Background: ASIC3 channels contribute to pain stimuli perception.
Results: A new compound (sevanol) was isolated from thyme extract and was shown to inhibit ASIC3 current components.
Conclusion: Sevanol could play a considerable role in thyme analgesic properties.
Significance: The data on the structure and potency of sevanol can make a contribution to understanding the function of ASIC3 and can be helpful for developing other pharmacological substances targeting ASIC3.
Keywords: Acid-sensing Ion Channels (ASIC), Electrophysiology, Mass Spectrometry (MS), Nuclear Magnetic Resonance, Plant Biochemistry, Lignan
Abstract
A novel compound was identified in the acidic extract of Thymus armeniacus collected in the Lake Sevan region of Armenia. This compound, named “sevanol,” to our knowledge is the first low molecular weight natural molecule that has a reversible inhibition effect on both the transient and the sustained current of human ASIC3 channels expressed in Xenopus laevis oocytes. Sevanol completely blocked the transient component (IC50 353 ± 23 μm) and partially (∼45%) inhibited the amplitude of the sustained component (IC50 of 234 ± 53 μm). Other types of acid-sensing ion channel (ASIC) channels were intact to sevanol application, except ASIC1a, which showed more than six times less affinity to it as compared with the inhibitory action on the ASIC3 channel. To elucidate the structure of sevanol, the set of NMR spectra in two solvents (d6-DMSO and D2O) was collected, and the complete chemical structure was confirmed by liquid chromatography-mass spectrometry with electrospray ionization (LC-ESI+-MS) fragmentation. This compound is a new lignan built up of epiphyllic acid and two isocitryl esters in positions 9 and 10. In vivo administration of sevanol (1–10 mg/kg) significantly reversed thermal hyperalgesia induced by complete Freund's adjuvant injection and reduced response to acid in a writhing test. Thus, we assume the probable considerable role of sevanol in known analgesic and anti-inflammatory properties of thyme.
Introduction
Plants of the genus Thymus are among the most popular in the world for their aromatic and medicinal properties (1). Leaf and flower extracts of Thymus species have sedative, antispasmodic, antioxidant (2), antimicrobial (3, 4), tonic, anti-inflammatory, and antiparasitic properties (5). Many components from essential Thymus oils are known, such as thymol, linalool, carvacrol, geranial, and γ-terpineol (6). There are a limited number of studies about the anti-inflammatory and analgesic activity of Thymus spp. extracts (7, 8), but up-to-date thyme analgesic compounds are still unknown in contrast to some components that possess anti-inflammatory activity (9, 10).
One of the promising targets for analgesic and anti-inflammatory drug development is the ASIC3 channel (11). This trimer channel, predominantly expressed in peripheral sensory neurons, belongs to a group of sodium-selective acid-sensing ion channels (ASIC)4 activated by extracellular acidosis (12–15). The homomeric ASIC3 channel, in contrast to channels constructed from other ASIC subunits, responds to a drop of extracellular pH by a transient inactivating current followed by a sustained component (12, 16). It is highly sensitive to protons, with half-maximal activation at pH 6.5 (12, 17). ASIC3 contributes to cutaneous sensing of acid, mediates prolonged anginal pain during myocardial ischemia, and takes part in primary inflammation pain (18). New selective pharmacological agents inhibiting ASIC3 can help to develop new drugs for treatment of acute and chronic pain (19).
Inhibition of ASIC3 activation by selective polypeptide antagonist APETx2 or siRNA revealed an analgesic effect in acid-induced pain, thermal and mechanical postoperative hyperalgesia, and complete Freund's adjuvant (CFA) inflammatory pain models (18, 20). Some nonsteroidal anti-inflammatory drugs (salicylic acid, acetylsalicylic acid, and diclofenac) are able to inhibit the sustained component of ASIC3 current but not the transient component in vitro (21). Also, non-amidine ASIC3 inhibitors were described, but they caused sedative or lethargic side effects in the rat CFA model (22). Therefore, there is a deficit of compounds suitable for both in vitro and in vivo experiments that can help to elucidate the physiological role of the ASIC3 channel.
We concentrated on a novel compound search to develop molecules capable of reducing the conductivity of the ASIC3 channel. In the present work, we describe the isolation and chemical structure elucidation of the active component from the acetate extract of Thymus armeniacus named “sevanol” that inhibits both sustained and transient currents of ASIC3.
EXPERIMENTAL PROCEDURES
Extraction from Plant Material
T. armeniacus plants were collected in the Lake Sevan region of Armenia in the summer of 2010. 2 g of dried herbs with flowers were crushed, and the first extraction was performed in 80 ml of 70% ethanol solution titrated to pH 8.0 by NaOH for 1 h at 24 °C with constant stirring. The extract was then centrifuged at 10,000 × g for 20 min, and supernatant was collected. The pellet was resuspended in 80 ml of 70% ethanol solution titrated to pH 4.0 by acetic acid. Extraction was done for 1 h at 24 °C with constant stirring, and after centrifugation at 10,000 × g for 20 min, the second portion of the supernatant was collected. The pellet was resuspended one more time in 80 ml of 10% acetic acid containing 10 mm EDTA and 1 mm PMSF. Extraction was done for 1 h at 24 °C with constant stirring, and after centrifugation 10,000 × g for 20 min, the last portion of the supernatant was obtained. Each supernatant was lyophilized and stored at −20 °C up to analysis.
Purification of the Active Component
The active component was purified from the third (acetic acid) supernatant by two-stage HPLC. First, separation was done on a reverse-phase Luna C18 column (Phenomenex, 150 × 4.6 mm, 5 μm) using a linear gradient from 0 to 60% acetonitrile in 0.1% TFA. A constant flow rate of 1 ml/min and the detection of eluted compounds at 210 and 280 nm were used. Finally, the active component was isolated in a Luna PFP(2) column (Phenomenex, 250 × 4.6 mm, 3 μm) using a linear gradient from 15 to 55% acetonitrile in 0.1% TFA. A constant flow rate 0.5 ml/min and the detection of eluted compounds at 210 and 280 nm were used.
UV Spectroscopy
The absorption spectra were registered on a Hitachi U-3210 spectra photometer. The coefficient of molar extinction was calculated from a set of quantity weighed for analysis.
Electrophysiological Study
Xenopus laevis oocytes were removed surgically, defolliculated, and injected with 2.5–10 ng of cRNA. cRNA transcripts were synthesized from linearized cDNA templates using a RiboMAXTM large-scale RNA production system T7 (Promega) according to a protocol for capped transcripts supplied by the manufacturer. Human ASIC3 cDNA (AF057711.1) was subcloned from EX-Q0260-B02 (GeneCopoeia, Inc.) to pcDNA3.1 and linearized by NaeI (Promega). By analogy, other cRNA were synthesized from pcDNA3.1-rat TRPV1, pcDNA3.1-human p2X3, and PCi plasmids containing rat ASIC1a, ASIC1b, and ASIC2a. After injection, the oocytes were kept for 2–3 days at 19 °C and then up to 7 days at 15 °C in a ND-96 medium containing (in mm) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES titrated to pH 7.4 with NaOH supplemented with gentamycin (50 μg/ml).
Two-electrode voltage clamp recordings were performed using a GeneClamp 500 amplifier (Axon Instruments), and data were filtered at 20 Hz and digitized at 100 Hz by an AD converter L780 (L-Card, Moscow, Russia) using homemade software. Microelectrodes were filled with 3 m KCl solution. An external ND96 solution with pH 7.8 or 7.3 was used. To induce specific currents, we employed ND-96 modified solutions (in which 5 mm HEPES was substituted to 10 mm acetic acid (pH 4.0) or 5 mm MES (pH 5.5)). All solutions of testing compounds were supplemented with 0.1% BSA. A computer-controlled valve system for fast solution application was used to switch solutions.
NMR Spectroscopy
NMR spectra of the active compound dissolved in d6-DMSO (4.5 mg) and D2O (3.4 mg, pH 2.51) were measured on a Bruker Avance III 600-MHz NMR spectrometer equipped with a pulsed-field gradient triple-resonance cryoprobe. The following spectra were used to identify chemical structures: 1H, 13C, 13C-DEPT-135, two-dimensional double-quantum filtered-COSY, two-dimensional NOESY (600 ms), two-dimensional ROESY (100 ms, DMSO only), two-dimensional 1H-13C HSQC, and two-dimensional 1H-13C HMBC (Jlong = 4Hz, 7Hz). To measure pKa values of carboxylic groups, 1H and two-dimensional 1H-13C HSQC spectra were acquired in D2O at 16 pH values in the range from 2.64 to 7.62.
Mass Spectrometry
Sample analysis was performed on an Agilent 6224 TOF LC/MS system (Agilent) equipped with a dual-nebulizer ESI source. MassHunter software was used for data acquisition and analysis. LC separation was performed on a ZORBAX RRHD Eclipse XDB-C18 column (Agilent, 2.1 × 100 mm, 1.8 μm) using a linear gradient from 0 to 28% acetonitrile with 0.1% TFA in 12 min at a flow rate of 0.3 ml/min.
Animals
Adult male CD-1 mice (Animal Breeding Facility Branch of Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Pushchino, Russia) weighing 20–25 g were used. Animals were housed at room temperature of 23 ± 2 °C subjected to a 12-h light-dark cycle with food and water available ad libitum. All experiments were performed after approval by the Animal Care and Use Committee of the Branch of the Institute of Bioorganic Chemistry, Russian Academy of Sciences (IBCh RAS) (Pushchino, Russia Federation). Sevanol or saline was administered intravenously 30 min before testing.
Complete Freund's Adjuvant-induced Thermal Hyperalgesia
CFA suspended in an oil/saline (1:1) emulsion was injected into the dorsal surface of the left hind paw of mice (20 μl/paw). Control mice received 20 μl of saline (intraplantar). Paw withdrawal latencies to thermal stimulation (53 °C) were measured 24 h after CFA injection.
Acetic Acid-induced Writhing (Abdominal Constriction Test of Visceral Pain)
Separate groups of mice were injected with 0.6% acetic acid in saline (10 ml/kg intraperitoneally). Mice were immediately placed inside transparent glass cylinders, and the number of writhes was recorded for 15 min.
Statistic
The significance of the data was determined by analysis of variance followed by Tukey's test. Data are presented as mean ± S.E.
RESULTS
Isolation of Active Compound
Different extraction compositions including ethanol and acetic acid have been described in the literature to isolate tannins, flavonoids, and peptides from plants. These classes of compounds are most attractive because their antinociceptive and anti-inflammatory activities are known (10, 23–26). Extraction procedures by three different solutions with subsequent electrophysiological tests on inhibition of ASIC3 currents were performed step-by-step to complete the separation of active compounds. The first alcohol extract had no effect on the ASIC3 channel, whereas both acid extracts did (Fig. 1). The second alcohol extract exerted a less pronounced effect than the acetate extract that almost completely blocked the ASIC3 currents. Thus, an acetic acid extract was chosen for further work.
FIGURE 1.
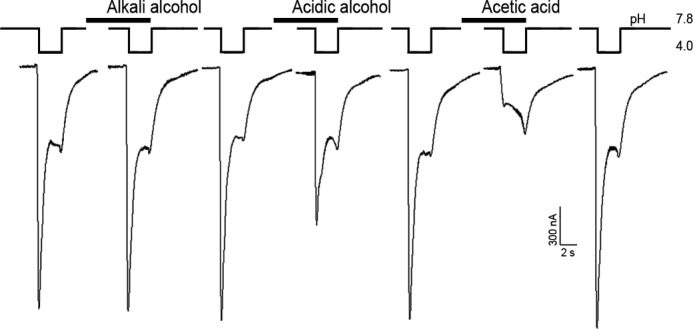
Action of thyme extracts on human ASIC3 channels expressed in X. laevis oocytes. Acid-induced currents were evoked by pH drop from 7.8 to 4.0 for 3 s; time intervals between recordings were about 1–2 min. Extracts were measured by 1 mg/ml concentration; holding potential was −50 mV.
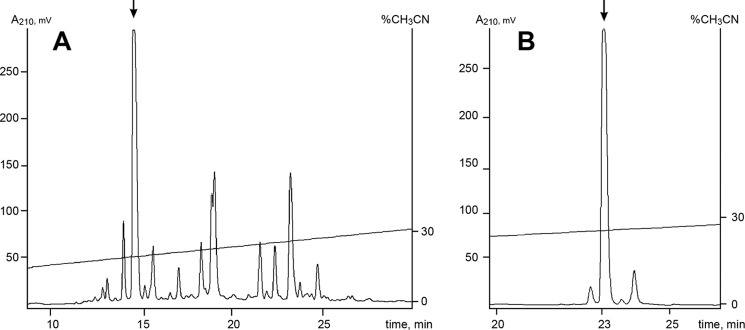
Separation of this extract by reverse-phase HPLC revealed a group of compounds, but only one most abundant that eluted on a 19-min inhibited ASIC3 pH-induced current (Fig. 2A). Finally, the pure active component named sevanol was isolated on a column with pentafluorophenyl groups as a stationary phase (Fig. 2B).
FIGURE 2.
Isolation of sevanol. A, the first separation stage of the acetic extract of thyme on a reverse-phase column Luna C18 (4.6 × 150 mm) in 0.1% TFA with a flow rate 1 ml/min using a linear gradient of acetonitrile concentration. B, final purification on a column Luna PFP(2) (4.6 × 250 mm) in 0.1% TFA with a flow rate of 0.5 ml/min using a linear gradient of acetonitrile concentration. Fractions containing the active component are marked by an arrow.
A second alcoholic extract HPLC separation revealed that sevanol was partially extracted by acetic alcohol. Its amount in the second alcoholic extract was five times less than in acetic acid extract. Further electrophysiological measures of the second extract compounds confirmed the inhibitory action on ASIC3 currents only for a single component: sevanol. Thus, T. armeniacus herbs and flowers contained only one ASIC3 inhibitor.
Sevanol is a major component in the acetate extract of T. armeniacus. Its content was estimated to be about 1.5% of crude dried plant weight. A UV spectrum of sevanol in an acidic solution has a maximum absorption at wavelengths (λ) 254 and 340 nm. Its molar extinction coefficient at λ = 254 nm was calculated as 4860 experimentally in a 0.1% TFA solution.
Structure Elucidation
The pure compound after HPLC isolation showed [M + H]+ ion 707.1092 Da and corresponding [M − H]− ion 705.0963 Da in LC-ESI+ and LC-ESI− mass spectra, respectively, that agreed with molecular formula C30H26O20. To resolve their structure, the set of NMR spectra was collected in two solvents: d6-DMSO and D2O. The carbon-13 NMR spectrum shows 8 carboxylic signals, 14 aromatic or olefinic signals, and 8 aliphatic carbon signals. Four singlet peaks in the 1H NMR spectrum in d6-DMSO (1H each) are not connected to any 13C atom (no corresponding two-dimensional peak in 13C-HSQC spectrum) and are absent in NMR spectra in D2O, indicating the presence of four hydroxyl groups. Other protons are divided into two groups: six aromatic or olefinic protons (connected carbon atoms have a 13C chemical shift 115.00 ppm and more) and 10 aliphatic protons (connected carbons atoms have a 13C chemical shift 73.07 ppm and less).
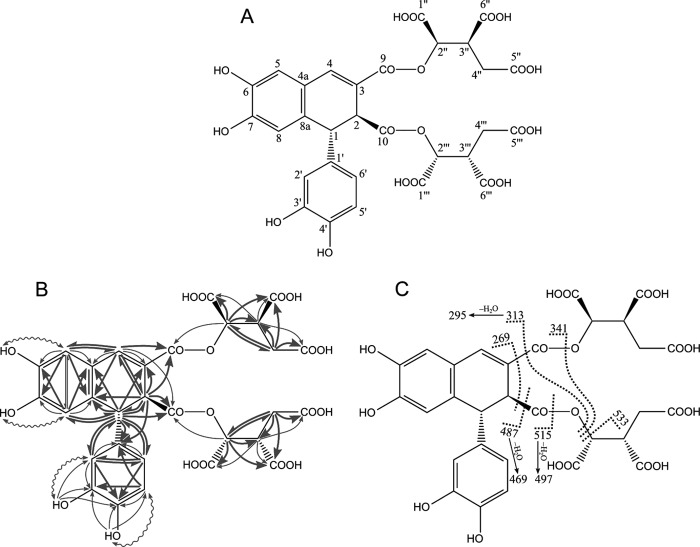
Assuming that the largest cross-peaks with participation of olefinic protons in the 1H-13C HMBC NMR spectrum should outline three-bond 1H-13C connectivity and that weak cross-peaks correspond to two- and four-bond connectivity, an epiphyllic acid moiety with the systematic name 2,3-dicarboxy-6,7-dihydroxy-1-(3′,4′-dihydroxy)-phenyl-1,2-dihydronaph-thalene (27, 28) was identified in the NMR spectrum (Fig. 3, A and B). The four hydroxyl groups of epiphyllic acid observed in d6-DMSO are unambiguously assigned due to ROESY and HMBC connectivity (Fig. 3B, Table 1). Additional HMBC connectivities outline the presence of 9,10-ester derivatives of the epiphyllic acid. The latter are better resolved in the NMR spectra acquired in D2O at pH 2.51 (note redundant chemical shifts, residual water, and DMSO overlap in Table 1). Both ester derivatives are isocitryl esters (Fig. 3B); consequently, the chemical structure of sevanol is epiphyllic acid 9, 10-diisocitryl ester (Fig. 3, Table 1). This compound is a new lignan derivative.
FIGURE 3.
Structure elucidation of sevanol as epiphyllic acid 9,10-diisocitryl ester. A, proposed structure of sevanol with numbering of carbon atoms. B, observed positive ROESY (wavy lines) and 1H → 13C HMBC connectivities (bold lines for strong peaks and thin lines for weak peaks in the HMBC NMR spectrum). C, observed fragmentation of sevanol in the LC-ESI+ mass spectrum. Fragments 295, 469, and 497 are speculated to be due to the formation of a double bond between atoms 1 and 2 followed by the release of one water molecule with the participation of an ester oxygen atom.
TABLE 1.
NMR data used for elucidation of the chemical structure
Chemical shifts, ROESY, and 1H → 13C HMBC connectivity of sevanol in d6-DMSO and D2O (303 K) are shown. —, not applicable.
| Atom |
d6-DMSO |
D2O, pH 2.51 |
Strong connectivitiesa | ||
|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | ||
| Epiphyllic acid | |||||
| 1 | 4.333 d(1.3) | 44.76 | 4.533 d(1.9) | 44.07 | 2,3,4a,8,8a, 10,1′,2′,6′ |
| 2 | 3.809 d(1.3) | 46.77 | 4.115 d(1.9) | 46.49 | 1,3,4,8a,9,10 |
| 3 | — | 119.68 | — | 119.80 | — |
| 4 | 7.600 s | 139.88 | 7.765 s | 141.44 | 2,5,8a,9 (3,4a,10) |
| 4a | — | 122.73 | — | 123.78 | — |
| 5 | 6.856 s | 116.90 | 7.026 s | 117.45 | 4,7,8a (4a,6,8) |
| 6 | 9.103 s (hydroxyl) | 144.96 | — | 143.38 | ROESY: H6 |
| 7 | 9.500 s (hydroxyl) | 148.76 | — | 147.53 | ROESY: H8 |
| 8 | 6.537 s | 116.80 | 6.746 s | 116.64 | 1,4a,6 (5,7,8a) |
| 8a | — | 129.54 | — | 130.79 | — |
| 9 | — | 165.55 | — | 166.86 | — |
| 10 | — | 170.82 | — | 172.83 | — |
| 1′ | — | 134.89 | — | 134.52 | — |
| 2′ | 6.336 d(1.5) | 115.00 | 6.640 d(1.7) | 115.33 | 1,4′,6′ (1′,3′) |
| 3′ | 8.741 s (hydroxyl) | 145.42 | — | 143.98 | (2′,3′,4′) ROESY: H2′ |
| 4′ | 8.682 s (hydroxyl) | 144.46 | — | 142.83 | (3′,4′,5′) ROESY: H5′ |
| 5′ | 6.574 d(8.1) | 115.84 | 6.724 d(8.3) | 116.22 | 1′,3′ (4′) |
| 6′ | 6.256 dd(1.5,8.1) | 118.11 | 6.472 dd(1.7,8.3) | 119.70 | 1,2′,4′ (1′) |
| 9-Isocitryl ester | |||||
| 1″ | — | 169.51 | — | 172.73 | — |
| 2″ | 5.183 d(2.7) | 72.25 | 5.325 d(4.0) | 73.07 | 1″,3″,4″,6″ (9) |
| 3″ | 3.263b | 43.17 | 3.538 m | 43.21 | (1″,5″,6″) |
| 4″ | 2.555c | 32.51 | 2.616 dd(5.2,17.2) | 32.34 | 2″,3″,5″,6″ |
| 2.487c | 2.787 dd(9.3,17.2) | ||||
| 5″ | — | 172.89 | — | 175.41 | — |
| 6″ | — | 171.93 | — | 174.21 | — |
| 10-Isocitryl ester | |||||
| 1‴ | — | 169.28 | — | 171.83 | — |
| 2‴ | 5.120 d(2.7) | 72.48 | 5.280 d(3.7) | 73.07 | 1‴,3‴,4‴,6‴(10) |
| 3‴ | 3.263b | 43.17 | 3.425 m | 42.96 | (1‴,5‴,6‴) |
| 4‴ | 2.318 | 32.51 | 2.262 dd(5.0,17.3) | 31.81 | 2‴,3‴,5‴,6‴ |
| 2.530c | 2.523 dd(9.6,17.3) | ||||
| 5‴ | — | 171.92 | — | 175.23 | — |
| 6‴ | — | 171.88 | — | 173.77 | — |
a Weak connectivities are in parentheses.
b Signal multiplicity is not resolved due to overlap with residual water signal (3.316 ppm).
c Signal multiplicity is not resolved due to overlap with residual DMSO signal (2.510 ppm).
The chemical structure was confirmed by LC-ESI+-MS fragmentation (Fig. 3C) as recommended by (29). Fragment 533.0920 Da follows the “+2” rule for esters (rule 12), confirmed by the loss of H2O (fragment 515.0827, rule 11). Fragment 487.0850 Da is due to rule 14 for esters. Three fragments appear in accordance with multiple cleavage rule MC2: 341.0648, 313.0699, and 269.0793 Da. The last three fragments (497.0703, 469.0747, and 295.0607 Da) do not follow rules described in Ref. 29, but they are easily identified as a loss of H2O from fragments 515.0827, 487.0850, and 313.0699 Da, respectively. The details of this process may be speculated as the formation of a double bond between atoms 1 and 2 followed by the release of the water molecule with the participation of one oxygen atom from the ester group(s).
Determination of pKa Values of Carboxyl Groups
Chemical shifts of four protons and three carbon atoms were measured at 16 pH values in the range 2.64–7.62 for both isocitryl moieties. To determine three pKa,m values for each isocitryl ester, seven chemical shifts of the ester were fitted to the equation.
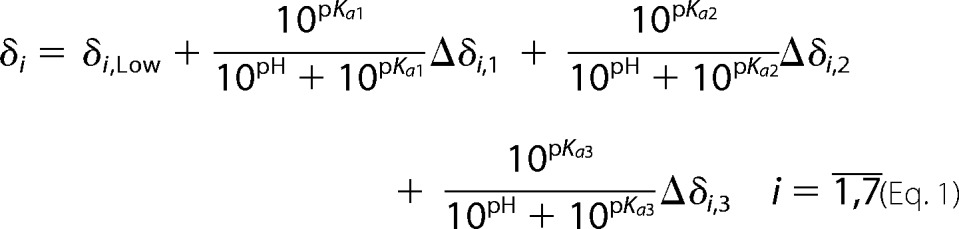 |
The nonlinear regression (fitting) was performed by the Levenberg-Marquardt method in Mathematica 8.0 software (Wolfram Research) under the assumption that all pKa,m values are equal for all seven chemical shifts. The errors of measured 1H and 13C chemical shifts were estimated as 0.013 and 0.19 ppm, respectively, as the cross-peak width at half-height. The nonlinear regression was made independently for each isocitryl ester moiety.
The determined pKa,m values are 3.0 ± 0.14, 5.0 ± 0.14, and 6.1 ± 0.2 for 9-isocitryl ester and 3.0 ± 0.17, 4.9 ± 0.18, and 6.1 ± 0.2 for 10-isocitryl ester. Relying on the chemical shift changes, these pKa values could be assigned to carboxyl groups with atoms 1″(1‴), 6″(6‴) and 5″(5‴) of isocitryl ester moieties, in this order.
Examination of ASIC3 Currents
The testing was performed by a standard two-electrode voltage clamp technique on X. laevis oocyte-expressed human ASIC3 channels. ASIC3 currents were evoked by rapid drop of pH in an external oocyte solution. The preliminary test for fractions was performed using the “general” protocol: pH drop from 7.8 to 4.0 for 3 s that induced both transient and sustained components of the current. Responses to repeated applications of low pH with 1–2-min intervals had good reproducibility, but sometimes a slow irreversible decrease in response over time (rundown) was detected. Only cells with no more than 1–2% difference in repeated pH 4.0 applications rundown were used.
All analyzed fractions were applied for 5 s before the low pH stimulation. The ratio of the amplitude transient (peak) component for analyzed compounds to a transient component for control solution was measured as transient ASIC3 current inhibition. The amplitude of the sustained component of the ASIC3 currents in control and sample experiments was calculated as a quasi-stationary value of current at the 3rd s.
Sevanol significantly inhibited the transient and sustained components of ASIC3 current in general protocol (Fig. 4A). The inhibitory effect on the transient component reached 100%, whereas on the sustained component, it did not exceed 50%. Response parameters recovered almost completely to the next control solution application; therefore, measured inhibition was completely reversible.
FIGURE 4.

Action of sevanol on ASIC3 channels. A, application traces of ASIC3 currents triggered by dropping the pH 7.8 to 4.0 without (first and third recording) and with purified sevanol. B and C, traces obtained for modified activation protocols. D and E, dose-response curves for sevanol inhibitory activity on ASIC3 currents separately calculated for transient (D) and sustained (E) components. Each point is the mean ± S.E. of 3–5 measurements. Data were fitted by the logistic equation.
We also checked out one well known component from Thymus essential oils, thymol, that is known to hold antibacterial and antioxidant activity. Up to 1 mm concentration of thymol did not show any effect on ASIC3 channels in our experimental system (data not shown).
To study the inhibitory effect of sevanol on the transient component of ASIC3 current separate from the sustained component, we used a protocol with a short (1-s) pH drop from 7.8 to 5.5. Sevanol was applied only before the pH step (Fig. 4B). The measured ratio of the response amplitudes with and without sevanol preincubation was estimated as transient ASIC3 current inhibition.
Because the transient component of ASIC3 current is fast and disappears about 2 s after activation by the acid step, the amplitude of the sustained component of the ASIC3 currents can be calculated as a quasi-stationary value of current on the 3rd s of activation in general protocol (Fig. 4A). To study only the sustained component inhibition, we used a pH drop from 7.3 to 4.0 for 5 s without sevanol preincubation (Fig. 4C).
The data on inhibition by specialized protocols suited well to general protocol data for both the transient and the sustained components of ASIC3 currents. The inhibitory effect on transient and sustained components of ASIC3 currents was concentration-dependent and fitted well by a logistic equation. The complete block of the transient current component was estimated with IC50 353 ± 23 μm and Hill coefficient (nH) 2.2 ± 0.28 (Fig. 4D). The sustained component block did not exceed 50% (calculated IC50 234 ± 53 μm and nH = 1.8 ± 0.61) (Fig. 4C).
The pH Dependence of Sevanol Action
To determine whether the sevanol inhibition of ASIC3 depends on pH level of activating stimuli, we evaluated sevanol-mediated inhibition of ASIC3 currents at different pH. As compared with sevanol inhibition of the ASIC3 transient current at pH 5.5, the percentage of inhibition at other pH values did not change. At a sevanol concentration of 1 mm, the amplitude of the transient component of the ASIC3 current was reduced by 81.5% ± 3.4 (n = 8) at pH 6.5; 83 ± 4.3% (n = 8) at pH 6.0; and 80 ± 5.3% (n = 8) at pH 5.5 (Fig. 5). These results indicate that the inhibition of ASIC3 currents by sevanol does not depend on the magnitude of the pH change.
FIGURE 5.
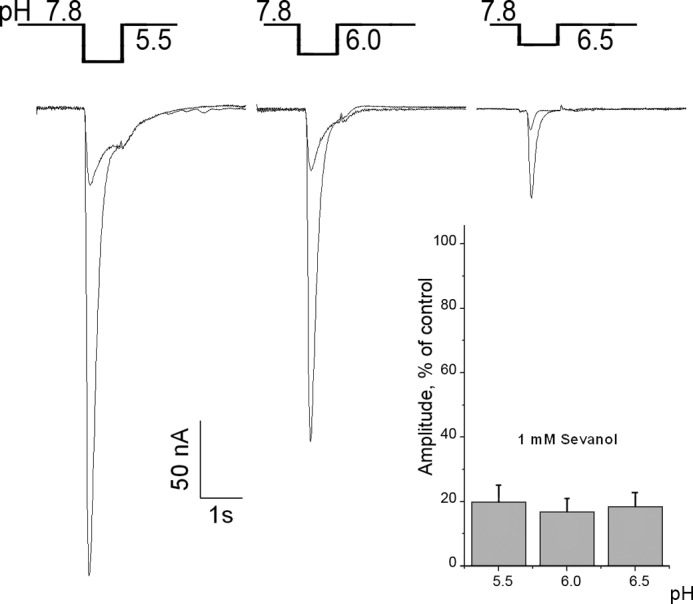
Sevanol inhibition of ASIC3 current is pH-independent. Current traces show the inhibitory effect of sevanol on pH-dependent activation of ASIC3 currents. Sevanol was applied only before the pH step, the same as shown on Fig. 4B. Statistical bars represent the relative amplitude of ASIC3 current inhibition by 1 mm of sevanol. Each point is mean ± S.E., n = 8.
Selectivity to Other Channels
To check sevanol selectivity, we examined its modulation activity on other channels (Fig. 6). Sevanol caused a complete inhibition of the ASIC1a current, but its action was more than six times weaker (IC50 2.2 ± 0.6 mm and nH = 2.4 ± 0.14) in comparison with its effect on the ASIC3 transient current. Sevanol at 1 mm showed neither agonistic nor antagonistic activity to ASIC1b, ASIC2a, and p2X3, but had a minor potentiating effect on TRPV1 capsaicin-induced currents (∼12%).
FIGURE 6.
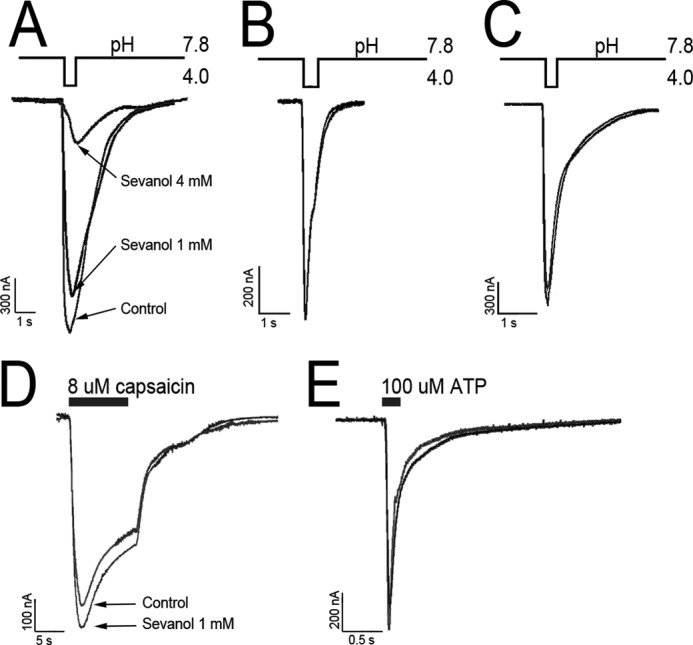
Sevanol action on other channels expressed in X. laevis oocytes. A–C, traces for ASIC1a, ASIC1b, and ASIC2a currents activated by pH decrease, respectively. D, traces of TRPV1 current generated by 8 μm capsaicin application. E, traces of p2X3 currents generated by 100 μm ATP application. All currents were recorded at −50 mV, and activation stimuli were loaded after a 5-s incubation of control solution or 1 mm sevanol solution. For A, a second sevanol concentration of 4 mm was measured.
Antinociceptive and Anti-inflammatory Properties of Sevanol
The intraplantar injection of CFA in the hind paw caused the development of the thermal hyperalgesia associated with inflammation. The group of mice treated with saline 24 h after CFA injection showed significantly reduced inflamed hind paw withdrawal latency (7.7 ± 0.7 s) on a hot plate in a comparison with the control group treated with saline twice (13.8 ± 0.6 s). Intravenous administration of sevanol (1 or 10 mg/kg) 30 min before testing significantly reversed thermal hyperalgesia. Latency to respond to the thermal stimulus was increased from 7.7 ± 0.7 s in the CFA/saline group to 10.9 ± 0.3 s following 1 mg/kg sevanol treatment and to 12.8 ± 0.6 s following 10 mg/kg sevanol treatment (Fig. 7A). The maximal effect, calculated as a percentage of hyperalgesia reduction, was 83 ± 12% for a 10 mg/kg dose and 53 ± 5% for a 1 mg/kg dose.
FIGURE 7.
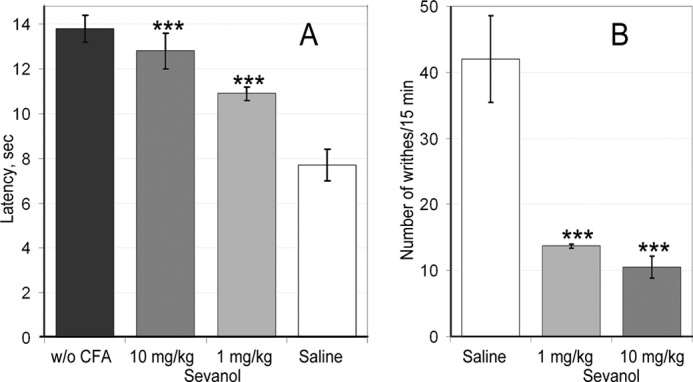
Efficacy of sevanol in pain models. A, sevanol significantly reversed CFA-induced thermal hyperalgesia, prolonging withdrawal latency of inflamed hind paw on a hot plate; n = 7. B, sevanol attenuated response to intraperitoneal administration of acetic acid estimated by the number of writhes occurring in 15 min; n = 7. Results are presented as mean ± S.E.; ***, p < 0.001 versus saline group.
An acetic acid writhing test was used as a second in vivo model. Intraperitoneal administration of acetic acid provoked a stereotyped behavior in the mouse that was characterized by abdominal contractions (acetic acid-induced writhes). Sevanol pretreatment (1 and 10 mg/kg) 30 min before testing significantly reduced the number of writhes (Fig. 7B). The number of writhes in the control group treated by saline was 42.0 ± 6.6, whereas the number of writhes decreased dramatically in both sevanol pretreated groups up to 10.5 ± 1.7 at a 10 mg/kg dose and up to 13.7 ± 0.3 at a 1 mg/kg dose. Thus, sevanol produced more than a 70% reduction of response to acid in this test.
Doses were chosen to reach a blood plasma concentration of sevanol ∼300 μm for the 10 mg/kg dose and ∼30 μm for the 1 mg/kg dose. Plasma concentrations were estimated with regard to blood volume (∼8% of weight) and blood plasma content (55–65% of volume) (30).
DISCUSSION
We identified a new component from acidic thyme extract named sevanol that exhibits inhibition of pH-induced ASIC3 currents. This is a new representative of lignans, a class of widespread natural phenolic compounds. Lignans are a class of molecules that have a diverse spectrum of biological activities. It is expected that they are promising organic molecules for new drug development. Lignans have shown cytotoxic, antitumor/anticancer, antiviral, antifungal, antileishmanial, antiangiogenic, antioxidant, antiulcer, antiallergen, antiplatelet, and antiosteoporotic hypolipidemic, hepatoprotective, and antirheumatic activities, and they are selective inhibitors of some enzymes such as 5-lipooxygenase and phosphodiesterases IV and V (31). The molecular targets of most lignans are still unknown. Sevanol is a novel lignan that predominantly inhibits the acid-sensing channel ASIC3.
Since the first evidence about an important role of ASIC3 channels in primary pain generation by tissue acidosis associated with ischemia, inflammation, or lesions was confirmed, these channels have been the subject of extensive research (11). We have already mentioned a number of ligands that can interact with varying degrees of efficiency toward the ASIC3 channel, but we would like to emphasize their different effects on the channel. Amiloride, a previously widely used K+-sparing diuretic agent, inhibits transient ASIC3 currents (IC50 ∼60 μm), but has no effect on the sustained component (12). In the same manner, a polypeptide toxin APETx2 specifically inhibits transient ASIC3 currents with IC50 63 nm, without affecting sustained ASIC3 currents (32). Another natural component, thalassiolin B, from the extract of sea grass Thalassia testudinum shows small inhibitory effects of about 30% only on the transient currents (IC50 27 μm), but produces significant antinociceptive effects (33). Other non-peptide compounds such as salicylic acid (IC50 260 μm) or diclofenac (IC50 92 μm) inhibit the sustained component of the ASIC3 current, but not the transient component (21).
In contrast to other compounds, sevanol completely blocked the transient component (IC50 353 μm) and inhibited ∼45% of the amplitude of the sustained component (IC50 234 μm) of ASIC3 current (Fig. 4). Thus, sevanol is the first natural compound that has an inhibition effect on both the transient and the sustained components of the ASIC3 current. In addition, the mode of action of this compound is absolutely new.
The similarity of IC50 values measured for transient and sustained components of ASIC3 currents suggests that the same binding site on the channel could regulate inhibitory effects and cover the components of both currents. Because dose-response analysis revealed nH values greater than 1, we assume a possible presence of more than one binding site of sevanol on the ASIC3 channel. We believe that sevanol is not a pore blocker because it does not completely inhibit the sustained current component. Probably, it interacts with regulatory sites of the channel and blocks activation of the transient component of the ASIC3 current more efficiently than a sustained component.
As sevanol did not produce a significant activation or inhibition effect on TRPV1, p2X3, ASIC1b, or ASIC2a channels expressed in oocytes (Fig. 6), an indirect influence on channels via intermediate membrane systems could be excluded. Because sevanol can inhibit rat ASIC1a pH-induced currents at high concentration, it was shown to be a rather specific inhibitor of ASIC3 channels with a less pronounced effect on ASIC1a channels.
Intravenous administration of sevanol (1–10 mg/kg) in mice significantly reversed CFA-induced thermal hyperalgesia and reduced the number of writhes in an acetic acid-induced writhing test (Fig. 7). Study of ASIC3 knock-out mice revealed involvement of this channel in the modulation of the response to acid in the writhing test (34). Also, knockdown of ASIC3 by siRNA and local administration of toxin APETx2 were reported to reverse thermal hyperalgesia in a CFA test (11). Thus, we can speculate that the analgesic effect of sevanol is mediated mostly by direct inhibition of ASIC3 channels.
Extracts of thyme (50–400 mg/kg) produced significant analgesic and anti-inflammatory effects in a variety of animal models of nociception (7, 8). Therefore, with consideration for the high content of sevanol in T. armeniacus (1.5% of dried plants), we assume the considerable role of this compound in the analgesic and anti-inflammatory properties of thyme.
In summary, we identified a new component with a novel structure from T. armeniacus extract that inhibits an important acid sensor of sensory neurons. The data on the structure and potency of this compound can make a contribution to understanding the function of the ASIC3 channel and can be helpful for developing other pharmacological substances targeting ASIC3, as well as for drug design.
Acknowledgments
We are sincerely grateful to Lilia V. Revasova for T. armeniacus collection and identification, Irina D. Konstantinova for assistance in obtaining mass spectra, and Sylvie Diochot (Institut de Pharmacologie Moleculaire et Cellulaire, Valbonne, France) for PCi plasmids containing cDNA of rat ASIC1a, ASIC1b, and ASIC2a.
This work was supported by the “Molecular and Cell Biology” and “Fundamental Sciences for Medicine” Programs of the Russian Academy of Sciences, by the Ministry of Education and Science of the Russian Federation, Contract 16.512.11.2195 from 14.03.2011, and by the Russian Foundation for Basic Research.
The structure has been deposited to the PubChem Compounds database under accession number CID 57411952.
- ASIC
- acid-sensing ion channel
- CFA
- complete Freund's adjuvant
- DMSO
- dimethyl sulfoxide
- LC-ESI-MS
- liquid chromatography-mass spectrometry with electrospray ionization
- p2X3
- purine-gated cation channel type 3
- TRPV1
- transient receptor potential vanilloid type 1
- HSQC
- heteronuclear single quantum correlation
- HMBC
- heteronuclear multiple bond correlation
- ROESY
- rotating-frame nuclear Overhauser effect correlation spectroscopy.
REFERENCES
- 1. Figueiredo A. C., Barroso J. G., Pedro L. G., Salgueiro L., Miguel M. G., Faleiro M. L. (2008) Portuguese Thymbra and Thymus species volatiles: chemical composition and biological activities. Curr. Pharm. Des. 14, 3120–3140 [DOI] [PubMed] [Google Scholar]
- 2. Miura K., Kikuzaki H., Nakatani N. (2002) Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. J. Agric. Food Chem. 50, 1845–1851 [DOI] [PubMed] [Google Scholar]
- 3. Marino M., Bersani C., Comi G. (1999) Antimicrobial activity of the essential oils of Thymus vulgaris L. measured using a bioimpedometric method. J. Food Prot. 62, 1017–1023 [DOI] [PubMed] [Google Scholar]
- 4. Hazzit M., Baaliouamer A., Faleiro M. L., Miguel M. G. (2006) Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 54, 6314–6321 [DOI] [PubMed] [Google Scholar]
- 5. Stahl-Biskup E., Saez F. (ed) (2002) Thyme: the Genus Thymus, pp. 263–316, Taylor and Francis, London [Google Scholar]
- 6. Kaloustian J., Abou L., Mikail C., Amiot Carlin M. J., Portugal H. (2005) Southern French thyme oils chromatographic study of chemotypes. J. Sci. Food Agric. 85, 2437–2444 [Google Scholar]
- 7. Mahmoudi M., Morteza-Semnani K., Mojra E. (2008) Anti-inflammatory and antinociceptive activity of Thymus pubescens extract. Fitoterapia 79, 361–365 [DOI] [PubMed] [Google Scholar]
- 8. Elhabazi K., Aboufatima R., Benharref A., Zyad A., Chait A., Dalal A. (2006) Study on the antinociceptive effects of Thymus broussonetii Boiss extracts in mice and rats. J. Ethnopharmacol. 107, 406–411 [DOI] [PubMed] [Google Scholar]
- 9. Baser K. H. (2008) Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 14, 3106–3119 [DOI] [PubMed] [Google Scholar]
- 10. Hotta M., Nakata R., Katsukawa M., Hori K., Takahashi S., Inoue H. (2010) Carvacrol, a component of thyme oil, activates PPARα and -γ and suppresses COX-2 expression. J. Lipid Res. 51, 132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deval E., Noël J., Lay N., Alloui A., Diochot S., Friend V., Jodar M., Lazdunski M., Lingueglia E. (2008) ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 27, 3047–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Escoubas P., De Weille J. R., Lecoq A., Diochot S., Waldmann R., Champigny G., Moinier D., Ménez A., Lazdunski M. (2000) Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J. Biol. Chem. 275, 25116–25121 [DOI] [PubMed] [Google Scholar]
- 13. Newcomb R., Szoke B., Palma A., Wang G., Chen X., Hopkins W., Cong R., Miller J., Urge L., Tarczy-Hornoch K., Loo J. A., Dooley D. J., Nadasdi L., Tsien R. W., Lemos J., Miljanich G. (1998) Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry 37, 15353–15362 [DOI] [PubMed] [Google Scholar]
- 14. Bässler E. L., Ngo-Anh T. J., Geisler H. S., Ruppersberg J. P., Gründer S. (2001) Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J. Biol. Chem. 276, 33782–33787 [DOI] [PubMed] [Google Scholar]
- 15. Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449, 316–323 [DOI] [PubMed] [Google Scholar]
- 16. Salinas M., Lazdunski M., Lingueglia E. (2009) Structural elements for the generation of sustained currents by the acid pain sensor ASIC3. J. Biol. Chem. 284, 31851–31859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hesselager M., Timmermann D. B., Ahring P. K. (2004) pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J. Biol. Chem. 279, 11006–11015 [DOI] [PubMed] [Google Scholar]
- 18. Deval E., Gasull X., Noël J., Salinas M., Baron A., Diochot S., Lingueglia E. (2010) Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol. Ther. 128, 549–558 [DOI] [PubMed] [Google Scholar]
- 19. Andreev Y. A., Vassilevski A. A., Kozlov S. A. (2012) Molecules to selectively target receptors for treatment of pain and neurogenic inflammation. Recent Pat. Inflamm. Allergy Drug Discov. 6, 35–45 [DOI] [PubMed] [Google Scholar]
- 20. Karczewski J., Spencer R. H., Garsky V. M., Liang A., Leitl M. D., Cato M. J., Cook S. P., Kane S., Urban M. O. (2010) Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br. J. Pharmacol. 161, 950–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Voilley N., de Weille J., Mamet J., Lazdunski M. (2001) Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J. Neurosci. 21, 8026–8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuduk S. D., Chang R. K., Di Marco C. N., Dipardo R. M., Cook S. P., Cato M. J., Jovanovska A., Urban M. O., Leitl M., Spencer R. H., Kane S. A., Hartman G. D., Bilodeau M. T. (2011) Identification of non-amidine inhibitors of acid-sensing ion channel-3 (ASIC3). Bioorg. Med. Chem. Lett. 21, 4255–4258 [DOI] [PubMed] [Google Scholar]
- 23. Ramezani M., Hosseinzadeh H., Daneshmand N. (2001) Antinociceptive effect of Elaeagnus angustifolia fruit seeds in mice. Fitoterapia 72, 255–262 [DOI] [PubMed] [Google Scholar]
- 24. Das S., Das D. K. (2007) Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 6, 168–173 [DOI] [PubMed] [Google Scholar]
- 25. Andreev Y. A., Kozlov S. A., Koshelev S. G., Ivanova E. A., Monastyrnaya M. M., Kozlovskaya E. P., Grishin E. V. (2008) Analgesic compound from sea anemone Heteractis crispa is the first polypeptide inhibitor of vanilloid receptor 1 (TRPV1). J. Biol. Chem. 283, 23914–23921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grishin E. V., Savchenko G. A., Vassilevski A. A., Korolkova Y. V., Boychuk Y. A., Viatchenko-Karpinski V. Y., Nadezhdin K. D., Arseniev A. S., Pluzhnikov K. A., Kulyk V. B., Voitenko N. V., Krishtal O. O. (2010) Novel peptide from spider venom inhibits P2X3 receptors and inflammatory pain. Ann. Neurol. 67, 680–683 [DOI] [PubMed] [Google Scholar]
- 27. Cullmann F., Adam K. P., Becker H. (1993) Moss Chemistry and Biology Research Group 64. Bisbibenzylis and Lignans from Pellia-Epiphylla. Phytochemistry 34, 831–834 [Google Scholar]
- 28. Cullmann F., Becker H. (1999) Lignans from the liverwort Lepicolea ochroleuca. Phytochemistry 52, 1651–1656 [Google Scholar]
- 29. Weissberg A., Dagan S. (2011) Interpretation of ESI(+)-MS-MS spectra-Towards the identification of “unknowns.” Int. J. Mass Spec. 299, 158–168 [Google Scholar]
- 30. Morton D. B., Abbot D., Barclay R., Close B. S., Ewbank R., Gask D., Heath M., Mattic S., Poole T., Seamer J., Southee J., Thompson A., Trussel B., West C., Jennings M. (1993) Removal of blood from laboratory mammals and birds: first report of the BVA/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim. 27, 1–22 [DOI] [PubMed] [Google Scholar]
- 31. Apers S., Vlietinck A., Pieters L. (2003) Lignans and neolignans as lead compounds. Phytochemistry Rev. 2, 201–217 [Google Scholar]
- 32. Diochot S., Baron A., Rash L. D., Deval E., Escoubas P., Scarzello S., Salinas M., Lazdunski M. (2004) A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 23, 1516–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garateix A., Salceda E., Menéndez R., Regalado E. L., López O., García T., Morales R. A., Laguna A., Thomas O. P., Soto E. (2011) Antinociception produced by Thalassia testudinum extract BM-21 is mediated by the inhibition of acid-sensing ionic channels by the phenolic compound thalassiolin B. Mol. Pain 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen C. C., Zimmer A., Sun W. H., Hall J., Brownstein M. J., Zimmer A. (2002) A role for ASIC3 in the modulation of high intensity pain stimuli. Proc. Natl. Acad. Sci. U.S.A. 99, 8992–8997 [DOI] [PMC free article] [PubMed] [Google Scholar]