Abstract
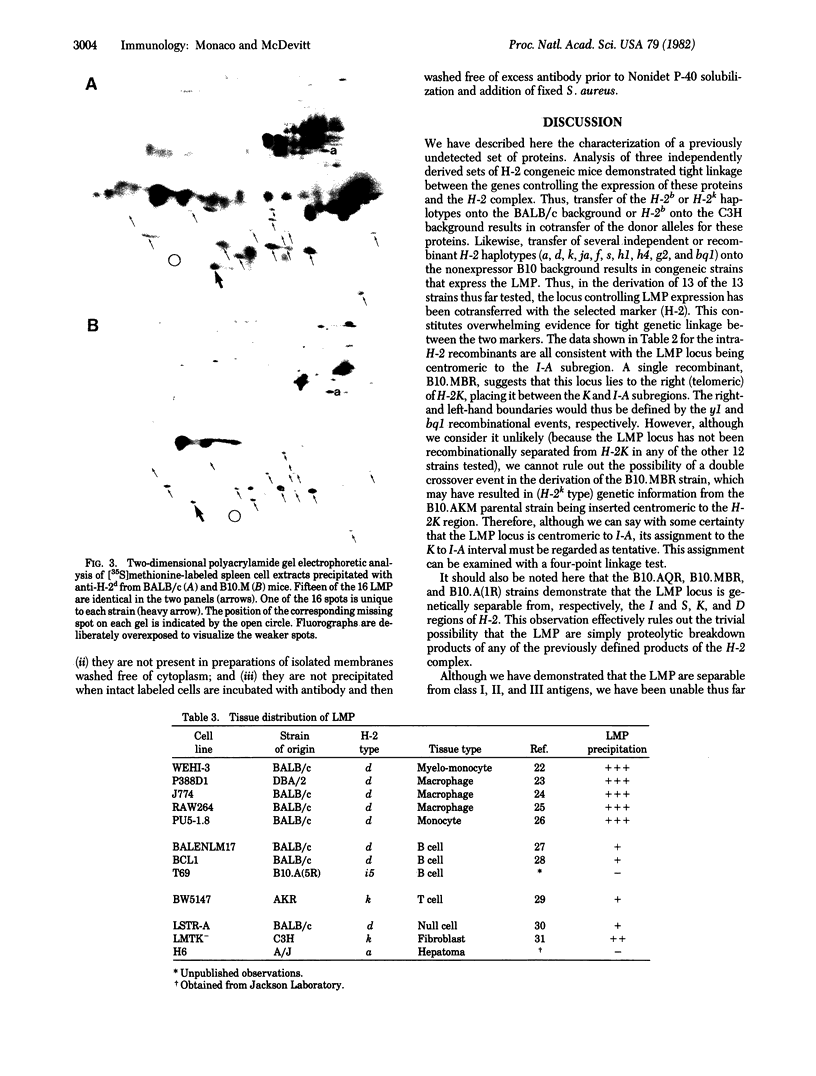
A series of proteins biochemically and genetically distinct from previously defined murine major histocompatibility complex class I and class II antigens is precipitated by a congeneic anti-H-2d antiserum. Sixteen such proteins have been defined, exhibiting a range of molecular weights (approximately 15,000-30,000) and isoelectric points (pI approximately 4-9). These proteins are not glycosylated, and they are probably not expressed at the cell surface. They are expressed most strongly in normal macrophages and macrophage cell lines and are also found in fibroblasts, B, T, and null cell lines. The genes controlling the expression of these proteins have been tentatively mapped within the H-2 complex, between the K and I-A subregions. Three alleles have been defined: mice of the H-2 haplotypes b and q possess a "null" allele, i.e., do not express any demonstrable protein product. Mice of the d haplotype cane be distinguished by their two-dimensional gel pattern from mice of all other positive H-2 types tested thus far (a, k, f, s, and ja).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J. Cytotoxic t-cell response to histocompatibility antigens: the role of H-2. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):519–527. doi: 10.1101/sqb.1977.041.01.060. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Chesebro K., Portis J. Characterization of Ia8 antigen, thy-1.2 antigen, complement receptors, and virus production in a group of murine virus-induced leukemia cell lines. J Immunol. 1976 Oct;117(4):1267–1274. [PubMed] [Google Scholar]
- Cullen S. E., Freed J. H., Nathenson S. G. Structural and serological properties of murine Ia alloantigens. Transplant Rev. 1976;30:236–270. doi: 10.1111/j.1600-065x.1976.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Jones P. P. Analysis of H-2 and Ia molecules by two-dimensional gel electrophoresis. J Exp Med. 1977 Nov 1;146(5):1261–1279. doi: 10.1084/jem.146.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., McDevitt H. O. Two-gene control of the expression of a murine Ia antigen. J Exp Med. 1978 Oct 1;148(4):925–939. doi: 10.1084/jem.148.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Klein J., Juretic A., Baxevanis C. N., Nagy Z. A. The traditional and a new version of the mouse H-2 complex. Nature. 1981 Jun 11;291(5815):455–460. doi: 10.1038/291455a0. [DOI] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Klein P. A. Anomalous reactions of mouse alloantisera with cultured tumor cells. I. Demonstration of widespread occurrence using reference typing sera. J Immunol. 1975 Nov;115(5):1254–1260. [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Klein P. A. Anomalous reactions of mouse alloantisera with cultured tumor cells. II. Cytotoxicity is caused by antibodies to leukemia viruses. J Immunol. 1975 Nov;115(5):1261–1268. [PubMed] [Google Scholar]
- Okumura K., Herzenberg L. A., Murphy D. B., McDevitt H. O., Herzenberg L. A. Selective expression of H-2 (i-region) loci controlling determinants on helper and suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):685–698. doi: 10.1084/jem.144.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Ralph P., Moore M. A., Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976 Jun 1;143(6):1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Antibody-dependent killing of erythrocyte and tumor targets by macrophage-related cell lines: enhancement by PPD and LPS. J Immunol. 1977 Sep;119(3):950–954. [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature. 1975 Oct 2;257(5525):393–394. doi: 10.1038/257393a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., David C. S., Sachs D. H., Paul W. E. T lymphocyte-enriched murine peritoneal exudate cells. III. Inhibition of antigen-induced T lymphocyte Proliferation with anti-Ia antisera. J Immunol. 1976 Aug;117(2):531–540. [PubMed] [Google Scholar]
- Shearer G. M., Schmitt-Verhulst A. M. Major histocompatibility complex restricted cell-mediated immunity. Adv Immunol. 1977;25:55–91. doi: 10.1016/s0065-2776(08)60931-1. [DOI] [PubMed] [Google Scholar]
- Shreffler D. C. The S region of the mouse major histocompatibility complex (H-2): genetic variation and functional role in complement system. Transplant Rev. 1976;32:140–167. doi: 10.1111/j.1600-065x.1976.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Singer A., Hathcock K. S., Hodes R. J. Cellular and genetic control of antibody responses. VIII. MHC restricted recognition of accessory cells, not B cells, by parent-specific subpopulations of normal F1 T helper cells. J Immunol. 1980 Mar;124(3):1079–1085. [PubMed] [Google Scholar]
- Slavin S., Strober S. Spontaneous murine B-cell leukaemia. Nature. 1978 Apr 13;272(5654):624–626. doi: 10.1038/272624a0. [DOI] [PubMed] [Google Scholar]
- Sung E., Jones P. P. The invariant chain of murine Ia antigens: its glycosylation, abundance and subcellular localization. Mol Immunol. 1981 Oct;18(10):899–913. doi: 10.1016/0161-5890(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Warner N. L., Moore M. A., Metcalf D. A transplantable myelomonocytic leukemia in BALB-c mice: cytology, karyotype, and muramidase content. J Natl Cancer Inst. 1969 Oct;43(4):963–982. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]