Background: Protective antigen (PA), a key component of anthrax toxin, is neutralized by the antibody cAb29 by an unknown mechanism.
Results: cAb29 binds monomeric or heptameric PA, preventing its ability to form the characteristic transmembranal pore.
Conclusion: cAb29 exerts its potent neutralizing activity in a novel and unique manner.
Significance: This study mechanistically demonstrates how cAb29 affects the hallmark steps in PA-based intoxication.
Keywords: Anthrax Toxin, Antibodies, Antibody Engineering, Bacillus, Bacterial Toxins
Abstract
Protective antigen (PA), a key component of anthrax toxin, mediates the entry of lethal factor (LF) or edema factor (EF) through a membranal pore into target cells. We have previously reported the isolation and chimerization of cAb29, an anti-PA monoclonal antibody that effectively neutralizes anthrax toxin in an unknown mechanism. The aim of this study was to elucidate the neutralizing mechanism of this antibody in vitro and to test its ability to confer post-exposure protection against anthrax in vivo. By systematic evaluation of the steps taking place during the PA-based intoxication process, we found that cAb29 did not interfere with the initial steps of intoxication, namely its ability to bind to the anthrax receptor, the consecutive proteolytic cleavage to PA63, oligomerization, prepore formation, or LF binding. However, the binding of cAb29 to the prepore prevented its pH-triggered transition to the transmembranal pore, thus preventing the last step of intoxication, i.e. the translocation of LF/EF into the cell. Epitope mapping, using a phage display peptide library, revealed that cAb29 binds the 2α1 loop in domain 2 of PA, a loop that undergoes major conformational changes during pore formation. In vivo, we found that 100% of anthrax-infected rabbits survived when treated with cAb29 12 h after exposure. In conclusion, these experiments demonstrate that cAb29 exerts its potent neutralizing activity in a unique manner by blocking the prepore-to-pore conversion process.
Introduction
Bacillus anthracis, the causative agent of anthrax, exerts its toxicity via the dissemination of a tripartite exotoxin composed of protective antigen (PA),2 lethal factor (LF), and edema factor (EF). Cell intoxication involves several steps, the first of which is the binding of PA83 to its cellular receptor (TEM8 or CMG2). Following binding, a 20-kDa region is proteolytically removed by a furin-like protease, whereas the remaining PA63 forms an oligomeric structure (heptamer or octamer, also referred to as a prepore) that binds EF or LF to form edema toxin or lethal toxin (LeTx), respectively. Following endocytosis of the prepore-EF/LF complex, an acid-driven prepore-to-pore conversion occurs, thus promoting the entry of EF/LF into the cytosol, where they exert their toxic effects (1).
Anthrax is considered a biological threat, and B. anthracis is classified as a category A agent by the Centers for Disease Control and Prevention. Currently, a three-dose vaccination schedule for anthrax using PA-based vaccine is indicated by the Food and Drug Administration as the post-exposure treatment together with a prolonged antibiotic regimen (2, 3). However, in cases where disease has progressed and a substantial amount of anthrax toxins has been delivered to the bloodstream, or when antibiotic-resistant B. anthracis strains are involved, these treatments will be less effective, highlighting the need for additional post-exposure treatment. To this end, passive transfer of neutralizing antibodies directed against either PA or LF was suggested as a complementary treatment that can provide immediate, specific, and low toxicity protection (4, 5). Indeed, over the past decade, extensive research was carried out to develop therapeutic antibodies that target anthrax toxins and can provide protection either when given alone or when given with antibiotic treatment (6). The neutralizing mechanisms of these antibodies were shown to span almost every step of the intoxication process, including the inhibition of PA-receptor interaction, proteolytic cleavage, heptamerization, internalization, and EF/LF binding.
We have previously isolated a monoclonal antibody, mab29, which possesses a highly potent LeTx neutralization activity, and converted it to a human IgG1-based chimeric antibody (cAb29), which was able to confer full protection to guinea pigs when given prior to infection with 40 LD50 B. anthracis spores (7, 8). The main goals of this study were to characterize the LeTx-neutralizing mechanism of cAb29 by systematic biochemical examination of the influence of this antibody on the hallmark steps in the PA-based intoxication process and to test its ability to confer post-exposure protection against anthrax in vivo.
EXPERIMENTAL PROCEDURES
Materials
PA and LF were purified by Q-Sepharose chromatography, essentially as described previously (9). Chimeric anti-PA monoclonal antibody (cAb29) was produced from a recombinant CHO cell line as described previously, and mouse IgG anti-PA monoclonal antibody (Ab33) was produced in ascetic fluid (8). Antibodies were purified by affinity chromatography on HiTrap protein G/A (GE Healthcare, Uppsala, Sweden) according to the manufacturer's instructions and dialyzed against PBS, pH 7.4.
Cellular Impedance Assay
The xCELLigence system (Roche Applied Science) was employed to measure the changes in cellular impedance following the addition of PA. Initial background measurements were carried out from 0.1% gelatin-coated E-plates (Roche Applied Science) filled with cell culture medium (EGM-2, Lonza Clonetics) followed by the addition of human vascular endothelial cells (Lonza Clonetics; 10,000 cells/well). Cells were grown overnight (37 °C, 5% CO2) until growth plateau was achieved. Cells were treated with the indicated compounds, and the cell index values were obtained immediately following ligand stimulation every 20 s. Cell index values are normalized to parallel values recorded in vehicle-treated wells.
Inhibition of Furin Cleavage
PA was incubated with furin (Sigma) for 30 min at room temperature in the absence or presence of neutralizing antibody (1:1 molar ratio). Samples were then resolved on a 4–15% SDS-PAGE and visualized using silver stain.
Prepore and Pore Formation
PA83 (5 μg, 60 pmol) was incubated with trypsin (5 ng) for 30 min at room temperature, and the reaction was stopped by the addition of a 5-fold excess of soybean trypsin inhibitor. The nicked PA was incubated with LF (60 pmol) for 20 min at room temperature, resolved on a 4–16% native gel (Novex), and visualized with Coomassie Blue. To obtain purified PA63 oligomers (prepore), nicked PA (2 mg) was desalted and loaded on a HiTrap Q-column (GE Healthcare) in a buffer containing 20 mm Tris-HCl, pH 8, and the prepore fraction was eluted by 1 m NaCl gradient (10). Prepore was converted into the SDS-resistant pore by incubation in low pH (50 mm MES buffer, pH 5.5, 150 mm NaCl), resolved on a 7% SDS-PAGE gel, and visualized with silver stain.
Binding Assays
Binding studies were carried out using the Octet RED system (ForteBio) that measures bio-layer interferometry. All steps were performed at 30 °C with shaking at 1500 rpm in a 96-well plate containing 200 μl of solution in each well. Streptavidin-coated biosensors were used throughout this study, and analytes (10 μg/ml) were diluted in PBS buffer, pH 7.4, containing 1 mg/ml BSA and 0.1% (v/v) Tween 20. Biosensors were loaded with biotinylated antibodies (50 μg/ml) for 300 s followed by wash. Binding and dissociation were measured as changes over time in light interference, and curves are presented after subtraction of parallel measurements from unloaded biosensors.
Neutralization Assay
Toxin cytotoxicity and neutralizing antibody activity were determined essentially as described previously (8, 9, 11) using the murine macrophage J774A.1 cells (ATCC). Cells (30 × 103/well) were incubated for 5 h with either PA83 or PA prepore (10 ng/ml) in the presence of LF (100 ng/ml) and different concentrations of neutralizing antibodies. Cell viability was determined using the XTT assay (Biological Industries, Beit Haemek, Israel), and the percentage of cell survival was plotted against antibody concentration.
Animal Studies
New Zealand white rabbits (2.5–3 kg) were obtained from Charles River Laboratories (Wilmington, MA). All animal experiments were performed in accordance with Israeli law and were approved by the Ethics Committee for Animal Experiments at the Israel Institute for Biological Research. Animals were maintained at 20–22 °C and a relative humidity of 50 ± 10% on a 12-h light/dark cycle, fed with commercial rodent chow (Koffolk Inc.), and provided with tap water ad libitum. Treatment of animals was in accordance with regulations outlined in the United States Department of Agriculture Animal Welfare Act and the conditions specified in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 1996).
Pharmacokinetic and Challenge Studies
For pharmacokinetics studies, rabbits were injected intravenously with 1 mg of purified cAb29, and blood samples were drawn at different time points. Antibody concentration in the blood samples was determined by ELISA using PA-coated 96-well microtiter plates (Nunc, Roskilde, Denmark) and alkaline phosphatase-conjugated secondary anti-human antibody for detection. The pharmacokinetic parameters were calculated using the PK Solutions software (Summit Research Services). For challenge experiments, animals were inoculated via the respiratory route by intranasal instillation of 5 × 106 cfu of fully virulent Vollum spores (200 LD50). The infected rabbits were treated with different doses of purified cAb29 antibody given intravenously at the indicated time points. Animal viability was monitored for at least 14 days.
Epitope Mapping
The Ph.D.-7 phage display library kit (New England Biolabs) was used for panning against immobilized cAb29 antibody, essentially as recommended by the manufacturer. After three rounds of selection, individual phage clones were isolated and tested for specific binding to cAb29 by ELISA. The positive clones were subjected to DNA sequencing to deduce the amino acid sequence of the presented peptides. The peptide sequences from the positive phages were aligned with ClustalW software.
RESULTS
Post-exposure Prophylaxis following Intranasal B. anthracis Challenge
cAb29 is a chimeric antibody that was selected from a large panel of PA-neutralizing monoclonal antibodies (8). It was shown that if given to guinea pigs prior to infection, cAb29 can provide full protection against anthrax. Here, we first sought to evaluate its ability to confer post-exposure protection in the stricter model of anthrax infection, namely infection by the respiratory route. Because the ability of the antibody to neutralize PA in vivo is a function of its residence time in the blood (8), we first determined the pharmacokinetic profile of the chimeric antibody following intravenous administration to rabbits. Blood samples were drawn at different time points after administration, antibody levels were determined by ELISA, and the obtained data were fitted to a one-compartment, first-order elimination model (Fig. 1A). The cAb29 antibody exhibited a biphasic elimination profile with mean resident time of 82 h. The first elimination phase (α) displayed a t½ value of 4 h (5% of area under the curve), whereas the second phase (β) was significantly longer, with t½ of 59 h.
FIGURE 1.
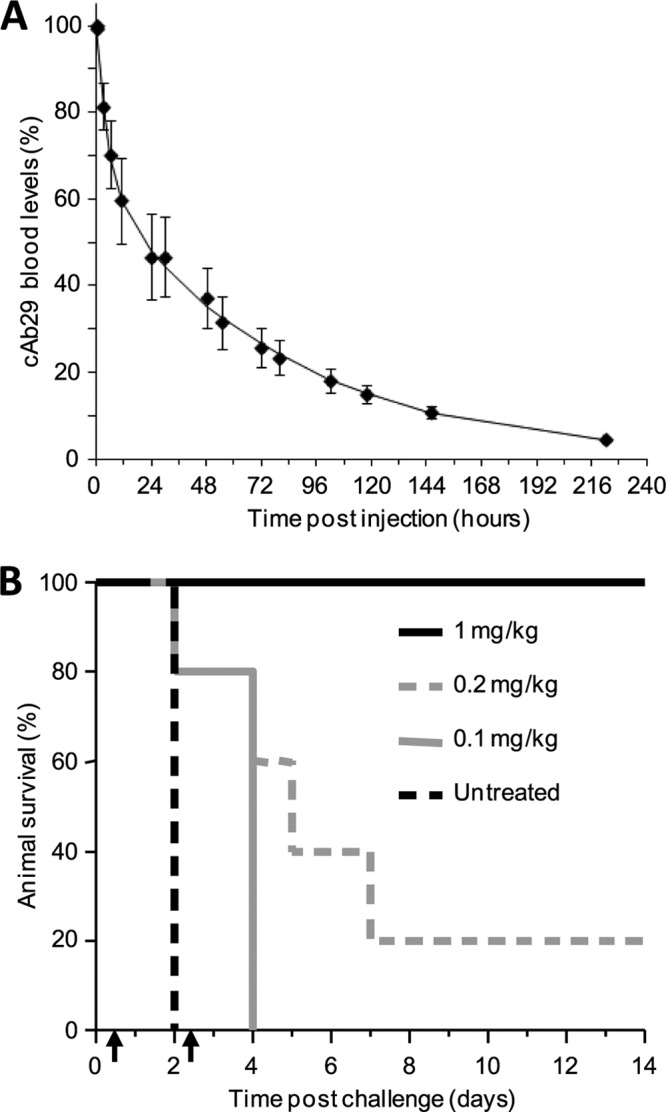
Post-exposure protection of rabbits against anthrax infection. A, circulatory clearance profile of cAb29 in rabbits. Rabbits (n = 3) were injected intravenously with 1 mg of cAb29. Blood samples drawn at various time points were assayed for antibody concentration by ELISA. Mean ± S.D. values are presented as percentages of maximum blood levels (Cmax). B, survival of rabbits following intranasal B. anthracis infection. Rabbits (n = 5 for each group) were intranasally infected with 5 × 106 cfu of Vollum spores (200 LD50) and treated with two successive treatments of cAb29 at the indicated doses, given intravenously 12 and 60 h after infection (indicated by arrows).
The prophylaxis efficacy of cAb29 was evaluated in rabbits that were intranasally infected with 5 × 106 cfu of Vollum spores (200 LD50). Under these conditions, all untreated rabbits rapidly developed respiratory anthrax and died within 2 days (Fig. 1B). Based upon the pharmacokinetic profile of the chimeric antibody, we decided to administer the antibody in two successive treatments, given 12 and 60 h after infection. Treatment with a low dose of antibody (0.1 mg/kg each) did not provide protection but prolonged the mean time to death. A slight increase in antibody to two doses of 0.2 mg/kg each resulted in 20% rabbit survival. All animals treated twice with 1 mg/kg survived the challenge with no evident disease within 14 days after challenge.
The finding that cAb29 is highly potent in vivo encouraged us to continue and explore its PA neutralization mechanism. To this end, we systematically examined the effect of cAb29 on the hallmark steps in the PA-based intoxication process.
cAb29 Does Not Hinder PA Binding to Cellular Receptors
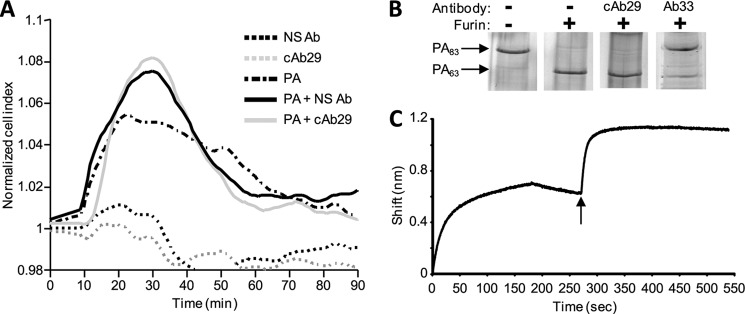
The first event in LeTx intoxication involves the binding of PA83 to one of its specific cellular receptors, TEM8 or CMG2 (12, 13). To evaluate whether cAb29 hampers PA83-cell receptor binding, we utilized the cellular impedance assay (using the xCELLigence system), which allows detection of physiological responses of cells, such as morphology, cell adhesion, and viability, expressing them as a change in cell index (14). Loss of cell adhesion in this system would generate a reduction in cell index, whereas increase in cell adhesion (usually seen after receptor binding) would result in an increase in cell index. Here, primary culture of human vascular endothelial cells was used as a model. These cells were found to obtain a minimal “transient negative phase,” which allows a more accurate monitoring of rapid changes in cell impedance following receptor binding. The addition of PA (25 nm) to these cells results in a typical change in cellular impedance starting 10 min following stimulation and returning to the resting cell index within 60 min (Fig. 2A). A similar pattern was observed when PA was added to the cells in the presence of a nonspecific antibody or in the presence of the neutralizing antibody cAb29 (125 nm), suggesting that this antibody does not interfere with the binding of PA to its receptor. In addition, cAb29 did not affect the binding of biotinylated PA to cultured cells, as judged by Western blot analysis (data not shown).
FIGURE 2.
cAb29 does not interfere with the initial steps of intoxication. A, PA induced cellular impedance. Measurements were carried out on human vascular endothelial cells following the addition of nonspecific antibody (NS Ab) or cAb29 in the absence or presence of PA. Impedance responses were normalized, and the baseline was corrected and expressed as the change in cell index. B, in vitro proteolysis of PA. PA was incubated with furin in the absence or presence of antibodies, and the samples were analyzed on SDS-PAGE. C, pairwise mapping analysis. Real time binding of antibodies to PA was measured using bio-layer interferometry. Biotinylated cAb29 was immobilized to streptavidin biosensor and then immersed in PA solution (0–180 s). Following a short wash (180–260 s), the sensor was dipped in Ab33 solution (indicated by the arrow, 260–440 s) followed by another wash step (440–550 s).
cAb29 Does Not Affect in Vitro Proteolytic Cleavage of PA
After PA83 binds to its cell surface receptor, a 20-kDa fragment (PA20) is cleaved by furin-like proteases, thus enabling the subsequent oligomerization of PA and the exposure of a large hydrophobic surface that later on facilitates binding of either LF or EF. In vitro, incubation of PA with furin results in the formation of PA63 that remains associated to PA20 (referred to as nicked PA), and the two fragments can be dissociated and resolved by SDS-PAGE (Fig. 2B). To determine whether cAb29 inhibits the proteolysis of PA by furin, the antibody was incubated with PA prior to furin addition. It was found that the presence of cAb29 has no effect on this process as judged by the appearance of PA63 (Fig. 2B). As a control, we used another anti-PA-neutralizing antibody, Ab33. This antibody was isolated from the same pool of PA-neutralizing antibodies as cAb29 and exhibited high neutralization potency both in vitro and in vivo (7, 8). Moreover, based on the binding pattern in ELISA, it was previously suggested that cAb29 and Ab33 bind PA at nonoverlapping epitopes (7, 8). Indeed, Ab33 significantly inhibited PA proteolysis by furin (Fig. 2B).
To use Ab33 as an internal control throughout the rest of this study, we performed a pairwise mapping analysis to verify that these two antibodies can bind different epitopes on PA. To this end, we used the Octet RED bio-layer interferometry system, in which binding of molecules to the biosensor causes a wavelength shift in the interference pattern, which can be measured in real time. In this experiment, binding of PA to a cAb29-coated biosensor resulted in a wavelength shift, and the addition of Ab33 to this complex resulted in an additional shift, indicating that these two antibodies indeed bind different, nonoverlapping epitopes on PA (Fig. 2C).
cAb29 Can Bind the Prepore Form
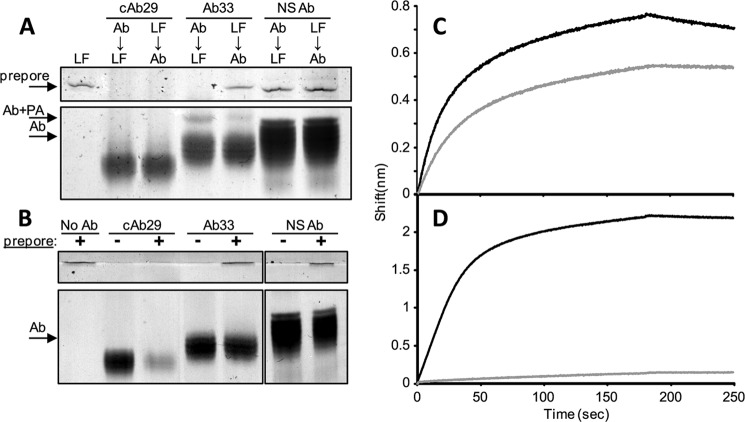
Although in vitro cleavage of PA results in the formation of PA63 and PA20, these two subunits do not readily dissociate from each other, therefore preventing the spontaneous oligomerization of PA63 (15). However, the addition of LF or EF to nicked PA releases PA20 and induces the spontaneous oligomerization of PA63 to its prepore form, which appears as a flat and discrete band in native PAGE analysis (Fig. 3A). The fact that the formation of the prepore can be initiated by the addition of LF was utilized as a tool for determining the role of the different antibodies in prepore formation. As can be expected, the formation of the prepore was not affected by the presence of a nonspecific antibody that was added either before or after LF addition. However, incubation of nicked PA with Ab33 prior to the addition of LF inhibited prepore formation. In this sample, a band with a somewhat higher molecular weight than the antibody appeared, probably representing the Ab33-PA complex, the formation of which prevented the oligomerization process. When Ab33 was added to the LF-induced oligomer, this band was much fainter, probably because most of the PA was already converted to the prepore form. When cAb29 was incubated with nicked PA prior to the addition of LF, prepore was not formed, suggesting that the presence of this antibody has also inhibited the ability of LF to interact with PA. However, no prepore was observed even when the cAb29 was added to the pre-existing prepore (after LF addition). These unexpected results led us to hypothesize that cAb29 does not interfere with the process of PA oligomerization, but rather binds the oligomer and produces a high molecular weight complex that cannot be resolved on the native PAGE.
FIGURE 3.
The role of cAb29 in prepore formation. A, nicked PA was incubated with LF before or after the addition of the indicated antibodies (Ab). LF-induced prepore formation was analyzed by native PAGE. NS Ab, nonspecific antibody. B, purified prepore (obtained by anion exchange chromatography of nicked PA) was mixed with an equimolar concentration of the indicated antibodies, and the mixture was analyzed by native PAGE. No Ab, no antibody. C and D, real time binding of PA (C) or purified prepore (D) to cAb29 (black line) or Ab33 (gray line) was measured using bio-layer interferometry (0–180 s for binding phase and 180–250 s for the wash phase).
To try and determine whether cAb29 can bind the prepore form of PA, another approach was taken. It was previously shown that a purified prepore fraction can be obtained without the need for LF/EF, merely by disrupting the electrostatic interactions between PA20 and PA63 using anion exchange chromatography (10). Here, nicked PA was loaded on a HiTrap Q-column, and a purified prepore form was obtained (Fig. 3B). Adding Ab33 or a nonspecific antibody to the prepore fraction had no effect on its appearance in the gel. However, when cAb29 was incubated with the purified prepore (in an equimolar concentration of antibody per calculated PA63 monomers), both bands of the prepore-associated antibody and the antibody itself were diminished, thus supporting the notion that cAb29 forms high molecular weight complexes with the prepore.
To further verify that cAb29 can bind the preformed oligomer, we again utilized the Octet RED apparatus. If PA83 monomer was used as the analyte and allowed to interact with cAb29- or Ab33-coated biosensors, classic 1:1 association/dissociation kinetics were obtained for both antibodies, with slight differences between them (cAb29 has a higher kon and a somewhat lower koff) (Fig. 3C). However, if the purified prepore fraction was used as the analyte, a binding curve was observed only for the cAb29-coated biosensor (Fig. 3D), supporting the overall findings presented so far.
cAb29 Does Not Interfere with LF Binding to Prepore
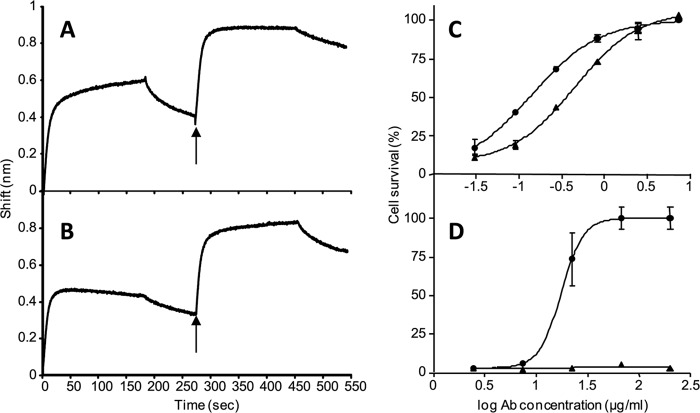
The next step of LeTx intoxication that follows the spontaneous oligomerization of PA63 on the cell surface is binding of three to four LF or EF units to the exposed hydrophobic subunit-subunit interface (16). Here, we took advantage of the fact that we can monitor the binding of the antibody to the purified prepore in the Octet RED system to determine whether its presence will affect the binding of LF to the prepore. To this end, a cAb29-coated Octet RED biosensor was saturated with the purified prepore, and after removal of prepore excess, a baseline was set. In theory, one molecule of fixed antibody is enough to bind one molecule of oligomer, hence leaving several antibody binding sites unoccupied on each oligomer. We therefore added another step in which the cAb29-prepore complex was incubated again with free cAb29 to saturate all antibody binding sites (Fig. 4A, 0–280 s). The complex was then immersed in LF, resulting in another significant wavelength shift (∼0.4 nm) that clearly indicates that LF can bind to the cAb29-prepore saturated complex. Similarly, adding LF to the bound prepore before saturating the cAb29 binding sites resulted in the same wavelength shift amplitude (∼0.4 nm) and interference pattern as seen before (compare Fig. 4B, 0–280 s with Fig. 4A, 280–550 s). Moreover, saturating the LF-bound complex with cAb29 also revealed a similar shift and pattern as seen earlier. It can be therefore concluded that the binding of cAb29 to the prepore complex does not interfere with the subsequent binding of LF, and vice versa, and that the two molecules bind different sites.
FIGURE 4.
cAb29 binds and neutralizes LF-prepore complexes. A and B, sequential binding of cAb29 and LF to the purified prepore was measured using bio-layer interferometry. Biotinylated cAb29 was immobilized on a streptavidin biosensor and reacted with the purified prepore. The biosensor was then immersed (0–180 s) in cAb29 solution (A) or LF (B) followed by a short wash (180–260 s). The sensor was then dipped (indicated by arrows) in a solution containing LF (A) or cAb29 (B) (260–440 s) followed by another wash step (440–550 s). C and D, the neutralization assay was performed using cultured J774A macrophages that were incubated for 5 h with fixed amounts of LF and PA83 (C) or purified prepore (D) in the presence of cAb29 (circles) or Ab33 (triangles) in the indicated concentrations. Cell survival was determined by XTT and plotted as the percentage of untreated control cells. Points are mean ± S.D. of triplicate determinants. Ab concentration, antibody concentration.
It was previously shown that PA can be cleaved in the bloodstream, thus forming soluble heptamers that complex with EF/LF and bind their cellular receptor (17). In vitro, this process can be simulated by incubating the purified prepore fraction with LF and then intoxicating the cells. Because cAb29 can bind to the prepore-LF complex, we asked whether the antibody will be able to neutralize the toxic activity of this preformed complex. In the classic LeTx neutralization assay, cultured J774A macrophages are exposed to a lethal dose of PA83 and LF in the presence of increasing antibody concentration, and cell survival is determined 5 h later. In this format, both cAb29 and Ab33 can protect the cells, and the effective concentration needed to neutralize 50% of LeTx activity (EC50) was 140 and 450 ng/ml for cAb29 and Ab33, respectively (Fig. 4C). In the second scenario, cells were exposed directly to the prepore-LF complex (calibrated to >90% cell killing) in the presence of increasing concentrations of cAb29 and Ab33 antibodies, and cell survival was monitored. It was found that under these conditions, only cAb29 could neutralize the toxicity of the preformed LF-oligomer complex, whereas antibody Ab33, which cannot bind the prepore form, had no effect on cell survival (Fig. 4D).
cAb29 Prevents Prepore-to-Pore Conversion
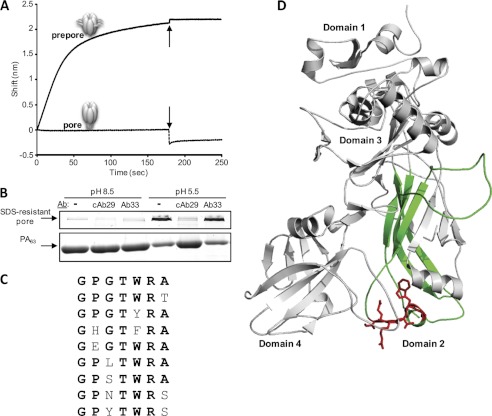
The internalization of the receptor-bound prepore oligomer into the acidic endosome enables the prepore to undergo a major conformational change, which mainly involves the large disordered flexible loop of domain 2 (2β2-2β3; residues 302–325) (15, 18). The resulting 14-stranded transmembranal β-barrel pore forms the channel through which LF or EF is translocated into the cytosol. In vitro, stable, irreversible pore structures can be obtained by exposing the purified prepore to low pH (19) without the need for membranal receptors or LF/EF. We therefore sought to determine whether these conformational changes will have any effect on the ability of cAb29 to bind the oligomer. Purified prepore was converted to the pore state by a pH drop (to pH 5.5), and the binding of cAb29 to the pore was determined in the Octet RED system. It was found that the antibody cannot bind the pore, as judged by the lack of change in wavelength interference (Fig. 5A). Even after readjusting the pH back to its original value (pH 8.5), no binding could be detected, suggesting that once the prepore converts to pore, the antibody recognition site is not accessible.
FIGURE 5.
Inhibition of prepore-to-pore conversion by cAb29. A, the binding of cAb29 to the prepore or pore forms was measured using bio-layer interferometry. Biotinylated cAb29 was immobilized to streptavidin biosensor and then immersed in a prepore containing solution (solid line; pH 8.5, 0–180 s) and then dipped in a pH 5.5 buffer (indicated by arrows) for another 70 s. In another set of experiments, the cAb29-coated biosensor was dipped in a pore containing solution (dashed line; pH 5.5, 0–180 s) and then dipped (indicated by arrows) in a pH 8.5 buffer for another 70 s. B, purified prepore (pH 8.5) was incubated with cAb29 or Ab33, and then the pH was either kept at 8.5 or changed to 5.5. The samples were then resolved by SDS-PAGE to monitor the formation of a SDS-resistant pore structure. Ab, antibody. C, alignment of the deduced amino acid sequences of phage-displayed peptide clones that bind cAb29. The consensus amino acids are highlighted in bold. D, crystal structure of PA63 (Protein Data Bank (PDB) 1TZO), emphasizing the cAb29 epitope (residues 342–348, plotted in red) and the Greek key motif (residues 262–368, plotted in green).
Interestingly, if the prepore was allowed to bind the antibody in a neutral pH (Fig. 5A, 0–180 s), and the cAb29-prepore complex was then immersed in an acidic solution (180–250 s), the bound oligomer did not dissociate from the antibody. These surprising results hinted that the binding of cAb29 to the prepore may inhibit the transition of the prepore into the pore form. To further explore this hypothesis, we took advantage of the fact that the pore form can be easily distinguished from the prepore form by SDS-PAGE analysis (19). Treatment of the prepore (kept at pH 8.5) with SDS results in its dissociation to its monomeric PA63 subunits (Fig. 5B). However, the pore form (equilibrated to pH 5.5) is SDS-resistant and appears mainly as a high molecular weight band in SDS-PAGE analysis. Here, the prepore was first incubated with the tested antibodies at pH 8.5, after which the pH was either kept at the same value or changed to pH 5.5 (supposedly resulting in the formation of the SDS-resistant pore). As established previously, our control antibody (Ab33) does not bind the purified prepore and indeed had no effect on the pH-dependent prepore-to-pore conversion as indicated by the appearance of the SDS-resistant high molecular weight band. Incubating the prepore with cAb29 (in pH 8.5) before exposing the complex to SDS did not alter its dissociation to its monomeric subunits. However, upon exposure of the prepore-cAb29 complex to low pH, no SDS-resistant form was formed, but rather, monomeric PA63 is seen in the gel analysis, clearly demonstrating that the presence of cAb29 inhibited prepore-to-pore conversion.
Mapping of the Epitope Recognized by cAb29
These unique findings have encouraged us to perform epitope mapping analysis using a random peptide (7-mer) phage display library to identify the residues that are recognized by the cAb29 antibody. After three rounds of panning, several phages that exhibited high selective binding toward cAb29 were isolated, and the sequence of the presented peptide was determined. Using ClustalW software, a consensus sequence was deduced with the common motif GPXTWRX, which could be best fitted with the PA sequence 342GERTWAE348 (Fig. 5C, plotted in red in the crystal structure of PA63). Interestingly, this epitope is part of the 2α1 loop, which is part of the “Greek key” motif (domain 2, residues 262–368, colored green) that together with 2β2 and 2β3 strands (residues 303–322) are predicted to peel away from the domain 2 core, thus forming the extended β-barrel pore (together with the 2β1, 2β4 strands) (20, 21). This finding may explain why the binding of cAb29 to PA prevents its conversion to pore and why upon conversion the epitope is no longer recognized by the antibody.
DISCUSSION
In this study, we provide evidence that cAb29 inhibits PA in a unique mechanism by blocking its ability to convert from prepore-to-pore state. Over the course of this study, we have systematically evaluated the steps taking place during the PA-based intoxication process in the presence of cAb29 or another PA-neutralizing antibody, Ab33, which served as a control. We found that cAb29 does not interfere with the initial step of anthrax intoxication, namely its ability to bind to the anthrax receptor (Fig. 2). Similarly, cAb29 does not inhibit the furin-based proteolytic cleavage of PA nor its ability to oligomerize and form the prepore structure, whereas these processes are inhibited by Ab33 (Figs. 2 and 3). It was also shown that cAb29 can bind the prepore simultaneously with LF (Fig. 4), yet the binding of cAb29 to the prepore prevents its pH-triggered transition to the SDS resistance pore (Fig. 5). Based on these results, we suggest the following model for the cAb29 neutralization mechanism (schematically illustrated in Fig. 6). cAb29 can bind PA either in solution (as a monomer or oligomer) or when it is bound to the cell membrane (in contrast to Ab33, which can bind PA only in its monomeric state; its neutralization mechanism is probably due to inhibition of PA cleavage and oligomerization). According to the proposed model, the binding of cAb29 to the prepore oligomer inhibits the pH-dependent prepore-to-pore conversion inside the acidic endosome, thus preventing the last step of intoxication, namely the translocation of LF/EF into the cell cytoplasm.
FIGURE 6.
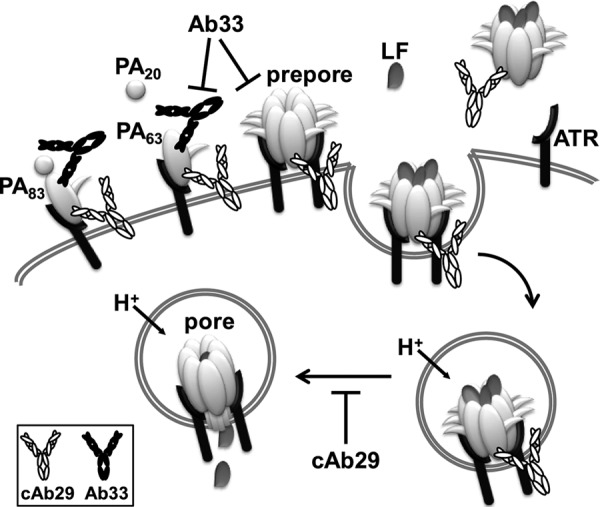
Suggested mechanism for PA neutralization by cAb29. The binding of cAb29 to PA83 does not interfere with the ability of the toxin to bind to the cellular receptor, to be cleaved by furin, or to form the prepore oligomer. In contrast, the control antibody, Ab33, does inhibit furin proteolysis and oligomerization. The binding of cAb29 to the prepore (either membrane-bound or soluble) does not prevent the concurrent binding of LF to the oligomer. However, this antibody blocks the pH-dependent prepore-to-pore conversion process that takes place in the endosomal compartment, thus preventing the translocation of LF (or EF) into the cell. ATR, anthrax receptor.
As recently reviewed (6), numerous PA-neutralizing monoclonal antibodies directed against all the four domains of PA were isolated and described. Those antibodies were found to neutralize almost each step of the intoxication process, yet to the best of our knowledge, this is the first study that demonstrates that PA intoxication can be neutralized by inhibiting the prepore-to-pore conversion process. Although several antibodies against domain 2 of PA were isolated before, all inhibiting PA proteolysis and/or heptamer formation, the 2β1 loop was not described as the target of any monoclonal antibody. Moreover, this epitope was not included in the repertoire of anti-PA polyclonal sera (22), suggesting that it is not highly immunogenic. As elaborated in our previous study (8), cAb29 was isolated in a stricter approach utilizing a neutralization functionality-based screen as the primary criterion for positive clones, rather than the classic method of selection of high PA binders by ELISA. In this screen, cAb29 was superior in its neutralizing efficacy as compared with other isolated antibodies, although its binding parameters were average, with a KD of 8 nm. It is therefore possible that the screening approach allowed us to reveal this otherwise negligible functional neutralizing antibody.
The epitope mapping analysis, which suggested residues 342–348 as the putative epitope for this antibody (Fig. 5), goes well with our observation that cAb29 can bind the prepore and not the soluble pore. These residues are part of the Greek key motif found in domain 2, which upon the acid-driven prepore-to-pore conversion undergoes major conformational changes and unfolds to form the β-hairpin amphipathic loop (residues 275–352) (20, 23), thereby disrupting the spatial structure that is recognized by the antibody.
The three-dimensional structures of PA83 and heptameric prepore bound to CMG2 revealed that the receptor binds primarily to domain 4 of PA (residues 596–735), yet another interaction occurs between residue 344 and the receptor (12, 18). It could therefore be expected that the binding of cAb29 to its epitope will affect PA binding to its receptor. However, we have found (by two independent methods) that the presence of the antibody did not interfere in this process (Fig. 2). It was previously shown that domain 4 constitutes the majority of the binding interface and that this domain alone is sufficient for binding both TEM8 and CMG2 (24). Indeed, several antibodies were found to neutralize PA via receptor binding inhibition and were all mapped to interact with residues within domain 4 (22). In addition, analysis of PA63 crystals produced at pH 6 or 7.5 shows that residues 342–348 (cAb29 epitope) are part of a flexible loop (15), altogether suggesting that the binding of cAb29 does not mask the three binding loops in domain 4 and therefore does not affect PA binding to the receptor. The importance of the residues recognized by cAb29 in the conversion process was demonstrated before by Mourez et al. (21) using scanning mutagenesis. In their study, replacement of individual PA residues (including Gly-342 and Trp-346) to cysteine blocked conversion of the prepore to the SDS-resistant pore, either by impairing their ability to contact with other residues in the transition intermediate form or by stabilizing the prepore, thus increasing the activation energy needed for the process. More studies will be needed to understand the exact mechanism by which the binding of cAb29 to the prepore is impeding its ability to convert to the pore form.
It is now well established that during anthrax progression, there are two potential mechanisms for cellular intoxication by LeTx. In the first scenario, PA can bind the receptor as a monomer, and then cleaved by furin to PA63 to form heptamers. However, PA can also be cleaved in the bloodstream, thus forming soluble heptamers that can bind the cellular receptor either alone or already bound to EF or LF (17). It can therefore be hypothesized that antibodies that bind to the heptamer form, even when it has already bound LF/EF, and inhibit the last stages of intoxication, i.e. receptor binding, internalization, and pore formation, will provide better protection against anthrax. Unfortunately, although numerous data exist regarding the potency of the numerous PA-neutralizing antibodies, it is impossible to compare the neutralizing potencies of antibodies that interfere at different stages of the intoxication process due to the large variations in the in vitro assays, animal models, infections, doses, etc. Here, we assayed two neutralizing antibodies (cAb29 and Ab33) that exhibit similar neutralizing potency in the “gold standard” in vitro LeTx assay and compared their ability to protect cells from the second intoxication mechanism (preformed oligomeric PA-LF complexes; Fig. 4). As expected, only cAb29, which retained its ability to bind the preformed prepore-LF complex, could protect the cells, whereas Ab33 was not effective at this stage. Similarly, in a recent study made by Crowe et al. (25), who compared the neutralizing activity of two sets of monoclonal antibodies directed either to the furin cleavage sites or to the receptor binding site in domain 4, it was found that although both antibodies possessed similar affinities and in vitro neutralization activities, the receptor binding site antibodies provided the best protection against in vivo LeTx challenge. It might be speculated that antibodies that will be able to inhibit the very last stage of the intoxication process, namely the prepore-to-pore conversion, will be able to confer similar if not better protection than antibodies that neutralize other intoxication stages, a point that should be addressed in the future. Moreover, anthrax toxins belong to the subgroup of bacterial AB binary toxins such as C2 toxin (Clostridium botulinum), iota toxin (Clostridium perfringens), and others, all possessing structurally conserved receptor-binding and pore-forming proteins (26). It is logical to assume that antibodies that will be able to bind the PA equivalent of the 2β2 and 2β3 strands and to inhibit the prepore-to-pore conversion will provide good protection. The lack of evidence as to the existence of such antibodies may imply (among other reasons) that these epitopes are not very immunogenic (as is probably also the case for PA). It therefore might be advantageous to develop epitope-based vaccines that will force the immune system to produce antibodies against this region in other toxins.
To summarize, we have illustrated a novel mechanism of antibody-based PA neutralization that presents new possibilities for the development of an effective epitope-based vaccine against anthrax. Moreover, cAb29 can offer post-exposure protection against B. anthracis infection, making it an attractive candidate for therapeutic preparations aimed at providing efficient and immediate anthrax toxin neutralization.
Acknowledgments
We thank Dr. Itai Glinert for providing useful discussions and Ron Alcalay and Yossi Shlomovitz for technical assistance.
Footnotes
- PA
- protective antigen
- LF
- lethal factor
- EF
- edema factor
- LeTx
- lethal toxin
- XTT
- sodium 2,3,-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)-carbonyl]-2H-tetrazolium inner salt.
REFERENCES
- 1. Young J. A., Collier R. J. (2007) Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76, 243–265 [DOI] [PubMed] [Google Scholar]
- 2. Friedlander A. M., Welkos S. L., Ivins B. E. (2002) Anthrax vaccines. Curr. Top Microbiol. Immunol. 271, 33–60 [DOI] [PubMed] [Google Scholar]
- 3. Grabenstein J. D. (2008) Vaccines: countering anthrax: vaccines and immunoglobulins. Clin. Infect. Dis. 46, 129–136 [DOI] [PubMed] [Google Scholar]
- 4. Albrecht M. T., Li H., Williamson E. D., LeButt C. S., Flick-Smith H. C., Quinn C. P., Westra H., Galloway D., Mateczun A., Goldman S., Groen H., Baillie L. W. (2007) Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect. Immun. 75, 5425–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karginov V. A., Robinson T. M., Riemenschneider J., Golding B., Kennedy M., Shiloach J., Alibek K. (2004) Treatment of anthrax infection with combination of ciprofloxacin and antibodies to protective antigen of Bacillus anthracis. FEMS Immunol. Med. Microbiol. 40, 71–74 [DOI] [PubMed] [Google Scholar]
- 6. Froude J. W, 2nd, Thullier P., Pelat T. (2011) Antibodies against anthrax: mechanisms of action and clinical applications. Toxins 3, 1433–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mazor O., Ben-Arie E., Mechaly A., Rosenfeld R., Marcus H., Ordentlich A. (2010) Combination of anthrax-toxin neutralizing antibodies: analysis of synergy/antagonism effect. in The challenge of highly pathogenic microorganisms (Shafferman A., Ordentlich A., Velan B., eds), pp. 275–285, Springer, Dordrecht, The Netherlands [Google Scholar]
- 8. Rosenfeld R., Marcus H., Ben-Arie E., Lachmi B. E., Mechaly A., Reuveny S., Gat O., Mazor O., Ordentlich A. (2009) Isolation and chimerization of a highly neutralizing antibody conferring passive protection against lethal Bacillus anthracis infection. PLoS ONE 4, e6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reuveny S., White M. D., Adar Y. Y., Kafri Y., Altboum Z., Gozes Y., Kobiler D., Shafferman A., Velan B. (2001) Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69, 2888–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mogridge J., Cunningham K., Collier R. J. (2002) Stoichiometry of anthrax toxin complexes. Biochemistry 41, 1079–1082 [DOI] [PubMed] [Google Scholar]
- 11. Singh Y., Chaudhary V. K., Leppla S. H. (1989) A deleted variant of Bacillus anthracis protective antigen is nontoxic and blocks anthrax toxin action in vivo. J. Biol. Chem. 264, 19103–19107 [PubMed] [Google Scholar]
- 12. Bradley K. A., Mogridge J., Mourez M., Collier R. J., Young J. A. (2001) Identification of the cellular receptor for anthrax toxin. Nature 414, 225–229 [DOI] [PubMed] [Google Scholar]
- 13. Scobie H. M., Rainey G. J., Bradley K. A., Young J. A. (2003) Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. U.S.A. 100, 5170–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu N., Atienza J. M., Bernard J., Blanc S., Zhu J., Wang X., Xu X., Abassi Y. A. (2006) Real time monitoring of morphological changes in living cells by electronic cell sensor arrays: an approach to study G protein-coupled receptors. Anal. Chem. 78, 35–43 [DOI] [PubMed] [Google Scholar]
- 15. Petosa C., Collier R. J., Klimpel K. R., Leppla S. H., Liddington R. C. (1997) Crystal structure of the anthrax toxin protective antigen. Nature 385, 833–838 [DOI] [PubMed] [Google Scholar]
- 16. Cunningham K., Lacy D. B., Mogridge J., Collier R. J. (2002) Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc. Natl. Acad. Sci. U.S.A. 99, 7049–7053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kintzer A. F., Sterling H. J., Tang I. I., Abdul-Gader A., Miles A. J., Wallace B. A., Williams E. R., Krantz B. A. (2010) Role of the protective antigen octamer in the molecular mechanism of anthrax lethal toxin stabilization in plasma. J. Mol. Biol. 399, 741–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lacy D. B., Wigelsworth D. J., Melnyk R. A., Harrison S. C., Collier R. J. (2004) Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc. Natl. Acad. Sci. U.S.A. 101, 13147–13151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller C. J., Elliott J. L., Collier R. J. (1999) Anthrax protective antigen: prepore-to-pore conversion. Biochemistry 38, 10432–10441 [DOI] [PubMed] [Google Scholar]
- 20. Bann J. G. (2012) Anthrax toxin protective antigen: insights into molecular switching from prepore to pore. Protein Sci. 21, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mourez M., Yan M., Lacy D. B., Dillon L., Bentsen L., Marpoe A., Maurin C., Hotze E., Wigelsworth D., Pimental R. A., Ballard J. D., Collier R. J., Tweten R. K. (2003) Mapping dominant-negative mutations of anthrax protective antigen by scanning mutagenesis. Proc. Natl. Acad. Sci. U.S.A. 100, 13803–13808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zarebski L. M., Vaughan K., Sidney J., Peters B., Grey H., Janda K. D., Casadevall A., Sette A. (2008) Analysis of epitope information related to Bacillus anthracis and Clostridium botulinum. Expert. Rev. Vaccines 7, 55–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santelli E., Bankston L. A., Leppla S. H., Liddington R. C. (2004) Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature 430, 905–908 [DOI] [PubMed] [Google Scholar]
- 24. Williams A. S., Lovell S., Anbanandam A., El-Chami R., Bann J. G. (2009) Domain 4 of the anthrax protective antigen maintains structure and binding to the host receptor CMG2 at low pH. Protein Sci. 18, 2277–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crowe S. R., Ash L. L., Engler R. J., Ballard J. D., Harley J. B., Farris A. D., James J. A. (2010) Select human anthrax protective antigen epitope-specific antibodies provide protection from lethal toxin challenge. J. Infect. Dis. 202, 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barth H., Aktories K., Popoff M. R., Stiles B. G. (2004) Binary bacterial toxins: biochemistry, biology, and applications of common Clostridium and Bacillus proteins. Microbiol. Mol. Biol. Rev. 68, 373–402 [DOI] [PMC free article] [PubMed] [Google Scholar]