Summary
Between 1992 and 2003, a total of2029 aneurysms in 1748 patients were treated by endovascular occlusion with electrolytically detachable coils. In this series, electrolytically detachable platinum coils with Nylon fibers (Sapphire Detachable Coil System, MTI, Irvine, CA, USA) were used in 474 aneurysms solely or in combination with bare coils from various manufacturers. To determine the safety and clinical efficacy of Nylon fibered coils for the endovascular treatment of intracranial aneurysms in comparison to bare platinum coils a thorough retrospective statistical analysis by means of logistic regression and matched pairs analysis was performed. Only treatments with data for all matching variables were used, resulting in 421 matched pairs. The analysis was performed with respect to clinical status and numerous parameters concerning individual aneurysm characteristics (e.g., location, neck width, fundus diameter). Treatment-related parameters included the use and percentage of fibered coils, occlusion rate, procedural complications, early clinical outcome and Glasgow Outcome Scale (GOS) scores. Finally, long-term follow-up results (particularly recurrence, cause of recurrence and post treatment haemorrhage) were evaluated.
Both logistic regression and matched pairs analysis showed a statistically improved occlusion rate if fibered coils had been used (96% largely occluded with the use of fibered coils vs. 84-85% with the exclusive use of bare coils). However, the amount of fibered coils calculated as percentage of coil length did not seem to have significant impact.
Procedures with fibered coils did not lead to a higher rate of thromboembolic events (8.0% for fibered vs. l0.5% for bare coils).The apparently better clinical outcome in the group treated with fibered coils determined by both postprocedural outcome and GOS, did not reach statistical significance. Analysis of the anatomical properties showed no differences between the groups treated with bare and fibered coils in terms of neck width, fundus diameter, and anatomic location. As expected, a higher occlusion rate was achieved in aneurysms with smaller neck and fundus independent from the type of coil used.
On follow up angiography, there was an apparently lower rate of recurrence secondary to coil compaction in the group treated with fibered coils, but these data were compromised by the fact that up to date only about one third of 474 aneurysms treated with fibered coils had undergone angiographic follow-up and this did not reach statistical significance.
From our experiences, we conclude that the use of fibered electrolytically detachable platinum coils in aneurysm treatment leads to significantly improved occlusion rates compared to the sole use of bare platinum coils. We hope that with increasing follow-up data we will be able to confirm that the apparently reduced recurrence rates for aneurysms treated with fibered coils can be proven with statistical significance.
Key words: aneurysms, embolization, fibered coils
Introduction
Since the proposal of electrolytically detachable platinum coils as an embolic agent for intracranial aneurysms in 1979 by Piton1 and the subsequent refinement by Guglielmi et Al in 19912,3 more than 100,000 patients worldwide have benefited from this technology. Despite the undoubtedly excellent clinical results4 aneurysm recurrence remains a concern5.
Various coil modifications have been proposed in order to improve the permanency of coil occlusion. Among these, collagen coating6, use of water absorbent gel coating 7, and biodegradable plastic surfaces8 are currently under evaluation.
Most of the published data on these coated coils is preclinical in vitro, animal model-based or refers to a limited number of patients. The proposed modifications are not only technically challenging and therefore costly but also alter the mechanical coil properties in terms of friction or stiffness. Also, the surface coating might be stripped off by coil or microcatheter manipulation, which could lead to thromboembolic adverse events.
Before the advent of electrolytically detachable coil systems, free pushable coils with Dacron fibers were readily available 9,10. With these systems, it was often reported that due to their high thrombogenicity, aneurysm occlusion by early thrombus formation could be obtained with loose packing, sometimes even with just one coil. However, only a few endovascular centers use electrolytically detachable fibered coils 11,12 and there are no systematic studies on long term results of aneurysms that have been treated with fibered or a combination of fibered and bare platinum coils. The purpose of this study was to evaluate the safety and acute as well as mid-term efficacy of electrolytically detachable fibered coils for the treatment of intracranial aneurysms.
Methods
Between November 6th 1992 and October 24th 2003 a total number of 2029 aneurysms in 1748 patients were treated by means of endovascular occlusion with detachable coils, at this single center. In this series, bare platinum coils from various manufacturers (GDC™, Boston Scientific/Target; Sapphire™ Detachable Coils, Dendron/MTI, Bochum - Germany; and ACT-MicroCoil™, Micrus, Sunnyvale, CA, USA) were used solely in 1536 aneurysms and in combination with fibered Sapphire Detachable Coils (SDCf, Dendron/MTI) in 452 aneurysms. Fibered coils were used exclusively in 22 aneurysms. In 19 aneurysms no information on the type of coils used could be obtained.
Most of the interventional procedures were performed on a Neurostar™ biplane digital angiography system (Siemens, Erlangen, Germany). The follow-up angiograms were obtained on a Angiostar™ monoplane DSA system (Siemens, Erlangen, Germany). Informed consent for registry and analysis of the data could not be obtained a priori on a regular basis due to the poor clinical status of a significant number of patients at the time of treatment. A posteriori patient allowance was limited due to logistic difficulties in reaching patients after several years due to patient migration and other factors.
The aspects of patient privacy and confidentiality were considered during all steps of data registry and analysis.
Data Analysis
The data registry comprised demographic features (name, age, and gender), clinical status (non-haemorrhagic signs and symptoms, Hunt and Hess score, Glasgow Outcome Scale score), and numerous parameters concerning individual aneurysm characteristics (e.g., anatomic location, previous rupture, neck width, maximum fundus diameter, partial thrombosis), treatment-related facts (date of first and subsequent treatment sessions, number of coils, use and percentage of fibered coils, occlusion rate, incidence and nature of procedural complications, early clinical outcome in relation to the treatment and additionally as plain GOS scores), and follow-up results (e.g., date of follow-up, angiographically determined occlusion rate, recurrence and cause of recurrence, incidence of post treatment haemorrhage, clinical long-term follow-up, cause and date of death).
The occlusion rate was graded as complete (100%) with full reconstruction of the contour of the parent artery, 90-99% in case of minor neck remnant, or less than 90% for all instances of visible opacification inside the aneurysm. The clinical result was rated on a 5-point scale according to the Glasgow Outcome Scale (GOS) 13 with grade V being unimpaired and grade I being dead.
Statistical analysis was carried out using a dedicated software package (Stata 8.0, StataCorp. 2003; Stata Statistical Software, release 8.0, college station, TX, USA) and included descriptive parameters (n, mean, standard deviation, median) for location, aneurysm neck width and fundus diameter, occlusion rate, frequency of thromboembolic complications, and occurrence of recurrence and coil compaction.
The percentage of fibered coils was calculated as a function of total applied coil length and ranged from 1 - 100% with a mean of 38.8%. To address the potential influence of different amounts of fibered coils, group were formed between <50%, 50-80%, and >80% of fibered coils. Thereafter, comparisons were made between aneurysms treated with either bare coils or fibered coils by means of logistic regression and matched pairs analysis.
Bivariate analysis: Categorical variables are described by frequencies (n,%). Continuous variables are described by mean, standard deviation as well as median, minimum and maximum. The null hypothesis of independence between two categorical variables was tested using Fischer's exact test or χ2-test of independence. To test for differences in the distribution of a continuous variable in two independent groups the Mann-Whitney-U-Test was used. All tests are two-sided. The significance level is set to 0.05.
Multivariate analysis: A matched pairs analysis and logistic regression test were used to identify the effect of fibered coils on occlusion rate and coil compaction. First, logistic regression was applied to find those variables that had a significant influence on the probability of the events: "occlusion rate ≥90%" and "coil compaction", respectively. This approach provided an estimated value for the effect of each identified factor. For identification of significant effects, backward selection was used. The first regression model includes all possible factors. Thereafter, stepwise exclusion of factors with a non-significant influence (p>0.05) on the probability of the target-event was done, starting with the variable with the largest p-value. At every step, it was decided whether a previously excluded variable should be included again to improve the model's fit. Odds ratios and 95%-confidence intervals are calculated as effect estimates. In addition, it was tested whether or not the odds ratio is significantly different from zero.
The estimated p-values may be wrong in case of strong dependencies between the factors used in the logistic regression approach. In order to control for this possibility, a matched pairs analysis was used. This approach does not provide estimates for all variables of interest but it offers control of their effects on the target events (occlusion rate and coil compaction). Matched pairs were formed with respect to aneurysm location, neck width, and fundus diameter compared toocclusion rate. For the analysis of coil compaction, previous rupture was used as an additional matching parameter. Irrespective of other anatomic details such as orientation of the longitudinal axis, groups were defined at 2 mm margins for the neck width and at measurements of ≤ 4 mm, 5-7 mm, 8-14 mm, 15-24 mm, and ≥ 25 mm for the fundus diameter. Only treatments with available data for all matching variables were included. In the event of more than one treatment available for matching in either bare-coils or fibered-coils, pairs were matched at random. This resulted in 421 matched pairs for analysis of the occlusion rate and in only 44 pairs for the coil compaction. The proportion of treatments with an occlusion rate of ≥ 90% and with subsequent coil compaction was compared between the groups treated with fibered-coils and those treated only with bare-coils.
Results
Illustrative Cases
The following cases were selected in order to emphasize individual aspects of the use of fibered coils, which are beyond the reach of a statistic validation.
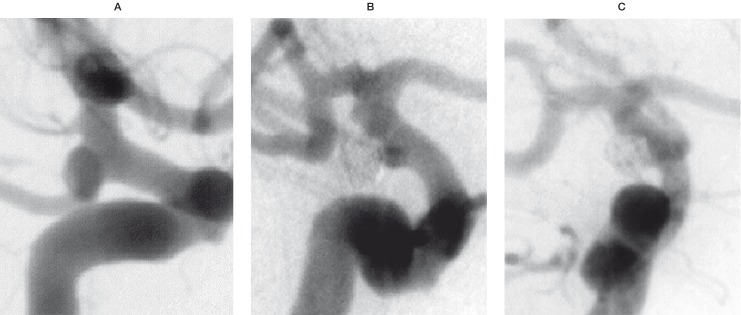
Case 1: Unruptured left sided aneurysm of the posterior communicating segment of the internal carotid artery in a 57-year-old woman. Despite the unfavorable neck-to-fundus ratio estimated at 4 mm by 5 mm, immediate complete occlusion was achieved with one fibered 3 mm / 10 cm T10 Sapphire coil together with a bare 2 mm / 4 cm coil. The main asset of the fibered coil in this case was the increased "grip" between the coil and the aneurysm wall, which allowed a stable deposition of the slightly undersized coil bridging the relatively wide neck. The follow-up angiography obtained after five months confirmed total occlusion of the aneurysm (figure 1).
Figure 1.
Pre treatment (A), final result (B) and 5 months follow-up (C) angiography (left to right) of an unruptured left ICA-PcomA aneurysm treatment with one fibered 3 mm /10 cm and one bare 2 mm / 4 cm coil.
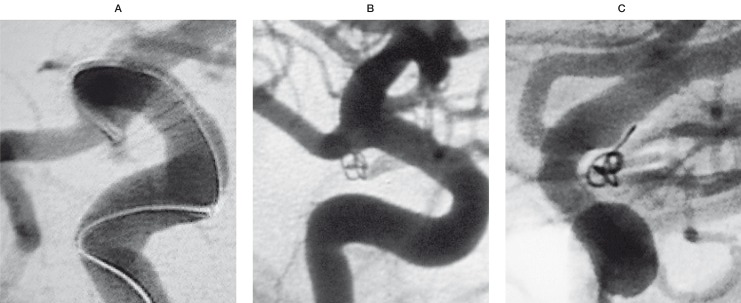
Case 2: A previously ruptured and partially clipped posterior communicating artery aneurysm. Placement of a single fibered 2 mm / 4 cm T10 Sapphire coil was possible. Further attempts to introduce more coils into the aneurysm failed due to the steep angle of the aneurysm in relation to the parent artery. At the end of the procedure, flow inside the aneurysm was still visible. However, the follow-up angiography four months later shows not only a complete occlusion but also significant bridging of the neck suggesting neointima formation (figure 2).
Figure 2.
Pre treatment (A) and 4 months follow-up angiography (B,C) of a previously ruptured right sided true PcomA aneurysm, treated with a single 2 mm / 4cm fibered coil. At the end of the procedure, flow could still be seen inside the aneurysm. Follow-up angiography confirmed complete exclusion of the aneurysm from blood circulation.
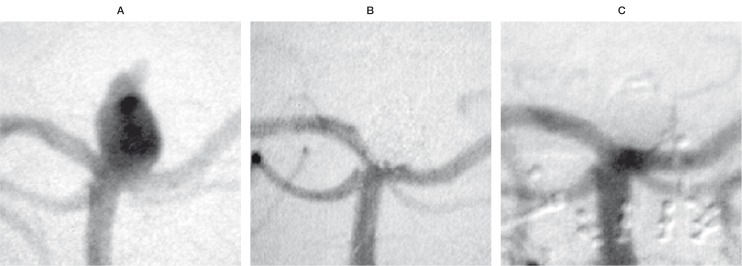
Case 3: Acutely ruptured aneurysm of the basilar tip with a 5 mm neck and 8 mm fundus. Complete occlusion could be obtained with 7 coils, including 14% fibered coils, which were used to seal the orifice. Follow-up angiography at 14 months confirmed complete exclusion of the aneurysm from the circulation. This observation suggests that a relatively small percentage of fibered coils might prevent a recurrence even in locations where high haemodynamic stress often leads to early coil compaction (figure 3).
Figure 3.
Pre treatment (A), final result (B) and 14 months follow-up angiography (C) of an acutely ruptured aneurysm of the basilar tip. The treatment was performed with 14% fibered coils as a fraction of total coil length, placed at the entrance zone.
Statistical Analysis
For the following statistical analysis, 1536 aneurysms treated with bare-coils exclusively were available for comparison with 474 aneurysms treated with various amounts of fibered-coils.
Occlusion Rate
Tables 1 and 2 summarize the occlusion rates of the first and second treatment sessions, respectively. The frequency of complete occlusion (100%) and subtotal occlusion (90-99%) after the first and second treatment was significantly higher if fibered coils had been used (p-values < 0.05). The percentage of fibered coils had no significant influence.
Table 1.
Occlusion rate of the first endovascular treatment session for bare and fibered coils
| Occl. rate |
bare coils (n =1536) |
fibered coils (n = 474) |
fibered coil fractions: | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <50% | (n = 332) | 50-80% | (n =96) | >80% | (n =31) | |||||
| 100% | 991 | (64.5%) | 338 | (71.3%) | 237 | (71.4%) | 68 | (70.8%) | 24 | (77.4%) |
| 90%-99% | 310 | (20.2%) | 114 | (24.1%) | 78 | (23.5%) | 26 | (27.1%) | 5 | (16.1%) |
| < 90%, failed | 235 | (15.3%) | 22 | (4.7%) | 17 | (5.1%) | 2 | (2.1%) | 2 | (6.5%) |
| p-values | p = 0.000 (χ2-test of independence) | p = 0.536 (χ2-test of independence) | ||||||||
Table 2.
Occlusion rate of the second endovascular treatment session for bare and fibered coils
| Occl. rate |
bare coils (n = 149) |
fibered coils (n = 63) <50% |
fibered coil fractions: | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=10) | 50-80% | (n =15) | >80% | (n = 6) | ||||||
| 100% | 70 | (47.0%) | 39 | (61.9%) | 7 | (70%) | 10 | (66.7%) | 2 | (33.3%) |
| 90%-99% | 42 | (28.2%) | 21 | (33,3%) | 2 | (20%) | 4 | (26.7%) | 3 | (50.0%) |
| < 90%, failed | 37 | (24.8%) | 3 | (4.8%) | 1 | (10%) | 1 | (6.7%) | 1 | (16.7%) |
| p-values | p = 0.001 (χ2-test of independence) | p = 0.623 (χ2-test of independence) | ||||||||
Thromboembolic Events
The frequency of all kinds of thromboembolic events and complications was determined in relation to the use of bare and fibered coils and are displayed in table 3. In general, procedures with fibered coils did not lead to a higher rate of thromboembolic events. The apparent trend towards a reduced frequency of this kind of complication with fibered coils did not reach statistical significance.
Table 3.
Comparison of the frequency of thromboembolic events during the first treatment session between bare and fibered coils
| bare coils (n =1536) |
fibered coils (n = 474) |
fibered coil fractions: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <50% | (n=332) | 50-80% | (n=96) | >80% | (n =31) | |||||
| No events | 1375 | (89.5%) | 436 | (92.0%) | 300 | (90.4%) | 91 | (94.8%) | 30 | (96.8%) |
| Events | 161 | (10.5%) | 38 | (8.0%) | 32 | (9.6%) | 5 | (5.2%) | 1 | (3.2%) |
| p-values | p = 0.134 (Fischer’s exact test) | p = 0.218 (χ2-test of independence) | ||||||||
Clinical Outcome
The apparently better clinical outcome for patients treated with fibered coils at the first treatment session proved not to be significantly different between the groups treated with fibered and bare coils as determined by both immediate postprocedural outcome (table 4) and GOS (table 5).
Table 4.
Clinical outcome after the first treatment session with respect to permanency and cause of neurological deterioration
| bare coils (n = 1533) |
fibered coils (n = 463) |
total (n = 1996) |
p-value | ||||
|---|---|---|---|---|---|---|---|
| No deficit | 1036 | (67.6%) | 330 | (71.3%) | 1366 | (68.4%) | |
| Neurological deficit | 497 | (32.4%) | 133 | (28.7%) | 630 | (31.6%) | 0.138 |
| Kind of deficit | |||||||
| - Coil-procedure related: | |||||||
| Transient | 84 | (5.5%) | 21 | (4.5%) | 105 | (5.3%) | 0.477 |
| Mild | 37 | (2.4%) | 6 | (1.3%) | 43 | (2.1%) | 0.200 |
| Severe | 43 | (2.8%) | 12 | (2.6%) | 55 | (2.8%) | 0.873 |
| Dead | 16 | (1.0%) | 7 | (1.5%) | 23 | (1.1%) | 0.455 |
| - Preexisting | 81 | (5.3%) | 4 | (0.9%) | 85 | (4.3%) | 0.000 |
| - Non coil-procedure related: | |||||||
| Mild | 43 | (2.8%) | 18 | (3.9%) | 61 | (3.1%) | 0.280 |
| Severe | 144 | (9.4%) | 48 | (10.4%) | 192 | (9.6%) | 0.530 |
| Dead | 47 | (3.1%) | 14 | (3.0%) | 61 | (3.1%) | 1.000 |
|
Procedural but non coil-procedure related |
2 | (0.1%) | 3 | (0.4%) | 5 | (0.1%) | 0.085 |
| For 23 treatments the clinical outcome was not yet defined. | |||||||
Table 5.
Clinical outcome after the first treatment session according to the Glasgow Outcome Scale
| bare coils (n = 1533) |
fibered coils (n = 463) |
total (n = 1996) |
||||
|---|---|---|---|---|---|---|
| Good | 1147 | (74.8%) | 352 | (76.0%) | 1499 | (75.1%) |
| Moderately disabled | 106 | (6.9%) | 26 | (5.6%) | 132 | (6.6%) |
| Severely disabled | 170 | (11.1%) | 48 | (10.4%) | 218 | (10.9%) |
| Vegetative state | 46 | (3.0%) | 15 | (3.2%) | 61 | (3.1%) |
| Dead | 64 | (4.2%) | 22 | (4.8%) | 86 | (4.3%) |
| p-value | p = 0.829 (χ2-test of independence) | |||||
| For 23 treatments the clinical outcome was not yet defined. | ||||||
Anatomical Aspects
Since the anatomical features of aneurysms supposedly have an influence on the occlusion rate and subsequent coil compaction, both treatment groups (bare and fibered coils) were analyzed with respect to neck width (table 6A), fundus diameter (table 7A) and aneurysm location (table 8A).
Table 6A.
Comparison of neck width of aneurysms treated with either bare or fibered coils concerning neck width
| coils | valid n | Neck Width mean ± SD mm |
Neck Width median mm |
Neck Width min-max mm |
|---|---|---|---|---|
| Bare coils | 1515 | 3.9 ± 2.7 | 3 | 1-30 |
| Fibered coils | 450 | 3.8 ± 1.9 | 3 | 1-13 |
| Total | 1965 | 3.9 ± 2.5 | 3 | 1-30 |
| p-value of the Mann-Whitney-U-Test: p = 0.1559 | ||||
Table 7A.
Comparison of aneurysms treated with either bare or fibered coils concerning their fundus diameters
| coils | valid n | Fundus Diameter mean ± SD mm |
Fundus Diameter median mm |
Fundus Diameter min-max mm |
|---|---|---|---|---|
| Bare coils | 1515 | 8.4 ± 6.3 | 6 | 1-50 |
| Fibered coils | 450 | 8.4 ± 5.1 | 7 | 2-32 |
| Total | 1965 | 8.4 ± 6.0 | 7 | 1-50 |
| p-value of the Mann-Whitney-U-Test: p = 0.0333 | ||||
Table 8A.
Comparison of aneurysms treated with either bare or fibered coils with respect to their anatomic locations
| Aneurysm location |
bare coils | fibered coils | p-value* | |||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| 1 ICA petrous segment | 3 | 100.0 | 0 | 0.0 | 0.579 | |
| 2 ICA cavernous segment | 54 | 80.6 | 13 | 19.4 | 0.775 | |
| 3 Superior hypophyseal artery | 34 | 85.0 | 6 | 15.0 | 0.259 | |
| 4 ICA ophthalmic segment | 117 | 84.2 | 22 | 15.8 | 0.0233 | |
| 5 ICA PcomA | 141 | 65.0 | 76 | 35.0 | 0.000 | |
| 6 ICA supra- / paraclinoidal | 22 | 84.6 | 4 | 15.4 | 0.484 | |
| 7 ICA AchoA | 32 | 78.0 | 9 | 22.0 | 1.000 | |
| 8 ICA bifurcation | 43 | 69.4 | 19 | 30.6 | 0.223 | |
| 9 Proximal MCA (M1-segment) | 24 | 68.6 | 11 | 31.4 | 0.314 | |
| 10 MCA bifurcation | 200 | 76.9 | 60 | 23.1 | 0.876 | |
| 11 Distal MCA (M2-segment) | 8 | 72.7 | 3 | 27.3 | 1.000 | |
| 12 Proximal ACA (A1-segment) | 18 | 85.7 | 3 | 14.3 | 0.324 | |
| 13 AcomA | 284 | 71.7 | 112 | 28.3 | 0.017 | |
| 14 Distal ACA | 40 | 90.9 | 4 | 9.1 | 0.050 | |
| 15 Vertebral artery | 24 | 82.8 | 5 | 17.2 | 0.091 | |
| 16 Vertebro-basilar junction | 30 | 100.0 | 0 | 0.0 | 0.001 | |
| 17 PICA | 63 | 84.0 | 12 | 16.0 | 0.277 | |
| 18 AICA | 5 | 100.0 | 0 | 0.0 | 0.597 | |
| 19 Superior cerebellar artery | 70 | 86.4 | 11 | 13.6 | 0.062 | |
| 20 Basilar trunk | 29 | 72.5 | 11 | 27.5 | 0.472 | |
| 21 Basilar tip | 226 | 80.4 | 55 | 19.6 | 0.096 | |
| 22 Proximal PCA (P1-segment) | 31 | 77.5 | 9 | 22.5 | 1.000 | |
| 23 Distal PCA (P2-segment) | 17 | 73.9 | 6 | 26.1 | 0.199 | |
| Total | 1515 | 77.1 | 451 | 22.9 | - | |
| *p-values of Fischer's Exact -Test | ||||||
The same parameters were subsequently evaluated for their impact on the achieved occlusion rate (tables 6B, 7B, and 8B) and the frequency of coil compaction (tables 6C, 7C, and 8C). The analysis showed no significant differences between the groups treated with bare and fibered coils in terms of neck width, fundus diameter, and anatomic location. As expected, a higher occlusion rate was achieved in aneurysms with smaller neck and fundus (table 6B and 7B)
Table 6B.
Comparison of aneurysms with < 90% vs. 90-100% occlusion rate concerning with respect to their neck widths irrespective of the type of coils used
| occlusion rate | valid n | Neck Width mean + SD mm |
Neck Width median mm |
Neck Width min-max mm |
|---|---|---|---|---|
| < 90% | 252 | 5.8 ±4.1 | 5 | 1-30 |
| 90-100% | 1720 | 3.6 ± 2.1 | 3 | 1-30 |
| Total | 1972 | 3.9 ± 2.5 | 3 | 1-30 |
| p-value of the Mann-Whitney-U-Test: p = 0.000 | ||||
Table 7B.
Comparison of aneurysms with <90% vs. 90-100% occlusion rate concerning with respect to their fundus diameter, irrespective of the type of coils used
| occlusion rate | valid n | Fundus Diameter mean ± SD mm |
Fundus Diameter median mm |
Fundus Diameter min-max mm |
|---|---|---|---|---|
| < 90% | 252 | 12.1 ± 9.3 | 10 | 1-50 |
| 90-100% | 1722 | 7.9 ± 5.2 | 6 | 2-35 |
| Total | 1974 | 8.4 ± 6 | 7 | 1-50 |
| p-value of the Mann-Whitney-U-Test: p = 0.000 | ||||
Table 8B.
Comparison of aneurysms with <90% vs. 90-100% occlusion rate with respect to their anatomic location, irrespective of the type of coils used
| occl. rate < 90% | occl. rate > 90% | p-value* | ||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| 1 ICA petrous segment | 1 | 25.0 | 3 | 75.0 | 0.424 | |
| 2 ICA cavernous segment | 20 | 27.8 | 52 | 72.2 | 0.000 | |
| 3 Superior hypophyseal artery | 7 | 17.5 | 33 | 82.5 | 0.344 | |
| 4 ICA ophthalmic segment | 29 | 20.6 | 112 | 79.4 | 0.009 | |
| 5 ICA PcomA | 20 | 9.0 | 202 | 91.0 | 0.071 | |
| 6 ICA supra- / paraclinoidal | 3 | 11.5 | 23 | 88.5 | 1.000 | |
| 7 ICA AchoA | 1 | 2.4 | 40 | 97.6 | 0.054 | |
| 8 ICA bifurcation | 6 | 9.7 | 56 | 90.3 | 0.565 | |
| 9 Proximal MCA (M1-segment) | 2 | 5.7 | 33 | 94.3 | 0.306 | |
| 10 MCA bifurcation | 37 | 14.2 | 224 | 85.8 | 0.489 | |
| 11 Distal MCA (M2-segment) | 1 | 8.3 | 11 | 91.7 | 1.000 | |
| 12 Proximal ACA (A1-segment) | 5 | 22.7 | 17 | 77.3 | 0.190 | |
| 13 AcomA | 43 | 10.8 | 354 | 89.2 | 0.182 | |
| 14 Distal ACA | 5 | 11.1 | 40 | 88.9 | 1.000 | |
| 15 Vertebral artery | 6 | 12.0 | 44 | 88.0 | 1.000 | |
| 16 Vertebro-basilar junction | 5 | 16.7 | 25 | 83.3 | 0.579 | |
| 17 PICA | 8 | 10.3 | 70 | 89.7 | 0.605 | |
| 18 AICA | 1 | 20.0 | 4 | 80.0 | 0.498 | |
| 19 Superior cerebellar artery | 8 | 9.8 | 74 | 90.2 | 0.500 | |
| 20 Basilar trunk | 9 | 20.9 | 34 | 79.1 | 0.110 | |
| 21 Basilar tip | 38 | 13.5 | 243 | 86.5 | 0.792 | |
| 22 Proximal PCA (P1-segment) | 3 | 7.5 | 37 | 92.5 | 0.472 | |
| 23 Distal PCA (P2-segment) | 2 | 6.7 | 28 | 93.3 | 0.417 | |
| Total | 260 | 12.9 | 1759 | 87.1 | - | |
| *p-values of Fischer's Exact -Test | ||||||
Table 6C.
Comparison of aneurysms with and without coil compaction as defined by angiographic follow-up concerning with respect to their neck widths, irrespective of the type of coils used
| coil compaction | valid n | Neck Width mean + SD mm |
Neck Width median mm |
Neck Width min-max mm |
|---|---|---|---|---|
| No compaction | 775 | 3.8 ± 2.3 | 3 | 1-20 |
| Compaction | 269 | 4.4 ± 2.6 | 4 | 1-20 |
| Total | 1044 | 4 ± 2.4 | 3 | 1-20 |
| p-value of the Mann-Whitney-U-Test: p = 0.0001 | ||||
Table 7C.
Comparison of aneurysms with and without coil compaction as defined by angiographic follow-up with respect to their fundus diameter, irrespective of the type of coils used
| coil compaction | valid n | Fundus Diameter mean + SD mm |
Fundus Diameter median mm |
Fundus Diameter min-max mm |
|---|---|---|---|---|
| No compaction | 775 | 8.1 ± 5.9 | 6 | 2-50 |
| Compaction | 269 | 10.1 ± 6.5 | 8 | 2-40 |
| Total | 1044 | 8.6 ± 6.1 | 7 | 2-50 |
| p-value of the Mann-Whitney-U-Test:p = 0.0000 | ||||
Table 8C.
Comparison of aneurysms with and without coil compaction as defined by angiographic follow-up with respect to their anatomic location, irrespective of the type of coils used
| no compaction | compaction | p-value* | ||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| 1 ICA petrous segment | 3 | 100.0 | 0 | 0.0 | 0.574 | |
| 2 ICA cavernous segment | 27 | 64.3 | 15 | 35.7 | 0.158 | |
| 3 Superior hypophyseal artery | 22 | 95.7 | 1 | 4.3 | 0.015 | |
| 4 ICA ophthalmic segment | 52 | 66.7 | 26 | 33.3 | 0.138 | |
| 5 ICA PcomA | 92 | 78.6 | 25 | 21.4 | 0.264 | |
| 6 ICA supra- / paraclinoidal | 11 | 68.8 | 5 | 31.3 | 0.779 | |
| 7 ICA AchoA | 14 | 58.3 | 10 | 41.7 | 0.095 | |
| 8 ICA bifurcation | 25 | 75.8 | 8 | 24.2 | 1.000 | |
| 9 Proximal MCA (Ml-segment) | 18 | 90.0 | 2 | 10.0 | 0.125 | |
| 10 MCA bifurcation | 92 | 71.9 | 36 | 28.1 | 0.518 | |
| 11 Distal MCA (M2-segment) | 3 | 100.0 | 0 | 0.0 | 0.574 | |
| 12 Proximal ACA (A1-segment) | 7 | 63.6 | 4 | 36.4 | 0.487 | |
| 13 AcomA | 143 | 73.7 | 51 | 26.3 | 0.856 | |
| 14 Distal ACA | 10 | 52.6 | 9 | 47.4 | 0.059 | |
| 15 Vertebral artery | 12 | 80.0 | 3 | 20.0 | 0.771 | |
| 16 Vertebro-basilar junction | 13 | 68.4 | 6 | 31.6 | 0.597 | |
| 17 PICA | 37 | 92.5 | 3 | 7.5 | 0.005 | |
| 18 AICA | 2 | 66.7 | 1 | 33.3 | 1.000 | |
| 19 Superior cerebellar artery | 35 | 77.8 | 10 | 22.2 | 0.728 | |
| 20 Basilar trunk | 17 | 63.0 | 10 | 37.0 | 0.183 | |
| 21 Basilar tip | 110 | 73.3 | 40 | 26.7 | 0.763 | |
| 22 Proximal PCA (P1-segment) | 20 | 95.2 | 1 | 4.8 | 0.023 | |
| 23 Distal PCA (P2-segment) | 10 | 76.9 | 3 | 23.1 | 1.000 | |
| Total | 775 | 74.2 | 269 | 25.8 | - | |
| *p-values of Fischer's Exact -Test | ||||||
Angiographic Follow-up
Angiographic follow-up data were available for 904 (58.9%) aneurysms treated with bare coils and for 156 (32.9%) aneurysms treated with fibered coils. After exclusion of aneurysms which showed recurrence for reasons other than coil compaction, 848 aneurysms treated with bare coils and 146 aneurysms treated with fibered coils were included for further analysis.
Table 9 shows a statistically non-significant trend towards a lower rate of recurrence secondary to coil compaction in the group treated with fibered coils. These data are largely compromised by the fact that only 156 out of 474 aneurysms underwent angiographic follow-up after fibered coil treatment. A comparison of the three subgroups in which different percentages of fibered coils had been used showed no difference between these groups. Again, these data are subject to small-number effects.
Table 9.
Frequency of coil compaction on follow-up angiography after the first treatment session
| bare coils (n = 848) |
fibered coils (n = 146) |
fibered coils percentage: | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <50% (n = 105) |
50-80% (n = 24) |
> 80% (n = 10) |
|||||||||
| No recurrence | 612 | (72.2%) | 111 | (76.0%) | 79 | (75.2%) | 19 | (79.2%) | 7 | (70.0%) | |
| Coil compaction | 236 | (27.8%) | 35 | (24.0%) | 26 | (24.8%) | 5 | (20.8%) | 3 | (30.0%) | |
| p-values p = 0.366 (Fischer’s Exact Test) p = 0.843 (χ2-test of independence) | |||||||||||
It is a common belief that those aneurysms, which had been treated after rupture show a higher liability to recurrence than unruptured aneurysms. This factor may overlap the effects of the use of bare vs. fibered coils. The data in table 10 confirm this concept. Irrespective of the type of implanted coils, coil compaction was found significantly more frequently in ruptured than in unruptured aneurysms.
Table 10.
Frequency of coil compaction on follow-up angiography after the first treatment session in unruptured and ruptured aneurysms, irrespective of the type of coils used
| previous rupture | no rupture (n = 527) | rupture (n = 535) | |||
|---|---|---|---|---|---|
| coil compaction | |||||
| No compaction | 412 | (78.2%) | 379 | (74.5%) | |
| Compaction | 115 | (21.8%) | 156 | (25.5%) | |
| p-values | p = 0.007 (Fischer’s Exact Test) | ||||
Multivariate Analysis – Occlusion Rate
a) Logistic Regression
Backward selection was used to find those variables, which have a significant influence on the probability of the event "occlusion rate of the 1st treatment session ≤90%".
The variables: "use of fibered coils", "diameter", "neck width", and "location at the A1-segment" had a significant effect on the probability of a high occlusion rate. High values for "neck width" (odds ratio = 0.81, p = 0.000) and "fundus diameter" (0.97, p = 0.032) are correlated to a lower occlusion rate. Also, A1- aneurysms showed a lower probability of a high occlusion rate than aneurysms in other locations (0.29, p = 0.019) (see tables 11 and 12 for detailed results and comments). As opposed to bare-coils, the use of fibered coils increased the probability of an occlusion rate of at least 90% (3.5, p = 0.000).
Table 11.
Results of the logistic regression for the event “occlusion rate of the 1st treatment session >90%”
| occlusion ≥ 90% | Odds Ratio | p-value | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|
| F-coil | 3.8 | 0.000 | 2.34 | 6.06 | ||
| Neck | ||||||
| 5-6 | 0.39 | 0.000 | 0.28 | 0.55 | ||
| 7-8 | 0.20 | 0.000 | 0.12 | 0.33 | ||
| 9-10 | 0.13 | 0.000 | 0.07 | 0.22 | ||
| >=11 | 0.11 | 0.000 | 0.05 | 0.26 | ||
| Diameter | >=15 | 0.39 | 0.002 | 0.21 | 0.71 | |
| A1 aneurysm | 0.31 | 0.027 | 0.11 | 0.87 | ||
Table 12A.
Frequency of locations of 421 aneurysms, matched as pairs concerning treatment with bare or fibered coils and occlusion rate
| Aneurysm location | n | % |
|---|---|---|
| ICA cavernous segment | 13 | 3.1 |
| ICA ophthalmic segment | 22 | 5.2 |
| Superior hypophyseal artery | 6 | 1.4 |
| ICA PcomA | 69 | 16.4 |
| ICA supra- /paraclinoidal | 1 | 0.2 |
| ICA AchoA | 8 | 1.9 |
| ICA bifurcation | 17 | 4.0 |
| Proximal MCA (M1-segment) | 9 | 2.1 |
| MCA bifurcation | 55 | 13.1 |
| Distal MCA (M2-segment) | 1 | 0.2 |
| Proximal ACA (A1-segment) | 2 | 0.5 |
| AcomA | 112 | 26.6 |
| Distal ACA | 4 | 1.0 |
| Vertebral artery | 5 | 1.2 |
| PICA | 12 | 2.9 |
| Superior cerebellar artery | 11 | 2.6 |
| Basilar trunk | 8 | 1.9 |
| Basilar tip | 54 | 12.8 |
| Proximal PCA (P1-segment) | 8 | 1.9 |
| Distal PCA (P2-segment) | 4 | 1.0 |
| 421 | 100 | |
The following variables turned out to have a significant influence (p ≤ 0.05) on the event "occlusion rate of the 1st treatment session ≥ 90%" (table 11).
The odds ratio of F-coil (3.8) is very close to the odds ratio of the matched pairs analysis (3.4, see below).
b) Matched Pairs Analysis
Taking the variables "aneurysm location", "neck diameter", and "fundus diameter" into account, a total of 421 aneurysm pairs were matched. Tables 12A, B, and C describe these matched pairs in terms of their locations (by frequency), neck- and fundus dimensions by means of descriptive statistics.
Table 12B.
Descriptive statistics of the neck width of 421 aneurysms, matched as pairs concerning treatment with bare or fibered coils
| valid n | mean ± SD | median | min-max | |
|---|---|---|---|---|
| Neck width (mm) |
421 | 3.3 ± 1.9 | 3 | 1-13 |
Table 12C.
Grouping of 421 aneurysms, matched as pairs concerning treatment with bare or fibered coils, concerning the fundus diameters
| fundus (mm) | n | (%) |
|---|---|---|
| ≤ 4 | 99 | 23.5 |
| 5-7 | 131 | 31.1 |
| 8-14 | 142 | 33.7 |
| 15-25 | 43 | 10.2 |
| ≤ 25 | 6 | 1.4 |
| Total | 421 | 100 |
The results of the matched pairs analysis for the occlusion rate being < 90% or 90-100% are given in table 13.
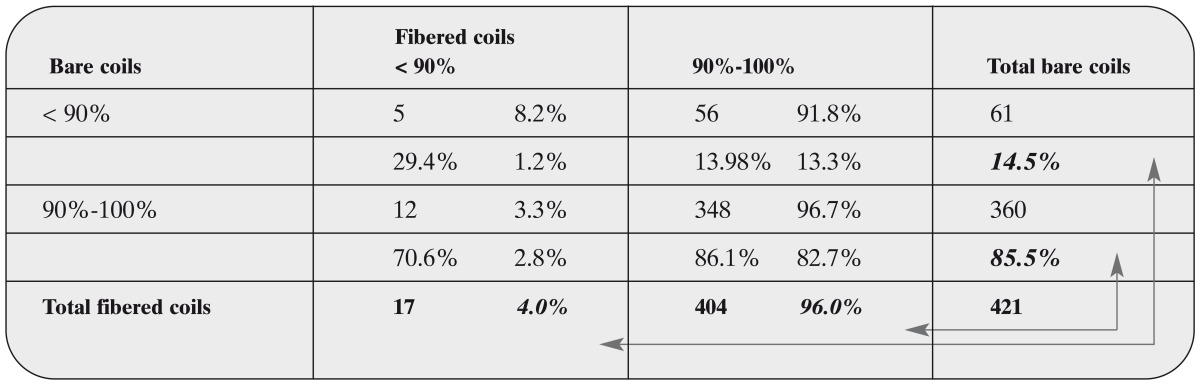
Table 13.
Matched pairs analysis of the occlusion rate of the first treatment session, comparing occlusion rates <90% vs. 90 100% and treatments with bare versus fibered coils and occlusion rate
How to read table 13: The percentage right of the absolute figure is with respect to the row, i.e. with respect to bare coils. It describes, how many of the aneurysms treated only with bare coils would exhibit the same outcome, had they been when treated with fibered coils. E.g. bare coil treatments resulted in an occlusion rate of < 90% in 61 cases. 91.8% of these cases would have exhibited an occlusion rate of 90%-100% if they had been treated with fibered coils and 8.2% of these would still show the same exclusion rate of < 90% with fibered coils.
The percentage beneath the absolute figure is with respect to the column, i.e. with respect to fi- bered coils. Again, 404 treatments with fibered coils resulted in an occlusion rate of 90%-100%. 86.1% of these treatments would have had the same occlusion rate when treated with bare coils, whereas 13.9% would have had a lower occlusion rate when treated with bare coils.
The percentages printed in bold and italic refer to the number of treatments with the same or a different occlusion rate respectively when a different coil hat been taken as indicated by the arrows. The third percentage in the lower right corner of each segment is with respect to the total number of treatments.
When treated with fibered coils, 96.0% of the aneurysms exhibited an occlusion rate of 90%-100% whereas only 85.5% of the aneu- rysms treated with bare coils showed this occlusion rate. These shares differ significantly (p = 0.000, binomial test). The odds ratio for 90-100% occlusion rate of fibered coils vs. bare coils equals 3.4 (95%-confidence interval: [2.8 - 4.0], p = 0.000). This confirms the results of the logistic regression approach.
Multivariate Analysis – Coil Compaction
a) Logistic Regression
Backward selection was used to find those variables that had a significant influence on the probability of the event "coil compaction". The variables "diameter", "previous rupture" and some of the locations had a significant effect on the probability of coil compaction. The use of fibered coils had no significant influence on the probability of coil compaction (p = 0.3885). All other selected factors had a positive effect on the probability of coil compaction (see tables 14 and 15 for detailed results
Table 14.
Results of the Logistic Regression for the Event “Coil Compaction”
| Coil compaction | Odds Ratio | p-value | 95% Confidence Interval | |
|---|---|---|---|---|
| Fundus diameter | 1.05 | 0.000 | 1.03 | 1.08 |
| Previous rupture | 1.91 | 0.000 | 1.37 | 2.66 |
| Aneurysm locations: | ||||
| ACA A1 segment | 5.96 | 0.011 | 1.51 | 23.47 |
| Vertebro-basilar junction | 3.29 | 0.040 | 1.05 | 10.26 |
| ICA cavernous segment | 4.25 | 0.001 | 1.75 | 10.34 |
| ICA ophthalmic segment | 4.44 | 0.000 | 2.09 | 9.43 |
| ICA PcomA | 2.16 | 0.035 | 1.05 | 4.41 |
| ICA ant. choroidal artery | 7.58 | 0.000 | 2.79 | 20.60 |
| ICA bifurcation | 3.35 | 0.016 | 1.25 | 8.97 |
| MCA bifurcation | 3.69 | 0.000 | 1.87 | 7.31 |
| AcomA | 2.73 | 0.002 | 1.43 | 5.23 |
| Vertebral artery | 8.17 | 0.000 | 2.80 | 23.79 |
| ICA supraclinoid segment | 4.32 | 0.023 | 1.23 | 15.19 |
| Sup. cerebellar artery | 2.67 | 0.033 | 1.08 | 6.60 |
| Basilar trunk | 3.63 | 0.010 | 1.36 | 9.74 |
| Basilar tip | 2.53 | 0.007 | 1.29 | 4.95 |
Table 15A.
Frequency of locations of 44 aneurysms, matched as pairs concerning with respect to treatment with bare or fibered coils and coil compaction
| Aneurysm location | N | % |
|---|---|---|
| ICA petrous segment | 0 | 0 |
| ICA cavernous segment | 0 | 0 |
| Superior hypophyseal artery | 0 | 0 |
| ICA ophthalmic segment | 0 | 0 |
| ICA PcomA | 13 | 29.6 |
| ICA supra- / paraclinoidal | 0 | 0 |
| ICA AchoA | 1 | 2.3 |
| ICA bifurcation | 1 | 2.3 |
| Proximal MCA (M1-segment) | 1 | 2.3 |
| MCA bifurcation | 3 | 0 |
| Distal MCA (M2-segment) | 0 | 0 |
| Proximal ACA (A1-segment) | 0 | 0 |
| AcomA | 12 | 27.3 |
| Distal ACA | 0 | 0 |
| Vertebral artery | 1 | 2.3 |
| Vertebro-basilar junction | 0 | 0 |
| PICA | 0 | 0 |
| AICA | 0 | 0 |
| Superior cerebellar artery | 2 | 0 |
| Basilar trunk | 0 | 0 |
| Basilar tip | 9 | 20.5 |
| Proximal PCA (P1-segment) | 1 | 2.3 |
| Distal PCA (P2-segment) | 0 | 0 |
| n = 44 | 100% | |
The following variables turned out to have a significant influence (p ≤ 0.05) on the event "coil compaction" (table 14).
b) Matched Pairs Analysis
For the matched pairs analysis of coil compaction, the following variables had been used for matching: "aneurysms location", "neck width" with tolerance of 2 mm, "fundus diameter" with a grouping of ≤ 4 mm, 5-7 mm, 8-14 mm, 15-24 mm, ≥ 25 and "previous rupture". This procedure resulted in 275 matched pairs. Unfortunately, in only 44 of these pairs was angiographic follow-up available. Tables 15A, B, and C describe these matched pairs in terms of their location (by frequency), neck and fundus dimensions by means of descriptive statistics.
Table 15B.
Descriptive statistics of the neck width of 44 aneurysms, matched as pairs concerning treatment with bare or fibered coils and coil compaction
| valid n | mean ± SD | median | min-max | |
|---|---|---|---|---|
| Neck (mm) | 44 | 3.0 ± 1.5 | 3 | 1-7 |
Table 15C.
Grouping of 44 aneurysms, matched as pairs concerning treatment with bare or fibered coils, concerning the fundus diameters and coil compaction.
| Fundus diameter mm | N | (%) |
|---|---|---|
| < = 4 | 8 | 18.2 |
| 5-7 | 19 | 43.2 |
| 8.-14 | 12 | 27.3 |
| 15-25 | 5 | 11.4 |
| > = 25 | 0 | 0 |
| Total | 44 | 100 |
Table 16.
Matched pairs analysis of the occurrence of coil compaction after the first treatment session, comparing occlusion treatments with bare versus fibered coils and occurrence of coil compaction
Previous rupture had been documented in 38 out of 44 (86.4%) of these matched pairs.
When treated with fibered coils 22.7% of the matched pairs do show coil compaction. The respective number is 29.6% after treatment with bare coils. These fractions do not differ significantly (p = 0.2814, binomial test).
Summary of the Results of the Multivariate Analysis
Occlusion Rate
The variables "use of fibered-coils", "fundus diameter", "neck width" and the "location at the A1 segment" had a significant effect on the probability of a high occlusion rate. Larger neck width and fundus diameter are negatively correlated with the occlusion rate: the larger the neck width, the smaller is the probability that the occlusion rate is at least 90% with a threshold value of 4 mm.
A neck width of less than or equal to 4 mm accounted for a positive odds ratio for 90-100% occlusion rate (table 11). A positive odds ratio for 90-100% occlusion was seen for fundus diameters between 1 and 14 mm with the highest value (2.42) for the range of 5-7 mm. Additionally, A1-aneurysms showed a lower probability of a high occlusion rate than aneurysms which were located elsewhere (0.31, p = 0.027) (see table 11 and 12 for detailed results and comments). In comparison with bare coils, the use of fibered coils significantly increased the probability of an occlusion rate of at least 90% (3.8, p = 0.000). It is noteworthy that the use of fibered coils has the highest positive odds ratio for an occlusion rate of 90-100%, outweighed only by the negatively correlated variables "neck width greater than 7 mm" (See table 11 above).
The odds for ratio for a 90-100% occlusion rate of the use of fibered coils (3.8) is very close to the odds ratio of the matched pairs analysis (3.4).
Matched pairs analysis yields to an occlusion rate of 90%-100% for 96.0% of the aneurysms treated with fibered coils whereas 85.5% of the aneurysms treated with bare coils show this occlusion rate. These shares differ significantly (p = 0.000, binomial test). The odds ratio of "fibered coils" vs. "bare coils" equals 3.4 (95%-confi- dence intervall: [2.8-4.0], p = 0.000). This confirms the logistic regression result.
Coil Compaction
The following variables turned out to have a significant influence (p ≤ 0.05) on the event "coil compaction" (table 17).
Table 17.
Results of the Logistic Regression for the Event “Coil Compaction”
| coil compaction | Odds Ratio | p-value | 95% Confidence Interval | |
|---|---|---|---|---|
| Fundus diameter | 1.05 | 0.000 | 1.03 | 1.08 |
| Previous rupture | 1.91 | 0.000 | 1.37 | 2.66 |
| Aneurysm locations: | ||||
| ACA A1 segment | 5.96 | 0.011 | 1.51 | 23.47 |
| Vertebro-basilar junction | 3.29 | 0.040 | 1.05 | 10.26 |
| ICA cavernous segment | 4.25 | 0.001 | 1.75 | 10.34 |
| ICA ophthalmic segment | 4.44 | 0.000 | 2.09 | 9.43 |
| ICA PcomA | 2.16 | 0.035 | 1.05 | 4.41 |
| ICA ant. choroidal artery | 7.58 | 0.000 | 2.79 | 20.60 |
| ICA bifurcation | 3.35 | 0.016 | 1.25 | 8.97 |
| MCA bifurcation | 3.69 | 0.000 | 1.87 | 7.31 |
| AcomA | 2.73 | 0.002 | 1.43 | 5.23 |
| Vertebral artery | 8.17 | 0.000 | 2.80 | 23.79 |
| ICA supraclinoid segment | 4.32 | 0.023 | 1.23 | 15.19 |
| Sup. cerebellar artery | 2.67 | 0.033 | 1.08 | 6.60 |
| Basilar trunk | 3.63 | 0.010 | 1.36 | 9.74 |
| Basilar tip | 2.53 | 0.007 | 1.29 | 4.95 |
For the matched pairs analysis of coil compaction, the following variables had been used for matching: "aneurysms location", "neck width" with tolerance of 2 mm, "fundus diameter" with a grouping of ≤ 4 mm, 5-7 mm, 8-14 mm, 15-24 mm, ≥ 25, and "previous rupture". This procedure resulted in 275 matched pairs but only for 44 of these aneurysm pairs angio- graphic follow-up data were available. When treated with fibered coils, 22.7% of the matched pairs do show coil compaction. The respective number is 29.6% after treatment with bare coils. These shares do not differ significantly (p = 0.2814, binomial test).
Discussion
Early descriptions of induced thrombosis for therapeutic occlusion of aneurysms by direct needle puncture and introduction of metallic wires or foreign bodies into the aneurysm date back as far as 1831. Generally, thrombogenicity of the embolic agent was considered to be beneficial 14,15. Together with the introduction of electrolytically detachable platinum coils for the endovascular treatment of intracranial aneu- rysms in 1991 2,3, numerous modifications of coil properties, materials, and especially coil surfaces have been proposed in order to improve early and long term occlusion rates 7,8,16-27. The concept of electrothrombosis (i.e., rapid thrombus formation induced by the application of a direct electric current) as a leading mechanism for aneurysm occlusion remained unconfirmed in animal studies28 and clinical practice 29. In fact, there is evidence that other mechanisms such as alteration of intra-aneurysmal flow, architecture and size of the aneurysm, coil packing density and coil surface properties are the main factors determining both short and long term occlusion rates. Furthermore, studies have shown that there is no significant difference between the results with electrolytically detached coil systems and systems that use other detachment mechanisms such as mechanical, elec- trothermic, or hydrodynamically pressure me- diated30-33.
In order to promote thrombus formation, to inhibit reperfusion, and to facilitate connective tissue formation and neointima proliferation across the neck, a number of strategies to alter the properties of the coils have been subject to in vitro studies, animal models, and clinical trials. We review some of these strategies in relation to our experiences with electrolytically detachable fibered platinum coils.
Improvement of mechanical properties: One of the main predictors of short term occlusion and prevention of reperfusion that is generally accepted to date is packing density. Increasing amounts of intra-aneurysmal platinum coils subsequently lead to reduction of the intra- aneurysmal pulse pressure amplitude, blood flow, and transmural pressure 34-36. In order to address this, modifications of coil shape and softness have been made by different manufacturers aiming for more dense packing of the aneurysm37. Although we did not calculate packing densities as a function of intra-aneu- rysmal volume displaced by platinum, the unchanged mechanical properties of the fibered coils when compared to their bare counterpart should allow the same packing density. Although on one hand it should be possible to produce fibered soft or ultrasoft coils, on the other hand further increase in packing density in the presence of fibered coils might have negligible influence on mid- and long-term recurrence rates. Early total occlusion due to a higher thrombogenicity with softer coils could actually lead to higher rates of recurrence secondary to coil compaction. However, this is hypothetical and remains to be proven, preferably by a prospective study with limited additional variables.
Increased thrombogenicity and improvement of early clot formation: Graves et Al27 have compared the thrombogenicty of pre-GDC simple curved coils, complex curved coils, and coils with silk fibers in a canine model with wide necked saccular aneurysms. In this experiment, only fibered coils exhibited significant thrombus formation and spatial stability.
Casasco et Al9 reported a series of 71 selected aneurysms that were treated with Dacron fibered platinum push coils gaining relatively high occlusion rates of 84.5% complete and 15.5% of 90-100% occlusion. A three month follow-up study was obtained in 54 cases, revealing three early recurrences but none after re-treatment. Besides the notably high occlusion rates, there was a remarkably highnumber of throm- boembolic events (with a morbidity of 4.2% and mortality of 11.3% due to major stroke).
A smaller series of 12 giant aneurysms treated with fibered coils is reported by Habboub12. He achieved 11 complete occlusions, and reported two thromboembolic complications.
A report of four cases of "high-flow neuro- vascular disorders" treated with varying numbers of electrolytically detachable Dacron-fibe- red GDC-coils, used alone in one case, or in conjunction with standard GDCs in another and with fibered pushable coils in two other cases was presented in 1998 by Halbach et Al38. The authors had used fibered coils specifically to treat "high flow situations". A case of a bilateral carotid cavernous fistula was successfully treated after three previous attempts with bare coils had failed in the same institution. The authors emphasize the increased safety of the detachment system. "In all four cases, a fibered electrolytically detachable coil was initially pushed to a position judged to be less than ideal and later repositioned into a more desirable site before being detached. This maneuver was accomplished quickly, with no evidence of thrombus formation and no increase in friction". However, the overall friction with this specific type of coil was judged to be considerably higher than with bare coils but also depended on the type of microcatheter used. No distal embolic occlusions were seen in four cases.
Bank11 used GDC fibered vortex coils for in- tracranial aneurysms in 60 out of 98 consecutive cases and reported a complete obliteration at six month follow-up in these cases even if the coil mesh initially appeared to be "loosely packed".
The important difference between the Nylon fibered Sapphire Detachable coils and the Dacron fibered GDC-coils used in most of the studies mentioned above is that in our experience they do not exhibit a noticeable increase in microcatheter friction, independent of effective coil length. Otherwise, we share a lot of the experiences reported in these publications, particularly the observation by Bank 11 that loose packing in the presence of fibered coils might lead to a surprisingly good follow-up result is exemplified by case-studies 1 and 2.
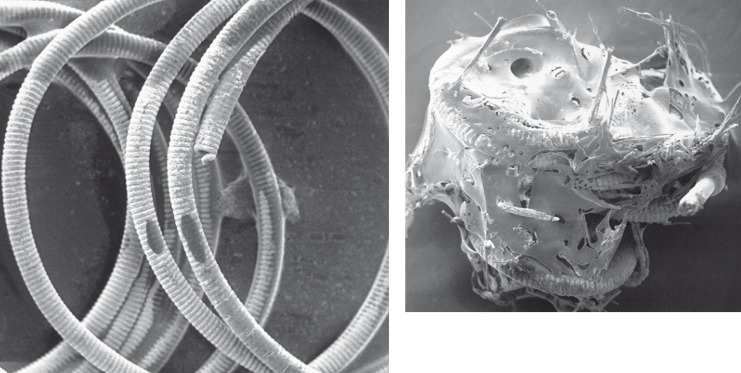
We did not observe an increased rate of thromboembolic complications in cases where fibered coils had been used. We hypothesize that the apparent trend towards a lower throm- boembolic rate in cases with fibered coils may be due to a reduction of thrombus dislodge- ment from the aneurysm by a "clot stabilizing effect" of the fibers interspersed into the thrombus. This is nicely demonstrated by electron microscopic photographs in figure 4 depicting a bare platinum and a fibered coil that were both exposed to human blood for 3 min with application of a 0.1 mA / 4 V DC-current.
Figure 4.
Electron microscopic image of a bare (left) andfibered (right) coil after exposure to blood for 3 min with application of 0.1 mA / 4 V DC.
The relatively high number of severe throm- boembolic events reported by Casasco 9 might be related to the much higher thrombogenicity of Dacron fibers when compared to Nylon or other surgical suture materials that have been reported by others in the past39-41.
Promotion of an inflammatory reaction, permanent scar or intraaneurysmal connective tissue formation, and neointimal bridging across the neck: A number of different investigators have modified electrolytically detachable coils by applying extracellular matrix proteins,growth factors, and polymers 6,19,21,42,43. In contrast to the type of fibered coils used in this study, most of these modifications lead to significant changes in the mechanical properties of the coil systems and altered their shape, trackability, and softness 6 as well as the coil thickness 19. Another problem that has been mentioned in these publications is that the coil coating could be stripped off during coil delivery and may become a potential source of thromboembolic complications.
A study published by Szikora et Al reports the initial experiences with collagen-filled GD- Cs that did not show improvement of the angio- graphic results. Besides, the modification again led to increased friction and coil stiffness 26. More recent studies suggest that problematic alterations of mechanical properties of protein- coated coils might be overcome by means of different ways of manufacturing such as ion implantation 24. It can be expected that some of these drawbacks will be overcome with further development of the coil systems.
Promotion of an inflammatory reaction by Nylon: Even though we are unable to directly prove that the attachment of Nylon fibers to platinum coils that are placed inside the vascular system, specifically inside an intracranial aneurysm, induces an inflammatory reaction that subsequently leads to the desired effect of a mild and prolonged inflammation with ingrowth of fibroblasts and collagen deposition, this effect has been observed by a number of authors in different locations in the past. The variety of applicable publications concerning tissue response or foreign body reaction secondary to incorporated or implanted Nylon is dominated by histological studies on surgical suture material. Most authors report moderate inflammatory reactions that appeared to be prolonged in comparison to biodegradable su- tures44. Kaletsch et Al have compared absor- bable and non-absorbable suture materials in microsurgical end-to-end-suture of the aorta in rats. The anastomoses were examined by light- and scanning electron microscopy. Both types of materials have shown a similar neo-intima. Non-absorbable suture materials, including Nylon, have shown greater tissue reactions which were also more persistent45. Witschel and Faul- born found that in contrast to Silicone implants, Silastic sponges, and Mersilene bands that were very well tolerated by the eye, Nylon threads caused a moderate and prolonged granuloma- tous inflammation 46.
In order to assess tissue reactivity to synthetic suture material in microsurgery, Ussia and coworkers conducted a comparative histologi- cal study in rabbits. The aorta of the animals was sectioned and subsequently anastomosed using polyglycolic acid in the first group and monofilament Nylon in the second group. Tissue reactivity during the initial 40 days had similar histological characteristics in both groups, evident as a peri-suture granuloma with ma- crophages and giant cells. In the second period, 40 to 90 days after surgery, only Nylon caused an inflammatory process that persisted due to the presence of non-absorbable monofilament, thus causing a fibrosclerotic transformation of the vessel wall47.
Using ultra thin Nylon threads in ocular surgery, Jongebloed et Al found that in contrast to stainless steel sutures an extensive tissue reaction could be observed around Nylon sutures within a few days and was still present after 4-6 weeks. On the endothelial side, covering of the Nylon with fibroblasts took place very slowly, this in contrast with stainless steel. Irritation of the tissue and an edematous appearance of the endothelium around the Nylon suture was the result. At first, a kind of collagen network, often mixed with inflammatory cells, was deposited on the Nylon material followed by the ingrowth of fibroblasts 48. A more recent paper by Gorbet et Al reports the result of flow cyto- metric measurements of neutrophil activation by foreign materials. All materials tested (cellophane, an acrylonitrile copolymer (AN69), Pel- lethane, Nylon, polyethylene terephthalate, low density polyethylene, and polydimethylsilox- ane) activated isolated human neutrophils.
The results suggest that upon contact with a material, neutrophil activation may occur through mechanisms that are not mediated by complement but the presence of plasma proteins such as fibrinogen at the interface which in turn may trigger activation and the release of other activating agents 49.
A study published in 1987 reported that after implantation of three different polymeric composite materials in the rat middle ear, a Nylon prosthesis was encapsulated more rapidly than the two polymethyl methacrylate based implants. Several PMMA based implants were only partially covered at the six month stage by a tenuous capsule made up of collagen and a mixed inflammatory cell infiltrate. The Nylon material was covered at the three months stage with a thin capsule and healthy fibroblasts 50.
However, other authors have reported that Nylon causes only mild inflammatory reactions when compared with silk, polyester, Perlon or even Dacron 51. These data were obtained from surgical implants or sutures in different parts of the body and overall suggest that silk causes the most intense inflammatory or foreign body reactions followed by Dacron. For intravascu- lar implants, comparable data exist only for Dacron and Gore-Tex. Dacron is known to sometimes provoke extensive peri-implant reactions when used for stent coverage that can lead to early stent obstruction 39,40,41, which, as mentioned above, might explain the differences between our own data and the experience reported by Casasco 9.
With the applied statistics we have proven that fibered electrolytically detachable platinum coils have a significant influence on the percentage of high (90-100%) occlusion rates in the initial, and second treatment session with an absolute improvement of roughly 10% in the group treated with fibered coils. The odds ratio for the event "occlusion greater than 90%" for the use of fibered coils was 3.4 in the matched pairs analysis and 3.8 with logistic regression, suggesting that the use of fibered coils has a high predictive value for a high occlusion rate. The fact that the amount of fibered coils used did not show a significant impact on the occlusion rate is likely to be due to the relatively small number of aneurysms (n = 31) that were treated with more than 80% fibered coils. Coil distribution inside the aneurysm may also be important. This aspect was not included in our statistical evaluation, though the sequence of coils is documented during the treatment sessions.
We could confirm a significant difference between the two groups with respect to postpro- cedural neurologic deficit and patient outcome. However, we could see a trend towards a reduced rate of recurrence secondary to coil compaction in the follow-up studies that were available at the time of this evaluation. These apparently improved mid-term occlusion rates might be related to the mechanical properties of fibered coils, which were only available as standard but not as soft coils. In comparison, the sole use of soft or ultrasoft fibered coils might be ambiguous. On one hand this would facilitate the concept of dense packing while holding the threat of coil compaction and early aneurysm recurrence on the other.
The already mentioned trend towards a reduction in the rate of thromboembolic events must be related to the fibers that are interspersed among the intraaneurysmal thrombus mass, thus stabilizing it.
Despite these encouraging results, we are aware that this study is subject to several limitations, only partly avoidable. The key parameters (e.g., aneurysm neck width, fundus diameter, degree of occlusion, nature of recurrence such as coil compaction) can only be assessed visually in a semi quantitative way of comparing aneurysmal diameter and neck width to the estimated diameter of neighboring vessels. This indeed may open a controversy on the reliability of the results. The angiograms and patient files were evaluated by two cooperating investigators (HH, SF). This was in the aim of improving the consistency of the evaluations, but could also have introduced bias.
Detachable fibered coils are provided only in a limited range of diameter and length. Large (≥ 4 mm), long (> 10 cm) or soft fibered coils are unfortunately not yet offered. Therefore, fibered coils were used for less than 50% of the total coil length in many aneurysms.
Other factors that might also have influenced our results and that cannot be completely controlled include the individual experience and the level of training and skills of the operator performing a procedure. This supposedly is of greater importance than apparently minor aspects such as the type of microcatheter used, and the actual geometry of the aneurysm. These and maybe other less obvious factors might also have contributed to both the early and long term results.
From a methodological point of view, the key limitation of this study is related to the fact that the two treatment subgroups (aneurysms treated with bare versus fibered coils) are different in many aspects. Aneurysm treatments with bare coils date back until 1992 whereas fibered coils have been used since 1999. Different operators used bare or fibered coils according to their personal preferences. In an ideal study a single operator would randomly treat aneu- rysms with either bare or fibered coils, having all possible dimensions and coil types available while using the same type of microcatheter for all procedures. Patients would undergo angio- graphic follow-up examinations after six, 12 and 24 months.
For obvious reasons such a study would cause logistic difficulties and quite a large number of patients would be required. With this in mind our data analysis may be regarded as a compromise dictated by the restrictions of clinical practice.
Conclusions
Fibered coils are more thrombogenic than bare coils, though thrombus formation will also occur with bare platinum coils.
This is due both to the surface properties of the coils and to the mechanical effect of coils on the intraaneurysmal haemodynamics, ultimately resulting in complete stagnation of blood flow. Thrombus around bare coils may form a more fragile mass, that can easily be dislodged from the aneurysmal sac either by the blood flow or by subsequent coil introduction. Interspersed fibers may act as a clot stabilizer and could therefore decrease the risk of thrombus displacement out of the aneurysm.
Apart from the fact that the design of these coils does not noticeably alter their mechanical properties or increase microcatheter friction, the lower thrombogenicity of Nylon fibered coils as opposed to dacron fibers might be beneficial in terms of a reduced rate of throm- boembolic events.
The induction of an intraaneurysmal moderate and prolonged inflammatory reaction is hypothetical, however, it can be based on a number of reports on short-, mid-, and long-term histological examinations after intravascular placement of Nylon threads and might account for better long-term occlusion rates. As we increase the number of follow-up studies, we hope to prove to statistical significance that the use of fibered coils is beneficial in terms of fewer recurrences due to coil compaction. At the beginning of our practice, the main indications for combining bare platinum with fibered coils included parent vessel occlusion, occlusion of large and/or wide necked aneurysms that appeared to be prone to recurrence, and occlusion of periprocedural rupture sites. Today, as a result of the experiences presented herein, we use fibered coils whenever technically feasible.
References
- 1.Piton J, Billerey J, et al. Embolization par courant électrique continu: ECEC. Application thérapeutic. J Radiol. 1979;80:799–808. [PubMed] [Google Scholar]
- 2.Guglielmi G, Vifluela F, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique and experimental results. J Neurosurg. 1991;75:1–7. doi: 10.3171/jns.1991.75.1.0001. [DOI] [PubMed] [Google Scholar]
- 3.Guglielmi G, Vifluela F, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous, (International Subarachnoid Aneurysm Trial - ISAT Collaborative Group) International Sub- arachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 5.Murayama Y, Nien YL, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98:959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja A, Hergenrother RW, et al. Platinum coil coating to increase thrombogenicity: a preliminary study in rabbits. Am J Neuroradiol. 1993;14:794–798. [PMC free article] [PubMed] [Google Scholar]
- 7.Kallmes DF, Fujiwara NH. New expandable hydrogel- platinum coil hybrid device for aneurysm embolization. Am J Neuroradiol. 2002;23:1580–1588. [PMC free article] [PubMed] [Google Scholar]
- 8.Murayama Y, Vifluela F, et al. Bioabsorbable polymeric material coils for embolization of intracranial aneurysms: a preliminary experimental study. J Neurosurg. 2001;94:454–463. doi: 10.3171/jns.2001.94.3.0454. [DOI] [PubMed] [Google Scholar]
- 9.Casasco AE, Aymard A, et al. Selective endovascular treatment of 71 intracranial aneurysms with platinum coils. J Neurosurg. 1993;79:3–10. doi: 10.3171/jns.1993.79.1.0003. [DOI] [PubMed] [Google Scholar]
- 10.Hilal SK, Khandji AG, et al. Synthethic fiber-coated platinum coils successfully used for endovascular treatment of arteriovenous malformations, aneurysms and direct arteriovenous fistulas of the CNS. Am J Neuroradiol. 1988;9:1030. [Google Scholar]
- 11.Bank WO. The use of GDC fibered vortex coils for intracranial aneurysms. J Neuroradiol. 2002;29:1S94–O-325. [Google Scholar]
- 12.Habboub H. Fibered coils in the management of giant intracranial aneurysms: thromboembolic incidence. J Neuroradiol. 2002;29:1S57–O-172. [Google Scholar]
- 13.Jennett B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet. 1975;7905:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 14.Philips B. Series of experiments for purpose of showing that arteries may be obliterated without ligature, compression, or knife. Med Gaz. 499:1831. [Google Scholar]
- 15.Gallagher JP. Pilojection for intracranial aneurysms. Report of progress. J Neurosurg. 1964;21:129–134. doi: 10.3171/jns.1964.21.2.0129. [DOI] [PubMed] [Google Scholar]
- 16.Abrahams JM, Forman MS, et al. Biodegradable polyglycolide endovascular coils promote wall thickening and drug delivery in a rat aneurysm model. Neurosurgery. 2001;49:1187–1195. [PubMed] [Google Scholar]
- 17.Abrahams JM, Diamond SL, et al. Topic review: surface modifications enhancing biological activity of Guglielmi detachable coils in treating intracranial aneurysms. Surg Neurol. 2000;54:34–41. doi: 10.1016/s0090-3019(00)00269-x. [DOI] [PubMed] [Google Scholar]
- 18.Abrahams JM, Forman MS, et al. Delivery of human vascular endothelial growth factor with platinum coils enhances wall thickening and coil impregnation in a rat aneurysm model. Am J Neuroradiol. 2001;22:1410–1417. [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson RC, Krisht AF, et al. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery. 1995;36:133–140. doi: 10.1227/00006123-199501000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Kallmes DF, Fujiwara NH, et al. A collagen-based coil for embolization of saccular aneurysms in a new zealand white rabbit model. Am J Neuroradiol. 2003;24:591–596. [PMC free article] [PubMed] [Google Scholar]
- 21.Kallmes DF, Borland MK, et al. In vitro proliferation and adhesion of basic fibroblast growth factor-producing fibroblasts on platinum coils. Radiology. 1998;206:237–243. doi: 10.1148/radiology.206.1.9423678. [DOI] [PubMed] [Google Scholar]
- 22.Murayama Y, Vifluela F, et al. Cellular responses of bioabsorbable polymeric material and Guglielmi detachable coil in experimental aneurysms. Stroke. 2002;33:1120–1128. doi: 10.1161/01.str.0000014423.20476.ee. [DOI] [PubMed] [Google Scholar]
- 23.Murayama Y, Vifluela F, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery. 1997;40:1233–1244. doi: 10.1097/00006123-199706000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Murayama Y, Vifluela F, et al. Development of the biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part II: An experimental study in a swine aneurysm model. Am J Neuroradiol. 1999;20:1992–1999. [PMC free article] [PubMed] [Google Scholar]
- 25.Raymond J, Desfaits AC, Roy D. Fibrinogen and vascular smooth muscle cell grafts promote healing of experimental aneurysms treated by embolization. Stroke. 1999;30:1657–1664. doi: 10.1161/01.str.30.8.1657. [DOI] [PubMed] [Google Scholar]
- 26.Szikora I, Wakhloo AK, et al. Initial experience with collagen-filled Guglielmi detachable coils for endovascular treatment of experimental aneurysms. Am J Neuroradiol. 1997;18:667–670. [PMC free article] [PubMed] [Google Scholar]
- 27.Graves VB, Partington CR, et al. Treatment of carotid artery aneurysms with platinum coils: An experimental study in dogs. Am J Neuroradiol. 1990;11:249–252. [PMC free article] [PubMed] [Google Scholar]
- 28.Reul J, Spetzger U, et al. The nature of early intraluminal thrombosis in terminal aneurysms occluded with guglielmi detachable coils. Interventional Neuroradiology. 1998;4:39–48. doi: 10.1177/159101999800400104. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz M, Samson D, Purdy P. Does electrothrombosis occur immediately after embolisation of an aneurysm with guglielmi detachable coils? Am J Neuroradiol. 1997;18:510–513. [PMC free article] [PubMed] [Google Scholar]
- 30.Lylyk P, Cerrato R, et al. Evolution of the mechanical treatment of intracranial aneurysms: the contribution of different coils. 5th joint meeting annual meeting of the AANS/CNS section on cerebrovascular surgery and the American Society of Interventional and Therapeutic Neuroradiology. Vol. 141. Addison (Dallas), TX, USA: Program Book, Poster; 2002. Feb, p. 78. [Google Scholar]
- 31.Cekirge HS, Saatci I, et al. Interlocking detachable coil occlusion in the endovascular treatment of intracranial aneurysms: preliminary results. Am J Neuroradiol. 1996;17:1651–1657. [PMC free article] [PubMed] [Google Scholar]
- 32.Koizumi K, Kawano T, et al. Histological findings in aneurysm treated with IDC: scanning electron microscopical study (Japanese, English abstract) Surg Neurol. 1997;25:1027–1031. [PubMed] [Google Scholar]
- 33.Manabe H, Fujita S, et al. Embolization of ruptured cerebral aneurysms with interlocking detachable coils in acute stage. Interventional Neuroradiology. 1997;3:49–63. doi: 10.1177/159101999700300106. [DOI] [PubMed] [Google Scholar]
- 34.Stiver SI, Porter PJ, et al. Acute human histopathology of an intracranial aneurysm treated using guglielmi detachable coils: case report and review of the literature. Neurosurgery. 1998;43:1203–1208. doi: 10.1097/00006123-199811000-00106. [DOI] [PubMed] [Google Scholar]
- 35.Sorteberg A, Sorteberg W, et al. Effect of Guglielmi detachable coil placement on intraaneurysmal pressure: experimental study in canines. Am J Neuroradiol. 2001;22:1750–1756. [PMC free article] [PubMed] [Google Scholar]
- 36.Sorteberg A, Sorteberg W, et al. Effect of Guglielmi detachable coil placement on intraaneurysmal flow: experimental study in canines. Am J Neuroradiol. 2002;23:288–294. [PMC free article] [PubMed] [Google Scholar]
- 37.Kawanabe Y, Sadato A, et al. Endovascular occlusion of intracranial aneurysms with Gulglielmi detachable coils: correlation between coil packing density and coil compaction. Acta Neurochirugica. 2001;143:451–455. doi: 10.1007/s007010170073. [DOI] [PubMed] [Google Scholar]
- 38.Halbach V, Dowd CF, et al. Preliminary experience with an electrolytically detachable fibered coil. Am J Neuroradiol. 1998;19:773–777. [PMC free article] [PubMed] [Google Scholar]
- 39.Schurmann K, Vorwerk D, et al. Perigraft inflammation due to Dacron-covered stent-grafts in sheep iliac arteries: correlation of MR imaging and histopathologic findings. Radiology. 1997;204:757–763. doi: 10.1148/radiology.204.3.9280255. [DOI] [PubMed] [Google Scholar]
- 40.Link J, Feyerabend B, et al. Dacron-covered stentgrafts for the percutaneous treatment of carotid aneurysms: effectiveness and biocompatibility-experimental study in swine. Radiology. 1996;200:397–401. doi: 10.1148/radiology.200.2.8685332. [DOI] [PubMed] [Google Scholar]
- 41.Paquay YC, de Ruijter JE, et al. Tissue reaction to Dacron velour and titanium fibrefiber mesh used for anchorage of percutaneous devices. Biomaterials. 1996;17:1251–1256. doi: 10.1016/0142-9612(96)84946-5. [DOI] [PubMed] [Google Scholar]
- 42.Kallmes DF, Williams AD, et al. Platinum coil-mediated implantation of growth factor-secreting endovascular tissue grafts: an in vivo study. Radiology. 1998;207:519–523. doi: 10.1148/radiology.207.2.9577504. [DOI] [PubMed] [Google Scholar]
- 43.Tamatani S, Ogawa T, et al. Histological interaction of cultered endothelial cells and endovascular embolic materials coated with extracellular matrix. J Neurosurg. 1997;86:109–112. doi: 10.3171/jns.1997.86.1.0109. [DOI] [PubMed] [Google Scholar]
- 44.Delbeke LO, Gomel V, et al. Histologic reaction to four synthetic microsutures in the rabbit. Fertil Steril. 1983;40:248–252. doi: 10.1016/s0015-0282(16)47245-6. [DOI] [PubMed] [Google Scholar]
- 45.Kaletsch B, Rehm KE, Stambolis C. Tissue reaction in microvessel anastomoses. Comparative study of resorbable and nonresorbable suture material. Handchir Mikrochir Plast Chir. 1982;14:99–102. [PubMed] [Google Scholar]
- 46.Witschel H, Faulborn J. Reaction of ocular tissues to scleral-implant and encircling material: polyamide-silicone-polyester [article in German] Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;206:217–226. doi: 10.1007/BF02387333. [DOI] [PubMed] [Google Scholar]
- 47.Ussia G, Feldmann D, Galletti M. Histopathological aspects of tissue reactivity to suture materials in microsurgical arterial, anastomosis. Ital J Surg Sci. 1985;15:287–292. [PubMed] [Google Scholar]
- 48.Jongebloed WL, van der Veen G, Kalicharan D. Reaction of the rabbit corneal endothelium to nylon sutures. A SEM study. Doc Ophthalmol. 1990;75:351–358. doi: 10.1007/BF00164850. [DOI] [PubMed] [Google Scholar]
- 49.Gorbet MB, Yeo EL, Sefton MV. Flow cytometric study of in vitro neutrophil activation by biomedical implants. J Biomed Mater Res. 1999;44:289–297. doi: 10.1002/(sici)1097-4636(19990305)44:3<289::aid-jbm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 50.Williams KR, Blayney AW. Tissue response of several polymeric materials implanted in the rat middle ear. Biomaterials. 1987;8:254–258. doi: 10.1016/0142-9612(87)90112-8. [DOI] [PubMed] [Google Scholar]
- 51.Abi Rached RS, de Toledo BE, et al. Reaction of the human gingival tissue to different suture materials used in periodontal surgery. Braz Dent J. 1992;2:103–113. [PubMed] [Google Scholar]