Abstract
Epidemics of soil-borne plant disease are characterized by patchiness because of restricted dispersal of inoculum. The density of inoculum within disease patches depends on a sequence comprising local amplification during the parasitic phase followed by dispersal of inoculum by cultivation during the intercrop period. The mechanisms that control size, shape, and persistence have received very little rigorous attention in epidemiological theory. Here we derive a model for dispersal of inoculum in soil by cultivation that takes account into the discrete stochastic nature of the system in time and space. Two parameters, probability of movement and mean dispersal distance, characterize lateral dispersal of inoculum by cultivation. The dispersal parameters are used in combination with the characteristic area and dimensions of host plants to identify criteria that control the shape and size of disease patches. We derive a critical value for the probability of movement for the formation of cross-shaped patches and show that this is independent of the amount of inoculum. We examine the interaction between local amplification of inoculum by parasitic activity and subsequent dilution by dispersal and identify criteria whereby asymptomatic patches may persist as inoculum falls below a threshold necessary for symptoms to appear in the subsequent crop. The model is motivated by the spread of rhizomania, an economically important soil-borne disease of sugar beet. However, the results have broad applicability to a very wide range of diseases that survive as discrete units of inoculum. The application of the model to patch dynamics of weed seeds and local introductions of genetically modified seeds is also discussed.
The problem of the movement of infectious material is central to epidemiology. Many botanical epidemics are characterized by patchiness because of restricted dispersal of inoculum, particularly those of soil-borne fungal diseases, in which most transmission occurs between contiguous plants. New foci arise from occasional long-distance transmission of inoculum by water movement, wind, or humans. Transmission distances within existing patches are frequently small relative to the expansion of patches observed between seasons, suggesting that mechanical dispersal of inoculum during harvest and cultivation is important. For many soil-borne diseases, parasitic activity therefore contributes to local amplification of inoculum, whereas mechanical cultivation governs dispersal. The balance between amplification and dispersal of inoculum leads to characteristic patch sizes and to shapes that range from circular through ellipsoidal to cross- and kite-shaped patches. Redistribution by cultivation also leads to local dilution of inoculum within an expanded patch. Many plant diseases have a threshold concentration of inoculum below which symptoms are not apparent, although the disease continues to amplify within the host (1). Cultivation may therefore lead to the disappearance of patches in successive seasons if the density of inoculum within a patch falls below the critical threshold. It may also increase the period for which a field is “infectious” without the infection being apparent.
In this paper, we propose a model for dispersal of inoculum in soil by cultivation. The model, developed from work by Brain and Marshall (2) on dispersal of weed seeds, takes into account the discrete stochastic nature of the system in time and space. In particular, we identify two simple parameters to characterize lateral dispersal of inoculum by cultivation. We use the model to analyze the size, shape, and persistence of disease patches in fields. Specifically, we address the following key questions: (i) Under what conditions are symptomatic patches produced, given that inoculum is present? (ii) How should patch size be defined, or equivalently, what constitutes the boundary of a symptomatic patch? (iii) How do dispersal mechanisms give rise to circular, ellipsoidal, cross- and kite-shaped patches that are frequently observed for soil-borne disease?
To focus the analyses, the work in this paper is motivated by the problem of the spread of rhizomania in sugar beet crops (3, 4). The causative agent of this disease is beet necrotic yellow vein virus, which is transmitted by the propagules of the soil-borne fungus Polymyxa betae. These propagules, or cystosori, are 50 to 100 μm in diameter and are highly infectious. However, a critical level of infection is required to generate symptoms in the host (1). One practical consequence of such a threshold is that the spread of disease within a field is underestimated by the observation of symptoms. The models, however, can be applied to a very wide range of diseases that survive as discrete units of inoculum, comprising fungal resting spores, sclerotia, and nematode eggs or as mycelium, bacterial cells, or virus particles in previously colonized fragments of host tissue. Cultivation is broadly defined as any mechanical process that redistributes inoculum, which can be characterized by a probability of redispersal and mean dispersal. Analogies also exist between the dynamics of soil-borne disease patches and the appearance, detection, and expansion of weed patches after periods of amplification within a season and redistribution between seasons. Accordingly, the broader implications of the models are discussed for the dynamics of weed patches and for the persistence of small patches of feral or genetically modified plants when they are introduced to field crops accidentally or in trial plots.
Theory and Model
We assume that the individual units of inoculum are independent of each other. To examine the properties of the overall distribution of inoculum, it is first necessary to construct the probability density function (pdf) for a single unit. This function is derived by examining the transport processes responsible for dispersal.
As discussed in the Introduction, the main dispersal mechanism with respect to soil-borne diseases and seeds is through the agency of cultivation equipment. A model for dispersal of this kind is developed by Brain and Marshall (2), in which a method of cultivation is characterized by a dispersal function, g(x). The probability that a unit of inoculum is dragged to an interval (x, x + dx), is given by g(x)dx. It is natural to assume that the probability of a particle detaching itself from the machinery is independent of position. With this assumption, the process becomes Poisson in nature and gives a decaying exponential form for g(x). Marshall and Brain (5) propose a form
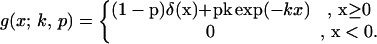 |
1 |
Particles are picked up by the cultivation implement with probability, p, and these are moved a mean distance of 1/k.
The position of a unit of inoculum in the field is represented by a probability distribution function, D(x, y). The pdf for an inoculum unit after cultivation is given by the convolution of the initial pdf with the characteristic function of the cultivation method,
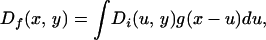 |
2 |
where cultivation is proceeding in the x direction. We can write the above convolution process by
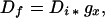 |
where the subscript, x, refers to the direction of cultivation. Brain and Marshall (2) discuss the fitting of this model to data. Here we use this description of the dispersal process as the basis of our analysis of inoculum redistribution.
The effect of successive cultivation passes can be modeled by successive convolutions with appropriate dispersal functions. As the convolution operation is associative (see Appendix A), it is possible to combine a sequence of cultivation procedures into one composite dispersal function, which can be applied to the initial inoculum pdf.
For present purposes, we assume that a field is subject to two mechanical cultivation events, or “passes,” between crops. Each of these events involves the dragging of the cultivating equipment across the field in a series of strips. As discussed in Appendix A, whether these strips overlap does not change the basic form of the distribution function. Successive cultivation passes are usually in different directions to avoid soil compaction, and these directions are usually approximately orthogonal, as dictated by the rectilinear nature of most fields. The means by which the theory is extended to nonorthogonal passes is summarized briefly at the end of Appendix A. We treat the two-pass case here as the simplest, which gives rise to two-dimensional redistribution and hence patterns.
The resulting dispersal function is derived in Appendix A. It has the form,
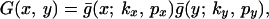 |
3 |
where the bar denotes a compound function involving multiple passes, and
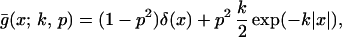 |
4 |
and subscripts from Eq. 3 indicate the direction of cultivation.
If the initial position of the particle is known, Di(x, y) = δ(x)δ(y), and Eq. 2 gives
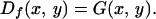 |
So if N inoculum units are initially found together at the origin, the pdf for each one will be G(x, y). The case in which the initial inoculum is located in a small patch is discussed in detail in Appendix B, which is published as supplemental data on the PNAS web site (www.pnas.org). We are primarily concerned with the redistribution of inoculum within a finite field, defined as rectangular with sides, Lx and Ly, and area Ω = LxLy, with the origin at the center. To avoid the complication of inoculum being taken beyond the perimeter of the field, we assume the mean dispersal distance to be much less than the dimension of the field, Lxkx, Lyky ≫ 1. To assess the impact of the redistributed inoculum on a subsequent crop, we need to calculate the likelihood of inoculum being found within some characteristic area, Ah, of a host plant.
For the inoculum of a soil-borne disease, this area depends primarily on the extent of the root structure and is here defined simply for discrete nonoverlapping root systems as the lateral extent of the root growth in the upper horizon of soil, within which inoculum usually occurs. For small discrete hosts, such as seedlings, this lateral distance may be extended to include the pathozone surrounding roots. The pathozone is defined as the region of soil surrounding the susceptible part of the plant in which a unit of inoculum has a chance of causing infection (6).
For a single unit of inoculum, the probability of being redistributed to Ah(x, y) is
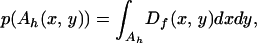 |
5 |
where Ah(x, y) is an area A centered on (x, y). The probability distribution for the number of inoculum units in Ah is binomial, so for N particles initially at the origin,
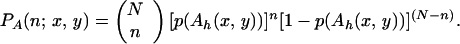 |
6 |
From the form of Df given in Eq. 3, a number of features of overall inoculum distribution are apparent. For those particles that move, the characteristic displacement of particles is given by 1/kx and 1/ky in the x and y directions, respectively. Hence Ac = 1/(kxky) gives an order-of-magnitude estimate for the area over which material is spread. For the case Ac ≤ Ah, the redistribution resulting from cultivation will not move inoculum significantly beyond the host plant at the origin. We therefore confine ourselves to the case Ac ≫ Ah. Under this assumption, we can assume that p(Ah(x, y)) ≪ 1, and hence the binomial distribution for the number of inoculum units in Ah(x, y) can be approximated by a Poisson distribution,
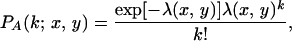 |
7 |
where
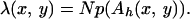 |
8 |
Parameter Ranges and Model Simulation.
For all the following simulated dispersal events, the initial inoculum distribution is assumed to be a point source, as discussed in Theory and Model. Hence the spatial probability distribution for a single particle after cultivation is given by Eq. 3. For geometric convenience, we take the characteristic area, Ah, of a host plant to be a square, lx = ly = l. As displacement in the x and y directions combines in a simple multiplicative manner in the dispersal function Eq. 3, we let kx = ky to keep parameters to a minimum. It is also assumed that host characteristic areas do not overlap.
Parameter ranges are derived from the literature. Values of the mean dispersal distance, 1/k, range from approximately 1 m (2) to 10.5 m (7). Values for the probability of movement, p, also show a wide range. Marinissen and van den Bosch (7) report values as low as 0.05. In experiments on a number of different cultivation methods, Brain and Marshall (5) found 0.47 < p < 0.97, often with considerable variation between replicates of the same method. These experiments were based on the movement of plastic or glass beads of similar dimensions to the inoculum units under consideration. For example, Marshall and Brain (2) use small plastic pellets, 3 mm by 1 mm, to represent weed seeds. However, it should be borne in mind that inoculum for some diseases may be confined within root structures at the time of harvest, and hence dispersal may depend on the movement of lateral roots, whose mean dispersal distances may be much greater than those of seeds, because of the ease with which they can become entangled in machinery.
Table 1 lists the key parameters and variables and their interpretations. In the following sections, we examine some of the properties of the resulting distribution.
Table 1.
Table of main parameters and variables and their interpretation
| Parameter/variable | Interpretation |
|---|---|
| (1 − p) | Probability of propagule remaining unaffected by cultivation pass. |
| 1/kx,y | Mean dispersal distance in x or y direction for transported propagules. |
| Ah(x, y), lx, ly | Characteristic area and dimensions of the host at (x, y). |
| Ac | Order of magnitude estimate for area over which material is moved. |
| Df(x, y) | Probability density function that a propagule is displaced to (x, y). |
| p(Ah(x, y)) | Probability that a single unit of inoculum is redistributed from the origin to Ah(x, y). |
| 2xc | Critical patch width. |
| r | Inoculum threshold for symptoms to be visible. |
| N | Total number of inoculum units. |
| Γ | Amplification of inoculum. |
| pf | Critical probability for the appearance of cross shapes. |
Analytical results and approximations are compared with stochastic simulations of dispersal from a point source of inoculum. The stochastic simulations were generated by using the basic probability distribution function Eq. 1. For each unit of inoculum, random deviates were generated for distance traveled in both the x and y directions. The numbers of inoculum units per plant were then calculated from these positions for plants placed on a regular grid.
Results
Patch Shape.
The probability distribution for the single particle, Df(x, y), is given by (from Eq. 3):
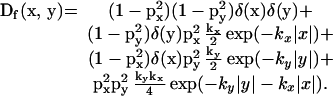 |
9 |
The terms on the right-hand side can be interpreted as a central spike, an exponentially decaying ridge in the x direction, an equivalent ridge in the y direction, and a background distribution, respectively. Depending on the relative sizes of these terms, the resulting distribution may be basically cross-shaped, with some material between the arms, or circular, with no apparent arm structure. Equal displacement in the x and y directions, of course, yields a circular patch, whereas a significant amount of material between the arms converts a cross to a kite shape. We can define the discriminating case as that in which the contribution to the inoculum in a host's characteristic area, Ah, is the same for the background distribution as for the ridge contribution.
Consider the mean number of inoculum units that a plant is exposed to at a point (x, 0), and hence lying on the x-direction arm,
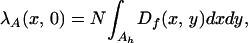 |
where N is the number of inoculum units. The function, Df(x, y), will contain only the second and fourth terms of Eq. 9 at a point on (x, 0). The assumption, Ac ≫ Ah, gives kxlx, kyly ≪ 1, and hence the exponentials can be taken as constant over the area of integration, Ah. The above expression can be thus reduced to
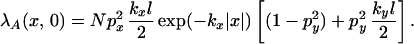 |
The two terms in square brackets represent the contribution from the ridge and background, respectively. We define the discriminant case to be when these terms are equal,
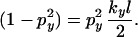 |
Note that this condition is independent of the position along the arm or N, the number of inoculum units. An analogous condition holds for the y direction. We can define a critical value of p,
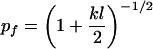 |
10 |
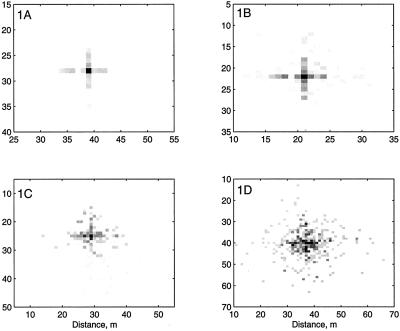
For p > pf, we would expect the cross shape to be obscured by the background distribution, but for p < pf, the arms of the cross should be enhanced. Fig. 1 shows a set of stochastically generated simulations with constant k = 0.2m−1, l = 1m but varying p. For these parameter values, pf = 0.95, which is the value of p in Fig. 1C. A cross is clearly visible in simulations A and B but is almost completely obscured in D. Case C appears to mark a qualitative boundary for the form of the distribution. From the assumption that Ac ≫ Ah (Theory and Model), it follows that generally, kl ≪ 1, and hence from Eq. 10 pf ≃ 1. Therefore, only cultivation methods with very high probabilities of moving material will fail to form cross-shaped patterns.
Figure 1.
Effect of changing the probability of movement of individual propagules on the spatial pattern of inoculum. Simulated distributions of inoculum levels per plant area, Ah for constant dispersal distance, k, but various movement probabilities, p. (A) p = 0.8; (B) p = 0.9; (C) p = 0.95; (D) p = 0.97. Other parameters: N = 400, k = 0.2 m−1. The critical probability at which there is a qualitative change in the shape of the patch from cross-shaped to radially diffuse is 0.95. The intensity of the grey level indicates the density of inoculum.
Patch Size.
We consider three criteria by which patch size is defined: (i) the standard deviation of the pdf for dispersal; (ii) the expected arm length for which contiguous plants are each exposed to inoculum; and (iii) the distance from the center of the patch at which probability of infection falls below a critical level.
The distribution of a single particle is governed by the pdf in Eq. 9. The measure of patch size could be related to the variance of this probability distribution. The distribution of particles in the x direction only is given by ḡ(x; kx, px) Eq. 4, which has SD
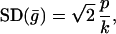 |
11 |
suggesting an expected patch size proportional to p/k.
A more accurate estimate can be constructed by considering the extent of symptomatic plants and hence the absolute number of inoculum units found in the vicinity of a host plant. Such a measure will have to include the area, Ah, and the number of inoculum units, N. A more practical approach is to try to define an “edge” to a patch in terms of the number of inoculum units encountered by a plant.
For the case of cross-shaped distributions, the size is given by the length of the arms of the cross. If we consider an arm to be an unbroken sequence of plants in contact with at least one inoculum unit, then the expected arm length will be
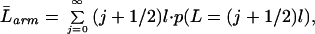 |
12 |
where p(L = (j + 1/2)l) is the probability of an arm having length (j + 1/2)l,
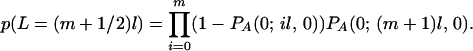 |
The width of a patch is then given by 2L̄arm. The additive factor of a half arises from the need to measure distance from the center of a host plant's characteristic area.
The sensitivity of mean arm length to changes in parameter values is hard to judge because of the complexity of the expressions in Eq. 12. A third approach gives a much simpler form for the dependence of arm length on parameter values at the expense of some of the accuracy.
As the probability distribution function for a single particle, D(x, y), decreases monotonically with distance from the origin, an estimate for the “edge” of a patch may be defined as the probability isocline at which the probability of a critical degree of infestation falls below some level. We could equally interpret it as the point at which the Poisson mean predicted by the distribution reached some critical value, λc. Considering only the “ridge” of the probability distribution (Eq. 9) in the x direction,
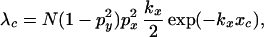 |
which gives
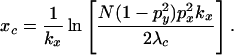 |
13 |
Because of the symmetry of the patches, the width is given by 2xc. To find the value of λc, the parameter was fitted to the output from the simulation, giving a value of λc = 1.5.
Comparisons of the three measures of patch size with the results of simulations can be seen in Table 2. The table clearly shows that Eq. 12, and the Poisson approximation underlying it, gives an accurate measure of the width of a patch for all parameter values (column 5). The predictions based on the SD (Eq. 11) of the underlying probability distribution are very poor (column 7). The SD greatly underestimates the arm length of the patches and, more importantly, does not exhibit consistent sensitivity with respect to the parameters N, p, k (Table 1). Column 6 shows the estimates based on Eq. 13 with λc = 1.5. They are in general within 10% of the mean values given by simulation and reflect the same sensitivity to N, p, k. The approximation loses accuracy for shorter arm lengths, but this must be expected from the form of Eq. 13. As the term inside the logarithm decreases below 1, it predicts negative arm lengths. Because the threshold number of inoculum units in this case is one, if N > 1, an arm length of zero is not possible. For threshold levels other than one, it will be necessary to recalculate the critical value, λc.
Table 2.
| N | p | k | Simulation
|
Theory
|
||
|---|---|---|---|---|---|---|
| Mean | 2L̄arm | 2xc | SD (ḡ) | |||
| 100 | 0.5 | 0.2 | 5.3 | 5.3 | 2.2 | 3.5 |
| 400 | 0.8 | 0.2 | 20.0 | 19.9 | 18.1 | 5.7 |
| 400 | 0.8 | 0.15 | 20.8 | 20.8 | 20.3 | 7.5 |
| 800 | 0.5 | 0.2 | 23.5 | 23.5 | 23.0 | 3.5 |
| 800 | 0.8 | 0.5 | 16.7 | 16.6 | 13.7 | 2.3 |
| 1,000 | 0.9 | 0.15 | 29.6 | 29.6 | 27.2 | 8.5 |
| 2,000 | 0.4 | 0.1 | 38.9 | 39.3 | 43.8 | 5.7 |
For circular patches, the probability distribution function is dominated by the fourth, “background” term from Eq. 9. Hence
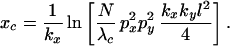 |
14 |
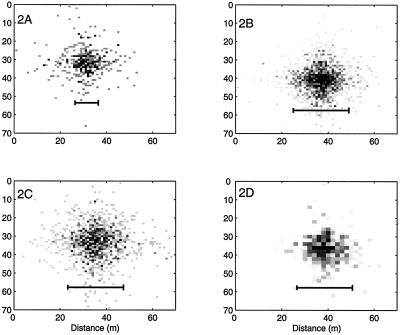
Fig. 1 shows four simulated distributions for different initial densities of inoculum, dispersion parameter, or characteristic area. All are symmetrical in x and y with respect to parameter values. Grey levels indicate the relative numbers of inoculum units per host. For distribution 2A, Eq. 13 gives a width of 10m, which is approximately the width of the central symptomatic patch. Distributions 2C–D all have the same width of 24m by Eq. 13, but with differing parameter values. Fig. 2C has a longer mean displacement than Fig. 2B but fewer inoculum units over all. Fig. 2D has a quarter of the inoculum units of 2B, but each host plant has four times the characteristic area. The sizes of these patches are clearly comparable.
Figure 2.
Effect of inoculum density displacement parameters and plant area on the pattern of inoculum and effective patch size. Bars represent predictions from theory. Simulated distributions of inoculum levels per plant area. (A) N = 400, kx = ky = 0.2, Ah = 1m2, 2xc = 10m. (B) N = 1600, kx = ky = 0.2, Ah = 1m2, 2xc = 24m. (C) N = 1100, kx = ky = 0.15, Ah = 1m2, 2xc = 24m. (D) N = 400, kx = ky = 0.2, Ah = 4m2, 2xc = 24m. The intensity of the grey scale indicates the relative densities of inoculum.
Asymptomatic Patches.
Given that some inoculum threshold, r, exists below which symptoms do not appear in the crop, it is possible that the dispersal process sufficiently dilutes the inoculum that the presence of infestation within the field is not detectable. A field in which the maximum inoculum level is above that necessary to cause symptoms in a subsequent crop could have its maximum diluted by cultivation to a point at which symptoms will no longer be visible. Because the underlying dispersal function, Eq. 3, is at a maximum at the origin, if the probability of r or more units within an area, Ah, is sufficiently low here, it will be lower everywhere else. Hence conditions at the origin act as a bound on those in the rest of the field. The critical probability at the origin, psymp, is the solution of
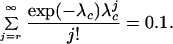 |
15 |
where λc = Npsymp, and a probability of 0.1 is chosen as indicating a rare event. For p < psymp, symptoms are very unlikely to be apparent. For values of r > 5, the Poisson formulation can be approximated by a normal distribution. Hence Eq. 15 can be expressed in terms of the error function,
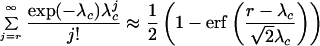 |
16 |
The value of psymp can then be equated with that given by the distribution function to give
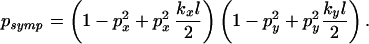 |
17 |
As with the analysis of patch shape in Patch Shape, the 1 − p2 and p2kl/2 terms represent contributions from the ridge and background of the probability distribution, respectively. Hence for a cross-shaped distribution, the probability of no detectable symptoms is dominated by the probability of movement of material, p, whereas for circular/ellipsoidal distributions, the probability also depends on the characteristic area of the host and the cultivation displacement.
Consider the scenario in which a point source of inoculum is first amplified by a host crop, and then the resultant inoculum is redistributed. If the initial concentration of inoculum is N0 and amplification is Γ, then N = N0Γ. Eq. 15 actually yields a solution in the form, Npsymp = K, where K is calculated from the error function approximation in Eq. 16. Hence we can write
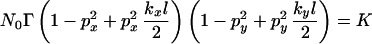 |
as representing the critical case in which the effects of amplification and dilution combine to give an asymptomatic subsequent crop. The effects of amplification are contained in the parameter, Γ, whereas the effects of dispersal are contained in the two bracketed terms. The small size of kxl and kyl ensures that the terms in brackets are always less than 1, whereas the parameter Γ is greater than 1 for an amplifying disease. The most diluting forms of cultivation are those for which the probability of movement, p = 1. In this case,
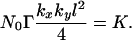 |
For values kx = ky = 0.2, l = 1m, the cultivation dilution term is 0.01. Hence the amplification, Γ, would have to be at least 100 for there to be any chance of symptoms showing in the subsequent crop.
Discussion
In this paper, we have extended the work of Brain and Marshall (2, 5) to analyze the consequences of cultivation on the size, shape, and persistence of disease patches in fields. The theory developed here allows discrimination between two broad classes of patch shapes, ellipsoid and cross-shaped, according to the relative magnitudes of inoculum redistribution along ridges parallel to the directions of cultivation, and the background redistribution between the ridges. The symmetry of cultivation and the magnitude of background infection may also lead to circular patches rather than ellipsoid and to kite-shaped rather than cross-shaped patches. Redistribution of inoculum is characterized by two parameters, the probability of movement and the mean dispersal distance. These are determined by the magnitude and type of cultivation, for example ploughing, harrowing, or tined cultivation. The appearance of symptoms, however, depends on the characteristic dimensions (lx, ly) of the crop plants that are exposed to the redistributed inoculum (see Eq. 9). It follows that a host with a small characteristic width may yield a cross-shaped disease patch after cultivation, whereas another crop or variety with a different rooting pattern that yields a larger characteristic width would yield a circular or ellipsoidal patch after the same cultivation processes. Such a patch would appear larger and would wrongly be taken to indicate a more extensive infestation.
The analysis of patch size underlines the importance of the amount of inoculum (N) as well as the cultivation (px, py, kx, ky) and host (lx and ly) parameters in controlling a patch expansion. Table 2 shows that the variance of the underlying probability of dispersal of a single propagule, which ignores N, is a very poor indicator of patch size or of its dependence on parameters. However, the definition of the edge of a patch as the expected arm length for which contiguous plants are exposed to inoculum or for which the probability of infection falls below a critical threshold both perform well. Both Larm and 2xc predict patch sizes to within 10% of those from simulations and exhibit the same sensitivity to parameters as the simulation. The use of the criterion involving a critical threshold for the probability of infection leads naturally to the consideration of asymptomatic patches and to the balance between net amplification of inoculum effected by parasitic activity and saprotrophic survival and dilution caused by redistribution by cultivation. The question remains why the critical value, λc, should represent a patch edge. For different inoculum threshold levels, it would be necessary to re-estimate this parameter. The usefulness of Eqs. 13 and 14, however, lies in the possibility of predicting how changes in cultivation practice, plant area, or inoculum loading will alter the size of the resulting disease patch. Of these, cultivation practice, which is characterized by p (the probability of movement of inoculum) and 1/k (the mean displacement), is under the control of the grower. We have shown that high values of p are necessary for certain patch shapes. We now propose that simple experiments with artificial or marked inoculum can be used to estimate p and 1/k for different cultivation methods.
Other questions that need to be addressed are the effects of finite initial patch sizes and multiple cultivation passes. Preliminary investigation of the redistribution of finite patches indicates that their effect on the distribution function can be represented by a multiplicative factor on each of the terms in Eq. 9. This factor reduces to unity as the patch size shrinks to zero. This is discussed in detail in Appendix B, which is published as supplemental data on the PNAS web site (www.pnas.org). Further cultivation passes rapidly complicate the calculation of the distribution function. However, it is possible to calculate the maximum of the distribution function for subsequent passes, showing that the diluting effect of cultivation decreases rapidly as the inoculum becomes more evenly distributed in the field.
The model rests on several assumptions, involving independent movement of propagules by machinery, nonoverlapping root systems during the period of host susceptibility and with the characteristic area over which material is moved Ac being greater than the characteristic area for Ah for host susceptibility. However, within these restrictions, the approach could be extended to other dispersal mechanisms, which could be represented as discrete events, such as splash dispersal. Continuous processes such as wind blow could also be treated as discrete events by integrating their effects over some finite time interval to generate an appropriate distribution function.
The spread of artificially introduced genes can also be described by this model. Pollen from crops containing such genes may hybridize with individual conventionally bred plants, producing hybrid progeny. During harvest, some seeds containing these genes will inevitably be distributed by machinery, giving rise to new hybrid plants in subsequent seasons. These plants can be seen as analogous to inoculum. However, detection will be more difficult, and hence their “symptomatic threshold” will be much higher.
The discrimination between symptomatic and asymptomatic patches is particularly important. Asymptomatic patches represent an “invisible” source of infestation for other fields on a farm and also other farms within a network of shared machinery. A grower's interests with regard to disease spread are best served by minimizing the number of invisible sources of infection, requiring that any disease present becomes visible as soon as possible. From Eq. 17, we can see that minimizing px,y and maximizing kx,y leads to the smallest amplification needed for symptoms to appear. Hence a cultivation technique is favored in which the probability of pickup and the mean displacement distance are at a minimum.
Given that the same process which transmits inoculum is also responsible for locally diluting its effect, it may be argued that the pdf for redistribution g(x) (Eq. 1) integrates to slightly less than one, and the shortfall is the small probability of material being transported beyond the confines of its parent field. Because each inoculum unit is considered to be independent, the probability of one being transmitted will be independent of the inoculum distribution in the field. The force of infection of a field would therefore depend on the total inoculum within a field, not on the inoculum distribution within it. From an epidemiological standpoint, such fields represent an invisible front extending beyond an invading wave of disease. From large-scale continuum models of invasion (8, 9), it is known that the shape of such fronts governs the dynamics of the spreading epidemic. To study such long-term phenomena, however, it will first be necessary to extend the model to incorporate multiple seasons.
Supplementary Material
Acknowledgments
This work was funded through a grant from the Ministry of Agriculture, Fisheries, and Food (MAFF) U.K. We gratefully acknowledge the support of the Biotechnology and Biological Sciences Research Council, the Royal Society, the Leverhulme Trust, and MAFF.
Abbreviation
probability density function
Convolution Theory.
The convolution operator is both commutative and associative. The associativity property means that several dispersal functions convoluted in turn with an initial inoculum distribution can, in fact, be convoluted with each other first to give a compound distribution function. The result can then be applied to the initial distribution.
A cultivation event consisting of two passes in opposite directions will be represented by the compound dispersal function,
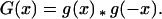 |
It is clear from the commutativity property that
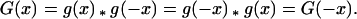 |
Hence all such dispersal functions will be symmetrical, about x = 0. For the case in which g(x) is defined as in Eq. 1, this gives a compound function, ḡ(x; k, p),
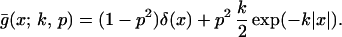 |
18 |
Because the underlying Poisson properties of the cultivation process, the resulting dispersal function is almost that which would be obtained by simply adding g(x) and g(−x). In terms of cultivation, the compound result is almost that obtained when half the inoculum is subject to cultivation in one direction and half in the opposite.
This result is significant when considering how cultivation of a field should be modeled. Ploughing is usually executed in a series of strips by a vehicle driven back and forth across the field. Hence material will be ploughed alternately in one direction or the other, or in both if the strips overlap. If the width of the strips is small in comparison to the dimensions of the inoculum patch, then the locally averaged effect of alternate direction ploughing will be
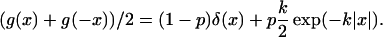 |
This is very similar to the expression for ḡ, given in Eq. 19, for material ploughed first in one direction and then in the other.
If we now include the effect of a second cultivation event perpendicular to the first, we recover the general expression for the dispersal function for two-pass cultivation along perpendicular axes,
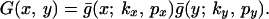 |
19 |
To illustrate the underlying shape of the isoclines of the distribution, we consider the case in which px, py = 1 and find
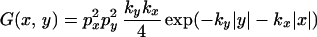 |
for a cultivation scheme in which all particles are moved. Probability isoclines are then given by ky|y| + kx|x| = constant. It is easy to show that such isoclines have a rhombic shape, with diagonal coincident with the x and y axes, where the ratio of x axis to y axis is ky:kx.
The case of nonorthogonal cultivation passes is a generalization of the orthogonal case. Nonorthogonal passes simply shear the underlying distribution function, as dictated by the directions of plowing. If k1 and k2 define the two directions of cultivation with magnitudes given by the inverses of the mean distances displaced, we can define a transformation matrix,
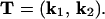 |
Then for any position (x′, y′) in the field, the probability density will be given by G(x, y) with
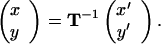 |
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tuitert G, Hofmeester Y. Neth J Plant Pathol. 1992;98:343–360. [Google Scholar]
- 2.Brain P, Marshall E J P. Aspects Appl Biol. 1996;46:173–179. [Google Scholar]
- 3.Tuitert G. Ph.D. thesis. Wageningen, The Netherlands: Wageningen Agricultural University; 1994. [Google Scholar]
- 4.Asher M J C. In: The Sugar Beet Crop: Science into Practice. Cooke D A, Scott R K, editors. London: Chapman & Hall; 1993. pp. 313–346. [Google Scholar]
- 5.Marshall E J P, Brain P. J Appl Ecol. 1999;36:443–454. [Google Scholar]
- 6.Gilligan C A. Phytopathology. 1985;75:61–67. [Google Scholar]
- 7.Marinissen J C Y, van den Bosch F. Oecologia. 1992;91:371–376. doi: 10.1007/BF00317626. [DOI] [PubMed] [Google Scholar]
- 8.Zawolek M W, Zadoks J C. A Physical Theory of Focus Development in Plant Disease. Wageningen Agricultural University, Wageningen, The Netherlands: Wageningen Agricultural University Papers; 1989. [Google Scholar]
- 9.Diekmann O. J Diff Eq. 1979;33:58–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.