Background: β4 integrin phosphorylation regulates hemidesmosome disassembly, a process necessary for migration and carcinoma invasion.
Results: Inhibition of calcineurin increases β4 phosphorylation, hemidesmosome disassembly, and migration. Activating calcineurin stabilizes hemidesmosomes.
Conclusion: The calcium/calcineurin pathway stabilizes hemidesmosomes and controls migration by regulating β4 phosphorylation.
Significance: These findings help understanding how cells modulate movement by assembling/disassembling anchorage structures, with interesting implications in calcineurin-inhibitor induced cancer.
Keywords: Calcineurin, Cell Adhesion, Cell Migration, Integrins, Phosphoprotein Phosphatase
Abstract
Cell migration depends on cells being able to create and disassemble adhesive contacts. Hemidesmosomes are multiprotein structures that attach epithelia to basal lamina and disassemble during migration and carcinoma invasion. Phosphorylation of the β4 integrin, a hemidesmosome component, induces disassembly. Although kinases involved in β4 phosphorylation have been identified, little is known about phosphatases countering kinase action. Here we report that calcineurin, a serine-threonine protein phosphatase, regulates β4 phosphorylation. Calcineurin inhibitor cyclosporin A (CsA) and calcineurin-siRNA increase β4 phosphorylation, induce hemidesmosome disassembly, and increase migration in HaCat keratinocytes, suggesting that calcineurin negatively regulates β4 phosphorylation. We found no direct dephosphorylation of β4 by calcineurin or association between β4 and calcineurin, suggesting indirect regulation of β4 phosphorylation. We therefore assessed calcineurin influence on MAPK and PKC, known to phosphorylate β4. CsA increased MAPK activity, whereas MAPK inhibitors reduced CsA-induced β4 phosphorylation, suggesting that calcineurin restricts β4 phosphorylation by MAPK. Calcineurin is activated by calcium. Increased [Ca2+]i reduces β4 phosphorylation and stabilizes hemidesmosomes, effects that are reversed by CsA, indicating that calcineurin mediates calcium effects on β4. However, MAPK activation is increased when [Ca2+]i is increased, suggesting that calcineurin activates an additional mechanism that counteracts MAPK-induced β4 phosphorylation. Interestingly, in some squamous cell carcinoma cells, which have reduced hemidesmosomes and increased β4 phosphorylation, an increase in [Ca2+]i using thapsigargin, bradykinin, or acetylcholine can increase hemidesmosomes and reduce β4 phosphorylation in a calcineurin-dependent manner. These findings have implications in calcineurin-inhibitor induced carcinoma, a complication of immunosuppressive therapy.
Introduction
Hemidesmosomes (HD)2 are specialized multiprotein complexes that mediate attachment of epithelial cells to the underlying basement membrane (1–3). These structures provide stability and mechanical strength to epithelia and are disassembled in migrating cells during wound healing and carcinoma invasion (3–5). HD are composed of several transmembrane and cytoplasmic proteins that connect the cytokeratin network to the extracellular matrix. Tissue fragility and blistering disease develop when hemidesmosomal components are genetically altered, indicating the significance of HD in maintaining epithelial tissue integrity (6, 7). The main organizer and nucleator of HD is the a6β4 integrin, a laminin receptor (8–10). This integrin recruits the HD component plectin, which then facilitates further association of the integrin with components BPAG1 and BPAG2 (8, 11). The cytokeratin network connects the HD through BPAG1 and plectin (11).
Although HD provides a stable anchor, its components undergo a rapid turnover, indicating a dynamic equilibrium (12, 13). This rapid turnover might allow epithelial cells to efficiently switch between a stationary and a migratory state by quickly changing the balance toward disassembly. Disassembly of HD becomes important during wound healing to allow cell migration and re-epithelialization (4). Importantly, disassembly of HD is also seen during carcinoma invasion, where a reduction of HD can predict metastatic potential (5).
Most of the efforts to understand the regulation of HD disassembly have focused on the main organizer of the HD, the a6β4 integrin. The phosphorylation of the β4 integrin subunit has been shown to be an important mechanism to induce HD disassembly (Refs. 14–21 and reviewed in Ref. 3). Growth factors that are secreted during wound healing or carcinoma invasion can induce the phosphorylation of the β4 integrin on serine and tyrosine residues and promote HD disassembly (16, 17, 22). Most of the phosphorylation on the β4 integrin occurs on serine residues of the connecting segment domain (17). A cluster of serines at the beginning of the connecting segment, Ser1356-Ser1360-Ser1364, as well as at a nearby site, Ser1424 (17–20), or a threonine at the C-tail (21), have been shown to play an important role in HD stability. Mutation on some of these serine residues to alanine to impede phosphorylation reduces the disruptive activity of EGF on HD, increases the interaction of β4 and plectin, and can inhibit cell migration by stabilizing HD, indicating a function of β4 serine phosphorylation in HD regulation (17–20). In contrast, mutation of these sites into aspartate, which mimics phosphorylation, increases HD disruption and plectin dissociation. Importantly, some of these sites are found hyperphosphorylated in invasive squamous cell carcinoma (SCC), suggesting that β4 phosphorylation plays a role in carcinoma invasion (23). In vitro, SCC cells show an increase in β4 phosphorylation when compared with keratinocytes. Interestingly, β4 seems to be preferentially phosphorylated in certain areas of the cells, such as trailing edges and retraction fibers, where previous studies have shown that HD disassemble as the cell moves forward (13, 18, 23). This distinct distribution of β4 phosphorylation suggests that local factors such as traction forces or calcium fluxes may be regulating β4 phosphorylation (24).
An important aspect of protein function regulation through phosphorylation is its reversibility. Although several kinases, such as PKC and the MAPK ERK1/2 and RSK2, have been shown to be involved in serine phosphorylation of β4 (16–20), little is known about the pathways that lead to dephosphorylation. Based on mutational studies, it can be asserted that dephosphorylation of the β4 integrin is necessary to stabilize HD (17–20). There is little information about serine-threonine protein phosphatases (PPP) that might be involved in this process. PPP inhibitors, such as okadaic acid and calyculin, have been shown to increase β4 phosphorylation as well as destabilize HD, implicating protein phosphatase 2a and or protein phosphatase 1 (18, 19). In the present study we addressed whether another major PPP, calcineurin (CN), is involved in the dephosphorylation and function of the β4 integrin. CN is a heterodimeric serine/threonine phosphatase, and it is the only PPP known to become highly stimulated in response to Ca2+ signals because of the direct binding of Ca2+ and calmodulin (25). CN is one of the key players directing the flow of information from local or global calcium signals to effectors that control immediate cellular responses and alter gene transcription. This may be of particular relevance to β4 function given that local factors seem to regulate β4 phosphorylation (18). Interestingly, CN inhibitors that are commonly used in immunosuppressive therapy, such as cyclosporin A (CsA), are known to increase the incidence of SCC (26), a type of tumor that, as mentioned above, shows increased migration through β4 phosphorylation and HD disassembly (23). Therefore, a study of the relationship between CN and β4 phosphorylation should improve our understanding of CN inhibitor-induced SCC. (24)
In this study, we have found that CN negatively regulates β4 phosphorylation and increases HD stability, whereas reducing cell migration. The effect of CN on β4 phosphorylation is indirect and occurs through two different pathways. In one case, CN inhibits the MAPK pathway, where ERK1/2 has been shown to directly phosphorylate β4. A second route is independent of MAPK, where CN seems to positively promote β4 dephosphorylation, probably regulating a downstream phosphatase. We also show that calcium flows affect β4 phosphorylation through CN and calmodulin and that membrane receptors modulating calcium, such as the bradykinin and acetylcholine receptors, can also regulate β4 phosphorylation and HD stability through CN. Interestingly, the activation of Ca/CN pathway can reduce the hyperphosphorylation of β4 in SCC cells and increase HD stability as well as reduce migration. Our results show that CN plays an important role in HD function through regulation of β4 phosphorylation and may be of particular relevance in SCC carcinoma induced by CN inhibitors.
EXPERIMENTAL PROCEDURES
Cells and Reagents
HaCat keratinocytes were obtained from Dr. S. La Flamme (Albany Medical College, Albany, NY). Squamous cell carcinoma cell lines were obtained as follows: A431 cells (vulvar SCC origin) were obtained from ATCC; Colo-16 (skin SCC origin) was obtained from Dr. N. Hail (University of Colorado, Denver, CO); and SCC-25 cells (head and neck SCC origin) were provided by Dr. A. M. Mercurio (University of Massachusetts Medical School, Worcester, MA). Cos-7 cells were provided by Dr. Pierre Kinet (Beth Israel Deaconess Medical Center, Boston, MA). Cells were maintained in DMEM with 10% fetal calf serum, except SCC25 cells, which were grown in DMEM/Ham's F12 (1:1) containing hydrocortisone (400 ng/ml) and 10% fetal bovine serum. Antibodies were as follows: 3E1 (β4, Chemicon); GoH3 (α6, Chemicon); rabbit anti-β4 (16); anti-BPAG1 (27); and anti-plectin (Santa Cruz Biotechnology). Affinity-purified phospho-specific rabbit polyclonal Ab (anti- phospho-Ser1356 Ab) was previously characterized (23). Anti-phospho ERK1/2 and anti-ERK1/2 Abs were from Cell Signaling Technology. Cyclosporin A, BAPTA-AM, thapsigargin, bradykinin, and acetylcholine were from Sigma. Inhibitors for PKC (Go6983), MEK (U0126), and calmodulin (W13) were from Enzo Biochemicals.
Plasmids and Transfections
Full-length cDNA calcineurin (PPP3CA, Open Biosystems) was used to produce a constitutively active form containing amino acids 1–390 (28) and fused to Myc using a Myc-pcDNA4 plasmid (Invitrogen). Transfection of the plasmid was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. ON-TARGETplus SMARTpool human PPP3CA (Dharmacon) was transfected using Lipofectamine® RNAiMAX, and cells were grown for 48 h before experiments. An AllStars negative control siRNA (Qiagen) was used to control siRNA transfections.
Indirect Immunofluorescence
Cells were stained as described previously (16, 29). Briefly, cells grown on coverslips were extracted or not with detergent buffer containing 0.5% Triton X-100, 100 mm KCl, 200 mm sucrose, 10 mm EGTA, 2 mm MgCl2, and 10 mm PIPES at pH 6.8 for 1 min and then fixed using paraformaldehyde or methanol. Cells were rinsed, blocked, and stained with the indicated Abs and Cy2/Cy3-conjugated secondary antibodies. Slides were analyzed using fluorescence microscopy. Quantitative analysis of HD-like structures was performed as described previously (23). Briefly, collected images of cells stained for β4 integrin were background-subtracted and thresholded, and fluorescence-integrated density per cell was calculated using the ImageJ software (National Institutes of Health). At least 100 cells were analyzed in each experiment.
In Vitro Wound Healing Assay
Cells grown to confluency were scratched with a yellow tip, and new medium containing inhibitors or not was added. Image records were collected at time 0. Wounded plates were incubated for 8–24 h. Images were collected, and the percentage of wound closure was determined by digital analysis.
Biochemical Analyses
Cells subjected to the treatments indicated in the figures were extracted directly using sample buffer, and aliquots were processed for Western analysis using standard techniques. To measure calcineurin activity, we used a calcineurin cellular activity assay kit (Enzo Biochemicals) and followed the manufacturer's instructions.
Statistics
Statistical analyses were performed using Student's t test. Statistical significance was set at a p value less than 0.05. Results are expressed as mean ± S.D. obtained from three or more independent experiments.
RESULTS
Calcineurin Inhibitors Induce the Phosphorylation of the β4 Integrin on Ser1356 in HaCat Keratinocytes
In previous work, we and others have determined that EGF induces the phosphorylation of the β4 integrin on Ser1356 in HaCat keratinocytes, resulting in the destabilization of HD (20, 23). This phosphorylation can be mediated by PKC and MAPK (20, 23). Because reversible protein phosphorylation catalyzed by protein kinases and protein phosphatases regulates numerous cellular processes including β4 function, we sought to determine the role of phosphatases in Ser1356 phosphorylation. The serine-threonine protein phosphatase calcineurin (CN, also known as protein phosphatase 2b) is known to play an important role in the dephosphorylation of a significant number of proteins and regulates a variety of signaling networks (25, 30). CN is regulated by Ca2+ through calmodulin, and it is inhibited by immunosuppressive drugs such as CsA and Tacrolimus (FK506) (25). We began by analyzing the effect of CN inhibitors on β4 phosphorylation and HD in HaCat keratinocytes. We found that CN inhibitor CsA can efficiently induce Ser1356 phosphorylation for relatively long periods of time (Fig. 1A). We then evaluated the effects of CsA on HD-like structures (HD-LS). HD-LS can be visualized by IF as characteristic plaques stained with anti-β4 Ab on the basal aspect of the cell as described previously (23). Moreover, by extracting unbound β4 with detergent, most of the detergent-resistant β4 colocalizes with plectin (23), and therefore, quantitation of detergent-resistant β4 provides a good estimate of HD-LS. Our results show that CsA induces disruption of HD-LS (Fig. 1, B and C), suggesting that CN is involved in stabilizing HD. Moreover, CsA can increase β4 phosphorylation induced by EGF (Fig. 1A). FK506 showed a similar effect (data not shown). CsA did not significantly affect Ser1424 phosphorylation, indicating specificity (data not shown). Interestingly, CsA can potentiate the effect of low concentrations of EGF (1 ng/ml) on keratinocyte migration (Fig. 1D), consistent with the idea that HD disassembly has a positive effect on migration and might be related to its potential to promote invasive SCCs. This may be particularly relevant in patients receiving CN inhibitors as the drug might enhance the migratory capability of SCC cells even when there is a low concentration of growth factors in the microenvironment.
FIGURE 1.
CN regulates β4 phosphorylation and HD stability. A, effects of CN inhibitor CsA on β4 phosphorylation (Ser1356). HaCat cells were treated for different lengths of time with CsA (1 μm), EGF (50 ng/ml), or a combination of both and were analyzed by Western blot. B, effects of CsA on HD stability. Quantitative IF analysis of detergent-resistant β4 was used to estimate HD-LS. Cells were treated with CsA (1 μm), EGF at low concentration (1 ng/ml), or a combination of both for the indicated time intervals and then detergent-extracted, fixed, and processed for IF using β4 Ab to measure fluorescence-integrated density per cell. C, representative IF images quantified in B. Bar = 10 μm. D, effects of CsA on EGF-induced migration. Cell migration was tested using wound healing assays in the presence or absence of EGF (1 ng/ml) and/or CsA (*, p < 0.05 versus control; **, p < 0.05 versus EGF or CsA). E, effects of CN-siRNA on β4 phosphorylation. HaCat keratinocytes were transfected with CN- or negative control siRNA and analyzed after 48 h for β4 phosphorylation by Western blot. F, effects of CN-siRNA on HD-LS. Control or CN-siRNA transfectants were analyzed for HD-LS abundance using quantitative IF (*, p < 0.05 versus control siRNA; **, p < 0.05 versus control siRNA+EGF or CN-siRNA). G, effects of CN-siRNA on keratinocyte migration using wound healing assays. HaCat keratinocytes transfected with control or CN-siRNA were grown to confluency for 48 h before performing wound healing assays in the presence of EGF (1 ng/ml) for 18 h. (*, p < 0.05 versus control siRNA; **, p < 0.05 versus control siRNA+EGF or CN-siRNA)
To corroborate that the effects of CsA on β4, HD disassembly, and cell migration are specifically mediated through inhibition of CN, we silenced the catalytic subunit of CN, PPP3CA, using siRNA technology. As shown in Fig. 1, E–G, the CN-siRNA, but not the negative control siRNA, was capable of reducing CN expression and increasing β4 phosphorylation, as well as reducing HD stability and enhancing cell migration induced by EGF in HaCat keratinocytes.
CN Regulates β4 Phosphorylation Indirectly through MAPK and PKC-dependent Pathways
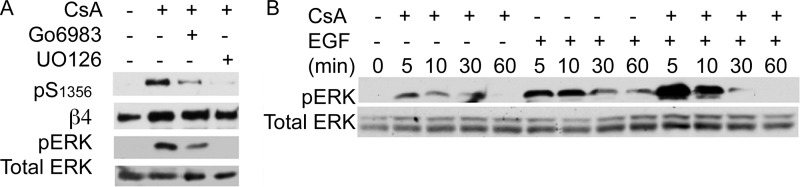
To understand the mechanism by which CN might be preventing β4 phosphorylation, we analyzed whether CN directly dephosphorylates the β4 integrin (on Ser1356 and Ser1424) or can associate with the integrin. Co-immunoprecipitation analyses could not detect association between CN and β4, and in vitro dephosphorylation analysis showed no direct dephosphorylation of β4 by CN-calmodulin complexes (data not shown), suggesting that CN inhibits the phosphorylation of β4 indirectly, by either negatively regulating the kinases involved in β4 phosphorylation or positively regulating the phosphatases that dephosphorylate β4. Both PKC and MAPK are activated by EGF and have been shown to directly phosphorylate β4 (17, 18, 20, 23). We therefore tested whether CsA effects on β4 phosphorylation are mediated through PKC or MAPK. We found that inhibitors of both PKC (Go6983) and MEK (U0126) are able to reduce the phosphorylating activity induced by CsA (Fig. 2A). However, the MEK inhibitor was much more efficient. This is consistent with previous findings that show ERK1/2 can phosphorylate Ser1356 (20). We therefore analyzed whether CsA may be inducing ERK1/2 activity. CsA induced ERK1/2 activity to a lesser extent than EGF, although it potentiated EGF effects when combined at some time points (Fig. 2B), suggesting that CN may be constantly guarding against kinase activity and counteracting EGF actions. This CsA-induced ERK1/2 phosphorylation was reduced with PKC inhibitor (Fig. 2A, bottom panels), suggesting that PKC mediates CsA effects upstream of the MAPK pathway. As expected, the MEK inhibitor abrogated phospho-ERK signal (Fig. 2A, bottom panels).
FIGURE 2.
CN regulates β4 phosphorylation through MAPK- and PKC-dependent pathways. A, HaCat keratinocytes were preincubated or not in the presence of MAPK inhibitor U0126 (10 μm) or PKC inhibitor Go6983 (1 μm) for 20 min before adding CsA for an additional 20 min and analyzed by Western blot using the indicated Abs. pS1356, Ser(P)1356; pERK, phospho-ERK. B, time course analysis of MAPK pathway activation. HaCat keratinocytes were treated with CsA (1 μm), EGF (50 ng/ml), or a combination and then stained with phospho-ERK1/2 or total ERK Abs and analyzed by Western blot.
Ca2+ Signaling Regulates β4 Phosphorylation and HD Stability through CN Regulation of a MAPK-independent Pathway
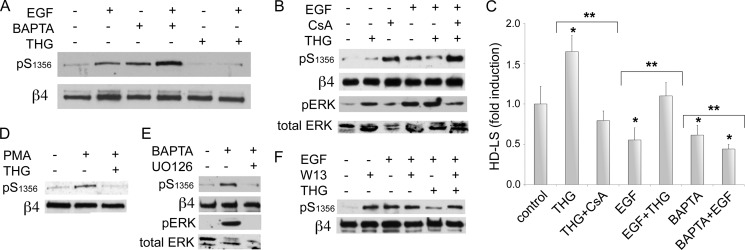
Our previous work on the distribution of β4 phosphorylation suggests that local factors such as tension or traction may be regulating β4 phosphorylation (18, 23). Ca2+ signaling is likely to be involved in this type of local process. Because CN activity is tightly regulated by Ca2+, we evaluated the influence of Ca2+ signaling on β4 phosphorylation and HD stability. As shown in Fig. 3, A and D, an increase in [Ca2+]i using thapsigargin (THG) significantly inhibited the phosphorylation of β4 induced by EGF or phorbol 12-myristate 13-acetate. Notably, the effect was reversed by CsA (Fig. 3B), suggesting that the effects of Ca2+ on β4 are mediated by CN. In contrast, the depletion of [Ca2+]i using BAPTA-AM had the opposite effect (Fig. 3A), increasing phospho-Ser1356 by itself, and had an additive effect on EGF-induced phosphorylation. To further support the involvement of Ca2+ and CN in β4 phosphorylation, we assessed whether inhibition of calmodulin, a Ca2+ sensor and partner/activator of CN (25), affects phosphorylation of β4. As shown in Fig. 3F, W13, a calmodulin inhibitor, recapitulated several of the effects of CN inhibition; it increased β4 phosphorylation by itself, and it reversed THG inhibition of EGF-induced phosphorylation of β4. However, it did not seem to enhance EGF-induced phosphorylation as CsA, suggesting that other calmodulin targets may be influencing β4 phosphorylation as well. Altogether, these data indicate the involvement of a Ca2+/calmodulin/CN pathway in the regulation of β4 phosphorylation.
FIGURE 3.
Ca2+ signaling regulates β4 phosphorylation and HD stability through CN. A, effects of [Ca2+]i modulation on EGF-induced phosphorylation of β4. HaCat keratinocytes were preincubated in the presence or not of THG (1 μm), which increases [Ca2+]i, or BAPTA-AM (10 μm), which reduces [Ca2+]i, and then stimulated or not with EGF for 20 min. Cell lysates were analyzed with the indicated Abs. pS1356, Ser(P)1356.B, inhibition of EGF-induced β4 phosphorylation by THG is reversed by CsA. Cells were preincubated with or without THG and/or CsA for 10 min before stimulation with EGF (50 ng/ml) for 20 min. pERK, phospho-ERK. C, effects of [Ca2+]i modulation on HD stability. HaCat keratinocytes were preincubated in the presence or not of THG, CsA, or BAPTA-AM, before stimulation with EGF for 20 min, then processed for quantitative IF analysis of HD-LS (*, p < 0.05 versus control; **, p < 0.05 between bracketed columns). D, effects of [Ca2+]i modulation on β4 phosphorylation induced by PKC. Cells were preincubated in the presence or not of THG for 10 min before stimulation with phorbol 12-myristate 13-acetate (PMA) (25 ng/ml) for 30 min. E, MAPK dependence of BAPTA effects on β4 phosphorylation. Cells were preincubated with MAPK inhibitor U0126 for 20 min and then treated with BAPTA-AM and analyzed by Western blot. F, analysis of calmodulin inhibition on β4 phosphorylation. Cells were preincubated in the presence or not of calmodulin inhibitor W13 (15 μg/ml) and/or THG for 20 min before stimulation with EGF for 20 min and analyzed by Western blot.
We next addressed how the MAPK pathway is affected by a [Ca2+]i rise. As shown in Fig. 3B (bottom panels), THG itself increased MAPK activation. These results indicate that although THG reverts EGF-induced phosphorylation of β4 through CN, it does not reduce EGF stimulation of MAPK activity, suggesting an alternative pathway to induce dephosphorylation, as for example, through promoting the activation of a CN-dependent phosphatase stimulated by Ca2+ that would counter β4 phosphorylation by ERK1/2. In contrast, BAPTA-induced phospho-Ser1356 is accompanied by ERK1/2 activation, and it is inhibited by UO126 (Fig. 3E), consistent with the idea that a reduction of calcium may disable the CN-mediated inhibition of MAPK.
Ca2+ effects on the stability of the HD were noteworthy (Fig. 3C), with THG substantially increasing the number of HD-LS and opposing the disruptive activity of EGF, whereas BAPTA-AM had the opposite effect, disrupting HD-LS by itself or enhancing the disruptive effects of EGF. Altogether, these results suggest that CN may use two pathways to prevent β4 phosphorylation: one by inhibiting the MAPK pathway and another that induces dephosphorylation, probably involving the activation of a phosphatase.
Ca2+/Calcineurin Pathway Reduces Hyperphosphorylation of β4 Integrin in Squamous Carcinoma Cells and Increases HD Stability
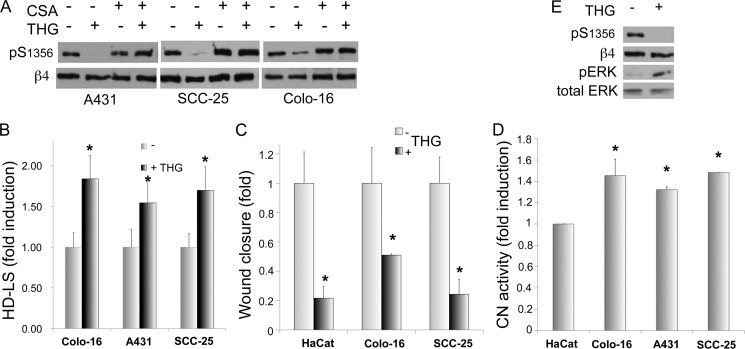
We have previously shown that β4 Ser1356 is hyperphosphorylated in SCC cells, explaining in part why in these cells HD are reduced and migration is increased (23). This increased phosphorylation of β4 is promoted by intrinsic factors as it can be detected in the absence of serum. We were therefore interested in assessing the effects of [Ca2+]i modulation on β4 phosphorylation in SCC cells. We used A431, SCC-25, and Colo-16 squamous cell carcinoma cells as a model. As shown in Fig. 4A, THG was highly inhibitory on phospho-Ser1356 in the three SCC cells lines tested, and this inhibition was completely reversed by CsA, suggesting that CN mediates the effects of Ca2+. Moreover, when treated with THG, SCC cells showed increased HD stability (Fig. 4B) and reduced migration potential (Fig. 4C). Because Ca2+-induced inhibition of β4 phosphorylation seems to be mediated by CN, we tested whether the high phosphorylation of β4 in SCC is due to a deficient activity of CN. As shown in Fig. 4D, CN activity was not only present but slightly elevated in SCC cells when compared with keratinocytes. Moreover, CsA did not increase β4 phosphorylation as much as in keratinocytes (Fig. 4A versus Fig. 1A), suggesting that in SCC cells, the inhibitory effect of CN is overpowered by the kinases responsible for β4 phosphorylation and that the slight increase in CN activity may be the result of a negative feedback loop trying to balance hyperphosphorylation.
FIGURE 4.
Increased [Ca2+]i in SCC cells stabilizes HD and reduces β4 phosphorylation and migration potential. A, SCC cells were incubated in the presence or absence of THG and/or CsA for 20 min as indicated and analyzed by Western blot. pS1356, Ser(P)1356. B and C, IF analysis of HD-LS (B) or wound healing assay (C) of SCC cells incubated in the presence or absence of THG. D, CN activity analysis of SCC cells. Cell lysates obtained from SCC cell lines were analyzed for CN activity using a colorimetric assay kit. E, THG effects on β4 phosphorylation and ERK1/2 activation in A431 cells. Cells were incubated in the presence or absence of THG for 20 min and analyzed by Western blot (*, p < 0.05 versus respective controls). pERK, phospho-ERK.
Because SCCs differ from keratinocytes in some of the mechanisms inducing β4 phosphorylation, we addressed whether THG is inhibiting β4 phosphorylation by affecting MAPK activity differently from keratinocytes. We analyzed phospho-ERK1 in A431 SCC cells treated with THG. As shown in Fig. 4E, THG increased ERK activity considerably, indicating that the inhibitory effect of Ca2+ on β4 phosphorylation does not go through inhibition of the MAPK pathway, but through a parallel pathway that counters the simultaneous activation of MAPK, such as the stimulation of a phosphatase.
Modulators of Calcium Influx, Such as Bradykinin and Acetylcholine, Can Regulate β4 Phosphorylation, HD Stability, and Cell Migration in Keratinocytes and SCC Cells
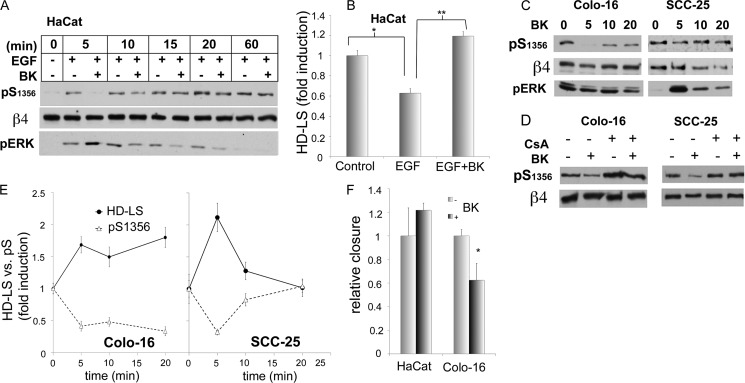
We were interested to see whether physiologic ligands that mostly modulate Ca2+ signaling would be able to recapitulate the effects of THG. We tested some of the Ca2+ agonists that are known to be elevated during wound healing, such as bradykinin (BK) and acetylcholine, both of which have receptors in keratinocytes that increase [Ca2+]i (31–34). Both were able to down-modulate EGF-induced β4 phosphorylation (Fig. 5A for BK, acetylcholine not shown). BK strongly inhibited EGF response mostly in the first few minutes after stimulation, and then the BK effect was gradually reduced. We then evaluated the effects of BK on EGF-induced HD disruption. At short times (10 min), BK was able to reverse EGF-induced disassembly of HD-LS in HaCat keratinocytes (Fig. 5B). However, this short inhibitory effect on β4 phosphorylation did not seem to affect the long-term EGF-induced HD disassembly (not shown).
FIGURE 5.
BK induces CN-dependent inhibition of β4 phosphorylation and stabilizes HD in keratinocytes and SCC; effects on migration are cell type-dependent. A, BK modulation of EGF effects on β4 phosphorylation. HaCat cells were preincubated in the presence or not of BK (0.1 μm) before stimulating with EGF(50 ng/ml) for the indicated times. pS1356, Ser(P)1356; pERK, phospho-ERK. B, BK modulation of EGF effects on HD-LS stability. HaCat cells were preincubated in the presence or not of BK before stimulating with EGF for 10 min and being processed for HD-LS analysis (*/**, p < 0.05 between bracketed columns). C, effects of BK on β4 phosphorylation in SCC cells. SCC cells were treated for the indicated times with BK and processed for Western analysis using the indicated Abs. D, dependence of BK effects on CN. SCC cells were preincubated in the presence or not of CsA for 10 min and then stimulated with BK for 5 min. E, effects of BK on HD-LS stability versus b4 phosphorylation in SCC cells. SCC cells were treated with BK for the indicated times and then processed for a HD-LS analysis using quantitative IF (relative fluorescence-integrated density) or processed for Western analysis (relative densitometric ratios). pS, phospho-serine. F, effects of BK on cell migration. Wound healing assays were performed on HaCat keratinocytes or SCC Colo-16 cells in the presence or absence of BK, and closure of the wound was measured as described under “Experimental Procedures” (*, p < 0.05 versus respective controls).
We then analyzed how BK affects β4 phosphorylation as well as HD stability in SCC cells Colo-16 and SCC-25. The results were varied. In SCC25, BK inhibited β4 phosphorylation for only a short period of time, whereas in Colo-16, there was a long-term inhibition (Fig. 5, C and E). In the case of SCC25, there was increased HD stabilization at early time intervals, which subsequently reverted to original levels (Fig. 5E). Interestingly, HD stability in Colo-16 increased with longer time intervals of BK, reflecting the long-term reduction of β4 phosphorylation (Fig. 5E). We evaluated whether the inhibitory effect of BK on β4 phosphorylation at early time intervals was mediated by CN. As shown in Fig. 5D, CsA reverted the inhibitory effects of BK in the two SCC cell lines. To assess whether BK effects on HD may have repercussions in cell migration, we performed in vitro wound healing assays on HaCat and Colo-16 cells (Fig. 5F). BK substantially reduced migration in Colo-16 cells, which is consistent with the long-term HD-stabilization effects of BK on these cells. In contrast, HaCat keratinocytes, which are inhibited only for a short period of time, did not show significant changes in migration. We also evaluated whether BK exerts its inhibitory effects on β4 phosphorylation by inhibiting MAPK through CN or, as seen with Ca2+, through an independent pathway. We assessed ERK1/2 activation after BK treatment in HaCat (Fig. 5A) and SCC (Fig. 5C). In all cases, BK induced ERK1/2 activity, suggesting that BK, through CN, inhibits β4 phosphorylation through stimulation of a MAPK-independent dephosphorylation pathway that counters the effects of a simultaneously activated ERK1/2. These results suggest that β4 phosphorylation can be modulated by extracellular signals that regulate [Ca2+]i. However, the data suggest that the effect of Ca2+ regulators on long-term behaviors (such as migration) might be cell type-specific.
Constitutively Activated CN Inhibits β4 Phosphorylation and Increases HD-LS
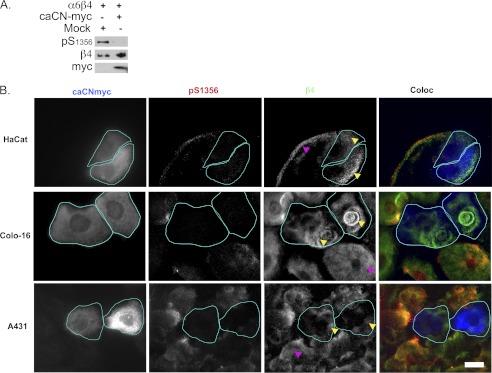
In view of these results, we hypothesized that overexpression of a Myc-tagged constitutively active form of CN (caCN-Myc (28)) might reduce β4 phosphorylation in EGF-stimulated normal or transformed cells and increase HD formation. We first co-expressed caCN-Myc with α6β4 in Cos7 cells, a model that allowed us to achieve a higher number of transfectants and perform a biochemical evaluation. As shown in Fig. 6A, when the transfected cells were stimulated with EGF, there was a strong inhibition of β4 phosphorylation.
FIGURE 6.
caCN-Myc inhibits β4 phosphorylation and increases HD stability. A, Cos7 cells cotransfected with α6, β4, + or − caCN-Myc, or mock plasmid (Mock) were treated with EGF and analyzed by Western blot. pS1356, Ser(P)1356. B, HaCat keratinocytes or SCC cells were transfected with caCN-Myc and processed for IF analysis using the indicated Abs (green, anti-β4; red, anti-Ser(P)1356; blue, anti-Myc). HaCat cells were incubated in serum-containing medium to induce phosphorylation of the β4 integrin, whereas SCC cells were in serum-free medium. Notice that Myc-positive cells (cyan outline) have reduced phosphorylation of the β4 integrin, whereas preserving or increasing HD-LS characteristic plaques (yellow arrowheads) when compared with the Myc-negative neighbor cells (magenta arrowheads). Bar = 10 μm.
We then transfected caCN-Myc into HaCat keratinocytes and SCC cells. HaCat keratinocytes expressing caCN-Myc showed a consistent reduction of β4 phosphorylation and well formed HD (Fig. 6B). In SCC cells, particularly Colo-16 cells, there was a reduction of β4 phosphorylation and an increase in HD, whereas in A431 cells, only a reduction of β4 phosphorylation was apparent (Fig. 6B). These results are consistent with the idea that CN can stabilize HD, although it may vary according to cell type.
DISCUSSION
A hallmark of many regulatory signal transduction pathways is the reversible phosphorylation of several of their components, often functioning as a binary switch that controls activation/deactivation. The opposing function of protein kinases and phosphatases balances and fine-tunes the flow of chemical messages through the signaling pathway. The phosphorylation of the β4 integrin plays an important role in HD stability and cell migration, and although we have knowledge of kinases involved in this process, there is little information about the phosphatases that balance β4 phosphorylation status. Here, we have studied the role of the Ser/Thr PPP CN on β4 phosphorylation and function. Our results show that CN controls the phosphorylation status of the β4 integrin, as indicated by the increase of β4 phosphorylation on Ser1356 produced by CN inhibitors and by the inhibitory effect of constitutively activated CN on β4 phosphorylation. The inhibition of CN promotes HD disruption, whereas the opposite is observed when constitutively active CN is expressed, which is consistent with the idea that serine phosphorylation of β4 controls HD stability, thereby affecting migration. Notably, the effect of lower concentration of EGF on cell migration is potentiated by CN inhibitors. This may be clinically relevant because CN inhibitors used during immunosuppressive therapy are known to increase up to 100 times the risk of SCC (26), and it has been shown that β4 phosphorylation correlates with SCC invasiveness, facilitating SCC migration in vitro (23). Previous work has identified a primary mechanism for CN inhibitors to induce SCC, namely an increase in ATF3 expression, which suppresses p53-dependent senescence and enhances tumorigenic potential (35). CN inhibitor-induced β4 phosphorylation and HD disruption may further contribute to the dissemination of the tumor by facilitating migration. Interestingly, β4 is known to activate p53, thus promoting apoptosis (36), and therefore, it would be valuable to determine whether phosphorylation can modify this capability and add to the effects of ATF3.
The interactions between integrins and calcineurin have been scarcely addressed. The most prominent study suggests that CN promotes migration in neutrophils in part by promoting detachment of the trailing edge by internalization of the αvβ3 (37). These findings contrast with our results where CN inhibitors increase cell migration and CN itself increases the stability of β4 in HD, suggesting that CN effects are cell type-specific.
CN is known to associate with several of its targets through two known consensus-binding sequences (25, 30). The β4 integrin has one of the consensus sites (LXVP) on the fourth fibronectin III repeat. However, we were unable to find any association between β4 and CN or a direct dephosphorylation of β4 by CN (data not shown), suggesting that β4 is not a direct target of CN and that the observed effects of CN on β4 phosphorylation are indirect. Two possible scenarios are that CN may be inhibiting the kinase or kinases involved in the phosphorylation of β4 or that it may be stimulating a specific phosphatase. Previous works have identified PKC and MAPK as kinases involved in Ser(P)1356 phosphorylation (17, 20). ERK1/2 has been shown to directly phosphorylate Ser1356 (20). Our results showed that β4 phosphorylation induced by CN inhibitors is inhibited by both PKC and MAPK inhibitors, suggesting that CN may be inhibiting the β4 phosphorylation activity of these kinases. MAPK inhibitors were more potent. Given that CN inhibitors strongly stimulate MAPK activity and that PKC inhibitors attenuate this effect, a possible scenario is that PKC mediates in part the CsA-dependent stimulation of MAPK, and therefore, CN may be inhibiting MAPK by inhibiting PKC. PKC can feed positive signals into the MAPK kinase pathway by activating Raf kinase (38). However, the PKC inhibitor effects are partial, and therefore, other pathways that contribute to MAPK activation may also be inhibited by CN.
The existence of an alternative pathway for CN to inhibit β4 phosphorylation is suggested by the fact that under certain circumstances, CN may inhibit β4 phosphorylation when MAPK is active. We derive this idea from the fact that influx of calcium by either THG or bradykinin can both inhibit β4 phosphorylation in a CN-dependent manner and at the same time activate MAPK in either keratinocytes or SCC cells. One possible scenario is that a phosphatase that dephosphorylates β4 is activated by CN, counteracting the β4-phosphorylating activity of the simultaneously activated MAPK. CN is known to activate some phosphatases such as Slingshot, although there is no evidence that this phosphatase targets anything other than cofilin (39). Future work will be needed to identify the phosphatase involved in Ser(P)1356 dephosphorylation and define its dependence on CN.
CN is primarily regulated by Ca2+ and calmodulin (25). We therefore made efforts to address whether modulation of calcium affected β4 in a CN-dependent manner. Our results show that raising [Ca2+]i by either THG or bradykinin results in inhibition of EGF-induced β4 phosphorylation in keratinocytes or inhibition of β4 hyperphosphorylation in SCC cells. This effect was highly dependent on the activity of CN because it was significantly reduced in the presence of CsA. Consistently, elevating calcium resulted in an increase in HD stability and inhibition of migration. Conversely, reduction of calcium using BAPTA-AM resulted in an increase of β4 phosphorylation and disruption of HD. BAPTA-AM increased EGF-induced phosphorylation β4. However, BAPTA-AM inhibited cell migration as well, suggesting that disruption of HD is not sufficient to promote migration and that, not surprisingly, there are other factors involved in migration that are calcium-dependent. Calcium effects on CN are mediated by calmodulin, which associates with CN (25). Our results show that by inhibiting calmodulin, there is an increase in the phosphorylation of β4. Once calmodulin is inhibited, β4 is no longer inhibited by THG, consistent with the idea that the calcium effect on β4 is conveyed by a calmodulin/CN pathway.
Interestingly, the intracellular activity of endogenous CN in SCC cells is slightly elevated when compared with HaCat keratinocytes. One possible scenario is that CN may be part of a feedback loop trying to compensate for a hyperactive signaling pathway that results in the phosphorylation of β4.
Calcium influx is regulated through a large number of membrane receptors, including the bradykinin and the acetylcholine receptor, which are present in keratinocytes (33, 34). When engaged, these receptors were able to inhibit β4 phosphorylation induced by EGF in a CN-dependent manner, as suggested by the fact that CsA reverted this inhibition. The effect was cell type-specific. Keratinocytes and SCC-25 responded in a relatively similar fashion, by inhibiting the phosphorylation of β4 induced by EGF only during the first few minutes. In contrast, Colo-16 cells showed a persistent inhibition of β4 phosphorylation. These time-related effects are reflected in HD disruption by EGF or cell migration, where keratinocytes and SCC-25 show a temporal inhibition of HD disruption induced by EGF and no effect on cell migration, whereas in Colo-16, HD disruption is inhibited for a long period of time and cell migration is inhibited as well. The precise role of BK receptors in keratinocyte function is not completely understood, although it is thought that they may play a role during wound healing, when the ligands are elevated (32). The reason why Colo-16 is different from the other cells needs further exploration, although it is important to mention that when compared with other SCC cells, Colo-16 shows a high rate of metastasis (40), and therefore, the observation that BK may hinder Colo-16 migration might be of value as BK could be potentially used as an inhibitor of tumor dissemination, thus warranting further investigation.
In summary, our data provide insight into the delicate balance of signals that regulate HD through β4 phosphorylation. Although the phosphatases that directly dephosphorylate β4 remain unidentified, we show that certain phosphatases, such as CN, may play an indirect but important role in this system that may have implications in those cancers produced by inhibition of CN.
Acknowledgment
We thank Don Senger for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant CA120202 (to I. R.).
- HD
- hemidesmosome(s)
- HD-LS
- hemidesmosome-like structures
- BK
- bradykinin
- BPAG
- bullous pemphigoid antigen
- CN
- calcineurin
- CsA
- cyclosporin A
- IF
- immunofluorescence analysis
- PPP
- serine-threonine protein phosphatases
- SCC
- squamous cell carcinoma
- BAPTA-AM
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- THG
- thapsigargin
- Ab
- antibody.
REFERENCES
- 1. Borradori L., Sonnenberg A. (1996) Hemidesmosomes: roles in adhesion, signaling, and human diseases. Curr. Opin. Cell Biol. 8, 647–656 [DOI] [PubMed] [Google Scholar]
- 2. Green K. J., Jones J. C. (1996) Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB J. 10, 871–881 [DOI] [PubMed] [Google Scholar]
- 3. Margadant C., Frijns E., Wilhelmsen K., Sonnenberg A. (2008) Regulation of hemidesmosome disassembly by growth factor receptors. Curr. Opin. Cell Biol. 20, 589–596 [DOI] [PubMed] [Google Scholar]
- 4. Gipson I. K., Spurr-Michaud S., Tisdale A., Elwell J., Stepp M. A. (1993) Redistribution of the hemidesmosome components α6β4 integrin and bullous pemphigoid antigens during epithelial wound healing. Exp. Cell Res. 207, 86–98 [DOI] [PubMed] [Google Scholar]
- 5. Herold-Mende C., Kartenbeck J., Tomakidi P., Bosch F. X. (2001) Metastatic growth of squamous cell carcinomas is correlated with up-regulation and redistribution of hemidesmosomal components. Cell Tissue Res. 306, 399–408 [DOI] [PubMed] [Google Scholar]
- 6. Borradori L., Sonnenberg A. (1999) Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Invest. Dermatol. 112, 411–418 [DOI] [PubMed] [Google Scholar]
- 7. Christiano A. M., Uitto J. (1996) Molecular complexity of the cutaneous basement membrane zone. Revelations from the paradigms of epidermolysis bullosa. Exp. Dermatol. 5, 1–11 [DOI] [PubMed] [Google Scholar]
- 8. Nievers M. G., Schaapveld R. Q., Sonnenberg A. (1999) Biology and function of hemidesmosomes. Matrix Biol. 18, 5–17 [DOI] [PubMed] [Google Scholar]
- 9. Mercurio A. M. (1995) Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 5, 419–423 [DOI] [PubMed] [Google Scholar]
- 10. Rabinovitz I., Mercurio A. M. (1996) The integrin α6β4 and the biology of carcinoma. Biochem. Cell Biol. 74, 811–821 [DOI] [PubMed] [Google Scholar]
- 11. Koster J., Geerts D., Favre B., Borradori L., Sonnenberg A. (2003) Analysis of the interactions between BP180, BP230, plectin, and the integrin α6β4 important for hemidesmosome assembly. J. Cell Sci. 116, 387–399 [DOI] [PubMed] [Google Scholar]
- 12. Geuijen C. A., Sonnenberg A. (2002) Dynamics of the α6β4 integrin in keratinocytes. Mol. Biol. Cell 13, 3845–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsuruta D., Hopkinson S. B., Jones J. C. (2003) Hemidesmosome protein dynamics in live epithelial cells. Cell Motil. Cytoskeleton 54, 122–134 [DOI] [PubMed] [Google Scholar]
- 14. Dans M., Gagnoux-Palacios L., Blaikie P., Klein S., Mariotti A., Giancotti F. G. (2001) Tyrosine phosphorylation of the β4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J. Biol. Chem. 276, 1494–1502 [DOI] [PubMed] [Google Scholar]
- 15. Mariotti A., Kedeshian P. A., Dans M., Curatola A. M., Gagnoux-Palacios L., Giancotti F. G. (2001) EGF-R signaling through Fyn kinase disrupts the function of integrin α6β4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J. Cell Biol. 155, 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabinovitz I., Toker A., Mercurio A. M. (1999) Protein kinase C-dependent mobilization of the α6β4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J. Cell Biol. 146, 1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rabinovitz I., Tsomo L., Mercurio A. M. (2004) Protein kinase C-α phosphorylation of specific serines in the connecting segment of the β4 integrin regulates the dynamics of type II hemidesmosomes. Mol. Cell. Biol. 24, 4351–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Germain E. C., Santos T. M., Rabinovitz I. (2009) Phosphorylation of a novel site on the β4 integrin at the trailing edge of migrating cells promotes hemidesmosome disassembly. Mol. Biol. Cell 20, 56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilhelmsen K., Litjens S. H., Kuikman I., Margadant C., van Rheenen J., Sonnenberg A. (2007) Serine phosphorylation of the integrin β4 subunit is necessary for epidermal growth factor receptor-induced hemidesmosome disruption. Mol. Biol. Cell 18, 3512–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frijns E., Sachs N., Kreft M., Wilhelmsen K., Sonnenberg A. (2010) EGF-induced MAPK signaling inhibits hemidesmosome formation through phosphorylation of the integrin β4. J. Biol. Chem. 285, 37650–37662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frijns E., Kuikman I., Litjens S., Raspe M., Jalink K., Ports M., Wilhelmsen K., Sonnenberg A. (2012) Phosphorylation of threonine 1736 in the C-terminal tail of integrin β4 contributes to hemidesmosome disassembly. Mol. Biol. Cell 23, 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mainiero F., Pepe A., Yeon M., Ren Y., Giancotti F. G. (1996) The intracellular functions of α6β4 integrin are regulated by EGF. J. Cell Biol. 134, 241–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kashyap T., Germain E., Roche M., Lyle S., Rabinovitz I. (2011) Role of β4 integrin phosphorylation in human invasive squamous cell carcinoma: regulation of hemidesmosome stability modulates cell migration. Lab. Invest. 91, 1414–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Almeida E. A., Huovila A. P., Sutherland A. E., Stephens L. E., Calarco P. G., Shaw L. M., Mercurio A. M., Sonnenberg A., Primakoff P., Myles D. G., White J. M. (1995) Mouse egg integrin α6β1 functions as a sperm receptor. Cell 81, 1095–1104 [DOI] [PubMed] [Google Scholar]
- 25. Rusnak F., Mertz P. (2000) Calcineurin: form and function. Physiol. Rev. 80, 1483–1521 [DOI] [PubMed] [Google Scholar]
- 26. Euvrard S., Kanitakis J., Claudy A. (2003) Skin cancers after organ transplantation. N. Engl. J. Med. 348, 1681–1691 [DOI] [PubMed] [Google Scholar]
- 27. Hieda Y., Nishizawa Y., Uematsu J., Owaribe K. (1992) Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. J. Cell Biol. 116, 1497–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clipstone N. A., Crabtree G. R. (1993) Calcineurin is a key signaling enzyme in T lymphocyte activation and the target of the immunosuppressive drugs cyclosporin A and FK506. Ann. N.Y. Acad. Sci. 696, 20–30 [DOI] [PubMed] [Google Scholar]
- 29. Rabinovitz I., Mercurio A. M. (1997) The integrin α6β4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J. Cell Biol. 139, 1873–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H., Rao A., Hogan P. G. (2011) Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 21, 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hepler J. R., Nakahata N., Lovenberg T. W., DiGuiseppi J., Herman B., Earp H. S., Harden T. K. (1987) Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J. Biol. Chem. 262, 2951–2956 [PubMed] [Google Scholar]
- 32. Schremmer-Danninger E., Heinz-Erian P., Töpfer-Petersen E., Roscher A. A. (1995) Autoradiographic localization and characterization of bradykinin receptors in human skin. Eur. J. Pharmacol. 283, 207–216 [DOI] [PubMed] [Google Scholar]
- 33. Talwar H. S., Fisher G. J., Voorhees J. J. (1990) Bradykinin induces phosphoinositide turnover, 1,2-diglyceride formation, and growth in cultured adult human keratinocytes. J. Invest. Dermatol. 95, 705–710 [DOI] [PubMed] [Google Scholar]
- 34. Grando S. A. (1997) Biological functions of keratinocyte cholinergic receptors. J. Investig. Dermatol. Symp. Proc. 2, 41–48 [DOI] [PubMed] [Google Scholar]
- 35. Wu X., Nguyen B. C., Dziunycz P., Chang S., Brooks Y., Lefort K., Hofbauer G. F., Dotto G. P. (2010) Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature 465, 368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bachelder R. E., Marchetti A., Falcioni R., Soddu S., Mercurio A. M. (1999) Activation of p53 function in carcinoma cells by the α6β4 integrin. J. Biol. Chem. 274, 20733–20737 [DOI] [PubMed] [Google Scholar]
- 37. Lawson M. A., Maxfield F. R. (1995) Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature 377, 75–79 [DOI] [PubMed] [Google Scholar]
- 38. Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. (1993) Protein kinase C-α activates RAF-1 by direct phosphorylation. Nature 364, 249–252 [DOI] [PubMed] [Google Scholar]
- 39. Wang Y., Shibasaki F., Mizuno K. (2005) Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin. J. Biol. Chem. 280, 12683–12689 [DOI] [PubMed] [Google Scholar]
- 40. Price J. E., Sauder D. N., Fidler I. J. (1988) Tumorigenicity and metastatic behavior in nude mice of two human squamous cell carcinoma lines that differ in production of the cytokine ETAF/IL-1. J. Invest. Dermatol. 91, 258–262 [DOI] [PubMed] [Google Scholar]