Abstract
BACKGROUND
Adrenal/intratumoral androgen biosynthesis contributes to ligand-dependent androgen receptor activation in metastatic castration-resistant prostate cancer (mCRCP). Compounds targeting CYP-17 hydroxylase and lyase, as ketoconazole and abiraterone, block adrenal/intratumoral androgen biosynthesis, and are used as sequential endocrine approaches in mCRCP. We aimed to describe contemporary experience and association of clinical factors with Prostate specific antigen (PSA) response and disease progression, in mCRPC progressing on GnRH-agonist, antiandrogen, antiandrogen withdrawal, and treated with ketoconazole.
METHODS
Data were retrospectively analyzed in all mCRPC patients treated with ketoconazole. Patients continued GnRH-agonist, and treated with ketoconazole 200–400 mg 3× a day until dose-limiting toxicity or disease progression. A multivariate cox regression model was used to identify clinical factors associated with PSA response and disease progression.
RESULTS
From 1999 to 2010, 114 mCRPC patients were treated with ketoconazole. With a median follow-up time of 31 months (range 5–129), 25 patients (22%) had grade 3/4 toxicity, most commonly fatigue, abdominal discomfort, nausea, and dizziness. Sixty-one patients (54%) had ≥50% PSA decline. Median time to progression was 8 months (range 1–129). Factors associated with PSA response and disease progression were response to prior antiandrogen (≥6 vs. <6 months), pre-treatment PSADT (≥3 vs. <3 months) and extent of disease (limited-axial skeleton and/or nodal vs. extensive-appendicular skeleton and/or visceral).
CONCLUSIONS
Ketoconazole is effective and safe in mCRPC. Prior response to antiandrogen, pre-treatment PSADT, and disease extent are associated with PSA response and disease progression, and further supports a therapeutic role in suppressing adrenal androgens in mCRPC.
Keywords: disease progression, ketoconazole, metastatic castration-resistant prostate cancer, PSA response
INTRODUCTION
Prostate cancer is the most common cancer affecting men and the second leading cause of male cancer mortality in the western world [1]. The initial treatment of metastatic prostate cancer consists of androgen deprivation therapy (ADT), usually using a luteinizing hormone-releasing hormone agonist and an antiandrogen [2]. Most patients will respond for 18–48 months, and eventually progress to a castration-resistant state [3].
Activation of the androgen receptor (AR) is central in the pathogenesis of prostate cancer. In the castration-resistant state the AR is exquisitely sensitive to low levels of androgens through its gene amplification, overexpression, activating mutation as well as enhancement of its responses and signaling by stimulation of kinases [4–6]. The source of androgens activating the androgen receptor in the castration-resistant state is the adrenal gland where 10–30% of total serum androgens are produced. More recently the role of intratumoral production of androgens through overexpression of enzymes including the CYP17AI, a key enzyme for de novo steroid and androgen biosynthesis has been emphasized [4–10].
The imidazole antifungal agent ketoconazole, which inhibits several cytochrome P450 enzymes including CYP17A1, suppresses adrenal and intratumoral steroidogenesis by inhibiting the conversion of cholesterol to pregnenolone [6,7,11]. It has been used for more than 30 years in the treatment of castration-resistant prostate cancer with reported >50% PSA decline rates and time to progression of 20–75% and 3–10 months, respectively [5–7,12–16]. Abiraterone acetate is a more potent and selective CYP 17 inhibitor, including both 17,20-lyase and 17-alpha-hydroxylase [6,7]. It blocks the synthesis of androgens in the testis, adrenal glands, and prostate, without causing adrenal insufficiency. Clinical trials in patients with metastatic castration-resistant prostate cancer (mCRCP) in chemotherapy naïve patients as well as post-docetaxel-based chemotherapy patients demonstrated that abiraterone acetate is safe and effective [6,17–20]. In a recent prospective randomized trial the combination of abiraterone plus prednisone demonstrated a survival advantage over placebo plus prednisone in men with mCRCP who had failed docetaxel [21].
Since many patients still benefit from treatments targeting androgen biosynthesis in the castrate state [6,15,16], we evaluated our experience with ketoconazole treatment and the association of various clinical factors with PSA response and time to disease progression, in patients with mCRCP.
MATERIALS AND METHODS
The study group consisted of an unselected cohort of all patients with evidence of mCRCP who were treated with ketoconazole, and followed by medical oncologists at the Johns Hopkins hospital Kimmel Cancer Center, between the years 1999 and 2010. Before initiation of ketoconazole treatment, patients were progressing biochemically (PSA) and/or clinically (scans) on GnRH-agonist, antiandrogen, and antiandrogen withdrawal. All patients had an ECOG performance status 0–2, and pretreatment serum testosterone <50 ng/ml [22]. Data were collected retrospectively from medical records. Outcome data was frozen on September 30, 2010.
Ketoconazole Treatment
Patients were maintained on gonadal suppression, staged with a CT scan and bone scan, and treated with ketoconazole 200 or 400 mg orally 3× a day, with replacement doses of hydrocortisone (30 mg morning and 10 mg night). The initial dose of ketoconazole was left at the discretion of treating physicians. Antacids, H-2 blockers, and proton pump inhibitors were avoided but not explicitly prohibited. Patients were evaluated for adverse events and response to therapy with history, physical examination, and laboratory analysis including liver function tests and PSA, initially around the first month and then every 3 months. An increase from 200 mg 3× a day to 400 mg 3× a day was deemed acceptable (left at the discretion of the treating physician) if a PSA decrease was not noted at three months or at the time of biochemical or clinical progression on the 200 mg 3× a day dose. Patients on 400 mg 3× a day had their treatment discontinued at the time of progression. Ketoconazole was discontinued and hydrocortisone was tapered and discontinued after 3 weeks followed by a clinical evaluation (including a serum PSA determination) after 4 weeks. Toxicity was defined according to the NCI Common Toxicity Criteria version 3.0 (http://ctep.info.nih.gov). Treatment was held for any grade 3/4 toxicity (except for hepatotoxicity—see below), at which point patients were followed until toxicity resolved to grade 1 or better, and then restarted. Treatment was discontinued upon occurrence of dose limiting toxicity defined as recurrence of a same grade 3/4 toxicity event or grade 3/4 hepatotoxicity at any time.
Statistical Analysis
Data were analyzed retrospectively. Follow-up time was defined as the time from initiation of ketoconazole treatment to September 30, 2010. Pre-ketoconazole treatment PSA doubling time (PSADT) was calculated by natural log of 2 (0.693) divided by the slope of the relationship between the log of PSA and time of PSA measurement for each patient [23], using all PSAs > 0.1 ng/ml that confirmed progressive castrate-resistant prostate cancer by PSA progression before initiating ketoconazole. Disease extent was defined as limited if involving only the axial skeleton and/or lymph nodes, or extensive if involving the appendicular skeleton and/or visceral organs [3]. Time to disease progression was defined as the time from ketoconazole treatment initiation until evidence of biochemical or clinical disease progression. Biochemical progression was defined as a PSA increase by ≥25% and ≥2 ng/ml from baseline or nadir as defined by the Prostate Cancer Clinical Trials Working Group criteria [24]. Clinical progression was defined as the new findings on physical examination or scans (measurable disease response and progression were evaluated according to the Response Evaluation Criteria in Solid Tumors). Bone scan progression was defined as two or more new lesions on bone scan). Univariate and multivariate regression models (Cox model for survival outcome and logistic model for binary outcome) were used to analyze clinical factors that are associated with PSA response and disease progression. Data were analyzed using S-Plus 8.0 for Windows Enterprise Developer.
Regulatory Considerations
The research was approved by the IRB committee of our institution.
RESULTS
Study Group
One hundred fourteen patients with mCRCP who were followed and treated by ketoconazole at Johns Hopkins Hospital between the years 1999 and 2010, were included in the present study. Baseline patient characteristics are listed in Table I.
TABLE I.
Baseline Patient Characteristics
| Parameter | Value |
|---|---|
| Age (years) at initiation of ketoconazole: mean ± SD (range); median | 68.14 ± 8.83 (50–88); 69 |
| Combined Gleason grade | |
| Low risk (≤6) | 7% (n = 8) |
| Intermediate risk [7] | 40% (n = 46) |
| High risk [8–10] | 49% (n = 56) |
| Unknown | 4% (n = 4) |
| Primary treatment | |
| Radical prostatectomy | 58% (n = 66) |
| Radiation therapy | 19% (n = 22) |
| Primary androgen deprivation therapy | 20% (n = 23) |
| Unknown | 3% (n = 3) |
| Response to antiandrogen (months) as second line androgen deprivation for metastatic disease: mean ± SD (range); median | 7.7 ± 7.38 (1–36); 5 |
| Baseline PSA doubling time (months) before the initiation of ketoconazole: mean ± SD (range); median | 4 ± 4.63 (0.4–29.3); 2.67 |
| Baseline PSA level (ng/ml) before the initiation of ketoconazole: mean ± SD (range); median | 167.58 ± 539.86 (0.4–4418); 14.83 |
| Disease extent | |
| Limited (axial skeleton and/or lymph nodes) | 33% (n = 38) |
| Extensive (appendicular skeleton and/or visceral organs) | 67% (n = 76) |
| ECOG performance status | |
| 0–1 | 94% (n = 108) |
| 2 | 4% (n = 4) |
| Unknown | 2% (n = 2) |
| Alkaline phosphatase (units/L) | 134.2 ± 132 (31–825), 89 |
| Hemoglobin (g/dl): mean ± SD (range); median | 13 ± 1.5 (8–15.4), 13.2 |
The median age at initiation of ketoconazole treatment was 69 years. Median baseline PSA was 14.8 ng/ml, and median pretreatment PSA doubling time was 2.7 months. Thirty-three percent (n = 38) of patients had limited disease and 67% (n = 76) had extensive disease.
Ketoconazole Treatment, PSA Response, and Disease Progression
Follow-up time was 40.1 ± 27.4 months (mean ± SD, range 5–129, median 31). The starting ketoconazole dose was 200 mg 3× a day in 82 patients (72%), of which 46 (40%) subsequently had their dose increased to 400 mg 3× a day dose (d/t lack of PSA response at 3 months, n = 13, or disease progression, n = 33). Thirty-two patients (28%) initiated treatment at a dose of 400 mg 3× a day. Overall, 61 patients (54%) had ≥50% PSA decline from baseline. Ten patients (22%) treated initially at 200 mg 3× and subsequently increased to 400 mg 3× a day had a subsequent PSA decline of ≥50% versus the PSA level before the increase of dose. Overall median time to PSA and or disease progression was 8 months (range 1–129). Forty-nine patients progressed biochemically, 49 clinically (scans), and 16 patients remained progression free with a median treatment time of 12 months (mean 29.9, range 6–122). Progression-free survival was 5 months in patients on 200 mg 3× a day, 8 months in patients that started with 200 mg 3× a day and then increased to 400 mg 3× a day, and 9 months in patients treated with 400 mg 3× a day initially (not statistically significant) (Table II).
TABLE II.
Ketoconazole Treatment, PSA Response, and Disease Progression
| Parameter | Value |
|---|---|
| Follow-up time (months): mean ± SD (range); median | 40.1 ± 27.4 (5–129); 31 |
| Ketoconazole dose | |
| 200 mg thrice daily dosage (tid) | 32% (n = 36) |
| 200 mg tid subsequently increased to 400 mg tid | 40% (n = 46) |
| 400 mg tid | 28% (n = 32) |
| PSA response | |
| PSA decrease (%) in the whole group: mean ± SD (range); median | 29 ± 81.5% (−400 to 99.9); 53% |
| Patients with PSA decrease ≥50% in the whole group | 54% (n = 61) |
| PSA decrease ≥50% in patients with limited vs. extensive disease | 84% (n = 32/38) vs. 36% (n = 27/76), OR 9.1, P < 0.001 |
| PSA decrease ≥50% in patients with pretreatment PSADT ≥3 vs. <3 | 62% (n = 31/50) vs. 44% (n = 28/64), OR 0.49, P = 0.01 |
| Patients with PSA decrease <50% | 21% (n = 24) |
| Patients refractory to treatment | 25% (n = 29) |
| Time to progression (months): mean ± SD (range), median | |
| Whole group | 12.8 ± 17.1 (1–129), 8 |
| In patients with limited vs. extensive disease | 20.2 ± 23.4 (1–129), 14 vs. 9.1 ± 11.3 (1–74), 5 |
| In patients with prior response to antiandrogen ≥6 vs. <6 months | 18.8 ± 11 (3–129), 18.5 vs. 6.5 ± 11.9 (1–74), 4 |
| In patients with pretreatment PSADT ≥3 vs. <3 months | 17.9 ± 23 (1–129), 12 vs. 8.8 ± 9.4 (1–47), 5 |
Ketoconazole Withdrawal
On follow-up 4 weeks after ketoconazole discontinuation in the 98 patients with disease progression, 11% (n = 11) had PSA stabilization (no rise ≥25% and ≥2 ng/ml vs. its level before ketoconazole cessation) and 8% (n = 8) had a PSA decline. Three of these patients were kept on steroids for pain control. The duration of the ketoconazole withdrawal effect was 6.4 ± 8.8 months (mean ± SD, range 1–40, median 4) before subsequent PSA progression (rise ≥25% and ≥2 ng/ml vs. its nadir level after discontinuation of ketoconazole) or another systemic treatment.
Factors Associated With PSA Response and Disease Progression
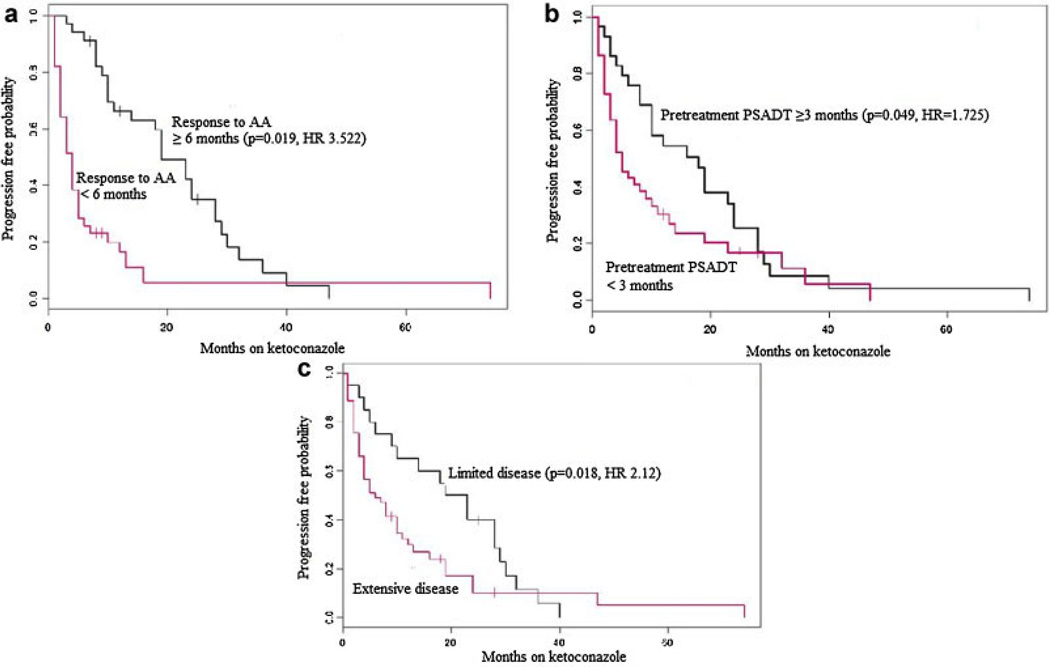
Pre-ketoconazole treatment PSADT (≥3 vs. <3 months; OR 0.49 with PSADT <3, P = 0.01) and extent of metastatic disease (limited-axial skeleton and/or nodal vs. extensive-appendicular skeleton and/or visceral; OR 9.1 with limited disease, P < 0.001) were associated with the probability of ≥50% PSA decline, whereas the duration of response to prior antiandrogen treatment (≥6 vs. <6 months; HR 3.522, P = 0.019; Table II; Fig. 1a), pre-ketoconazole treatment PSADT (≥3 vs. <3 months; HR 1.725, P = 0.049; Table II; Fig. 1b), and extent of metastatic disease (limited-axial skeleton and/or nodal vs. extensive-appendicular skeleton and/or visceral; HR 2.12, P = 0.018; Table II; Fig. 1c) were associated with progression free survival with ketoconazole. Age, primary treatment modality (surgery, radiation, and androgen deprivation), Gleason score of the primary tumor, the presence of pain, elevated serum level of alkaline phosphatase, the presence of anemia, and initial dose/subsequent increase in dose were not found to be independently associated with PSA response or disease progression.
Fig. 1.
a–c: Kaplan–Meier curves showing progression-free survival (using biochemical and/or clinical criteria), stratified by (a) prior response to antiandrogen (AA), (b) pretreatment PSADT, and (c) disease extent (limited vs. extensive). [Color figure can be viewed in the online issue, which is available atwileyonlinelibrary.com.]
Toxicity
Ketoconazole treatment-associated toxicity is described in Table III. Twenty-two percent of the patients (n = 25) had severe grade 3/4 treatment related toxicity, including mainly fatigue, abdominal discomfort, nausea, and dizziness. Fifty-two percent of the patients (n = 59) had grade 1 or 2 adverse events. Nine patients (8%) discontinued treatment due to dose limiting toxicity (fatigue n = 7, hepatotoxicity/thrombocytopenia n = 1). There were no treatment related fatal or irreversible toxicities.
TABLE III.
Ketoconazole Treatment-Associated Adverse Events
| Type of adverse event |
Number of events: all grades |
Number of events: grade 3/4 |
|---|---|---|
| Fatigue | 50 | 14 |
| Abdominal pain | 17 | 4 |
| Hepatotoxicity | 17 | 1 |
| Nausea | 16 | 4 |
| Mucositis | 8 | 2 |
| Rash | 8 | 0 |
| Diarrhea | 7 | 2 |
| Decrease of appetite | 6 | 1 |
| Change of taste | 5 | 0 |
| Vomiting | 4 | 2 |
| Dizziness | 3 | 3 |
| Abdominal gas | 2 | 0 |
| Thrombocytopenia | 1 | 1 |
| Hyperglycemia | 1 | 0 |
| Depression | 1 | 0 |
DISCUSSION
The present study summarizes a single institution’s clinical experience with ketoconazole therapy in patients with mCRPC. The efficacy and safety of ketoconazole treatment (with the 600 or 1,200 mg/day) in the present study is similar to what was described in prior trials [5,12] and similar to current compounds with similar mechanism of action. The incidence and severity of adverse drug related reactions were usually modest and reversible, and indicate that long-term treatment is feasible.
The present study suggests that clinical factors associated with PSA response and disease progression in mCRPC patients treated with ketoconazole are prior response to antiandrogen, pre-ketoconazole treatment PSADT, and metastatic disease extent. Given the increasing treatment choices in the mCRPC state, these data, albeit retrospective, may help in the selection of patients who are more likely to benefit from sequential CYP17 inhibition therapy.
Interestingly, our results revealed a 19% PSA stabilization or decrease after discontinuation of ketoconazole. This has not been previously described. The incidence and duration of this observation suggests a similar pattern in terms of frequency and duration previously reported with the withdrawal of antiandrogens, and suggests that a period of observation after discontinuation of treatment is warranted before the initiation of subsequent treatment, especially if this involves a clinical trial.
Our study has limitations. This is a retrospective study which limits our ability to exclude the possibility that unequal distribution of unidentified clinical-pathologic parameters in this patient cohort may have biased the observed results, and the sample size is relatively small. Furthermore, this study represents a single-center experience and may not reflect national or international practice patterns. Finally, the median follow-up time is relatively short and therefore we could not examine clinical factors influencing overall survival. Despite these limitations, the strong associations of various factors with PSA response and disease progression observed herein could potentially contribute to treatment decisions, patient selection, and clinical trials design.
CONCLUSIONS
Ketoconazole is effective and safe in mCRCP. Prior response to antiandrogen, pre-treatment PSADT, and disease extent are associated with PSA response and disease progression, and further supports a therapeutic role for suppressing adrenal androgens in mCRCP.
Footnotes
Previous presentations: The data were presented at the GU Cancers Symposium: ASCO, Orlando, FL, CA, USA, February 2011.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, Middleton R, Sharp SA, Smith TJ, Talcott J, Taplin M, Vogelzang NJ, Wade JL, III, Bennett CL, Scher HI. American Society of Clinical Oncology. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 Update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, Wilding G, Sears K, Culkin DJ, Thompson IM, Jr, Bueschen AJ, Lowe BA. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 4.Reid AH, Attard G, Barrie E, de Bono JS. CYP17 inhibition as a hormonal strategy for prostate cancer. Nat Clin Pract Urol. 2008;5:610–620. doi: 10.1038/ncpuro1237. [DOI] [PubMed] [Google Scholar]
- 5.Taplin ME, Regan MM, Ko YJ, Bubley GJ, Duggan SE, Werner L, Beer TM, Ryan CW, Mathew P, Tu SM, Denmeade SR, Oh WK, Sartor O, Mantzoros CS, Rittmaster R, Kantoff PW, Balk SP. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7099–7105. doi: 10.1158/1078-0432.CCR-09-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang JE, Olmos D, de Bono JS. CYP17 blockade by abiraterone: Further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br J Cancer. 2009;100:671–675. doi: 10.1038/sj.bjc.6604904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yap TA, Carden CP, Attard G, de Bono JS. Targeting CYP17: Established and novel approaches in prostate cancer. Curr Opin Pharmacol. 2008;8:449–457. doi: 10.1016/j.coph.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 11.Dreicer R, Carducci M. E-1899: An Eastern Cooperative Oncology Group study comparing ketoconazole plus hydrocortisone with docetaxel plus estramustine for asymptomatic, androgen-independent, nonmetastatic prostate cancer patients with rising PSA levels. Rev Urol. 2003;5(Suppl 2):S35–S41. [PMC free article] [PubMed] [Google Scholar]
- 12.Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, Gable P, Torti FM, Kaplan E, Vogelzang NJ. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–1033. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Ryan CJ, Weinberg V, Rosenberg J, Fong L, Lin A, Kim J, Small EJ. Phase II study of ketoconazole plus granulocyte-macrophage colony-stimulating factor for prostate cancer: Effect of extent of disease on outcome. J Urol. 2007;178:2372–2376. doi: 10.1016/j.juro.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Small EJ, Baron AD, Fippin L, Apodaca D. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J Urol. 1997;157:1204–1207. [PubMed] [Google Scholar]
- 15.Scholz M, Jennrich R, Strum S, Brosman S, Johnson H, Lam R. Long-term outcome for men with androgen independent prostate cancer treated with ketoconazole and hydrocortisone. J Urol. 2005;173:1947–1952. doi: 10.1097/01.ju.0000158449.83022.40. [DOI] [PubMed] [Google Scholar]
- 16.Ryan CJ, Halabi S, Ou SS, Vogelzang NJ, Kantoff P, Small EJ. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: Results from a cancer and leukemia group B study. Clin Cancer Res. 2007;13:2030–2037. doi: 10.1158/1078-0432.CCR-06-2344. [DOI] [PubMed] [Google Scholar]
- 17.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 18.Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, Messiou C, Molife LR, Maier G, Thompson E, Olmos D, Sinha R, Lee G, Dowsett M, Kaye SB, Dearnaley D, Kheoh T, Molina A, de Bono JS. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, Martins V, Lee G, Kheoh T, Kim J, Molina A, Small EJ. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, Taplin ME, Bubley GJ, Kheoh T, Haqq C, Molina A, Anand A, Koscuiszka M, Larson SM, Schwartz LH, Fleisher M, Scher HI. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Bono J, Logothetis CJ, Fizazi K, North S, Chu L, Chi KN, Kheoh T, Haqq C, Molina A, Scher HI. Abiraterone acetate (AA) plus low dose prednisone (P) improves overall survival (OS) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) who have progressed after docetaxel-based chemotherapy (chemo): Results of COU-AA-301, a randomized, double-blind, placebo-controlled phase III study; 35th ESMO Congress; 2010. Abstract LBA5. [Google Scholar]
- 22.Gomella LG. Effective testosterone suppression for prostate cancer: Is there a best castration therapy? Rev Urol. 2009;11:52–60. [PMC free article] [PubMed] [Google Scholar]
- 23.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 24.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]