Abstract
Antimicrobial photodynamic therapy (aPDT) is an emerging alternative to antibiotics motivated by growing problems with multi-drug resistant pathogens. aPDT uses non-toxic dyes or photosensitizers (PS) in combination with harmless visible of the correct wavelength to be absorbed by the PS. The excited state PS can form a long-lived triplet state that can interact with molecular oxygen to produce reactive oxygen species such as singlet oxygen and hydroxyl radical that kill the microbial cells. To obtain effective PS for treatment of infections it is necessary to use cationic PS with positive charges that are able to bind to and penetrate different classes of microbial cells. Other drug design criteria require PS with high absorption coefficients in the red/near infra-red regions of the spectrum where light penetration into tissue is maximum, high photostability to minimize photobleaching, and devising compounds that will selectively bind to microbial cells rather than host mammalian cells. Several molecular classes fulfill many of these requirements including phenothiazinium dyes, cationic tetrapyrroles such as porphyrins, phthalocyanines and bacteriochlorins, cationic fullerenes and cationic derivatives of other known PS. Larger structures such as conjugates between PS and cationic polymers, cationic nanoparticles and cationic liposomes that contain PS are also effective. In order to demonstrate in vivo efficacy it is necessary to use animal models of localized infections in which both PS and light can be effectively delivered into the infected area. This review will cover a range of mouse models we have developed using bioluminescent pathogens and a sensitive low light imaging system to non-invasively monitor the progress of the infection in real time. Effective aPDT has been demonstrated in acute lethal infections and chronic biofilm infections; in infections caused by Gram-positive, Gram-negative bacteria and fungi; in infections in wounds, third degree burns, skin abrasions and soft-tissue abscesses. This range of animal models also represents a powerful aid in antimicrobial drug discovery.
Keywords: Antimicrobial photodynamic therapy, cationic tetrapyrroles, phenothiazinium dyes, reactive oxygen species, mouse models of localized infections, bioluminescence imaging, drug discovery
1. INTRODUCTION
Photodynamic therapy (PDT) is an established modality for the treatment of cancer. It has also been extended for the treatment of noncancerous conditions such as age related macular degeneration and other dermatological applications [1, 2]. Besides these applications, there has also been a growing interest in the application of PDT for the treatment of infectious diseases [1]. PDT involves the use of non-toxic dyes that act as photoactive drugs called photosensitizers (PS) in combination with visible light of the appropriate wavelength to excite the PS. The excited state PS, in the presence of the oxygen, transfers energy or electrons to ground state molecular oxygen producing reactive oxygen species (ROS) such as singlet oxygen and hydroxyl radical which are responsible for the killing of cells [3]. When the cells to be killed are pathogenic microorganisms the procedure is termed photodynamic inactivation (PDI) or antimicrobial PDT (aPDT). A diversity of different models of infectious has been used in research for the testing the efficacy of different antimicrobial PS. Before discussing these models, a brief history and overview of antimicrobial PDT and antimicrobial PS will be discussed.
The potential of PDT as an antimicrobial therapy was recognized at the start of the twentieth century when Raab noticed the killing of the paramecia with acridine orange in presence of light [4]. However earlier results showed that the commonly used PS for cancer were poorly effective for the photodynamic killing of some well known pathogens [5]. Moreover it was assumed that the invention of antibiotics would have the lasting potential to combat infectious diseases. Quite the reverse of this, in present times effective therapy for infectious diseases is challenged by the emergence of multidrug-resistant pathogens which is leading to increased morbidity [6]. The difficulty is further aggravated due to a number of mechanisms adopted by microbes to fight against the external insults. These include, thickening of their outer wall, encoding of new proteins which prevent the penetration of drugs or actively efflux them, generation of mutants deficient in the porin channels which permit the entry of externally added chemicals, etc. As a result, it is difficult to identify a broadly applicable approach to overcome this problem [7]. In the 1990s there were reports showing that cationic PS such as phthalocyanines [8], porphyrins [9] and phenothiaziniums [10] induce a rapid and extensive light-mediated killing of typical Gram-negative bacteria, such as Escherichia coli and Pseudomonas aeruginosa, in addition to the PDI of fungi and Gram-positive bacteria.
Some of the advantages of aPDT are: (A) It is broad-spectrum and can kill a wide range of microbes such as Gram-positive and Gram-negative bacteria, yeasts, fungi and parasitic protozoa as well as inactivate viruses. (B) There is a low chance of any possibility of developing photoresistant species even after multiple treatments. (C) PS and drug-light intervals can be designed that exhibit selectivity for microbes over host cells and tissue. (D) There is a low risk of inducing mutagenic effects. (E) aPDT kills microbial cells rapidly (minutes) while antibiotics can take days to work. (F) Because PS are topically delivered into infected areas, aPDT can be effective in traumatic infections where the blood supply is compromised preventing antibiotics reaching the microbes. (G) It has been demonstrated that aPDT can be effective in biofilm infections that are resistant to antibiotics. (H) Last but not the least it is inexpensive.
1.1. The General Features of PDI of Microbial cells
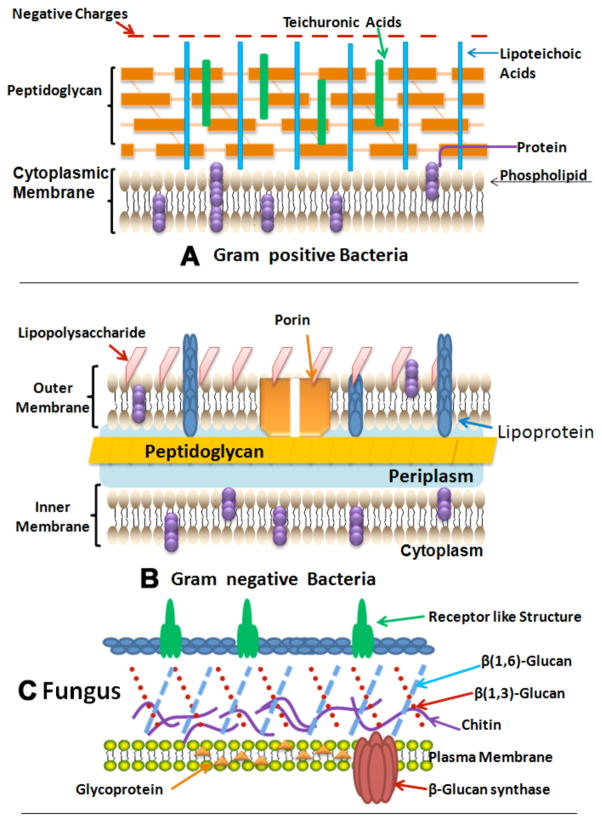
Due to the marked difference regarding the size and composition of various microbes there occurs a difference in the susceptibility for various organisms. In the 1990s it was found that basic differences in susceptibility to PDT exist between Gram (+) and Gram (−) bacteria. This was due to difference in morphology: the Gram (+) bacteria are surrounded by a layer of only peptidoglycan and lipoteichoic acid that is comparatively porous, while Gram (−) bacteria have a somewhat more intricate, non-porous cell wall structure consisting of an inner cytoplasmic membrane and an outer membrane, which are separated by the peptidoglycan-containing periplasm (Fig. 1). Fungal cells have intermediate permeability between Gram-positive and Gram-negative bacteria. Besides this, cysts formed by protozoa also represent challenging targets. Thus the procedure adopted for the treatment of infections cannot be focused on just one type of pathogen; rather it must be characterized by the possibility to efficiently act on microbial pathogens with very different characteristics.
Fig. (1). Structures of the cell walls of three different classes of microbial pathogens.
A) Gram-positive bacterium showing porous layer of peptidoglycan and single lipid bilayer. B) Gram-negative bacterium showing double lipid bilayer sandwiching peptidoglycan layer and an outer layer of lipopolysaccharide. C) Fungal cell with a less porous layer of beta-glucan and chitin surrounding a single lipid bilayer.
1.2. Photobiological Processes
The photodynamic action occurs by two mechanisms Type I and Type II. The ground state photosensitizer on absorption of a photon is converted into its long-lived triplet state via a short-lived singlet state. This triplet state is the reactive intermediate. In type I mechanism the triplet state PS transfers an electron to ground state molecular oxygen to produce reactive oxygen species (ROS) such as superoxide, hydroxyl radicals and peroxides. While in the type II mechanism the triplet state of the PS reacts undergoes energy transfer to the ground state of oxygen which is in triplet state to give another ROS very reactive species i.e. singlet oxygen (Fig. 2). This singlet oxygen then further reacts with the surrounding bio-molecules. The main molecules targeted by both the mechanisms are certain amino acids, pyrimidine and purine bases of DNA/RNA, and unsaturated lipids. The wide range of biomolecules damaged by ROS means that the spectrum of microbial targets of PDT is very broad.
Fig. (2). Schematic mechanism of antimicrobial PDT.
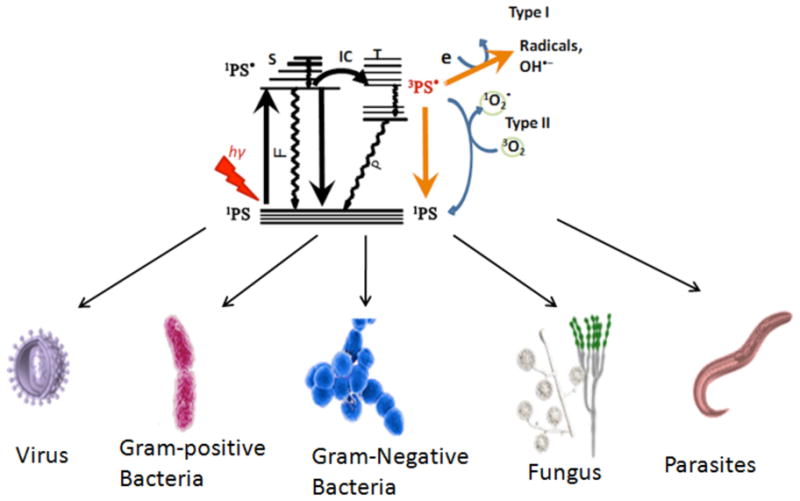
Type 1 and Type 2 photochemical mechanisms operate from photosensitizer triplet state producing ROS that are able to destroy all known microorganisms.
Some of the properties considered to be favorable for ideal antimicrobial PS: (1) The photosensitizer should have long-lived excited triplet state; and a high quantum yield for the generation of ROS on excitation with visible light. (2) It should have high extinction coefficient mainly in the red and far-red region where light transmission through tissue is maximal. Though for the treatment of superficial infections also the intensely absorbed blue light (400–420 nm) is useful. (3) A large affinity for the broadest possible range of microbial cells. (4) The PS should bind selectively to the cytoplasmic membrane, due to which the cell death will be mainly due to damage of the membrane rather than the genetic material. (5) The mechanisms involved in photodynamic inactivation should have no mutagenic effect. (6) A broad spectrum of action on bacteria, fungi, yeasts and parasitic protozoa (Figs. 2 and 3), to help the treatment of those infectious diseases which are considered to be due to the presence of a varied flora of pathogens. (7) The cell-selective binding conferred by the molecular structure should be such that there is maximum damage to the microbes with minimal damage to the host tissue.
Fig. (3). Broad spectrum of effect of antimicrobial PDT.
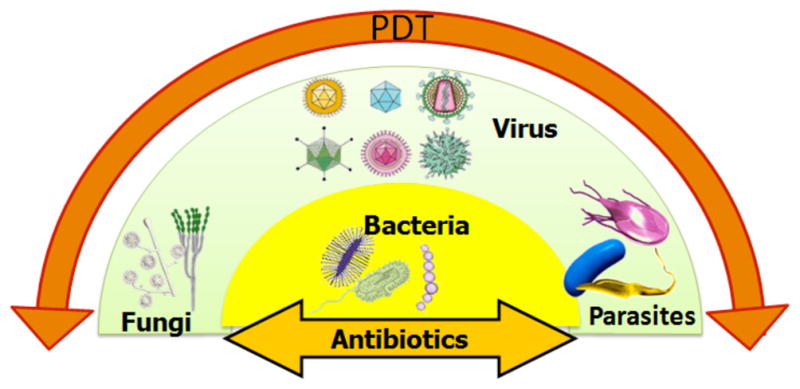
Many antibiotics, antibacterial and antifungal drugs have a relatively narrow spectrum of action, while antimicrobial PDT has an extremely broad spectrum of that takes effect rapidly.
The advantage of the broad-spectrum exhibited by PDT (see Fig. 3) means that it could be used to treat a localized infection before the clinical microbiology laboratory identified the culprit microbe, and the appropriate antibiotic was selected. Moreover a more limited range of antimicrobial photosensitizers could be stocked in pharmacies compared to the wide range of antibiotics needed now.
2. DRUG DISCOVERY AND ANTIMICROBIAL PDT
It has been known for many decades that Gram-positive bacteria are highly susceptible to killing by traditional PS with the same molecular features as those PS used to kill cancer cells (such as porphyrins) as well as other types of photoactive dyes [11]. In the 1990s it was discovered that PS with cationic charges could kill Gram-positive bacteria, which had previously been thought to be resistant to many aPDT regimens [8, 9, 12]. Other classes of pathogens such as viruses (both enveloped and non-enveloped) [13], yeasts [14, 15], filamentous fungi [16], protozoa [17], parasites [18, 19] etc, have been reported to be susceptible to aPDT mediated by cationic PS. We will give some examples of molecular structures that have been investigated as antimicrobial PS.
2.1. Phenothiazinium Dyes
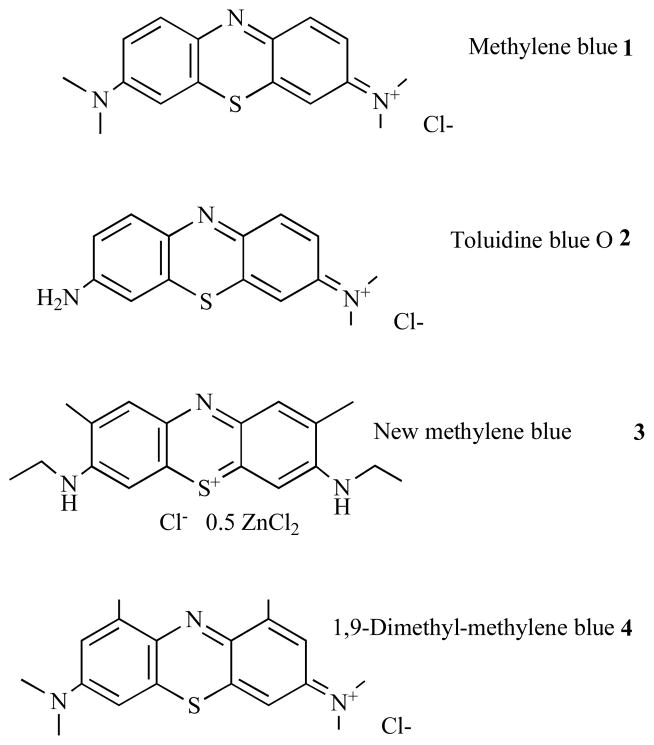
Structures of members of this class are shown in Fig. 4; these compounds have a single cationic charge that is delocalized over the three-ring structure. Methylene blue (MB) 1, and toluidine blue O (TBO) 2, are probably the most-widely studied members of this class [20–22]. Compounds such as these have the additional advantage that MB is clinically approved as an injectable IV therapy for methemoglobinemia [22] and both MB and TBO are generally accepted as safe for topical application to living human tissue [23]. Other members of the class that have been used as antimicrobial PS include new methylene blue 3 [24] and dimethyl-methylene blue 4 [25]. It is generally accepted that these latter compounds are more powerful antimicrobial PS than MB and TBO [26]. Interestingly we previously showed that members of this class of phenothiazinium dyes were substrates of microbial drug-efflux systems [27], and that aPDT could be potentiated by combining the phenothiazinium dye with an inhibitor of the drug-efflux pump [28]. Related structures are the benzophenoxazines and their sulfur and selenium analogs [29].
Fig. (4). Structures of phenothiazinium dyes.
Methylene blue, 1; Toluidine blue O, 2; New methylene blue, 3; Dimethyl-methylene blue, 4.
2.2. Cationic Porphyrins
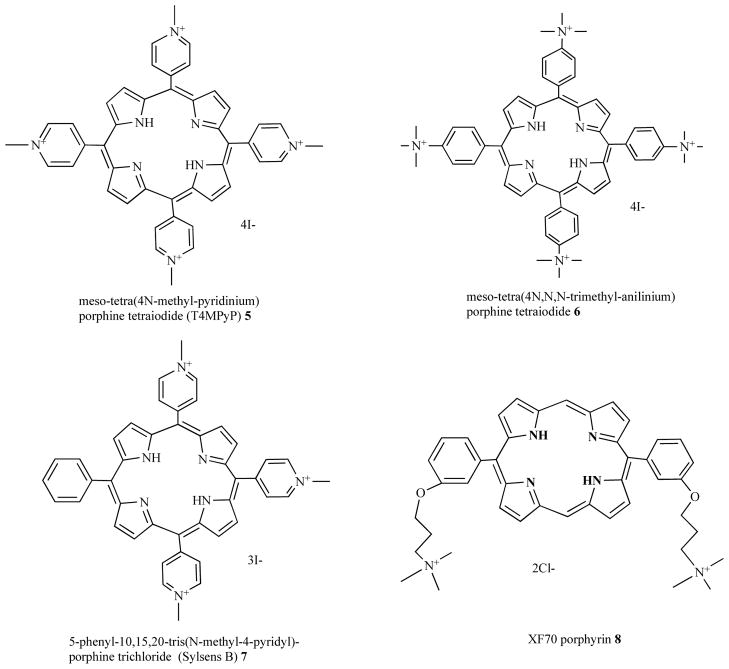
Porphyrins can be synthesized bearing cationic groups that are usually attached to phenyl groups substituted in the meso-position of the porphyrin macrocycle (Fig. 5). Merchat et al [9] reported that cationic meso-substituted porphyrins, namely tetra(4N-methyl-pyridyl) porphine tetraiodide (T4MPyP) 5, and tetra(4N,N,N-trimethyl-anilinium) porphine tetraiodide (T4MAP) 6, effectively mediated aPDT Gram-negative bacteria. Another cationic porphyrin, 5-phenyl-10,15,20-tris(N-methyl-4-pyridyl)-porphyrin chloride (PTMPP) or Sylsens B, 7 was shown to be to an effective and versatile antimicrobial PS that was able to kill bacteria, Candida, and the dermatophyte Trichophyton rubrum [30–32]. Maisch et al reported [33] that bis-cationic porphyrins such as XF70 8 were broad spectrum antimicrobial PS, giving good photokilling of methicillin-resistant and methicillin-sensitive S. aureus strains, methicillin-resistant Staphylococcus epidermidis and E. coli. Subsequent reports suggested that these porphyrins could even be used as antibacterial compounds in the dark [34, 35]. Other reports have used a wide range of substituted cationic porphyrins to mediate a PDT of diverse species of pathogens [36–40].
Fig. (5). Structures of cationic porphyrins.
Tetra(4N-methyl-pyridyl) porphine tetraiodide (T4MPyP) 5, tetra(4N,N,N-trimethyl-anilinium) porphine tetraiodide (T4MAP) 6; 5-phenyl-10,15,20-tris(N-methyl-4-pyridyl)-porphine chloride (PTMPP or Sylsens B), 7; bis-cationic porphyrin, XF70, 8.
2.3. Cationic Phthalocyanines
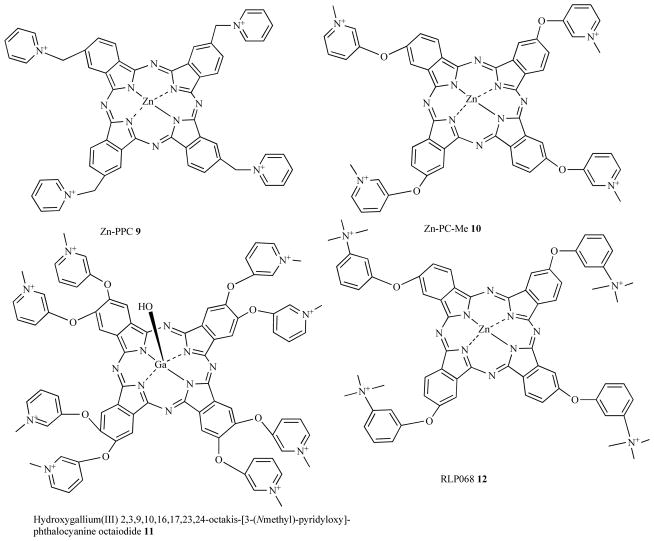
Phthalocyanines are another class of tetrapyrrole dyes that have been synthesized with cationic substituents to render them suitable for aPDT (see Fig. 6). Unlike porphyrins, phthalocyanines are usually prepared with central coordinated metal atoms to prevent aggregation and enhance photochemical properties. The diamagnetic metal ions such as zinc(II) impart a high fluorescence quantum yield, long triplet lifetimes and high triplet quantum yields which lead to a high probability of energy or electron transfer. Phthalo-cyanine zinc(II) molecules with different charges were evaluated as PS to kill bacteria by Minnock et al [8] who showed that Gram-negative bacteria could be photoinactivated when illuminated in the presence of a tetra-cationic water-soluble zinc pyridinium phthalo-cyanine, Zn-PPC, 9.
Fig. (6). Structures of cationic phthalocyanines.
Tetrakis cationic zinc pyridinium phthalocyanine, Zn-PPC, 9; tetrakis cationic phthalocyanine 10; octakis-cationic Ga(III)-PC, 11; tetrakis cationic Zn-PC, RLP068, 12.
Mantareva et al. [41] showed that another cationic phthalo-cyanine with four cationic groups 10 was able to photoinactivate Gram-positive Staphylococcus aureus, the Gram-negative Pseudomonas aeruginosa, and the fungal species Candida albicans.
A recent paper [42] from the same group described a Ga(III)-substituted phthalocyanine with eight cationic groups 11 that was able to mediate photoinactivation of Gram-positive, Gram-negative bacteria and Candida species in planktonic form an in biofilms.
Roncucci et al showed [43] that another tetracationic Zn-PC 12 designated as RLP068 [17] could photoinactivate S. aureus, P. aeruginosa and C. albicans and moreover did not generate any resistance even after 20 successive cycles of sub-lethal PDT and regrowth.
2.4. Cationic Bacteriochlorins
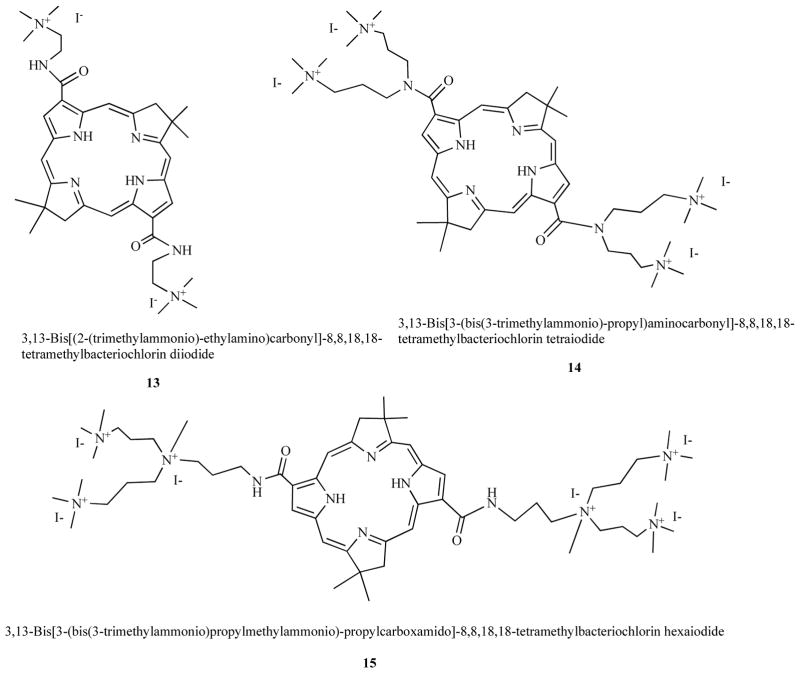
Bacteriochlorins are porphyrins with two opposed reduced pyrrole rings in the macrocycle; a molecular feature that imparts an intense absorption band in the NIR spectrum (>700-nm). In a similar manner to porphyrins they can be synthesized with peripheral cationic groups to give antimicrobial PS. Using completely synthetic methodology we prepared compounds that were rendered stable by introduction of gem-dimethyl groups that prevented adventitious re-oxidation of the reduced rings [44]. Compounds such as bis-cationic 13, tetrakis cationic 14, and hexakis cationic 15 shown in Fig. 7 were effective in killing Gram-positive, Gram-negative bacteria and fungi. Moreover they showed good selectivity for microbial cells over host
Fig. (7). Structures of cationic bacteriochlorins.
Bis-cationic BC 13, Tetrakis-cationic BC 14, Hexakis-cationic BC 15.
2.5. Cationic Fullerenes
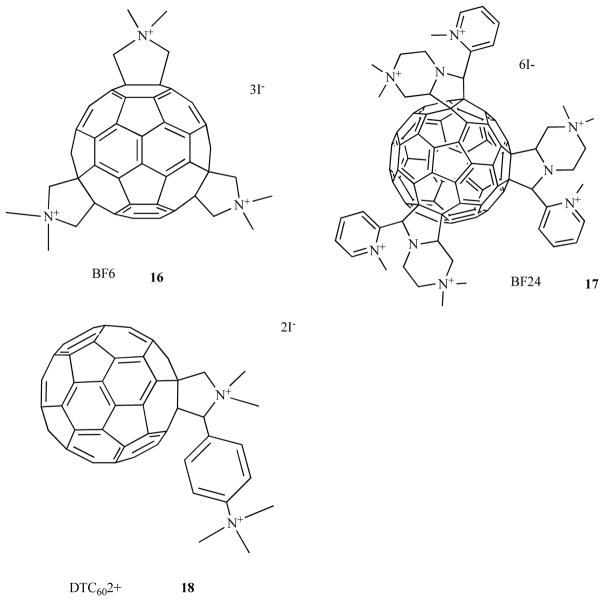
Fullerenes are closed cage molecules composed entirely of carbon atoms, and the most widely studied member of this class is C60, was originally named buckminsterfullerene [45]. The extended system of conjugated double bonds present in the spherical molecule mean that these molecules absorb extensively in the visible region of the spectrum as well as the UV region. Moreover they have a triplet yield approaching unity and hence no fluorescence. The long-lived triplet state can undergo either energy transfer to produce singlet oxygen or electron transfer to produce superoxide and subsequently hydroxyl radical. Pristine C60 is highly insoluble but functionalization of the periphery with the appropriate groups imparts water solubility and if these groups are cationic, the fullerenes can act as highly effective antimicrobial PS as shown in Fig. 8. The tris-cationic fullerene 16 was shown by our laboratory [46] to be a broad spectrum PS able to mediate photokilling of Gram-positive, Gram-negative bacteria and fungi. Another study from our group [47] showed that cationic fullerenes such as 17 with 6 cationic groups was also highly effective. Other laboratories [48] have also shown that cationic fullerenes such as 18 (2 cationic groups) are also effective against Gram-negative bacteria.
Fig. (8). Structures of cationic fullerenes.
Tris-methyl pyrrolidinium fullerene 16; hexakis-cationic fullerene, 17; bis-cationic fullerene, 18.
2.6. Miscellaneous Cationic PS
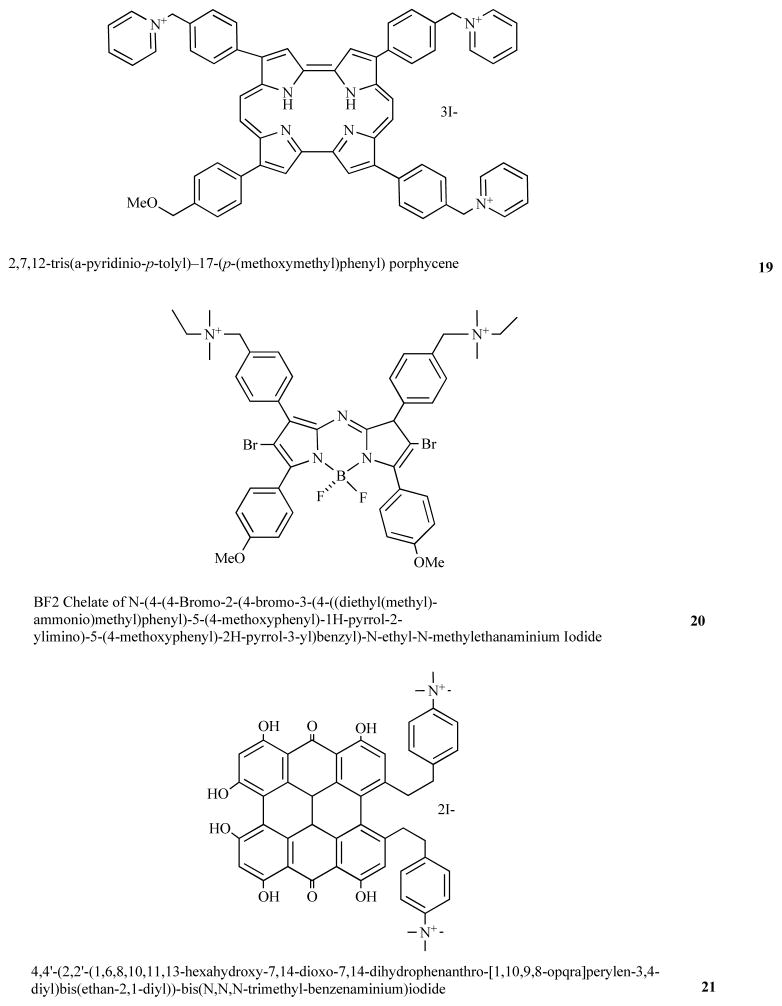
Nonell’s laboratory in Barcelona, Spain has developed PS based on the porphyrin-structural isomer backbone known as porphycenes [49]. By synthesizing a porphycene 19 with 3 cationic groups a broad-spectrum antimicrobial PS was obtained [50], that could kill Gram-positive and Gram-negative bacteria, as well as a fungal yeast. Moreover it was also able to effectively treat an in vivo mouse infection model using aPDT.
A group from Dublin, Ireland has developed a new class of PS based on brominated BF2 chelated tetraarylazadipyrromethane dyes [51]. By adding two cationic groups to this backbone to give compound 20 a broad-spectrum antimicrobial PS was obtained [52].
Hypericin is a natural produce isolated from St John’s Wort and has been used as a PS for PDT of cancer. Hager et al [53] synthesized bis-cationic derivates of hypericin such as 21 and demonstrated these PS could be used for PDI of Propionibacterium acnes.
2.7. Conjugates Between PS and Cationic Polymers
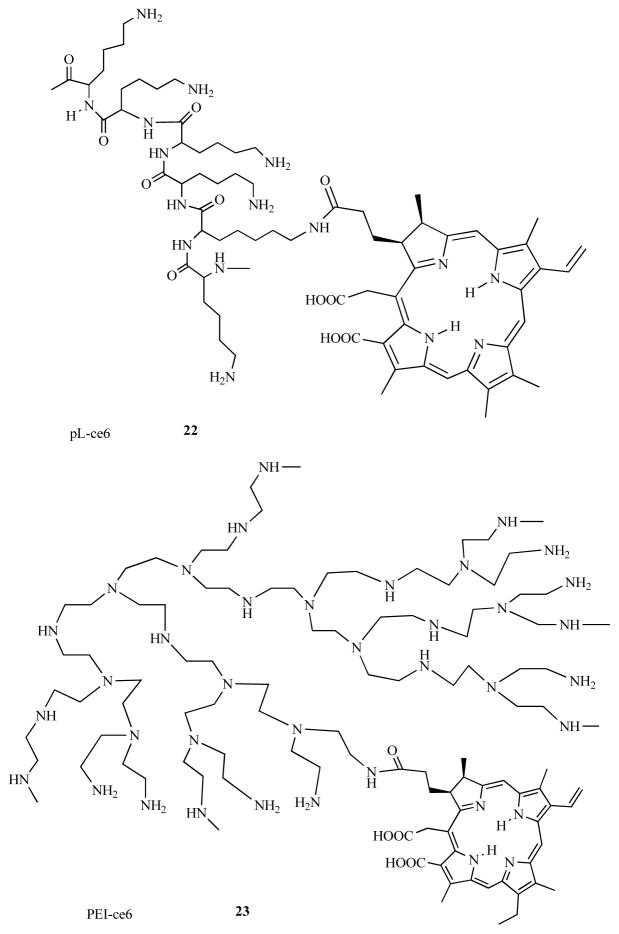
In 1997 we formed the hypothesis [54] that by covalently attaching a non-cationic PS (chlorin(e6), ce6) to amino-groups present on a cationic polymer such as poly-L-lysine (pL) to form pL-ce6 conjugates, interesting microbial-targeted PS could be prepared (Fig. 9). Various forms of the cationic pL-ce6 22 was shown to be effective in photoinactivating both Gram-positive and Gram-negative bacteria [55, 56]. A similar synthetic approach using poly-ethylenimine (PEI) allowed the formation of PEI-ce6 243 that had the additional advantages of being protease stable and also more cost effective [57]. The concept of polycationic PS conjugates proved to be fruitful and has been extensively studied both in our laboratory [58–66] and by others [67–69].
Fig. (9). Structures of miscellaneous cationic PS.
Tris-cationic porphycene 19; bis-cationic brominated BF2 chelated tetraarylazadipyrromethene dye, 20; bis-cationic derivative of hypericin, 21.
2.8. PS Encapsulated Into Cationic Liposomes and Nanoparticles
It has been shown that by encapsulation of PS in cationic liposomes rather than the more usual liposomes composed of anionic or neutral lipids, the ability of non-cationic PS such as m-tetrahydroxyphenylchlorin (mTHPC) to kill microbial cells can be enhanced [70]. A similar report [71] used cationic liposomes composed with DOTAP (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate) and containing either hematoporphyrin or chlorophyll a to mediated aPDT of MRSA.
Likewise the explosion of interest in nanoparticles has led to researchers preparing nanoparticles containing both PS and cationic charges for antimicrobial photoinactivation. Schwiertz et al [72] found that calcium phosphate nanoparticles containing PS and bearing cationic charges were superior PS delivery vehicles especially for Gram-negative P. aeruginosa. Ferro and coworkers [73] compared a monocationic porphyrin (5-[4-(1-dodecanoylpyridinium)]-10,15,20-triphenyl-porphine) (TDPyP) complexed into supramolecular aggregates of cationic amphiphilic beta-cyclodextrin with the same porphyrin encapsulated into cationic liposomes [74]. The cationic cyclodextrin porphyrin was more effective in mediating photoinactivation of both Gram-positive and Gram-negative bacteria than the cationic liposome formulation.
2.9. Conjugates Between PS and Antibodies
One exception to the rule that cationic groups are required to produce PS that can efficiently photoinactivate Gram-negative bacteria appears to be the use of monoclonal antibodies (mAb) conjugated to PS. A conjugate between mAb NO76 and Sn-ce6 [75] was used by Yarmush et al to kill the resistant Gram-negative species Pseudomonas aeruginosa both in vitro [76] and in vivo [77]. The conjugate did not kill S. aureus to which the mAb did not bind. This finding (lack of cationic charge) may be explained by the tight binding between antibody and bacterial cell.
3. BIOLUMINESCENCE IMAGING OF INFECTION MODELS
3.1. Rationale for Bioluminescence Imaging of Infection Models
Animal models have become standard tools for the study a wide array of antimicrobial therapies of wound infections, including antimicrobial PDT. Mice are by far the most frequently used species in wound infection models. The principal disadvantages of mouse models relate to the small size of the animals. Hence, for example, one is limited to the number of sequential sampling of blood, other fluids, and tissues that can be performed without compromising the mouse. As a result, in vivo studies of PDT on mouse infection models suffer from difficulties in monitoring the development of an infection in mice and its response to treatment. Standard microbiological techniques used to follow infections in animal models frequently involve sacrifice of the animals, removal of the infected tissue, homogenization, serial dilution, plating and colony counting. These assays use a large number of animals, are time consuming, and often are not statistically reliable.
In order to facilitate the non-invasive monitoring of animal models of infection, we have developed a procedure that uses bioluminescent genetically-engineered bacteria and a light sensitive imaging system to allow real-time visualization of infections. When these bacteria are treated with PDT either in vitro or in vivo, the loss of luminescence parallels the loss of colony-forming ability. We have developed several mouse models of localized infections that can be followed by bioluminescence imaging (BLI) [78].
BLI can be used either to track the course of an infection or monitor the efficacy of antimicrobial therapies (see Fig. 11). Bacterial pathogenesis appeared to be unaffected by the presence of the luciferase genes, and bioluminescence can be detected throughout the study period in animals. Further, the intensity of the bioluminescence measured from the living animal correlated well with the bacterial burden subsequently determined by standard protocols [79–81]. Transposon-mediated integration of the luciferase operon into the bacterial chromosome to make stable transformants means that reduction of luminescence from sites of infection in animals can be attributed to reduction of bacterial numbers rather than loss of luciferase-encoding plasmids.
Fig. (11). Schematic illustration of bioluminescence imaging to monitor PDT response in infection models.
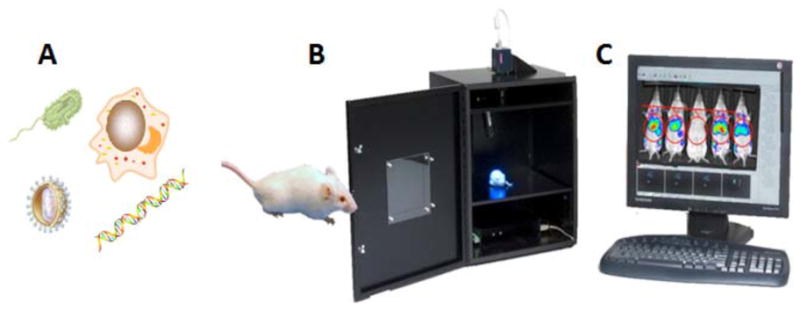
A) Different classes of pathogenic microorganisms have been rendered bioluminescent. B) Low light imaging camera consists of a light tight box with a sensitive CCD camera to collect emitted photons. C) Software allows the luminescence signal to be analyzed and the spread and intensity of the infection can be quantified over time.
3.2. Tracking the Course of Infections Using Bioluminescence Imaging
Different localized-infection models in various experimental animals have been used by different groups to demonstrate the utility of bioluminescence imaging using bioluminescent pathogens and repetitive imaging over the course of hours, days or weeks. A broad range of variables must be taken into account when designing these protocols. These variables are: (A) The species and strain of pathogen taking into account its rate of growth, and its specific virulence and pathogenicity characteristics that may on occasion be species specific to the animal model being employed. (B) The number of cells (CFU) innoculated into the infection together with the precise composition of the liquid in which the microbes are suspended. (C) The type of injury or trauma appplied to damage the tissue that allows entry of the pathogens. (D) The immune staus of the animals; in many cases specific immunosuppression strategies must be applied that can either be pharmacological or genetic. (E) The presence of a foreign body in the infected area; foreign bodies can dramatically potentiate infection.
When the bioluminescent microbes have been introduced into the lesion it is frequently found that a large number of the cells die in the hours following their introduction. This phenomenon is probably due to evaporation of liquid present in the innoculum and the fact that the pathogens need time to establish a source of nutrients from the tissue. When the parameters have been selected correctly it will be found that the surviving microbes then proceed to regrow and a true infection is then established. Depending on the microbes and the tissue involved a biofilm may be formed; this occurrence often leads to a chronic infection being formed that can last for several weeks. Again depending on the virulence and invasiveness of the microbes, it is possible that the microbes may reach the systemic circulation of the animal and then bacteremia or sepsis ocurs that can often be fatal. This event is easily established by culture of bioluminescent microbes from the blood or internal organs such as heart or liver. In many cases when the infection does not become systemic, as the tissue heals the bioluminescent microbes become confined in a scab or in an abscess due to the body’s natural response to wall-off infection, and this scab can eventually fall of taking the last traces of bioluminescence with it.
3.3. Bioluminescence Imaging to Demonstrate the Efficacy of PDT
To bring aPDT towards a clinical treatment it is necessary to show its effectiveness in treating actual infections rather than just inactivating pathogens in vitro. In contrast to PDT of cancer where delivery of PS is generally carried out be intravenous injection of the PS frequently associated with an appropriate delivery vehicle, adminstration of the PS for a PDT of infections is different. In this case the PS is delivered into or onto the infected area. For superficial infections this may simply be accomplished by pipetting a solution of PS onto the surface of the tissue, but for deeper infections it may involve injection or infiltration of the PS into the infected area. The delivery vehicle employed is also likely to be different for PS used for cancer and PS used for infections. For cancer the appropriate PS are much more hydrophobic and delivery vehciles such as liposomes, detergent micelles or orgaic solvent mixtures are common. For aPDT the PS employed are hydrophilic and generally water soluble. In some cases adding a proportion of a water miscible organic solvent to the aqueous solution of PS does help penetration into the infected tissue.
The drug-light interval employed in aPDT is also very different from that which is usual in PDT for cancer. In cancer applications 24 hours or even longer is a common interval, although some PS do have shorter intervals such as a few hours. By contrast in aPDT applications it has been found that drug-light intervals as short as a few minutes are optimal. The reasons for this are the fact that binding and penetration of PS to microbial cells is relatively rapid process. Uptake of the PS into mammalian cells that compose the infected tissue is a much slower process, so the desirable selectivity for microbes over host tissue is a time-dependent process and can be maximized at short drug-light intervals.
Light delivery is often as simple as shining the light as a spot that covers the infected area. One finding that impacts the methodology of carrying out aPDT is the discovery that photobleaching is an important limiting factor in the efficay of the antimicrobal PDI [82]. Photobleaching is the chemical degradation of the molecular structure of the PS by the ROS produced during PDT, that can reduce its effectiveness in killing microbes. However this limitation can be overcome by repeating the addition of the PS at intervals during the illumination [58].
Another consideration that is important in aPDT, is the realization that the relative masses of microbial cells and host cells in the infected tissue is very different. This means that the total dose of PDT (PS concentration and fluence of light) is much larger (100–1000 times larger) than the dose that is needed to kill microorganisms in vitro. A recent publication [83] provided a perspective on why this should be. Since the definition of infection is 10(5) CFU/g tissue and 10(5) bacteria have a total mass of only 1 microgram, it is evident that there is 1 million times more mass of tissue than there is mass of bacteria and this provides a real challenge for selective binding of the PS to the microbial cells rather than the host cells. Even if the infection is very severe (10(8) CFU/g tissue) the ratio is still 1000:1.
In actual practice the experimental application of aPDT for localized infections comprises the following steps. (A) Establish the infection and monitor it by BLI as described in section 3.2. (B) Apply the PS to the infected area and monitor by a second BLI procedure, any dark toxicity that may happen when the PS kills some microbial cells by its innate ability to penetrate and permeabilize the cells. (C) After a short time begin illumination and after a suitable amount of light has been delivered (for instance 10–50 J/cm2) carry out a third BLI. (D) Repeat PS addition and light delivery enough times to ensure maximum eradication of bioluminescence signal. (E) It may be necessary to deliver even more PDT than appears to be necessary judging by BLI in order to reduce the possible regrowth of microbes after the procedure. (F) Monitor any regrowth of microbes within the infection in succeeding days by daily BLI procedures. In some cases when regrowth does occur, a second aPDT procedure after some days may give a second useful reduction in bioluminescence signal. (G) When regrowth of microbes in succeeding days is problematic, it may be possible to combine aPDT with an antibiotic or other traditional antimicrobial that is microbistatic rather than microbicidal and can prevent re-growth [84].
4. PDT FOR WOUND INFECTIONS
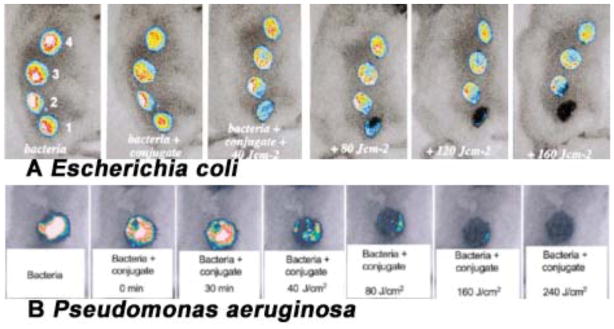
The first mouse model of localized infection using bioluminescent bacteria to be utilized for aPDT was a model of simple excisional wounds that were superficially inoculated with bacteria. Infected wounds can be problematic in elderly patients, for some surgical patients, for non-healing leg ulcers and for battlefield combat casualties such as open fractures. Hamblin et al [66] developed a mouse model of excisional wound infections. In that model, four rectangular full-thickness excisional wounds were made in a line along the back of shaved male BALB/c mice. Wounds measured 8-mm × 12.5-mm and had at least 5 mm of unbroken skin between them. The bottom of the wound was panniculus carnosus with no visible bleeding. A suspension (50 μL PBS) containing 5 ×106 cells of mid-log phase bioluminescent E. coli was inoculated into each wound, and the mouse was imaged with the luminescence camera to ensure equal bacterial loading into each wound. The next day, infected wounds in living mice had lost, on an average, 90% of the original luminescence signal but with considerable inter-animal variability. A rapid light dose-dependent loss of luminescence was observed as measured by image analysis after PDT with polycationic photosensitizer pL-ce6 conjugate 22. Fig. 12A shows dose response bacterial luminescence to PDT from a representative mouse in which bacteria were inoculated in all wounds, pL-ce6 22 was added to wounds 1 and 4, and wounds 3 and 4 were illuminated with red light. Therefore, wound 1 was the dark control with conjugate, wound 2 was the absolute control, wound 3 was the light-alone control and wound 4 was PDT treated. Topical application of pL-ce6 22 followed by laser illumination at 665 nm led to a 99% reduction in bacterial luminescence.
Fig. (12). PDT for infected excisional wounds.
(A) Successive overlaid luminescence images of a mouse with four excisional wounds infected with equal numbers of E. coli (5 ×106). Wounds 1 (nearest tail) and 4 (nearest head) received topical application of pL-ce6 conjugate 22. Wounds 1 and 2 (two nearest tail) were then illuminated with successive fluences (40–160 J/cm2) of 665 nm light. (B) Successive overlaid luminescence false-color images of mice bearing excisional wounds infected with 5×106 luminescent P. aeruginosa treated with pL-ce6 conjugate 22 and increasing doses of 660 nm light.
The mouse model was also used by the same group [65] to test the efficacy of aPDT against the infections induced by a more invasive species, P. aeruginosa. In this case the highly virulent bacteria will rapidly reach the blood stream of the mice and then death will ensue. Mice with single wounds measuring 8-mm312.5-mm received 5×106 mid-log phase P. aeruginosa suspended in 50 mL of PBS. The pL-ce6 conjugate 22 was added as 50 uL of a 200-uM ce6 equivalent concentration. To allow the conjugate to bind to and penetrate the bacteria, illumination at 665 nm was commenced at 30 minutes after the inoculation of bacteria. As can be seen from a set of luminescence images from a representative mouse, shown in Fig. 12B, PDT produced a fluence-dependent loss of luminescence, until only a trace remained, after 240 J/cm2 had been delivered. Ninety % of the PDT-treated mice survived (9 out of 10), in contrast, all of non-treated control mice (n=10) died within 5 days. Furthermore the wounds treated with PDT healed better than wounds that were treated with an alternative topical antimicrobial (silver nitrate). This improvement in healing was attributed to the fact that PDTR can also destroy protease enzymes responsible for slowing down wound healing.
5. PDT FOR BURN INFECTIONS
Skin is the first line of defense providing body with a physical barrier against several pathogens including bacteria, viruses and fungi. Impairment of this important defensive function renders the skin susceptible to infections from otherwise harmless microorganisms. One of the injuries that compromise skin’s protective role is the burn injury. Not only do the burns breach the cutaneous barrier, but severely burned sites are rendered avascular, immunosuppressed, and are rich in bacterial nutrients. Consequently burns are highly susceptible to infections and large burns that occupied a high % of total body surface area often proved to be fatal in the past due to infectious complications. The microbial species responsible for these invasive burn infections include the ubiquitous pathogens P. aeruginosa, S. aureus, Candida spp., and filamentous fungi. With the use of techniques like early excision, grafting, topical antibiotics and antimicrobials, there has been a dramatic improvement in the survival rates following burn infection. However, development of microbial resistance to antibiotics and other antimicrobials has led to a renewed search for alternative approaches to prevent and combat burn infections. Antimicrobial PDT has emerged as a promising alternative to antimicrobial agents. Photodynamic inactivation of S. aureus, A. baumannii, and MRSA has been shown to be effective in animal models of infected burns in mouse and guinea pig.
aPDT of S aureus in a mouse burn model has been evaluated [85]. To create the burn injury, ends of two preheated brass rods (≈95 °C) were pressed against opposite sides of a raised dorsal skin fold for 10 s (see Fig. 13). This created a third degree burn covering 2cm2 or 5% of the body surface area. Ten minutes later, burn wounds were infected with bioluminescent S. aureus and allowed to multiply in the wound for 24 h to establish an infection. At the end of 24 h, 5-phenyl-10,15, 20-tris(N-methyl-4-pyridyl)-porphyrin chloride (PTMPP, Sylsens B, 7), a cationic porphyrin that has proved to be an effective and versatile antimicrobial PS, was applied both topically and injected under the burn. The burns were illuminated directly after the application of the PTMPP with red light and periodic imaging of the mice using a sensitive camera to detect the bioluminescence signal. More than 98% of the bacteria were eradicated after a light dose of 210 J/cm2 in the presence of PTMPP. However, bacterial re-growth was observed. Collectively these data suggested that PDT had the potential to rapidly reduce the bacterial load in infected burns but treatment needed to be optimized to reduce wound damage and prevent recurrence [85].
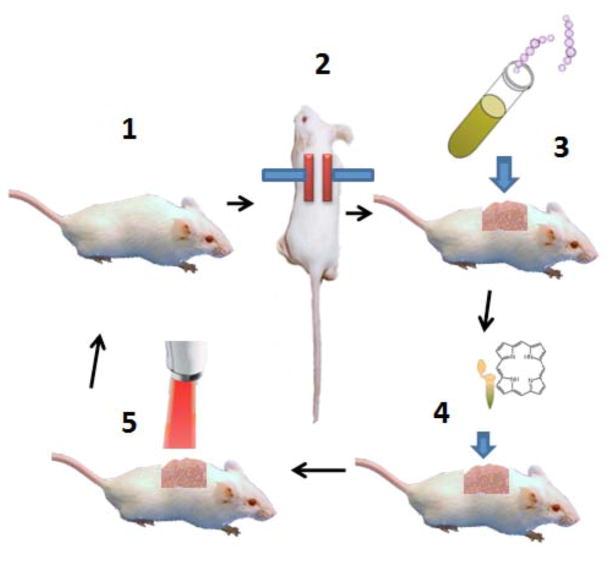
Fig. (13). Schematic illustration of procedures involved in carrying out PDT for burn infection.
1. Shave and anesthetize a mouse. 2. Press two heated brass blocks against an elevated skin fold. 3. Add suspension of bioluminescent bacteria with a pipette tip. 4. Add photosensitizer with a pipette tip. 5. Deliver red light from a suitable light source.
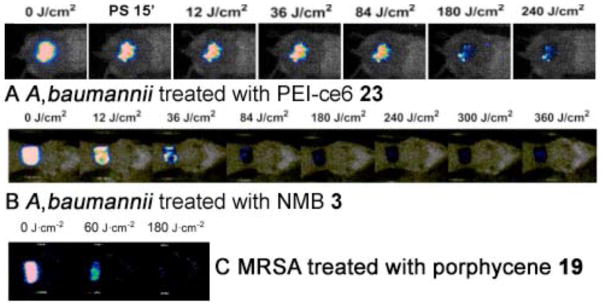
PDT of Acinetobacter baumannii in a burn wound infection was studied by Dai et al [59] using polyethylenimine chlorine (e6) (PEI-ce6) conjugate 23 and non-coherent red light at 660-nm. The burn wounds were created as described by Lambrechts et al [85]. Five minutes after the creation of the burns, a suspension of luminescent A. baumannii containing 108 cells was inoculated onto the eschar of each burn. This led to chronic infections that lasted, on average, 22 days and was characterized by a remarkably stable bacterial bioluminescence. Starting PDT on day 0 was more effective in reducing bacterial luminescence (3-log10 units) than on day 1 or day 2 (approximately 1.7-log10 reduction). Fig. 12A shows the PDT dose response of bacterial luminescence of a representative mouse burn infected with A. baumannii and treated with PDT on day 1 (24 hours) after infection. PDT induced approximately 1.8 logs reduction of bacterial luminescence from the mouse burn (Fig. 14A). Bacterial re-growth in the treated burn was observed but was generally modest. Also the PDT did not lead to inhibition of wound healing. The data suggest that PDT may be an effective new treatment for multi-drug resistant localized A. baumannii infections.
Fig. (14).
A) PDT dose response of bacterial luminescence from a representative mouse burn infected with A. baumannii and treated with PDT using PEI-ce6 conjugate 23. B) PDT dose response of bacterial luminescence from a representative mouse burn infected with A. baumannii and treated with PDT using NMB 3. C) PDT dose response of bacterial luminescence from a representative mouse burn infected with MRSA and treated with PDT using PEI-ce6 conjugate 23.
The same model was used by Ragas et al [86] to demonstrate PDT mediated by the phenothiazinium dye NMB 3. NMB was applied 30 minutes after infection followed by illumination with 180 J/cm2 of red light at 635-nm. As shown in Fig. 14B, PDT of A. baumannii led to a 3.2-log10 reduction of the bacterial luminescence after 360 J/cm2 had been delivered.
The third degree mouse burn model as described above was also used by Ragas et al to test the photodynamic efficacy of a cationic porphycene 19 against drug resistant MRSA (methicillin resistant Staphylococcus aureus). 5 minutes after creation of burns, 50 ul of bacterial suspension (108 cells) was inoculated onto the surface of each burn with a pipette tip and then smeared onto the burn surface with an inoculating loop. Porphycene 19, applied thirty minutes later followed by illumination with 180 J/cm2 of red light, led to a 2.6-log10 reduction of MRSA bioluminescence [50] as seen in Fig. 14C.
6. PDT FOR ABRASION INFECTION MODEL
Contact sports such as American football, basketball, wresting, and rugby inevitably lead to skin and soft-tissue injuries that place athletic population at increased risk for infection. Skin infections, particularly those caused by Methicillin-resistant Staphylococcus aureus (MRSA), are common among the athletes with a prevalence of over 10% [87]. The skin injuries occurred in contact sports are mostly cutaneous traumas such as cuts, abrasions, turf burns etc. In some cases, significant morbidity can occur, and in some other cases infections result in life threatening conditions [88]. Not only do infections present a public health concern, they can also disrupt or potentially eliminate a team’s chance to compete at the highest level [89].
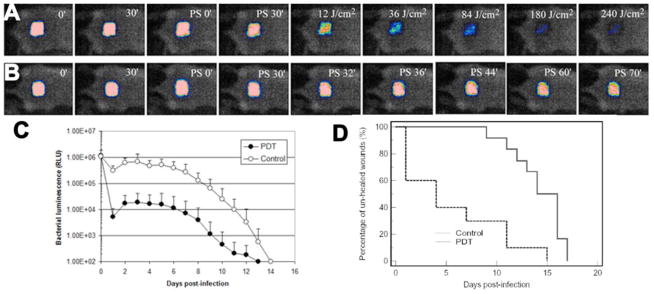
A mouse model of skin abrasion wound infected with MRSA was developed by Dai et al. [58]. Bioluminescent strain of MRSA was used to allow the real time monitoring of the extent of infection in mouse wounds. Skin abrasions were made within defined 1×1 cm2 areas on the backs of mice using 28 gauge needles. The abrasions were made in such a manner that they only damaged the stratum corneum and upper-layer of the epidermis but not the dermis. Ten minutes after wounding, an aliquot of 50 μL suspension containing 108 CFU of bioluminescent MRSA was inoculated to each wound. Fig. 15B shows the successive bioluminescence images of a representative abrasion wound in a mouse treated with PEI-ce6 23 in the dark. As indicated by the bacterial luminescence, the infection remained strong and stable until day 5 post-infection and detectable until day 12. Fig 15A shows the corresponding reduction in bioluminescence image intensity observed when red light was added to PEI-ce6 23. Fig. 15C quantifies the bioluminescence signals and presents the data as a time course over the whole course of the observation period until both treated and untreated abrasions healed (day 14). Fig 15D shows the Kaplan-Meier wound healing curves of the non-treated mice (n=12) and the mice treated with PDT (n=10). Statistical analysis indicated that PDT treated mice had a significant advantage in wound healing over the non-treated mice. The average wound healing times of the PDT treated mice and non-treated ones were 5.6±5.1 and 14.2±2.6 days (p=0.0002), respectively. In 6 out of 10 of the PDT treated mice, complete wound healing was achieved within 4 days post-infection. The reason for the large advantage of wound healing in the PDT group probably reflects the fact that an abrasion has unbroken skin between the scratches that can form a nucleus for wound healing. However this normal skin can be destroyed by the bacteria if the infection is untreated, leading to a uniform large wound that takes much longer to heal. Findings from this study demonstrated that PDT significantly reduced the bio-burden of MRSA in the mouse wounds, which would otherwise develop severe infections. In addition, wound healing and morbidity (body weight loss) were greatly benefited by the eradication of MRSA from the wounds. Photodynamic therapy may represent an alternative approach for the treatment of MRSA skin infections.
Fig. (15). PDT for skin abrasion infected with MRSA.
A) Successive bacterial luminescence images showing dose response with PDT using PEI-ce6 conjugate 23 of a representative mouse abrasion wound infected with luminescent MRSA. B) Successive bacterial luminescence images showing dark response with PEI-ce6 conjugate 23 of a representative mouse abrasion wound infected with luminescent MRSA. C). Time courses of bacterial luminescence of the infected abrasion wounds in the PDT treated mice (n=10) and non-treated mice (n=12). D) Kaplan-Meier wound healing curves of MRSA infected mouse abrasion wounds without treatment (neither PS nor light was applied) and treated with PDT, respectively.
7. PDT FOR CANDIDA INFECTION MODEL
A genus of fungi, Candida spp, is a common commensal inhabitant on human mucosal surfaces and skin, yet when that outer layer of protection is compromised, Candida can cause local infection, and in immunocompromised individuals it can infect deeper layers and if it becomes systemic, can be lethal. Doyle et al. [90, 91] created C. albicans strains expressing the firefly luciferase gene under the control of the strong C. albicans ENO1 promoter and showed the fungal cells could be detected in animals with induced vulvovaginal candidiasis that had been subjected to a vaginal lavage with a solution containing luciferin. However, this in vivo reporter system only allowed limited bioluminescent detection of C. albicans in vivo probably due to the limited permeability of hyphal cells to luciferin. This is a drawback since the yeast-to-hyphal transition is a major virulence determinant in this species [92]. D’Enfert’s laboratory recently overcame this limitation by stably transforming a synthetic codon optimized Gaussia princeps luciferase gene fused to C. albicans PGA59, which encodes a glycosylphosphatidylinositol-linked cell wall protein. Expression of the luciferase was localized at the C. albicans cell surface, allowing efficient detection of luciferase in intact cells without the necessity of the substrate, coelenterazine, penetrating into the cell.
Fungal cells prefer relatively low temperatures for growth compared to bacteria. While most pathogenic bacteria grow well at body temperature (37°C), this property is rare amongst fungi and yeasts [93]. The requirement for lower temperatures means that fungal infections on skin, mucosa, and burns tend to be superficial in nature and therefore more susceptible to aPDT.
We have tested aPDT in a mouse model of localized C. albicans infection. After screening several antimicrobial PS we settled on the phenothiazinium dye, new methylene blue (NMB) 3. Since Candida spp are eukaryotic cells, the advantage provided by PS structures with multiple cationic charges that has been clearly demonstrated in prokaryotic bacterial cells, is less pronounced, and more lipophilic less cationic molecules perform better.
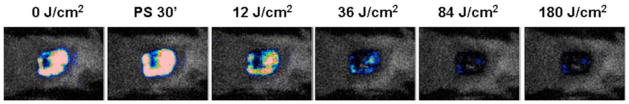
We developed a new mouse model of C. albicans in a skin abrasion formed by scraping the surface of the skin with a blade. Then a suspension (40 μL) of C. albicans in sterile phosphate buffered saline (PBS) containing 106 cells was inoculated onto the surface of the abrasion. Twenty μL coelenterazine (Gaussia princeps luciferase substrate) was topically applied to the surface of each infected abrasion. Mice were then placed in the bioluminescence imaging camera. Twenty-four hours later topical application of NMB 3 solution followed by illumination with red light produced a light-dose dependent reduction of bioluminescence as seen in Fig 16.
Fig. (16). PDT of a Candida albicans infection.
Dose response of fungal luminescence from a representative mouse skin abrasion wound infected with 106 CFU luminescent C. albicans and treated with new methylene blue 3 and 635-nm light at 24 hours after infection.
8. CONCLUSION AND FUTURE PROSPECTS
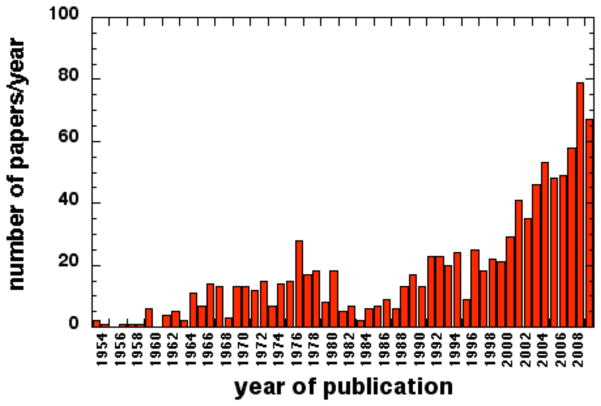
Judging by the exponential growth of published studies in antimicrobial PDT both in vitro and in vivo (see Fig. 17), the field is only going to expand further in years to come. The clinical approval in Europe and Canada of the Periowave System made by Ondine Biopharma for treating periodontitis by applying MB dye into the dental pocket followed by light delivery into the pocket using a fine fiber optic has led to over 50,000 clinical procedures being performed. The number of small companies entering this field combined with growing antibiotic resistance and the public’s tendency to distrust big pharmaceutical companies also suggests that the growth of a PDT will continue. Good animal models of localized infections suitable for testing aPDT will continue to be in demand. Bioluminescence imaging dramatically facilitates this animal testing.
Fig. (17). Antimicrobial PDT is a rapidly growing field.
The number of papers per year published in the general area of antimicrobial PDT obtained by a search of the MedLine database between 1954 and 2010.
Fig. (10). Structures of conjugates between PS and cationic polymers.
Conjugate between poly-L-lysine and ce6, pL-ce6, 22; conjugate between polyethylenimine and ce6, PEI-ce6, 23.
Acknowledgments
Research in the Hamblin laboratory is supported by NIH grants (R01A1050875 and R01CA/AI838801to MRH; R01CA137108 to Long Y Chiang), US Air Force MFEL Program (FA9550-04-1-0079), Center for Integration of Medicine and Innovative Technology (DAMD17-02-2-0006), CDMRP Program in TBI (W81XWH-09-1-0514).
References
- 1.Levy JG, Obochi M. New applications in photodynamic therapy. Introduction Photochem Photobiol. 1996;64:737–9. doi: 10.1111/j.1751-1097.1996.tb01828.x. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B. 1997;39:1–18. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 4.Raab O. Über die Wirkung fluoresceinder Stoffe and Infusorien. Zeit für Biol. 1900;39:524–546. [Google Scholar]
- 5.Wainwright W. Photoantimicrobials - a PACT against resistance and infection. Drug Future. 2004;29:85–93. [Google Scholar]
- 6.Gleckman RA, Borrego F. Adverse reactions to antibiotics. Clues for recognizing, understanding, and avoiding them. Postgrad Med. 1997;101:97–8. 101–4, 107–8. doi: 10.3810/pgm.1997.04.198. [DOI] [PubMed] [Google Scholar]
- 7.Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, Tenover FC, Zervos MJ, Band JD, White E, Jarvis WR. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- 8.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown ST. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J Photochem Photobiol B. 1996;32:159–64. doi: 10.1016/1011-1344(95)07148-2. [DOI] [PubMed] [Google Scholar]
- 9.Merchat M, Bertolini G, Giacomini P, Villanueva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J Photochem Photobiol B. 1996;32:153–7. doi: 10.1016/1011-1344(95)07147-4. [DOI] [PubMed] [Google Scholar]
- 10.Wilson M, Burns T, Pratten J, Pearson GJ. Bacteria in supragingival plaque samples can be killed by low-power laser light in the presence of a photosensitizer. J Appl Bacteriol. 1995;78:569–74. doi: 10.1111/j.1365-2672.1995.tb03101.x. [DOI] [PubMed] [Google Scholar]
- 11.Malik Z, Hanania J, Nitzan Y. Bactericidal effects of photoactivated porphyrins--an alternative approach to antimicrobial drugs. J Photochem Photobiol B. 1990;5:281–93. doi: 10.1016/1011-1344(90)85044-w. [DOI] [PubMed] [Google Scholar]
- 12.Malik Z, Ladan H, Nitzan Y. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J Photochem Photobiol B. 1992;14:262–6. doi: 10.1016/1011-1344(92)85104-3. [DOI] [PubMed] [Google Scholar]
- 13.Wainwright M. Photoinactivation of viruses. Photochem Photobiol Sci. 2004;3:406–11. doi: 10.1039/b311903n. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs BB, Tegos GP, Hamblin MR, Mylonakis E. Susceptibility of Cryptococcus neoformans to photodynamic inactivation is associated with cell wall integrity. Antimicrob Agents Chemother. 2007;51:2929–36. doi: 10.1128/AAC.00121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bliss JM, Bigelow CE, Foster TH, Haidaris CG. Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob Agents Chemother. 2004;48:2000–6. doi: 10.1128/AAC.48.6.2000-2006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales FP, da Silva SH, Roberts DW, Braga GU. Photodynamic inactivation of conidia of the fungi Metarhizium anisopliae and Aspergillus nidulans with methylene blue and toluidine blue. Photochem Photobiol. 2010;86:653–61. doi: 10.1111/j.1751-1097.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- 17.Kassab K, Dei D, Roncucci G, Jori G, Coppellotti O. Phthalocyanine-photosensitized inactivation of a pathogenic protozoan, Acanthamoeba palestinensis. Photochem Photobiol Sci. 2003;2:668–72. doi: 10.1039/b300293d. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb P, Shen LG, Chimezie E, Bahng S, Kenney ME, Horowitz B, Ben-Hur E. Inactivation of Trypanosoma cruzi trypomastigote forms in blood components by photodynamic treatment with phthalocyanines. Photochem Photobiol. 1995;62:869–74. doi: 10.1111/j.1751-1097.1995.tb09149.x. [DOI] [PubMed] [Google Scholar]
- 19.Akilov OE, Kosaka S, O’Riordan K, Song X, Sherwood M, Flotte TJ, Foley JW, Hasan T. The role of photosensitizer molecular charge and structure on the efficacy of photodynamic therapy against Leishmania parasites. Chem Biol. 2006;13:839–47. doi: 10.1016/j.chembiol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Phoenix DA, Sayed Z, Hussain S, Harris F, Wainwright M. The phototoxicity of phenothiazinium derivatives against Escherichia coli and Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:17–22. doi: 10.1016/S0928-8244(03)00173-1. [DOI] [PubMed] [Google Scholar]
- 21.Wainwright M, Phoenix DA, Marland J, Wareing DR, Bolton FJ. A study of photobactericidal activity in the phenothiazinium series. FEMS Immunol Med Microbiol. 1997;19:75–80. doi: 10.1111/j.1574-695X.1997.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 22.Wainwright M, Crossley KB. Methylene Blue--a therapeutic dye for all seasons? J Chemother. 2002;14:431–43. doi: 10.1179/joc.2002.14.5.431. [DOI] [PubMed] [Google Scholar]
- 23.Wainwright M. ‘Safe’ photoantimicrobials for skin and soft-tissue infections. Int J Antimicrob Agents. 2010;36:14–8. doi: 10.1016/j.ijantimicag.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Ragas X, Dai T, Tegos GP, Agut M, Nonell S, Hamblin MR. Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: in vitro and in vivo studies. Lasers Surg Med. 2010;42:384–90. doi: 10.1002/lsm.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neill J, Wilson M, Wainwright M. Comparative antistreptococcal activity of photobactericidal agents. J Chemother. 2003;15:329–34. doi: 10.1179/joc.2003.15.4.329. [DOI] [PubMed] [Google Scholar]
- 26.Wainwright M, Phoenix DA, Rice L, Burrow SM, Waring J. Increased cytotoxicity and phototoxicity in the methylene blue series via chromophore methylation. J Photochem Photobiol B. 1997;40:233–9. doi: 10.1016/s1011-1344(97)00061-4. [DOI] [PubMed] [Google Scholar]
- 27.Tegos GP, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob Agents Chemother. 2006;50:196–203. doi: 10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tegos GP, Masago K, Aziz F, Higginbotham A, Stermitz FR, Hamblin MR. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob Agents Chemother. 2008;52:3202–9. doi: 10.1128/AAC.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley JW, Song X, Demidova TN, Jalil F, Hamblin MR. Synthesis and properties of benzo[a]phenoxazinium chalcogen analogues as novel broad-spectrum antimicrobial photosensitizers. J Med Chem. 2006;49:5291–9. doi: 10.1021/jm060153i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smijs TG, Pavel S, Talebi M, Bouwstra JA. Preclinical studies with 5,10,15-Tris(4-methylpyridinium)-20-phenyl-[21H,23H]-porphine trichloride for the photodynamic treatment of superficial mycoses caused by Trichophyton rubrum. Photochem Photobiol. 2009;85:733–9. doi: 10.1111/j.1751-1097.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- 31.Lambrechts SA, Aalders MC, Van Marle J. Mechanistic study of the photodynamic inactivation of Candida albicans by a cationic porphyrin. Antimicrob Agents Chemother. 2005;49:2026–34. doi: 10.1128/AAC.49.5.2026-2034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambrechts SA, Aalders MC, Langeveld-Klerks DH, Khayali Y, Lagerberg JW. Effect of monovalent and divalent cations on the photoinactivation of bacteria with meso-substituted cationic porphyrins. Photochem Photobiol. 2004;79:297–302. doi: 10.1562/sa-03-15.1. [DOI] [PubMed] [Google Scholar]
- 33.Maisch T, Bosl C, Szeimies RM, Lehn N, Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob Agents Chemother. 2005;49:1542–52. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ooi N, Miller K, Randall C, Rhys-Williams W, Love W, Chopra I. XF-70 and XF-73, novel antibacterial agents active against slow-growing and non-dividing cultures of Staphylococcus aureus including biofilms. J Antimicrob Chemother. 2010;65:72–8. doi: 10.1093/jac/dkp409. [DOI] [PubMed] [Google Scholar]
- 35.Ooi N, Miller K, Hobbs J, Rhys-Williams W, Love W, Chopra I. XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J Antimicrob Chemother. 2009;64:735–40. doi: 10.1093/jac/dkp299. [DOI] [PubMed] [Google Scholar]
- 36.Banfi S, Caruso E, Buccafurni L, Battini V, Zazzaron S, Barbieri P, Orlandi V. Antibacterial activity of tetraaryl-porphyrin photosensitizers: an in vitro study on Gram negative and Gram positive bacteria. J Photochem Photobiol B. 2006;85:28–38. doi: 10.1016/j.jphotobiol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Alves E, Costa L, Carvalho CM, Tome JP, Faustino MA, Neves MG, Tome AC, Cavaleiro JA, Cunha A, Almeida A. Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009;9:70. doi: 10.1186/1471-2180-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvalho CM, Gomes AT, Fernandes SC, Prata AC, Almeida MA, Cunha MA, Tome JP, Faustino MA, Neves MG, Tome AC, Cavaleiro JA, Lin Z, Rainho JP, Rocha J. Photoinactivation of bacteria in wastewater by porphyrins: bacterial beta-galactosidase activity and leucine-uptake as methods to monitor the process. J Photochem Photobiol B. 2007;88:112–8. doi: 10.1016/j.jphotobiol.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Costa L, Carvalho CM, Faustino MA, Neves MG, Tome JP, Tome AC, Cavaleiro JA, Cunha A, Almeida A. Sewage bacteriophage inactivation by cationic porphyrins: influence of light parameters. Photochem Photobiol Sci. 2010;9:1126–33. doi: 10.1039/c0pp00051e. [DOI] [PubMed] [Google Scholar]
- 40.Grinholc M, Kawiak A, Kurlenda J, Graczyk A, Bielawski KP. Photodynamic effect of protoporphyrin diarginate (PPArg2) on methicillin-resistant Staphylococcus aureus and human dermal fibroblasts. Acta Biochim Pol. 2008;55:85–90. [PubMed] [Google Scholar]
- 41.Mantareva V, Kussovski V, Angelov I, Borisova E, Avramov L, Schnurpfeil G, Wohrle D. Photodynamic activity of water-soluble phthalocyanine zinc(II) complexes against pathogenic microorganisms. Bioorg Med Chem. 2007;15:4829–35. doi: 10.1016/j.bmc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 42.Mantareva V, Kussovski V, Angelov I, Wohrle D, Dimitrov R, Popova E, Dimitrov S. Non-aggregated Ga(iii)-phthalocyanines in the photodynamic inactivation of planktonic and biofilm cultures of pathogenic microorganisms. Photochem Photobiol Sci. 2010 doi: 10.1039/b9pp00154a. [DOI] [PubMed] [Google Scholar]
- 43.Giuliani F, Martinelli M, Cocchi A, Arbia D, Fantetti L, Roncucci G. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob Agents Chemother. 2010;54:637–42. doi: 10.1128/AAC.00603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang L, Huang YY, Mroz P, Tegos GP, Zhiyentayev T, Sharma SK, Lu Z, Balasubramanian T, Krayer M, Ruzie C, Yang E, Kee HL, Kirmaier C, Diers JR, Bocian DF, Holten D, Lindsey JS, Hamblin MR. Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers. Antimicrob Agents Chemother. 2010;54:3834–41. doi: 10.1128/AAC.00125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura E, Isobe H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc Chem Res. 2003;36:807–15. doi: 10.1021/ar030027y. [DOI] [PubMed] [Google Scholar]
- 46.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, Hamblin MR. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12:1127–35. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang L, Terakawa M, Zhiyentayev T, Huang YY, Sawayama Y, Jahnke A, Tegos GP, Wharton T, Hamblin MR. Innovative cationic fullerenes as broad-spectrum light-activated antimicrobials. Nanomedicine. 2010;6:442–52. doi: 10.1016/j.nano.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spesia MB, Milanesio ME, Durantini EN. Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C60 derivatives. Eur J Med Chem. 2008;43:853–61. doi: 10.1016/j.ejmech.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Stockert JC, Canete M, Juarranz A, Villanueva A, Horobin RW, Borrell JI, Teixido J, Nonell S. Porphycenes: facts and prospects in photodynamic therapy of cancer. Curr Med Chem. 2007;14:997–1026. doi: 10.2174/092986707780362934. [DOI] [PubMed] [Google Scholar]
- 50.Ragas X, Sanchez-Garcia D, Ruiz-Gonzalez R, Dai T, Agut M, Hamblin MR, Nonell S. Cationic Porphycenes as Potential Photosensitizers for Antimicrobial Photodynamic Therapy. J Med Chem. 2010 doi: 10.1021/jm1009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDonnell SO, Hall MJ, Allen LT, Byrne A, Gallagher WM, O’Shea DF. Supramolecular photonic therapeutic agents. J Am Chem Soc. 2005;127:16360–1. doi: 10.1021/ja0553497. [DOI] [PubMed] [Google Scholar]
- 52.Frimannsson DO, Grossi M, Murtagh J, Paradisi F, O’Shea DF. Light induced antimicrobial properties of a brominated boron difluoride (BF(2)) chelated tetraarylazadipyrromethene photosensitizer. J Med Chem. 2010;53:7337–43. doi: 10.1021/jm100585j. [DOI] [PubMed] [Google Scholar]
- 53.Hager B, Strauss WS, Falk H. Cationic hypericin derivatives as novel agents with photobactericidal activity: synthesis and photodynamic inactivation of Propionibacterium acnes. Photochem Photobiol. 2009;85:1201–6. doi: 10.1111/j.1751-1097.2009.00587.x. [DOI] [PubMed] [Google Scholar]
- 54.Soukos NS, Hamblin MR, Hasan T. The effect of charge on cellular uptake and phototoxicity of polylysine chlorin(e6) conjugates. Photochem Photobiol. 1997;65:723–9. doi: 10.1111/j.1751-1097.1997.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 55.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42:2595–601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamblin MR, O’Donnell DA, Murthy N, Rajagopalan K, Michaud N, Sherwood ME, Hasan T. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49:941–951. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]
- 57.Tegos GP, Anbe M, Yang C, Demidova TN, Satti M, Mroz P, Janjua S, Gad F, Hamblin MR. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50:1402–10. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med. 2010;42:38–44. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, Baer DG, Hamblin MR. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53:3929–34. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcez AS, Nunez SC, Hamblin MR, Ribeiro MS. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. J Endod. 2008;34:138–42. doi: 10.1016/j.joen.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcez AS, Ribeiro MS, Tegos GP, Nunez SC, Jorge AO, Hamblin MR. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med. 2007;39:59–66. doi: 10.1002/lsm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Demidova TN, Hamblin MR. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother. 2005;49:2329–35. doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gad F, Zahra T, Hasan T, Hamblin MR. Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob Agents Chemother. 2004;48:2173–8. doi: 10.1128/AAC.48.6.2173-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gad F, Zahra T, Francis KP, Hasan T, Hamblin MR. Targeted photodynamic therapy of established soft-tissue infections in mice. Photochem Photobiol Sci. 2004;3:451–8. doi: 10.1039/b311901g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003;187:1717–25. doi: 10.1086/375244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75:51–7. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 67.Polo L, Segalla A, Bertoloni G, Jori G, Schaffner K, Reddi E. Polylysine-porphycene conjugates as efficient photosensitizers for the inactivation of microbial pathogens. J Photochem Photobiol B. 2000;59:152–8. doi: 10.1016/s1011-1344(01)00114-2. [DOI] [PubMed] [Google Scholar]
- 68.Lauro FM, Pretto P, Covolo L, Jori G, Bertoloni G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem Photobiol Sci. 2002;1:468–70. doi: 10.1039/b200977c. [DOI] [PubMed] [Google Scholar]
- 69.Rovaldi CR, Pievsky A, Sole NA, Friden PM, Rothstein DM, Spacciapoli P. Photoactive porphyrin derivative with broad-spectrum activity against oral pathogens In vitro. Antimicrob Agents Chemother. 2000;44:3364–7. doi: 10.1128/aac.44.12.3364-3367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bombelli C, Bordi F, Ferro S, Giansanti L, Jori G, Mancini G, Mazzuca C, Monti D, Ricchelli F, Sennato S, Venanzi M. New cationic liposomes as vehicles of m-tetrahydroxyphenylchlorin in photodynamic therapy of infectious diseases. Mol Pharm. 2008;5:672–9. doi: 10.1021/mp800037d. [DOI] [PubMed] [Google Scholar]
- 71.Ferro S, Ricchelli F, Mancini G, Tognon G, Jori G. Inactivation of methicillin-resistant Staphylococcus aureus (MRSA) by liposome-delivered photosensitising agents. J Photochem Photobiol B. 2006;83:98–104. doi: 10.1016/j.jphotobiol.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 72.Schwiertz J, Wiehe A, Grafe S, Gitter B, Epple M. Calcium phosphate nanoparticles as efficient carriers for photodynamic therapy against cells and bacteria. Biomaterials. 2009;30:3324–31. doi: 10.1016/j.biomaterials.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 73.Ferro S, Jori G, Sortino S, Stancanelli R, Nikolov P, Tognon G, Ricchelli F, Mazzaglia A. Inclusion of 5-[4-(1-dodecanoylpyridinium)]-10,15,20-triphenylporphine in supramolecular aggregates of cationic amphiphilic cyclodextrins: physicochemical characterization of the complexes and strengthening of the antimicrobial photosensitizing activity. Biomacromolecules. 2009;10:2592–600. doi: 10.1021/bm900533r. [DOI] [PubMed] [Google Scholar]
- 74.Ferro S, Ricchelli F, Monti D, Mancini G, Jori G. Efficient photoinactivation of methicillin-resistant Staphylococcus aureus by a novel porphyrin incorporated into a poly-cationic liposome. Int J Biochem Cell Biol. 2007;39:1026–34. doi: 10.1016/j.biocel.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 75.Lu XM, Fischman AJ, Stevens E, Lee TT, Strong L, Tompkins RG, Yarmush ML. Sn-chlorin e6 antibacterial immunoconjugates. An in vitro and in vivo analysis. J Immunol Methods. 1992;156:85–99. doi: 10.1016/0022-1759(92)90014-k. [DOI] [PubMed] [Google Scholar]
- 76.Friedberg JS, Tompkins RG, Rakestraw SL, Warren SW, Fischman AJ, Yarmush ML. Antibody-targeted photolysis. Bacteriocidal effects of Sn (IV) chlorin e6-dextran-monoclonal antibody conjugates. Ann N Y Acad Sci. 1991;618:383–93. doi: 10.1111/j.1749-6632.1991.tb27258.x. [DOI] [PubMed] [Google Scholar]
- 77.Berthiaume F, Reiken SR, Toner M, Tompkins RG, Yarmush ML. Antibody-targeted photolysis of bacteria in vivo. Biotechnology (N Y) 1994;12:703–6. doi: 10.1038/nbt0794-703. [DOI] [PubMed] [Google Scholar]
- 78.Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J Photochem Photobiol B. 2005;81:15–25. doi: 10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rocchetta HL, Boylan CJ, Foley JW, Iversen PW, LeTourneau DL, McMillian CL, Contag PR, Jenkins DE, Parr TR., Jr Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob Agents Chemother. 2001;45:129–37. doi: 10.1128/AAC.45.1.129-137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Francis KP, Joh D, Bellinger-Kawahara C, Hawkinson MJ, Purchio TF, Contag PR. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun. 2000;68:3594–600. doi: 10.1128/iai.68.6.3594-3600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Francis KP, Yu J, Bellinger-Kawahara C, Joh D, Hawkinson MJ, Xiao G, Purchio TF, Caparon MG, Lipsitch M, Contag PR. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect Immun. 2001;69:3350–8. doi: 10.1128/IAI.69.5.3350-3358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Latorre-Esteves E, Akilov OE, Rai P, Beverley SM, Hasan T. Monitoring the efficacy of antimicrobial photodynamic therapy in a murine model of cutaneous leishmaniasis using L. major expressing GFP. J Biophotonics. 2010;3:328–35. doi: 10.1002/jbio.201000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamblin MR, Dai T. Can surgical site infections be treated by photodynamic therapy? Photodiagnosis Photodyn Ther. 2010;7:134–6. doi: 10.1016/j.pdpdt.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu Z, Dai T, Huang L, Kurup DB, Tegos GP, Jahnke A, Wharton T, Hamblin MR. Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine (UK) 2010;5 doi: 10.2217/nnm.10.98. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambrechts SA, Demidova TN, Aalders MC, Hasan T, Hamblin MR. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochem Photobiol Sci. 2005;4:503–9. doi: 10.1039/b502125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ragas X, Dai T, Tegos GP, Agut M, Nonell S, Hamblin MR. Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: in-vitro and in-vivo studies. Lasers Surg Med. 2010 doi: 10.1002/lsm.20922. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bowers AL, Huffman GR, Sennett BJ. Methicillin-resistant Staphylococcus aureus infections in collegiate football players. Med Sci Sports Exerc. 2008;40:1362–7. doi: 10.1249/MSS.0b013e31816f1534. [DOI] [PubMed] [Google Scholar]
- 88.Turbeville SD, Cowan LD, Greenfield RA. Infectious disease outbreaks in competitive sports: a review of the literature. Am J Sports Med. 2006;34:1860–5. doi: 10.1177/0363546505285385. [DOI] [PubMed] [Google Scholar]
- 89.Kirkland EB, Adams BB. Methicillin-resistant Staphylococcus aureus and athletes. J Am Acad Dermatol. 2008;59:494–502. doi: 10.1016/j.jaad.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 90.Doyle TC, Nawotka KA, Purchio AF, Akin AR, Francis KP, Contag PR. Expression of firefly luciferase in Candida albicans and its use in the selection of stable transformants. Microb Pathog. 2006;40:69–81. doi: 10.1016/j.micpath.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Doyle TC, Nawotka KA, Kawahara CB, Francis KP, Contag PR. Visualizing fungal infections in living mice using bioluminescent pathogenic Candida albicans strains transformed with the firefly luciferase gene. Microb Pathog. 2006;40:82–90. doi: 10.1016/j.micpath.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Saville SP, Lazzell AL, Chaturvedi AK, Monteagudo C, Lopez-Ribot JL. Use of a genetically engineered strain to evaluate the pathogenic potential of yeast cell and filamentous forms during Candida albicans systemic infection in immunodeficient mice. Infect Immun. 2008;76:97–102. doi: 10.1128/IAI.00982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robert VA, Casadevall A. Vertebrate endothermy restricts most fungi as potential pathogens. J Infect Dis. 2009;200:1623–6. doi: 10.1086/644642. [DOI] [PubMed] [Google Scholar]