Abstract
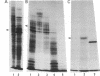
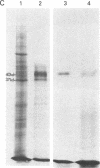
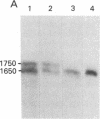
cDNA clones of Adh1, one of two genes encoding alcohol dehydrogenase (ADH; alcohol:NAD+ oxidoreductase, EC 1.1.1.1) in the maize genome, have been isolated. They were derived from mRNA extracted from anaerobically treated roots of maize seedlings. Identification was initially made on the basis of molecular weight and electrophoretic properties of the in vitro polypeptide obtained in hybridization-release-translation experiments. The identification was confirmed by antibody precipitation and by the use of maize stocks having different genetic constitutions at the Adh1 locus. The sequence of the longest cDNA segment, ≈900 base pairs, was determined and appears to code for 168 COOH-terminal amino acids and to have a 3′ nontranslated region of 364 base pairs. Reverse Southern hybridizations established that two different Adh1-S stocks produce a mRNA of 1,650 nucleotides, whereas an additional mRNA of 1,750 nucleotides is produced in three Adh1-F stocks. A 50-fold increase in Adh1 mRNA level occurs during anaerobiosis, reaching a maximum at 5 hr. Return to aerobic conditions indicates a half-life of more than 18 hr for the anaerobically induced Adh1 mRNA.
Keywords: anaerobic stress, DNA sequence determination, reverse Southern hybridization, hybrid release translation, genetic variants
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Ferl R. J., Brennan M. D., Schwartz D. In vitro translation of maize ADH: evidence for the anaerobic induction of mRNA. Biochem Genet. 1980 Aug;18(7-8):681–691. doi: 10.1007/BF00484585. [DOI] [PubMed] [Google Scholar]
- Freeling M. Allelic variation at the level of intragenic recombination. Genetics. 1978 May;89(1):211–224. doi: 10.1093/genetics/89.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling M., Schwartz D. Genetic relationships between the multiple alcohol dehydrogenases of maize. Biochem Genet. 1973 Jan;8(1):27–36. doi: 10.1007/BF00485554. [DOI] [PubMed] [Google Scholar]
- Freeling M. Simultaneous induction by anaerobiosis or 2,4-D of multiple enzymes specificed by two unlinked genes: differential Adh1-Adh2 expression in maize. Mol Gen Genet. 1973 Dec 31;127(3):215–227. doi: 10.1007/BF00333761. [DOI] [PubMed] [Google Scholar]
- Geiser M., Döring H. P., Wöstemeyer J., Behrens U., Tillmann E., Starlinger P. A cDNA clone from Zea mays endosperm sucrose synthetase mRNA. Nucleic Acids Res. 1980 Dec 20;8(24):6175–6188. doi: 10.1093/nar/8.24.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Whitfeld P. R., Matthews R. E. Size distribution and in vitro translation of the RNAs isolated from turnip yellow mosaic virus nucleoproteins. Virology. 1978 Jan;84(1):153–161. doi: 10.1016/0042-6822(78)90227-1. [DOI] [PubMed] [Google Scholar]
- Kelly J., Freeling M. Purification of maize alcohol dehydrogenase-1 allozymes and comparison of their tryptic peptides. Biochim Biophys Acta. 1980 Jul 24;624(1):102–110. doi: 10.1016/0005-2795(80)90229-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Bracker C. E., Tsai C. Y. Storage Protein Synthesis in Maize: Isolation of Zein-synthesizing Polyribosomes. Plant Physiol. 1976 May;57(5):740–745. doi: 10.1104/pp.57.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Schwartz D. Genetic control of alcohol dehydrogenase--a competition model for regulation of gene action. Genetics. 1971 Mar;67(3):411–425. doi: 10.1093/genetics/67.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. Regulation of expression of Adh genes in maize. Proc Natl Acad Sci U S A. 1976 Feb;73(2):582–584. doi: 10.1073/pnas.73.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. The genetic control of alcohol dehydrogenase in maize: gene duplication and repression. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1431–1436. doi: 10.1073/pnas.56.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Tosi M., Young R. A., Hagenbüchle O., Schibler U. Multiple polyadenylation sites in a mouse alpha-amylase gene. Nucleic Acids Res. 1981 May 25;9(10):2313–2323. doi: 10.1093/nar/9.10.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Woodman J. C., Freeling M. Identification of a genetic element that controls the organ-specific expression of adh1 in maize. Genetics. 1981 Jun;98(2):357–378. doi: 10.1093/genetics/98.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]