Background: Telomerase up-regulation is found in about 90% of human cancer specimens and contributes actively to carcinogenesis.
Results: β-Catenin·TCF4 plays an important role in telomerase activation.
Conclusion: hTERT is a direct transcriptional target of the Wnt/β-catenin pathway.
Significance: Identifying the function of the Wnt/β-catenin pathway in telomerase regulation provides better insight into the role of Wnt pathway in carcinogenesis.
Keywords: Carcinogenesis, Cancer Biology, Signaling, Telomerase, Telomeres, Wnt/beta-Catenin Pathway, Telomerase Regulation
Abstract
Telomerase activation plays a critical role in human carcinogenesis through the maintenance of telomeres, but the activation mechanism during carcinogenesis remains unclear. The human telomerase reverse transcriptase (hTERT) promoter has been shown to promote hTERT gene expression selectively in tumor cells but not in normal cells. Deregulation of the Wnt/β-catenin signaling pathway is reported to be associated with human carcinogenesis. However, little is known about whether the Wnt/β-catenin pathway is involved in activating hTERT transcription and inducing telomerase activity (TA). In this study, we report that hTERT is a novel target of the Wnt/β-catenin pathway. Transient activation of the Wnt/β-catenin pathway either by transfection of a constitutively active form of β-catenin or by LiCl or Wnt-3a conditioned medium treatment induced hTERT mRNA expression and elevated TA in different cell lines. Furthermore, we found that silencing endogenous β-catenin expression by β-catenin gene-specific shRNA effectively decreased hTERT expression, suppressed TA, and accelerated telomere shortening. Of the four members of the lymphoid-enhancing factor (LEF)/T-cell factor (TCF) family, only TCF4 showed more effective stimulation on the hTERT promoter. Ectopic expression of a dominant negative form of TCF4 inhibited hTERT expression in cancer cells. Through promoter mapping, electrophoretic mobility shift assay, and chromatin immunoprecipitation assay, we found that hTERT is a direct target of β-catenin·TCF4-mediated transcription and that the TCF4 binding site at the hTERT promoter is critical for β-catenin·TCF4-dependent expression regulation. Given the pivotal role of telomerase in carcinogenesis, these results may offer insight into the regulation of telomerase in human cancer.
Introduction
Telomeres are highly specialized structures at chromosome ends that are essential for genome stability (1). Telomerase is a ribonucleoprotein that catalyzes de novo synthesis of repetitive telomeric DNA after cell division and maintains chromosomal stability, leading to cellular immortalization (2, 3). Telomere dysfunction and telomerase activation have been implicated in human cancer progression (4). A high level of telomerase activity (TA)2 is detected in about 90% of human cancer specimens, whereas most somatic cells do not display TA or express it only at very low levels in a cell cycle-dependent manner (5, 6). Overexpression of telomerase can stabilize telomeres in normal human cells and extend their replicative life span by at least 20 doublings (7). Conversely, inhibition of TA in cancer cells leads to reduction in telomere length and death of tumor cells (8). These findings establish an important role of telomerase-mediated telomere maintenance in human cells and suggest that telomerase up-regulation may contribute actively to cellular immortalization and carcinogenesis (9). Therefore, telomerase can be considered as a prime target for cancer diagnosis, and telomerase repression may be a tumor-suppressive mechanism (10, 11).
The expression level of human telomerase reverse transcriptase (hTERT), a catalytic subunit bearing the enzymatic activity of telomerase, is the rate-limiting determinant of human TA and is thought to be a sensitive indicator of telomerase function and activity, whereas the other subunits are constitutively expressed both in normal and cancer cells (12–14). Therefore, there is no doubt that hTERT expression plays a key role in cancer-specific telomerase activation. Numerous studies suggest that TA and the expression of telomerase components are regulated at multiple levels, including transcription and post-transcription, accurate assembly, and proper localization; however, hTERT expression level is considered primarily under transcriptional control (15–17). Thus, investigation of transcriptional regulation of hTERT should be essential for elucidating molecular mechanisms of telomerase regulation, cellular senescence, immortalization, and carcinogenesis in humans.
Many factors have been implicated in direct or indirect regulation of hTERT in cancer and normal cells, including cellular transcriptional activators (like c-Myc, Sp1, HIF-1, and AP2) (18–21) as well as the repressors, most of which comprise tumor suppressor gene products, such as p53 and WT1 (22, 23). Recently, Zhou et al. (24) reported that inhibition of PinX1 can contribute to carcinogenesis by activating telomerase and inducing chromosome instability. However, it remains largely unknown how hTERT is inactivated during development and how it is reactivated during carcinogenesis. Given that most cancer cells exhibit high TA, we hypothesized that certain cancer-specifically activated signaling pathways play critical roles in telomerase activation. In this study, by using qTRAP, a real-time PCR-based version of the telomere repeat amplification protocol (TRAP) (5), we sought to identify novel TA inhibitors from well known Wnt, epidermal growth factor receptor, and JAK/STAT pathway inhibitor libraries. Moreover, this screen will unveil novel signaling pathways implicated in telomerase regulation. We have identified one of the Wnt signaling pathway inhibitors, FH535 (β-catenin·TCF complex inhibitor), which could significantly inhibit TA in all cell lines used in this study, suggesting that the Wnt pathway may play a critical role in telomerase regulation in cancer cells.
Signaling by the Wnt family is one of the fundamental mechanisms that regulate cell proliferation, cell polarity, and cell fate determination during embryonic development and tissue homeostasis (25). As a result, abnormalities in Wnt signaling are reported to promote cancer development (26, 27). β-catenin, a central effector of the Wnt pathway, is involved in diverse cellular processes, including cell adhesion, growth, differentiation, and transcription of Wnt-responsive genes (28, 29). In the absence of the Wnt signaling, β-catenin is tightly regulated by a multiprotein degradation complex, in which β-catenin is phosphorylated by glycogen synthase kinase-3 (GSK3β), leading to its degradation via the ubiquitin-proteasome pathway (30). This continual elimination of β-catenin prevents it from reaching the nucleus, and Wnt target genes are thereby repressed by the DNA-bound lymphoid-enhancing factor (LEF)/T-cell factor (TCF) transcription factors. In the presence of Wnt signaling, β-catenin is uncoupled from the degradation complex and translocates to the nucleus to form complexes with LEF/TCF, thus activating Wnt target gene expression. Deregulation of β-catenin leads to the formation of β-catenin·TCF complexes and altered expression of oncogenes, such as c-MYC (31), cyclin D1 (32), and NOS2 (33), which can then contribute to the development of cancer (34). However, the involvement of the Wnt/β-catenin pathway in regulating telomerase gene expression has not yet been elucidated.
Here, we demonstrate for the first time that the hTERT gene is a novel target of the Wnt pathway. We employed a combination of electrophoretic mobility shift assay (EMSA), chromatin immunoprecipitation (ChIP), and luciferase reporter gene assays that led to the identification of hTERT as a direct transcriptional target of the β-catenin·TCF4 complex. Moreover, knockdown of β-catenin by shRNA largely repressed hTERT gene expression and TA in cancer cells. Our findings highlight the significance of the Wnt/β-catenin pathway in telomerase regulation and aid in the advancement of our understanding of the role of the canonical Wnt pathway in carcinogenesis.
EXPERIMENTAL PROCEDURES
Cell Lines
AGS, MCF7, 293T, MCF10A, HCT116, and LS174T were used in this study. HTC116, LS174T, and 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen), whereas AGS was cultured in Roswell Park Memorial Institute-1640 (RPMI 1640; Sigma-Aldrich). Both media contain 10% heat-inactivated fetal bovine serum. (FBS; Invitrogen) and 1% penicillin/streptomycin mixtures (Invitrogen). MCF10A was cultured in DMEM/F-12 with 15 mm HEPES buffer, 5% horse serum, 10 μg/ml insulin, 20 ng/ml EGF, 100 ng/ml cholera toxin, and 1% penicillin/streptomycin mixtures.
L-cells (control) and Wnt-3a-expressing L-cells (ATCC) were maintained in DMEM supplemented with 10% FBS. Wnt-3a-expressing L-cells were additionally supplemented with 400 μg/ml G418. For the preparation of L-control medium and Wnt-3a conditioned medium (CM), CM was collected from cultured parental L-cells or Wnt-3a-expressing L-cells and was diluted to 50% in serum-free DMEM. All cells were cultured in an incubator with 5% CO2 and 37 °C.
Inhibitor Library
The InhibitorSelectTM inhibitor library, consisting of well defined inhibitors from Wnt, epidermal growth factor receptor, and JAK/STAT signaling pathway inhibitor libraries, was purchased from Merck. Wnt pathway inhibitors A–O are listed in supplemental Table S1.
qTRAP
qTRAP is a real-time PCR-based method that measures the ability of telomerase to add telomeric repeats to a substrate. The real-time PCR-based version of the TRAP assay allows the estimation of TA in real time via fluorescence measurements. The qTRAP assay was modified from a conventional TRAP assay for use on the Rotor-Gene 6000 system (Qiagen) as described previously (35). Briefly, cells were treated with various signaling pathway inhibitors, and samples were lysed in 0.5% (v/v) CHAPS buffer (pH 7.5) supplemented with 10 mm Tris-HCl, 1 mm MgCl2, 1 mm EGTA, 0.1 mm benzamidine, 5 mm 2-mercaptoethanol, and 10% glycerol for 30 min on ice. Following lysis, the samples were centrifuged for 20 min at 12,000 × g at 4 °C to remove cell debris. The telomerase reaction was carried out in 1× TRAP buffer (20 mm Tris-HCl, pH 8.3, 1.5 mm MgCl2, 63 mm KCl, 0.05% Tween 20, 1 mm EGTA, 0.1 mg/ml BSA) and 50 μm each of the four dNTPs and 80 ng/μl TS primer (5′-AAT CCG TCG AGC AGA GTT-3′) and 2 μg (protein amount) of cell lysate in a total volume of 10 μl for 30 min at 30 °C and was stopped by incubation at 94 °C for 10 min. The qTRAP was subsequently carried out by adding 10 μl of the following 2× PCR mixture (2× TRAP buffer, 1 mg/μl BSA, 40 ng/μl ACX primer (5′-GCG CGG CTT ACC CTT ACC CTT ACC CTA ACC-3′), 15% glycerol, 1:10,000 SYBR Green, 0.08 unit/μl Taq polymerase). The PCR conditions used were as follows: 10-min incubation at 94 °C and 40 cycles of PCR at 94 °C for 30 s and 60 °C for 90 s. All of the samples were quantified using the Rotor-Gene quantification software and then compared with the standard curve generated using 293T cells and activity expressed as relative TA, relative to the control.
Real-time PCR
Reverse transcription was performed using the Promega RT-PCR kit and oligo(dT) primer as per the manufacturer's protocol (Promega). Real-time PCR was performed using Brilliant SYBR Green qPCR Master Mix on the Rotor-Gene 6000 system (Qiagen). The following primers were used for real-time PCR: 5′-CATGAGGTGGTAGCCTGGAT-3′ and 5′-ACCACTGTGCCCTGTCTTCT-3′ (human DKC1), 5′- GCGAAGAGTTGGGCTCTGTC-3′ and 5′-CATGTGTGAGCCGAGTCCTG-3′ (human telomerase RNA), 5′-ATGCGACAGTTCGTGGCTCA-3′ and 5′-ATCCCCTGGCACTGGACGTA-3′ (hTERT), and 5′-GTGGACCTGACCTGCCGTCT-3′ and 5′-GGAGGAGTGGGTGTCGCTGT-3′ (human glyceraldehydes-3-phosphate dehydrogenase (GAPDH)). Data were analyzed using the ΔΔCT method.
Generating Stable Cell Line
Lentiviral pLKO.1 short hairpin RNA (shRNA) against human β-catenin and control scramble shRNA lentiviral particles (Sigma) and retroviral vector pSUPER-retro containing human β-catenin sequence, retroviral vector pBabe-hTERT-hygro, were used to generate lentivirus and retrovirus in 293T or phoenix cells. The viruses were then used to infect cells, followed by 2 μg/ml puromycin or 100 μg/ml hygromycin (AG Scientific, Inc.) selection 24 h after infection. After 14 days of selection, stable pools of puromycin- or hygromycin-resistant cells were obtained and further expanded.
Telomere Length Assay
DNA was extracted from the cells using the DNeasy blood and tissue kit (Qiagen). Telomere length analysis was carried out using a non-radioactive TeloTAGGG telomere length assay (Roche Applied Science) as described by the manufacturer. Approximately 1 μg of DNA of each sample was digested with HinfI/RsaI enzyme mix and separated by gel electrophoresis. DNA fragments were transferred to a nylon membrane (GE Healthcare) by Southern transfer and hybridized to digoxigenin (digoxigenin-labeled probe), specific for telomeric repeats. A digoxigenin-specific antibody conjugated to alkaline phosphatase was used to incubate the membrane, and the probe was visualized by chemiluminescence detection and subsequent exposure to x-ray film (GE Healthcare). Mean telomeric repeat binding factor lengths were determined by comparison with the molecular weight standard.
Generation of Mutant Promoter Reporter Vectors
Luciferase vector pGL3–88bp, pGL3–385bp, and pGL3–949bp driven by various length of wild-type hTERT promoter were kind gifts of Prof. Horikawa, Izumi (NCI, National Institutes of Health). The putative TCF4 binding element (TBE) (5′-TGCAAAG-3′) contained in the hTERT promoter (between −659 and −653 bp) was mutated to 5′-TGCGGAG-3′ (Mut1) and 5′-TGCAAGA-3′ (Mut2) using site-directed mutagenesis according to the manufacturer's recommendations (QuikChangeTM site-directed mutagenesis kit, Stratagene). The mutations were confirmed by sequencing. The following primers were used for generating the mutants: 5′-CCG CCT GAG AAC CTG CGG AGA GAA ATG ACG GGC C-3′ and 5′-GGC CCG TCA TTT CTC TCC GCA GGT TCT CAG GCG G-3′ (Mut1), 5′-GCC GCC TGA GAA CCT GCA AGA AGA AAT GAC GGG CC-3′ and 5′-GGC CCG TCA TTT CTT CTT GCA GGT TCT CAG GCG GC-3′ (Mut2), and 5′-GCG AGA TCT GCG AGA AAT GAC GGG CCT GTG TCA AGG-3′ and 5′-GGC CAG GGC TTC CCA CGT GCG CAG TAT CCA TGG TAT-3′ (Truncate).
Transient Transfections and Luciferase Reporter Assays
All transient transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommendations. Respective amounts of cells (2 × 105/ml for MCF7, 4 × 105/ml for HCT116 and 293T cells) were seeded in a 24-well plate. 1 μg of plasmid encoding the protein ΔN β-catenin, ΔN TCF4, LEF1, TCF1, TCF3, TCF4, TAK1, or TAB or pCDNA control were transfected into cells. 2 days after transfection, cells were harvested and analyzed by real-time PCR and qTRAP. In a luciferase reporter assay, wild-type pGL3–88bp, pGL3–385bp and pGL3–949bp or mutant reporter plasmids were transfected into cells. After 24 h, cells were harvested, and whole cell lysates were used to measure luciferase activities using the Dual-Luciferase reporter assay system (Promega). To examine the effect of the activated Wnt pathway on the hTERT promoter, cells were treated with Wnt-3a CM or LiCl after transfection. In another assay, pGL3–88bp, pGL3–385bp, and pGL3–949bp reporter plasmids were co-transfected with TAK1 and TAB expression vector to study TAK1 negative regulation on the hTERT promoter. All firefly luciferase readings were normalized with the Renilla luciferase readings. Reporter assays were conducted in duplicates and repeated three times.
EMSA
Sense and antisense oligonucleotides containing TCF4 binding sites were labeled by the Biotin 3′ End DNA labeling kit (Thermo Scientific). The binding reactions contain 10 μg of crude 293T nuclear extract, 1 μg of double-stranded poly(dI-dC)-poly(dI-dC), and 10 fmol of biotin-labeled probe in 20 μl of 1× EMSA buffer (25 mm HEPES, pH 7.9, 62.5 mm KCl, 0.05% Nonidet P-40, 2 mm MgCl2, 8% Ficoll 400, 500 μg/ml bovine serum albumin, and protease inhibitors (0.5 mm PMSF, 5 μg/ml each of antipain, leupeptin, aprotinin, chymostatin, and pepstatin A), and 3.75 μl of H2O). The incubation was carried out for 30 min at room temperature. Unlabeled probes in 500-fold molar excess were included at the time of reaction for competitive binding assays. Anti-TCF4 and c-Myc antibodies were used for supershift analysis. 20 μl of the reaction was loaded onto a 6% non-denaturing polyacrylamide gel and transferred onto a nylon membrane. DNA was cross-linked to the membrane under UV light. The biotin-end-labeled DNA was detected using the streptavidin-horseradish peroxide conjugate and chemiluminescent substrate.
ChIP Assays
The assays for ChIP were performed using reagents commercially obtained from Upstate Biotechnology and conducted according to the manufacturer's instructions. Briefly, formaldehyde-cross-linked chromatin was isolated from 5 × 107 of HCT116 cells and sonicated into 500–1000-bp fragments. Immunoprecipitation was performed with anti-TCF4, anti-β-catenin (Millipore), and normal mouse IgG (Santa Cruz Biotechnology, Inc.). ChIP-enriched DNA was quantified by quantitative real-time PCR, and primer sets were designed flanking the putative TBE: hTERT 1, forward (5′-CCGGTGGGTGATTAACAGAT-3′) and reverse (5′-GGACGTGAAGGGGAGGAC-3′); hTERT 2, forward (5′- CCCCGGTGGGTGATTAACAG-3′) and reverse (5′-GGAGTGCCTCCCTGCAACAC-3′); hTERT 3, forward (5′-CTCCATTTCCCACCCTTTCT-3′) and reverse (5′-ACTTGGGCTCCTTGACACAG-3′; hTERT 4, forward (5′-AGGCCGTTGTGGCTGGTGTGAG-3′) and reverse (5′-CCGTCATTTCTCTTTGCAGGT-3′); hTERT 5, forward (5′- TTGCTCATGGTGGGGACCC-3′) and reverse (5′- GACGAACCCGAGGACGCAT-3′). Data are expressed as percentage of input.
Search for Consensus TCF/LEF Site
TCF binding sites (A/T)(A/T)CAAAG in the hTERT promoter were identified using an in silico tool named TFExplorer. TFExplorer is a database of regulatory elements in the genomic sequences of humans, mice, and rats. We obtained a TCF4 binding sequence 653 bp upstream of the transcription start site of the hTERT promoter from the human genome annotation files provided by NCBI.
Senescence-associated β-Galactosidase Staining
BJ fibroblast cells were cultured to the same population doubling level in the presence or absence of LiCl or Wnt-3a CM. Cell staining was performed using a kit from U.S. Biological. After removing growth medium from the cells, the cells were washed with PBS and fixed with the manufacturer's fixative solution for 15 min at room temperature. The cells were then washed twice with PBS and stained with X-gal staining solution overnight at 37 °C following the manufacturer's protocol.
Statistical Analysis
All experiments were independently performed at least three times, with similar results. All data are presented as mean ± S.D. of data obtained and were analyzed using either one-way analysis of variance or Student's t test, and p < 0.05 was considered statistically significant.
RESULTS
Identification of Signaling Pathway Inhibitors That Preferentially Inhibit TA
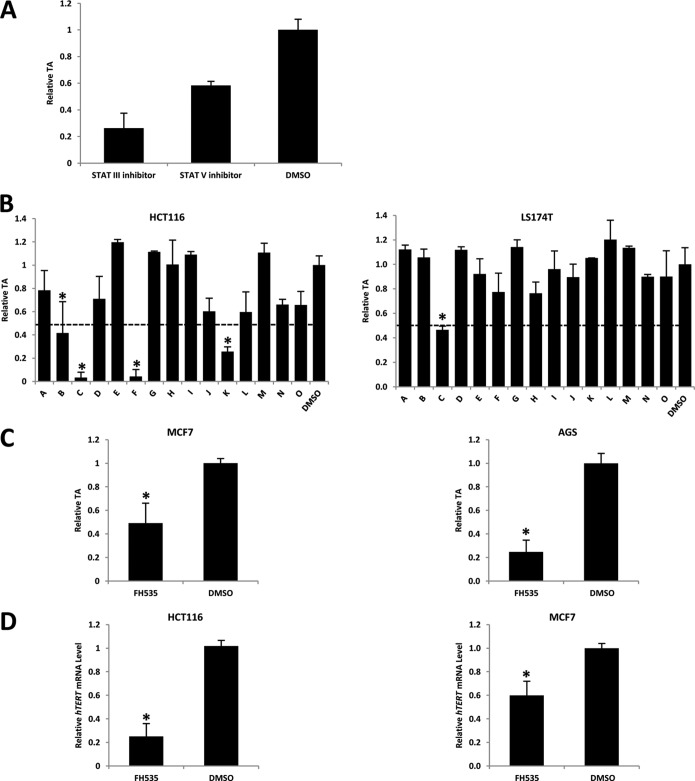
To identify telomerase inhibitors from the InhibitorSelectTM inhibitor library, consisting of Wnt, epidermal growth factor receptor, and JAK/STAT inhibitor libraries, we modified the existing qTRAP to suit the screen (36). TA was quantitated using qTRAP, and all data obtained were normalized against the vehicle control, DMSO. The concentration of each inhibitor was chosen based on its half-maximal inhibitory concentration (IC50). Screen and validation were carried out in a wide range of cancer cell lines (gastric cancer, AGS; breast cancer, MCF7; colorectal cancer, HCT116 and LS174T) that have similarly reactivated telomerase enzymatic activity but differ in genetic backgrounds. Inhibitors shown to be able to reduce TA by at least 50% in the initial screen were considered as potent telomerase inhibitors and further validated. It has been shown that STAT III and V are implicated in transcriptional activation of the hTERT promoter (36, 37); thus, their respective inhibitors served as positive controls in our screen platform. As shown in Fig. 1A, STAT III and V inhibitors were able to significantly reduce TA in HCT116 cells by 60 and 40% respectively, suggesting that the screen platform is workable.
FIGURE 1.
Screening telomerase inhibitor from well known signaling pathway inhibitor libraries. A, STAT III/V inhibitors inhibit TA in HCT116 cells. 24 h before drug treatment, 4 × 105 HCT116 cells were seeded into 12-well plates. The seeding density was chosen to obtain the optimum 60% confluence prior to drug treatment. Cells were treated with inhibitors or DMSO (vehicle control) and incubated for 48 h. After treatment, cells were harvested for qTRAP to measure TA. B, effect of Wnt pathway inhibitors on TA in HCT116 and LS174T cells. FH535, inhibitor C, is the most effective TA inhibitor. Inhibitors named A–O are listed in supplemental Table S1. The dotted line marks 50% TA inhibition, which was normalized with DMSO control. C, FH535 inhibitory effect on TA is validated in MCF7 and AGS cell lines. D, FH535 treatment leads to reduction of hTERT expression. Data were normalized against DMSO treatment. Data are the average of three independent experiments. *, p < 0.05. Error bars, S.D.
The screen result showed that four Wnt pathway inhibitors (B, casein kinase II inhibitor III, TBCA; C, β-catenin·TCF complex inhibitor, FH535; F, protein kinase A H-89, dihydrochloride; K, TAK1 inhibitor, (5Z)-7-oxozeaenol; supplemental Table S1) were able to inhibit TA by at least 50% compared with vehicle controls after 2 days of treatment in HCT116 cells (Fig. 1B, left). To further confirm the result and avoid cell type specificity, the same screen was performed with LS174T cells (Fig. 1B, right). We also found that FH535 could efficiently reduce TA by 50%, whereas other hits did not show an inhibitory effect in LS174T cells. Moreover, FH535 also demonstrated a strong inhibitory effect on TA in MCF7 and AGS cells (Fig. 1C). Our data showed that FH535 treatment led to significant reduction in hTERT expression (Fig. 1D), suggesting that the inhibition of TA was through inhibition of the telomerase component, hTERT transcription. Taken together, these results promoted us to hypothesize that hTERT is a novel Wnt target gene, and β-catenin·TCF complexes may play a critical role in telomerase regulation in cancer cells.
hTERT Is Up-regulated by Activated Wnt/β-Catenin Signaling
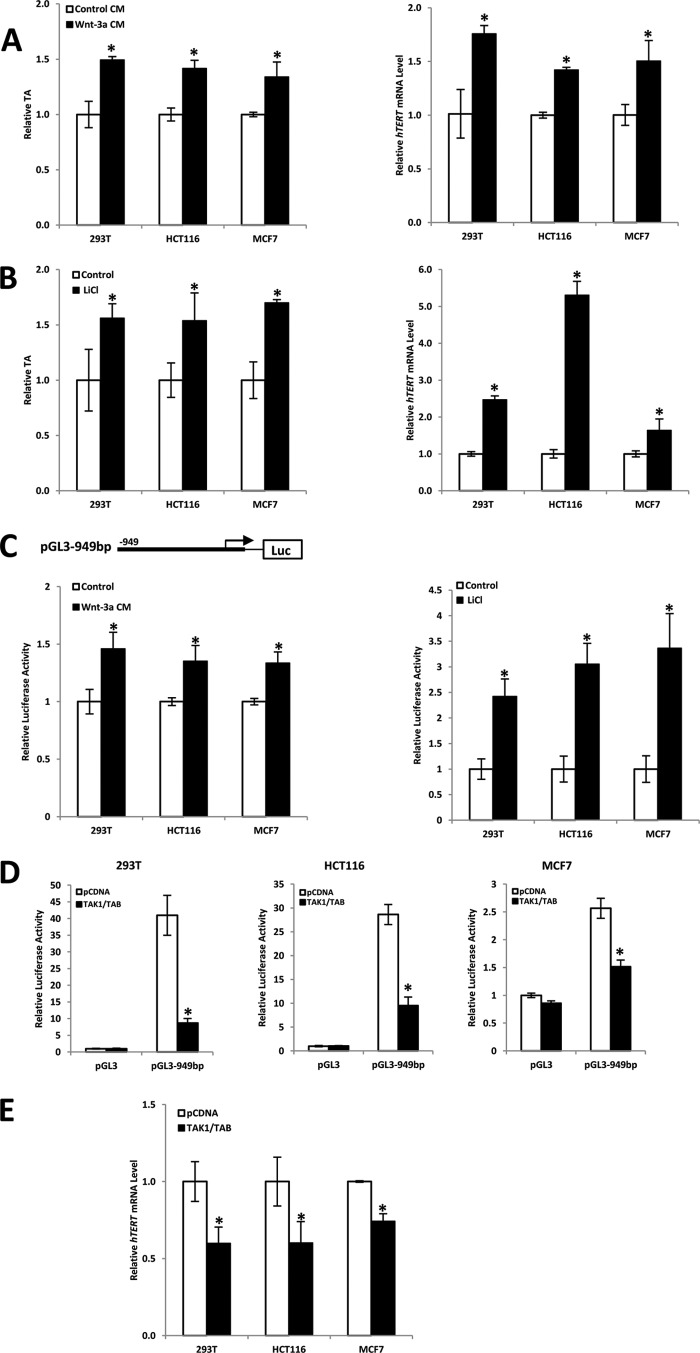
To identify whether the hTERT gene is regulated by the Wnt pathway, 293T, HCT116, and MCF7 cells were exposed to Wnt-3a CM or control medium for 48 h. TA and hTERT gene transcription were measured by qTRAP and real-time PCR, respectively. Compared with controls, we found that TA was increased 1.5-, 1.41-, and 1.34-fold in 293T, HCT116, and MCF7 cells, respectively, following Wnt-3a CM treatment (Fig. 2A, left). Consistent with this result, we found that Wnt-3a CM treatment could largely elevate the hTERT mRNA level (Fig. 2A, right), suggesting that the activated Wnt pathway is able to stimulate hTERT expression and hence enhance TA. To support this result, we examined the effect of LiCl on the expression of the hTERT gene. LiCl, a small molecular activator of Wnt signaling that works by inhibiting GSK-3β, hence increases and stabilizes β-catenin protein levels (38). As shown in Fig. 2B, 15 mm LiCl treatment for 24 h in multiple human cell lines that we have tested efficiently elevated TA (left) as well as hTERT mRNA level (right).
FIGURE 2.
Activated Wnt/β-catenin directly affects hTERT gene expression and TA. A and B, Wnt-3a and LiCl stimulation of 293T, HCT116, and MCF7 cells increases hTERT gene expression and TA. Cells were treated with Wnt-3a CM or control CM (A) for 48 h or 15 mm LiCl for 24 h (B), respectively, and assayed for real-time PCR and qTRAP to measure TA (left) and hTERT expression level (right). C, activated Wnt pathway elevates hTERT promoter activities. A luciferase reporter construct, containing 0.95 kb upstream of the hTERT gene transcription initiation site, was transiently transfected into 293T, HCT116, and MCF7 cells. 24 h after transfection, cells were treated with Wnt-3a CM (left) or 15 mm LiCl (right), respectively. Wnt-3a CM or 15 mm LiCl could significantly activate hTERT promoter compared with controls. D and E, Wnt pathway negative regulator TAK1 represses hTERT promoter activities. 293T (Wnt-3a CM-treated), HCT116, and MCF7 cells were co-transfected with a 949-bp hTERT promoter-driven reporter construct and the indicated expression constructs for TAK1 and TAB (D). In another assay, after transfection with TAK1, cells were subjected to real-time PCR to measure hTERT expression (E). Empty expression vector pCDNA was used as a control. Expression of TAK1/TAB abolished hTERT promoter activities compared with control. Relative luciferase activity was standardized to Renilla luciferase activities. Data are the average of three independent experiments. *, p < 0.05. Error bars, S.D.
To further confirm these results, a luciferase reporter assay was carried out to examine the effect of Wnt signaling on activation of the hTERT gene promoter. A luciferase reporter construct containing 0.95 kb upstream of the hTERT gene transcription initiation site (39) was transiently transfected into 293T, HCT116, and MCF7 cells. As shown in Fig. 2C, Wnt-3a CM (left) or LiCl (right) treatment significantly enhanced hTERT gene promoter activity as compared with controls.
Additionally, the involvement of β-catenin·TCF complexes in hTERT gene transactivation is suggested by the inhibition of hTERT promoter activation using TAK1 (transforming growth factor-β-activated kinase 1). The TAK1-NLK (Nemo-like kinase) pathway plays a negative role in the canonical Wnt/β-catenin signaling through regulating the LEF/TCF family transcriptional factors. When activated by TAK1, NLK can directly phosphorylate LEF/TCFs to prevent the β-catenin·LEF/TCF complex from binding to DNA (40). It is known that β-catenin is able to accumulate in the nucleus of cancer cells but not in 293T cells. To check the biological function of β-catenin·LEF/TCF complex in hTERT gene regulation, 293T cells were treated with Wnt-3a CM to stimulate the accumulation of β-catenin in the nucleus. Luciferase reporter assay results demonstrated that transactivation of the hTERT promoter by endogenous β-catenin·TCF complexes was strongly repressed by co-transfection of TAK1/TAB (TAK1-binding protein) compared with the empty vector pCDNA3 (Fig. 2D). Consistent with this result, our data demonstrated that exogenous TAK1/TAB could reduce hTERT expression in all cell lines tested (Fig. 2E). In conclusion, these data indicate that the expression of the hTERT gene can be regulated by Wnt/β-catenin pathway in human cancer cells.
Knockdown of β-Catenin or Its Overexpression Dramatically Affects hTERT Expression and Telomerase Activity
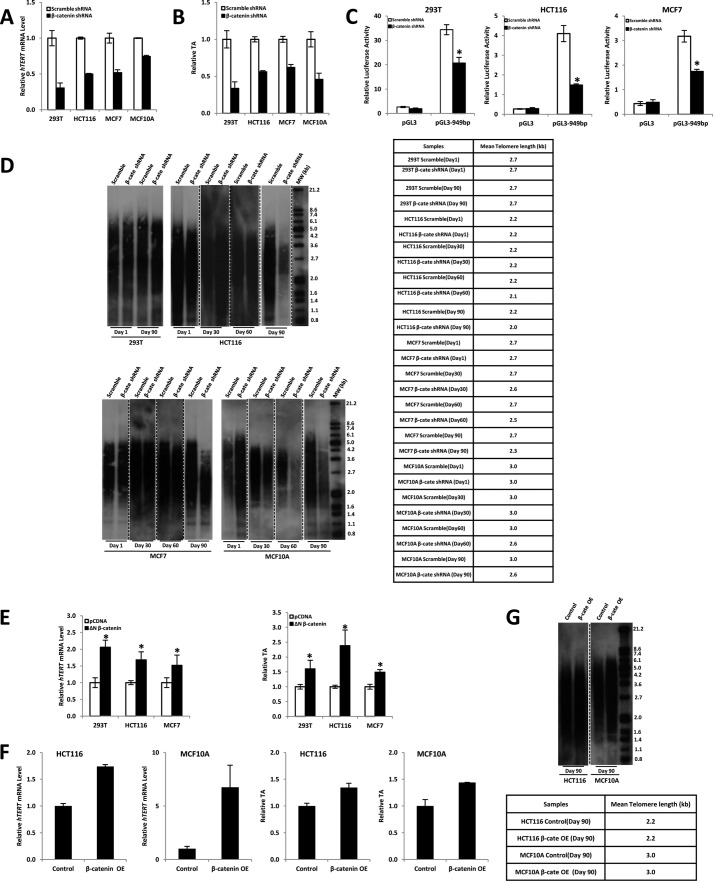
To study the possible role of β-catenin·TCF complexes in telomerase regulation, we examined whether endogenous β-catenin·TCF signaling activates the endogenous hTERT gene. Here, we focused on β-catenin, which is a central player in the Wnt signaling pathway. We established stable β-catenin knockdown in 293T, HCT116, MCF7, and MCF10A cell lines with lentiviral shRNA against β-catenin. These β-catenin knockdown cell lines were used to examine the effect of β-catenin on telomerase regulation. Western blot results showed that β-catenin protein expression levels were significantly decreased in the β-catenin knockdown stable cell lines (supplemental Fig. S1). Analysis of hTERT expression in such stable cell lines, where β-catenin levels were suppressed, revealed a major decrease in hTERT mRNA level (Fig. 3A). In addition, qTRAP assay results indicated that silencing the β-catenin gene markedly reduced TA (Fig. 3B), which is consistent with real-time PCR results in Fig. 3A. Moreover, the luciferase reporter assay results showed that repressing endogenous β-catenin expression could significantly inhibit hTERT promoter activity in 293T, HCT116, and MCF7 cells (Fig. 3C).
FIGURE 3.
Effects of β-catenin knockdown on the hTERT gene and TA. A, real-time PCR analysis of hTERT expression in β-catenin knockdown stable 293T (Wnt-3a CM-treated), HCT116, MCF7, and MCF10A cells. hTERT mRNA levels were normalized by comparison with GAPDH as internal controls. -Fold change was calculated relative to the control. B, TRAP was carried out to measure TA in β-catenin knockdown stable cells and control cells. C, 949-bp hTERT promoter-driven luciferase reporter construct was transiently transfected into β-catenin knockdown stable cells: 293T (Wnt-3a CM-treated), HCT116, and MCF7. 24 h after transfection, cells were collected for luciferase activity measurement. β-Catenin knockdown could significantly inhibit hTERT promoter activity compared with controls. Relative luciferase activity was standardized to Renilla luciferase activities. Data are the average of three independent experiments. *, p < 0.05. D, telomere length was measured by Southern blotting in β-catenin knockdown stable cells and control cells. The table beside the Southern blot indicates the mean telomere length measured. E, expression construct containing the constitutively active form of β-catenin (ΔN β-catenin) was transiently transfected into 293T, HCT116, and MCF7 cells. 48 h after transfection, cells were harvested and subjected to real-time PCR or qTRAP to measure hTERT mRNA expression level (left) or TA (right). Empty expression vector pCDNA was used as a control. Data are the average of three independent experiments. *, p < 0.05. F, β-catenin overexpression (OE) stable HCT116 and MCF10A cell lines were obtained as described under “Experimental Procedures.” These stable cells were harvested and subjected to real-time PCR or qTRAP to measure hTERT mRNA expression level (top) or TA (bottom). Empty retroviral expression vector was used as a control. G, telomere length was measured by Southern blotting in β-catenin overexpression stable HCT116 and MCF10A cell lines. The table beside the Southern blot indicates the mean telomere length measured. Error bars, S.D.
The activation of hTERT is required for the maintenance of telomere length in cancer cells. To better understand the biologic function of β-catenin in regulating activation of hTERT, we determined the effects of inhibition of β-catenin expression on telomere length in stable β-catenin knockdown cells. We continuously cultured stable β-catenin knockdown cells over 90 days through ∼30 cell passages. Cells were collected at day 1, day 30, day 60, and day 90, and the effect of β-catenin on telomere length was determined by Southern blotting. Telomere signals appeared as a broad smear of densities. As shown in Fig. 3D, β-catenin knockdown effectively induced significant telomere shortening in HCT116, MCF7, and MCF10A cells compared with stable cells infected with scramble shRNA lentivirus. However, silencing β-catenin gene in 293T cells had no effect on telomere length maintenance. It is possible that β-catenin knockdown may take a longer time to affect the telomere length in 293T cells.
Next, we examined whether the expression of hTERT is regulated in a β-catenin-dependent manner. We exogenously provided 293T, HCT116, and MCF7 cells with a constitutively active form of β-catenin (ΔN β-catenin) as described (41) and examined the β-catenin dependence of the hTERT gene expression and TA by real-time PCR or qTRAP analysis, respectively. As shown in Fig. 3E, ectopic expression of the stable active β-catenin form enhanced transcriptional activation of the hTERT gene (left) and subsequently elevated TA (right). To further confirm this result, we established stable β-catenin overexpression in HCT116 and MCF10A cell lines with retroviral expression vector. Real-time PCR results demonstrated that β-catenin expression levels were significantly enhanced in the β-catenin overexpression stable cell lines (supplemental Fig. S2). Compared with the controls, real-time PCR analysis showed that hTERT gene expression was elevated by 1.7- and 6.7-fold in stable β-catenin overexpression HCT116 and MCF10A cell lines, respectively (Fig. 3F, top). Consistent with this result, TA was correspondingly enhanced in both cell lines (Fig. 3F, bottom). Although hTERT gene expression and TA were increased in β-catenin overexpression stable cell lines, we did not find obvious telomere length extension (Fig. 3G). It could be that β-catenin overexpression may take a longer time to affect the telomere length or that immortalized cells just need telomerase to maintain telomere length rather than extend telomere length. Overall, these results suggest that β-catenin plays an important role in regulating the expression of hTERT.
β-Catenin and TCF4 Up-regulate Promoter Activity of hTERT
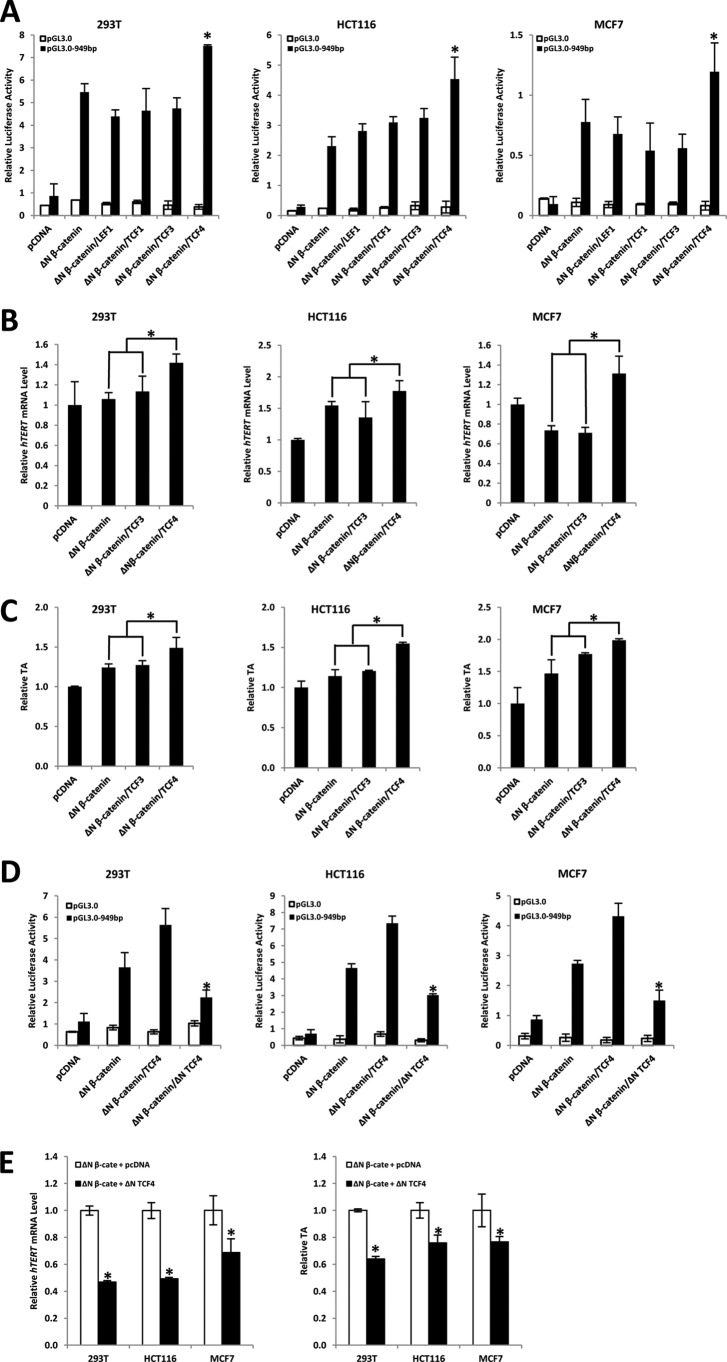
LEF/TCFs are sequence-specific DNA binding transcription factors that bind to Wnt target gene promoters and repress gene transcription. Upon β-catenin binding to LEF/TCFs, the complex will activate target gene transcription (42). Higher organisms have four family members: LEF-1, TCF-1, TCF-3, and TCF-4. To further evaluate the function of β-catenin·TCF complexes in telomerase regulation, we sought to identify which LEF/TCF member can bind to the hTERT promoter and synergistically activate hTERT expression with β-catenin. To this end, we transiently co-transfected the ΔN β-catenin construct alone or with different LEF/TCF members and a 949-bp hTERT promoter-driven luciferase construct into 293T, HCT116, and MCF7 cells to analyze the effect on promoter activity of hTERT. As shown in Fig. 4A, compared with ΔN β-catenin alone, the co-transfection of ΔN β-catenin and TCF4 could significantly activate the hTERT promoter in 293T, HCT116, and MCF7, whereas ΔN β-catenin·TCF1/3 or LEF1 complexes had no effect on activating the hTERT promoter. To further confirm this result, we carried out real-time PCR to examine the effect of ΔN β-catenin·TCF3/4 complexes on hTERT regulation. The result demonstrated that the exogenous β-catenin·TCF4 complex could slightly elevate hTERT expression and TA compared with ΔN β-catenin alone or ΔN β-catenin·TCF3 (Fig. 4, B and C). To further investigate whether hTERT is a transcriptional target of the β-catenin·TCF4 complex and whether activation of the hTERT promoter is due to a synergistic effect of β-catenin and TCF4, we performed a luciferase reporter assay utilizing the dominant negative form of TCF4 (ΔN TCF4), which is known to diminish the transcriptional activity of TCF-responsive promoters (43). We observed that the stimulation of the hTERT promoter upon ΔN β-catenin overexpression was significantly suppressed by co-overexpression of ΔN TCF4 (Fig. 4D). In addition, ectopic expression of ΔN TCF4 could inhibit ΔN β-catenin-induced hTERT expression (Fig. 4E, left) and hence TA (Fig. 4E, right). Thus, β-catenin regulation on hTERT occurs via a TCF4-dependent pathway. These data imply that TCF4 may bind to hTERT promoter and activate hTERT gene transcription upon forming a transcriptional complex with β-catenin.
FIGURE 4.
β-Catenin·TCF4 specifically up-regulates promoter activity of hTERT. A, 293T, HCT116, and MCF7 cells were co-transfected with a 949-bp hTERT promoter-driven reporter construct, the indicated expression construct for ΔN β-catenin, and/or LEF1, TCF1, TCF3, and TCF4 expression constructs. pGL3.0 reporter plasmid was used as a control. Co-transfection of β-catenin and TCF4 specifically activates the hTERT promoter. B, 293T, HCT116, and MCF7 cells were transfected with the expression construct for ΔN β-catenin alone or with TCF3 or TCF4 expression constructs, respectively. Empty expression vector pCDNA was used as a control. 48 h after transfection, cells were harvested and subjected to real-time PCR to measure hTERT mRNA. Co-transfection of β-catenin and TCF4 could more efficiently increase hTERT expression. C, 293T, HCT116, and MCF7 cells were transfected with expression construct for ΔN β-catenin alone or with TCF3 or TCF4 expression constructs, respectively. Empty expression vector pCDNA was used as a control. 48 h after transfection, cells were harvested and subjected for qTRAP to measure TA. Co-transfection of β-catenin and TCF4 could more efficiently elevate TA. D, 293T, HCT116, and MCF7 cells were co-transfected with a 949-bp hTERT promoter-driven reporter construct or the indicated expression constructs for ΔN β-catenin, TCF4, or ΔN TCF4. pGL3.0 reporter plasmid was used as a control. Co-transfection of ΔN TCF4 significantly inhibited hTERT promoter activation. Relative luciferase activity was standardized to Renilla luciferase activities. E, 293T, HCT116, and MCF7 cells were co-transfected with expression construct for ΔN β-catenin and ΔN TCF4. pcDNA plasmid was used as a control. Co-transfection of ΔN TCF4 was able to reduce β-catenin-induced hTERT expression (left) and TA (right). Data are the average of three independent experiments. *, p < 0.05. Error bars, S.D.
Characterization of the Distal TBE in the hTERT Promoter
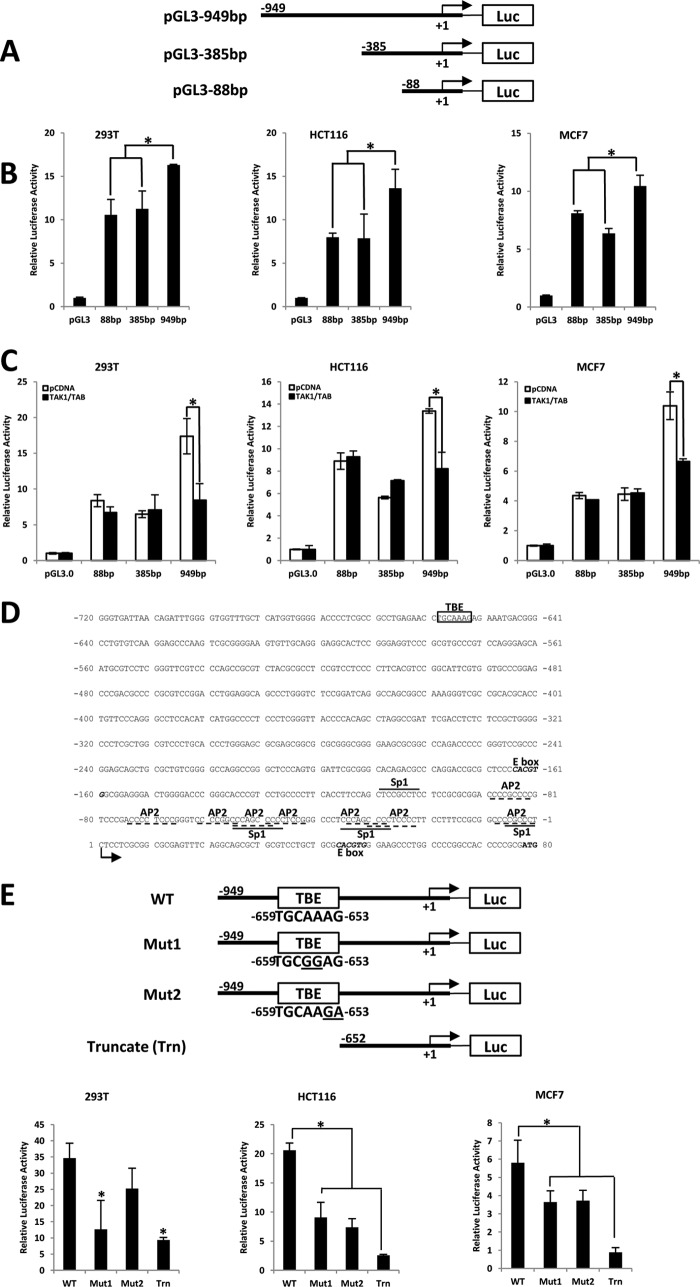
We next sought evidence of functional binding of the β-catenin·TCF4 complex on the hTERT promoter. To this end, a luciferase reporter assay was carried out to examine the effect of the β-catenin·TCF4 complex on various lengths of the hTERT promoter (88, 385, and 949 bp) (Fig. 5A) in multiple cell lines. As shown in Fig. 5B, the 949-bp promoter could induce relatively higher luciferase activity when compared with the 88- or 385-bp hTERT promoter in the presence of β-catenin·TCF4 in 293T, HCT116, and MCF10A. The TAK1-NLK pathway phosphorylates LEF/TCFs and, hence, inhibits β-catenin-dependent transcription. We therefore proposed that if β-catenin·TCF4 can directly regulate hTERT gene expression at the 949-bp promoter, co-transfection of TAK1/TAB will only reduce the 949-bp length hTERT promoter activity but not that of the 88- and 385-bp promoters. As expected, we found that co-transfection of TAK1/TAB significantly reduced luciferase activity with the 949-bp hTERT promoter, whereas it had no effect on the 88- or 385-bp hTERT promoter (Fig. 5C). These results imply that β-catenin·TCF4 can directly regulate hTERT expression, and one or more distal TCF4 binding sites exist between position −386 and −949 bp in the hTERT promoter.
FIGURE 5.
Identification of TBE in the hTERT promoter region. A, schematic representation of various luciferase reporter plasmids of hTERT promoter (88, 385, and 949 bp). B, 949-bp hTERT promoter is most responsive to β-catenin·TCF4. 293T, HCT116, and MCF7 cells were transiently transfected with various luciferase reporter constructs containing 88, 385, and 949 bp (upstream of the transcriptional start site) of the hTERT promoter and β-catenin·TCF4 expression constructs. C, TAK1/TAB reduces promoter activity of the hTERT by β-catenin·TCF4. 293T, HCT116, and MCF7 cells were co-transfected with the indicated various hTERT promoter luciferase reporters and β-catenin·TCF4 expression constructs. TAK1/TAB overexpression leads to a significant reduction in 949-bp hTERT promoter activity only. D, the sequence of the distal and proximal promoter of hTERT. Putative protein binding sites for various transcription factors in the first 181 bp are indicated. The +1 indicates the first nucleotide of the hTERT mRNA. Consensus motifs for Sp1 and AP2 are underlined by solid and broken lines, respectively, and the E box (c-Myc binding site) consensus motif is in italic type. The putative TBE consensus motif is boxed. The initiating ATG codon is shown in boldface type. E, TBE is important for β-catenin·TCF4 function on the hTERT promoter. Schematic representation of various luciferase reporter plasmids of the hTERT promoter. The putative TCF4 binding element is located between −659 and −653 bp from the transcription initiation site. Construct WT contains the wild-type binding element; Mut1 has a 2-bp substitution (AA → GG, underlined); Mut2 has another 2-bp substitution (AG → GA, underlined); and Truncate (Trn) only consists of the 652-bp sequence upstream of the transcriptional start site of the hTERT promoter, which does not contain the putative TBE. 293T, HCT116, and MCF7 cells were transiently transfected with various luciferase reporter constructs (WT, Mut1, Mut2, and Truncate) and β-catenin·TCF4 expression constructs. The promoter reporter activity was normalized with Renilla luciferase reporter. Data are the average of three independent experiments. *, p < 0.05. Error bars, S.D.
To further investigate the functional interaction between β-catenin·TCF4 signaling and hTERT gene expression, we analyzed the transcriptional control sequences of the hTERT gene for TCF protein binding sites by using TESS (Transcription Element Search System). The search result revealed the presence of a putative consensus TBE between positions −659 and −653 bp (5′-TGCAAAG-3′) upstream of the transcription start site of hTERT (Fig. 5D) that showed a high degree of homology to the core consensus sequence (CTTTG or CAAAG) for TCF4 binding (44). To test whether the putative TCF4 binding site could affect the promoter activity of hTERT, we introduced mutations in the TBE (Fig. 5E) by site-directed mutagenesis and constructed a shorter hTERT promoter (652 bp) without the TBE. We transiently co-transfected various mutant luciferase reporter constructs with β-catenin·TCF4 expression constructs into 293T, HCT116, and MCF7 cells. Introduction of the mutations into the putative TBE reduced the stimulatory effect of β-catenin·TCF4 by more than 50% compared with the wild-type promoter in all of the three cell lines (Fig. 5E), and the deletion of the TBE (Truncate) significantly repressed the hTERT promoter activity by ∼80% (Fig. 5E). These results suggest that the putative TBE motif is responsible for the β-catenin·TCF4-dependent promoter activity of hTERT.
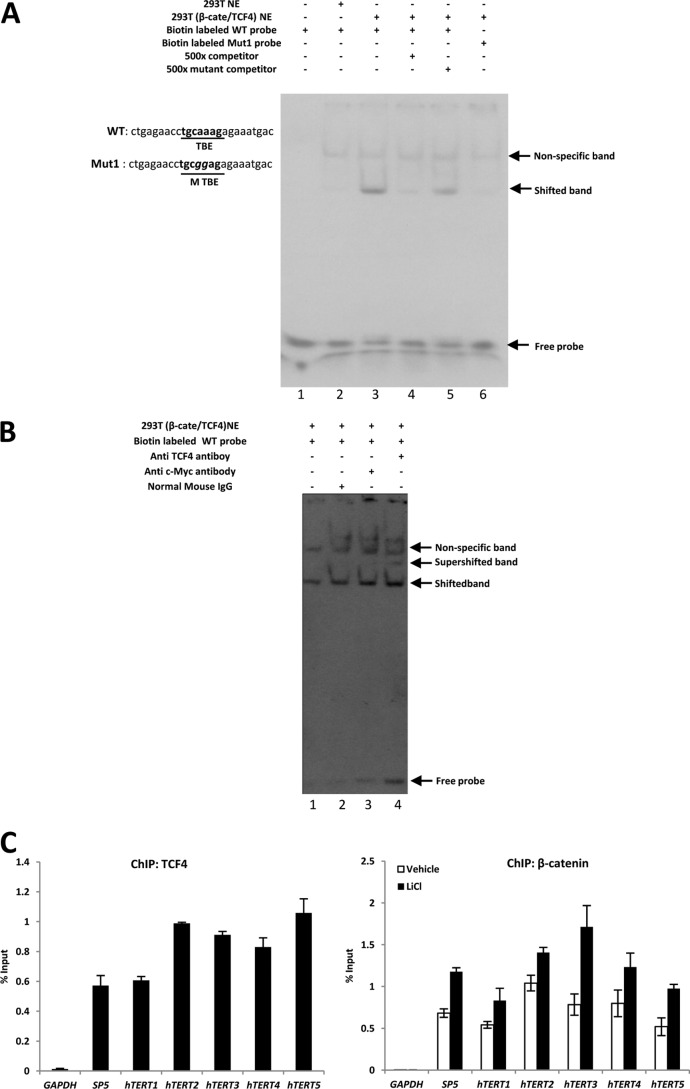
TCF4 Directly Binds to the TBE in the hTERT Promoter
To verify whether TCF4 could bind to the putative distal TBE, we carried out an EMSA with a probe containing the potential TBE. As shown in Fig. 6A, complexes were formed between the biotin-labeled wild-type TBE probe and proteins in 293T nuclear extracts with β-catenin·TCF4 overexpression (Fig. 6A, lane 3), whereas normal 293T nuclear extracts could not form a visible complex with biotin-labeled wild-type TBE probe (Fig. 6A, lane 2). The specificity of the interaction between β-catenin·TCF4 and wild-type TBE probe was confirmed when a mutant probe (Mut1), with a two-nucleotide substitution in the core of TBE, dramatically decreased complex formation (Fig. 6A, lane 6), which is consistent with the luciferase reporter assay results (Fig. 5E). The addition of 500-fold competitor (unlabeled wild-type TBE probe) could abrogate the complex formation between the biotin-labeled probe and the nuclear extract (Fig. 6A, lane 4), but the addition of 500-fold cold mutant probe was unable to inhibit formation of the specific complexes (Fig. 6A, lane 5), validating that the binding of β-catenin·TCF4 to the labeled probe was specific. Moreover, the addition of anti-TCF4 antibody could result in formation of the supershifted band between nuclear proteins and TBE probe (Fig. 6B, lane 4), providing evidence that TCF4 is one of the transcription factors that binds to the TBE in the hTERT promoter. Neither normal IgG nor anti-c-Myc antibody could result in formation of a supershifted band (Fig. 6B, lanes 2 and 3). We also examined whether TCF3 could bind to TBE. The EMSA data showed that TCF3 is unable to form complexes with TBE probe (supplemental Fig. S3). Taken together, the putative TBE between −659 and −653 bp in the hTERT promoter is indeed a TCF4 binding element. EMSAs provided direct evidence for the physical interaction between TCF4 and the TBE probes in vitro. To show the in vivo occupancy of TCF4 on the hTERT promoter, we performed ChIP with TCF4 antibody in HCT116 cells. The quality of the ChIP DNA was confirmed using the SP5 promoter, a well known Wnt/β-catenin target gene, as a positive control, and a region in the GAPDH coding region as a negative control. As shown in Fig. 6C (left), our data showed a specific enrichment of the hTERT promoter in TCF4 ChIP DNA using five pairs of hTERT primers, implying that TCF4 is likely to directly interact with the hTERT promoter. To further examine whether hTERT is directly regulated by β-catenin·TCF4, another ChIP assay was performed using anti-β-catenin antibody. As shown in Fig. 6C (right), anti-β-catenin antibody was able to specifically precipitate chromatin-DNA complexes containing an hTERT promoter fragment. In addition, LiCl treatment enhanced binding of β-catenin on the TBE of the hTERT promoter. Collectively, our data confirmed that endogenous TCF4 and β-catenin bound to hTERT TBE in vivo, consistent with the in vitro results in Fig. 6, A and B.
FIGURE 6.
Binding of TCF-4 and β-catenin to a putative TBE in the hTERT promoter. A, TBE is important and is the minimal sequence for TCF4 binding. Biotin-labeled wild-type TBE probe (lanes 1–5) and mutant TBE probe (lane 6) were incubated with 10 μg of 293T nuclear extracts or 293T nuclear extracts with β-catenin·TCF4 overexpression for 30 min. Competition experiments were performed by preincubating with a 500-fold molar excess of the unlabeled TBE probes (competitor; lane 4), or 500-fold molar excess of the unlabeled mutant TBE (mutant competitor; lane 5). The arrow indicates the position of specific transcription factor complexes. B, biotin-labeled TBE (lanes 1–4) were incubated with 10 μg of 293T cell nuclear extracts with β-catenin·TCF4 overexpression for 30 min. Antibody binding experiments were carried out following the vendor's instruction with anti-TCF4 antibody (lane 4), normal mouse IgG as control (lane 2), or anti-c-Myc antibody (lane 3). The arrows indicate the position of shifted and supershifted bands. C, TCF4 binds to the hTERT promoter in vivo. A ChIP assay was performed using anti-TCF4 or β-catenin antibodies and analyzed by real-time PCR. GAPDH was used as a negative control, and SP5 was used as a positive control. Error bars, S.D.
Wnt/β-Catenin Pathway Is Involved in Regulation of hTERT in Somatic Cells
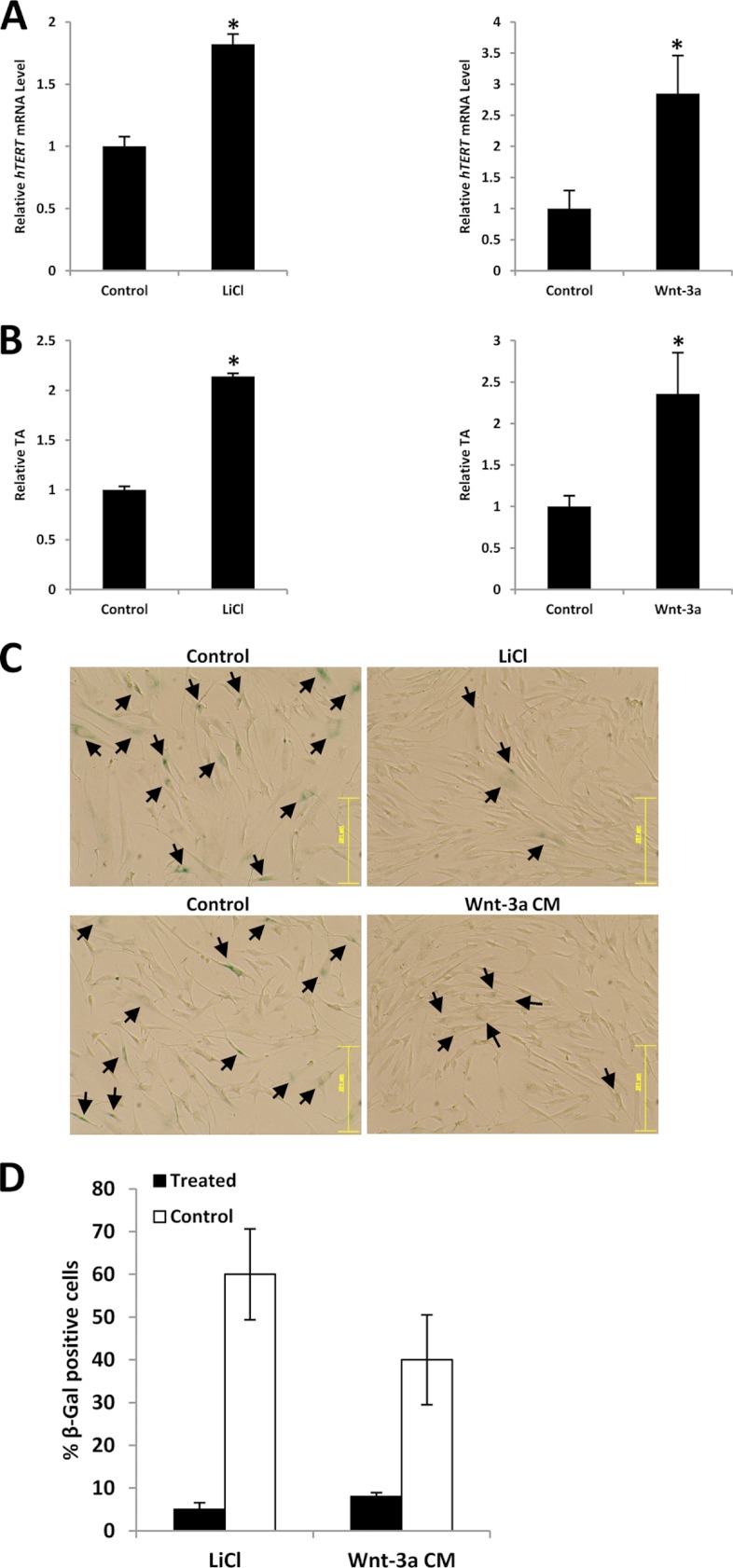
Association of deregulated Wnt/β-catenin signaling with cancer has been well documented, whereas the Wnt pathway is tightly regulated in normal somatic cells. We questioned whether activation of Wnt/β-catenin signaling could stimulate hTERT transcription and even reactivate telomerase in somatic cells. Very interestingly, we found LiCl or Wnt-3a CM treatment could significantly increase hTERT gene expression and hence reactivate telomerase in BJ cells (human normal fibroblast cells) compared with the control (Fig. 7, A and B).
FIGURE 7.
The Wnt/β-catenin signaling pathway is involved in telomerase reactivation in somatic cells. A and B, LiCl or Wnt-3a CM stimulation of BJ cells increases hTERT gene expression and reactivates telomerase. BJ cells were treated with LiCl or Wnt-3a and subjected to real-time PCR or TRAP to measure hTERT expression level (A) or TA (B), respectively. C, activation of the Wnt pathway could extend the life span of BJ cells. BJ cells were treated with LiCl or Wnt-3a CM for 20 population doublings, and the morphological changes were monitored under a microscope. Blue coloration in cells indicates senescence, indicated by arrows. Bar, 251 μm. D, bars represent the percentage of β-galactosidase-positive cells. Data are mean ± S.D. (error bars) from five images each.
Human somatic cells gradually lose telomeric sequences as a result of incomplete replication (45). Telomere shortening can induce replicative senescence, which may play an important role in suppression of cancer emergence, although inheriting shorter telomeres probably does not protect against cancer (46). To check whether activation of Wnt/β-catenin signaling could affect cellular senescence via reactivating telomerase, we performed a β-galactosidase assay to determine the senescence status of BJ cells in the presence or absence of LiCl and Wnt-3a CM. Cells were cultured to the same population doubling level (20 population doublings) and stained. Morphologic examination of the cells without activating Wnt/β-catenin pathway showed an increased proportion of flat and giant cells with phenotypic characteristics of senescence and overexpression of β-galactosidase activity, suggesting that the active Wnt/β-catenin pathway may lead to extension of the life span of somatic cells via reactivating telomerase (Fig. 7C). Taken together, these results lead us to hypothesize that Wnt/β-catenin plays a critical role in telomerase reactivation in carcinogenesis. However, further mechanistic studies are necessary to explain the phenomenon.
DISCUSSION
In this study, we showed for the first time that hTERT is a direct transcriptional target of the Wnt/β-catenin signaling pathway.
Previous studies have demonstrated that hTERT expression is highly specific to cancer cells and tightly associated with telomerase activity; therefore, it is the main protein that holds the key to one of the important hallmarks of cancer, the infinite proliferative capacity (14, 47–49). Despite the tremendous attention to defining telomerase regulation in cancer cells, it remains unclear how cancer cells gain the ability to reactivate telomerase and whether genetic variations affect hTERT expression in cancer cells. The almost universal presence of telomerase in human cancers and cancer stem cells, together with its near absence in most normal tissues make telomerase an attractive therapeutic target. Consequently, it is rather reasonable to hypothesize that the tumor-specific activation of the hTERT promoter may be regulated by various cellular factors, such as transcription factors and effectors, which are differentially activated in tumor cells or repressed in normal cells. These tumor-specific cellular factors can either specifically bind to the hTERT promoter or interact with its effectors to differentially regulate hTERT transcription. A large class of oncogenes can be assigned to the DNA-binding proteins, the function of which is controlled not just by their abundance or expression level but mainly at the level of their activity in terms of their interactions with DNA and protein targets.
Evidence to date indicates that the activity of the Wnt pathway is frequently deregulated, resulting in the activation of target genes whose dysregulation has significant effects on the development and progression of cancer, but the molecular details remain unclear. Elevated levels of β-catenin, the central player of the canonical Wnt pathway, have been observed in most common forms of human malignancies (50). Therefore, identifying the target genes of Wnt signaling is important for understanding β-catenin-associated carcinogenesis. Recently, Park et al. (51) showed that hTERT plays an essential role in the Wnt/β-catenin signaling pathway by serving as a cofactor in a β-catenin transcriptional complex. However, whether the Wnt/β-catenin signaling pathway is involved in telomerase regulation was not investigated.
In this study, we have demonstrated that the real-time PCR-based qTRAP assay coupled with well defined signaling pathway inhibitor libraries is a powerful approach for discovering and detecting signaling pathways that may be involved in telomerase regulation. Using the qTRAP assay, we clearly detected a β-catenin·TCF complex inhibitor, FH535, which could effectively inhibit TA in gastric cancer, breast cancer, and colorectal cancer cells, suggesting that the Wnt pathway is widely implicated in telomerase regulation in human cancers.
To assess the function of the Wnt pathway on telomerase activation, Wnt-3a CM and LiCl were used to activate the Wnt pathway in 293T, HCT116, and MCF7 cells. We found that the activated Wnt pathway is indeed able to up-regulate hTERT expression and increase TA. Conversely, the Wnt pathway negative regulatory factor TAK1 could eliminate hTERT promoter activation. These results led us to postulate that the activated hTERT promoter in those tumor cells was due to an increase in the activated form of β-catenin. The results from β-catenin knockdown and overexpression experiments confirmed this notion and illustrate the critical role of β-catenin in telomerase regulation. Moreover, expression of the constitutively activated form of β-catenin could stimulate hTERT expression. On the other hand, silencing β-catenin gene expression resulted in down-regulation of hTERT expression and significant reduction of TA, which consequently led to telomere length shortening in multiple cells tested. The data from β-catenin shRNA knockdown experiments further confirmed the role of β-catenin in regulating TA and altering telomere length. Telomerase is a unique reverse transcriptase (RT) that contains a catalytic protein subunit, the telomerase RT, a telomerase RNA, and species-specific accessory proteins. Telomerase accessory protein components are known to play important roles in regulating telomerase biogenesis, subcellular localization, and function in vivo. For example, dyskerin is required for the stability and accumulation of human telomerase RNA in vivo (52), and the mutation of dyskerin is associated with dyskeratosis congenital, a human stem cell disorder (53). Thus, it would be very interesting to examine whether other telomerase component genes are also under the Wnt pathway regulation. Our data demonstrated that β-catenin knockdown also decreased human telomerase RNA and DKC1 expressions in human cancer cells (HCT116 and MCF7) and normal stable cell lines (293T and MCF10A) (supplemental Fig. S4). Taken together, these results suggest that the Wnt/β-catenin pathway may play more important roles in regulating the whole telomerase holoenzyme level in vivo.
It is known that human embryonic stem (ES) cells also present high TA, which is essential for the cells' self-renewal characteristics. Previous studies demonstrated that modulation of Wnt signaling by controlling the dose of adenomatous polyposis coli or by treatment with a GSK3-β inhibitor can enhance self-renewal of both mouse and human ES cells (54, 55). These results led us to question whether the Wnt pathway is also involved in telomerase regulation in human ES cells. In the present study, we found that silencing β-catenin was capable of inhibiting hTERT, telomerase RNA, and DKC1 expressions and repressing TA in human ES cell line hES3 (supplemental Figs. S5 and S6), suggesting that the Wnt pathway may play a pivotal role in telomerase regulation not only in human cancer cells but also in ES cells. This is supported by the fact that the β-catenin·TCF4 complex drives the same genetic program in colorectal cancer cells as in crypt stem and progenitor cells (56). Moreover, we demonstrated that the activated Wnt pathway could increase hTERT expression and reactivate telomerase, leading to an extended life span in BJ cells. Somatic cells do not display TA, or they express it only at very low levels in a cell cycle-dependent manner (5, 6). However, telomerase is reactivated and TA is largely increased during carcinogenesis and reprogramming from somatic cells to iPS (induced pluripotent stem) cells. The molecular mechanism of how telomerase is reactivated still remains unclear. Our data may suggest that the Wnt pathway could be involved in telomerase reactivation in carcinogenesis and reprogramming. However, further studies are required. Moreover, other signaling cascades can also impinge on the expression of telomerase (10). For instance, STAT III selectively stimulates hTERT expression through the JAK/STAT signaling pathway (37). Therefore, several signaling cascades can contribute to the control of the hTERT gene expression, which might be used differentially in distinct types of tumors. Our findings indicated that β-catenin knockdown significantly inhibited telomerase genes expression in Wnt pathway deregulated cells (i.e. HCT116), whereas silencing β-catenin just moderately repressed telomerase gene expression in human ES cells and MCF10A, in which Wnt pathway is tightly regulated. It seems like, in Wnt pathway deregulated cells, the Wnt pathway plays a predominant role in telomerase regulation.
Activation of the Wnt pathway leads to the accumulation of stabilized cytosolic β-catenin, which then translocates to the nucleus to form complexes with members of the DNA-bound LEF/TCF family of proteins and activates Wnt target gene expression. Within the functional complex, LEF/TCF contributes the DNA binding, and β-catenin confers the transcription activation (57). The LEF/TCF transcription factor family has four major members, LEF1, TCF1, TCF3, and TCF4, which recognize the consensus sequence 5′-CTTTGWW-3′ (or, in reverse orientation, 5′-WWCAAAG-3′) through the high mobility group (HMG) domain (58–61). DNA binding by LEF/TCF alone is not sufficient to cause transcription activation. Promoter activation is accomplished only after a functional bipartite transcriptional factor is created through complex formation between the LEF/TCF transcription factor and β-catenin (62). In this study, our data suggest that the β-catenin·TCF4 complex, but not β-catenin·TCF1/3 or LEF1, is an effective activator of hTERT expression in immortalized cells. This observation was extended using overexpression of β-catenin·TCF4, which could stimulate hTERT expression in 293T, HCT116, and MCF7 cells. Furthermore, β-catenin-induced hTERT transcription was suppressed by the dominant negative form of TCF4. Although LEF/TCFs share remarkable ∼95–99% amino acid sequence conservation in the HMG domain and bind to the consensus sequence 5′-CTTTGWW-3′, much genetic evidence shows that LEF/TCFs play diverse roles in cell growth, development, and differentiation (42), suggesting that the complexity of LEF/TCF action must be due to context-dependent actions and differential recognition of endogenous target genes.
In the present study, we have identified one TBE in the hTERT promoter between positions −659 and −653 (5′-TGCAAAG-3′) upstream of transcription start site of hTERT. The luciferase reporter assay showed that mutations in TBE could significantly repress β-catenin·TCF4-mediated activation of the hTERT promoter, whereas deletion of TBE almost abolished the effect of β-catenin·TCF4 complex on hTERT, implying that the TBE is essential for hTERT promoter activities. Transcriptional factor binding sites usually exist in the sequence of the proximal promoter of target genes; for example, known hTERT activators, such as c-Myc, Sp1, and AP-1 binding sites, mostly locate between position −200 bp and the transcription start site (Fig. 5D) (47). Our finding is supported by a previous study that suggests that most of the TBEs in β-catenin·TCF4 target genes are located at large distances from transcription start sites (63). Finally, we provide evidence that hTERT is a direct transcriptional target of the β-catenin·TCF4 complex by showing that TCF4 physically occupies the TBE in vitro and in vivo using EMSA and ChIP assays. Moreover, the ChIP result demonstrated that activated Wnt signaling could enhance β-catenin binding on the TBE of the hTERT promoter, suggesting the critical role of Wnt signaling in hTERT regulation. The identification of TBE in the hTERT promoter will provide valuable information for the understanding of hTERT expression in human cancer cells.
The present study offers evidence that the Wnt/β-catenin pathway regulates the expression of hTERT. hTERT has been shown to serve as a cofactor in the β-catenin transcriptional complex (51), so we are curious whether hTERT drives a positive feedback loop to reinforce the Wnt/β-catenin signaling. To this end, we established stable hTERT overexpression in 293T, HCT116, MCF7, and MCF10A cell lines (supplemental Fig. 7). However, our data showed that overexpression of hTERT had no effect on expression of Wnt pathway target genes, c-MYC and NOS2 (supplemental Fig. 8), suggesting that hTERT cannot form a positive feedback loop with Wnt/β-catenin signaling in these cell lines we studied.
In conclusion, we show that hTERT is a novel target gene of the Wnt pathway and that the hTERT TBE is the critical transcriptional element controlling hTERT expression in cancer cells. Our results demonstrate that the β-catenin·TCF4 complex is capable of activating the hTERT promoter and increasing TA. Furthermore, our findings shed light on the biological roles of β-catenin in regulating activation of the hTERT promoter and facilitate our understanding of the telomerase regulation in various human cancers. Because high TA is most common in human cancers, understanding the role of the β-catenin·TCF4 complex in telomerase regulation is of interest and may lead to new therapeutic perspectives.
Supplementary Material
Acknowledgments
We thank Linglee Tay and Linming Lee for technical assistance. We thank Dr. Horikawa Izumi for hTERT promoter constructs; Dr. Bradley J. Merrill for ΔN β-catenin, LEF1, TCF1, TCF3, and TCF4 expression plasmids; Dr. Jun Ninomiya-Tsuji for PCMV-TAK1 and TAB1 constructs; and Dr. Jeong K. Yoon for ΔN TCF4 expression plasmid. We are grateful to Dr. Thilo Hagen and Dr. Takao Inoue for reading the manuscript.
This work is supported by funding from the MOE-Academic Research Fund (AcRF) Tier 1 Faculty Research Committee (FRC) grant.

This article contains supplemental Table S1 and Figs. S1–S8.
- TA
- telomerase activity
- TRAP
- telomere repeat amplification protocol
- qTRAP
- real-time PCR-based version of TRAP
- LEF
- lymphoid-enhancing factor
- TCF
- T-cell factor
- CM
- culture medium
- ES
- embryonic stem
- HMG
- high mobility group.
REFERENCES
- 1. Blackburn E. H. (1991) Telomeres. Trends Biochem. Sci. 16, 378–381 [DOI] [PubMed] [Google Scholar]
- 2. Perrem K., Bryan T. M., Englezou A., Hackl T., Moy E. L., Reddel R. R. (1999) Repression of an alternative mechanism for lengthening of telomeres in somatic cell hybrids. Oncogene 18, 3383–3390 [DOI] [PubMed] [Google Scholar]
- 3. Greider C. W., Blackburn E. H. (1989) A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337, 331–337 [DOI] [PubMed] [Google Scholar]
- 4. Blasco M. A., Hahn W. C. (2003) Evolving views of telomerase and cancer. Trends Cell Biol. 13, 289–294 [DOI] [PubMed] [Google Scholar]
- 5. Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 6. Cesare A. J., Reddel R. R. (2010) Alternative lengthening of telomeres. Models, mechanisms, and implications. Nat. Rev. Genet. 11, 319–330 [DOI] [PubMed] [Google Scholar]
- 7. Bodnar A. G., Ouellette M., Frolkis M., Holt S. E., Chiu C. P., Morin G. B., Harley C. B., Shay J. W., Lichtsteiner S., Wright W. E. (1998) Extension of life span by introduction of telomerase into normal human cells. Science 279, 349–352 [DOI] [PubMed] [Google Scholar]
- 8. Hahn W. C., Stewart S. A., Brooks M. W., York S. G., Eaton E., Kurachi A., Beijersbergen R. L., Knoll J. H., Meyerson M., Weinberg R. A. (1999) Inhibition of telomerase limits the growth of human cancer cells. Nat. Med. 5, 1164–1170 [DOI] [PubMed] [Google Scholar]
- 9. Shay J. W., Keith W. N. (2008) Targeting telomerase for cancer therapeutics. Br J. Cancer 98, 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kyo S., Takakura M., Fujiwara T., Inoue M. (2008) Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 99, 1528–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meyerson M. (2000) Role of telomerase in normal and cancer cells. J. Clin. Oncol. 18, 2626–2634 [DOI] [PubMed] [Google Scholar]
- 12. Nakayama J., Tahara H., Tahara E., Saito M., Ito K., Nakamura H., Nakanishi T., Tahara E., Ide T., Ishikawa F. (1998) Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat. Genet. 18, 65–68 [DOI] [PubMed] [Google Scholar]
- 13. Poole J. C., Andrews L. G., Tollefsbol T. O. (2001) Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT). Gene 269, 1–12 [DOI] [PubMed] [Google Scholar]
- 14. Kyo S., Kanaya T., Takakura M., Tanaka M., Inoue M. (1999) Human telomerase reverse transcriptase as a critical determinant of telomerase activity in normal and malignant endometrial tissues. Int. J. Cancer 80, 60–63 [DOI] [PubMed] [Google Scholar]
- 15. Aisner D. L., Wright W. E., Shay J. W. (2002) Telomerase regulation: not just flipping the switch. Curr. Opin. Genet. Dev. 12, 80–85 [DOI] [PubMed] [Google Scholar]
- 16. Nakamura T. M., Morin G. B., Chapman K. B., Weinrich S. L., Andrews W. H., Lingner J., Harley C. B., Cech T. R. (1997) Telomerase catalytic subunit homologs from fission yeast and human. Science 277, 955–959 [DOI] [PubMed] [Google Scholar]
- 17. Meyerson M., Counter C. M., Eaton E. N., Ellisen L. W., Steiner P., Caddle S. D., Ziaugra L., Beijersbergen R. L., Davidoff M. J., Liu Q., Bacchetti S., Haber D. A., Weinberg R. A. (1997) hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90, 785–795 [DOI] [PubMed] [Google Scholar]
- 18. Kirkpatrick K. L., Ogunkolade W., Elkak A. E., Bustin S., Jenkins P., Ghilchick M., Newbold R. F., Mokbel K. (2003) hTERT expression in human breast cancer and non-cancerous breast tissue. Correlation with tumor stage and c-Myc expression. Breast Cancer Res. Treat. 77, 277–284 [DOI] [PubMed] [Google Scholar]
- 19. Bilsland A. E., Stevenson K., Atkinson S., Kolch W., Keith W. N. (2006) Transcriptional repression of telomerase RNA gene expression by c-Jun-NH2-kinase and Sp1/Sp3. Cancer Res. 66, 1363–1370 [DOI] [PubMed] [Google Scholar]
- 20. Deng W. G., Jayachandran G., Wu G., Xu K., Roth J. A., Ji L. (2007) Tumor-specific activation of human telomerase reverses transcriptase promoter activity by activating enhancer-binding protein-2β in human lung cancer cells. J. Biol. Chem. 282, 26460–26470 [DOI] [PubMed] [Google Scholar]
- 21. Yatabe N., Kyo S., Maida Y., Nishi H., Nakamura M., Kanaya T., Tanaka M., Isaka K., Ogawa S., Inoue M. (2004) HIF-1-mediated activation of telomerase in cervical cancer cells. Oncogene 23, 3708–3715 [DOI] [PubMed] [Google Scholar]
- 22. Xu D., Wang Q., Gruber A., Björkholm M., Chen Z., Zaid A., Selivanova G., Peterson C., Wiman K. G., Pisa P. (2000) Down-regulation of telomerase reverse transcriptase mRNA expression by wild type p53 in human tumor cells. Oncogene 19, 5123–5133 [DOI] [PubMed] [Google Scholar]
- 23. Oh S., Song Y., Yim J., Kim T. K. (1999) The Wilms' tumor 1 tumor suppressor gene represses transcription of the human telomerase reverse transcriptase gene. J. Biol. Chem. 274, 37473–37478 [DOI] [PubMed] [Google Scholar]
- 24. Zhou X. Z., Huang P., Shi R., Lee T. H., Lu G., Zhang Z., Bronson R., Lu K. P. (2011) The telomerase inhibitor PinX1 is a major haploinsufficient tumor suppressor essential for chromosome stability in mice. J. Clin. Invest. 121, 1266–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Logan C. Y., Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 26. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 27. Barker N., Clevers H. (2006) Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 5, 997–1014 [DOI] [PubMed] [Google Scholar]
- 28. Morin P. J. (1999) β-Catenin signaling and cancer. BioEssays 21, 1021–1030 [DOI] [PubMed] [Google Scholar]
- 29. Nusse R. (2005) Wnt signaling in disease and in development. Cell Res. 15, 28–32 [DOI] [PubMed] [Google Scholar]
- 30. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling. Components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He T. C., Sparks A. B., Rago C., Hermeking H., Zawel L., da Costa L. T., Morin P. J., Vogelstein B., Kinzler K. W. (1998) Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 [DOI] [PubMed] [Google Scholar]
- 32. Tetsu O., McCormick F. (1999) β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–426 [DOI] [PubMed] [Google Scholar]
- 33. Du Q., Park K. S., Guo Z., He P., Nagashima M., Shao L., Sahai R., Geller D. A., Hussain S. P. (2006) Regulation of human nitric-oxide synthase 2 expression by Wnt β-catenin signaling. Cancer Res. 66, 7024–7031 [DOI] [PubMed] [Google Scholar]
- 34. van Es J. H., Barker N., Clevers H. (2003) You Wnt some, you lose some. Oncogenes in the Wnt signaling pathway. Curr. Opin. Genet. Dev. 13, 28–33 [DOI] [PubMed] [Google Scholar]
- 35. Betts D. H., Perrault S., Harrington L., King W. A. (2006) Quantitative analysis of telomerase activity and telomere length in domestic animal clones. Methods Mol. Biol. 325, 149–180 [DOI] [PubMed] [Google Scholar]
- 36. Chai J. H., Zhang Y., Tan W. H., Chng W. J., Li B., Wang X. (2011) Regulation of hTERT by BCR-ABL at multiple levels in K562 cells. BMC Cancer 11, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Konnikova L., Simeone M. C., Kruger M. M., Kotecki M., Cochran B. H. (2005) Signal transducer and activator of transcription 3 (STAT3) regulates human telomerase reverse transcriptase (hTERT) expression in human cancer and primary cells. Cancer Res. 65, 6516–6520 [DOI] [PubMed] [Google Scholar]
- 38. Stambolic V., Ruel L., Woodgett J. R. (1996) Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signaling in intact cells. Curr. Biol. 6, 1664–1668 [DOI] [PubMed] [Google Scholar]
- 39. Horikawa I., Cable P. L., Mazur S. J., Appella E., Afshari C. A., Barrett J. C. (2002) Downstream E-box-mediated regulation of the human telomerase reverse transcriptase (hTERT) gene transcription. Evidence for an endogenous mechanism of transcriptional repression. Mol. Biol. Cell 13, 2585–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamada M., Ohnishi J., Ohkawara B., Iemura S., Satoh K., Hyodo-Miura J., Kawachi K., Natsume T., Shibuya H. (2006) NARF, a Nemo-like kinase (NLK)-associated ring finger protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF). J. Biol. Chem. 281, 20749–20760 [DOI] [PubMed] [Google Scholar]
- 41. Yi F., Pereira L., Hoffman J. A., Shy B. R., Yuen C. M., Liu D. R., Merrill B. J. (2011) Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat. Cell Biol. 13, 762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arce L., Yokoyama N. N., Waterman M. L. (2006) Diversity of LEF/TCF action in development and disease. Oncogene 25, 7492–7504 [DOI] [PubMed] [Google Scholar]
- 43. Kolligs F. T., Nieman M. T., Winer I., Hu G., Van Mater D., Feng Y., Smith I. M., Wu R., Zhai Y., Cho K. R., Fearon E. R. (2002) ITF-2, a downstream target of the Wnt/TCF pathway, is activated in human cancers with β-catenin defects and promotes neoplastic transformation. Cancer Cell 1, 145–155 [DOI] [PubMed] [Google Scholar]
- 44. Alexander-Bridges M., Ercolani L., Kong X. F., Nasrin N. (1992) Identification of a core motif that is recognized by three members of the HMG class of transcriptional regulators. IRE-ABP, SRY, and TCF-1α. J. Cell Biochem. 48, 129–135 [DOI] [PubMed] [Google Scholar]
- 45. Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. (1992) Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 11, 1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eisenberg D. T. (2011) An evolutionary review of human telomere biology. The thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am. J. Hum. Biol. 23, 149–167 [DOI] [PubMed] [Google Scholar]
- 47. Takakura M., Kyo S., Kanaya T., Tanaka M., Inoue M. (1998) Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 58, 1558–1561 [PubMed] [Google Scholar]
- 48. Hanahan D., Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 49. Harley C. B., Kim N. W., Prowse K. R., Weinrich S. L., Hirsch K. S., West M. D., Bacchetti S., Hirte H. W., Counter C. M., Greider C. W. (1994) Telomerase, cell immortality, and cancer. Cold Spring Harb. Symp. Quant. Biol. 59, 307–315 [DOI] [PubMed] [Google Scholar]
- 50. Luu H. H., Zhang R., Haydon R. C., Rayburn E., Kang Q., Si W., Park J. K., Wang H., Peng Y., Jiang W., He T. C. (2004) Wnt/β-catenin signaling pathway as a novel cancer drug target. Curr. Cancer Drug Targets 4, 653–671 [DOI] [PubMed] [Google Scholar]
- 51. Park J. I., Venteicher A. S., Hong J. Y., Choi J., Jun S., Shkreli M., Chang W., Meng Z., Cheung P., Ji H., McLaughlin M., Veenstra T. D., Nusse R., McCrea P. D., Artandi S. E. (2009) Telomerase modulates Wnt signaling by association with target gene chromatin. Nature 460, 66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fu D., Collins K. (2007) Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol. Cell 28, 773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walne A. J., Dokal I. (2008) Dyskeratosis congenita. A historical perspective. Mech. Ageing Dev. 129, 48–59 [DOI] [PubMed] [Google Scholar]
- 54. Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A. H. (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10, 55–63 [DOI] [PubMed] [Google Scholar]
- 55. Kielman M. F., Rindapää M., Gaspar C., van Poppel N., Breukel C., van Leeuwen S., Taketo M. M., Roberts S., Smits R., Fodde R. (2002) Apc modulates embryonic stem cell differentiation by controlling the dosage of β-catenin signaling. Nat. Genet. 32, 594–605 [DOI] [PubMed] [Google Scholar]
- 56. van de Wetering M., Sancho E., Verweij C., de Lau W., Oving I., Hurlstone A., van der Horn K., Batlle E., Coudreuse D., Haramis A. P., Tjon-Pon-Fong M., Moerer P., van den Born M., Soete G., Pals S., Eilers M., Medema R., Clevers H. (2002) The β-catenin·TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 [DOI] [PubMed] [Google Scholar]
- 57. Barker N., Morin P. J., Clevers H. (2000) The Yin-Yang of TCF/β-catenin signaling. Adv. Cancer Res. 77, 1–24 [DOI] [PubMed] [Google Scholar]
- 58. Brantjes H., Barker N., van Es J., Clevers H. (2002) TCF. Lady Justice casting the final verdict on the outcome of Wnt signaling. Biol. Chem. 383, 255–261 [DOI] [PubMed] [Google Scholar]
- 59. Oosterwegel M. A., van de Wetering M. L., Holstege F. C., Prosser H. M., Owen M. J., Clevers H. C. (1991) TCF-1, a T cell-specific transcription factor of the HMG box family, interacts with sequence motifs in the TCRβ and TCRδ enhancers. Int. Immunol. 3, 1189–1192 [DOI] [PubMed] [Google Scholar]
- 60. Travis A., Amsterdam A., Belanger C., Grosschedl R. (1991) LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor α enhancer function [corrected]. Genes Dev. 5, 880–894 [DOI] [PubMed] [Google Scholar]
- 61. Waterman M. L., Fischer W. H., Jones K. A. (1991) A thymus-specific member of the HMG protein family regulates the human T cell receptor C α enhancer. Genes Dev. 5, 656–669 [DOI] [PubMed] [Google Scholar]
- 62. Hsu S. C., Galceran J., Grosschedl R. (1998) Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with β-catenin. Mol. Cell Biol. 18, 4807–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hatzis P., van der Flier L. G., van Driel M. A., Guryev V., Nielsen F., Denissov S., Nijman I. J., Koster J., Santo E. E., Welboren W., Versteeg R., Cuppen E., van de Wetering M., Clevers H., Stunnenberg H. G. (2008) Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol. Cell Biol. 28, 2732–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.