Background: Insulin suppresses lipolysis through direct effects on adipocytes and indirectly through its neuronal receptors by reducing sympathetic outflow to adipose tissue.
Results: Three-day voluntary overfeeding impairs the ability of brain insulin to suppress lipolysis in vivo.
Conclusion: Unrestrained lipolysis that is commonly seen in obesity and diabetes can be caused by impaired brain insulin action.
Significance: Enhancing brain insulin action potentially restores lipolytic control.
Keywords: Endocannabinoids, Glucose Metabolism, Hypothalamus, Insulin, Lipolysis, Overfeeding
Abstract
Insulin controls fatty acid (FA) release from white adipose tissue (WAT) through direct effects on adipocytes and indirectly through hypothalamic signaling by reducing sympathetic nervous system outflow to WAT. Uncontrolled FA release from WAT promotes lipotoxicity, which is characterized by inflammation and insulin resistance that leads to and worsens type 2 diabetes. Here we tested whether early diet-induced insulin resistance impairs the ability of hypothalamic insulin to regulate WAT lipolysis and thus contributes to adipose tissue dysfunction. To this end we fed male Sprague-Dawley rats a 10% lard diet (high fat diet (HFD)) for 3 consecutive days, which is known to induce systemic insulin resistance. Rats were studied by euglycemic pancreatic clamps and concomitant infusion of either insulin or vehicle into the mediobasal hypothalamus. Short term HFD feeding led to a 37% increase in caloric intake and elevated base-line free FAs and insulin levels compared with rats fed regular chow. Overfeeding did not impair insulin signaling in WAT, but it abolished the ability of mediobasal hypothalamus insulin to suppress WAT lipolysis and hepatic glucose production as assessed by glycerol and glucose flux. HFD feeding also increased hypothalamic levels of the endocannabinoid 2-arachidonoylglycerol after only 3 days. In summary, overfeeding impairs hypothalamic insulin action, which may contribute to unrestrained lipolysis seen in human obesity and type 2 diabetes.
Introduction
WAT3 plays a critical role in lipid homeostasis. It acts as the primary storage site for excess energy in the form of triglycerides and releases FAs through lipolysis during fasting or times of high energy demand. The dynamic switching between energy storage and release according to metabolic needs is critical for lipid homeostasis. WAT dysfunction is characterized by impaired lipid deposition and unrestrained lipolysis as is seen in lipodystrophy (1) and obesity and diabetes (2–5). WAT dysfunction results in increased FA flux in the nonfasted state, which leads to lipotoxicity, i.e., ectopic lipid accumulation in nonadipose tissue organs that has been linked to insulin resistance in muscle and liver (6–10). Insulin is the key signal in the switch from a FA-releasing to a FA-storing mode of WAT during the fasting to fed transition by inhibiting lipolysis (11) and inducing de novo lipogenesis (12). In states of high energy demand, such as fasting or exercise, insulin levels are low, and increased sympathetic nervous system (SNS) outflow induces lipolysis in WAT. The antilipolytic and prolipogenic effects of insulin are commonly attributed to the direct effects of insulin on adipocytes. We have recently demonstrated that in addition to its direct effects, insulin suppresses lipolysis and increases de novo lipogenesis in WAT also indirectly by reducing SNS outflow to WAT through hypothalamic insulin signaling (13).
The antilipolytic properties of insulin are important in regulating hepatic glucose production (GP), because lipolysis provides the gluconeogenic precursor glycerol and energy substrates in the form of FAs to the liver (14, 15). Thus, unrestrained lipolysis increases gluconeogenesis and thereby hepatic glucose output. Because impaired hypothalamic insulin action can unrestrain WAT lipolysis (13), the increased FA flux could in turn contribute to a failure of insulin to suppress hepatic GP.
Short term voluntary overfeeding in rats and humans impairs systemic insulin action (16–19). This early insulin resistance in terms of carbohydrate metabolism seems to be exclusively attributable to hepatic insulin resistance, whereas peripheral insulin action, i.e., glucose utilization in muscle and WAT as assessed through glucose disposal during a clamp, is not impaired (16–19). Because increased lipolytic flux from WAT to the liver can drive gluconeogenesis, we asked whether short term overfeeding alters WAT and hepatic insulin action and whether this is associated with impaired hypothalamic insulin action.
EXPERIMENTAL PROCEDURES
Animals
Experiments were performed in 10-week-old, standard chow-fed (Rodent Diet 5001; LabDiet, St. Louis, MO), male Sprague-Dawley (SD) rats (Charles River Breeding Laboratories, Wilmington, MA), which were housed in a temperature- and light-controlled facility in separate cages. Prior to the clamp studies, we stereotaxically fit rats with indwelling cannulae targeting the mediobasal hypothalamus (MBH) as previously described (13). We routinely injected food dye to verify placement of the cannulae. After a 1-week recovery period, we implanted carotid and jugular catheters for blood sampling and infusion during the pancreatic clamp studies. We allowed rats to recover for an additional 4–6 days and required them to return to within 10% of their presurgery body weight. After recovery we assigned rats to a HFD (Modified Lab w/10% Lard 57IR; LabDiet) or RC (Rodent Diet 5001; LabDiet) ad libitum for 3 consecutive days preceding the clamp studies. According to the manufacturer, the ingredients of the HFD are 90% Rodent Diet 5001 supplemented with 10% lard (w/w). Daily food intake was monitored by manually weighing the food pellets. All of the animal protocols were approved by the Institutional Animal Care and Use Committee of the Mount Sinai School of Medicine.
Pancreatic Clamp Studies
Rat clamp experiments and [3-3H]glucose and [2H-5]glycerol tracer studies were performed in conscious nonrestrained rats as previously described (13). The rats were either fed RC or HFD for three consecutive days prior to the basal 1 milliunit·kg−1·min−1 pancreatic clamp studies. During the 6-h study protocol (see Fig. 1A), we either infused artificial cerebrospinal fluid (aCSF, vehicle) (Harvard Apparatus, Holliston, MA) or human insulin (2 microunits; Humulin R diluted in aCSF (Lilly, Indianapolis IN)) at a rate of 0.18 μl/h per side for 6 h into the MBH. For the RC MBH insulin-infused group, we used controls from studies described in Ref. 13.
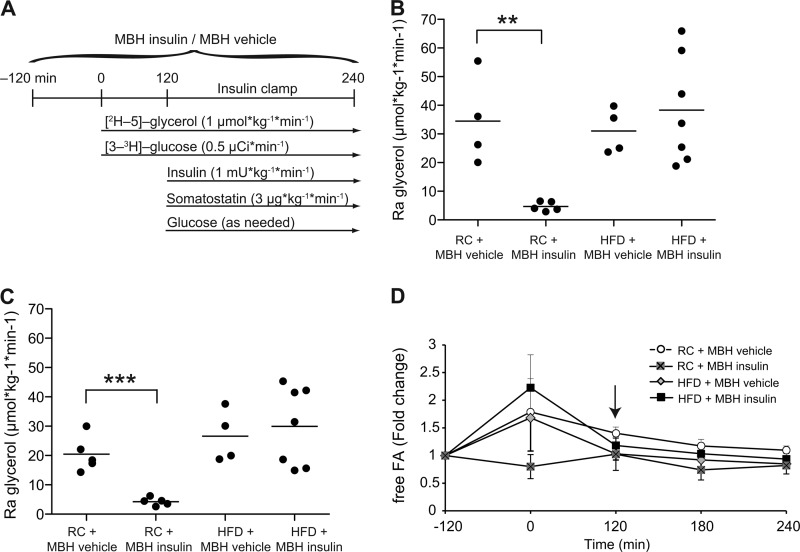
FIGURE 1.
Brain insulin fails to suppress lipolysis after 3-day HFD feeding. A, experimental protocol. B and C, Ra glycerol at base line (B) and the 1 milliunit·kg−1·min−1 clamp (C) (n ≥ 4/group). D, fold change of plasma free FA levels from base line. Pancreatic clamp was started at the 120-min time point (indicated by the arrow; n ≥ 5/group). All error bars represent S.E. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 versus RC MBH vehicle + 1 milliunit·kg−1·min−1 clamp group.
WAT Insulin Signaling Studies
Conscious male rats, which were either fed a RC diet or a HFD for 3 consecutive days, were infused with either vehicle (aCSF) or insulin (2 microunits) directly into the MBH for 6 h. In this series the animals were not clamped. 15 min before the end of the study, the animals were injected with an intraperitoneal bolus of insulin (1 unit/kg of bodyweight in 5% glucose) or saline control, after which the animals were anesthetized and killed. Epidydimal fat pads were harvested, snap frozen in liquid nitrogen, and later analyzed by Western blot.
Rimonabant Studies
Rats overfed for 3 days were subjected to 3 milliunits·kg−1·min−1 hyperinsulinemic clamp studies, during which [2H-5]glycerol tracer and either rimonabant (0.2 μg/μl dissolved in aCSF, 0.35% Me2SO, and 0.02% Tween 80) or vehicle (aCSF plus 0.35% Me2SO and 0.02% Tween 80) was infused directly into the third ventricle (intracerebroventricularly) as described in Ref. 20. Supportive data including confirmation of glucose control, insulin levels, and glucose fluxes, as well as a detailed clamp protocol, are published elsewhere (20).
MBH Endocannabinoid Extraction
Endocannabinoid levels in MBH samples were analyzed after a Folch extraction as described in Ref. 21.
Analytic Procedures
Blood glucose levels during the clamp were measured with an Analox GM9D enzymatic oxygen rate glucose analyzer (Analox Instruments, Lunenburg, MA). We analyzed glucose fluxes and Ra glycerol as described elsewhere (13, 22–25). We measured plasma free glycerol and triglycerides with a colorimetric assay from Sigma-Aldrich and plasma nonesterified FA with a kit from Wako Chemicals (Richmond, VA). Rat plasma insulin and adiponectin were analyzed by radioimmunoassay at the hormone assay core of Albert Einstein Medical College (New York, NY). Rat plasma glucagon and leptin levels were measured with Luminex MultiAnalyte Profiling (Millipore, Billerica, MA).
Western Blot Analyses
of WAT samples were performed as previously described (13, 26). Two different lysis buffers (pH 7.4) were used: 1) 20 mm MOPS, 2 mm EGTA, 5 mm EDTA, 30 mm sodium fluoride, 40 mm β-glycerophosphate, 10 mm sodium pyrophosphate, 2 mm sodium orthovanadate, 0.5% Nonidet P-40, and complete protease inhibitor mixture (Roche Applied Sciences) for Fig. 2 (A and B) and 2) 50 mm Tris, 150 mm NaCl (Cellgro, Manassas, VA), 1.25% CHAPS (Affymetrix, Cleveland, OH), 1 mm EDTA, 1 mm sodium orthovanadate, 2 mm sodium fluoride, 10 mm sodium pyrophosphate, 8 mm β-glycerophosphate (Sigma-Aldrich), and a Complete Protease Inhibitor Mixture (Roche Applied Sciences) for Fig. 2 (C and D). This latter extraction seems to result in better extraction of large proteins such as fatty acid synthase in WAT, which was further enhanced by a 45-min solubilization step where homogenates were shaken at 4 °C before centrifugation. Primary antibodies against phospho-Akt 473 and 308, phospho-Hsl (Ser-563, Ser-565, and Ser-660) (all from Cell Signaling Technology, Beverly, MA), and β-actin (Abcam, Cambridge, MA) were used. The membranes were scanned with the LI-COR Odyssey infrared scanner (LI-COR, Lincoln, NE), and band intensity was quantified with Odyssey 3.0 software on the basis of direct fluorescence measurement.
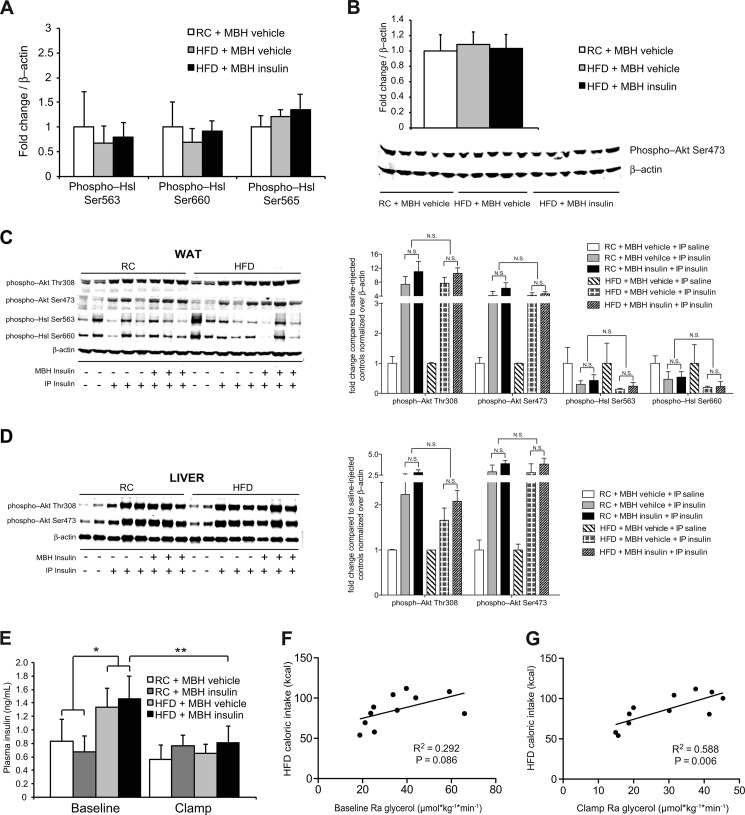
FIGURE 2.
Brain insulin fails to suppress Hsl activation after 3-day HFD feeding. A and B, Western blot analyses and quantification of epidydimal fat pads harvested after euglycemic pancreatic clamps (n ≥ 5/group). A, quantification of WAT Hsl phosphorylation. B, Western blot and quantification of WAT Akt signaling. C and D, WAT (C) and liver (D) insulin signaling as assessed by Western blot analysis. Epidydimal fat and liver samples were harvested 15 min after an intraperitoneal (IP) bolus of insulin or saline. Before the intraperitoneal injection of insulin, RC- and HFD-fed rats were infused with MBH insulin or vehicle for 6 h (n = 3/group; n = 2/group for saline injected controls). N.S., not statistically significant, as indicated by brackets. E, insulin levels at base line and during the clamp (n ≥ 4/group). F and G, correlation of caloric intake on HFD and Ra glycerol at base line (F) and during the clamp period (G) (both n = 11). All error bars represent S.E.
Statistics
All of the data are represented as the means ± S.E. Comparisons among groups were made using one-way analysis of variance followed by unpaired two-tailed Student's t tests. Repeated measures within the same group were compared by paired two-tailed Student's t tests. Differences were considered statistically significant at p < 0.05. For Figs. 2 (F and G) and 4A, we used Pearson correlation and a two-tailed t test performed in Graphpad Prism 5.0b for Mac (GraphPad Software, San Diego, CA).
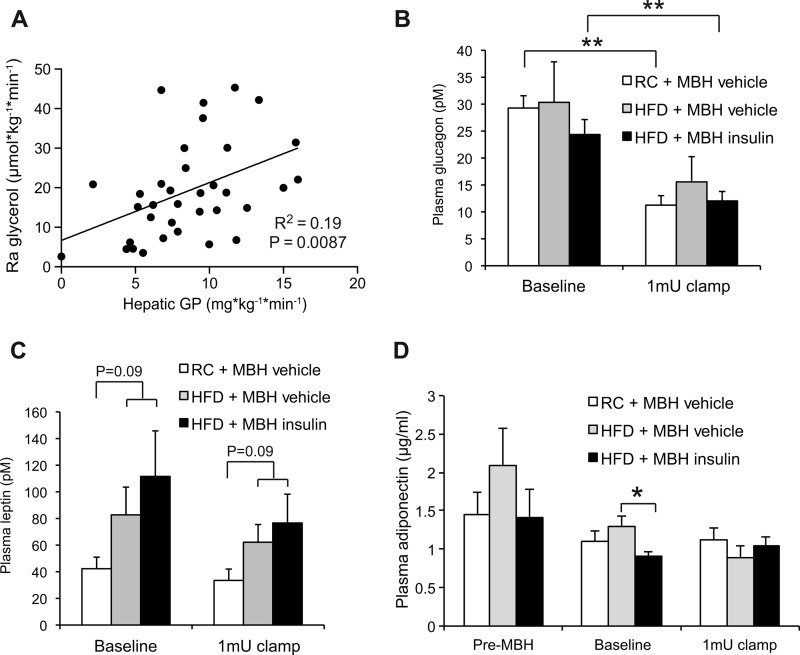
FIGURE 4.
Lipolytic flux correlates with the degree of hepatic GP. A, correlation of Ra glycerol and hepatic GP during clamps of HFD- and RC-fed rats infused with either MBH insulin or vehicle (n = 34). B–D, plasma glucagon (B), leptin (C), and adiponectin (D) levels during the base line and clamp periods. Adiponectin levels were also measured prior to MBH insulin or vehicle infusion (pre-MBH) (n ≥ 3/group). All error bars represent S.E. *, p < 0.05; and **, p < 0.01 as indicated.
RESULTS
Short Term Overfeeding Obliterates the Ability of MBH Insulin to Suppress WAT Lipolysis
To test whether short term overfeeding impairs the ability of MBH insulin to suppress lipolysis, we fed male SD rats either a highly palatable HFD (w/10% lard) or RC for 3 consecutive days. Rats fed a HFD increased their daily caloric intake by ∼30% (61 versus 83 kcal/day) compared with controls. Body weight, base-line plasma triglycerides, and free glycerol levels were not different between treatment groups because of the short duration of overfeeding (Table 1). Previous studies have shown that a similar increase in caloric intake impairs hepatic insulin action (16, 17, 27) and increases insulin levels in this model (16). Although in our studies insulin levels tended to be higher, this failed to reach statistical significance (Table 1). The fact that FA levels are not lower in the HFD group despite higher insulin levels indicates impaired WAT insulin action, which is an important cause of WAT dysfunction. To assess hypothalamic insulin action in this model, we performed basal 1 milliunits·kg−1·min−1 euglycemic clamp studies and simultaneously assessed lipolytic flux using a [2H-5]glycerol tracer, while infusing insulin or vehicle directly into the MBH (protocol outlined in Fig. 1A). In RC-fed rats, MBH insulin markedly suppressed lipolytic flux as assessed by the rate of appearance (Ra) of glycerol at base line and while using a euglycemic clamp (Fig. 1, B and C). However, voluntary overfeeding almost completely abolished the ability of MBH insulin to suppress WAT lipolysis (Fig. 1, B and C). This inability of MBH insulin to suppress lipolysis was also reflected in the lack of suppression of free FA levels during the MBH insulin infusions (Fig. 1D). We have previously shown that MBH insulin decreases the activation state of WAT hormone-sensitive lipase (Hsl) by suppressing phosphorylation on serine 563 and 660 as assessed by Western blot (13). After 3 days of overfeeding, phosphorylation of Hsl in WAT was unchanged in epidydimal WAT harvested at the end of the clamp (Fig. 2A). However, despite the impaired ability of brain insulin to suppress WAT lipolysis, insulin signaling in WAT was not decreased (Fig. 2B). This is in agreement with our previous studies where under RC conditions central insulin infusion did not affect peripheral insulin signaling in the adipose tissue (13). One can argue that insulin signaling is not acutely induced at the end of a euglycemic basal insulin clamp, and therefore one may miss subtle differences of insulin signaling. We therefore acutely stimulated insulin signaling after a 6-h MBH infusion in an additional cohort of rats. Notably, we observed no differences in WAT (Fig. 2C) and liver (Fig. 2D) insulin signaling between RC and overfed rats as suggested by equal stimulability of Akt and suppressibility of Hsl phosphorylation. This indicates that insulin signaling in peripheral organs such as WAT and liver is still intact after 3 days of overfeeding and that hypothalamic insulin signaling does not alter peripheral insulin signaling, which is in agreement with our prior studies and those of others (13, 28).
TABLE 1.
General characteristics of experimental groups
Blood was collected in the morning after ad libitum feeding (n ≥ 5/group). All of the values are represented as the means ± S.E.
| RC | HFD | |
|---|---|---|
| Bodyweight (g) | 278 ± 8 | 298 ± 14 |
| Daily caloric intake (kcal) | 61 ± 3 | 83 ± 5a |
| Plasma glucose (mg/dl) | 142 ± 2 | 144 ± 4 |
| Plasma insulin (ng/ml) | 0.8 ± 0.23 | 1.4 ± 0.21 |
| Plasma free FAs (mm) | 0.39 ± 0.01 | 0.57 ± 0.04a |
| Plasma free glycerol (μg/μl) | 0.014 ± 0.002 | 0.016 ± 0.001 |
| Plasma triglycerides (μg/μl) | 0.17 ± 0.03 | 0.21 ± 0.02 |
a p < 0.05 versus RC.
Thus, the effects of central insulin on WAT metabolism can occur independently of alterations of WAT insulin signaling, and thus WAT insulin action can be compromised in the absence of a peripheral insulin signaling defect. Protein expression of Atgl (adipose tissue triglyceride lipase) and ABHD5 (α/β hydrolase domain containing protein 5; also known as CGI-58, for comparative gene identification-58), an activator of Atgl, was not different between groups (data not shown), although this does not rule out the possibility that Atgl activity was altered by MBH insulin.
Furthermore, overfeeding resulted in elevated base-line free FA levels (Table 1), despite comparable lipolytic rates. Plasma free FAs are only an indirect measure of lipolysis, because free FA levels are also a function of differences in FA utilization and uptake. Because it has been shown in mice that brain insulin is also able to increase FA uptake into WAT (29), our data therefore suggest that free FA utilization and/or uptake may be impaired after short term overfeeding as has been demonstrated for obese humans and mice (29, 30). Insulin levels in the HFD-fed rats were moderately increased during the base-line period, consistent with systemic insulin resistance (Figs. 2E). Notably, at base-line and during the clamp period (during which insulin levels were controlled and not different between groups), the Ra glycerol positively correlated with the caloric intake in the HFD-fed rats (Fig. 2, F and G). Thus, short term overfeeding is associated with higher rates of lipolysis in this animal model.
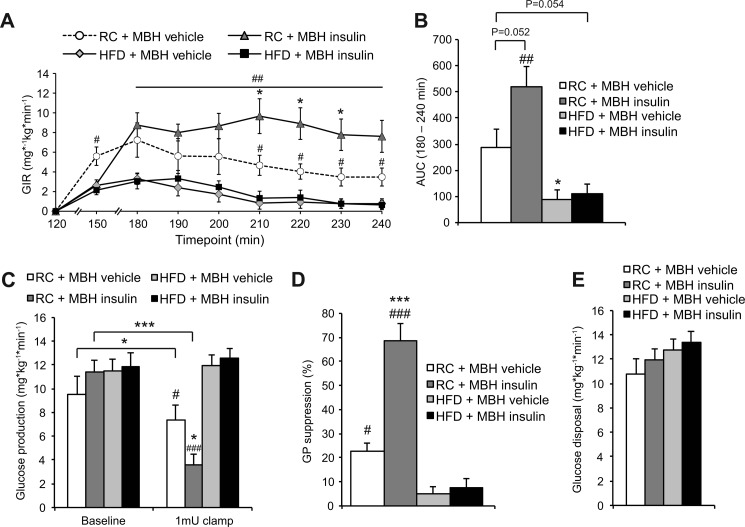
Overfeeding Abolished the Ability of MBH Insulin to Suppress Hepatic GP
Hypothalamic insulin signaling, at least in rodents, is essential for insulin-mediated suppression of hepatic GP (22, 23, 31). Short term overfeeding is sufficient to induce hepatic insulin resistance (16, 17, 32). Thus, we tested whether the ability of MBH insulin to suppress hepatic GP is impaired in this model of voluntary overfeeding. During a basal 1 milliunit·kg−1·min−1 clamp, the glucose infusion rate required to prevent hypoglycemia was lower in both HFD groups compared with MBH vehicle-infused controls, whereas the MBH insulin-infused rats required markedly higher glucose infusion rates to maintain euglycemia (Fig. 3, A and B). Under RC conditions, MBH insulin infusion suppressed hepatic GP, whereas short term overfeeding abolished the ability of MBH insulin to suppress hepatic GP (Fig. 3, C and D). Peripheral glucose utilization was not impaired in the HFD group (Fig. 3E), which is consistent with prior reports (16, 27). In our previous MBH insulin infusion studies, performed in RC-fed rats, lipolytic flux correlated with hepatic GP (13), supporting the notion that lipolytic flux is a driver of hepatic glucose production. After combining these data with our short term overfeeding studies, this correlation persisted (Fig. 4A). Unrestrained lipolytic flux from WAT could therefore account for the inability of MBH insulin to suppress hepatic GP in this model. Glucagon levels were equally suppressed during the clamp and not different between treatments (Fig. 4B). Short term overfeeding causes moderate hyperleptinemia in the 3-day overfed SD rat (33), and although leptin levels tended to be higher in our study, this did not reach statistical significance. Plasma leptin levels were unaffected by the clamp and the central insulin infusions (Fig. 4C) consistent with prior studies (13, 33). Adiponectin levels were decreased by MBH insulin infusion during the base-line period (Fig. 4D). However, because adiponectin levels usually do not change during central infusions (13, 34, 35) and the HFD vehicle group trended toward increased adiponectin levels even before the MBH insulin infusion was started, this finding is of unclear physiological relevance because we have not observed this in prior studies.
FIGURE 3.
Short term overfeeding impairs the ability of MBH insulin to suppress hepatic GP. A, glucose infusion rate (GIR) required to maintain euglycemia (n ≥ 5/group). B, area under the curve (AUC) of graph in A (n ≥ 5/group). C, GP at base line and during the 1 milliunit·kg−1·min−1 clamp period (n ≥ 4/group). D, GP suppression (n ≥ 4/group). E, glucose disposal during the clamp period (n ≥ 4/group). All error bars represent S.E. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 versus RC MBH vehicle + 1 milliunit·kg−1·min−1 clamp group unless otherwise indicated. #, p < 0.05; ##, p < 0.01; and ###, p < 0.001 versus HFD groups.
Overfeeding Increases Endocannabinoid Levels in the MBH
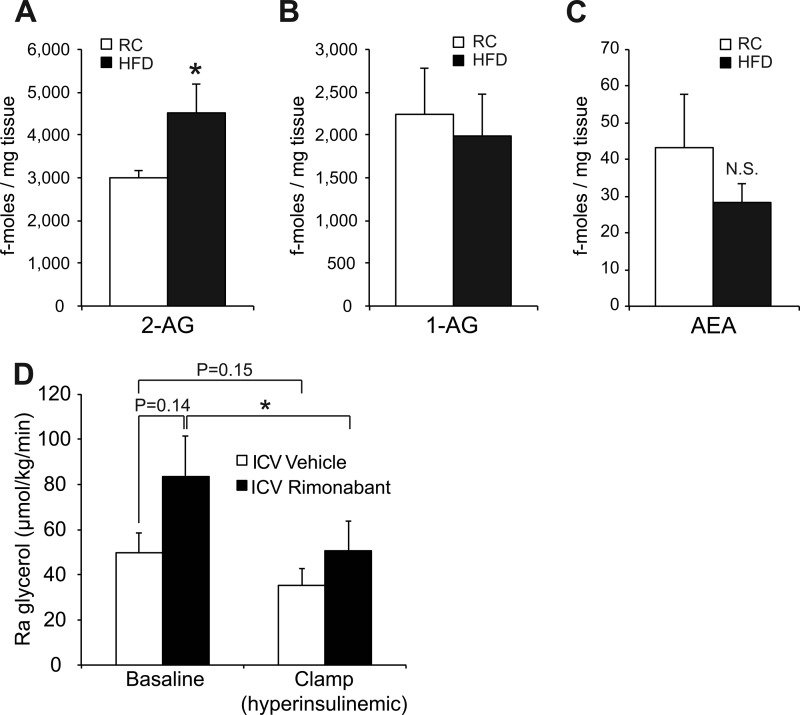
We have recently shown that endocannabinoid (EC) receptor agonists administered directly into the third ventricle of SD rats can induce hepatic insulin resistance, and the degree of hepatic insulin resistance is similar to that seen in the 3-day HFD model. Increased brain EC signaling also impaired the ability of systemic insulin to suppress lipolysis (20). Furthermore, it is known that long term overfeeding and genetic obesity increase hypothalamic EC tone (36), but it is unknown whether 3-day overfeeding is sufficient to increase hypothalamic EC levels in rats. Indeed, in the present study 3-day overfeeding increased hypothalamic 2-AG (Fig. 5A) to a degree comparable with that described in Zucker rats (36), a genetic model of obesity, whereas 1-AG and anandamide levels were not significantly changed (Fig. 5, B and C). Intracerebroventricular infusion of the EC receptor antagonist rimonabant reinstalls the ability of systemic insulin to suppress hepatic GP (20). Thus, we next tested whether brain infusion of rimonabant acutely improves the antilipolytic effects of systemic insulin by analyzing glycerol flux in the exact same 3-day overfed male rats from our prior studies (20). In fact, when rimonabant was infused intracerebroventricularly in overfed rats, the ability of hyperinsulinemia to suppress lipolysis was improved compared with vehicle-infused control rats (Fig. 5D). This improvement in insulin action occurred despite a trend of intracerebroventricular rimonabant administration to acutely increase lipolytic flux, although this failed to reach statistical significance. The increase in lipolytic flux is probably due to the fact that rimonabant has been shown to acutely stimulate the sympathoadrenal system (37). Thus, overfeeding rapidly increases hypothalamic 2-AG levels, which in part accounts for the impaired hypothalamic insulin action, a very early mechanism through which excessive caloric intake impairs systemic insulin action (20).
FIGURE 5.
HFD feeding increases 2-AG levels in rat hypothalami after only 3 days. A–C, measurements of key endocannabinoids in MBH tissue samples (n ≥ 7/group). D, Ra glycerol flux of 3-day HFD-fed male rats, which were intracerebroventricularly (ICV) infused with either rimonabant or vehicle for 6 h. Ra glycerol was measured at base line and after induction of a 3 milliunits·kg−1·min−1 hyperinsulinemic clamp. All error bars represent S.E. *, p < 0.05 versus RC-fed group. N.S., not statistically significant.
DISCUSSION
Hypothalamic insulin action regulates WAT lipolysis in addition to and in concert with hepatic glucose production (13). Here we show that only 3 days of overfeeding in SD rats is sufficient to severely impair the ability of hypothalamic insulin to suppress WAT lipolysis. We chose this established short term overfeeding protocol in rats, because short term overfeeding in humans produces comparable insulin resistance. Thus, our protocol mirrors common forms of human insulin resistance (16–19, 32). Furthermore, there is emerging evidence that obesity in humans is associated with functional brain insulin resistance (38). Therefore, we speculate that unrestrained lipolysis, as is seen in early forms of human insulin resistance (39), may in part be explained by impaired hypothalamic insulin action.
The finding that the antilipolytic effects of brain insulin are lost, even though insulin signaling in WAT is not altered, might appear surprising. However, there are now several studies consistent with the concept that brain insulin signaling does not acutely alter peripheral insulin signaling, despite the fact that central insulin does regulate insulin action in WAT and liver (13, 28). Thus, hypothalamic insulin uses signaling pathways other than insulin signaling in target organs such as WAT and liver to regulate insulin action, i.e., lipolysis and hepatic glucose production. In the case of WAT, our previous studies demonstrated that central insulin suppresses sympathetic outflow to WAT, resulting in altered cAMP signaling (13), the principal signaling pathway regulating lipolysis (40). In the liver, the signaling pathway mediating brain insulin action is less clear, although it seems to require parasympathetic but not muscarinic input (41). Therefore, the discrepancy between insulin action and insulin signaling in peripheral tissues such as WAT and liver is not a real paradox but reflects the complex physiology of insulin action that regulates nutrient fluxes in part through controlling organ cross-talk and/or through regulating autonomic nervous system activity in addition to signaling in target organs such as WAT. It follows that in the early state of overfeeding-induced insulin resistance, insulin signaling can still be intact in target organs but, because of impaired brain insulin action, results in impaired insulin action in WAT and liver as demonstrated in these studies.
Because lipolytic flux from WAT drives hepatic gluconeogenesis and therefore hepatic GP, one implication of our findings is that the lack of antilipolytic effects of brain insulin after overfeeding contributes to hepatic insulin resistance (18, 19). Thus, our studies suggest that one of the mechanism through which the brain controls hepatic GP is regulation of WAT lipolysis, which highlights the potential importance of organ cross-talk in the central regulation of nutrient partitioning.
Obese individuals have increased circulating 2-AG levels that correlate with visceral adiposity and therefore the degree of insulin resistance (42, 43). EC tone is increased within the CNS of obese or leptin-resistant or -deficient rodents (36). In our short term overfeeding model, we detected a 30% increase in 2-AG levels after only 3 days. It is surprising that the relative increase in hypothalamic 2-AG levels is comparable with that seen in leptin-resistant obese Zucker rats, whose metabolic phenotype is severe and chronic (36). Consistent with this prior report performed in Zucker rats, we could not detect a significant change in hypothalamic anandamide levels (36). Hypothalamic ECs could impair brain insulin action through several mechanisms. First, ECs can inhibit insulin signaling by directly interfering with the insulin receptor and its downstream signaling (44). Second, a recent report suggested that 2-AG may serve as a precursor for neuroinflammatory prostaglandins (45). Because inflammatory processes within the hypothalamus have been linked to impaired metabolic control and energy imbalance, the increased 2-AG pool may disrupt brain control of lipid metabolism by promoting neuroinflammation (46, 47). Third, 2-AG can act as a retrograde inhibitor of synaptic transmission within the CNS (48) and thereby block the propagation of insulin-induced activation of neuronal pathways. Studies are underway to examine these intracellular pathways that link increased EC tone with impaired hypothalamic insulin action.
EC receptors are highly abundant throughout the CNS. The effects of central ECs on SNS outflow are rather complex and may be organ-specific. Although injection of EC agonists, for example, in the posterior periaqueductal gray or intracisternally can activate renal SNS activity and increase blood pressure (49, 50), EC activation in the nucleus tractus solitarius decreased renal SNS outflow (51). From our previous studies, it appears that the increased hypothalamic EC tone in the overfed state seems to antagonize the ability of MBH insulin to dampen SNS outflow to WAT and thereby unrestrain lipolysis. Furthermore, systemic leptin injections into rodents that are leptin-sensitive reduce their hypothalamic EC levels (36).
Of note, central leptin administration to 3-day overfed rats can restore hepatic insulin action (33). Whether central leptin infusion reduces EC tone in the MBH of these animals remains to be determined, but antagonizing EC signaling in overfed rats has similar effects and restores hepatic insulin sensitivity (20) and the ability of insulin to suppress lipolytic flux during a hyperinsulinemic clamp (Fig. 5D).
Overfeeding has also been shown to impair nutrient sensing in the brain. FAs fail to suppress hepatic glucose production when infused centrally (52, 53). Whether nutrient sensing also participates in the hypothalamic control of WAT lipolysis remains to be determined.
Taken together, our results demonstrate that short term overfeeding abolishes the ability of hypothalamic insulin to suppress WAT lipolytic flux, which coincides with an increase in hypothalamic EC tone. Therefore, the loss of brain insulin action could be an underlying cause for the unrestrained lipolysis that is seen early on in the development of diabetes mellitus type 2. Restoration or enhancement of brain insulin action may therefore be able to ameliorate lipotoxicity in obesity and type 2 diabetes.
Acknowledgments
We thank Rui Chang for excellent technical assistance, Michelle Puchowicz at Case Western University Mouse Metabolic Phenotyping Center for the glycerol mass spectrometry analyses, George Kunos and Judith Harvey-White for the EC determinations, and Lakshmi Devi and Ivone Gomes for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants DK074873, DK083568, and DK082724. This work was also supported by an ADA basic research award (to C. B.) and a European Foundation for the Study of Diabetes grant (to T. S.).
- WAT
- white adipose tissue
- aCSF
- artificial cerebrospinal fluid
- AG
- arachidonoylglycerol
- EC
- endocannabinoid
- GP
- glucose production
- HFD
- high fat diet
- Hsl
- hormone-sensitive lipase
- MBH
- mediobasal hypothalamus
- FA
- fatty acid
- Ra
- rate of appearance
- RC
- regular chow
- SD
- Sprague Dawley
- SNS
- sympathetic nervous system.
REFERENCES
- 1. Garg A. (2004) Acquired and inherited lipodystrophies. N. Engl. J. Med. 350, 1220–1234 [DOI] [PubMed] [Google Scholar]
- 2. Mittendorfer B., Magkos F., Fabbrini E., Mohammed B. S., Klein S. (2009) Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity 17, 1872–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Groop L. C., Saloranta C., Shank M., Bonadonna R. C., Ferrannini E., DeFronzo R. A. (1991) The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 72, 96–107 [DOI] [PubMed] [Google Scholar]
- 4. Roust L. R., Jensen M. D. (1993) Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes 42, 1567–1573 [DOI] [PubMed] [Google Scholar]
- 5. Groop L. C., Bonadonna R. C., DelPrato S., Ratheiser K., Zyck K., Ferrannini E., DeFronzo R. A. (1989) Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Invest. 84, 205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boden G. (2006) Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr. Diab Rep. 6, 177–181 [DOI] [PubMed] [Google Scholar]
- 7. Boden G., Chen X., Ruiz J., White J. V., Rossetti L. (1994) Mechanisms of fatty acid-induced inhibition of glucose uptake. J. Clin. Invest. 93, 2438–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roden M., Price T. B., Perseghin G., Petersen K. F., Rothman D. L., Cline G. W., Shulman G. I. (1996) Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Invest. 97, 2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. (1983) Effect of fatty acids on glucose production and utilization in man. J. Clin. Invest. 72, 1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boden G., Cheung P., Stein T. P., Kresge K., Mozzoli M. (2002) FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am. J. Physiol. Endocrinol. Metab. 283, E12–E19 [DOI] [PubMed] [Google Scholar]
- 11. Degerman E., Landström T. R., Holst L. S., Göransson O., Härndahl L., Ahmad F., Choi Y.-H., Masciarelli S., Liu H., Manganiello V. (2003) Role for phosphodiesterase 3B in regulation of lipolysis and insulin secretion, in Diabetes Mellitus: A Fundamental and Clinical Text (LeRoith D., Olefsky J. M., Taylor S. I., eds) 3rd Ed., pp. 374–381, Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 12. Assimacopoulos-Jeannet F., Brichard S., Rencurel F., Cusin I., Jeanrenaud B. (1995) In vivo effects of hyperinsulinemia on lipogenic enzymes and glucose transporter expression in rat liver and adipose tissues. Metabolism 44, 228–233 [DOI] [PubMed] [Google Scholar]
- 13. Scherer T., O'Hare J., Diggs-Andrews K., Schweiger M., Cheng B., Lindtner C., Zielinski E., Vempati P., Su K., Dighe S., Milsom T., Puchowicz M., Scheja L., Zechner R., Fisher S. J., Previs S. F., Buettner C. (2011) Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 13, 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rebrin K., Steil G. M., Mittelman S. D., Bergman R. N. (1996) Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J. Clin. Invest. 98, 741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rebrin K., Steil G. M., Getty L., Bergman R. N. (1995) Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes 44, 1038–1045 [DOI] [PubMed] [Google Scholar]
- 16. Wang J., Obici S., Morgan K., Barzilai N., Feng Z., Rossetti L. (2001) Overfeeding rapidly induces leptin and insulin resistance. Diabetes 50, 2786–2791 [DOI] [PubMed] [Google Scholar]
- 17. Kraegen E. W., Clark P. W., Jenkins A. B., Daley E. A., Chisholm D. J., Storlien L. H. (1991) Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 40, 1397–1403 [DOI] [PubMed] [Google Scholar]
- 18. Cornier M. A., Bergman B. C., Bessesen D. H. (2006) The effects of short-term overfeeding on insulin action in lean and reduced-obese individuals. Metabolism 55, 1207–1214 [DOI] [PubMed] [Google Scholar]
- 19. Brøns C., Jensen C. B., Storgaard H., Hiscock N. J., White A., Appel J. S., Jacobsen S., Nilsson E., Larsen C. M., Astrup A., Quistorff B., Vaag A. (2009) Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J. Physiol. 587, 2387–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Hare J. D., Zielinski E., Cheng B., Scherer T., Buettner C. (2011) Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes 60, 1055–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang L., Liu J., Harvey-White J., Zimmer A., Kunos G. (2003) Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc. Natl. Acad. Sci. U.S.A. 100, 1393–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Obici S., Zhang B. B., Karkanias G., Rossetti L. (2002) Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 8, 1376–1382 [DOI] [PubMed] [Google Scholar]
- 23. Pocai A., Lam T. K., Gutierrez-Juarez R., Obici S., Schwartz G. J., Bryan J., Aguilar-Bryan L., Rossetti L. (2005) Hypothalamic K(ATP) channels control hepatic glucose production. Nature 434, 1026–1031 [DOI] [PubMed] [Google Scholar]
- 24. Liu L., Karkanias G. B., Morales J. C., Hawkins M., Barzilai N., Wang J., Rossetti L. (1998) Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J. Biol. Chem. 273, 31160–31167 [DOI] [PubMed] [Google Scholar]
- 25. Kang L., Chen X., Sebastian B. M., Pratt B. T., Bederman I. R., Alexander J. C., Previs S. F., Nagy L. E. (2007) Chronic ethanol and triglyceride turnover in white adipose tissue in rats. Inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J. Biol. Chem. 282, 28465–28473 [DOI] [PubMed] [Google Scholar]
- 26. Buettner C., Muse E. D., Cheng A., Chen L., Scherer T., Pocai A., Su K., Cheng B., Li X., Harvey-White J., Schwartz G. J., Kunos G., Rossetti L. (2008) Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat. Med. 14, 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buettner C., Pocai A., Muse E. D., Etgen A. M., Myers M. G., Jr., Rossetti L. (2006) Critical role of STAT3 in leptin's metabolic actions. Cell Metab. 4, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramnanan C. J., Saraswathi V., Smith M. S., Donahue E. P., Farmer B., Farmer T. D., Neal D., Williams P. E., Lautz M., Mari A., Cherrington A. D., Edgerton D. S. (2011) Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J. Clin. Investig. 121, 3713–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coomans C. P., Geerling J. J., Guigas B., van den Hoek A. M., Parlevliet E. T., Ouwens D. M., Pijl H., Voshol P. J., Rensen P. C., Havekes L. M., Romijn J. A. (2011) Circulating insulin stimulates fatty acid retention in white adipose tissue via KATP channel activation in the central nervous system only in insulin-sensitive mice. J. Lipid Res. 52, 1712–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McQuaid S. E., Hodson L., Neville M. J., Dennis A. L., Cheeseman J., Humphreys S. M., Ruge T., Gilbert M., Fielding B. A., Frayn K. N., Karpe F. (2011) Downregulation of adipose tissue fatty acid trafficking in obesity. A driver for ectopic fat deposition? Diabetes 60, 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Könner A. C., Janoschek R., Plum L., Jordan S. D., Rother E., Ma X., Xu C., Enriori P., Hampel B., Barsh G. S., Kahn C. R., Cowley M. A., Ashcroft F. M., Brüning J. C. (2007) Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 5, 438–449 [DOI] [PubMed] [Google Scholar]
- 32. Samuel V. T., Liu Z. X., Qu X., Elder B. D., Bilz S., Befroy D., Romanelli A. J., Shulman G. I. (2004) Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279, 32345–32353 [DOI] [PubMed] [Google Scholar]
- 33. Pocai A., Morgan K., Buettner C., Gutierrez-Juarez R., Obici S., Rossetti L. (2005) Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54, 3182–3189 [DOI] [PubMed] [Google Scholar]
- 34. Gutiérrez-Juárez R., Obici S., Rossetti L. (2004) Melanocortin-independent effects of leptin on hepatic glucose fluxes. J. Biol. Chem. 279, 49704–49715 [DOI] [PubMed] [Google Scholar]
- 35. Lam T. K., Pocai A., Gutierrez-Juarez R., Obici S., Bryan J., Aguilar-Bryan L., Schwartz G. J., Rossetti L. (2005) Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat. Med. 11, 320–327 [DOI] [PubMed] [Google Scholar]
- 36. Di Marzo V., Goparaju S. K., Wang L., Liu J., Bátkai S., Járai Z., Fezza F., Miura G. I., Palmiter R. D., Sugiura T., Kunos G. (2001) Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410, 822–825 [DOI] [PubMed] [Google Scholar]
- 37. Mølhøj S., Hansen H. S., Schweiger M., Zimmermann R., Johansen T., Malmlöf K. (2010) Effect of the cannabinoid receptor-1 antagonist rimonabant on lipolysis in rats. Eur. J. Pharmacol. 646, 38–45 [DOI] [PubMed] [Google Scholar]
- 38. Hallschmid M., Benedict C., Schultes B., Born J., Kern W. (2008) Towards the therapeutic use of intranasal neuropeptide administration in metabolic and cognitive disorders. Int. J. Obes. (Lond.) 32, 275–28217848936 [Google Scholar]
- 39. Paolisso G., Tataranni P. A., Foley J. E., Bogardus C., Howard B. V., Ravussin E. (1995) A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia 38, 1213–1217 [DOI] [PubMed] [Google Scholar]
- 40. Bartness T. J., Shrestha Y. B., Vaughan C. H., Schwartz G. J., Song C. K. (2010) Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol. Cell. Endocrinol. 318, 34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li J. H., Gautam D., Han S. J., Guettier J. M., Cui Y., Lu H., Deng C., O'Hare J., Jou W., Gavrilova O., Buettner C., Wess J. (2009) Hepatic muscarinic acetylcholine receptors are not critically involved in maintaining glucose homeostasis in mice. Diabetes 58, 2776–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cote M., Matias I., Lemieux I., Petrosino S., Almeras N., Despres J. P., Di Marzo V. (2007) Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. (Lond.) 31, 692–699 [DOI] [PubMed] [Google Scholar]
- 43. Blüher M., Engeli S., Klöting N., Berndt J., Fasshauer M., Bátkai S., Pacher P., Schön M. R., Jordan J., Stumvoll M. (2006) Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 55, 3053–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim W., Doyle M. E., Liu Z., Lao Q., Shin Y. K., Carlson O. D., Kim H. S., Thomas S., Napora J. K., Lee E. K., Moaddel R., Wang Y., Maudsley S., Martin B., Kulkarni R. N., Egan J. M. (2011) Cannabinoids inhibit insulin receptor signaling in pancreatic β-cells. Diabetes 60, 1198–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nomura D. K., Morrison B. E., Blankman J. L., Long J. Z., Kinsey S. G., Marcondes M. C., Ward A. M., Hahn Y. K., Lichtman A. H., Conti B., Cravatt B. F. (2011) Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334, 809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. (2008) Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thaler J. P., Yi C. X., Schur E. A., Guyenet S. J., Hwang B. H., Dietrich M. O., Zhao X., Sarruf D. A., Izgur V., Maravilla K. R., Nguyen H. T., Fischer J. D., Matsen M. E., Wisse B. E., Morton G. J., Horvath T. L., Baskin D. G., Tschöp M. H., Schwartz M. W. (2012) Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 122, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanimura A., Yamazaki M., Hashimotodani Y., Uchigashima M., Kawata S., Abe M., Kita Y., Hashimoto K., Shimizu T., Watanabe M., Sakimura K., Kano M. (2010) The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase α mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327 [DOI] [PubMed] [Google Scholar]
- 49. Dean C. (2011) Endocannabinoid modulation of sympathetic and cardiovascular responses to acute stress in the periaqueductal gray of the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R771–R779 [DOI] [PubMed] [Google Scholar]
- 50. Niederhoffer N., Szabo B. (2000) Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J. Pharmacol. Exp. Ther. 294, 707–713 [PubMed] [Google Scholar]
- 51. Brozoski D. T., Dean C., Hopp F. A., Hillard C. J., Seagard J. L. (2009) Differential endocannabinoid regulation of baroreflex-evoked sympathoinhibition in normotensive versus hypertensive rats. Auton. Neurosci. 150, 82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morgan K., Obici S., Rossetti L. (2004) Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J. Biol. Chem. 279, 31139–31148 [DOI] [PubMed] [Google Scholar]
- 53. Lam T. K. (2010) Neuronal regulation of homeostasis by nutrient sensing. Nat. Med. 16, 392–395 [DOI] [PubMed] [Google Scholar]