Background: The γ-secretase complex is a drug target in the treatment of Alzheimer disease (AD).
Results: Two novel second generation γ-secretase modulators (GSMs) modulate both Nβ and Aβ but not Notch intracellular domain (NICD) production.
Conclusion: Second generation and NSAID-based GSMs have different modes of action regarding Notch processing.
Significance: GSMs that do not affect NICD signaling are essential for the development of tolerable AD therapeutics.
Keywords: Alzheimer Disease, Amyloid Precursor Protein, Enzyme Processing, Neurodegenerative Diseases, Notch, MALDI-TOF MS, Gamma-Secretase Modulators
Abstract
The γ-secretase complex is an appealing drug target when the therapeutic strategy is to alter amyloid-β peptide (Aβ) aggregation in Alzheimer disease. γ-Secretase is directly involved in Aβ formation and determines the pathogenic potential of Aβ by generating the aggregation-prone Aβ42 peptide. Because γ-secretase mediates cleavage of many substrates involved in cell signaling, such as the Notch receptor, it is crucial to sustain these pathways while altering the Aβ secretion. A way of avoiding interference with the physiological function of γ-secretase is to use γ-secretase modulators (GSMs) instead of inhibitors of the enzyme. GSMs modify the Aβ formation from producing the amyloid-prone Aβ42 variant to shorter and less amyloidogenic Aβ species. The modes of action of GSMs are not fully understood, and even though the pharmacology of GSMs has been thoroughly studied regarding Aβ generation, knowledge is lacking about their effects on other substrates, such as Notch. Here, using immunoprecipitation followed by MALDI-TOF MS analysis, we found that two novel, second generation GSMs modulate both Notch β and Aβ production. Moreover, by correlating S3-specific Val-1744 cleavage of Notch intracellular domain (Notch intracellular domain) to total Notch intracellular domain levels using immunocytochemistry, we also demonstrated that Notch intracellular domain is not modulated by the compounds. Interestingly, two well characterized, nonsteroidal anti-inflammatory drugs (nonsteroidal anti-inflammatory drug), R-flurbiprofen and sulindac sulfide, affect only Aβ and not Notch β formation, indicating that second generation GSMs and nonsteroidal anti-inflammatory drug-based GSMs have different modes of action regarding Notch processing.
Introduction
Amyloid-β precursor protein (APP)3 is processed into the Alzheimer-related amyloid-β peptide (Aβ) via a proteolytic event that is sequentially mediated by the membrane-integral β- and γ-secretases (1). After ectodomain shedding of APP, γ-secretase processes the membrane-bound stub, C99, generating Aβ peptides of different lengths (γ cleavage), and simultaneously, the APP intracellular domain is released into the cytosol (ϵ cleavage) (1). Aβ is manifested in many different forms (2), although Aβ40 and Aβ42 are among the most abundant species (3–5). The longer Aβ42 peptide is more prone to aggregate into toxic soluble oligomers (6–8), before eventually forming the insoluble plaques observed in the brains of Alzheimer disease (AD)-affected patients (9).
γ-Secretase is an unusual intramembrane-cleaving protease, composed of presenilin 1 or 2, which harbors the catalytic site, Aph-1-a or -b, nicastrin, and Pen-2 (10–13). The γ-secretase complex processes several type I membrane-bound proteins, including the Notch receptor. Notch is an important signaling molecule in cell differentiation during development as well as adulthood. Notch is processed in a manner similar to APP, generating Nβ peptides (S4 cleavage) and the Notch intracellular domain (NICD) (S3 cleavage) (14, 15), which translocates to the nucleus and acts as an essential regulator of transcription factors (16).
The γ-secretase complex is an appealing drug target when the therapeutic strategy is to alter the metabolism of Aβ. It is directly involved in the Aβ formation, and it also determines the pathogenic potential of Aβ. Because γ-secretase mediates cleavage of many substrates involved in cell signaling, such as the Notch receptor, it is crucial to sustain these pathways while inhibiting toxic Aβ-secretion. However, investigations attempting to find γ-secretase inhibitors (GSIs) with a sufficient therapeutic window between APP and Notch processing has been extremely challenging (17). Moreover, severe side effects, probably caused by abrogated Notch signaling, were present, causing a recent large clinical phase 3 trial to be interrupted (reviewed in Ref. 18). Alternative strategies to combat Aβ production is therefore clearly needed, and γ-secretase modulators (GSMs) represent a growing and promising class of anti-amyloidogenic drugs. Importantly, Notch inhibition is avoided using GSMs instead of GSIs. Typically, GSMs do not affect the overall rate of Notch and APP processing (ϵ and S3 cleavage). Instead, by shifting the cleavage preference of the enzyme from producing the amyloid-prone Aβ42 variant to shorter and less toxic Aβ species, GSMs change the proportions of various Aβ peptides that are formed (19, 20). The first GSMs, subsets of nonsteroidal anti-inflammatory drugs (NSAIDs), such as sulindac sulfide and ibuprofen, were identified in 2001 (19). Since then, many second generation GSMs have brought improvement. These compounds, which are generally structurally distinct from the NSAID family by the absence of an acidic carboxyl group, are more potent and efficient in the central nervous system than were the early GSMs (21–24). Some studies reported NSAID-based GSMs interacting with APP-derived C99 peptides (25–27), but other groups have challenged this implied substrate targeting hypothesis (28–31). During the last year, we and others identified γ-secretase instead of APP as the principal target of second generation GSMs (21, 32–36). However, even though the pharmacology of Aβ generation with respect to first and second generation GSMs has been thoroughly studied, little is known about their overall effect on γ-secretase-mediated cleavage of other substrates, such as the Notch receptor. In this study, we have developed an assay studying Nβ production and investigated, head to head, Notch and APP processing in presence of both first and second generation GSMs. These studies provide compelling evidence that it is possible to obtain selectivity between Nβ and Aβ production, a feature that may be important in development of GSMs for chronic treatment in AD.
EXPERIMENTAL PROCEDURES
Compounds
The GSIs dibenzazepine (DBZ) and L-685,458, as well as GSMs R-flurbiprofen and sulindac sulfide, were obtained from Sigma-Aldrich. AZ1136 was prepared as previously described (32), as was AZ4126, according to patent number WO2010132015.
Ethical Permission
All animal experiments were performed in accordance with relevant guidelines and regulations provided by the Swedish Board of Agriculture. The ethical permission was provided by the Stockholm Södra Animal Research Ethical Board.
Cell Culture
HEK293 cells stably expressing human FLAG-Notch1-ΔE (FLAG-NΔE) (32) or APPswe were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, nonessential amino acids, 10 μm Hepes, and 300 μg/ml hygromycin or 100 μg/ml Zeocin, respectively. For each experiment, the cells were counted and plated in T75 flasks, 6- or 384-well plates (for Nβ, Aβ, and NICD experiments, respectively) the day before treatment. On the following day, the GSM, R-flurbiprofen (200 μm), sulindac sulfide (125 μm), AZ1136 (25 μm), AZ4126 (400 nm), or vehicle control (Me2SO) was separately added to fresh cell media and incubated for 24, 16, or 5 h (for Nβ, Aβ, and NICD experiments, respectively) before conditioned media or cells were analyzed.
Immunoblotting
The cells were treated as described above and lysed in cell lysis buffer (10 mm Tris, pH 8.1, 1 mm EDTA, 150 mm NaCl, 0.65% Igepal CA-630) supplemented with protease inhibitor mixture (Roche Applied Science). The protein levels were determined by the BCA protein assay kit (Pierce), separated on NuPAGE 4–12% gradient Bis-Tris gels with MES buffer (both Invitrogen), and transferred to nitrocellulose membranes (Bio-Rad), which were probed with α-FLAG M2 (Sigma), Val-1744 (Cell Signaling), or α-GAPDH (Acris GmbH) primary antibodies. The blots were developed using horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) followed by Immobilon Western chemiluminescent HRP substrate (Millipore) using Amersham Biosciences HyperfilmTM ECL (GE Healthcare).
Immunoprecipitation (IP) and MS Analysis of Nβ and Aβ
After a clarifying spin and addition of complete protease inhibitor (Roche Applied Science), collected media were immunoprecipitated with 40 μl of α-FLAG M2-agarose (Sigma) or 5 μg of 4G8 (Covance) that were preincubated with a mixture of protein A- and G-Sepharose beads (GE Healthcare) for FLAG-tagged Nβ (F-Nβ) and Aβ, respectively. The samples containing Nβ were incubated at 4 °C, washed, and eluted using a FLAG-tagged protein immunoprecipitation kit (Sigma) according to the manufacturer's instructions. The Aβ-containing samples were incubated at room temperature for 2 h and washed three times for 10 min at 4 °C in PBS (pH 7.4). The immunoprecipitated peptides were then eluted in 100 μl of 0.1% TFA in 20% acetonitril. Eluted Nβ and Aβ peptides were concentrated using C18ZipTips (Millipore) and re-eluted with 0.1% TFA in 50% acetonitril saturated with α-cyano-4-hydroxy-cinnamic acid matrix and analyzed using MALDI-TOF MS. The experiments were performed in triplicate for Nβ and duplicate for Aβ and repeated five or six times. Control experiments included IP of media from cells without FLAG-NΔE (HEK293 cells) or beads without 4G8 antibodies attached, as well as cell media treated with two different γ-secretase inhibitors, L-685,458, and DBZ. MALDI-TOF MS measurements were performed using a Micromass M@LDI instrument (Waters). Each spectrum represents an average of 1000 shots acquired 10 at a time. In the spectrum a total peak height of +5 m/z, relative to the monoisotopic peak, was calculated for each isoform. Prior to analysis the peak heights were normalized to the sum of the peak height of all isoforms in the spectra, and an average was obtained from duplicated and triplicated samples, respectively. The data show relative changes among all the different isoforms in response to GSM treatment. It should be noted that a relative quantification cannot be interpreted as a direct reflection of an absolute or relative abundance of a species because the ionization efficiency might be different for different isoforms and because different isoforms are more hydrophobic than others.
For tandem mass spectrometry (MS/MS) analysis, F-Nβ peptides were immunoprecipitated using the α-FLAG M2 antibody (Sigma) coupled to magnetic beads (2). Briefly, 10 μg of the antibody was added to 50 μl of magnetic Dynabeads M-280 sheep anti-mouse IgG (Invitrogen). Then antibody-coated beads were added to cell media and incubated at 4 °C for 2 h. After washing using the KingFisher magnetic particle processor, the F-Nβ peptides were eluted using 100 μl of 0.5% formic acid. Mass spectrometry measurements were performed using a Bruker Daltonics UltraFleXtreme MALDI-TOF/TOF instrument, and all of the samples were analyzed in duplicate. By result of MS analysis, several compounds were selected for tandem MS/MS, and 2000 single shot spectra were recorded of the precursor ions and 10000 of the fragment ions.
Quantification of Secreted Aβ from Cells
HEK/APPSwe cells were plated in 384-well plates and on the following day exposed to GSMs or vehicle control for 5 h, before conditioned media were analyzed as described previously (32). Briefly, Aβ levels were determined using Meso Scale Discovery (MSD) technology with C-terminally specific antibodies measuring Aβ1-X (where X indicates 37–42). For total Aβ levels, the 4G8 antibody was used.
Animals and Animal Handling
Female C57BL/6 mice (Harlan Laboratories), 10 weeks old at the time of drug administration, were kept in conventional housing and fed standard rodent chew (RM1(E)SQC, SDS, Scanbur) and tap water ad libitum. The mice were randomized into different treatment groups and after acclimatization, AZ4126 (10, 25, 50, or 75 μmol/kg) or vehicle (0.4–3% dimethylacetamide in 2.7–20% HPβCD in water) was administered once by oral gavage. The animals were anesthetized with isoflurane 2 h after treatment. Plasma was isolated from blood collected by cardiac puncture into EDTA tubes by centrifugation for 10 min at 3000 × g at 4 °C within 20 min from sampling. The plasma samples were frozen on dry ice and stored at −80 °C until analysis. The animals were sacrificed by decapitation, and the brains were dissected into hemispheres, frozen on dry ice, and stored at −80 °C until analysis of Aβ40 and Aβ42 and drug concentration
Quantification of Aβ and Drug Concentrations in Animal Samples
Drug concentration in plasma and brain samples was determined by reversed phase liquid chromatography and electrospray MS/MS (32). Soluble Aβ was extracted from mouse brain tissue in diethylamine according to an earlier described protocol (32). Aβ40 and Aβ42 levels in brain were measured by a validated commercial ELISA (BioSource) according to the manufacturer's instructions. All samples were analyzed in duplicate, and data analysis was performed using GraphPad Prism 4, one-way analysis of variance followed by Dunnett's multiple comparison test. The level of significance was set at p ≤ 0.05.
Autoradiography Competition Study
Autoradiography binding on 10-μm frozen brain sagittal sections from rats were performed according to previously described protocols (32, 37). Briefly, rat brains were sectioned, air-dried, and stored at −80 °C upon analysis. Prior to the addition of 5 nm [3H]AZ8349 or [3H]AZ8349 together with unlabeled GSMs (500 μm of R-flurbiprofen or 10 μm of AZ4800 or AZ4126), prewarmed adjacent sections were incubated in 50 mm Tris-buffer (pH 7.4). The sections were incubated at room temperature for 40 min, followed by three 7-min washes in buffer at 1 °C to reduce unspecific binding and then exposed to imaging plates (Fuji BAS-TR2040) for 4 days together with a plastic tritium standard (Amersham Biosciences). The plates were processed with a FLA7000 Imaging Reader (Fujifilm), and the binding was analyzed with Multigauge software V3.0 (Fujifilm).
In Vitro Cellular NICD Formation Assay
The NICD formation assay using ImageXpress scanner (Molecular Devices) was carried out as described earlier with minor modifications (32). Briefly, the cells were treated, washed, fixed with 4% paraformaldehyde, and then stained using primary α-NICD antibodies, C-20 (Santa Cruz Biotechnology) and Val-1744 (Cell Signaling). Thereafter, the secondary antibody, Alexa Flour 594 (Invitrogen), was added. By automatic measurements, the average fluorescence was determined and the percentage of NICD formation for Val-1744 was expressed relative to 0.5% Me2SO (100% control) and 500 nm L-685,458 (0% control). For NICD modulation, Val-1744 fluorescence was normalized to total C20 fluorescence and expressed relative to Me2SO.
RESULTS
γ-Secretase-mediated Notch Processing Results in Many Different Notch β Peptides
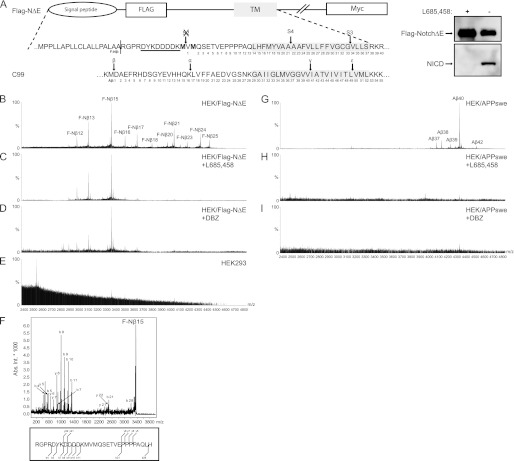
To investigate how different structural classes of GSMs affect Notch processing, we used human embryonic kidney cells (HEK293) stably expressing an N-terminally FLAG-tagged human Notch1 ΔE (FLAG-NΔE) variant (Fig. 1A). Our first goal was to confirm that the FLAG-NΔE variant is a γ-secretase substrate by examining the well characterized S3 cleavage. Using α-FLAG M2 and neo-specific Val-1744 antibodies on immunoblotting, we obtained a robust expression of FLAG-NΔE, as well as of NICD, which was abolished in the presence of the GSI, L-685,458 (Fig. 1A, right panel). To study the production of secreted FLAG-tagged Notch-β peptides (F-Nβ), conditioned media from HEK/FLAG-NΔE cells were immunoprecipitated using the α-FLAG M2 antibody and then subjected to MALDI-TOF MS analysis. The MS spectrum revealed a range of different M2-immunoprecipitated F-Nβ peptides (Fig. 1B), consistent with previous results (15). By culturing the cells in the presence of two different GSIs, L-685,458 and DBZ, we found that the majority of the F-Nβ peptides were generated in a γ-secretase-dependent manner (Fig. 1, C and D). Interestingly, the generation of some shorter peptides, below mass to charge (m/z) 3400, were not inhibited by the GSIs and thus were probably generated through other enzymatic reactions (Fig. 1, C and D). Moreover, no peptides precipitated by M2-agarose were identified in the media of HEK293 cells that were lacking expression of FLAG-NΔE, which displayed the specificity of the precipitation protocol (Fig. 1E).
FIGURE 1.
Detection of secreted FLAG-Nβ and Aβ peptides. A, schematic representation of the human Notch1-ΔE protein containing an N-terminal FLAG epitope (FLAG-NΔE) to facilitate detection of the secreted FLAG-Nβ peptides. The two methionine residues depicted in bold are incorporated to prevent S2 cleavage and thus removal of the FLAG epitope. The α-FLAG antibody M2 recognition site is underlined. The part of C99 that is cleaved by β- and γ-secretase is aligned with FLAG-NΔE in respect to transmembrane domains. Arrows show the S2, S3, S4, β, α, γ, and ϵ cleavage sites, and numbering is shown for F-Nβ and Aβ. There is protein expression of HEK293 cells stably expressing human FLAG Notch1-ΔE and the generation of NICD, using a α-FLAG or the Val-1744 antibody. The NICD formation was abolished in the presence of the GSI L-685,458. B, MALDI-TOF MS spectrum of F-Nβ using conditioned medium from HEK/FLAG-NΔE cells that was immunoprecipitated with α-FLAG M2-agarose. F-Nβ numbering, using the nomenclature set by Okochi et al. (40) of the individual peaks are indicated. C and D, MALDI-TOF MS spectrum of F-Nβ using conditioned medium from HEK/FLAG-NΔE cells treated with the GSIs L-685,458 or DBZ, respectively. Spectra analysis reveals that only F-Nβ16–25 is inhibited by GSIs. E, MALDI-TOF MS spectrum of F-Nβ using conditioned medium from HEK293 cells. F, all F-Nβ peaks were identified by MS/MS, and a representative spectrum of F-Nβ15 is shown. G, MALDI-TOF MS spectrum of Aβ using 4G8 immunoprecipitated conditioned medium from HEK/APPswe cells. H and I, MALDI-TOF MS spectrum of Aβ using conditioned medium from HEK/APPswe cells treated with the GSIs L-685,458 or DBZ, respectively.
Next, we identified which peptide sequence corresponded to which peak in the MALDI spectrum. To confirm peptide identities, the immunoprecipitates were analyzed by MALDI-TOF/TOF. Given the natural cleavage site in the signal peptide, FLAG-NΔE, we matched peptides, starting with RGPR before the FLAG sequence, and identified 11 different peptides corresponding to a range of species between F-Nβ12 to F-Nβ25 (Fig. 1F). In addition, we could not observe peptides longer than F-Nβ25 (Fig. 1C), which is in line with data reported by Okochi et al. (15).
First Generation NSAID Class GSMs Affect Aβ but Not Nβ Production
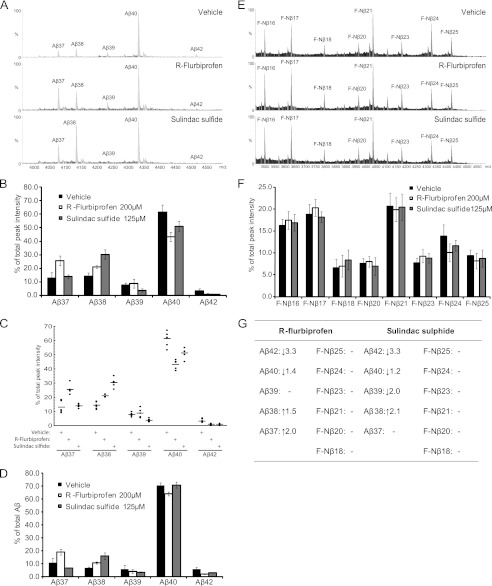
To explore how the NSAID class GSMs modulate the γ-secretase complex, we first studied their effect on APP processing by measuring the Aβ peptide generation in HEK293 cells stably expressing the APPswe mutation, HEK/APPswe. Conditioned media were subjected to IP-MALDI-TOF MS analysis, resulting in detection of Aβ37, Aβ38, Aβ39, Aβ40, and Aβ42 (Fig. 1G). Like F-Nβ, the generation of the Aβ peptides was abolished in the presence of either of two GSIs, L-685,458 or DBZ (Fig. 1, H and I). We treated the cells with two well characterized NSAIDs, R-flurbiprofen and sulindac sulfide or vehicle, and studied the Aβ peptide profile. Both GSMs are known to modulate γ-secretase activity by shifting the amino acid cleavage from positions 42 to 38 (19, 38), and as expected, we observed a decrease in Aβ42 accompanied by a relative increase in Aβ38 compared with untreated cells (Fig. 2, A–C). In line with previous findings, treatment with sulindac sulfide resulted in a relative decrease of Aβ39 (19). Interestingly, we also observed an increase in Aβ37 in cells treated with R-flurbiprofen, but not with sulindac sulfide. These results were confirmed by Aβ peptide analysis using MSD technology (Fig. 2D).
FIGURE 2.
The effect of first generation NSAID class GSMs on Aβ and F-Nβ formation. A, MALDI-TOF MS spectrum displaying the Aβ pattern in conditioned medium from HEK/APPswe cells treated with R-flurbiprofen, sulindac sulfide, or vehicle. The intensities of the highest peak were set to 100% in the spectrum. B, Aβ peak distribution under the influence of first generation GSMs. Each Aβ peak is plotted as a percentage of total Aβ (i.e., the sum of Aβ37–42). The bars represent the means of five or six experiments with error bars indicating S.D. C, scatter plots of the Aβ peptide distribution under the influence of first generation GSMs. The data are from five or six experiments and plotted as percentages of total Aβ (i.e., the sum of Aβ37–42). D, detection of secreted Aβ peptides in conditioned medium from HEK/APPswe cells treated with R-flurbiprofen, sulindac sulfide, or vehicle by MSD technology. Aβ peptide formation is determined as a percentage of total Aβ (i.e., the sum of Aβ37–42). The bars represent the means of two experiments with error bars indicating S.D. E, MALDI-TOF MS spectrum of F-Nβ using α-FLAG immunoprecipitated conditioned medium from HEK/FLAG-NΔE cells treated with R-flurbiprofen, sulindac sulfide, or vehicle. The intensities of the highest peak were set to 100% in the spectrum. F, F-Nβ peak distribution under the influence of first generation GSMs. Because only F-Nβ16–25 could be inhibited by GSIs and not the shorter F-Nβ12–15, the latter were excluded from peak analysis. Each F-Nβ peak is plotted as a percentage of total F-Nβ (i.e., the sum of F-Nβ16–25). The bars represent the means of five or six experiments with error bars indicating S.D. G, a summary of the effect of first generation GSMs on Aβ and F-Nβ peptide formation.
To further study how NSAID class GSMs affect Nβ generation, we treated HEK/FLAG-NΔE cells with R-flurbiprofen, sulindac sulfide, or vehicle and monitored the generation of F-Nβ peptides using IP-MALDI-TOF. Because only F-Nβ16–25 could be inhibited by GSIs and not the shorter F-Nβ12–15 (Fig. 1, C and D), the latter was excluded from peak analysis. Importantly, we did not detect any differences, neither increases nor decreases, in any F-Nβ peptide level between treated and untreated cells (Fig. 2, E and F). Collectively, these results suggest that first generation NSAID class GSMs seem to affect Aβ but not Nβ production (summarized in Fig. 2G).
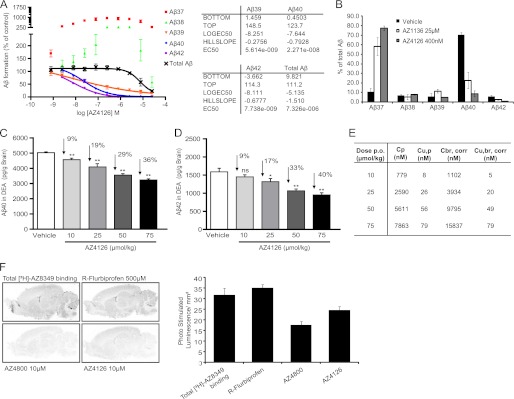
AZ4126 Is a Novel Second Generation GSM That Modulates Aβ Formation Both in Vitro and in Vivo
We next characterized the pharmacology of a novel and chemically distinct second generation GSM, AZ4126, in both in vitro and in vivo assays. In a first series of experiments, HEK/APPswe cells were exposed to AZ4126, as well as the previously described GSM AZ1136 (32), and their effect on secreted Aβ production was investigated. Both AZ1136 and AZ4126 caused a reduction in Aβ42 and Aβ40, although the latter more potently than the former (AZ1136: IC50Aβ42 = 990 ± 150 nm, IC50Aβ40 = 1400 ± 50 nm; AZ4126: IC50Aβ42 = 6.0 ± 3.1 nm, IC50Aβ40 = 22.5 ± 8.7 nm; Fig. 3, A and B, and Ref. 32). This pattern of Aβ42 and Aβ40 inhibition is consistent with the behavior of second generation GSMs (21, 32). Importantly, total Aβ was not affected at the concentration range at which modulation occurred, suggesting that these molecules are bona fide GSMs (Fig. 3A and Ref. 32). Further, the effect of AZ4126 was also investigated in vivo by administration of a single dose at different concentrations by oral gavage to wild type (C57BL/6) mice. Two hours after drug administration, both Aβ40 and Aβ42 levels were measured in diethylamine-extracted brain homogenates. AZ4126 reduced both Aβ40 and Aβ42 in a dose-dependent manner, with up to 36 and 40% for Aβ40 and Aβ42, respectively (Fig. 3, C and D). The concentration of AZ4126 in both plasma and brain followed a dose-dependent pattern (Fig. 3E). Thus, AZ4126 readily reaches the brain and exhibits the expected CNS Aβ lowering activity.
FIGURE 3.
Characterization of novel AZ second generation GSMs in vitro and in vivo. A, dose-response curve of AZ4126 displaying Aβ modulation, where Aβ formation is set relative to 0.5% Me2SO (100%) and 0.5 μm L-685,458 (0%) controls. The curve represents the means of two experiments with error bars indicating S.D. B, detection of secreted Aβ peptides in conditioned medium from HEK/APPswe cells treated with AZ1136, AZ4126, or vehicle using MSD technology. Aβ peptide formation is determined as a percentage of total Aβ (i.e., the sum of Aβ37–42). The bars represent the means of three experiments with error bars indicating S.D. C and D, AZ4126 reduces brain Aβ40 (C) and Aβ42 (D) levels in female C57BL/6 mice in a dose-dependent manner. Levels of Aβ in the brain were measured in mice 2 h after peroral administration of AZ4126 (10, 25, 50, or 75 μmol/kg) or vehicle (n = 7/group). Statistical analysis was one-way analysis of variance followed by Dunnett's multiple comparison test. *, p ≤ 0.05; **, p ≤ 0.01. E, plasma and brain exposure levels of AZ4126 in C57BL/6 mice 2 h after peroral dosing. Cp, plasma concentration; Cu,p, unbound plasma concentration; Cbr,corr, brain concentration corrected for plasma; Cu,br,corr, unbound brain concentration corrected for plasma. F, displacement of [3H] AZ8349 GSM by different GSMs on sagittal rat brain sections. 5 nm of [3H] AZ8349 was incubated in the absence or presence of 500 μm R-flurbiprofen, 10 μm of the reference compound AZ4800 (32), or 10 μm AZ4126, and the binding in the autoradiograms was quantified as optical density (photo-stimulated luminescence/mm2). AZ4126 as well as the reference compound AZ4800 displace [3H] AZ8349 but not R-flurbiprofen, indicating distinct interaction sites. The bars represent the means of two experiments with error bars indicating S.D.
Moreover, we have previously shown that several identified compounds, including AZ1136, display a competitive interaction to established second generation GSMs but not to first generation GSMs. To confirm that AZ4126 belongs to the second generation GSMs, we performed radioligand displacement binding experiments with an established tritiated second generation GSM, [3H]AZ8349, as tracer (32). Although AZ4800, a structural derivative to AZ4126, and AZ4126 caused a displacement of [3H]AZ8349, R-Flurbiprofen did not (Fig. 3F). Combined, the Aβ profile of displacement results both in vitro and in vivo together clearly manifests AZ4126 as a second generation GSM.
Second Generation GSMs Affect Both Nβ and Aβ Production
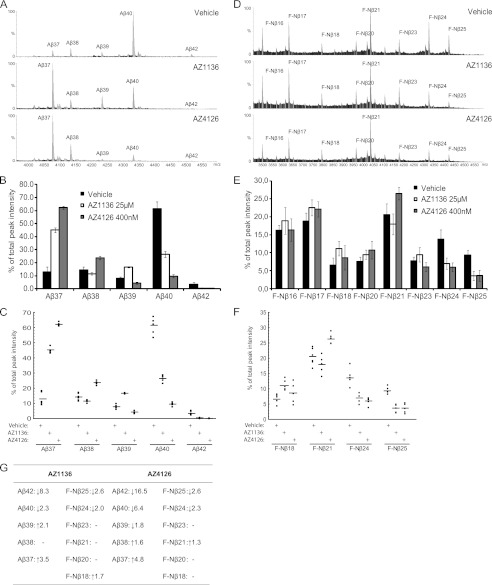
Because we had found that the first generation GSM displays a clear differentiation with regard to Aβ and Nβ generation, we wanted to compare the effect of second generation GSM on the generation of these peptides. First, we analyzed the effect of AZ GSMs on Aβ peptide formation in HEK/APPswe cells using IP-MALDI-TOF MS. In line with MSD data (Fig. 3B), both AZ1136 and AZ4126 caused a differential reduction in Aβ40 and Aβ42 (2.3- and 6.4-fold decreases for Aβ40 and 8.3- and 16.5-fold decreases for Aβ42, respectively). The MALDI-TOF MS also revealed a unique Aβ pattern in response to the two AZ GSMs (Fig. 4, A–C): AZ1136 relatively increased both Aβ37 and Aβ39 (3,5 and 2.1 times, respectively), whereas Aβ38 was unaffected, consistent with previously published results (32). AZ4126 also increased Aβ37 but with a higher magnitude (4.8-fold), decreased Aβ39 (1.8-fold), and marginally induced the levels of Aβ38 (1.6-fold). Importantly, MSD analysis resulted in the same Aβ pattern as the MS results (Fig. 3B).
FIGURE 4.
The effect of second generation GSMs on Aβ and F-Nβ generation. A, MALDI-TOF MS spectrum of Aβ using 4G8 immunoprecipitated conditioned medium from HEK/APPswe cells treated with AZ1136, AZ4126, or vehicle. The intensities of the highest peak were set to 100% in the spectrum. B, Aβ peak distribution under the influence of second generation GSMs. Each Aβ peak is plotted as a percentage of total Aβ (i.e., the sum of Aβ37–42). The bars represent the means of five or six experiments with error bars indicating S.D. C, scatter plots of the Aβ peptide distribution under the influence of second generation GSMs. The data are from five or six experiments and plotted as percentages of total Aβ (i.e., the sum of Aβ37–42). D, MALDI-TOF MS spectrum of F-Nβ using α-FLAG immunoprecipitated conditioned medium from HEK/FLAG-NΔE cells treated with AZ1136, AZ4126, or vehicle. The intensities of the highest peak were set to 100% in the spectrum. E, F-Nβ peak distribution under the influence of second generation GSMs. We excluded F-Nβ12–15 peaks from peak analysis because they were not γ-secretase-dependent. Each F-Nβ peak is plotted as a percentage of total F-Nβ (i.e., the sum of F-Nβ16–25). The bars represent the means of five or six experiments with error bars indicating S.D. F, scatter plots of the F-Nβ18, 21, 24, and 25 peptide distributions under the influence of second generation GSMs. The data are from five or six experiments and plotted as percentages of total F-Nβ (i.e., the sum of F-Nβ16–25). G, a summary of the effect of second generation GSMs on Aβ and F-Nβ peptide formation.
The effect of AZ GSMs on Nβ formation was studied using HEK/FLAG-NΔE cells and IP-MALDI-TOF. Interestingly, both AZ1136 and AZ4126 caused a relative decrease in F-Nβ24 (2.0 and 2.3 times, respectively) and F-Nβ25 (2.6-fold for both) levels, in comparison with untreated cells. However, we did not observe as distinct differences in the production of shorter F-Nβ, as observed for shorter Aβ peptides in response to the same compounds. AZ1136 increased F-Nβ18 1.7-fold, whereas AZ4126 increased F-Nβ21 only 1.3-fold. Examined together, these data suggest that second generation AZ GSMs modulate Nβ production, although to a lower extent compared with their effect on Aβ production (summarized in Fig. 4G).
Second Generation GSMs Do Not Modulate NICD Formation
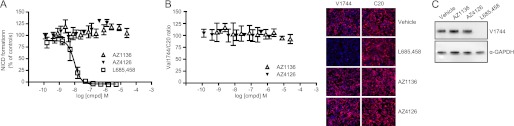
We finally explored whether the Notch S3 cleavage, liberating the more biologically relevant NICD peptide, also was affected by second generation GSMs, because we observed that they modulate Nβ production. To address this question, we used a previously developed immunocytochemistry assay based on HEK/FLAG-NΔE cells and the C20 antibody that recognizes total NICD (32). Here, we included an anti-NICD antibody that specifically recognizes NICD beginning at position Val-1744 (V1744-NICD). We performed dose-response experiments and analyzed the amount of V1744-NICD in the nucleus relative to untreated cells and confirmed that AZ GSMs do not inhibit NICD formation (Fig. 5A). L-685,458 inhibited V1744-NICD formation with similar potency (IC50 Val-1744 = 10.0 nm) as previously determined for its effect on total NICD formation (IC50 C20 = 6.0 nm (32)). By calculating the ratio of the signal from both NICD antibodies, we could determine whether the relative amount of V1744-NICD to total NICD was affected. If GSMs modulate NICD formation, the amount of V1744-NICD and hence the ratio would change. None of the GSMs showed any change of V1744-NICD to total NICD ratio (Fig. 5B), suggesting that AZ GSMs do not modulate NICD formation. These results were also confirmed by Western blot analysis (Fig. 5C).
FIGURE 5.
The effect of AZ1136 and AZ4126 on NICD formation and modulation. A, in vitro cellular V1744-NICD formation assay. Neither AZ1136 nor AZ4126 inhibit NICD formation. The GSI L-685,458 serves as positive control for inhibition. The curves represent the means of three experiments with error bars indicating S.D. B, the ratio of the S3 specific V1744-NICD/total NICD is unaltered by AZ GSMs compared with vehicle, as determined by measuring the fluorescent intensity from both Val-1744 and C20 antibodies. The curves represent the means of three experiments with error bars indicating S.D. Representative immunocytochemistry images of vehicle-, L-685,458-, AZ1136-, and AZ4126-treated cells are shown. C, Western blot analysis was performed to confirm the in vitro cellular NICD formation assay. The cells were treated with AZ1136, AZ4126, or vehicle and analyzed with the Val-1744 and α-GAPDH antibodies.
DISCUSSION
The insights in the mode of action of first and second generation GSMs are not fully understood, even though they represent a prioritized therapeutic approach in AD drug discovery. However, a growing body of evidence suggests that many second generation GSMs have a mode of action that is different from first generation NSAID class GSMs, because the former appear to target γ-secretase instead of APP (21, 32–36). Even though the pharmacology of Aβ generation associated with GSMs has been thoroughly studied, less is known about their effect on other substrates, such as the Notch receptor. Here, we describe a head-to-head comparison of first and second generation GSMs affecting γ-secretase processing of APP and Notch. Whereas both the first generation GSMs R-flurbiprofen and sulindac sulfide and the second generation GSMs AZ4126 and AZ1136 display Aβ modulation, only the second generations GSMs modify Nβ production. The modulatory effect of AZ4126 and AZ1136 on Aβ and Nβ differs both with regard to efficacy and profile, and the overall impact is more pronounced on Aβ generation compared with Nβ production. These data suggest that γ-secretase-targeting GSMs are not only modulating Aβ but also modify Nβ production and possibly also other γ-secretase-dependent Aβ-like peptides.
To compare the effect of GSMs on Nβ and Aβ formation, we established an antibody pulldown approach that we followed with MALDI-TOF MS analysis. This strategy enabled detection of the expected Aβ peptides, as well as 11 different F-Nβ species, ranging from F-Nβ12 to F-Nβ25, of which F-Nβ16–25 were generated in a γ-secretase-dependent manner. These observations are well in line with previously reported results (15). MALDI-TOF analysis of Aβ peptides revealed some clear commonalities but also differences in the pharmacological profile among the compounds examined. Both AZ1136 and AZ4126 cause a very potent reduction in Aβ42 and Aβ40 and a parallel increase in Aβ37. In contrast, R-flurbiprofen and sulindac sulfide are associated with a clear relative reduction in Aβ42 but not Aβ40 and a concomitant increase of Aβ38, suggesting that these different classes of GSM compounds modulate Aβ production through different mechanisms. In line with our previous study, we also found that AZ1136 treatment increases the relative levels of Aβ39, whereas AZ4126 causes an increase in Aβ38 (32). The pharmacological profiles of R-flurbiprofen and sulindac sulfide display differently; R-flurbiprofen primarily increases Aβ37, whereas sulindac sulfide reduces Aβ39. Thus, besides the differences in Aβ modulation between the first and second generation GSMs, clear differences are found within each class of GSMs with regard to Aβ modulation. Further studies are needed to explain the mechanism that gives rise to these differences and thus shed more light on the pharmacology of these different GSMs with respect to Aβ modulation.
The fact that both AZ1136 and AZ4126, but neither R-flurbiprofen nor sulindac sulfide, affect Nβ production is interesting. Similar to their effect on Aβ40 and Aβ42, AZ1136 and AZ4126 reduce both F-Nβ24 and F-Nβ25, but the relative effects are not as discriminating. Strikingly, even though AZ4126 decreases Aβ40 and Aβ42 approximately twice as much as AZ1136 at their respective tested concentration, both compounds appear to have the same efficacy in reducing F-Nβ24 and F-Nβ25. Regarding the shorter F-Nβ peptides, we observed that most of these peptides were similarly unaffected by the AZ GSMs. In contrast to their very clear relative elevation of Aβ37, AZ41126 and AZ1136 do not cause a clear increase in the same F-Nβ peptide. However, AZ1136 increases F-Nβ18 slightly, whereas F-Nβ21 is elevated by AZ4126. Thus, it seems like both compounds share a general pharmacological profile on APP and Notch processing, specifically, a decrease in the longer Aβ and F-Nβ peptides (Aβ40/Aβ42 and F-Nβ24/F-Nβ25) and an increase in some of the shorter peptides, but that disparities do exist between the compounds, pertaining to the efficacy of the process and the specificity of which peptides are to be modulated. Our data therefore suggest that it is possible to generate γ-secretase targeting GSMs that are preselective for Aβ over Nβ production. γ-Secretase targeting is not restricted to these compounds, because there are several well characterized GSIs of different chemical classes that have been shown to bind to the γ-secretase complex and exhibit different potencies in APP and Notch processing. Such features are assigned the Notch-sparing GSIs, such as BMS-708163, PF-3084014, Begacestat, ELND-006, and Semagacestat, which differ in their magnitude of selectivity of APP toward Notch with 3–1473 times (see review in Ref. 18).
γ-Secretase-mediated NICD formation is a key event in Notch receptor activation. Generating GSMs that do not affect NICD signaling is crucial toward developing tolerable AD therapeutics. The observation that many GSMs are Notch-sparing, that is, they do not affect the total amount of NICD generated, is applicable to the GSMs used in this study (Fig. 4A) (32). However, it may not be only the total amount of NICD generated that ought to be considered but also the specificity of the γ-secretase cleavage event at the S3 site. Indeed, Tagami et al. (39) recently reported that NICD exists in two distinct forms at the N terminus, which results in NICDs with quite different stabilities and thus different signaling properties. In this study we could not find evidence for modulatory effect by AZ1136 and AZ4126 on Notch S3 cleavage, suggesting that the AZ GSMs are selective for S4 cleavage modulation.
Our finding that second generation GSMs modulate both Aβ and Nβ supports the theory that the γ-secretase complex is the molecular target of second generation GSMs (21, 32–36). In contrast, there has been no consensus as to what is the binding site of NSAID-based GSMs. Some studies report that these compounds interact with APP-derived C99 fragment (25–27), whereas others have challenged this hypothesis (28–31). Our data, showing that R-flurbiprofen and sulindac sulfide affect Aβ but not Nβ production, indicate that APP is the target of NSAID-based GSMs. These data contrast with a previous report showing that sulindac sulfide decreases the ratio of Nβ25/(Nβ21 + Nβ25) (40). These discrepancies could be explained by differences in technical strategy. However, our results fit very well with a previous finding that NSAIDs such as sulindac sulfide interact and interfere with dimerization of the APP transmembrane domain, thereby affecting Aβ generation (27). APP forms dimers with the help of three different dimerization sites: two in the ectodomain and a third formed by three GXXXG motifs in the APP transmembrane domain. Recently, it was reported that a slight decrease in the dimerization strength of the GXXXG motifs gave rise to an enormous decline of Aβ42 formation (41). Although it has been claimed that Notch can dimerize through its epidermal growth factor repeats in the ectodomain, Vooijs et al. (42) also report that most surface Notch molecules are monomeric. Thus, it is plausible that NSAIDs interact with mechanisms affecting dimerization of APP, resulting in no modulation in Nβ. Further studies with competitive experiments and photo-probe labeling on NSAID-based GSMs are needed to fully understand the binding target of the NSAIDs.
Modulation by second generation GSMs may also affect other substrate-releasing Aβ-like peptides, such as APP-like proteins 1 and 2, CD44, and interleukin-1 receptor II (43–45). Therefore, it will be of great interest to monitor these peptides during the research development of this class of drugs. However, the biological relevance of the Aβ-like peptides is currently unclear. However, the APP-like protein 1 Aβ-like peptide, which is less amyloidogenic than Aβ42, is present in human cerebrospinal fluid and is purported to function as a surrogate marker for Aβ42 in response to γ-secretase-targeting drugs (46). Thus, the selectivity pattern of a given GSM should be a major consideration in biomarker development.
In summary, we report that second generation but not first generation GSMs affect Nβ production, although to a much lower level than they affect Aβ formation. However, second generation GSMs do not affect the Notch S3 cleavage site or the NICD formation, which is a crucial factor toward developing safe and tolerable AD therapeutics.
This work was supported by grants from Swedish Brain Power, Wallenberg's Foundation, Loo och Hans Ostermans Stiftelse, Stiftelsen Gamla Tjänarinnor, Karolinska Institutet Stiftelsen för Åldersforskning, Swedish Medical Society, Gun och Bertil Stohnes Stiftelse, and Strategic Research Area: Neuroscience under Regulation 2009-1077.
- APP
- amyloid precursor protein
- Aβ
- amyloid-β peptide
- GSM
- γ-secretase modulator
- Nβ
- Notch β
- NICD
- Notch intracellular domain
- NSAID
- nonsteroidal anti-inflammatory drug
- GSI
- γ-secretase inhibitor
- FLAG-NΔE
- FLAG-Notch1-ΔE
- DBZ
- dibenzoazepine
- IP
- immunoprecipitation
- MS/MS
- tandem mass spectrometry
- AD
- Alzheimer disease
- F-Nβ
- FLAG-tagged Nβ
- MSD
- Meso Scale Discovery.
REFERENCES
- 1. Wolfe M. S. (2010) Structure, mechanism and inhibition of γ-secretase and presenilin-like proteases. Biol. Chem. 391, 839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Portelius E., Tran A. J., Andreasson U., Persson R., Brinkmalm G., Zetterberg H., Blennow K., Westman-Brinkmalm A. (2007) Characterization of amyloid β peptides in cerebrospinal fluid by an automated immunoprecipitation procedure followed by mass spectrometry. J. Proteome Res. 6, 4433–4439 [DOI] [PubMed] [Google Scholar]
- 3. Czirr E., Cottrell B. A., Leuchtenberger S., Kukar T., Ladd T. B., Esselmann H., Paul S., Schubenel R., Torpey J. W., Pietrzik C. U., Golde T. E., Wiltfang J., Baumann K., Koo E. H., Weggen S. (2008) Independent generation of Aβ42 and Aβ38 peptide species by γ-secretase. J. Biol. Chem. 283, 17049–17054 [DOI] [PubMed] [Google Scholar]
- 4. Kakuda N., Funamoto S., Yagishita S., Takami M., Osawa S., Dohmae N., Ihara Y. (2006) Equimolar production of amyloid β-protein and amyloid precursor protein intracellular domain from β-carboxyl-terminal fragment by γ-secretase. J. Biol. Chem. 281, 14776–14786 [DOI] [PubMed] [Google Scholar]
- 5. Qi-Takahara Y., Morishima-Kawashima M., Tanimura Y., Dolios G., Hirotani N., Horikoshi Y., Kametani F., Maeda M., Saido T. C., Wang R., Ihara Y. (2005) Longer forms of amyloid β protein. Implications for the mechanism of intramembrane cleavage by γ-secretase. J. Neurosci. 25, 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lesné S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., Gallagher M., Ashe K. H. (2006) A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 [DOI] [PubMed] [Google Scholar]
- 7. Shankar G. M., Li S., Mehta T. H., Garcia-Munoz A., Shepardson N. E., Smith I., Brett F. M., Farrell M. A., Rowan M. J., Lemere C. A., Regan C. M., Walsh D. M., Sabatini B. L., Selkoe D. J. (2008) Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 9. Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 82, 4245–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Francis R., McGrath G., Zhang J., Ruddy D. A., Sym M., Apfeld J., Nicoll M., Maxwell M., Hai B., Ellis M. C., Parks A. L., Xu W., Li J., Gurney M., Myers R. L., Himes C. S., Hiebsch R., Ruble C., Nye J. S., Curtis D. (2002) aph-1 and pen-2 are required for Notch pathway signaling, γ-secretase cleavage of βAPP, and presenilin protein accumulation. Dev. Cell 3, 85–97 [DOI] [PubMed] [Google Scholar]
- 11. Goutte C., Tsunozaki M., Hale V. A., Priess J. R. (2002) APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc. Natl. Acad. Sci. U.S.A. 99, 775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398, 513–517 [DOI] [PubMed] [Google Scholar]
- 13. Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., Tandon A., Song Y. Q., Rogaeva E., Chen F., Kawarai T., Supala A., Levesque L., Yu H., Yang D. S., Holmes E., Milman P., Liang Y., Zhang D. M., Xu D. H., Sato C., Rogaev E., Smith M., Janus C., Zhang Y., Aebersold R., Farrer L. S., Sorbi S., Bruni A., Fraser P., St George-Hyslop P. (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature 407, 48–54 [DOI] [PubMed] [Google Scholar]
- 14. De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J. S., Schroeter E. H., Schrijvers V., Wolfe M. S., Ray W. J., Goate A., Kopan R. (1999) A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 [DOI] [PubMed] [Google Scholar]
- 15. Okochi M., Steiner H., Fukumori A., Tanii H., Tomita T., Tanaka T., Iwatsubo T., Kudo T., Takeda M., Haass C. (2002) Presenilins mediate a dual intramembranous γ-secretase cleavage of Notch-1. EMBO J. 21, 5408–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schroeter E. H., Kisslinger J. A., Kopan R. (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393, 382–386 [DOI] [PubMed] [Google Scholar]
- 17. Lundkvist J., Naslund J. (2007) γ-Secretase. A complex target for Alzheimer's disease. Curr. Opin. Pharmacol. 7, 112–118 [DOI] [PubMed] [Google Scholar]
- 18. Imbimbo B. P., Giardina G. A. (2011) γ-Secretase inhibitors and modulators for the treatment of Alzheimer's disease. Disappointments and hopes. Curr. Top. Med. Chem. 11, 1555–1570 [DOI] [PubMed] [Google Scholar]
- 19. Weggen S., Eriksen J. L., Das P., Sagi S. A., Wang R., Pietrzik C. U., Findlay K. A., Smith T. E., Murphy M. P., Bulter T., Kang D. E., Marquez-Sterling N., Golde T. E., Koo E. H. (2001) A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216 [DOI] [PubMed] [Google Scholar]
- 20. Weggen S., Eriksen J. L., Sagi S. A., Pietrzik C. U., Golde T. E., Koo E. H. (2003) Aβ42-lowering nonsteroidal anti-inflammatory drugs preserve intramembrane cleavage of the amyloid precursor protein (APP) and ErbB-4 receptor and signaling through the APP intracellular domain. J. Biol. Chem. 278, 30748–30754 [DOI] [PubMed] [Google Scholar]
- 21. Kounnas M. Z., Danks A. M., Cheng S., Tyree C., Ackerman E., Zhang X., Ahn K., Nguyen P., Comer D., Mao L., Yu C., Pleynet D., Digregorio P. J., Velicelebi G., Stauderman K. A., Comer W. T., Mobley W. C., Li Y. M., Sisodia S. S., Tanzi R. E., Wagner S. L. (2010) Modulation of γ-secretase reduces β-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron 67, 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oehlrich D., Berthelot D. J., Gijsen H. J. (2011) γ-Secretase modulators as potential disease modifying anti-Alzheimer's drugs. J. Med. Chem. 54, 669–698 [DOI] [PubMed] [Google Scholar]
- 23. Portelius E., Van Broeck B., Andreasson U., Gustavsson M. K., Mercken M., Zetterberg H., Borghys H., Blennow K. (2010) Acute effect on the Aβ isoform pattern in CSF in response to γ-secretase modulator and inhibitor treatment in dogs. J. Alzheimers Dis. 21, 1005–1012 [DOI] [PubMed] [Google Scholar]
- 24. Tomita T., Iwatsubo T. (2006) γ-Secretase as a therapeutic target for treatment of Alzheimer's disease. Curr. Pharm. Des. 12, 661–670 [DOI] [PubMed] [Google Scholar]
- 25. Botev A., Munter L. M., Wenzel R., Richter L., Althoff V., Ismer J., Gerling U., Weise C., Koksch B., Hildebrand P. W., Bittl R., Multhaup G. (2011) The amyloid precursor protein C-terminal fragment C100 occurs in monomeric and dimeric stable conformations and binds γ-secretase modulators. Biochemistry 50, 828–835 [DOI] [PubMed] [Google Scholar]
- 26. Kukar T. L., Ladd T. B., Bann M. A., Fraering P. C., Narlawar R., Maharvi G. M., Healy B., Chapman R., Welzel A. T., Price R. W., Moore B., Rangachari V., Cusack B., Eriksen J., Jansen-West K., Verbeeck C., Yager D., Eckman C., Ye W., Sagi S., Cottrell B. A., Torpey J., Rosenberry T. L., Fauq A., Wolfe M. S., Schmidt B., Walsh D. M., Koo E. H., Golde T. E. (2008) Substrate-targeting γ-secretase modulators. Nature 453, 925–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richter L., Munter L. M., Ness J., Hildebrand P. W., Dasari M., Unterreitmeier S., Bulic B., Beyermann M., Gust R., Reif B., Weggen S., Langosch D., Multhaup G. (2010) Amyloid β 42 peptide (Aβ42)-lowering compounds directly bind to Aβ and interfere with amyloid precursor protein (APP) transmembrane dimerization. Proc. Natl. Acad. Sci. U.S.A. 107, 14597–14602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beel A. J., Barrett P., Schnier P. D., Hitchcock S. A., Bagal D., Sanders C. R., Jordan J. B. (2009) Nonspecificity of binding of γ-secretase modulators to the amyloid precursor protein. Biochemistry 48, 11837–11839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Page R. M., Gutsmiedl A., Fukumori A., Winkler E., Haass C., Steiner H. (2010) β-Amyloid precursor protein mutants respond to γ-secretase modulators. J. Biol. Chem. 285, 17798–17810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barrett P. J., Sanders C. R., Kaufman S. A., Michelsen K., Jordan J. B. (2011) NSAID-based γ-secretase modulators do not bind to the amyloid-β polypeptide. Biochemistry 50, 10328–10342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clarke E. E., Churcher I., Ellis S., Wrigley J. D., Lewis H. D., Harrison T., Shearman M. S., Beher D. (2006) Intra- or intercomplex binding to the γ-secretase enzyme. A model to differentiate inhibitor classes. J. Biol. Chem. 281, 31279–31289 [DOI] [PubMed] [Google Scholar]
- 32. Borgegard T., Juréus A., Olsson F., Rosqvist S., Sabirsh A., Rotticci D., Paulsen K., Klintenberg R., Yan H., Waldman M., Stromberg K., Nord J., Johansson J., Regner A., Parpal S., Malinowsky D., Radesater A. C., Li T., Singh R., Eriksson H., Lundkvist J. (2012) First and second generation γ-secretase modulators (GSMs) modulate amyloid-β (Aβ) peptide production through different mechanisms. J. Biol. Chem. 287, 11810–11819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crump C. J., Fish B. A., Castro S. V., Chau D. M., Gertsik N., Ahn K., Stiff C., Pozdnyakov N., Bales K. R., Johnson D. S., Li Y. M. (2011) Piperidine acetic acid based γ-secretase modulators directly bind to Presenilin-1. ACS Chem. Neurosci. 2, 705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ebke A., Luebbers T., Fukumori A., Shirotani K., Haass C., Baumann K., Steiner H. (2011) Novel γ-secretase enzyme modulators directly target presenilin protein. J. Biol. Chem. 286, 37181–37186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohki Y., Higo T., Uemura K., Shimada N., Osawa S., Berezovska O., Yokoshima S., Fukuyama T., Tomita T., Iwatsubo T. (2011) Phenylpiperidine-type γ-secretase modulators target the transmembrane domain 1 of presenilin 1. EMBO J. 30, 4815–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jumpertz T., Rennhack A., Ness J., Baches S., Pietrzik C. U., Bulic B., Weggen S. (2012) Presenilin is the molecular target of acidic γ-secretase modulators in living cells. PLoS One 7, e30484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jureus A., Swahn B. M., Sandell J., Jeppsson F., Johnson A. E., Johnstrom P., Neelissen J. A., Sunnemark D., Farde L., Svensson S. P. (2010) Characterization of AZD4694, a novel fluorinated Aβ plaque neuroimaging PET radioligand. J. Neurochem. 114, 784–794 [DOI] [PubMed] [Google Scholar]
- 38. Eriksen J. L., Sagi S. A., Smith T. E., Weggen S., Das P., McLendon D. C., Ozols V. V., Jessing K. W., Zavitz K. H., Koo E. H., Golde T. E. (2003) NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Aβ 42 in vivo. J. Clin. Invest. 112, 440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tagami S., Okochi M., Yanagida K., Ikuta A., Fukumori A., Matsumoto N., Ishizuka-Katsura Y., Nakayama T., Itoh N., Jiang J., Nishitomi K., Kamino K., Morihara T., Hashimoto R., Tanaka T., Kudo T., Chiba S., Takeda M. (2008) Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1. Mol. Cell Biol. 28, 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Okochi M., Fukumori A., Jiang J., Itoh N., Kimura R., Steiner H., Haass C., Tagami S., Takeda M. (2006) Secretion of the Notch-1 Aβ-like peptide during Notch signaling. J. Biol. Chem. 281, 7890–7898 [DOI] [PubMed] [Google Scholar]
- 41. Munter L. M., Voigt P., Harmeier A., Kaden D., Gottschalk K. E., Weise C., Pipkorn R., Schaefer M., Langosch D., Multhaup G. (2007) GXXXG motifs within the amyloid precursor protein transmembrane sequence are critical for the etiology of Aβ42. EMBO J. 26, 1702–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vooijs M., Schroeter E. H., Pan Y., Blandford M., Kopan R. (2004) Ectodomain shedding and intramembrane cleavage of mammalian Notch proteins is not regulated through oligomerization. J. Biol. Chem. 279, 50864–50873 [DOI] [PubMed] [Google Scholar]
- 43. Lammich S., Okochi M., Takeda M., Kaether C., Capell A., Zimmer A. K., Edbauer D., Walter J., Steiner H., Haass C. (2002) Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Aβ-like peptide. J. Biol. Chem. 277, 44754–44759 [DOI] [PubMed] [Google Scholar]
- 44. Eggert S., Paliga K., Soba P., Evin G., Masters C. L., Weidemann A., Beyreuther K. (2004) The proteolytic processing of the amyloid precursor protein gene family members APLP-1 and APLP-2 involves α-, β-, γ-, and ϵ-like cleavages. Modulation of APLP-1 processing by N-glycosylation. J. Biol. Chem. 279, 18146–18156 [DOI] [PubMed] [Google Scholar]
- 45. Kuhn P. H., Marjaux E., Imhof A., De Strooper B., Haass C., Lichtenthaler S. F. (2007) Regulated intramembrane proteolysis of the interleukin-1 receptor II by α-, β-, and γ-secretase. J. Biol. Chem. 282, 11982–11995 [DOI] [PubMed] [Google Scholar]
- 46. Yanagida K., Okochi M., Tagami S., Nakayama T., Kodama T. S., Nishitomi K., Jiang J., Mori K., Tatsumi S., Arai T., Ikeuchi T., Kasuga K., Tokuda T., Kondo M., Ikeda M., Deguchi K., Kazui H., Tanaka T., Morihara T., Hashimoto R., Kudo T., Steiner H., Haass C., Tsuchiya K., Akiyama H., Kuwano R., Takeda M. (2009) The 28-amino acid form of an APLP1-derived Aβ-like peptide is a surrogate marker for Aβ42 production in the central nervous system. EMBO Mol. Med. 1, 223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]