Background: One of the major hurdles hindering the clinic development of hESC-based therapy is the teratoma risk.
Results: A genetic modification via homologous recombination can effectively eliminate hESCs without killing their derivatives.
Conclusion: A scalable and safe approach to eliminate the teratoma risk associated with hESCs.
Significance: Our approach improves the feasibility to develop hESCs into human cell therapy.
Keywords: Cell Death, Cell Differentiation, Cell Therapy, Embryonic Stem Cell, Transplantation, Teratomas
Abstract
As the renewable source of all cell types in the body, human embryonic stem cells (hESCs) hold great promise for human cell therapy. However, one major bottleneck that hinders the clinic application of hESCs is that hESCs remaining with their differentiated derivatives pose cancer risk by forming teratomas after transplantation. NANOG is a critical pluripotency factor specifically expressed in hESCs but rarely in their differentiated derivatives. By introducing a hyperactive variant of herpes simplex virus thymidine kinase gene into the 3′-untranslated region of the endogenous NANOG gene of hESCs through homologous recombination, we developed a safe and highly scalable approach to efficiently eliminate the teratoma risk associated with hESCs without apparent negative impact on their differentiated cell types. As thymidine kinase is widely used in human gene therapy trials and is the therapeutic target of U. S. Food and Drug Administration-approved drugs, our strategy could be effectively applied to the clinic development of hESC-based human cell therapy.
Introduction
Human embryonic stem cells (hESCs)3 can undergo unlimited self-renewal and retain the pluripotency to differentiate into all cell types in the body. Therefore, as a renewable source of various cell types, hESCs hold great promise for the cell replacement therapy of many human diseases that currently have no cure (1, 2). However, several hurdles must be overcome before hESC-based therapies can enter the clinic such as genetic stability and immunogenicity, and the serious teratoma risk that even a few contaminating undifferentiated hESCs within their differentiated derivatives may form benign and potentially malignant teratomas after transplantation (3–5).
Despite the significant progress in establishing conditions to reproducibly differentiate hESCs into various functional cell types of therapeutic value, the differentiation process is rarely complete with some undifferentiated hESCs remaining among their differentiated derivatives. Because hESCs can form teratomas in vivo, this raises serious cancer risk when differentiated cells contaminated with hESCs are transplanted into patients (4). To address this concern, antibody-based strategy has been under development to remove the remaining hESCs before transplantation. For example, a cytotoxic antibody specific for PODXL (podocalyxin-like protein-1) can kill hESCs via oncosis (6, 7). However, PODXL is widely expressed in human tissues, making it impractical to use this antibody to selectively eliminate the hESCs within the differentiating culture (8). Recent studies have shown that immunodepletion of hESCs with a combination of antibodies against multiple hESC-specific surface markers (SSEA5, CD9, CD30, CD90, and CD200) can remove hESCs from differentiating cultures and reduce their associated teratoma risk (9). Although the expression of SSEA5 is relatively specific for hESCs, the other surface markers CD9, CD30, CD90, and CD200 are broadly expressed in differentiated tissues (10–13). Therefore, the antibody-based strategy could be limited by the specificity of the expression of their recognized surface markers in hESCs. Another limitation is that some progenitor cells could spontaneously dedifferentiate into pluripotent state after transplantation, leading to teratoma formation (14).
To efficiently eliminate the teratoma risk associated with hESCs in a scalable manner, we designed a strategy to introduce a suicide gene into a genetic locus that is highly specifically expressed in pluripotent stem cells but not in their differentiated derivatives. The most widely used suicide gene in the human imaging trials and gene therapy is the HSV-thymidine kinase (TK) gene (15). It is the target of the U. S Food and Drug Administration-approved drugs that kill TK-expressing cells infected by herpesvirus. Using Oct4 promoter to drive the expression of TK, previous studies have shown that the transgenic mouse ESCs can be eliminated in vitro without killing their neural derivatives (16). In addition, the expression of TK in hESCs under the control of a ubiquitous promoter leads to the elimination of teratoma formation of the transgenic hESCs in SCID mice (17). Recently, it was reported that GCV treatment could prevent the teratoma formation of hESCs with a lentivirus-delivered transgenic TK gene driven by a 406-bp mouse Nanog promoter (18). However, these approaches are not suitable for the clinic application due to two reasons. First, the PGK promoter is ubiquitously expressed and OCT4 promoter also has relatively widespread expression in differentiated cell types such as adult stem cells (19). Second, the random integration of the transgene in the genome can modulate the promoter activity and also increase cancer risk. To improve the feasibility for clinic application, we chose the pluripotency gene NANOG locus that is highly specifically expressed in pluripotent stem cells and rapidly down-regulated post-epiblast stage during embryonic development (20, 21), although the expression of NANOG is found in very restricted cell lineages (germ line stem cells, mesenchymal stem cells, and endothelial cells) (20–25). Therefore, the NANOG locus would be the best choice for introducing the suicide gene via homologous recombination so the expression of the suicide gene is restricted to hESCs, leading to the selective elimination of hESCs. The knock-in approach also eliminates the locus-specific modulation of gene expression and the cancer risk associated with random genomic integration. Our findings indicate that this scalable approach can specifically eliminate hESCs in vitro and in vivo without affecting their differentiated derivatives.
EXPERIMENTAL PROCEDURES
Construction of BAC-based Targeting Vector
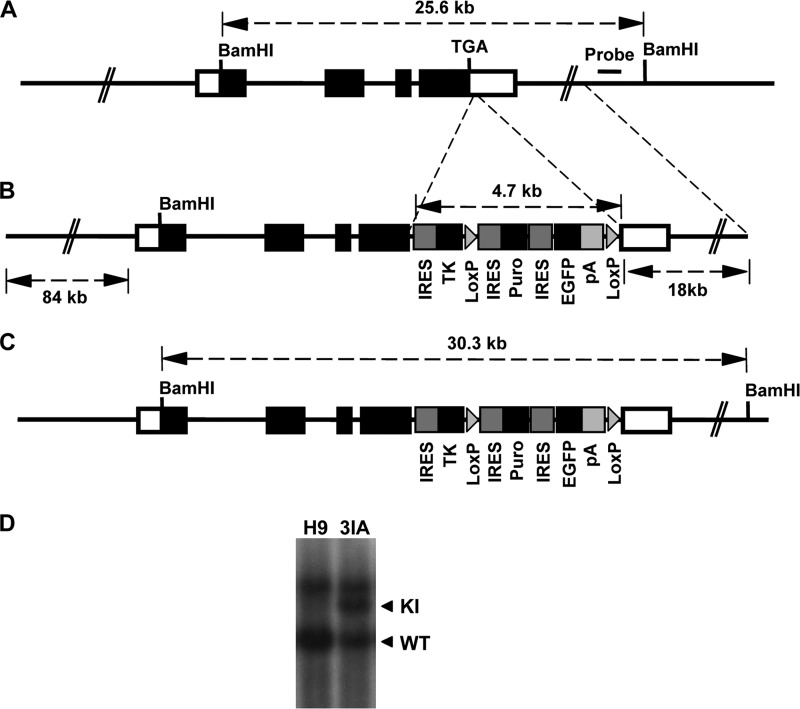
The NANOG bacterial artificial chromosome (BAC) clone RP11-277J24 was purchased from Invitrogen, and the targeting vector was constructed by recombineering as described previously (26). Briefly, one of the homologous arms was shortened to 18 kb by recombineering to allow screening of homologous recombinants by Southern blotting analysis (27). The IRES-TKSR39-IRES-Puro-IRES-EGFP expression cassette was inserted ∼100 bp downstream of the stop codon. The IRES-Puro-IRES-EGFP cassette was flanked with two LoxP sites. Because NANOG is expressed in hESCs, the BAC targeting vector will confer puromycin resistance to the transfected cells.
Cell Culture
The hESCs were cultured on mouse embryonic fibroblast feeder layer in DMEM/F12 supplemented with 20% knock-out serum replacement, 1 mm glutamine, 0.1 mm nonessential amino acids, 10 ng/ml bFGF, and 100 μm β-mercaptoethanol. For the feeder-free culture, hESCs were plated on Matrigel-coated plates in mTesR-1 medium (STEMCELL Tech.). To passage hESCs, confluent culture was washed with PBS, trypsinized for 5 min with TrypLE, and resuspended into single cells. All tissue culture reagents were purchased from Invitrogen unless indicated otherwise.
Teratoma Formation Assay in SCID Mice
All animal work was approved by the Institutional Animal Care and Use Committee. hESCs were harvested, washed twice with PBS, suspended in PBS with 30% Matrigel, and subcutaneously injected into the hind leg region of SCID mice. About four million cells were used for each injection. Teratomas were surgically removed from the euthanized mice, fixed in 10% buffered formalin, sectioned, and stained with hematoxylin and eosin for histological assessment or analyzed by TUNEL assay for apoptotic cells. To test whether GCV treatment can abolish the teratoma formation by TK-hESCs in SCID mice, 1 day after cell injection, the mice were consecutively administered with daily intraperitoneal injection of various dosages of GCV (Sigma) for 1 or 2 weeks (10 mg/kg/day for 1 or 2 weeks, 5 mg/kg/day for 2 weeks). To test the sensitivity of the cell lineages derived from TK-hESCs to GCV treatment, when the teratomas were established 6 weeks after implantation of TK-hESCs in SCID mice, the SCID mice were consecutively administered with daily intraperitoneal injections of GCV (10 mg/kg/day) for 2 weeks. All animal work has been reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
Derivation of Human Fibroblasts from Teratomas
To derive fibroblast-like cells from teratomas, teratomas were chopped into tiny pieces and cultured in DMEM supplemented with 10% serum, 1% Pen-Strep, and 55 μm β-mecaptoethanol. After several passages, the adherent culture becomes homogenous with fibroblast-like cells that express a panel of fibroblast-specific markers.
Differentiation of hESCs into Neural Progenitor Cells
Human ESC colonies were gently scraped from the dishes and transferred to ultra low cluster plate in embryoid body formation media (DMEM/F12-Glutamax, 10% characterized FBS, 1% Pen-Strep, 55 μm β-mercaptoethanol). Six days later, the media were changed to neural progenitor cells media (NPC medium: DMEM/F12-Glutamax, 0.5% N2, 1% B27, 1% Pen-Strep, 10 ng/ml bFGF). Two days later, the embryoid bodies were replated onto poly-l-ornithine/laminin-coated 6-cm dishes. After neural progenitor cells migrated away from the embryoid bodies, the culture was treated with 0.05% trypsin for 1–2 min at room temperature, resuspended into single cells, and replated onto poly-l-ornithine/laminin-coated plates at a dilution of 1:5–7.
Retinoic Acid-induced Differentiation of hESCs
Human ESCs were trypsinized and transferred to Matrigel-coated plates in the differentiation media (DMEM/F12 supplemented with 20% FBS, 1 mm glutamine, 0.1 mm nonessential amino acids, 100 μm β-mercaptoethanol, and 1 μm all trans-retinoic acid). Three days later, the cells were harvested, resuspended in PBS with 30% Matrigel, and subcutaneously injected into the hindleg region of SCID mice at a dosage of four million cells per injection site.
Quantitative Real-time PCR Analysis
Total RNA from hESCs, fibroblasts, neural progenitor cells, retinoic acid-induced differentiated cells, and teratomas were isolated using an RNeasy mini kit (Qiagen). One microgram total RNA was reversely transcribed to cDNA, which was analyzed by quantitative real-time PCR as described previously (28). The primers used were as described previously (9, 22, 26).
Immunofluorescence Staining
Neural progenitor cells grown on poly-l-ornithine/laminin-coated four-well chamber plate were fixed in 4% paraformaldehyde for 15 min at room temperature, permeabilized with 0.3% Triton X-100 in TBS for 10 min, blocked with 2% FBS in TBST (0.05% Tween 20) for 20 min, and stained with NESTIN and SOX2 antibodies (BD Stemflow human neural lineage analysis kit) in blocking buffer at 4 °C overnight. The next day, the cells were stained with the second antibody in blocking buffer for 45 min, and the nuclei were counterstained with DAPI.
TUNEL Assay
TUNEL assay was used to detect apoptotic cells in sections of teratomas as described previously (29). Briefly, deparaffinized tissue sections were digested with proteinase K and quenched in 0.3% hydrogen peroxide. After treatment in equilibration buffer, the slides were incubated in terminal deoxynucleotidyl transferase enzyme solution for 1 h at 37 °C and then probed with anti-digoxin antibody for 30 min at room temperature in a humidified chamber. The positive signal was developed using peroxidase substrate 3-amino-9-ethylcarbazole (Vector Laboratories) for 15–30 min. Finally, the slides were counterstained with hematoxylin and mounted with coverslips.
Flow Cytometric Analysis
About 5 × 105 hESCs were washed with PBS, stained for 40 min at room temperature in the dark with phycoerythrin-conjugated anti-TRA 1–61, anti-TRA 1–81, anti-SSEA3, or anti-SSEA4 antibodies (BD Phamingen). Isotype-matched IgG was used as negative control. The stained cells were washed once with PBS and analyzed by a BD LSR-II machine using FACS Diva software (Becton Dickinson). Neural progenitor cells were trypsinized with 0.05% trypsin for 1–2 min at room temperature. Media containing 10% FBS were added to stop trypsin. After washing with PBS once, the cells were stained with propidium iodide for 15 min and analyzed by a BD LSR-II machine.
RESULTS
To knock-in the TK Expression Cassette into the Endogenous NANOG Gene in hESCs
Because NANOG is required for the self-renewal of hESCs and its haplodeficiency induces spontaneous differentiation of ESCs (20, 21, 30), we designed the knock-in strategy to introduce the TK gene expression cassette into the 3′-untranslated region of the endogenous NANOG gene locus of hESCs to allow the normal expression of the NANOG gene in hESCs (Fig. 1, A and B). We have recently optimized the BAC-based gene targeting strategy to achieve high efficiency of homologous recombination in hESCs (26). Despite the large size of the homologous arms of the BAC vector, we found no random integration of pieces of the BAC vector in the targeted hESCs (26). The strategy to knock-in IRES-TKSR39-IRES-Puro-IRES-EGFP expression cassette into the 3′-untranslated region of the endogenous NANOG gene is described in Fig. 1. We chose the enhanced mutant version of HSV-TK (TKSR39) that is much more sensitive to TK-targeting drug ganciclovir (GCV) than wild-type (31). TK and has been widely used in human imaging studies and gene therapy (32). The IRES-Puro-IRES-EGFP cassette is flanked by two LoxP sites so that it can be eliminated from the knock-in allele by transient expression of the Cre enzyme (Fig. 1B). The homologous recombinants were screened by Southern blotting analysis with BamHI digestion and hybridization to the probe, giving a 30.3-kb mutant band and a 25.6-kb WT band (Fig. 1, A, C, and D). The knock-in hESCs, denoted TK-hESCs, were resistant to puromycin but expressed very low levels of EGFP likely due to that it was translated from the third IRES element on the transcript. The knock-in construct worked well in various hESC lines, and we obtained TK-hESC lines in both H9 and HUES8 hESCs (Fig. 1D, data not shown). TK-hESCs expressed similar levels of pluripotency genes and hESC-specific surface markers and could form well differentiated teratomas in SCID mice, confirming the pluripotent state of TK-hESCs (supplemental Fig. S1).
FIGURE 1.
Generation of TK-hESCs. A, the endogenous NANOG locus in hESCs. Open boxes indicate the 5′ and 3′ UTRs of the human NANOG gene. Filled boxes indicate the NANOG open reading frame. The stop codon (TGA) of the NANOG gene and the location of the probe for Southern blotting are indicated. The size of the WT BamHI restriction fragment is shown. B, BAC-based targeting vector. The IRES-TKSR39-IRES-Puro-IRES-EGFP expression cassette was inserted into the BAC vector ∼100 bp downstream of NANOG TGA stop codon by recombineering. The sizes of homologous arms are indicated. C, the configuration of the knock-in allele. The size of mutant BamHI restriction fragment is indicated. D, Southern blotting analysis of the knock-in TK-hESC (3IA) clone in H9 hESC line. Genomic DNA was digested with BamHI and hybridized to the probe outside of the targeting construct. The BamHI restriction fragments derived from WT and the knock-in alleles are indicated.
TK-hESCs Are Hypersensitive to GCV Both in Vitro and in Vivo
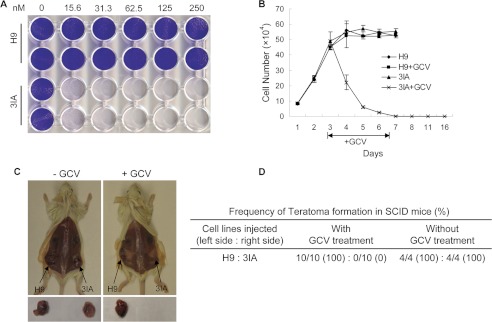
As expected, although the parental hESCs were resistant to GCV, TK-hESCs were hypersensitive to GCV and were eliminated rapidly after GCV treatment in culture (Fig. 2, A and B). In addition, transient treatment with GCV for 4 days can eliminate the hESCs as indicated by the lack of hESC growth even after 2 weeks (Fig. 2B). Once transplanted into SCID mice, hESCs can form well differentiated teratomas that contain cells derived from each of the three germ layers (33). Because our goal is to eliminate the teratoma risk associated with hESCs, we tested the efficacy of GCV in suppressing teratoma formation by TK-hESCs in SCID mice. Parental H9 hESCs and TK-hESCs were implanted into the left and right side of the same SCID mouse, respectively. One day after implantation, the transplanted mice were intraperitoneally injected with either GCV or PBS daily for one or 2 weeks (10 mg/kg/day for 1 or 2 weeks and 5 mg/kg/day for 1 or 2 weeks). The dosage of GCV used is similar to that used for human patients (The Clinician's Ultimate Reference (GLOBALRPH)) instead of the high dosage of 50 mg/kg/day usually used in mouse model. Although both H9 hESCs and TK-hESCs efficiently formed teratomas in mock-treated SCID mice, GCV treatment selectively abolished the teratoma formation by TK-hESCs in SCID mice (Fig. 2, C and D).
FIGURE 2.
GCV eliminates TK-hESCs (3IA) in vitro and abolishes the teratoma formation of TK-hESCs in vivo. A, TK-hESCs are hypersensitive to GCV. H9 and 3IA TK-hESCs were treated with increasing concentrations of GCV. Three days after GCV treatment, cells were fixed and stained with crystal violet. The genotypes are indicated on the left, and the concentrations of GCV (nm) are indicated on the top. B, TK-hESCs were rapidly eliminated by GCV in culture. H9 hESCs and TK-hESCs were plated, mock-treated, or treated with 0.25 μm GCV for 4 days. GCV was removed from the medium, and the cells were cultured for additional 9 days. Cell numbers were counted at the indicated time points. C, GCV selectively abolishes the teratoma formation of TK-hESCs in SCID mice. SCID mice were subcutaneously injected with H9 hESCs and TK-hESCs around the left and right hind legs, respectively. One day after implantation, mice were intraperitoneally injected with GCV of various dosages for one or 2 weeks. Six-to-8 weeks after implantation, SCID mice were euthanized, and teratomas were examined. Representative images are shown. D, the summary of the frequency of teratoma formation of hESCs and TK-hESCs in SCID mice with or without GCV treatment.
To better mimic the therapeutic setting, TK-hESCs and control parental hESCs were partially differentiated by RA treatment as described (9). The expression of several cell lineage-specific genes such as PAX6 (ectoderm), PECAM (mesoderm), 3SCL, and alpha fetoprotein (endoderm) was increased after RA treatment for 3 days, indicating some differentiation of hESCs (supplemental Fig. S2A). The RA-treated differentiated cultures were transplanted into the SCID mice, which subsequently were intraperitoneally injected with either GCV or PBS daily for 1 week. Although the RA-treated hESC culture formed teratomas in all mock-treated SCID mice, GCV treatment abolishes the teratoma formation by these partially differentiated cultures from TK-hESCs (supplemental Fig. S2, B and C). Therefore, GCV can efficiently eliminate the TK-hESCs in vitro and abolish their capability to form teratomas in vivo.
Cells Differentiated From TK-hESCs Are Resistant to GCV
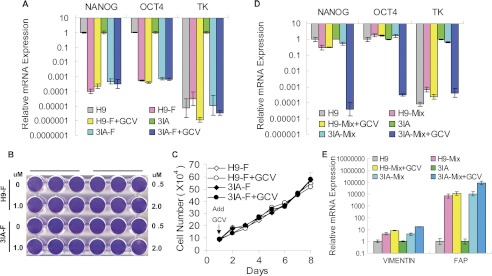
To be applicable for pluripotent stem cell-based therapy, our strategy was designed to eliminate the hESCs without affecting their derivatives that are used for transplantation in human cell replacement therapy. To confirm this notion, we derived fibroblasts from TK-hESCs and tested their sensitivity to GCV. The expression of the pluripotency factors NANOG and OCT4 was essentially undetectable in the fibroblasts derived from TK-hESCs (Fig. 3A). Consistent with this finding, the expression of TK was also undetectable in the fibroblasts derived from TK-hESCs (Fig. 3A). As expected, fibroblasts derived from TK-hESCs were completely resistant to GCV even at high concentrations (Fig. 3, B and C). To test the efficiency to eliminate the hESCs remaining in the differentiating culture, fibroblasts derived from TK-hESCs were spiked with TK-hESCs, followed by GCV treatment or mock treatment for 4 days. Comparison of the expression of NANOG and TK genes in the GCV-treated and mock-treated cultures demonstrated that treatment of differentiating culture with GCV can eliminate the contaminating TK-hESCs (Fig. 3D). In addition, based on the expression of the fibroblast-specific genes, the GCV treatment did not affect the survival of fibroblasts (Fig. 3E).
FIGURE 3.
Fibroblasts (indicated by F) differentiated from TK-hESCs (3IA) are insensitive to GCV. A, the relative mRNA levels of NANOG, OCT4 and TK in fibroblasts derived from the teratomas formed by H9 and TK-hESCs. Fibroblasts were treated with or without 1.0 μm GCV for 4 days. The mRNA levels in fibroblasts are compared with those in hESCs. Mean value are presented with S.D. (n = 3). B, fibroblasts derived from H9 and TK-hESCs were treated with increasing concentrations of GCV for 4 days and subsequently stained with crystal violet. C, the proliferation curve of fibroblasts derived from H9 hESCs and TK-hESCs with or without 1.0 μm GCV treatment. Cells were treated with GCV, and duplicate wells were counted at the indicated time points. D and E, GCV treatment selectively eliminates undifferentiated TK-hESCs when spiked into fibroblast culture. hESCs were plated onto a six-well plate with 1 × 105 cells per well together with 2 × 105 fibroblasts and treated with 1.0 μm GCV for 4 days. The mRNA levels of the NANOG, OCT4, TK, as well as vimentin and fibroblast activation protein (FAP) by the remaining cells were analyzed by qPCR and compared with those in hESCs. Mean value are presented with S.D. (n = 3).
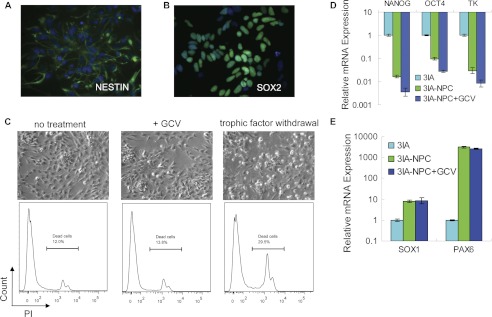
To further evaluate the toxicity of GCV on the hESC derivatives of therapeutic value, the TK-hESCs were differentiated into neural progenitor cells (Nestin+ Sox2+) (Fig. 4, A and B). GCV treatment did not induce apparent cell death of TK-hESC-derived neural progenitor cells (Fig. 4C). Consistent with this notion, GCV treatment had no effect on the expression levels of the neuroepithelial markers SOX1 and PAX6 but reduced the expression levels of TK and pluripotency genes NANOG and OCT4, indicating that the residual undifferentiated hESCs remaining with the neural progenitor cells were killed by GCV (Fig. 4, D and E).
FIGURE 4.
Neural progenitor cells differentiated from TK-hESCs (3IA) are insensitive to GCV. A and B, immunostaining assay reveals the expression of neural progenitor cells (NPC) marker genes NESTIN and SOX2. C, after treatment with or without 1.0 μm GCV for 2 days, neural progenitor cells were trypsinized, stained with phosphatidylinositol (PI), and analyzed by FACS. The sample with trophic factor withdrawn for 12 h was used as a positive control for cell death. D and E, the relative mRNA levels of TK, pluripotency markers NANOG and OCT4, and the neuroepithelial markers SOX1 and PAX6 in NPCs treated with or without 1.0 μm GCV for 2 days were analyzed by real-time PCR and compared with those in TK-hESCs. Mean values are presented with S.D. (n = 3).
To examine the impact of GCV on various cell lineages differentiated from TK-hESCs, we again took advantage of capability of hESCs to form teratomas, which is consisted of various cell types of the three germ layers but with very few undifferentiated hESCs. In this context, TK-hESCs were implanted into SCID mice, and 6 weeks later, GCV (10 mg/kg/day) was intraperitoneally injected into these SCID mice already harboring the established teratomas daily for 2 weeks. The GCV treatment did not stop the growth of the established teratomas formed by TK-hESCs in SCID mice (Fig. 5A). Consistent with this finding, the expression levels of NANOG and TK mRNA in the teratomas formed by TK-hESCs are essentially identical to those in the teratomas formed by the parental hESCs, indicating the presence of very few TK-hESCs in the teratomas (Fig. 5B). In addition, there was no apparent difference in the apoptosis in the teratomas treated with GCV and mock treated with PBS, both of which contain live cells of each of the three germ layers such as neuronal cells and muscle (Fig. 5, C–E). To rule out the possibility that the insensitivity of differentiated cells within the large teratomas formed by TK-hESCs to GCV is due to the inaccessibility of the drug, we treated the SCID mice with GCV for 1 week at different time points (2 and 4 weeks) after transplantation with TK-hESCs. Two weeks after transplantation, the teratomas remained impalpable; 4 weeks after transplantation, the teratomas were quite small and just became palpable. After the GCV treatment at these earlier time points, the teratomas formed by TK-hESCs continued to grow, further supporting our conclusion that cells differentiated from TK-hESCs are insensitive to GCV. Therefore, we conclude that GCV does not kill cells of various lineages differentiated from TK-hESCs. This finding also indicates that GCV should be applied to the patients immediately after transplantation to eliminate any residual contaminating hESCs.
FIGURE 5.
Various cell types differentiated from TK-hESCs (3IA) are insensitive to GCV. A, established teratomas formed by TK-hESCs are not sensitive to GCV. SCID mice were subcutaneously injected with hESCs and TK-hESCs around left and right hind legs, respectively. When teratomas were established after 6 weeks, mice were intraperitoneally injected with GCV (10 mg/kg/day) for 2 weeks. Mice were sacrificed, and teratomas were recovered. B, The mRNA levels of NANOG, OCT4, and TK in teratomas (indicated by −T) formed by both H9 hESCs and TK-hESCs with or without GCV treatment. The mRNA levels in the teratomas were determined by qPCR and compared with those in hESCs. Mean value are shown with S.D. (n = 3). C, cell lineages representing each of the three germ layers are presented in the established teratomas formed by TK-hESCs after GCV treatment as revealed by H&E staining. SE, squamous epithelium; R, rosette; PE, pigment epithelium; B, bone; C, cartilage; SM, smooth muscle; AT, adipose tissue; P, pancreas; GE, gut-like epithelium; SG, salivary gland. D, identification of cells of various lineages in the established teratomas formed by TK-hESCs after GCV treatment as revealed by immunostaining analyses. NeuN, neuronal marker; tropnin I, muscle marker. E, apoptotic cells in teratomas formed by TK-hESCs with or without GCV treatment as determined by TUNEL assay. The arrowheads indicate the apoptotic cells.
DISCUSSION
With their capability to undergo unlimited self-renewal and differentiate into all cell types in human body, hESCs hold great potential for cell therapy of many untreatable human diseases. Significant progress has been achieved to establish the conditions to propagate and differentiate hESCs into various lineages of functional cells for human cell replacement therapy (34). Such progress is further underscored by recent U. S Food and Drug Administration approval of clinic trials to use hESC-derived cells to treat spinal cord injury and macular degeneration (34). However, one major concern that remains difficult and very costly to overcome is the teratoma risk associated with hESCs remaining with their differentiated derivatives. This problem is further aggregated by the intrinsic variability associated with lineage-specific differentiation of hESCs. For example, the kinetics of the teratoma formation would depend on the number of remaining pluripotent stem cells, and thus, it could take a long time for a small number of hESCs to form teratomas. Therefore, the batch-to-batch variation in the lineage-specific differentiation will make it a lengthy and sometimes inconclusive effort to evaluate the teratoma risk of the hESC-derived cells prepared for human therapy. Even if the data from the ongoing clinical trials support the efficacy and safety of the hESC-based therapy, the teratoma risk will have to be continuously evaluated when the hESC-based therapy is applied to human patients. Therefore, an efficient and scalable approach to eliminate the teratoma risk associated with hESCs would greatly facilitate the development of hESC-based cell replacement therapy.
To resolve this major hurdle in developing hESC-based therapy, we developed a highly scalable approach that allows efficient elimination of the hESCs both in the differentiating culture in vitro and in the transplants in vivo. Therefore, our approach offers two safety checkpoints: the remaining hESCs can be efficiently eliminated from the differentiation culture before transplantation; any hESCs remaining in the transplant or dedifferentiated from the progenitor cells in the transplants can be eliminated by treating the patients with GCV immediately after transplantation. By expressing the TK gene in ESCs as a transgene under either the constitutive promoter or the Oct4 promoter, previous studies have shown that the transgenic expression of TK can eliminate the pluripotent stem cells (16, 17). Compared with these transgenic approaches, our approach significantly improves the feasibility for the clinic development. For example, the PGK promoter is ubiquitinously expressed and OCT4 promoter also has relatively widespread expression in differentiated cell types such as various adult stem cells (19). Therefore, in addition to the transgenic pluripotent stem cells, many cell types differentiated from the transgenic pluripotent stem cells will be eliminated by TK as well. Second, the random integration of the transgene in the genome of pluripotent stem cells can change the promoter activity and the expression pattern of TK. Third, the random integration of the transgene into the genome increases the cancer risk. Lastly, we used a hyperactive variant of TK gene (TKSR39) instead of wild type TK gene, leading to the efficient killing of hESCs at 1/10 dose of GCV (5 mg/kg/day compared with 50 mg/kg/day) normally used. It is known that GCV has some cytotoxic effects on human cells (35), our strategy should improve the safety in clinic development.
Although the successful generation of TK-hESCs in various hESC lines further supports the feasibility to genetically modify any clinic-grade hESCs under the good manufacturing practice conditions, the genetic manipulation itself poses a safety concern in human therapy due to possible random integration of the exogenous DNA into the human genome. This concern can be minimized in the context of the knock-in approach because homologous recombination can be achieved without any random integration of the exogenous DNA (26, 36). To further address this concern, the entire genome of TK-hESCs generated under the good manufacturing practice condition for clinic development can be sequenced to confirm that no other mutations or random insertion of exogenous DNA are introduced during the homologous recombination. In summary, the genetic approach described here could resolve one of the major hurdles associated with teratoma risk and greatly facilitates the development of hESC-based human cell replacement therapy.
Supplementary Material
Acknowledgments
We thank Dr. Hoseok Song for help with the BAC targeting strategy and Professor Nissi Varki for histologic analysis.
This work was supported by Grant 81172828 from National Natural Science Foundation of China (to X. F.) and Early Translational Grant ET-01277 from California Institute for Regenerative Medicine (to Y. X.).

This article contains supplemental Figs. S1 and S2.
- hESC
- human embryonic stem cell
- GCV
- ganciclovir
- RA
- retinoic acid
- BAC
- bacterial artificial chromosome
- EGFP
- enhanced GFP
- IRES
- internal ribosomal entry site.
REFERENCES
- 1. Scadden D., Srivastava A. (2012) Advancing stem cell biology toward stem cell therapeutics. Cell Stem Cell 10, 149–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singec I., Jandial R., Crain A., Nikkhah G., Snyder E. Y. (2007) The leading edge of stem cell therapeutics. Annu. Rev. Med. 58, 313–328 [DOI] [PubMed] [Google Scholar]
- 3. Goldring C. E., Duffy P. A., Benvenisty N., Andrews P. W., Ben-David U., Eakins R., French N., Hanley N. A., Kelly L., Kitteringham N. R., Kurth J., Ladenheim D., Laverty H., McBlane J., Narayanan G., Patel S., Reinhardt J., Rossi A., Sharpe M., Park B. K. (2011) Assessing the safety of stem cell therapeutics. Cell Stem Cell 8, 618–628 [DOI] [PubMed] [Google Scholar]
- 4. Ben-David U., Benvenisty N. (2011) The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 11, 268–277 [DOI] [PubMed] [Google Scholar]
- 5. Fong C. Y., Gauthaman K., Bongso A. (2010) Teratomas from pluripotent stem cells: A clinical hurdle. J. Cell. Biochem. 111, 769–781 [DOI] [PubMed] [Google Scholar]
- 6. Tan H. L., Fong W. J., Lee E. H., Yap M., Choo A. (2009) mAb 84, a cytotoxic antibody that kills undifferentiated human embryonic stem cells via oncosis. Stem Cells 27, 1792–1801 [DOI] [PubMed] [Google Scholar]
- 7. Choo A. B., Tan H. L., Ang S. N., Fong W. J., Chin A., Lo J., Zheng L., Hentze H., Philp R. J., Oh S. K., Yap M. (2008) Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells 26, 1454–1463 [DOI] [PubMed] [Google Scholar]
- 8. Kershaw D. B., Beck S. G., Wharram B. L., Wiggins J. E., Goyal M., Thomas P. E., Wiggins R. C. (1997) Molecular cloning and characterization of human podocalyxin-like protein. Orthologous relationship to rabbit PCLP1 and rat podocalyxin. J. Biol. Chem. 272, 15708–15714 [DOI] [PubMed] [Google Scholar]
- 9. Tang C., Lee A. S., Volkmer J. P., Sahoo D., Nag D., Mosley A. R., Inlay M. A., Ardehali R., Chavez S. L., Pera R. R., Behr B., Wu J. C., Weissman I. L., Drukker M. (2011) An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat. Biotechnol. 29, 829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradley J. E., Ramirez G., Hagood J. S. (2009) Roles and regulation of Thy-1, a context-dependent modulator of cell phenotype. BioFactors 35, 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horie R., Watanabe T. (1998) CD30: Expression and function in health and disease. Semin. Immunol. 10, 457–470 [DOI] [PubMed] [Google Scholar]
- 12. Rubinstein E., Billard M., Plaisance S., Prenant M., Boucheix C. (1993) Molecular cloning of the mouse equivalent of CD9 antigen. Thromb. Res. 71, 377–383 [DOI] [PubMed] [Google Scholar]
- 13. Wright G. J., Jones M., Puklavec M. J., Brown M. H., Barclay A. N. (2001) The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology 102, 173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujikawa T., Oh S. H., Pi L., Hatch H. M., Shupe T., Petersen B. E. (2005) Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am. J. Pathol. 166, 1781–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen Y., Nemunaitis J. (2006) Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 13, 975–992 [DOI] [PubMed] [Google Scholar]
- 16. Hara A., Aoki H., Taguchi A., Niwa M., Yamada Y., Kunisada T., Mori H. (2008) Neuron-like differentiation and selective ablation of undifferentiated embryonic stem cells containing suicide gene with Oct-4 promoter. Stem Cells Dev. 17, 619–627 [DOI] [PubMed] [Google Scholar]
- 17. Schuldiner M., Itskovitz-Eldor J., Benvenisty N. (2003) Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells 21, 257–265 [DOI] [PubMed] [Google Scholar]
- 18. Cheng F., Ke Q., Chen F., Cai B., Gao Y., Ye C., Wang D., Zhang L., Lahn B. T., Li W., Xiang A. P. (2012) Protecting against wayward human induced pluripotent stem cells with a suicide gene. Biomaterials 33, 3195–3204 [DOI] [PubMed] [Google Scholar]
- 19. Liedtke S., Stephan M., Kögler G. (2008) Oct4 expression revisited: Potential pitfalls for data misinterpretation in stem cell research. Biol. Chem. 389, 845–850 [DOI] [PubMed] [Google Scholar]
- 20. Mitsui K., Tokuzawa Y., Itoh H., Segawa K., Murakami M., Takahashi K., Maruyama M., Maeda M., Yamanaka S. (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631–642 [DOI] [PubMed] [Google Scholar]
- 21. Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643–655 [DOI] [PubMed] [Google Scholar]
- 22. Hart A. H., Hartley L., Parker K., Ibrahim M., Looijenga L. H., Pauchnik M., Chow C. W., Robb L. (2005) The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer 104, 2092–2098 [DOI] [PubMed] [Google Scholar]
- 23. Arumugam S. B., Trentz O. A., Arikketh D., Senthinathan V., Rosario B., Mohandas P. V. (2011) Detection of embryonic stem cell markers in adult human adipose tissue-derived stem cells. Indian J. Pathol. Microbiol. 54, 501–508 [DOI] [PubMed] [Google Scholar]
- 24. Kohler E. E., Cowan C. E., Chatterjee I., Malik A. B., Wary K. K. (2011) NANOG induction of fetal liver kinase-1 (FLK1) transcription regulates endothelial cell proliferation and angiogenesis. Blood 117, 1761–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Potdar P. D., D'souza S. B. (2011) Isolation of Oct4+, Nanog+, and SOX2- mesenchymal cells from peripheral blood of a diabetes mellitus patient. Hum. Cell 24, 51–55 [DOI] [PubMed] [Google Scholar]
- 26. Song H., Chung S. K., Xu Y. (2010) Modeling disease in human ESCs using an efficient BAC-based homologous recombination system. Cell Stem Cell 6, 80–89 [DOI] [PubMed] [Google Scholar]
- 27. Copeland N. G., Jenkins N. A., Court D. L. (2001) Recombineering: A powerful new tool for mouse functional genomics. Nat. Rev. Genet. 2, 769–779 [DOI] [PubMed] [Google Scholar]
- 28. Song H., Hollstein M., Xu Y. (2007) p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat. Cell Biol. 9, 573–580 [DOI] [PubMed] [Google Scholar]
- 29. Liu D., Ou L., Clemenson G. D., Jr., Chao C., Lutske M. E., Zambetti G. P., Gage F. H., Xu Y. (2010) Puma is required for p53-induced depletion of adult stem cells. Nat. Cell Biol. 12, 993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hatano S. Y., Tada M., Kimura H., Yamaguchi S., Kono T., Nakano T., Suemori H., Nakatsuji N., Tada T. (2005) Pluripotential competence of cells associated with Nanog activity. Mech. Dev. 122, 67–79 [DOI] [PubMed] [Google Scholar]
- 31. Black M. E., Kokoris M. S., Sabo P. (2001) Herpes simplex virus-1 thymidine kinase mutants created by semi-random sequence mutagenesis improve prodrug-mediated tumor cell killing. Cancer Res. 61, 3022–3026 [PubMed] [Google Scholar]
- 32. Serganova I., Ponomarev V., Blasberg R. (2007) Human reporter genes: Potential use in clinical studies. Nucl. Med. Biol. 34, 791–807 [DOI] [PubMed] [Google Scholar]
- 33. Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., Jones J. M. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 [DOI] [PubMed] [Google Scholar]
- 34. Fu X., Xu Y. (2011) Self-renewal and scalability of human embryonic stem cells for human therapy. Regen. Med. 6, 327–334 [DOI] [PubMed] [Google Scholar]
- 35. McGavin J. K., Goa K. L. (2001) Ganciclovir: An update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs 61, 1153–1183 [DOI] [PubMed] [Google Scholar]
- 36. Howden S. E., Gore A., Li Z., Fung H. L., Nisler B. S., Nie J., Chen G., McIntosh B. E., Gulbranson D. R., Diol N. R., Taapken S. M., Vereide D. T., Montgomery K. D., Zhang K., Gamm D. M., Thomson J. A. (2011) Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proc. Natl. Acad. Sci. U.S.A. 108, 6537–6542 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.