Abstract
BACKGROUND
Since the beginning of the conflicts in the Middle East, US Army physicians have noted a high rate of multidrug-resistant Acinetobacter baumannii infections among US soldiers wounded and initially treated in Iraq. In this study, we investigated the use of ultraviolet C (UVC) light for prevention of multidrug-resistant A. baumannii wound infections using mouse models.
METHODS
Partial-thickness skin abrasions and full-thickness burns in mice were infected with a multidrug-resistant A. baumannii isolate recovered from a wounded US soldier deployed to Iraq. The luxCDABE operon, which was contained in plasmid pMF 385, was cloned into the A. baumannii strain. This allowed real-time monitoring of the extent of infection in mice using bioluminescence imaging. UVC light was delivered to the mouse wounds at 30 minutes after the inoculation of A. baumannii. Groups of infected mouse wounds without being exposed to UVC served as the controls.
RESULTS
In vitro studies demonstrated that A. baumannii cells were inactivated at UVC exposures much lower than those needed for a similar effect on mammalian cells. It was observed in animal studies that UVC (3.24 J/cm2 for abrasions and 2.59 J/cm2 for burns) significantly reduced the bacterial burdens in UVC-treated wounds by approximately 10-fold compared with nontreated controls (p = 0.004 for abrasions, p = 0.019 for burns). DNA lesions were observed by immunofluorescence in mouse skin abrasions immediately after a UVC exposure of 3.24 J/cm2; however, the lesions were extensively repaired within 72 hours.
CONCLUSION
These results suggested that UVC may be useful in preventing combat-related wound infections.
Keywords: Skin abrasion, burn, Acinetobacter baumannii, ultraviolet C, combat-related infection
Acinetobacter baumannii is a gram-negative bacterium that can cause infectious outbreaks in critically ill patients often with limited treatment options because of drug resistance.1–3 Since the beginning of the Middle East conflicts, the outbreak of A. baumannii infections has been a threat to the health of wounded US soldiers.4–7 The primary measures used in the military evacuation system to prevent wound infections are antibiotics and surgical management. Although the use of antibiotics is feasible across all levels of care in the military evacuation system, surgical management is typically only available at levels III to V (i.e., the use of antibiotics is the only option at levels I to II).5,8 However, current evidence strongly suggests that the routine use of antibiotics may be an important factor in building resistant bacterial strains.7,9 As a result, there is a pressing need for the development of alternative treatment regimens.
Although it has been known for the last 100 years that ultraviolet C (UVC) light (wavelength 240–280 nm) is highly germicidal, the use of UVC for wound infections remains at an early stage of development. The mechanism of UVC inactivation of microorganisms is to cause cellular damage by inducing changes in the chemical structure of DNA chains.10 The consequence is the production of cyclobutane pyrimidine dimers (CPDs), causing distortion of DNA molecule, which causes malfunctions in cell replication and leads to cell death. Bacteria are particularly vulnerable to UVC damage because their small size limits effective cellular shading, and their genetic material comprises a significant portion of their cellular volume.11,12 In this study using mouse models, we explored the potential of UVC light for prevention of multidrug-resistant A. baumannii infections in partial-thickness skin abrasions or full-thickness thermal burns.
MATERIALS AND METHODS
Bacterial Strain and Culture Conditions
The A. baumannii strain that we used was a multidrug-resistant clinical isolate recovered from a wounded US soldier deployed to Iraq. The luxCDABE operon, which was contained in plasmid pMF 385, was cloned into the A. baumannii strain, as previously described.13 This allowed real-time monitoring of the extent of infection in mice using bioluminescence imaging.14 A. baumannii cells were grown in brain heart infusion (BHI) medium in an orbital shaking incubator (37°C, 100 rpm) to an optical density of 0.6 to 0.8 at 600 nm, which corresponds to 108 colony forming units (CFUs)/mL (mid-log phase). This suspension was then centrifuged, washed with phosphate-buffered saline (PBS), and resuspended in PBS at the appropriate cell density for the in vitro and animal studies.
Confluent Keratinocyte Monolayer Cultures
PAM 212 cells, a mouse keratinocyte cell line, were used. To obtain keratinocyte monolayer cultures, keratinocytes were inoculated into 35-mm Petri dishes (≈200 cells/dish) in RPMI-1640 medium (Sigma-Aldrich Co., St. Louis, MO). The dishes were then incubated at 37°C in a humidified atmosphere of 5% CO2 for 18 to 24 hours before the experiment.
UVC Light Source
The UVC light was delivered using a low-pressure mercury vapor lamp (American Ultraviolet Co., Lebanon, IN). Emission spectrum measurement of this lamp using a spectroradiometer (SPR-01; Luzchem Research, Inc., Ottawa, Ontario, Canada) showed a peak emission at 254 nm ± 2 nm. The UVC irradiance on the target surface was adjusted by manipulating the distance between the UVC lamp and the target and was measured using a model IL-1700 research radiometer/photometer (International Light, Inc., Newburyport, MA).
UVC Irradiation of Keratinocytes in Monolayer Cultures
Before UVC exposure, the culture medium was replaced with PBS and lids were removed. For each trial, five culture dishes were exposed to UVC for 0, 3, 6, 9, and 12 seconds, respectively, at an irradiance of 1.6 mW/cm2. After UVC exposure, PBS was removed from the 35-mm Petri dishes. The keratinocyte cells were then detached using 2mL of trypsin–0.25% ethylenediaminetetraacetic acid (EDTA) solution (Sigma-Aldrich, St. Louis, MO) and recultured to 150-mm Petri dishes inoculated with 50 mL of fresh culture medium. The 150-mm dishes were then incubated at 37°C for 10 days for growing keratinocyte colonies to visible sizes. Culture medium in the 150-mm dishes was refreshed every 3 days. After 10 days of incubation, the culture medium was removed from the 150-mm dishes, and keratinocyte viability was assessed by staining the keratinocyte colonies in the dishes with crystal violet solution. The stained keratinocyte colonies were then counted.
UVC Inactivation of A. baumannii In Vitro
The experiments were performed using two different types of bacterial culture: monolayer cell culture on agar plates and suspension in PBS. Bacterial monolayers were a direct comparison with cellular monolayers; since bacteria are smaller in size than mammalian cells, the susceptibilities to UVC between bacteria and keratinocytes were also compared in a fairer manner (bacterial suspension vs. keratinocytes monolayer).
For the experiments using monolayer bacterial cell cultures, before UVC exposure, bacterial suspensions with the density of at least 108 CFU/mL were subject to five serial 10-fold dilutions in PBS. Aliquots (10 μL) from the serial dilutions were streaked on square BHI plates in an order of most (1:105) to least (1:1) diluted.15 This procedure resulted in monolayer bacterial cell cultures with five serial 10-fold dilutions on BHI plates. The BHI plates were then exposed to UVC at an irradiance of 0.072 mW/cm2 for 0, 10, 20, 30, 40, and 50 seconds, respectively. After UVC exposure, the BHI plates were incubated at 37°C for 18 to 24 hours before the surviving cells were counted.
For the experiments using bacterial suspensions, 3 mL of A. baumannii suspension containing 108 CFU/mL in PBS was placed into a 35-mm Petri dish at room temperature to give a 3-mm depth liquid. The suspension then was exposed to UVC at an irradiance of 1.6 mW/cm2 with the lid removed. During UVC exposure, the bacterial suspension was stirred by a mini-magnetic bar. Aliquots (40 μL) of the bacterial suspension were withdrawn at 0, 3, 6, 9, and 12 seconds, respectively. Post-UVC CFU were then analyzed by serial dilutions on BHI plates.15 Colonies were allowed to grow for 18 to 24 hours at 37°C before counting.
All in vitro experiments were performed in triplicate.
Wound Infections in Mice
Seven-week old female BALB/c mice (Charles River Laboratories, Wilmington, MA) were used. The mice were housed singly to prevent cross-contamination, fighting, and chewing on the wounds and had access to food and water ad libitum. They were maintained on a 12-hour light and dark cycle at room temperature.
For skin abrasion infections, 19 mice were randomly divided into two groups: UVC-treated group (n = 10) and non-treated control group (n = 9). All mice were given two intraperitoneal (i.p.) injections of cyclophosphamide before the infection on day 0. The first dose, 150 mg of cyclophosphamide per kilogram mouse body weight (150 mg/kg), at day –4 was followed by the second dose of 100 mg/kg at day –1. This treatment reduced peripheral blood neutrophils to less than 100/μL of blood, fostering a more vulnerable environment in the mice to infection. Before the creation of skin abrasions, mice were anesthetized by i.p. injection of a ketamine-xylazine cocktail (80 and 10 mg/kg) and then shaved on the dorsal surfaces using an electric fur clipper. Mouse skin was then scraped with no. 15 scalpel blades until a reddened area appeared (just short of drawing blood).16,17 This procedure resulted in first-degree skin abrasions, with most of the epidermis removed. Each wound measured approximately 1.2 cm × 1.2 cm. One drop (60 μL) of the prepared bacterial suspension containing 3 ×107 CFU was then smeared onto the wound surface.
For burn infections (for which no cyclophosphamide was necessary), 21 mice were also randomly divided into 2 groups: UVC-treated group (n = 12) and nontreated control group (n = 9). Mice were anesthetized and shaved, and burns were incurred by applying a preheated (≈95°C) brass block to the dorsal surface of each mouse for 7 seconds, resulting in nonlethal, full-thickness, third-degree burns17,18 measuring approximately 1 cm × 1.5 cm. Five minutes after burn incurrence, 60-μL bacterial suspension containing 3 × 106 CFU was topically applied on to the eschar of each burn.
The bacterial inocula for both skin abrasion and burn infections were the minimum numbers of bacterial cells to develop infections in mice and were analyzed by preexperiments.
UVC Prophylaxis of Wound Infections in Mice
UVC light was delivered at 30 minutes after bacterial inoculation with the irradiance of 2.7 mW/cm2. Mice were given a total UVC exposure of 3.24 J/cm2 (20 minutes illumination, for skin abrasion infection) or 2.59 J/cm2 (16 minutes illumination, for burn infection) in aliquots with bioluminescence imaging taking place after each aliquot of UVC exposure. During UVC exposure, the unwounded areas surrounding the abrasions or burns were masked using aluminum foil. Bacterial luminescence from mouse wounds was measured daily after UVC exposure until the infections were cured (characterized by the disappearance of bacterial luminescence).
Bioluminescence Imaging
The setup consisted of an ICCD camera (model C2400-30H; Hamamatsu Photonics, Bridgewater, NJ), a camera controller, a light-tight imaging chamber, an image processor (C5510-50, Hamamatsu Photonics), and a color monitor (PVM 1454Q, Hamamatsu Photonics). Using photon-counting mode, an image can be obtained by detecting and integrating individual photons emitted by the bacterial cells.
Anesthetized mice were placed on an adjustable stage in the imaging chamber, and the infected wounds were positioned directly under the camera. A grayscale background image of each wound was made, and this was followed by a bioluminescence image of the same region displayed in a false color scale ranging from pink (most intense) to blue (least intense) and superimposed on the grayscale image. The signal from the bioluminescence image was quantified as relative luminescence units (RLUs) using Argus software (Hamamatsu Photonics).
Immunohistochemical Analyses of UVC-Induced DNA Lesions in Mouse Skin Abrasions
The UVC light was delivered to mouse skin abrasions at the antimicrobial dose of 3.24 J/cm2. Skin biopsies from the irradiated wounds were taken before, immediately after, 24 hours, 48 hours, and 72 hours after UVC exposure. They were fixed in 10% phosphate-buffered formalin at 4°C for 18 to 24 hours and then embedded in paraffin. For immunohistochemical analyses, serial sections of skin (4 μm) were made, deparaffinized, and rehydrated with distilled water. Skin sections were incubated for 30 minutes at room temperature with antithymine dimer monoclonal antibody (Kaniya Biomedical Company, Seattle, WA), which reacts specifically with CPD produced by UV irradiation in double- or single-stranded DNA. Antibody dilution was 1:50. Skin sections were then incubated with M.O.M. bio-tinylated antimouse IgG reagent (second antibody; Vector Laboratories, Burlingame, CA) for 10 minutes at room temperature. Then the skin sections were incubated in the dark for 5 minutes at room temperature with Fluorescein Avidin DCS (Vector Laboratories). Between the incubations, the skin sections were extensively washed in PBS. The skin sections were then mounted with coverslips using mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories) and observed using an Olympus Fluoview FV1000 MPE multiphoton microscope (Olympus Co., Tokyo, Japan).
Statistical Analysis
Differences between the in vitro cell inactivation rates of A. baumanni and keratinocytes were analyzed using a Student’s t test. The area-under-the-curve (AUC) data, which represent the time courses of bacterial luminescence of the skin abrasions or burns and also the overall bacterial burden of the skin abrasions/burns, were calculated using numerical integration. Differences in the AUC between the nontreated control and UVC-treated mouse wounds were compared for statistical significance using a Student’s t test. Values of p < 0.05 were considered significant.
RESULTS
In Vitro Susceptibilities to UVC of A. baumannii Cells and Keratinocytes
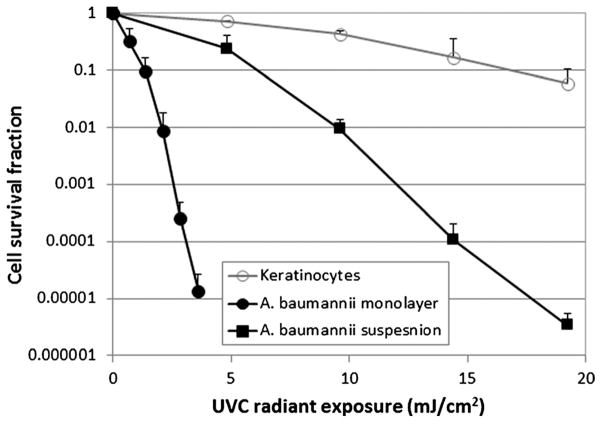
Figure 1 compares the susceptibilities to UVC inactivation in vitro between A. baumannii cells and keratinocytes. All the inactivation curves approximately followed first-order kinetics,19 a linear relation between the log-transformed cell survival fraction log10 (N/N0) and UVC exposure H, that is,
Figure 1.
In vitro cell survival fractions of A. baumannii and keratinocytes in response to UVC irradiation. Bars indicate SD.
| (1) |
where N is the CFU count at the UVC exposure H, N0 is the initial CFU count, and kH is the cell inactivation rate coefficient (or the slope of the inactivation curve).20 It can be seen that the UVC exposures required to induce a 90% (1-log10-cycle) reduction in CFU count were 1.4, 6.1, and 16.8 mJ/cm2 for bacterial monolayer cell culture, bacterial suspension, and keratinocyte monolayer culture, respectively. The mean kH of A. baumannii monolayer, A. baumannii suspension, and keratinocyte monolayer were 1.35, 0.28, and 0.06cm2/mJ, respectively(A. baumannii monolayer vs. keratinocyte monolayer, p = 0.002; A. baumannii suspension vs. keratinocytes monolayer, p = 0.0017).
UVC Prophylaxis for Partial-Thickness Mouse Skin Abrasions Infected With A. baumannii
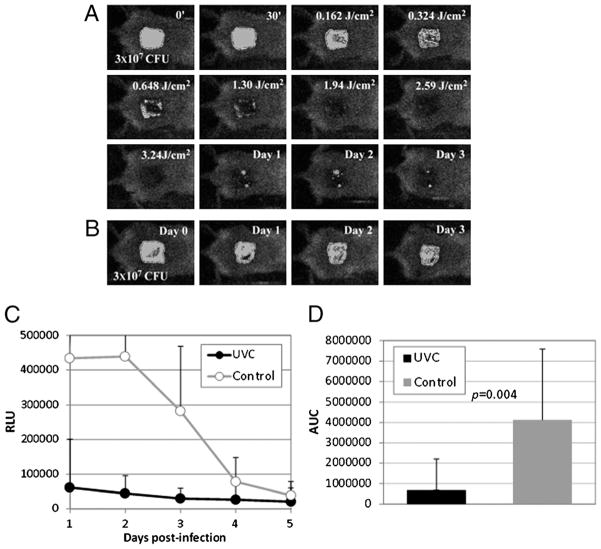
Panels A and B of Figure 2 show the successive bacterial luminescence images of representative A. baumannii–infected mouse skin abrasions with and without UVC prophylaxis, respectively. After exposure to 3.24 J/cm2 UVC at 30 minutes after bacterial inoculation, a greater than 2-log10-cycle reduction of bacterial luminescence (proportional to CFU count) was observed in the UVC-treated wound, and only a modest reoccurrence of bacterial luminescence was observed from day 1 to day 3 (Fig. 2A), since strong bacterial luminescence remained in the untreated wound during the same period (Fig. 2B). Figure 2C shows the time courses of the mean bacterial luminescence (RLU) from day 1 to day 5 of the infected mouse wounds treated with UVC (n = 10) and without treatment (n = 9). Statistical comparison of the AUC of bioluminescence time course in Figure 2C demonstrated that UVC significantly reduced the bacterial burden of the infected wounds (Fig. 2D). The mean AUC of the UVC-treated and untreated wounds were 1.42 × 105 and 1.04 × 106, respectively—almost a 10-fold difference (p = 0.004).
Figure 2.
UVC prophylaxis of A. baumannii infections in partial-thickness mouse skin abrasions. (A) Successive bacterial luminescence images of a representative mouse skin abrasion infected with 3 × 107 CFU of A. baumannii and exposed to 3.54 J/cm2 UVC at 30 minutes after bacterial inoculation. (B) Successive bacterial luminescence images of a representative mouse skin abrasion infected with 3 × 107 CFU of A. baumannii and without being exposed to UVC. (C) Time courses of mean bacterial luminescence of the infected skin abrasions with (n = 10) and without (n = 9) UVC prophylaxis. Bars indicate SD. (D) Mean areas under the bioluminescence versus time curves (in the two-dimensional coordinate system in Fig. 3C), representing the overall bacterial burden of the mouse wounds. Bars indicated SD. Color figure available online as Supplemental Digital Content 1, http://links.lww.com/TA/A179.
UVC Prophylaxis for Full-Thickness Mouse Burns Infected With A. baumannii
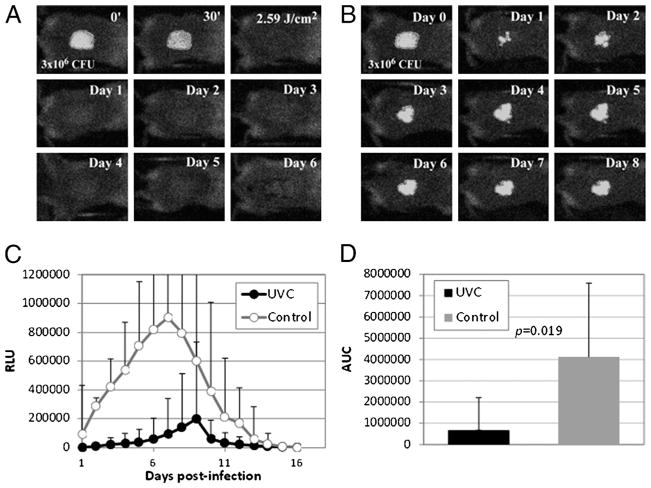
Figure 3A is a set of bacterial luminescence images from a representative mouse burn infected with A. baumannii and exposed to 2.59 J/cm2 UVC at 30 minutes after bacterial inoculation. The bacterial luminescence was almost completely eradicated after UVC exposure, and no bacterial regrowth was observed on the successive days. In contrast, stable infection was developed in the burn without UVC prophylaxis (Fig. 3B). Figure 3C shows the time courses of the mean bacterial luminescence RLU from day 1 to day 16 of A. baumannii–infected mouse burns with (n = 12) and without UVC prophylaxis (n = 9). Statistical comparison of the AUC of bioluminescence time courses in Figure 3C demonstrated that UVC significantly decreased the bacterial burden of the infected burns (Fig. 3D). The mean AUC of the UVC-treated and untreated burns were 3.25 × 106 and 4.12 × 107, respectively, indicating a more than 10-fold reduction as a result of UVC prophylaxis (p = 0.019).
Figure 3.
UVC prophylaxis of A. baumannii infections in full-thickness mouse burns. (A) Successive bacterial luminescence images of a representative mouse burn infected with 3 × 106 CFU of A. baumannii and exposed to 2.59 J/cm2 UVC at 30 minutes after bacterial inoculation. (B) Successive bacterial luminescence images of a representative mouse burn infected with A. baumannii but without being exposed to UVC. (C) Time courses of bacterial luminescence of the infected mouse burns with (n = 12) and without (n = 9) UVC prophylaxis. Bars indicate SD. (D) Mean areas under the bioluminescence versus time curves (in the two-dimensional coordinate system in Fig. 4C), representing the overall bacterial burden of mouse burns. Bars indicate SD. Color figure available online as Supplemental Digital Content 2, http://links.lww.com/TA/A182.
Effects of UVC on Mouse Skin at the Antimicrobial Dose
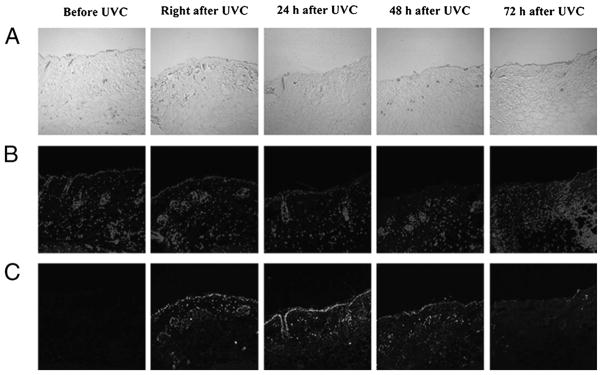
Figure 4 depicts the effects of UVC at the antimicrobial dose (3.24 J/cm2) on host tissue in mouse skin abrasion. CPD-positive nuclei were observed in the immunofluorescence micrograph of the skin biopsy taken immediately after UVC exposure. However, the damage was extensively repaired within 72 hours, as shown in the immunofluorescence micrograph of the skin biopsy taken at 72 hours after UVC exposure, where only traces of fluorescence remained.
Figure 4.
Immunohistochemical analyses of DNA lesions in a representative mouse skin abrasions after UVC exposure. Biopsies were taken before, immediately after, 24 hours, 48 hours, and 72 hours after UVC irradiation. (A) Micrographs of the morphologies of skin abrasion. (B) Micrographs of 4′,6-diamidino-2-phenylindole counterstaining of cell nuclei. (C) Immunofluorescence micrographs of CPDs in skin cell nuclei. Color figure available online as Supplemental Digital Content 3, http://links.lww.com/TA/A183.
DISCUSSION
The results obtained from the present study demonstrated that the multidrug-resistant clinical isolate of A. baumannii is highly susceptible to UVC inactivation. In addition, the UVC inactivation rate of A. baumannii is significantly faster than that of keratinocytes. The UVC inactivation rates of A. baumannii monolayer and suspension cultures are 22.5 and 4.67 times faster than that of keratinocyte monolayer culture, suggesting that the UVC may selectively inactivate microorganisms while having little cytotoxic effect on mammalian cells. In vivo studies showed that both the infections in partial-thickness mouse skin abrasions and full-thickness mouse burns could be well controlled by a single exposure to UVC.
It was also observed that when mouse skin abrasions were exposed to UVC at the antimicrobial exposure, UVC did cause DNA lesions in host tissue. However, the DNA lesions were almost completely repaired within 72 hours after UVC exposure. In general, we think that there are situations where the risk-benefit ratio is favorable for the use of UVC for treating infectious diseases particularly when the microorganisms responsible are antibiotic resistant. For wound infections, we expect that only a limited number of repetitions of UVC would be required, whereas the UV-induced carcinogenic mutation is a long-term effect.21 Furthermore, the cells in the upper epidermis that receive the nonpenetrating UVC are already en route to being shed, whereas the basal epidermal cells that could undergo malignant transformation are shielded from UVC. In situations such as abrasions, where the epidermis has been removed, the host cell density is much sparser in the dermis and long-lived “stem or progenitor” cells that could serve as originators of malignancy are absent.
Similar to all topical antimicrobials, the efficacy of topical light delivery is limited for deeply located infections (e.g., open facture infections). However, with the advancement of techniques in optics, this limitation could be overcome by the use of optical fibers to interstitially deliver light to the infected wound sites. Interstitial light delivery has been extensively investigated in photothermal therapy22,23 and photodynamic therapy.24–27 For UVC prevention of deeply located infections, tissues including bone, nerves, and blood vessels would be exposed to UVC. The effects of UVC on these tissues need to be investigated in future studies.
In the present study, we only investigated the use of UVC for prophylaxis of A. baumannii infections, that is, applying UVC before the infections are established or biofilms are formed. Clearly, it is of great interest to elucidate the efficacy of UVC on biofilm or established infections. Future studies in this regard are warranted.
In recent years, infections caused by multidrug-resistant microorganisms have complicated the care of US soldiers wounded in Middle East conflicts.7,28,29 The uniquely austere environment encountered in the combat zone raises the issue of how best to prevent infections in wounded soldiers. It is suggested that wound care and the use of prophylactic antimicrobials should occur as soon as possible in the evacuation process.5 As a result, the use of UVC light for combat-related wound infections is compelling, and it is conceivable that portable UVC devices could be distributed to the US soldiers and used without medical training. This technology could be implemented on the battlefield and could delay the onset or progression of infection until medical intervention is available. As a pilot study, we only tested the efficacy of UVC light for infections in partial-thickness skin abrasions and full-thickness burns in mice. To explore the full use of UVC light for combat-related wound decontaminations, future studies on investigating UVC for infections in open fractures and large soft tissue defects are warranted.
CONCLUSIONS
The multidrug-resistant A. baumannii cells were inactivated at UVC exposures much lower than those needed for a similar effect on mammalian cells. UVC significantly reduced the bacterial burdens in partial-thickness skin abrasions and full-thickness burns in mice infected with A. baumannii. UVC-induced DNA lesions in skin abrasions were extensively repaired within 72 hours after UVC exposure. These results suggested that UVC may be used to prevent combat-related wound infections.
Acknowledgments
This study was supported in part by an Airlift Research Foundation Extremity Trauma Research Grant (grant 109421 to T.D.), a COTA/Smith & Nephew Grant (Grant 2012-16 to T.D.), and the National Institutes of Health (grant RO1AI050875 to M.R.H.). G.P.T. was supported by the National Institutes of Health (grant 5U54MH084690-02).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
AUTHORSHIP
T.D. and M.R.H. conducted the literature search for this study, which T.D., C.K.M., M.S.V., and M.R.H. designed. T.D. and M.R.H. collected data; T.D., C.K.M., D.G.B., G.P.T., and M.R.H. participated in data interpretation. T.D., C.K.M, D.G.B, G.P.T., and M.R.H. wrote the manuscript, for which T.D. and M.R.H. contributed figures.
DISCLOSURE
The authors declare no conflicts of interest.
References
- 1.Murray CK, Hospenthal DR. Treatment of multidrug-resistantAcinetobacter. Curr Opin Infect Dis. 2005;18:502–506. doi: 10.1097/01.qco.0000185985.64759.41. [DOI] [PubMed] [Google Scholar]
- 2.Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis. 2010;23:332–339. doi: 10.1097/QCO.0b013e32833ae38b. [DOI] [PubMed] [Google Scholar]
- 3.Mihu MR, Martinez LR. Novel therapies for treatment of multidrug resistant Acinetobacter baumannii skin infections. Virulence. 2011;2:97–102. doi: 10.4161/viru.2.2.15061. [DOI] [PubMed] [Google Scholar]
- 4.Scott P, Deye G, Srinivasan A, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 5.D’Avignon LC, Chung KK, Saffle JR, et al. Prevention of infections associated with combat-related burn injuries. J Trauma. 2011;71:S282–S289. doi: 10.1097/TA.0b013e318227adc2. [DOI] [PubMed] [Google Scholar]
- 6.Murray CK, Obremskey WT, Hsu JR, et al. Prevention of infections associated with combat-related extremity injuries. J Trauma. 2011;71:S235–S257. doi: 10.1097/TA.0b013e318227ac5f. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun JH, Murray CK, Manring MM. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res. 2008;466:1356–1362. doi: 10.1007/s11999-008-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray CK, Hsu JR, Solomkin JS, et al. Prevention and management of infections associated with combat-related extremity injuries. J Trauma. 2008;64:S239–S251. doi: 10.1097/TA.0b013e318163cd14. [DOI] [PubMed] [Google Scholar]
- 9.Eardley WG, Brown KV, Bonner TJ, et al. Infection in conflict wounded. Philos Trans R Soc Lond B Biol Sci. 2011;366:204–218. doi: 10.1098/rstb.2010.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang JC, Ossoff SF, Lobe DC, et al. UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol. 1985;49:1361–1365. doi: 10.1128/aem.49.6.1361-1365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippini M, Ortelli C, Svercel M, et al. Interspecies variation in survival and growth of filamentous heterotrophic bacteria in response to UVC radiation. J Photochem Photobiol B. 2011;103:234–242. doi: 10.1016/j.jphotobiol.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Agogue H, Joux F, Obernosterer I, et al. Resistance of marine bacterioneuston to solar radiation. Appl Environ Microbiol. 2005;71:5282–5289. doi: 10.1128/AEM.71.9.5282-5289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai T, Tegos GP, Lu Z, et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53:3929–3934. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamblin MR, O’Donnell DA, Murthy N, et al. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75:51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Jett BD, Hatter KL, Huycke MM, et al. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 16.Kraft WG, Johnson PT, David BC, et al. Cutaneous infection in normal and immunocompromised mice. Infect Immun. 1986;52:707–713. doi: 10.1128/iai.52.3.707-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai T, Kharkwal GB, Tanaka M, et al. Animal models of external traumatic wound infections. Virulence. 2011;2:296–315. doi: 10.4161/viru.2.4.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens EJ, Ryan CM, Friedberg JS, et al. A quantitative model of invasive Pseudomonas infection in burn injury. J Burn Care Rehabil. 1994;15:232–235. doi: 10.1097/00004630-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Xiong R, Xie G, Edmondson AE, et al. A mathematical model for bacterial inactivation. Int J Food Microbiol. 1999;46:45–55. doi: 10.1016/s0168-1605(98)00172-x. [DOI] [PubMed] [Google Scholar]
- 20.Sinton LW, Hall CH, Lynch PA, et al. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl Environ Microbiol. 2002;68:1122–1131. doi: 10.1128/AEM.68.3.1122-1131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisby S, Gniadecki R, Wulf HC. UV-induced DNA damage in human keratinocytes: quantitation and correlation with long-term survival. Exp Dermatol. 2005;14:349–355. doi: 10.1111/j.0906-6705.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 22.Kosoglu MA, Hood RL, Rossmeisl JH, Jr, et al. Fiberoptic microneedles: novel optical diffusers for interstitial delivery of therapeutic light. Lasers Surg Med. 2011;43:914–920. doi: 10.1002/lsm.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosoglu MA, Hood RL, Chen Y, et al. Fiber optic microneedles for transdermal light delivery: ex vivo porcine skin penetration experiments. J Biomech Eng. 2010;132:091014. doi: 10.1115/1.4002192. [DOI] [PubMed] [Google Scholar]
- 24.Svanberg K, Bendsoe N, Axelsson J, et al. Photodynamic therapy: superficial and interstitial illumination. J Biomed Opt. 2010;15:041502. doi: 10.1117/1.3466579. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie AL. How may external and interstitial illumination be compared in laser photodynamic therapy? Phys Med Biol. 1985;30:455–460. doi: 10.1088/0031-9155/30/5/008. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka H, Hashimoto K, Yamada I, et al. Interstitial photodynamic therapy with rotating and reciprocating optical fibers. Cancer. 2001;91:1791–1796. doi: 10.1002/1097-0142(20010501)91:9<1791::aid-cncr1198>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Lowdell CP, Ash DV, Driver I, et al. Interstitial photodynamic therapy: clinical experience with diffusing fibres in the treatment of cutaneous and subcutaneous tumours. Br J Cancer. 1993;67:1398–1403. doi: 10.1038/bjc.1993.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hospenthal DR, Crouch HK, English JF, et al. Response to infection control challenges in the deployed setting: Operations Iraqi and Enduring Freedom. J Trauma. 2010;69(suppl 1):S94–S101. doi: 10.1097/TA.0b013e3181e44b3f. [DOI] [PubMed] [Google Scholar]
- 29.Keen EF, 3rd, Robinson BJ, Hospenthal DR, et al. Incidence and bacteriology of burn infections at a military burn center. Burns. 2010;36:461–468. doi: 10.1016/j.burns.2009.10.012. [DOI] [PubMed] [Google Scholar]