication process in mice, and it appears that GM1a plays an important role in the pathogenesis of alpha-toxin. In addition, alpha-toxin-induced death of GalNAcT−/− mice was not completely inhibited. The C-domain of alpha-toxin has ability of binding to phospholipids of cell membranes (33, 39, 40). It, therefore, appears that the binding of phospholipids via C-domain of alpha-toxin also participated in the pathogenesis of the toxin.
Clostridial neurotoxins such as TeNT and BoNT have a lactose- or ganglioside-binding site that is characterized by the presence of the peptide motif H … SXWY … G (41–45), and a similar motif is conserved in alpha-toxin. We have generated single-point mutants of six amino acid residues (Asp-81, Asn-82, Ser-83, Trp-84, Tyr-85, Tyr-88) to clarify the molecular interaction between alpha-toxin and GM1a. Substitution of Trp-84 and Tyr-85 residues located in positions comparable to the ganglioside binding pockets of TeNT and BoNTs dramatically affected the binding to GM1a-liposome and the release of IL-8. In addition, we showed that the W84A and Y85A variants were significantly reduced in their ability to activate TrkA. GM1a associates with TrkA on cell membranes and activates TrkA and ERK1/2 (46–48). Cholera toxin, which is a high affinity ligand for GM1, activated TrkA in PC12 cells (47). Ichikawa et al. (27) reported that GM1 clustering promotes the enrichment of TrkA in the lipid raft and activation of downstream signal transduction pathways. We also found the alpha-toxin-induced clustering of BODIPY-GM1a and the colocalization of BODIPY-GM1a and Cy3-alpha-toxin on the cell membranes (Fig. 3). The high concentration (more than 5 mol%) of BODIPY-GM1a indicates the red fluorescence. We could not detect the red fluorescence of BODIPY-GM1a in A549 cells treated with non-labeled alpha-toxin under our experimental conditions (data not shown). These results suggest that the binding of alpha-toxin to GM1a plays an important role in the clustering and activation of TrkA.
The results of our current in silico docking analyses show that Trp-84 interacts with the sialic acid of GM1a through a hydrophobic ring stacking mechanism and a hydrogen bond, and Tyr-85 interacts with the galactosamine of GM1a through a hydrogen bond. The structures of BoNT and ganglioside complexes show that the indole ring of the tryptophan residue in the loop motif is crucial for the structural integrity of the sialic acid-binding site (49–51). Thus, our results support the hypothesis that Trp-84 of alpha-toxin plays an important role in the interaction with the sialic acid of GM1a. The C-domain (C2-like domain) of alpha-toxin contains a phospholipid-binding site (33, 39, 40), and our results suggest that the ganglioside-binding site in the loop domain is a second binding site and that binding of alpha-toxin to gangliosides is the first step in its cytotoxic mechanism.
In conclusion, Trp-84 and Tyr-85 of alpha-toxin specifically interact with GM1a, leading to the clustering of GM1a at the cell membrane and activation of TrkA in A549 cells. Thus, GM1a can be a potential target for the development of drugs against this pathogen.
Supplementary Material
Acknowledgments
We thank Riko Hayashi and Kota Igarashi for technical assistance.
This work was supported in part by Grant-in-aid for Scientific Research 21790431 from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of the Japanese Government and MEXT-supported Program for the Strategic Research Foundation at Private Universities, 2008–2012 (S0801078).

This article contains supplemental Fig. S1.
- PLC
- phospholipase C
- TrkA
- tyrosine kinase A
- TeNT
- tetanus neurotoxin
- BoNT
- botulinum neurotoxin
- PPMP
- d-threo-1-phenyl-2-hexadecanoylamino-3-morpholino-1-propanol.
REFERENCES
- 1. Flores-Díaz M., Alape-Girón A., Clark G., Catimel B., Hirabayashi Y., Nice E., Gutiérrez J. M., Titball R., Thelestam M. (2005) A cellular deficiency of gangliosides causes hypersensitivity to Clostridium perfringens phospholipase C. J. Biol. Chem. 280, 26680–26689 [DOI] [PubMed] [Google Scholar]
- 2. Urbina P., Collado M. I., Alonso A., Goñi F. M., Flores-Díaz M., Alape-Girón A., Ruysschaert J. M., Lensink M. F. (2011) Unexpected wide substrate specificity of C. perfringens alpha-toxin phospholipase C. Biochim. Biophys. Acta 1808, 2618–2627 [DOI] [PubMed] [Google Scholar]
- 3. Songer J. G. (1997) Bacterial phospholipases and their role in virulence. Trends Microbiol. 5, 156–161 [DOI] [PubMed] [Google Scholar]
- 4. Vázquez-Boland J. A., Kuhn M., Berche P., Chakraborty T., Domínguez-Bernal G., Goebel W., González-Zorn B., Wehland J., Kreft J. (2001) Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14, 584–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stonehouse M. J., Cota-Gomez A., Parker S. K., Martin W. E., Hankin J. A., Murphy R. C., Chen W., Lim K. B., Hackett M., Vasil A. I., Vasil M. L. (2002) A novel class of microbial phosphocholine-specific phospholipases C. Mol. Microbiol. 46, 661–676 [DOI] [PubMed] [Google Scholar]
- 6. Shimizu K., Kawasaki Y., Hiraga S., Tawaramoto M., Nakashima N., Sugino A. (2002) The fifth essential DNA polymerase φ in Saccharomyces cerevisiae is localized to the nucleolus and plays an important role in synthesis of rRNA. Proc. Natl. Acad. Sci. U.S.A. 99, 9133–9138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oda M., Ikari S., Matsuno T., Morimune Y., Nagahama M., Sakurai J. (2006) Signal transduction mechanism involved in Clostridium perfringens alpha-toxin-induced superoxide anion generation in rabbit neutrophils. Infect. Immun. 74, 2876–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ochi S., Oda M., Matsuda H., Ikari S., Sakurai J. (2004) Clostridium perfringens alpha-toxin activates the sphingomyelin metabolism system in sheep erythrocytes. J. Biol. Chem. 279, 12181–12189 [DOI] [PubMed] [Google Scholar]
- 9. Oda M., Saito Y., Morimune Y., Nagahama M., Sakurai J. (2011) Induction of neurite-outgrowth in PC12 cells by alpha-toxin from Clostridium perfringens. Biochem. Biophys. Res. Commun. 411, 241–246 [DOI] [PubMed] [Google Scholar]
- 10. Ochi S., Miyawaki T., Matsuda H., Oda M., Nagahama M., Sakurai J. (2002) Clostridium perfringens alpha-toxin induces rabbit neutrophil adhesion. Microbiology 148, 237–245 [DOI] [PubMed] [Google Scholar]
- 11. Sakurai J., Nagahama M., Oda M. (2004) Clostridium perfringens alpha-toxin. Characterization and mode of action. J. Biochem. 136, 569–574 [DOI] [PubMed] [Google Scholar]
- 12. Bryant A. E., Stevens D. L. (1996) Phospholipase C and perfringolysin O from Clostridium perfringens up-regulate endothelial cell-leukocyte adherence molecule 1 and intercellular leukocyte adherence molecule 1 expression and induce interleukin-8 synthesis in cultured human umbilical vein endothelial cells. Infect. Immun. 64, 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oda M., Kihara A., Yoshioka H., Saito Y., Watanabe N., Uoo K., Higashihara M., Nagahama M., Koide N., Yokochi T., Sakurai J. (2008) Effect of erythromycin on biological activities induced by Clostridium perfringens alpha-toxin. J. Pharmacol. Exp. Ther. 327, 934–940 [DOI] [PubMed] [Google Scholar]
- 14. Titball R. W., Naylor C. E., Basak A. K. (1999) The Clostridium perfringens alpha-toxin. Anaerobe 5, 51–64 [DOI] [PubMed] [Google Scholar]
- 15. Sakurai J., Nagahama M., Oda M., Tsuge H., Kobayashi K. (2009) Clostridium perfringens ι-toxin. Structure and function. Toxins 1, 208–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keyburn A. L., Sheedy S. A., Ford M. E., Williamson M. M., Awad M. M., Rood J. I., Moore R. J. (2006) Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 74, 6496–6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bunting M., Lorant D. E., Bryant A. E., Zimmerman G. A., McIntyre T. M., Stevens D. L., Prescott S. M. (1997) Alpha-toxin from Clostridium perfringens induces proinflammatory changes in endothelial cells. J. Clin. Invest. 100, 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oda M., Matsuno T., Shiihara R., Ochi S., Yamauchi R., Saito Y., Imagawa H., Nagahama M., Nishizawa M., Sakurai J. (2008) The relationship between the metabolism of sphingomyelin species and the hemolysis of sheep erythrocytes induced by Clostridium perfringens alpha-toxin. J. Lipid Res. 49, 1039–1047 [DOI] [PubMed] [Google Scholar]
- 19. Oda M., Shiihara R., Ohmae Y., Kabura M., Takagishi T., Kobayashi K., Nagahama M., Inoue M., Abe T., Setsu K., Sakurai J. (2012) Clostridium perfringens alpha-toxin induces the release of IL-8 through a dual pathway via TrkA in A549 cells. Biochim. Biophys. Acta 1822, 1581–1589 [DOI] [PubMed] [Google Scholar]
- 20. Tettamanti G. (2004) Ganglioside/glycosphingolipid turnover. New concepts. Glycoconj. J. 20, 301–317 [DOI] [PubMed] [Google Scholar]
- 21. Holmgren J., Elwing H., Fredman P., Strannegård O., Svennerholm L. (1980) Gangliosides as receptors for bacterial toxins and Sendai virus. Adv. Exp. Med. Biol. 125, 453–470 [DOI] [PubMed] [Google Scholar]
- 22. Kitamura M., Iwamori M., Nagai Y. (1980) Interaction between Clostridium botulinum neurotoxin and gangliosides. Biochim. Biophys. Acta 628, 328–335 [DOI] [PubMed] [Google Scholar]
- 23. Heyningen S Van. (1974) Cholera toxin. Interaction of subunits with ganglioside GM1. Science 183, 656–657 [DOI] [PubMed] [Google Scholar]
- 24. Mutoh T., Tokuda A., Miyadai T., Hamaguchi M., Fujiki N. (1995) Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. U.S.A. 92, 5087–5091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mutoh T., Hamano T., Tokuda A., Kuriyama M. (2000) Unglycosylated Trk protein does not co-localize nor associate with ganglioside GM1 in stable clone of PC12 cells overexpressing Trk (PCtrk cells). Glycoconj. J. 17, 233–237 [DOI] [PubMed] [Google Scholar]
- 26. Pitto M., Mutoh T., Kuriyama M., Ferraretto A., Palestini P., Masserini M. (1998) Influence of endogenous GM1 ganglioside on TrkB activity in cultured neurons. FEBS Lett. 439, 93–96 [DOI] [PubMed] [Google Scholar]
- 27. Ichikawa N., Iwabuchi K., Kurihara H., Ishii K., Kobayashi T., Sasaki T., Hattori N., Mizuno Y., Hozumi K., Yamada Y., Arikawa-Hirasawa E. (2009) Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J. Cell Sci. 122, 289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagahama M., Okagawa Y., Nakayama T., Nishioka E., Sakurai J. (1995) Site-directed mutagenesis of histidine residues in Clostridium perfringens alpha-toxin. J. Bacteriol. 177, 1179–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitamura M., Takamiya K., Aizawa S., Furukawa K. (1999) Gangliosides are the binding substances in neural cells for tetanus and botulinum toxins in mice. Biochim. Biophys. Acta 1441, 1–3 [DOI] [PubMed] [Google Scholar]
- 30. Kitamura M., Igimi S., Furukawa K. (2005) Different response of the knockout mice lacking b-series gangliosides against botulinum and tetanus toxins. Biochim. Biophys. Acta 1741, 1–3 [DOI] [PubMed] [Google Scholar]
- 31. Takamiya K., Yamamoto A., Furukawa K., Yamashiro S., Shin M., Okada M., Fukumoto S., Haraguchi M., Takeda N., Fujimura K., Sakae M., Kishikawa M., Shiku H., Furukawa K., Aizawa S. (1996) Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc. Natl. Acad. Sci. U.S.A. 93, 10662–10667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okada M., Itoh Mi M., Haraguchi M., Okajima T., Inoue M., Oishi H., Matsuda Y., Iwamoto T., Kawano T., Fukumoto S., Miyazaki H., Furukawa K., Aizawa S. (2002) b-series ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J. Biol. Chem. 277, 1633–1636 [DOI] [PubMed] [Google Scholar]
- 33. Naylor C. E., Eaton J. T., Howells A., Justin N., Moss D. S., Titball R. W., Basak A. K. (1998) Structure of the key toxin in gas gangrene. Nat. Struct. Biol. 5, 738–746 [DOI] [PubMed] [Google Scholar]
- 34. Arimitsu H., Tsukamoto K., Ochi S., Sasaki K., Kato M., Taniguchi K., Oguma K., Tsuji T. (2009) Lincomycin-induced overexpression of mature recombinant cholera toxin B subunit and the holotoxin in Escherichia coli. Protein Expr. Purif. 67, 96–103 [DOI] [PubMed] [Google Scholar]
- 35. Clark G. C., Briggs D. C., Karasawa T., Wang X., Cole A. R., Maegawa T., Jayasekera P. N., Naylor C. E., Miller J., Moss D. S., Nakamura S., Basak A. K., Titball R. W. (2003) Clostridium absonum alpha-toxin. New insights into clostridial phospholipase C substrate binding and specificity. J. Mol. Biol. 333, 759–769 [DOI] [PubMed] [Google Scholar]
- 36. Alape-Girón A., Flores-Díaz M., Guillouard I., Naylor C. E., Titball R. W., Rucavado A., Lomonte B., Basak A. K., Gutiérrez J. M., Cole S. T., Thelestam M. (2000) Identification of residues critical for toxicity in Clostridium perfringens phospholipase C, the key toxin in gas gangrene. Eur. J. Biochem. 267, 5191–5197 [DOI] [PubMed] [Google Scholar]
- 37. Walker N., Holley J., Naylor C. E., Flores-Díaz M., Alape-Girón A., Carter G., Carr F. J., Thelestam M., Keyte M., Moss D. S., Basak A. K., Miller J., Titball R. W. (2000) Identification of residues in the carboxyl-terminal domain of Clostridium perfringens alpha-toxin (phospholipase C) that are required for its biological activities. Arch. Biochem. Biophys. 384, 24–30 [DOI] [PubMed] [Google Scholar]
- 38. Jepson M., Bullifent H. L., Crane D., Flores-Diaz M., Alape-Giron A., Jayasekeera P., Lingard B., Moss D., Titball R. W. (2001) Tyrosine 331 and phenylalanine 334 in Clostridium perfringens alpha-toxin are essential for cytotoxic activity. FEBS Lett. 495, 172–177 [DOI] [PubMed] [Google Scholar]
- 39. Nagahama M., Mukai M., Morimitsu S., Ochi S., Sakurai J. (2002) Role of the C-domain in the biological activities of Clostridium perfringens alpha-toxin. Microbiol. Immunol. 46, 647–655 [DOI] [PubMed] [Google Scholar]
- 40. Jepson M., Howells A., Bullifent H. L., Bolgiano B., Crane D., Miller J., Holley J., Jayasekera P., Titball R. W. (1999) Differences in the carboxyl-terminal (putative phospholipid binding) domains of Clostridium perfringens and Clostridium bifermentans phospholipases C influence the hemolytic and lethal properties of these enzymes. Infect. Immun. 67, 3297–3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsukamoto K., Kozai Y., Ihara H., Kohda T., Mukamoto M., Tsuji T., Kozaki S. (2008) Identification of the receptor-binding sites in the carboxyl-terminal half of the heavy chain of botulinum neurotoxin types C and D. Microb. Pathog. 44, 484–493 [DOI] [PubMed] [Google Scholar]
- 42. Emsley P., Fotinou C., Black I., Fairweather N. F., Charles I. G., Watts C., Hewitt E., Isaacs N. W. (2000) The structures of the H(C) fragment of tetanus toxin with carbohydrate subunit complexes provide insight into ganglioside binding. J. Biol. Chem. 275, 8889–8894 [DOI] [PubMed] [Google Scholar]
- 43. Fotinou C., Emsley P., Black I., Ando H., Ishida H., Kiso M., Sinha K. A., Fairweather N. F., Isaacs N. W. (2001) The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J. Biol. Chem. 276, 32274–32281 [DOI] [PubMed] [Google Scholar]
- 44. Rummel A., Bade S., Alves J., Bigalke H., Binz T. (2003) Two carbohydrate binding sites in the H(CC) domain of tetanus neurotoxin are required for toxicity. J. Mol. Biol. 326, 835–847 [DOI] [PubMed] [Google Scholar]
- 45. Rummel A., Karnath T., Henke T., Bigalke H., Binz T. (2004) Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J. Biol. Chem. 279, 30865–30870 [DOI] [PubMed] [Google Scholar]
- 46. Mutoh T., Tokuda A., Inokuchi J., Kuriyama M. (1998) Glucosylceramide synthase inhibitor inhibits the action of nerve growth factor in PC12 cells. J. Biol. Chem. 273, 26001–26007 [DOI] [PubMed] [Google Scholar]
- 47. Yamazaki Y., Horibata Y., Nagatsuka Y., Hirabayashi Y., Hashikawa T. (2007) Fucoganglioside α-fucosyl(α-galactosyl)-GM1. A novel member of lipid membrane microdomain components involved in PC12 cell neuritogenesis. Biochem. J. 407, 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duchemin A. M., Ren Q., Mo L., Neff N. H., Hadjiconstantinou M. (2002) GM1 ganglioside induces phosphorylation and activation of Trk and Erk in brain. J. Neurochem. 81, 696–707 [DOI] [PubMed] [Google Scholar]
- 49. Stenmark P., Dupuy J., Imamura A., Kiso M., Stevens R. C. (2008) Crystal structure of botulinum neurotoxin type A in complex with the cell surface co-receptor GT1b-insight into the toxin-neuron interaction. PLoS Pathog. 4, e1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Strotmeier J., Lee K., Völker A. K., Mahrhold S., Zong Y., Zeiser J., Zhou J., Pich A., Bigalke H., Binz T., Rummel A., Jin R. (2010) Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. Biochem. J. 431, 207–216 [DOI] [PubMed] [Google Scholar]
- 51. Karalewitz A. P., Kroken A. R., Fu Z., Baldwin M. R., Kim J. J., Barbieri J. T. (2010) Identification of a unique ganglioside binding loop within botulinum neurotoxins C and D-SA. Biochemistry 49, 8117–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.