Background: TIPE2, a newly identified protein, is essential for maintaining immune homeostasis.
Results: Genetic ablation of the Tipe2 gene significantly increased the cerebral volume of infarction and neurological dysfunction in experimental stroke.
Conclusion: TIPE2 is involved in the pathogenesis of stroke.
Significance: TIPE2 plays an essential role in a signal transduction pathway that links the inflammatory immune response to specific conditions after cerebral ischemia.
Keywords: Immunology, Inflammation, Ischemia, NADPH Oxidase, Neuroimmunology, Reactive Oxygen Species (ROS), Stroke
Abstract
The inflammatory responses accompanying stroke are recognized to contribute to secondary ischemic injury. TIPE2 is a very recently identified negative regulator of inflammation that maintains immune homeostasis. However, it is unknown whether TIPE2 is expressed in the brain and contributes to the regulation of cerebral diseases. In this study, we explored the potential roles of TIPE2 in cerebral ischemia/reperfusion injury. TIPE2−/− mice were used to assess whether TIPE2 provides neuroprotection following cerebral ischemia/reperfusion induced by middle cerebral artery occlusion (MCAO), and in vitro primary cerebral cell cultures were used to investigate the expression and regulation of TIPE2. Our results show that genetic ablation of the Tipe2 gene significantly increased the cerebral volume of infarction and neurological dysfunction in mice subjected to MCAO. Flow cytometric analysis revealed more infiltrating macrophages, neutrophils, and lymphocytes in the ischemic hemisphere of TIPE2−/− mice. The responses to inflammatory cytokines and chemokines were significantly increased in TIPE2−/− mouse brain after MCAO. We further observed that TIPE2 was highly induced in WT mice after cerebral ischemia and was expressed mainly in microglia/macrophages, but not in neurons and astrocytes. Finally, we found that regulation of TIPE2 expression was associated with NADPH oxidase activity. These findings demonstrate, for the first time, that TIPE2 is involved in the pathogenesis of stroke and suggest that TIPE2 plays an essential role in a signal transduction pathway that links the inflammatory immune response to specific conditions after cerebral ischemia. Targeting TIPE2 may be a new therapeutic strategy for stroke treatment.
Introduction
TIPE2 (TNF-α-inducible protein 8-like 2, or TNFAIP8L2), a newly identified protein, is essential for maintaining immune homeostasis (1). TIPE2 shares considerable sequence homology with members of the TNFAIP8 (TNF-α-inducible protein 8) family, which are thought to regulate cellular and immune homeostasis. TNFAIP8, the first identified member in this family, is able to enhance cell survival and inhibit apoptosis (2, 3). TIPE2 is a recently identified negative regulator of innate and adaptive immunity and is preferentially expressed in lymphoid tissues (1, 4). TIPE2 deletion in the mouse 129 strain leads to multiorgan inflammation, splenomegaly, and premature death. TIPE2-deficient cells are hyper-responsive to Toll-like receptor (TLR)2 activation, indicating that TIPE2 is an essential regulator of TLR-mediated innate immunity. Interestingly, although TIPE2 is not expressed in the NIH 3T3 fibroblast cell line, following stimulation with TNF-α, NIH 3T3 fibroblasts express detectable levels of TIPE2 mRNA, suggesting that TIPE2 may be expressed in other cell types to establish equilibrium during an inflammatory response (1, 5). Besides TNFAIP8 and TIPE2, two additional uncharacterized members of the TNFAIP8 family may exist and are designated TNFAIP8L1 (TIPE1) and TNFAIP8L3 (TIPE3) in the GenBank.
Stroke is one of the most common vascular diseases of the central nervous system. The inflammatory responses accompanying stroke are recognized to contribute to secondary ischemic injury (6–8), including induced microglial and astrocyte activity; increased production of cytokines, chemokines, adhesion molecules, and metalloproteinases; and infiltration of monocytes and leukocytes into injured cerebral regions. Recent studies have indicated that the activation of the immune system as a result of disturbances in the redox state of cells seems to contribute to the brain damage and cerebrovascular diseases in these conditions. However, to date, it is unclear whether the TNFAIP8 family is expressed in the brain and contributes to the regulation of cerebral functions. Given the role of TIPE2 in the regulation of TLR function and the link between TLRs and ischemic cerebral injury, in this study, we used an in vivo middle cerebral artery occlusion (MCAO) model and in vitro primary cerebral cell cultures to determine the expression and functions of TIPE2 in cerebral ischemia-induced injury.
EXPERIMENTAL PROCEDURES
Animals
TIPE2-deficient (TIPE2−/−) mice were generated as described (1). All TIPE2−/− mice used in these studies had been backcrossed to the C57BL/6 genetic background for 10 generations and were <10 weeks old. These mice do not develop spontaneous inflammatory diseases at this age. All procedures were preapproved by the Institutional Animal Care and Use Committee.
Model for Transient Focal Cerebral Ischemia
Transient MCAO was induced in both TIPE2−/− and WT C57BL/6 mice (22–25 g) as described previously (9). After 2 h of MCAO, the filament was removed, and blood flow was restored. Mice were killed after reperfusion at 12, 24, 48, 72, or 96 h. The sham groups were subjected to the same procedure except for the occlusion of the middle cerebral artery.
Infract Volume and Neurological Function Assessment
Stroke outcomes were assessed at 12, 24, 48, or 72 h after reperfusion using both cerebral infarct volume and a four-tiered neurological scoring system as described previously (10).
Primary Cell Cultures and Treatments
Primary microglia and astrocytes were isolated and cultured as described previously (11). The purity of microglia and astrocytes was evaluated by immunofluorescence staining using antibodies against CD11b (Pharmingen) and glial fibrillary acidic protein (GFAP; Invitrogen), respectively. Primary microglial cells were pretreated with apocynin (100 μm) for 30 min and then stimulated with LPS (0.1 μg/ml) or by the model of oxygen/glucose deprivation (OGD).
RNA Extraction, RT-PCR, and ELISA
Total RNA was isolated from brain or cells using TRIzol reagent (Invitrogen) as described previously (12). The mRNA levels were analyzed by RT-PCR or real-time quantitative RT-PCR using a Bio-Rad iCycler system. Proinflammatory cytokines were measured by ELISA as described (13).
Western Blot Analysis
Western blotting was performed as described (14). Anti-TIPE2 and anti-NOX2 primary antibodies (Proteintech Group Inc., Chicago, IL) were used in this study. To document the loading controls, the membrane was reprobed with a primary antibody against the housekeeping protein β-actin.
Immunofluorescence Staining
Staining was performed on tissue sections or cultured cells as described (10). To determine the lineage of TIPE2-positive cells, additional staining of neurons was performed using anti-NeuN antibody (Invitrogen), and that of astrocytes was performed using anti-GFAP antibody, whereas anti-CD11b antibody was used to stain microglia/macrophages.
Cresyl Violet and TUNEL Staining
Cresyl violet staining was performed as described previously (15), and TUNEL staining was performed using an ApopTag peroxidase in situ apoptosis detection kit (EMD Millipore, Billerica, MA) according to the manufacturer's protocol.
Preparation of Infiltrating Cells and Flow Cytometry
The infiltrating cells in the brain were prepared according to the procedure described previously (16). Rat monoclonal antibodies specific for mouse surface markers (Pharmingen) were as follows. FITC-conjugated anti-CD11b antibody recognizes monocytes/macrophages and microglia. Peridinin-chlorophyll-protein complex-conjugated anti-CD45 antibody identifies leukocyte common antigen expressed in all leukocytes and at lower levels in resting microglia. Phycoerythrin-conjugated anti-CD3 antibody is a T cell-specific marker. Allophycocyanin-conjugated anti-Ly-6G antibody is a specific marker for neutrophils. Recovered cells were stained with fluorescently conjugated antibodies. Flow cytometric analysis was performed with a FACScan (BD Biosciences). Data were analyzed (FlowJo software, Tree Star Inc.) using isotype control antibodies to set quadrants before calculating the percentage of positive cells.
Measurements of NADPH Oxidase Activity
NADPH oxidase activity was determined by measurement of superoxide (O2˙̄) production in brain tissue homogenates. Fluorescence spectrometry for O2˙̄ production was performed using a modified dihydroethidium-based fluorescence spectrometric assay as described previously (17).
Statistics
All data are expressed as means ± S.E. Statistical analysis was performed by two-way analysis of variance, followed by the Student-Newman-Keuls test. When only two groups were compared, Student's t test was used. Significance was placed at p < 0.05.
RESULTS
Expression Patterns of the TNFAIP8 Family in the Brain
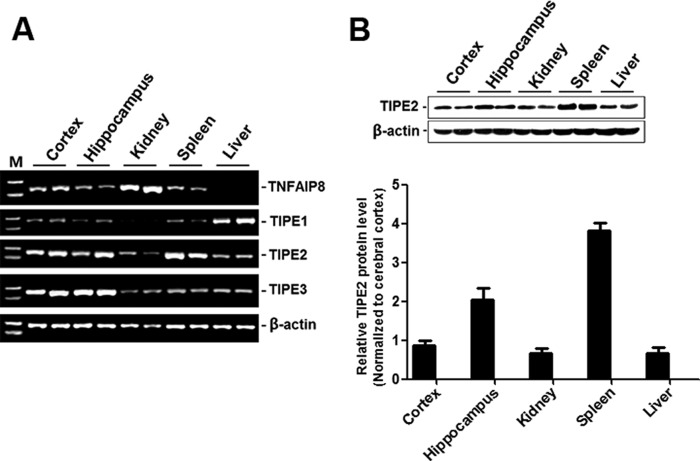
To determine the expression patterns of the TNFAIP8 family in the brain, total RNA was extracted from tissues of C57BL/6 mice, and RT-PCR was performed. As shown in Fig. 1A, of the brain (cortex and hippocampus), kidney, liver, and spleen, TNFAIP8 was the most abundant in the kidney and was hardly detectable in the liver. TIPE1 was detected in these tissues with a relatively high level in the liver. TIPE2 was rich in the spleen, present in the brain and liver, and weak in the kidney, and TIPE3 was abundant in the brain. Because we wanted to focus on the role of TIPE2 in cerebral ischemia injury, we further confirmed the protein expression patterns of TIPE2 in these tissues by Western blot analysis as shown in Fig. 1B.
FIGURE 1.
Expression patterns of the TNFAIP8 family in the brain. A, RT-PCR analysis of total RNAs extracted from freshly harvested organs of C57BL/6 mice, including the brain (cortex and hippocampus), kidney, liver, and spleen. B, representative Western blot and summarized data showing the protein levels of TIPE2 in the selected tissues (n = 6).
TIPE2 Deficiency Exacerbates Stroke Outcomes
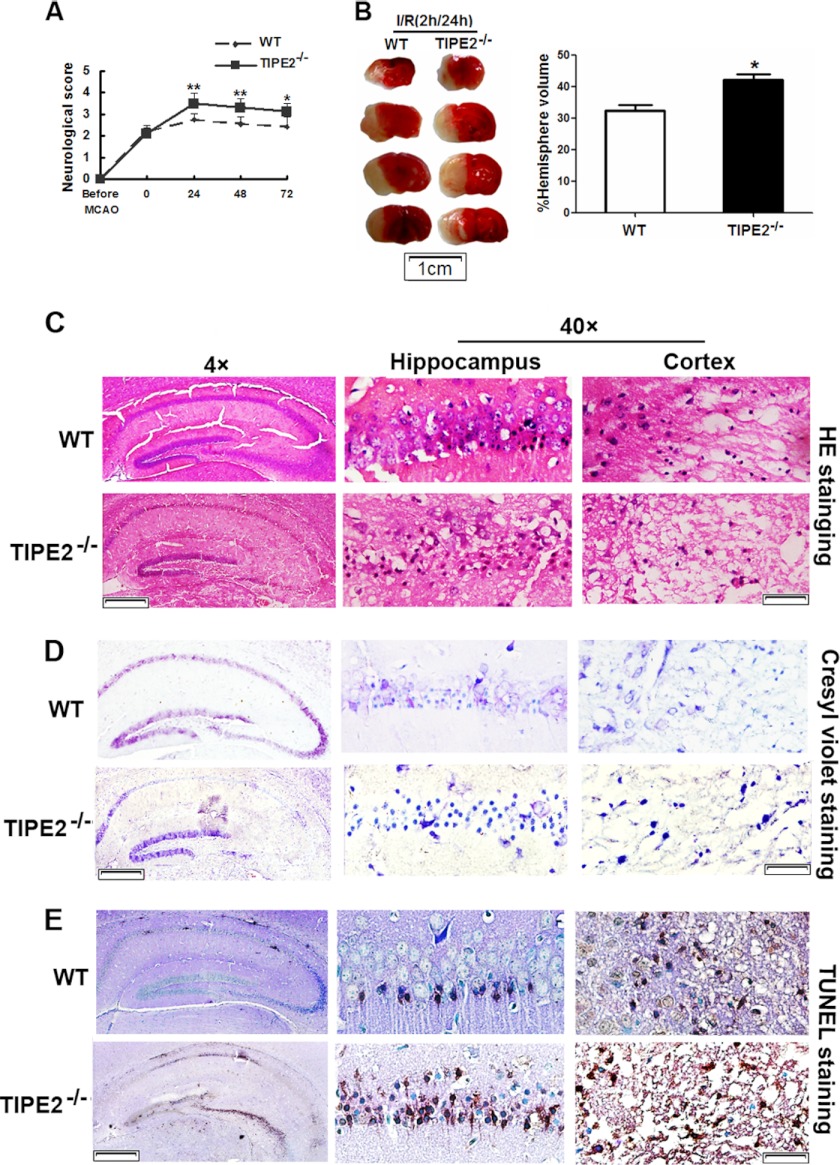
The neurological deficit score was used to evaluate neurological function after MCAO. TIPE2−/− mice showed worse neurological dysfunctions compared with WT mice at different time points after reperfusion (Fig. 2A). To confirm that the ischemic insult was equivalent among all groups, relevant physiological parameters were assessed before and during MCAO. As an example in Table 1, physiological variables were not significantly different between WT and TIPE2−/− mice before MCAO, during MCAO, or at 24 h after reperfusion. However, infarction volume at 24 h after reperfusion was significantly larger in TIPE2−/− mice (42.1 ± 3.8%) compared with WT mice (31.5 ± 3.3% (n = 8/group), p = 0.0016) (Fig. 2B). The increase in infarct size in TIPE2−/− mice was associated with more severe neurological impairment (Fig. 2A). To further assess the role of TIPE2 in ischemia-evoked neuronal apoptosis, H&E staining was used to show morphological features of injured neurons in the hippocampus and cerebral cortex (Fig. 2C). Cresyl violet staining was used to examine the surviving neurons (Fig. 2D). We found that ischemia/reperfusion induced severe neuron death in TIPE2−/− mice. TUNEL staining was used to assess the apoptosis-like neurons (Fig. 2E); a significant number of TUNEL-positive neurons manifested as brownish staining in the nuclei of TIPE2−/− mice. Collectively, these results indicate that a lack of TIPE2 is detrimental to the brain during ischemia.
FIGURE 2.
TIPE2 deficiency exacerbates stroke outcomes. A, neurological deficit scores in WT and TIPE2−/− mice after cerebral ischemia/reperfusion (I/R). B, representative photographs of 2,3,5-triphenyltetrazolium chloride staining (left panel) and calculated infarct volume (right panel) showing increased cerebral volume at 24 h of reperfusion after MCAO in WT and TIPE2−/− mice. C, representative photomicrographs of H&E staining in the hippocampus and cerebral cortex after ischemia/reperfusion. D, representative photomicrographs of cresyl violet staining in the hippocampus and cerebral cortex. E, representative photomicrographs of TUNEL staining and counterstaining with methyl green. For magnification ×4, scale bars = 200 μm; for magnification ×40, scale bars = 20 μm. *, p < 0.05; **, p < 0.01 for WT mice versus TIPE2−/− mice (n = 8).
TABLE 1.
Comparison of physiological variables
Relevant physiological parameters were assessed before, during, and after MCAO. The physiological variables were not significantly different between WT and TIPE2−/− mice before MCAO, during MCAO, or at 24 h after reperfusion, confirming that the ischemic insult was equivalent among all groups. Values are means ± S.E. SpO2, oxygen saturation.
| WT mice | TIPE2−/− mice | |
|---|---|---|
| Before MCAO | ||
| Heart rate (beats/min) | 455.13 ± 5.72 | 459.36 ± 6.12 |
| Arterial blood pH | 738 ± 0.10 | 7.40 ± 0.08 |
| SpO2 (%) | 95.75 ± 0.54 | 93.74 ± 0.89 |
| Temperature (°C) | 36.47 ± 0.21 | 36.41 ± 0.11 |
| Glucose (mg/dl) | 215.25 ± 5.22 | 213.16 ± 5.13 |
| During MCAO | ||
| Heart rate (beats/min) | 451.33 ± 4.59 | 449.28 ± 5.98 |
| Arterial blood pH | 7.38 ± 0.27 | 7.41 ± 0.31 |
| SpO2 (%) | 94.97 ± 0.71 | 94.51 ± 0.57 |
| Temperature (°C) | 36.35 ± 0.26 | 36.29 ± 0.31 |
| Glucose (mg/dl) | 222.13 ± 10.20 | 217.67 ± 7.21 |
| After MCAO | ||
| Heart rate (beats/min) | 451.49 ± 12.21 | 453.57 ± 10.29 |
| Arterial blood pH | 7.37 ± 0.19 | 7.43 ± 0.35 |
| SpO2 (%) | 94.27 ± 0.63 | 94.51 ± 0.92 |
| Temperature (°C) | 36.28 ± 052 | 36.11 ± 0.32 |
| Glucose (mg/dl) | 211.22 ± 27.88 | 203.27 ± 24.33 |
TIPE2 Deficiency Increases the Levels of Cytokines and Alters Cerebral Inflammatory Cell Infiltration
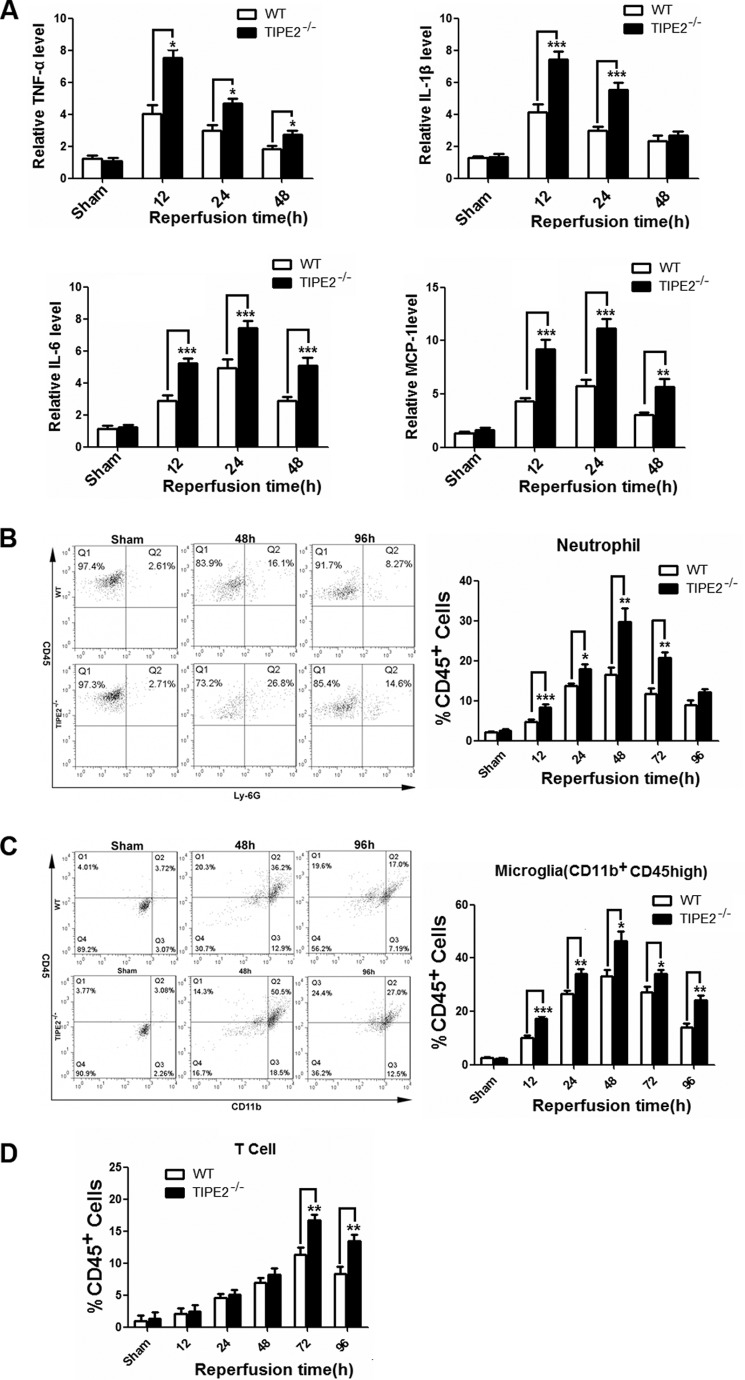
To elucidate the roles of TIPE2 in vivo, we measured the production of cytokines in ischemic brains of TIPE2−/− mice. We found that the levels of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6, in the TIPE2−/− group were significantly enhanced compared with the ischemic brains of WT mice. The up-regulation of TNF-α peaked at 12 h after reperfusion, and the extent of the post-ischemic response was approximately the same as IL-1β, but a delayed peak expression of IL-6 was observed at 24 h after reperfusion. Expression of the chemokine MCP-1 was also dramatically increased and reached a peak value at 24 h after reperfusion (Fig. 3A).
FIGURE 3.
TIPE2 deficiency increases cytokine levels and alters cerebral inflammatory cell infiltration. A, levels of proinflammatory mediators, including TNF-α, IL-1β, IL-6, and MCP-1, in ischemic brains as determined by ELISA at 12, 24, 48, or 72 h after reperfusion. B, leukocytes are major effectors of inflammatory damage after cerebral ischemia. The number of infiltrating Gr1+ neutrophils was evaluated in ischemic brain by flow cytometric analysis. C, number of infiltrating CD11b+CD45high macrophages in ischemic brain. D, number of infiltrating CD3+ T cells in ischemic brain. *, p < 0.05; **, p < 0.01; ***, p < 0.001 for WT mice versus TIPE2−/− mice (n = 8).
Leukocytes are major effectors of inflammatory damage after cerebral ischemia (18, 19). To determine whether the TIPE2 deficiency altered leukocyte composition in the brain after MCAO, the numbers of infiltrating Gr1+ neutrophils, CD11b+CD45high macrophages, and CD3+ T cells were evaluated by flow cytometry after 2 h of ischemia at different reperfusion times. We found that the accumulation of these leukocyte subtypes was significantly greater in the TIPE2−/− group after reperfusion. (Fig. 3, B–D). TIPE2 deficiency significantly increased in the percentage of neutrophils and microglia/macrophages especially during the early reperfusion period. It seems that the threshold of the leukocytes lacking TIPE2 was decreased. The infiltration patterns of neutrophils and macrophages were similar and reached a peak value at 48 h after reperfusion. On the other hand, T cell infiltration peaked at 72 h. Overall, the absence of TIPE2 has a profound effect on the inflammatory response after reperfusion.
Increased Expression of TIPE2 after Cerebral Ischemia/Reperfusion
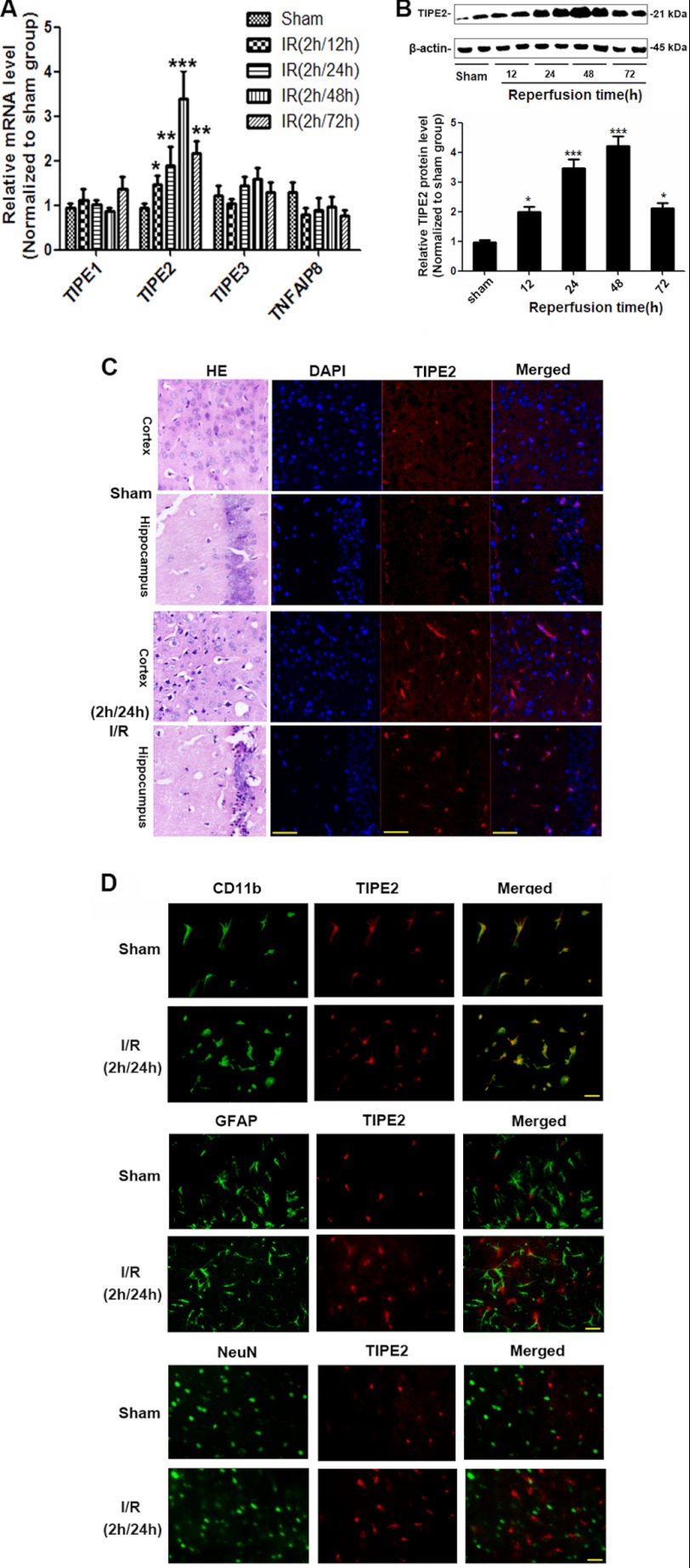
By real-time RT-PCR analysis, we found that, among the TNFAIP8 family members, TIPE2 mRNA levels were markedly enhanced in the brains from ischemic WT mice. TNFAIP8, TIPE1, and TIPE3 had no significant change compared with the sham groups, although TNFAIP8 had a tendency to decrease (Fig. 4A). We further confirmed the up-regulation of TIPE2 in post-ischemic brain by Western blot analysis (Fig. 4B) and immunofluorescence analysis (Fig. 4C). To identify cell types that express TIPE2 in the brain after ischemia/reperfusion, dual immunofluorescence staining was performed. We identified neurons by staining for the neuronal nuclear marker NeuN, astrocytes by staining for GFAP, and activated microglia/macrophages by staining for CD11b. We found that TIPE2 is expressed in microglia from ischemic brain. However, our results did not reveal a colocalization of TIPE2 and GFAP (NeuN), despite expression of GFAP and TIPE2 in the direct vicinity (Fig. 4D), indicating that neither astrocytes nor neurons express TIPE2 before and after cerebral ischemia. Collectively, microglia are a major source of TIPE2 in regions vulnerable to ischemic insult.
FIGURE 4.
Increased expression levels of TIPE2 in WT mice after cerebral ischemia/reperfusion. A, relative quantitation of mRNA levels in the TNFAIP8 family by real-time RT-PCR analysis in ischemic brain. B, Western blot analysis of TIPE2 protein levels in brains from ischemic WT mice. C, immunofluorescence staining showing the expression of TIPE2 in ischemic WT mouse hippocampus and cortex. H&E-stained images are included with these parallel sections for histological details of the areas. Scale bars = 50 μm. D, cellular localization of TIPE2 after cerebral ischemia/reperfusion (I/R) showing that TIPE2 is expressed in microglia rather than in astrocytes and neurons of ischemic brain. Scale bars = 20 μm. *, p < 0.05; ***, p < 0.001 for ischemic WT mice versus sham-operated WT mice (n = 8).
Enhanced Expression Levels of Cytokines in TIPE2-deficient Microglial Cells upon OGD
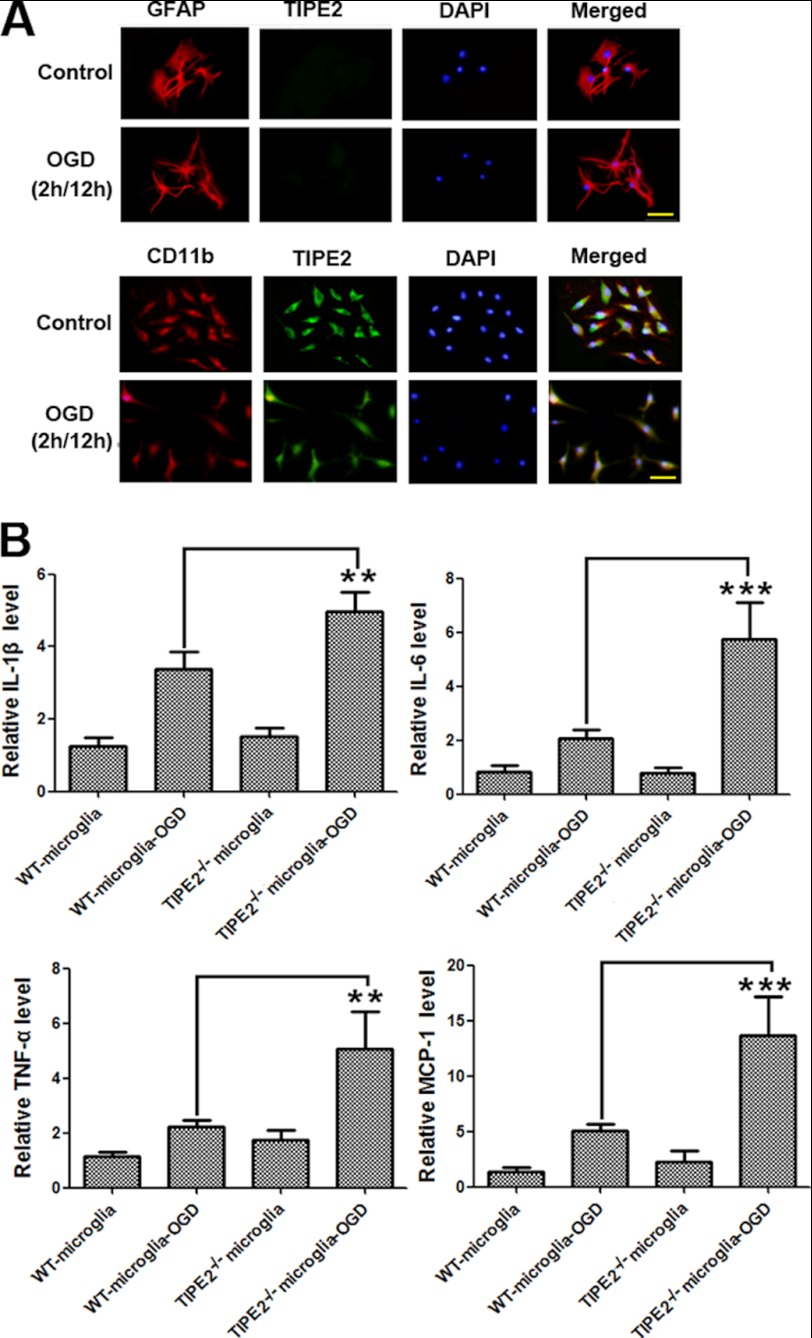
To provide evidence to establish the relationship between TIPE2 expression in microglia and the altered immune response in the brain, we isolated microglia and astrocytes, and purity was confirmed by staining with the specific markers. By immunofluorescence staining, we found that TIPE2 was expressed and reduced by OGD in microglia but not in astrocytes (Fig. 5A). We then examined the expression of cytokines in isolated microglia from both WT and TIPE2−/− mice. We found that TIPE2−/− microglia expressed significantly more cytokines, including IL-1β, TNF-α, IL-6, and MCP-1, than WT microglia upon OGD (Fig. 5B). Similar results were also obtained by knocking down TIPE2 expression in BV-2 cells (data not shown). Our results indicate that immune responses in TIPE2−/− microglia are augmented.
FIGURE 5.
Enhanced expression of cytokines in TIPE2-deficient microglial cells upon OGD. A, immunofluorescence staining indicating that TIPE2 is expressed and reduced by OGD in microglia but not in astrocytes. Scale bars = 20 μm. B, expression of cytokines, including IL-1β, IL-6, TNF-α, and MCP-1, in microglia isolated from WT and TIPE2−/− mice. **, p < 0.01; ***, p < 0.001 for TIPE2−/− microglia versus WT microglia (n = 8).
TIPE2 Expression Is Associated with NADPH Oxidase Activity in Vivo
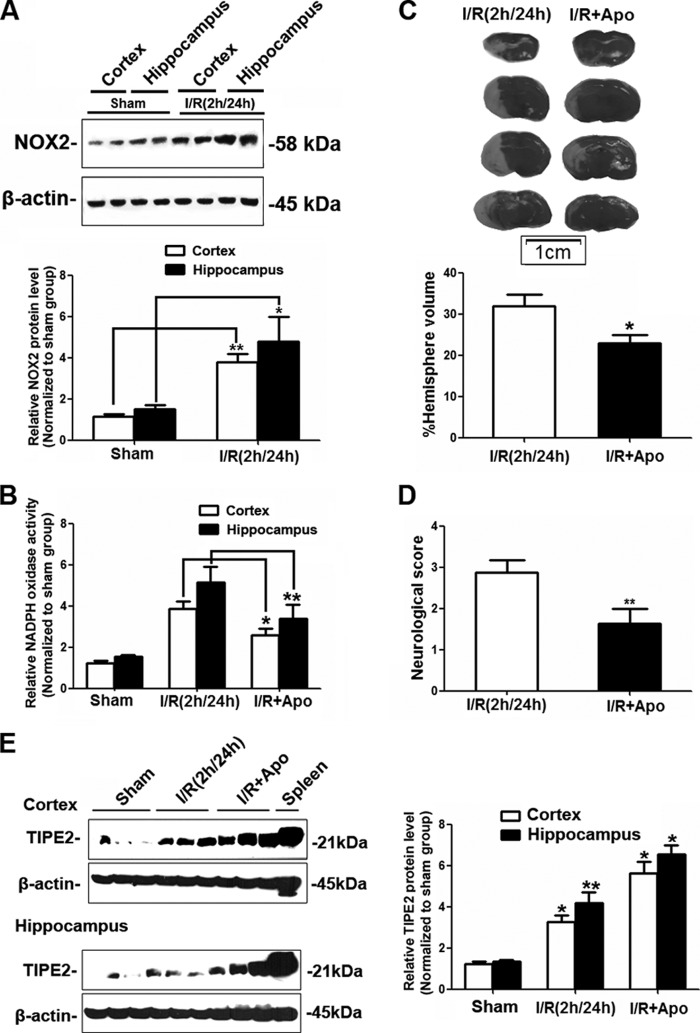
In this study, we treated C57BL/6 mice with apocynin, a well known NADPH oxidase inhibitor, at a dose of 3.0 mg/kg 30 min before reperfusion as described (20). Cerebral ischemia induced expression of the NADPH oxidative subunit NOX2 (Fig. 6A), and its activity was markedly attenuated by apocynin treatment (Fig. 6B). Inhibition of NADPH oxidase activity significantly reduced infarct volume (Fig. 6C) and the neurological deficit score (Fig. 6D). Interestingly, we further found that apocynin significantly induced more TIPE2 expression in ischemic WT mice (Fig. 6E). Together with our preliminary studies showing that apocynin had no effect on TIPE2 expression in WT mice under the sham-operated conditions, our results suggest that NADPH oxidase activity is involved in the regulation of TIPE2 expression after cerebral ischemia/reperfusion.
FIGURE 6.
Effects of inhibition of NADPH oxidase activity on cerebral ischemia/reperfusion injury and expression of TIPE2. A, Western blot analysis of NOX2 protein levels in brains from ischemic WT mice. B, summarized data showing the effect of the NADPH oxidase inhibitor apocynin (Apo) on superoxide production in ischemic brain. C, calculated infarct volume in ischemic WT mice with apocynin treatment after cerebral ischemia/reperfusion (I/R). D, neurological deficit scores in WT mice with apocynin treatment after cerebral ischemia/reperfusion. E, representative Western blots and quantified data showing the relative protein levels of TIPE2 in ischemic WT mouse cerebral cortex and hippocampus with apocynin treatment. *, p < 0.05; **, p < 0.01 for ischemic WT mice versus sham-operated WT mice (n = 8).
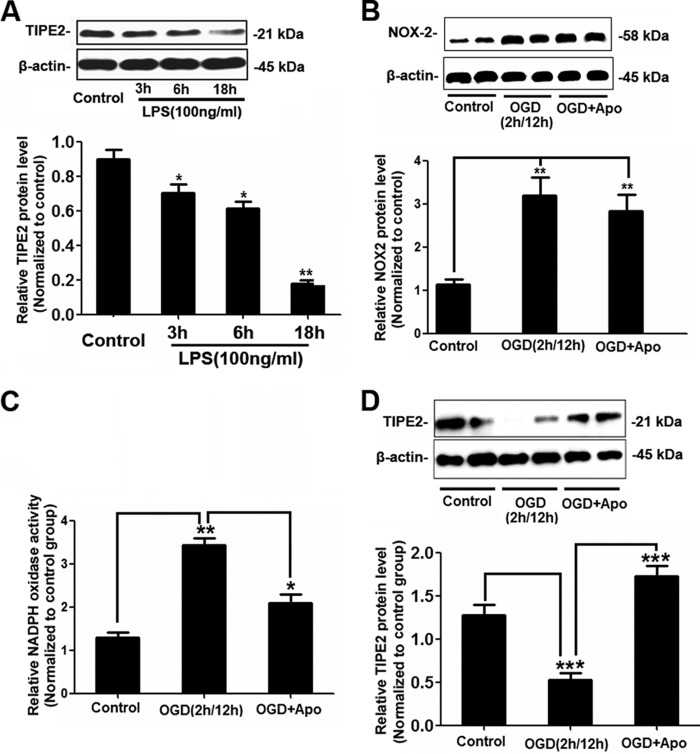
Apocynin Attenuates TIPE2 Down-regulation in Response to LPS/OGD in Primary Microglial Cells
WT microglia were treated with LPS, and we found that LPS significantly reduced TIPE2 expression in a time-dependent manner (Fig. 7A). To further elucidate the role of NADPH oxidase in the regulation of TIPE2 expression, apocynin was used in this study. As shown in Fig. 7 (B and C), NOX2 expression was markedly enhanced by OGD, and apocynin inhibited OGD-induced NADPH oxidase activity. Moreover, we found that OGD decreased TIPE2 expression, which could be recovered by apocynin (Fig. 7D).
FIGURE 7.
Apocynin attenuates TIPE2 down-regulation in response to LPS/OGD in microglia. A, representative Western blots and quantified data showing that LPS significantly reduced TIPE2 expression in a time-dependent manner. *, p < 0.05; **, p < 0.01 for the LPS-treated group versus the control group. B, Western blot analysis of NOX2 protein levels in microglia upon OGD. C, summarized data showing the effect of the NADPH oxidase inhibitor apocynin (Apo) on superoxide production in microglia upon OGD. **, p < 0.01 for the OGD group versus the control group; *, p < 0.05 for the OGD group versus the OGD + apocynin group. D, representative Western blots and quantified data showing that OGD-reduced TIPE2 expression could be recovered by apocynin. ***, p < 0.001 for the OGD group versus the control group or the OGD + apocynin group (n = 8).
DISCUSSION
Increasing evidence indicates that the post-ischemic inflammatory response plays a detrimental role in the secondary progression of stroke injury (21, 22). Inflammatory cell recruitments and activation have been implicated in the progression of cerebral ischemia/reperfusion injury. Studies have demonstrated that the infiltration of T cells into the brain, as well as the cytokines IL-23 and IL-17, has a pivotal role in the evolution of brain infarction and the accompanying neurological deficits (23), and T and B cell-deficient mice with experimental stroke have reduced lesion size and inflammation (24). A major conundrum in the immunology of stroke is how to enhance the early immunoregulation that limits excessive inflammation of the central nervous system. This is the first report to characterize TIPE2, a newly identified regulator of immunity, in the brain and to evaluate the role of TIPE2 in cerebral ischemia/reperfusion injury. Our results show that genetic ablation of the Tipe2 gene increased the cerebral volume of infarction, neurological dysfunction, inflammatory cytokine production, and infiltration of inflammatory cells in mice subjected to MCAO, suggesting a neuroprotective role of TIPE2 via anti-inflammatory mechanisms in the pathogenic processes of stroke. We further found that TIPE2 was highly induced after cerebral ischemia and that regulation of TIPE2 expression was associated with NADPH oxidase activity. These data indicate that TIPE2 is an immunoregulator that prevents deleterious inflammatory responses after stroke.
A subfamily of proteins that contribute to cellular homeostasis possesses a hexahelical bundle motif called the death effector domain (DED) (25). Proteins with DED are key signal transducers involved in cell death and inflammation (26–28). The TNFAIP8 family is normally considered as a new subfamily of DED-containing proteins, although the three-dimensional structure of the TIPE2 DED-like domain is distinct (4). Recent reports have demonstrated that TIPE2 is an essential regulator of inflammation and immune homeostasis (1, 4). TIPE2 governs immune homeostasis by negatively regulating signaling by T cell receptors and TLRs. The most important findings of this study are that TIPE2 deficiency increases the cerebral volume of infarction in ischemic mice and that TIPE2 is significantly induced after cerebral ischemia, indicating that TIPE2 is involved in the pathogenesis of stroke. TIPE2 deficiency increasing the cerebral volume of infarction could account for inflammation. TIPE2 influences the production of proinflammatory cytokines and immune cell infiltration, which are required for the detrimental post-ischemic inflammatory response contributing to secondary brain damage.
Although these data clearly implicate TIPE2 as a critical mediator of cerebral injury following stroke, the source and distribution of TIPE2 in ischemic brain remain to be further characterized. The brain responds to ischemic injury with acute and prolonged inflammatory phases, which are characterized by rapid activation of resident cells (mainly microglia), production of proinflammatory mediators, and infiltration of various types of inflammatory cells into the ischemic brain tissue (29). These cellular events collaboratively contribute to ischemic brain injury. It is not surprising that infiltration of inflammatory cells into ischemic brain contributes to the up-regulation of TIPE2 expression in ischemic brain as we reported in this study. We wondered whether TIPE2 is also induced in cerebral resident cells after ischemia to establish equilibrium during an inflammatory response; we then detected the expression of TIPE2 in three major types of cerebral cells, including microglia, astrocytes, and neurons. Microglial cells, the resident macrophages of the brain, play a critical role as resident immunocompetent and phagocytic cells in the brain. Resident microglia are activated within minutes of ischemia onset and produce a plethora of proinflammatory mediators to exacerbate tissue damage (29). In this study, we identified that TIPE2 is expressed in microglia in the ischemic cerebral cortex and hippocampus rather than in astrocytes and neurons, although like microglia, astrocytes are also capable of secreting inflammatory factors. We isolated microglia from both WT and TIPE2−/− mice and found that TIPE2−/− microglia expressed significantly more cytokines upon OGD, indicating that TIPE2 from microglia may play an important role in the regulation of immune responses in cerebral ischemia injury.
Recent studies have indicated that activation of the immune system as a result of disturbances in the redox state of cells seems to contribute to brain damage (30–32). A number of studies have demonstrated that NADPH oxidase-derived reactive oxygen species are central to cerebral ischemia-induced oxidative stress in the brain (33–36). Inhibition of NADPH oxidase activity is neuroprotective after ischemia/reperfusion (20, 34). In this study, we found that expression of the NADPH oxidase subunit NOX2 (gp91phox) and NADPH oxidase activity were dramatically increased after reperfusion. Therefore, it is important to determine whether the regulation of TIPE2 expression is associated with NADPH oxidase activity. Indeed, our results show that inhibition of NADPH oxidase activity further increased TIPE2 expression under cerebral ischemia conditions. These results suggest that the neuroprotective effects of inhibition of NADPH oxidase activity after ischemia/reperfusion are associated with the regulation of TIPE2-related signaling pathways. In an in vitro study, the expression of TIPE2 was significantly decreased, and apocynin increased the expression of TIPE2 to protect against OGD in microglia. It is known that resting microglia are in a quiescent state and that their ability to induce inflammation is low. Upon cerebral ischemia/reperfusion injury, activation, recruitment, and accumulation of inflammatory cells are significantly enhanced. The results reported here indicate that TIPE2 serves as a regulator of this process. TIPE2 is constitutively expressed at relatively high levels in microglial cells to prevent their activation. Although OGD reduces TIPE2 levels in microglia, leading to enhanced immune responses as in vitro studies indicated, which is inconsistent with our in vivo study, the overall higher expression of TIPE2 may be due to more inflammatory cells infiltrating into the ischemic penumbra. Inhibition of NADPH oxidase activity further induced TIPE2 expression in microglia to protect against this damage. Thus, TIPE2 serves as a negative regulator of inflammation to maintain immune homeostasis by mediating oxidative stress.
It should be noted that we did not further explore the regulatory mechanisms of TIPE2 expression by NADPH oxidase activity. Further studies need to address this issue. We investigated the expression and action of TIPE2 in the brain and addressed the role of TIPE2 in cerebral ischemia/reperfusion injury. These findings demonstrate, for the first time, that TIPE2 is involved in the pathogenesis of stroke and suggest that TIPE2 may play an essential role in a signal transduction pathway that links the inflammatory immune response to specific conditions after cerebral ischemia.
This work was supported by the Scientific Research Foundation for the National Nature Science Foundation of China (Grants 30901551, 81070918, 81070572, and 81170772), the National 973 Basic Research Program of China (Grant 2010CB732605), the Research Fund for the Doctoral Program of Higher Education, State Education Ministry, China (Grant 20090131120081), the Shandong Natural Science Fund for Distinguished Young Scholars (Grant JQ201121), the Foundation for Excellent Young and Middle-Aged Scientists of Shandong Province, China (Grants BS2009SW005 and BS2009YY017), the Independent Innovation Foundation of Shandong University (Grants IIFSDU, 2010JC17, and 2012TS116), the China Postdoctoral Science Foundation (Grant 2012M511041), and the Foundation of Program for New Century Excellent Talents in University (Grant NCET-11-0311).
- TLR
- Toll-like receptor
- MCAO
- middle cerebral artery occlusion
- GFAP
- glial fibrillary acidic protein
- OGD
- oxygen/glucose deprivation
- DED
- death effector domain.
REFERENCES
- 1. Sun H., Gong S., Carmody R. J., Hilliard A., Li L., Sun J., Kong L., Xu L., Hilliard B., Hu S., Shen H., Yang X., Chen Y. H. (2008) TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133, 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang C., Chakravarty D., Sakabe I., Mewani R. R., Boudreau H. E., Kumar D., Ahmad I., Kasid U. N. (2006) Role of SCC-S2 in experimental metastasis and modulation of VEGFR-2, MMP-1, and MMP-9 expression. Mol. Ther. 13, 947–955 [DOI] [PubMed] [Google Scholar]
- 3. Kumar D., Gokhale P., Broustas C., Chakravarty D., Ahmad I., Kasid U. (2004) Expression of SCC-S2, an anti-apoptotic molecule, correlates with enhanced proliferation and tumorigenicity of MDA-MB-435 cells. Oncogene 23, 612–616 [DOI] [PubMed] [Google Scholar]
- 4. Zhang X., Wang J., Fan C., Li H., Sun H., Gong S., Chen Y. H., Shi Y. (2009) Crystal structure of TIPE2 provides insights into immune homeostasis. Nat. Struct. Mol. Biol. 16, 89–90 [DOI] [PubMed] [Google Scholar]
- 5. Zhang S., Zhang Y., Wei X., Zhen J., Wang Z., Li M., Miao W., Ding H., Du P., Zhang W., He M., Yi F. (2010) Expression and regulation of a novel identified TNFAIP8 family is associated with diabetic nephropathy. Biochim. Biophys. Acta 1802, 1078–1086 [DOI] [PubMed] [Google Scholar]
- 6. Amantea D., Nappi G., Bernardi G., Bagetta G., Corasaniti M. T. (2009) Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 276, 13–26 [DOI] [PubMed] [Google Scholar]
- 7. Xia W., Han J., Huang G., Ying W. (2010) Inflammation in ischemic brain injury: current advances and future perspectives. Clin. Exp. Pharmacol. Physiol. 37, 253–258 [DOI] [PubMed] [Google Scholar]
- 8. Suzuki S., Tanaka K., Suzuki N. (2009) Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J. Cereb. Blood Flow Metab. 29, 464–479 [DOI] [PubMed] [Google Scholar]
- 9. Jasmin J. F., Malhotra S., Singh Dhallu M., Mercier I., Rosenbaum D. M., Lisanti M. P. (2007) Caveolin-1 deficiency increases cerebral ischemic injury. Circ. Res. 100, 721–729 [DOI] [PubMed] [Google Scholar]
- 10. Mocco J., Mack W. J., Ducruet A. F., Sosunov S. A., Sughrue M. E., Hassid B. G., Nair M. N., Laufer I., Komotar R. J., Claire M., Holland H., Pinsky D. J., Connolly E. S., Jr. (2006) Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ. Res. 99, 209–217 [DOI] [PubMed] [Google Scholar]
- 11. Halim N. D., Mcfate T., Mohyeldin A., Okagaki P., Korotchkina L. G., Patel M. S., Jeoung N. H., Harris R. A., Schell M. J., Verma A. (2010) Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia 58, 1168–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Z., Wei X., Zhang Y., Ma X., Li B., Zhang S., Du P., Zhang X., Yi F. (2009) NADPH oxidase-derived ROS contributes to up-regulation of TRPC6 expression in puromycin aminonucleoside-induced podocyte injury. Cell. Physiol. Biochem. 24, 619–626 [DOI] [PubMed] [Google Scholar]
- 13. Manhas N., Shi Y., Taunton J., Sun D. (2010) p90 activation contributes to cerebral ischemic damage via phosphorylation of Na+/H+ exchanger isoform 1. J. Neurochem. 114, 1476–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yi F., Xia M., Li N., Zhang C., Tang L., Li P. L. (2009) Contribution of guanine nucleotide exchange factor Vav2 to hyperhomocysteinemic glomerulosclerosis in rats. Hypertension 53, 90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao J., Pei D. S., Zhang Q. G., Zhang G. Y. (2007) Down-regulation Cdc42 attenuates neuronal apoptosis through inhibiting MLK3/JNK3 cascade during ischemic reperfusion in rat hippocampus. Cell. Signal. 19, 831–843 [DOI] [PubMed] [Google Scholar]
- 16. Campanella M., Sciorati C., Tarozzo G., Beltramo M. (2002) Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke 33, 586–592 [DOI] [PubMed] [Google Scholar]
- 17. Yi F., Zhang A. Y., Janscha J. L., Li P. L., Zou A. P. (2004) Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int. 66, 1977–1987 [DOI] [PubMed] [Google Scholar]
- 18. Gee J. M., Kalil A., Shea C., Becker K. J. (2007) Lymphocytes: potential mediators of post-ischemic injury and neuroprotection. Stroke 38, 783–788 [DOI] [PubMed] [Google Scholar]
- 19. Wang Q., Tang X. N., Yenari M. A. (2007) The inflammatory response in stroke. J. Neuroimmunol. 184, 53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang X. N., Cairns B., Cairns N., Yenari M. A. (2008) Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience 154, 556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becker K. J. (2010) Modulation of the post-ischemic immune response to improve stroke outcome. Stroke 41, S75–S78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yilmaz G., Arumugam T. V., Stokes K. Y., Granger D. N. (2006) Role of T lymphocytes and interferon-γ in ischemic stroke. Circulation 113, 2105–2112 [DOI] [PubMed] [Google Scholar]
- 23. Shichita T., Sugiyama Y., Ooboshi H., Sugimori H., Nakagawa R., Takada I., Iwaki T., Okada Y., Iida M., Cua D. J., Iwakura Y., Yoshimura A. (2009) Pivotal role of cerebral interleukin-17-producing γδ T cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950 [DOI] [PubMed] [Google Scholar]
- 24. Hurn P. D., Subramanian S., Parker S. M., Afentoulis M. E., Kaler L. J., Vandenbark A. A., Offner H. (2007) T and B cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J. Cereb. Blood Flow Metab. 27, 1798–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Freundt E. C., Bidere N., Lenardo M. J. (2008) A different TIPE of immune homeostasis. Cell 133, 401–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang J., Cado D., Chen A., Kabra N. H., Winoto A. (1998) Fas-mediated apoptosis and activation-induced T cell proliferation are defective in mice lacking FADD/Mort1. Nature 392, 296–300 [DOI] [PubMed] [Google Scholar]
- 27. Tibbetts M. D., Zheng L., Lenardo M. J. (2003) The death effector domain protein family: regulators of cellular homeostasis. Nat. Immunol. 4, 404–409 [DOI] [PubMed] [Google Scholar]
- 28. Walsh C. M., Wen B. G., Chinnaiyan A. M., O'Rourke K., Dixit V. M., Hedrick S. M. (1998) A role for FADD in T cell activation and development. Immunity 8, 439–449 [DOI] [PubMed] [Google Scholar]
- 29. Jin R., Yang G., Li G. (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 87, 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arumugam T. V., Okun E., Tang S. C., Thundyil J., Taylor S. M., Woodruff T. M. (2009) Toll-like receptors in ischemia/reperfusion injury. Shock 32, 4–16 [DOI] [PubMed] [Google Scholar]
- 31. Gill R., Tsung A., Billiar T. (2010) Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 48, 1121–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Q. W., Li J. C., Lu F. L., Wen A. Q., Xiang J., Zhang L. L., Huang Z. Y., Wang J. Z. (2008) Up-regulated expression of Toll-like receptor 4 in monocytes correlates with severity of acute cerebral infarction. J. Cereb. Blood Flow Metab. 28, 1588–1596 [DOI] [PubMed] [Google Scholar]
- 33. Kim G. S., Jung J. E., Niizuma K., Chan P. H. (2009) CK2 is a novel negative regulator of NADPH oxidase and a neuroprotectant in mice after cerebral ischemia. J. Neurosci. 29, 14779–14789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen H., Song Y. S., Chan P. H. (2009) Inhibition of NADPH oxidase is neuroprotective after ischemia/reperfusion. J. Cereb. Blood Flow Metab. 29, 1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun G. Y., Horrocks L. A., Farooqui A. A. (2007) The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J. Neurochem. 103, 1–16 [DOI] [PubMed] [Google Scholar]
- 36. Kahles T., Luedike P., Endres M., Galla H. J., Steinmetz H., Busse R., Neumann-Haefelin T., Brandes R. P. (2007) NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 38, 3000–3006 [DOI] [PubMed] [Google Scholar]