Background: Heat shock protein 90 (HSP90) has previously been co-purified with P2X1 receptors for ATP.
Results: P2X1 receptor trafficking and responses (currents and calcium rises) were reduced by HSP90 inhibitors.
Conclusion: P2X1 receptors are constitutively regulated by HSP90.
Significance: In addition to a role in cancer treatment, HSP90 inhibitors may provide protection from thrombosis.
Keywords: Collagen, Electrophysiology, HSP90, Platelets, Purinergic Receptor, Trafficking, Calcium, Patch Clamp
Abstract
We have used selective inhibitors to determine whether the molecular chaperone heat shock protein 90 (HSP90) has an effect on both recombinant and native human P2X1 receptors. P2X1 receptor currents in HEK293 cells were reduced by ∼70–85% by the selective HSP90 inhibitor geldanamycin (2 μm, 20 min). This was associated with a speeding in the time course of desensitization as well as a reduction in cell surface expression. Imaging in real time of photoactivatable GFP-tagged P2X receptors showed that they are highly mobile. Geldanamycin almost abolished this movement for P2X1 receptors but had no effect on P2X2 receptor trafficking. P2X1/2 receptor chimeras showed that the intracellular N and C termini were involved in geldanamycin sensitivity. Geldanamycin also inhibited native P2X1 receptor-mediated responses. Platelet P2X1 receptors play an important role in hemostasis, contribute to amplification of signaling to a range of stimuli including collagen, and are novel targets for antithrombotic therapies. Platelet P2X1 receptor-, but not P2Y1 receptor-, mediated increases in intracellular calcium were reduced by 40–45% following HSP90 inhibition with geldanamycin or radicicol. Collagen stimulation leads to ATP release from platelets, and calcium increases to low doses of collagen were also reduced by ∼40% by the HSP90 inhibitors consistent with an effect on P2X1 receptors. These studies suggest that HSP90 inhibitors may be as effective as selective antagonists in regulating platelet P2X1 receptors, and their potential effects on hemostasis should be considered in clinical studies.
Introduction
In blood, ATP is released into the extracellular space in a variety of ways including from damaged cells and platelet granules as well as from endothelial cells in response to shear stress (1). ATP can activate cell surface G protein-coupled P2Y receptors and P2X receptor ion channels (2). P2X receptors comprise a distinct family of ligand-gated ion channels with two transmembrane segments, an extracellular ligand binding loop, and intracellular N and C termini. Seven mammalian P2X receptor subunits (P2X1–7) have been identified, and they assemble to form homo- or heterotrimeric receptors with a range of properties (3). P2X1 receptors are emerging as important regulators of blood cell function. Platelet P2X1 receptors amplify signaling through multiple major stimuli (4), including collagen, thrombin, and Toll-like receptors (5). Activation of platelet P2X1 receptors is particularly important in vivo at high levels of shear because P2X1−/− mice are resistant to thrombosis within small arteries and arterioles (6). Furthermore, overexpression of the P2X1 receptor in platelets resulted in increased mortality due to thromboembolism following intravenous injection of collagen and adrenaline (7). In neutrophils, P2X1 receptor activation promotes chemotaxis through Rho kinase activation (8) and provides a protective role in endotoxemia (9). In T lymphocytes P2X1 receptors contribute to activation at the immune synapse (10). The activity of P2X1 receptors can therefore have an important impact on cardiovascular health, and P2X1 receptor-selective antagonists have therapeutic potential as antithrombotic agents and in stroke prevention.
An understanding of the cellular mechanisms of regulation of P2X1 receptor activity is developing. We have shown that P2X1 receptors preferentially associate with cholesterol-rich lipid rafts in arteries as well as platelets, and depletion of cellular cholesterol inhibits calcium influx and downstream responses (11, 12). Our recent proteomic analysis of P2X1 receptor-interacting proteins has identified a regulatory role of the actin cytoskeleton in P2X1 receptor signaling (13). Interestingly, these studies also identified heat shock protein 90 (HSP90) as part of the P2X1 receptor signaling complex (13). HSP90 acts as a molecular chaperone and has been shown to have a role in regulating ion channel function and expression, e.g. for ATP-sensitive potassium channels (14, 15). A potential role of HSP90 in regulation of the P2X1 receptor is suggested from studies on P2X3-like receptors in dorsal root ganglion neurons (16) and recombinant P2X7 receptors (17) where HSP90 inhibitors potentiated responsiveness. In this study we have determined the contribution of HSP90 to P2X1 receptor signaling for both recombinant and native platelet P2X1 receptors. We show that HSP90 plays a significant role in both gating of the receptor channel and trafficking of the receptor to the cell surface and that HSP90 inhibitors reduce P2X1 receptor-mediated responses.
MATERIALS AND METHODS
Cell Culture and Transfection of HEK293 Cells
Native HEK293 cells were maintained in minimal essential medium with Earle's salts (with GlutaMAXTM I; Invitrogen) supplemented with 10% fetal bovine serum and 1% nonessential amino acids (Invitrogen) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. A monolayer of cells at 80–90% confluence in a 24-well culture dish was transiently transfected using 0.5 μg of DNA and 1 μl of Lipofectamine 2000 (Invitrogen) in 500 μl of serum-free Opti-MEM1. After 24-h incubation, cells were plated onto 13-mm No. 1 coverslips for electrophysiological experiments and left to grow in DMEM. Cells were subjected to experiments 24–48 h after transfection. Cells were transfected with wild type or mutant human P2X receptors. Chimeric P2X1/2 receptors receptors were as described previously (18, 19). Photoactivatable GFP (PAGFP)4 (20) C-terminally tagged P2X1 and P2X2 receptor DNA was constructed by PCR and cloning; the PAGFP vector was a kind gift from Dr. Lippincott-Schwartz, National Institutes of Health. Cells transfected with P2X1-PAGFP or P2X2-PAGFP were maintained in standard medium that contained Geneticin (1 mg/ml) over 4 weeks. Random cell testing by electrophysiological means showed that >80% cells were positive for the targeted protein.
Electrophysiological Recordings
Whole cell and permeabilized patch voltage clamp recordings were made from HEK293 cells using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Membrane currents were recorded at a holding potential of −70 mV (corrected for tip potential). Data were low pass-filtered at 1 kHz, digitized at a sampling interval of 200 μs, and acquired using a Digidata 1200 analog-to-digital converter with pClamp 9.2 acquisition software (Axon Instruments). Patch pipettes were filled with internal solution composed of 140 mm potassium gluconate, 10 mm EGTA, 10 mm HEPES, 5 mm NaCl, 5 (pH 7.3, adjusted with KOH) and had resistances in the range of 2–6 megohms. For perforated patch pipette recordings amphotericin B was added to the internal solution at a final concentration of 200 μg ml−1. The bath was continuously perfused with extracellular solution containing 150 mm NaCl, 5 mm KCl, 2.5 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, 10 mm glucose (pH 7.3, adjusted with NaOH). Agonists were applied with a U-tube perfusion system. For P2X1 receptors repeated applications of agonist α,β-methylene ATP (α,β-meATP) were separated by 5 min to allow recovery from receptor desensitization. P2X2 receptors and chimeric P2X1/2 receptors were stimulated with 100 μm ATP. In the permeabilized patch configuration experiments geldanamycin was perfused over the cells. For whole cell experiments we used cells pretreated in drug-containing solution before recordings. Control untreated cells were recorded from every day, and comparisons between nontreated and treated cells were made between the cells from the same batch. All chemicals were purchased from Sigma.
Cell Surface Biotinylation
HEK293 cells expressing P2X1 receptors were cultured in 6-well plates and treated with geldanamycin (2 μm, 30 min). Surface proteins were biotinylated by the addition of 0.5 mg/ml sulfo-NHS-LC-biotin (sulfo-biotin; Pierce) diluted in PBS (30 min at 4 °C). Excess biotin was quenched with two 20-min (4 °C) washes with 100 mm glycine in PBS. Cells were lysed in 300 μl of buffer H (100 mm NaCl, 20 mm Tris-Cl, pH 7.4, 1% Triton X-100, and 10 μl/ml protease inhibitor mixture (P8340; Sigma)), incubated on ice for 20 min and cleared by centrifugation (4 °C at 16,000 × g for 10 min). For isolation of biotinylated proteins, 30 μl of streptavidin-agarose beads (Sigma) were added to 200 μl of supernatant and mixed on a rolling shaker (4 °C, 3 h). The rest of the supernatant was kept to assess total protein for each sample. Beads were washed four times in buffer H, and 30 μl of 2× gel sample loading buffer was added. Samples were separated on 10% SDS-polyacrylamide gels and transferred onto nitrocellulose. Membranes were then processed with the primary anti-P2X1 receptor antibodies (1:1000) (Alomone, Jerusalem, Israel). Protein bands were visualized using an ECL Plus kit and Hyperfilm MP (Amersham Biosciences).
Fluorescence Imaging
Cells expressing photoactivatable C-terminally tagged P2X1 and P2X2 receptors were plated on 35-mm tissue culture μ-Dishes (Ibidi; Thistle Scientific, Glasgow, UK) 12 h before experiments. Confocal fluorescence measurements were made on an Olympus inverted microscope with a confocal laser scanning module (Olympus FluoView1000). Cells were observed with a 60× oil immersion objective lens (UPLSAPO 60×, NA 1.35). The PAGFP fluorescence was activated with a 405-nm laser diode for 5 ms (97% power, 6-μm2 area), and then the whole field excited with 488-nm argon laser at low power and emission was detected and collected from 500 to 600 nm at a rate of 1 Hz. Each experiment started with collection of 60 base-line control images of the cell followed by 5-ms-long activation of the region of interest. Typically, 250 images were recorded at low laser power after photoactivation of PAGFP. Average fluorescence intensities of activated and background regions were recorded for each time point. Fluorescence signals were background-subtracted and expressed as F/Fmax ratios to normalize fluorescence levels (F) against maximum fluorescence (Fmax). Fluorescence intensities of the region of interest were obtained using FluoView software and plotted.
Preparation of Washed Platelet Suspensions
Blood was obtained by standard phlebotomy from informed, consenting donors into acid citrate dextrose anticoagulant (85 mm trisodium citrate, 78 mm citric acid, 111 mm glucose). The study was approved by University of Leicester Committee for Research Ethics concerning human subjects. The blood and acid citrate dextrose mixture (6:1 by volume) was centrifuged at 700 × g for 5 min. Platelet-rich plasma was removed and treated with aspirin (100 μm) and apyrase type VII (0.32 unit ml−1) and loaded with the calcium indicator fura-2 by incubation of platelet-rich plasma with 2 μm fura-2/AM for 45 min at 37 °C. Washed platelet suspensions were then prepared by centrifugation for 20 min at 350 × g and resuspension of the pellet in nominally calcium-free saline (145 mm NaCl, 5 mm KCl, 1 mm MgCl2, 10 mm HEPES, 10 mm glucose, pH 7.35) with type VII apyrase (0.32 unit ml−1). All platelet experiments were performed in the presence of 2 mm CaCl2 added 30 s prior to agonist stimulation.
For platelet cell surface biotinylation 5 × 108 platelets were treated with either buffer or geldanamycin (2 μm final) for 30 min. Platelets were biotinylated and lysed (150 μl of buffer H), and streptavidin-agarose beads (75 μl) were used to isolate surface proteins essentially as for the HEK surface biotinylation experiment.
Intracellular Calcium Measurements on Platelets
Ratiometric fluorescence measurements were conducted at 37 °C in a Cairn spectrofluorometer system (Cairn Research Limited, Faversham, Kent, UK) in response to 1 μm α,β-meATP, 1 μm ADP, or 0.5 μg ml−1 type I collagen horm (Nycomed, Austria) following 1-min or 20-min incubation with 2 μm geldanamycin, 1 μg ml−1 radicicol, or 0.2% dimethyl sulfoxide. In experiments where P2X1 receptors were desensitized, 0.6 μm α,β-meATP was added 1 min prior to the addition of CaCl2. Responses to fura-2 fluorescence signals at 340-and 380-nm excitation (>490-nm emission) were calibrated using a dissociation constant of 224 nm as described previously (21).
Data Analysis
Data were analyzed with CLAMPFIT (Axon Instruments) or ORIGIN 6.0 (Microcal Software, Northampton MA). Data in the text and graphs are shown as means ± S.E. of mean from n determinations as indicated and analyzed using the appropriate Student's t test, except for data with the chimeras that were analyzed by one-way ANOVA followed by Dunnett's multiple comparisons test, and p < 0.05 was considered significant.
RESULTS
Regulatory Role of HSP90 on P2X1 Receptor Currents
In protein pulldown studies we recently identified HSP90 as a P2X1 receptor-interacting protein (13). To determine whether this association is functionally important we tested the effects of the selective HSP90 inhibitor geldanamycin (22) on recombinant P2X1 receptors expressed in HEK293 cells. In the permeabilized patch recording configuration (to maintain the integrity of intracellular signaling pathways) application of α,β-meATP (10 μm, 3 s) evoked rapidly desensitizing inward currents that were reproducible when a 5-min interval was given between applications as reported previously (23). Application of geldanamycin (2 μm) caused a time-dependent reduction of P2X1 receptor-mediated currents (Fig. 1A), reaching a maximal inhibition of ∼85% after 15 min (Fig. 1, B and C). As well as reducing the peak current amplitude, geldanamcyin also increased the rate of P2X1 receptor current desensitization (decay constant 204.5 ± 10.1 and 154.9 ± 11.4 ms for control and geldanamycin, respectively, p < 0.01, n = 48,20), suggesting that the ≈25% more rapid desensitization (i.e. an effect on channel gating) contributes to the reduction in peak current amplitude.
FIGURE 1.
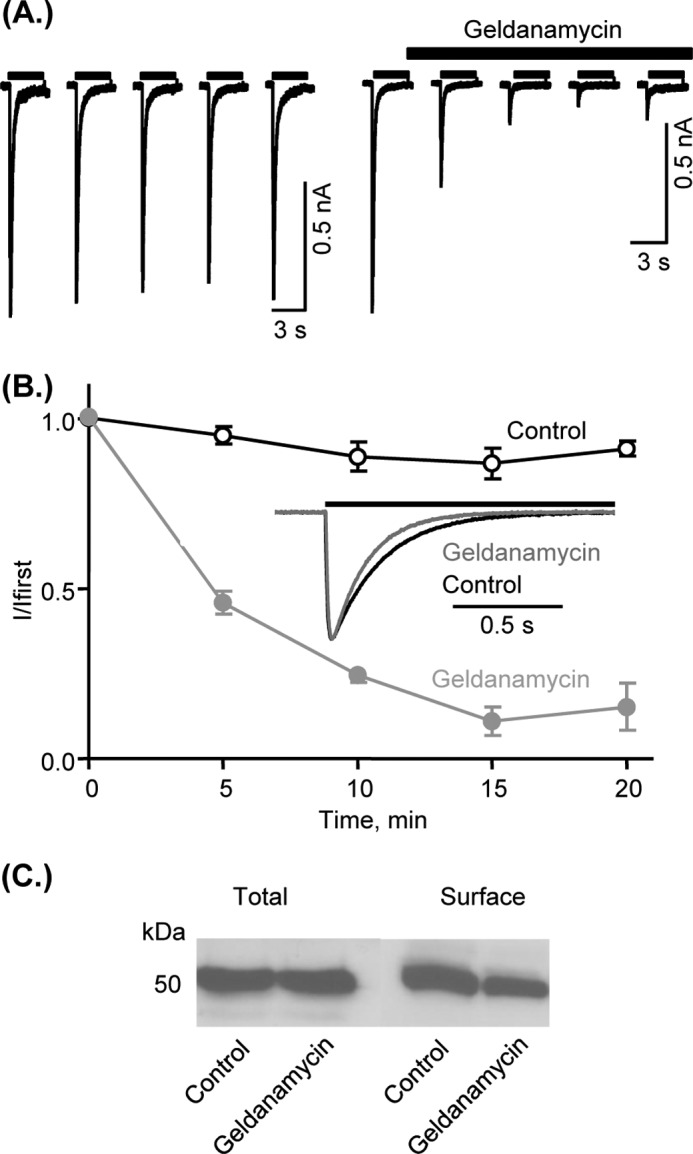
Effects of the HSP90 inhibitor geldanamycin on P2X1 receptor-mediated currents and receptor expression. A, α,β-meATP-evoked (10 μm, application indicated by bar) reproducible inward currents in the permeabilized patch recording configuration from human P2X1 receptors expressed in HEK293 cells (left). The HSP90 inhibitor geldanamycin (2 μm) reduced the peak amplitude of the currents with maximal effect after 15 min. B, mean normalized data of P2X1 receptor-mediated currents in control conditions and in the presence of geldanamycin (n = 5–7). Inset, time course of the P2X1 receptor current in control following geldanamycin. Traces have been normalized to the peak current amplitude to show the increased rate of decay with geldanamycin. Values are shown as means ± S.E. (error bars). C, Western blotting of P2X1 receptors expressed in HEK293 cells. Results show that 2 μm geldanamycin (30 min) has no effect on total levels of the receptor, but reduced surface expression of the P2X1 receptor assessed by cell surface biotinylation results in reduction of P2X1 receptors on membrane surface (by 36.3 ± 6.7%, n = 8).
HSP90 is a molecular chaperone that has been shown to contribute to trafficking of a range of receptors and ion channels. We therefore used a cell surface biotinylation assay and Western blotting to determine whether geldanamycin had any effect on the expression and distribution of P2X1 receptors. Treatment with geldanamycin had no effect on the total levels of P2X1 receptor expression in HEK293 cells but reduced cell surface expression of the receptor by 36.3 ± 6.7% (Fig. 1C). These results suggest that the HSP90 inhibitor geldanamycin inhibits P2X1 receptor-mediated responses by an effect on both surface expression and gating of the channel.
HSP90 Plays an Important Role in P2X1 Receptor Trafficking
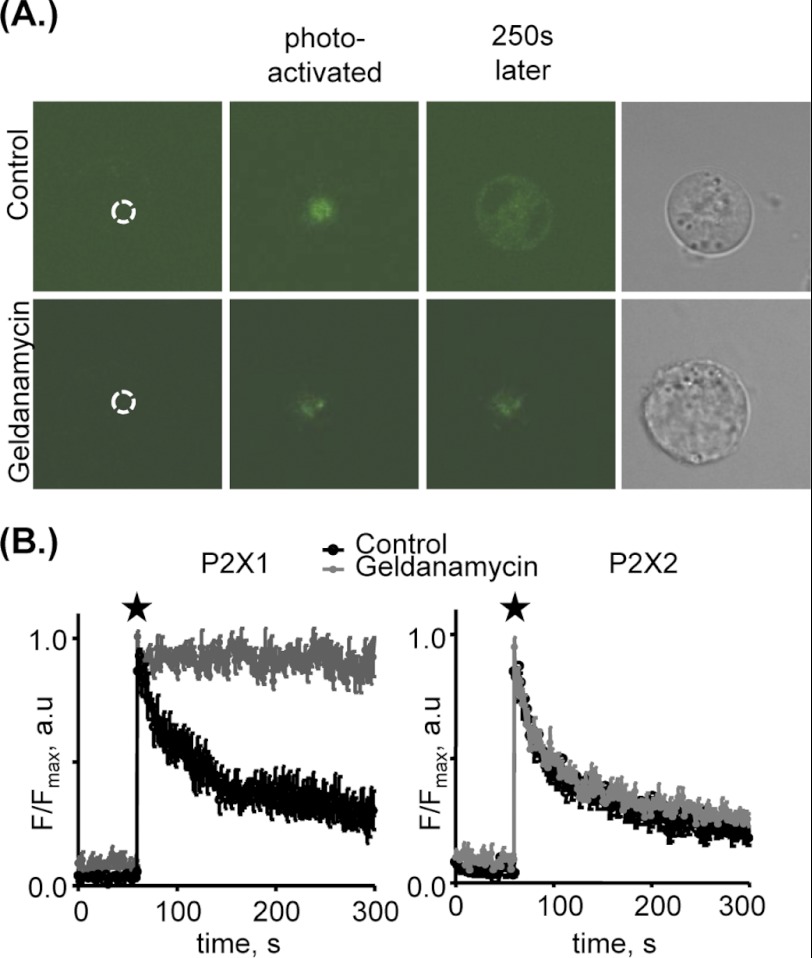
To look at mobility of the P2X1 receptors in real time we have used a P2X1 receptor that has been C-terminally tagged with photoactivatable GFP (PAGFP) (20). Fluorescence of PAGFP can be increased up to 100-fold when excited with ∼405-nm light and can be used to visualize protein trafficking (20, 24). The advantage of this technique is that GFP within a specific region of the cell can be selectively activated, allowing P2X1 receptor movements to be tracked over time. We illuminated a 6-μm2 area at the center of the cell. Following illumination of the region of interest there was an increase in fluorescence, and with time P2X1-PAGFP-labeled receptors redistributed throughout the cell (Fig. 2A and supplemental Movie 1). To quantify this movement we measured the change in fluorescence at the site of illumination (24). This increased fluorescence then decayed back to base-line levels with ≈70% of the fluorescence moving away from the area after 3 min. This clearly indicates that the P2X1 receptor is highly mobile in the cell. However, following treatment with geldanamycin (2 μm, 30 min) the P2X1-PAGFP fluorescence remained predominantly within the area of illumination with only an ≈10% decrease in level over 3 min (Fig. 2 and supplemental Movie 2). P2X2 receptors tagged with PAGFP showed similar mobility under control conditions, although this movement was unaffected by geldanamycin (Fig. 2B). This demonstrates that the geldanamycin effects are specific to the P2X1 receptor and suggest that HSP90 plays a central role in trafficking of the P2X1 receptor.
FIGURE 2.
HSP90 promotes trafficking of P2X1 but not P2X2 receptors. A, representative snapshots of HEK293 cells expressing P2X1 receptors C-terminally tagged with PAGFP. Fluorescent images represent the cell in control conditions or treated with 2 μm geldanamycin (>30 min) before P2X1-PAGFP activation (dotted circle indicates region to be photoactivated), immediately after activation (indicated by star), and 250 s after photoactivation. Right panel, brightfield images of the cell. Under control conditions the P2X1-PAGFP fluorescence moves away from the site of illumination; however, following geldanamycin treatment the P2X1-PAGFP remains predominantly within the area of photoillumination. B, averaged changes in fluorescence in control cells and cells treated with geldanamycin as fraction of maximal fluorescence observed immediately after activation of P2X1-PAGFP (indicated by star) or P2X2-PAGFP, within the cell; size of activated area, 6 μm2 (n = 5–9). Images were taken on an Olympus inverted microscope with a confocal laser scanning module (OlympusFluoView1000). Cells were imaged with a 60× oil immersion objective (UPLASAPO 60×, NA 1.35). Cells were bathed in standard extracellular solution at room temperature. Fluorescent intensities of regions of interest were obtained using FluoView software. Values are shown as means ± S.E.
Molecular Basis of HSP90 Effects on the P2X1 Receptor
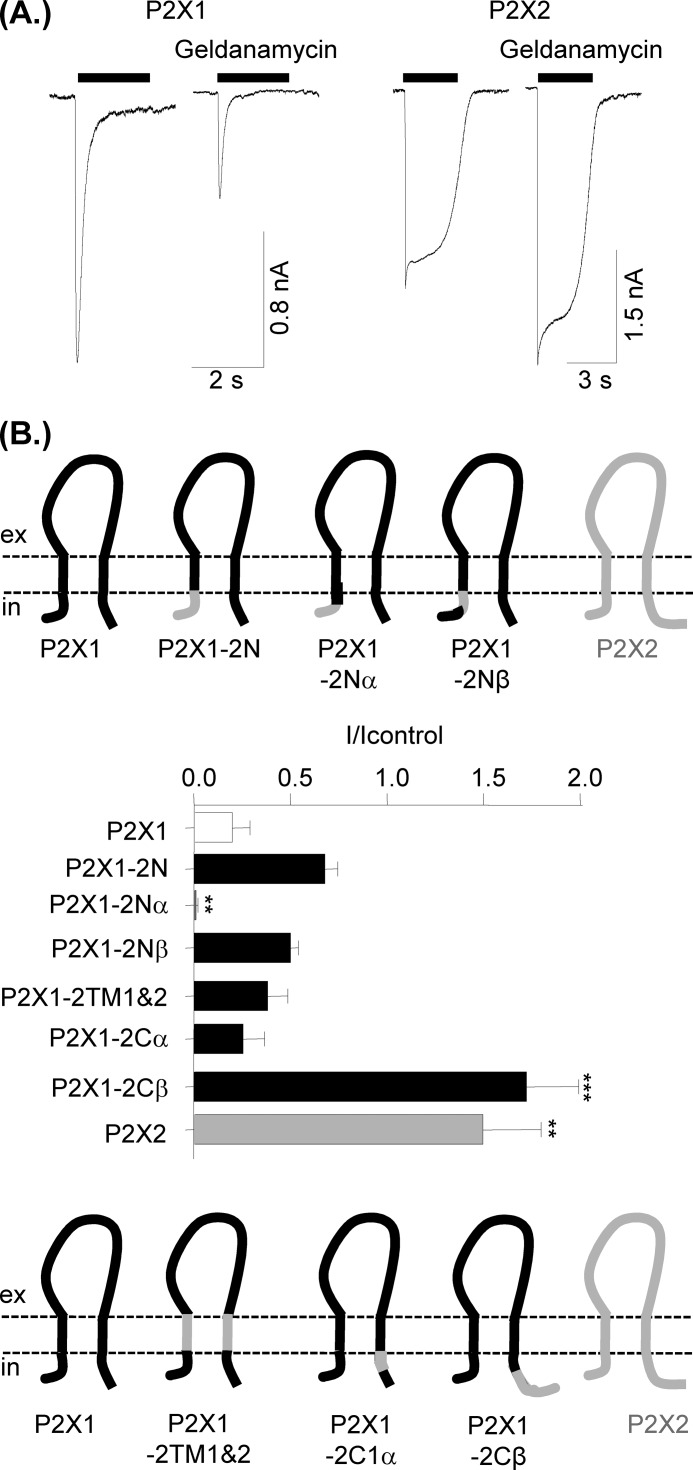
Proteomic studies have shown that HSP90 is also associated with the P2X2 receptor (25). Geldanamycin (2 μm >30-min treatment) potentiated 100 μm ATP-evoked P2X2 receptor currents (150 ± 30% of control, n = 10, p < 0.001) (Fig. 3), demonstrating that the effects of HSP90 are dependent on the P2X receptor subtype. We have previously used chimeras between the human P2X1 and P2X2 receptors to understand the molecular basis of their properties and shown that the transmembrane segments as well as the intracellular N and C termini can play an important role in channel regulation (26). We have now used these chimeras to determine whether these regions are important for imparting sensitivity to geldanamycin (Fig. 3). Inhibition by geldanamycin was unaffected by replacing transmembrane segments 1 and 2 of the P2X1 receptor with those from the P2X2 receptor (P2X1-2TM1/2), the N terminus (P2X1-2N), (amino acids 16–30, P2X1-2Nβ) or the first 12 amino acids of the C terminus (P2X1-2Cα). Responses were essentially abolished by geldanamycin when the first 16 amino acids were swapped (P2X1-2Nα). For the C terminus chimeras swapping the first 16 amino acids (P2X1-2Cα, incorporating the conserved trafficking motif (27)) had no effect on geldanamycin inhibition. In contrast, swapping the remainder of the C terminus (P2X1-2Cβ) resulted in a receptor that showed potentiation by geldanamycin similar to that of the parent P2X2 receptor. These results suggest that both the intracellular N and C termini of the P2X1 receptor play a role in regulation by HSP90.
FIGURE 3.
Effects of the HSP90 inhibitor geldanamycin on P2X1 and P2X2 receptor currents and identification of the region of P2X1 receptor responsible for sensitivity to HSP90 inhibition. A, representative traces of P2X1 and P2X2 currents in control conditions and after treatment with 2 μm geldanamycin (>30 min). Bar represents period of agonist application, 10 μm α,β-meATP and 100 μm ATP for P2X1 and P2X2, respectively. B, schematic representation of P2X1/2 chimeras swapping portions of the intracellular N terminus (upper panel) and transmembrane segments and C terminus (lower panels). The histogram shows the effects of 2 μm geldanamycin treatment (30 min) treatment on currents mediated by P2X1, P2X2, and P2X1/P2X2 chimeric receptors expressed as a fraction of the response to 100 μm ATP in control nontreated cells. Values are shown as means ± S.E. (error bars; n = 6–14). **, p < 0.01; ***, p < 0.001 significantly different from P2X1.
HSP90 Inhibitors Selectively Modulate P2X1 but Not P2Y1 Receptor-mediated Calcium Influx in Human Platelets
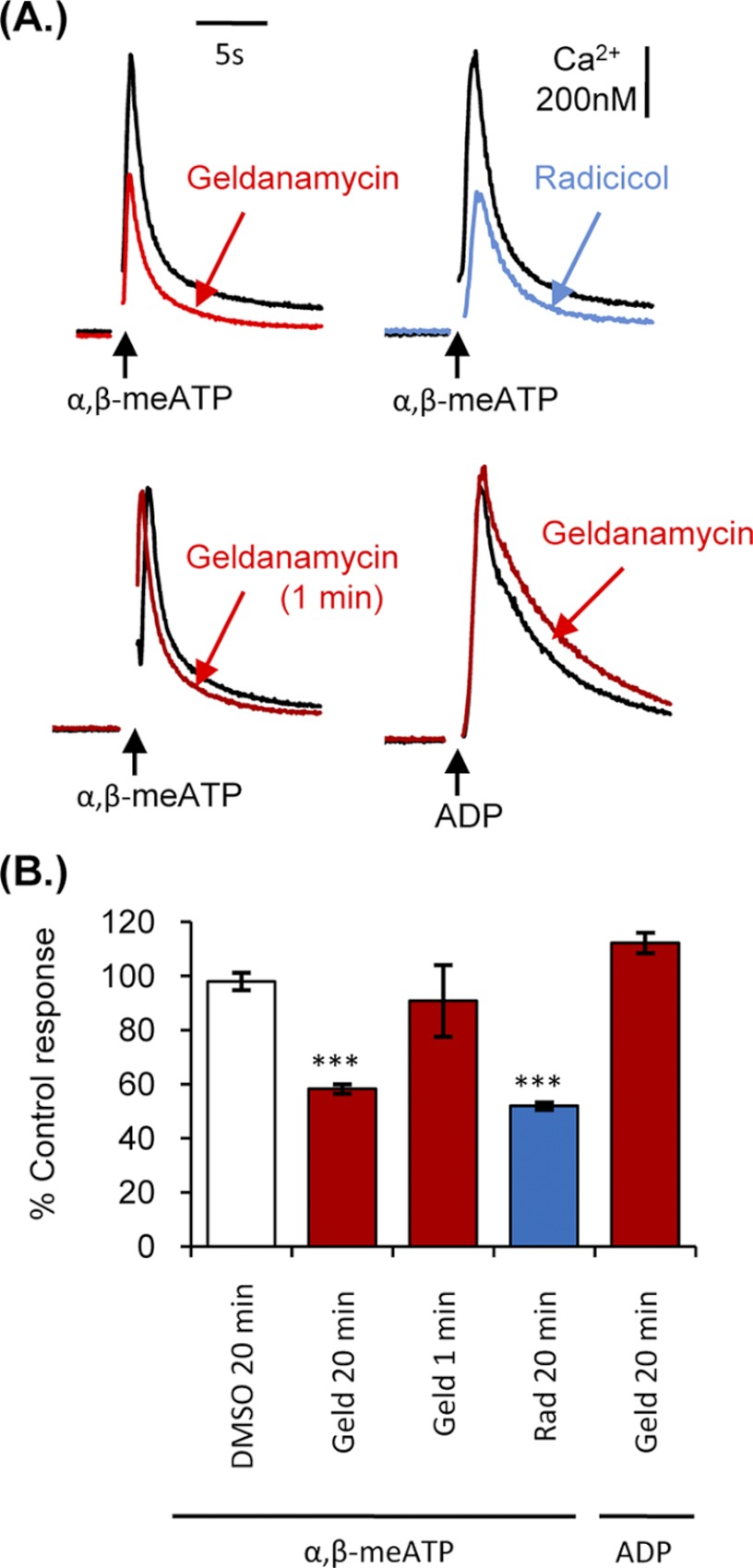
The studies on recombinantly expressed P2X1 receptors in HEK293 cells suggest a role of HSP90 in regulation of the receptor, and it was therefore important to determine whether similar effects were seen for native P2X1 receptors. Given the important role of P2X1 receptors in platelet responsiveness we determined the effects of HSP90 inhibition on P2X1 receptor-mediated responses in human platelets (Fig. 4). α,β-MeATP (1 μm) can be used to selectively activate P2X1 receptors in platelets (21) and evoked a transient increase in intracellular calcium (521.8 ± 101 nm). Geldanamycin (20-min preincubation, 2 μm) reduced the α,β-meATP-evoked calcium transient by 41.8. ± 1.9% (p < 0.001, n = 4). This was associated with a 14.6 ± 5.7% reduction in the surface expression of the P2X1 receptor on platelets (n = 6 from three donors, p < 0.05; total P2X1 receptor levels were unaffected by geldanamycin treatment, 100 ± 5.7% of control). Geldanamycin acts by blocking the ATP binding site of HSP90, raising the possibility that it could interfere with agonist binding at the P2X1 receptor. However, geldanamycin had no significant effect on P2X1 function when applied only 1 min prior to agonist stimulation (90.8 ± 13.8% of control; p > 0.05) (Fig. 4), indicating that geldanamycin does not act as a direct antagonist of the P2X receptor. Geldanamycin pretreatment (20 min, 2 μm) also had no effect on 1 μm ADP-evoked P2Y1 receptor-mediated calcium responses (112 ± 3.8% of control) (Figs. 1B and 4B), indicating that the HSP90 inhibitor does not have a general inhibitory effect on calcium signaling in platelets. Radicicol (a macrocyclic antibiotic) is structurally unrelated to geldanamycin and is another commonly used HSP90 inhibitor (28). Radicicol (20-min preincubation, 1 μg ml−1) reduced the α,β-meATP-evoked calcium transient by 48.1 ± 1.4% (n = 4, p < 0.001) (Fig. 4B). Taken together, these results show that HSP90 inhibitors regulate P2X1 receptor signaling.
FIGURE 4.
HSP90 inhibitors reduced P2X1-dependent calcium transients in platelets. Washed platelets loaded with fura-2 were treated with 0.2% dimethyl sulfoxide, 2 μm geldanamycin, or 1 μg ml−1 radicicol for 20 min prior to stimulation with 1 μm α,β-meATP or 1 μm ADP; in addition the bottom left panel shows effects of 1-min preincubation with geldanamycin on α,β-meATP responses. Intracellular calcium concentration was measured at 37 °C under stirring conditions in the presence of 2 mm CaCl2. A, representative calcium traces. B, summary data displayed as a percentage of the control calcium response. Values are shown as means ± S.E. (error bars; n = 3–4; ***, p < 0.001).
HSP90 Inhibitors Reduce Collagen-evoked Calcium Responses in Platelets by Reducing P2X1 Receptor-mediated Calcium Influx
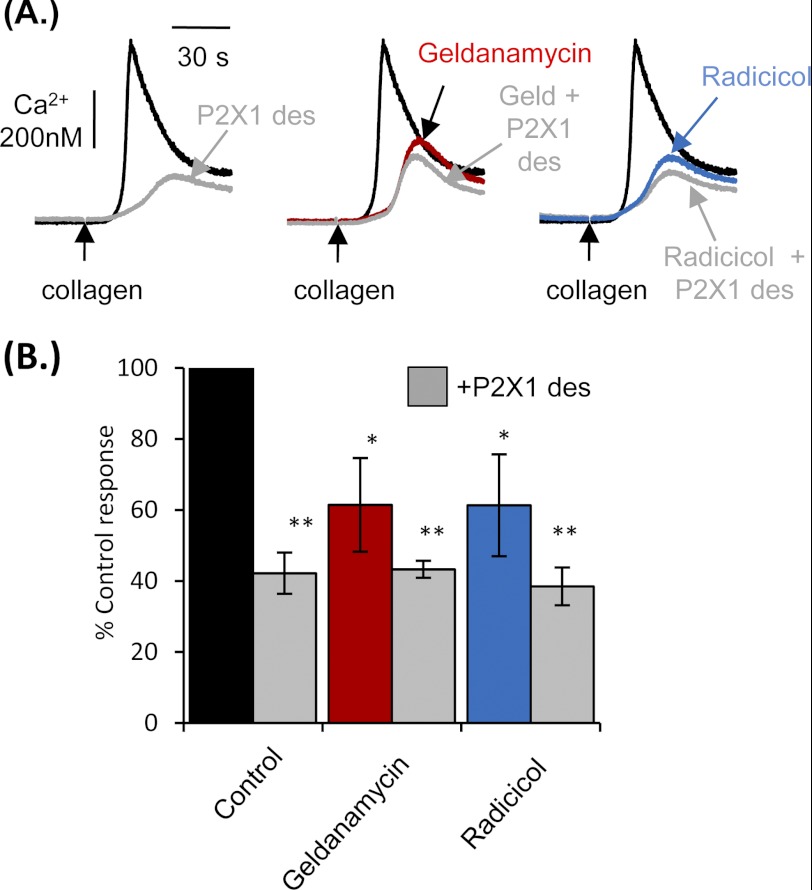
The P2X1 receptor plays a central role in enhancing platelet function, particularly following stimulation of glycoprotein VI by collagen (7, 29). A large proportion of the calcium response stimulated by collagen is dependent upon the secondary activation of P2X1 receptors through the secretion of ATP from platelet-dense granules (30). Consistent with this, desensitization of P2X1 receptors markedly reduced collagen-evoked calcium responses (609.2 ± 87.8 nm) by 57.8 ± 5.8% (n = 4, p < 0.01) (Fig. 5). The residual response corresponds to direct calcium elevation mediated by GPVI receptor signaling and phospholipase Cγ (5). Geldanamycin (2 μm) reduced collagen-mediated calcium responses by 38.5 ± 13.2% and 1 μg ml−1 radicicol by 38.7 ± 14.3% (n = 4, p < 0.05) (Fig. 5B). Calcium transients were further reduced by desensitization of the P2X1 receptor but not to a significant extent and not below control levels following P2X1 desensitization. This demonstrates that HSP90 is crucially involved in modulating the P2X1-dependent component of the collagen response.
FIGURE 5.
Collagen-evoked platelet calcium responses were perturbed by HSP90 inhibitors through disruption of P2X1 function. A, washed platelets loaded with fura-2 were treated with 0.2% dimethyl sulfoxide, 2 μm geldanamycin, or 1 μg ml−1 radicicol for 20 min, and calcium transients measured in response to 0.5 μg ml−1 collagen before and after P2X1 desensitization in the presence of 2 mm CaCl2. B, summary data are displayed as a percentage of the control Ca2+ response. Values are shown as means ± S.E. (error bars) response (n = 4; *, p < 0.05; **, p < 0.01).
It is possible that HSP90 inhibitors could reduce P2X1-dependent calcium influx stimulated by collagen by altering dense granule secretion, thereby reducing the amount of ATP secreted. To examine this hypothesis, 0.5 μg ml−1 collagen-evoked ATP secretion was measured following treatment with 0.2% dimethyl sulfoxide, 2 μm geldanamycin, or 1 μg ml−1 radicicol. ATP secretion (108.9 ± 13.6 nm) was not affected by the inhibitors (109.8 ± 15.7 nm and 122.83 ± 15.6 nm for geldanamycin and radicicol, respectively), indicating that HSP90 does not play a role in dense granule secretion and that the reduced calcium responses observed upon collagen stimulation were the result of altered P2X1 receptor function.
DISCUSSION
Activation of P2X1 receptors by ATP opens the integral receptor cation channel, leading to calcium influx and membrane depolarization. In platelets this signaling makes a significant contribution to responsiveness to a range of stimuli giving P2X1 receptors an important role in thrombosis and hemostasis. We recently identified HSP90 as a P2X1 receptor-interacting protein (13) and now show that the activity of the P2X1 receptor is reduced by HSP90 inhibitors by a dual action first to modulate surface expression of the receptor and second by an effect on channel gating.
The present study demonstrates for the first time that P2X1 receptor currents and calcium responses are reduced by HSP90 inhibitors. Following geldanamycin treatment HSP90 still co-purified with the P2X1 receptor (mass spectroscopic determination from three purifications; data not shown), indicating that the interaction is not dependent on active HSP90. Geldanamycin and radicicol, in the low micromolar range used in this study (and supplemental Fig. 1), act at the ATP binding site to inactivate HSP90 and have been used extensively to study the contribution of HSP90 to cell signaling (31). This raised the possibility that the HSP90 inhibitors could simply be acting as antagonists for the ATP binding site of the P2X receptor. However, four compelling lines of evidence demonstrate that this is not the case. (i) 1-min preapplication of geldanamycin or radicicol did not have an effect on P2X1 receptor signaling in platelets that would be expected if it had a direct antagonist action at the receptor. (ii) The time course of P2X1 receptor-mediated currents is concentration-dependent; if geldanamycin was a P2X1 receptor antagonist it would slow the time course of the response, but the time course was actually faster in the presence of geldanamycin. Similarly, the level of inhibition by geldanamycin was the same for 10 and surpramaximal 100 μm α,β-meATP, 64.1 ± 16.9 and 68.5 ± 11.7% inhibition, respectively (n = 6,4). (iii) Geldanamycin potentiated P2X2 receptor responses that share the core features of ATP binding (32, 33). (iv) The effects of geldanamycin were abolished in chimeras where the intracellular regions of the P2X1 were replaced with those from P2X2, demonstrating that the extracellular ligand binding domain of the P2X1 receptor is not the site of geldanamycin action.
We have shown previously that P2X1 receptor currents are inhibited following cholesterol depletion or disruption of the actin cytoskeleton (11, 13, 18). Interestingly, stabilization of the cytoskeleton with jasplakinolide abolished the inhibitory effects of cholesterol depletion (13), suggesting that the lipid rafts may play a role in stabilizing an interaction of the P2X1 receptor with the cytoskeleton. The inhibition of P2X1 receptor currents by the HSP inhibitor geldanamycin, however, was unaffected by jasplakinolide (69.5 ± 10.8 and 76.0 ± 7.6% inhibition in response to geldanamycin or pretreatment with jasplakinolide, 30 nm, 1 h, and co-application of geldanamycin with jasplakinolide, respectively, n = 16,14). This suggests that HSP90 is not reducing P2X1 receptor responsiveness through an effect on cytoskeletal polymerization. It is also unlikely that the geldanamycin treatment results in ATP release and receptor desensitization because the platelet studies were all carried out in apyrase to break down any released ATP. Similarly, in whole cell patch clamp studies the inhibition of P2X1 receptor currents by geldanamycin was the same when cells were also treated with apyrase (64.1 ± 16.9 and 68.6 ± 9.3% inhibition for geldanamycin and apyrase pretreatment and co-application with geldanamycin, respectively, n = 6,4).
One of the key functional roles of HSP90 is as a molecular chaperone facilitating the movement of proteins in the cell. We have shown that inhibiting HSP90 reduced the cell surface expression of the receptor. The lack of effect of geldanamycin on total levels of P2X1 receptor expression suggested a role of HSP90 in targeting the P2X1 receptor to the cell surface. The 20-min incubation for maximal inhibition of P2X1 receptor responses and reduction in surface expression is similar to that reported for the inhibition of the ClC-2 chloride channels (34) and ATP-sensitive potassium channels (15), suggesting either similar levels of receptor turnover and/or the time required for HSP90 to equilibrate in the cell. The imaging of photoactivated P2X1-PAGFP clearly demonstrates the normally highly mobile nature of the P2X1 receptor. We have previously shown that the P2X1 receptor is constitutively internalized/recycled (19). Taken together with the P2X1-PAGFP data, this suggests that the reduction in cell surface expression following geldanamycin treatment results from reduced trafficking of P2X1 receptors to the cell surface by the molecular chaperone HSP90.
Analysis of the time course of P2X1 receptor-mediated currents suggests that HSP90 is not only involved in trafficking the receptor to the cell surface but also in regulating the gating of the P2X1 receptor ion channel. One of the characteristic features of the P2X1 receptor is that the activity of the receptor rapidly desensitizes (time constant of ≈200 ms) during continued stimulation with ATP. This was further speeded by ≈25% following HSP90 inhibition. This speeding will not only reduce the peak current amplitude but also the duration of the P2X1 receptor response. Our recent studies have shown that the intracellular N and C termini can contribute to the regulation of the P2X1 receptor time course (26). This is consistent with the present study with the P2X1/2 receptor chimeras demonstrating the importance of these regions for HSP90 sensitivity. This indicates that the interaction of HSP90 with the intracellular domains of the P2X1 receptor has a stabilizing role on channel gating. Our studies therefore suggest that HSP90 has a dual role in P2X1 receptor responsiveness, regulating not only the expression of the receptor at the cell surface but also the activity of the channels once there.
The reduction in P2X1 receptor-mediated calcium responses by geldanamycin and radicicol in platelets demonstrates an important functional role for HSP90 in regulation of the receptor. A previous study on platelets had suggested that geldanamycin inhibits ADP responses (35). However, this was at a 10-fold higher concentrations than used in the present study and resulted in membrane damage (35). In the current study HSP90 inhibitors had no effect on either P2Y1- or P2X1-independent collagen-evoked calcium increases. This shows that other modes of calcium signaling in platelets are not regulated, at least in the short term, by HSP90.
It is clear that the P2X1 receptor can make a significant contribution to signaling in platelets not only in response to direct activation by ATP released from damaged cells but also as a result of ATP release from platelets themselves for example in response to collagen, thrombin, thromboxane A2, and Toll-like receptor stimulation (5). In vivo studies have clearly demonstrated the importance of P2X1 receptor in thromboembolism and the protective nature of P2X1 receptor inhibition, e.g. by the selective P2X1 receptor antagonist NF449 (36). The current study now highlights another molecular approach by which P2X1 receptor activity may be targeted with HSP90 inhibitors. These inhibitors are currently being tested for their potential as anticancer agents (37) and have been shown to attenuate inflammatory responses in atherosclerosis (38). Our results suggest that a reduction in P2X1 receptor platelet signaling may contribute to these therapeutic roles as there is substantial evidence suggesting important roles for platelets in tumor metastasis (39) and development of atherosclerotic plaques (5). Given the important role of P2X1 receptors in platelet activation, particularly at high shear, our work suggests that the potential antithrombotic/platelet action of HSP90 inhibitors should be a consideration in clinical trials.
Supplementary Material
Acknowledgments
We thank Dr. Lippincott-Schwartz, National Institutes of Health, for the PAGFP construct; Dr. R. C. Allsopp, University of Leicester, for generation of the P2X1-PAGFP plasmid; Dr. K. Straatman for help setting up the imaging protocols; and Dr. S. Mistry, Dr. A. R. Bottrill, and Shairbanu Y. Ashra for mass spectroscopy analysis and Kirk A. Taylor for help with platelet preparation.
This work was supported by the Wellcome Trust.

This article contains supplemental Fig. 1 and Movies 1 and 2.
- PAGFP
- photoactivatable GFP
- α,β-meATP
- α,β-methylene ATP.
REFERENCES
- 1. Burnstock G. (2006) Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 58, 58–86 [DOI] [PubMed] [Google Scholar]
- 2. Abbracchio M. P., Burnstock G. (1994) Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol. Ther. 64, 445–475 [DOI] [PubMed] [Google Scholar]
- 3. North R. A. (2002) Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 [DOI] [PubMed] [Google Scholar]
- 4. Fung C. Y., Cendana C., Farndale R. W., Mahaut-Smith M. P. (2007) Primary and secondary agonists can use P2X1 receptors as a major pathway to increase intracellular Ca2+ in the human platelet. J. Thromb. Haemost. 5, 910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fung C. Y., Jones S., Ntrakwah A., Naseem K. M., Farndale R. W., Mahaut-Smith M. P. (2012) Platelet Ca2+ responses coupled to glycoprotein VI and Toll-like receptors persist in the presence of endothelial-derived inhibitors. Blood 119, 3613–3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hechler B., Lenain N., Marchese P., Vial C., Heim V., Freund M., Cazenave J. P., Cattaneo M., Ruggeri Z. M., Evans R., Gachet C. (2003) A role of the fast ATP-gated P2X1 cation channel in the thrombosis of small arteries in vivo. J. Exp. Med. 198, 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oury C., Kuijpers M. J., Toth-Zsamboki E., Bonnefoy A., Danloy S., Vreys I., Feijge M. A., De Vos R., Vermylen J., Heemskerk J. W., Hoylaerts M. F. (2003) Overexpression of the platelet P2X1 ion channel in transgenic mice generates a novel prothrombotic phenotype. Blood 101, 3969–3976 [DOI] [PubMed] [Google Scholar]
- 8. Lecut C., Frederix K., Johnson D. M., Deroanne C., Thiry M., Faccinetto C., Marée R., Evans R. J., Volders P. G., Bours V., Oury C. (2009) P2X1 ion channels promote neutrophil chemotaxis through Rho kinase activation. J. Immunol. 183, 2801–2809 [DOI] [PubMed] [Google Scholar]
- 9. Lecut C., Faccinetto C., Delierneux C., van Oerle R., Spronk H. M., Evans R. J., El Benna J., Bours V., Oury C. (2012) ATP-gated P2X1 ion channels protect from endotoxemia by dampening neutrophil activation. J. Thromb. Haemost. 10, 453–465 [DOI] [PubMed] [Google Scholar]
- 10. Woehrle T., Yip L., Elkhal A., Sumi Y., Chen Y., Yao Y., Insel P. A., Junger W. G. (2010) Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 116, 3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vial C., Evans R. J. (2005) Disruption of lipid rafts inhibits P2X1 receptor-mediated currents and arterial vasoconstriction. J. Biol. Chem. 280, 30705–30711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vial C., Fung C. Y., Goodall A. H., Mahaut-Smith M. P., Evans R. (2006) Differential sensitivity of human platelet P2X1 and P2Y1 receptors to disruption of lipid rafts. Biochem. Biophys. Res. Commun. 343, 415–419 [DOI] [PubMed] [Google Scholar]
- 13. Lalo U., Roberts J. A., Evans R. J. (2011) Identification of human P2X1 receptor-interacting proteins reveals a role of the cytoskeleton in receptor regulation. J. Biol. Chem. 286, 30591–30599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiao J. D., Garg V., Yang B., Hu K. (2008) Novel functional role of heat shock protein 90 in ATP-sensitive K+ channel-mediated hypoxic preconditioning. Cardiovasc. Res. 77, 126–133 [DOI] [PubMed] [Google Scholar]
- 15. Yan F. F., Pratt E. B., Chen P. C., Wang F., Skach W. R., David L. L., Shyng S. L. (2010) Role of Hsp90 in biogenesis of the beta-cell ATP-sensitive potassium channel complex. Mol. Biol. Cell 21, 1945–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McDowell T. S., Yukhananov R. Y. (2002) HSP90 inhibitors alter capsaicin- and ATP-induced currents in rat dorsal root ganglion neurons. Neuroreport 13, 437–441 [DOI] [PubMed] [Google Scholar]
- 17. Adinolfi E., Kim M., Young M. T., Di Virgilio F., Surprenant A. (2003) Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J. Biol. Chem. 278, 37344–37351 [DOI] [PubMed] [Google Scholar]
- 18. Allsopp R. C., Lalo U., Evans R. J. (2010) Lipid raft association and cholesterol sensitivity of P2X1–4 receptors for ATP: chimeras and point mutants identify intracellular amino-terminal residues involved in lipid regulation of P2X1 receptors. J. Biol. Chem. 285, 32770–32777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lalo U., Allsopp R. C., Mahaut-Smith M. P., Evans R. J. (2010) P2X1 receptor mobility and trafficking; regulation by receptor insertion and activation. J. Neurochem. 113, 1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patterson G. H., Lippincott-Schwartz J. (2002) A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873–1877 [DOI] [PubMed] [Google Scholar]
- 21. Rolf M. G., Brearley C. A., Mahaut-Smith M. P. (2001) Platelet shape change evoked by selective activation of P2X1 purinoceptors with α,β-methylene ATP. Thromb. Haemost. 85, 303–308 [PubMed] [Google Scholar]
- 22. Ficker E., Dennis A. T., Wang L., Brown A. M. (2003) Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ. Res. 92, e87–100 [DOI] [PubMed] [Google Scholar]
- 23. Lewis C. J., Evans R. J. (2000) Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilised patch technique: physiological and pharmacological characterisation of the properties of mesenteric artery P2X receptor ion channels. Br. J. Pharmacol. 131, 1659–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lippincott-Schwartz J., Patterson G. H. (2003) Development and use of fluorescent protein markers in living cells. Science 300, 87–91 [DOI] [PubMed] [Google Scholar]
- 25. Chaumont S., Compan V., Toulme E., Richler E., Housley G. D., Rassendren F., Khakh B. S. (2008) Regulation of P2X2 receptors by the neuronal calcium sensor VILIP1. Sci. Signal. 1, ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Allsopp R. C., Evans R. J. (2011) The intracellular amino terminus plays a dominant role in desensitization of ATP gated P2X receptor ion channels. J. Biol. Chem. 286, 44691–44701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaumont S., Jiang L. H., Penna A., North R. A., Rassendren F. (2004) Identification of a trafficking motif involved in the stabilization and polarization of P2X receptors. J. Biol. Chem. 279, 29628–29638 [DOI] [PubMed] [Google Scholar]
- 28. Roe S. M., Prodromou C., O'Brien R., Ladbury J. E., Piper P. W., Pearl L. H. (1999) Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 42, 260–266 [DOI] [PubMed] [Google Scholar]
- 29. Oury C., Toth-Zsamboki E., Thys C., Tytgat J., Vermylen J., Hoylaerts M. F. (2001) The ATP-gated P2X1 ion channel acts as a positive regulator of platelet responses to collagen. Thromb. Haemost. 86, 1264–1271 [PubMed] [Google Scholar]
- 30. Fung C. Y., Brearley C. A., Farndale R. W., Mahaut-Smith M. P. (2005) A major role for P2X1 receptors in the early collagen-evoked intracellular Ca2+ responses of human platelets. Thromb. Haemost. 94, 37–40 [DOI] [PubMed] [Google Scholar]
- 31. Panaretou B., Prodromou C., Roe S. M., O'Brien R., Ladbury J. E., Piper P. W., Pearl L. H. (1998) ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 17, 4829–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang L. H., Rassendren F., Surprenant A., North R. A. (2000) Identification of amino acid residues contributing to the ATP binding site of a purinergic P2X receptor. J. Biol. Chem. 275, 34190–34196 [DOI] [PubMed] [Google Scholar]
- 33. Roberts J. A., Digby H. R., Kara M., El Ajouz S., Sutcliffe M. J., Evans R. J. (2008) Cysteine substitution mutagenesis and the effects of methanethiosulfonate reagents at P2X2 and P2X4 receptors support a core common mode of ATP action at P2X receptors. J. Biol. Chem. 283, 20126–20136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hinzpeter A., Lipecka J., Brouillard F., Baudoin-Legros M., Dadlez M., Edelman A., Fritsch J. (2006) Association between Hsp90 and the ClC-2 chloride channel up-regulates channel function. Am. J. Physiol. Cell Physiol. 290, C45–56 [DOI] [PubMed] [Google Scholar]
- 35. Suttitanamongkol S., Gear A. R., Polanowska-Grabowska R. (2000) Geldanamycin disrupts platelet-membrane structure, leading to membrane permeabilization and inhibition of platelet aggregation. Biochem. J. 345, 307–314 [PMC free article] [PubMed] [Google Scholar]
- 36. Hechler B., Magnenat S., Zighetti M. L., Kassack M. U., Ullmann H., Cazenave J. P., Evans R., Cattaneo M., Gachet C. (2005) Inhibition of platelet functions and thrombosis through selective or nonselective inhibition of the platelet P2 receptors with increasing doses of NF449 [4,4′,4″,4‴-(carbonylbis(imino-5,1,3-benzenetriylbis-(carbonylimino)))tetrakis-benzene-1,3-disulfonic acid octasodium salt]. J. Pharmacol. Exp. Ther. 314, 232–243 [DOI] [PubMed] [Google Scholar]
- 37. Dolgin E., Motluk A. (2011) Heat shock and awe. Nat. Med. 17, 646–649 [DOI] [PubMed] [Google Scholar]
- 38. Madrigal-Matute J., López-Franco O., Blanco-Colio L. M., Muñoz-García B., Ramos-Mozo P., Ortega L., Egido J., Martín-Ventura J. L. (2010) Heat shock protein 90 inhibitors attenuate inflammatory responses in atherosclerosis. Cardiovasc Res 86, 330–337 [DOI] [PubMed] [Google Scholar]
- 39. Gay L. J., Felding-Habermann B. (2011) Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 11, 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.