Abstract
Formation of the enteric nervous system (ENS) from migratory neural crest-derived cells that colonize the primordial gut involves a complex interplay among different signaling molecules. The bone morphogenetic proteins (BMPs), specifically BMP2 and BMP4, play a particularly important role in virtually every stage of gut and ENS development. BMP signaling helps to pattern both the anterior-posterior axis and the radial axis of the gut prior to colonization by migratory crest progenitor cells. BMP signaling then helps regulate the migration of enteric neural crest-derived precursors as they colonize the fetal gut and form ganglia. BMP2 and -4 promote differentiation of enteric neurons in early fetal ENS development and glia at later stages. A major role for BMP signaling in the ENS is regulation of responses to other growth factors. Thus BMP signaling first regulates neurogenesis by modulating responses to GDNF and later gliogenesis through its effects on GGF-2 responses. Furthermore, BMPs promote growth factor dependency for survival of ENS neurons (on NT-3) and glia (on GGF-2) by inducing TrkC (neurons) and ErbB3 (glia). BMP signaling limits total neuron numbers, favoring the differentiation of later born neuronal phenotypes at the expense of earlier born ones thus influencing the neuronal composition of the ENS and the glia/neuron ratio. BMP2 and -4 also promote gangliogenesis via modification of neural cell adhesion molecules and promote differentiation of the circular and then longitudinal smooth muscles. Disruption of BMP signaling leads to defects in the gut and in ENS function commensurate with these complex developmental roles.
Keywords: neural crest, p75NTR, pSmad1/5/8, noggin, TrkC, NT-3, ErbB3, GGF-2, transgenic mice, neurotransmitters, PSA-NCAM, enteric nervous system
FOREWORD
Dr. Ira Black was a major leader in the study of neural development. His early work focused on development of neural crest derivatives with a particular emphasis on specification of neurotransmitter phenotype (Black et al., 1971); (Cochard et al., 1978) and work in this area continued throughout his career. We thus feel that the topic of this review is particularly fitting to honor his fundamental contributions and his memory.
INTRODUCTION
The enteric nervous system (ENS) is derived from migratory vagal and sacral neural crest (Yntema, 1954); (Le Douarin and Teillet, 1973) and the spatiotemporal migratory routes taken by the neural crest-derived precursors of the ENS within the primordial gut are now well understood (Burns and Thapar, 2006); (Hao et al., 2009). Many of the signaling molecules controlling the differentiation and diversity of enteric neuronal phenotypes and enteric glia have now also been identified (Hao and Young, 2009); (Gershon, 2010). The present review focuses on the roles of the Bone Morphogenetic Proteins (particularly BMP2 and BMP4) as dominant signaling molecules in shaping the fetal gut and in regulating its subsequent intrinsic innervation by enteric neural crest-derived cells (ENCDC).
Why the BMPs?
The BMP subclass of the TGFβ superfamily of growth factors is highly conserved from Drosophila to mammals and is involved in all stages of neural development from neurulation through embryonic and post-natal development, and continuing in adulthood (Hogan, 1996). BMPs act in dorso-ventral patterning of the neural tube, formation of the neural crest (Selleck et al., 1998) and onset of neural crest migration (Sela-Donenfeld and Kalcheim, 1999). They influence the shaping of the neural crest-derived peripheral nervous system (PNS) and act as gradient morphogens on subventricular zone (SVZ) progenitor cells in the central nervous system (CNS). They promote neuronal lineage commitment during early CNS development but later promote astroglial lineage at the expense of neuronal or oligodendroglial fates (Mehler et al., 1997).
BMP2 and BMP4 bind to a type II receptor subunit (BMPRII) to form a heterotetrameric complex with a type I subunit (BMPRIA or BMPRIB) (Yamashita et al., 1996). The type I subunits are phosphorylated by BMPRII and in turn recruit and phosphorylate the downstream signal transduction molecules, Smad 1, 5 or 8 (Attisano and Wrana, 2000). BMP actions are regulated in vivo in a time and location-dependent manner by proteins such as noggin, follistatin, gremlin and chordin that antagonize BMP signaling by directly binding BMPs and blocking ligand activity (Cho and Blitz, 1998).
BMPs HELP TO SPECIFY GUT SUBDIVISIONS PRIOR TO COLONIZATION BY ENCDC
Roberts, Tabin and collaborators in the late 1990s demonstrated the importance of BMP signaling in formation of the chick gut (Roberts et al., 1995); (Roberts et al., 1998) (Roberts, 2000). Expression of BMP 2, -4 and -7 varies temporarily and spatially within the primordial gut (Goldstein et al., 2005). BMP4 is expressed in the mesoderm of the entire gut except in the gizzard/stomach and in conjunction with Bapx-1/Nkx3.2 regulates the thickness of the smooth muscle layer in the stomach (Nielsen et al., 2001). Sonic hedgehog (Shh) induces BMP4 in the splanchnic mesoderm through an endoderm-mesoderm interaction. BMP4 in the stomach controls smooth muscle hypertrophy and promotes differentiation. Subsequently BMP2 promotes glandular formation in the stomach epithelium through a mesoderm-endoderm interaction. Thus BMP4 and-2 control regional specification of the antero-posterior axis of the gut into foregut, midgut and hindgut (Smith et al., 2000) ; (De Santa Barbara et al., 2005). Sonic hedgehog (Shh) in endodermal epithelium is responsible for patterning of the radial axis of the gut. Shh activates its receptor Patched as well as BMP4 in adjacent non-smooth muscle mesenchyme, where differentiation of lamina propria and submucosa will occur. Shh and BMP4 act along a decreasing concentration gradient from epithelium to the outer gut regions; as concentrations of Shh decrease towards the outer regions, smooth muscle and enteric neuron differentiation occur, thus determining the outer location of the ENS intercalated with the circular and longitudinal smooth muscle layers (Sukegawa et al., 2000).
SHH AND BMPs ALSO INFLUENCE ENCDC PROLIFERATION AND MIGRATION WITHIN THE FETAL GUT
Enteric murine neural crest-derived cells express the Shh receptor Patched and the transcription factor Gli1. Shh promotes ENCDC proliferation in explants of murine gut but suppresses both their migration and their differentiation and coalescence into ganglia induced by glial cell-line derived neurotrophic factor, GDNF (Fu et al., 2004). In the chick gut, inhibition of BMP signaling by over-expression of the antagonist noggin starting at E2 delayed the migration of ENCDC in vivo, and by E12 hypoganglionosis occurred in the hindgut with reduction in ganglion size. However, the timing of neural differentiation was unaffected and there was no change in the rate of cell death (Goldstein et al., 2005).
In contrast to the delayed migration of the crest-cells promoted by inhibition of BMP signaling in the chick hindgut, enhancement of migration of the crest-cells was observed in the mouse hindgut at comparable developmental stages, resulting in abnormal location of the neural crest cells towards the epithelium rather than towards the bowel outer wall where the myenteric plexus is normally located (Fu et al., 2006). The opposite effects on neural crest cells migratory behavior in chick vs mouse, caused by inhibition of BMP signaling, underscores the importance of the location and concentration of BMP ligands encountered by the neural crest cells. It may also reflect the fact that the normal migratory behavior of these cells differs between the two species.
ENCDC FROM RAT or MICE FETAL GUT CAN BE ISOLATED AND STUDIED
ENCDC express selectively the pan-neurotrophin binding receptor p75NTR, (Baetge et al., 1990);(Chalazonitis et al., 1997);(Chalazonitis et al., 1998a);(Chalazonitis et al., 1998b) and highly purified preparations of ENCDC can be prepared by immunoisolation based on p75NTR expression. In rat fetal gut at E12 colonization is not completed in the hindgut, whereas by E14 and E16 colonization is nearly completed throughout the gut and ENCDC proliferation is declining while neuronal and glial differentiation is increasing. The expression of BMP signaling molecules and the effects of BMP2 and -4 were examined using cultures of ENCDC immunoisolated from three distinct stages of fetal rat gut development, E12, E14 and E16 (equivalent to E11, E13 and E15 in mice).
BMP2 and -4, their transducing receptors subunits and inhibitors such as noggin, are expressed both by ENCDC p75NTR+ and by non p75NTR− populations
In intact rat fetal gut, BMP2 and −4, but not BMP7, are expressed from E12 onwards and expression declines in the adult. qPCR analysis shows that transcripts for BMP2 are more abundant than for BMP4 at all stages with a peak at E12-E14. BMP4 expression surges at E14 and again at E19-P2 (Chalazonitis et al., 2004). Similarly in avian gut BMP7 is not expressed in the ENS and is restricted to the epithelial layer (Goldstein et al., 2005). BMP2 and -4 are each expressed both by isolated ENCDC (p75NTR+cells) and by the remaining population of gut cells (p75NTR− depleted, Hand-1+ cells) which is comprised predominantly of smooth muscle precursors of mesodermal origin (Chalazonitis et al., 2004). Both populations also express BMPRII, BMPRIA and BMPRIB. By contrast in the developing and adult human ENS, BMPRIA is expressed only by ganglion cells, and co-localizes with the neural crest marker HNK-1 and the neuronal marker Hu. It is thus a reliable marker of human colonic enteric ganglion cells at all stages of their development (Brewer et al., 2005). ENCDC and non-ENCDC populations both translocate the phosphorylated form of Smad (pSmad1/5/8) to the nucleus in response to a 1 hr in vitro exposure to BMP2 or -4 (Chalazonitis et al., 2004). The BMP inhibitors gremlin, noggin, chordin and follistatin are all also expressed by fetal and mature rat gut. Noggin is expressed by both ENCDC and the non-ENCDC populations at E14, suggesting that it modulates BMP signaling in both populations. By contrast, in the avian hindgut noggin is strongly expressed in the submucosal layer with only minimal expression in the ENS (Goldstein et al., 2005). In toto these observations suggest that by the end of the migratory stage through the post-natal period, BMP2 and -4 exert paracrine as well as autocrine influences on both ENCDC and non-ENCDC populations.
BMP2 and -4 promote optimal neuronal differentiation of rat ENCDC precursors only at specific concentrations and for a limited time of treatment. With prolonged exposure (e.g.6 days) of cultured p75NTR+ ENCDC to BMP2 or -4, the maximal degree of neuronal differentiation occurs at relatively low concentrations (up to 1 ng/ml) and declines at higher concentrations (up to 100 ng/ml) of ligand. Conversely, maximal neuronal differentiation in response to high concentrations of BMPs occurs with a short exposure (2 days), suggesting that longer treatments compromise neuronal survival. (Chalazonitis et al., 2004) (see diagram Fig1). In fact the total number of cells is reduced in cultures of ENCDC treated with high concentrations of BMP2 or -4 and the proportions of cells undergoing apoptosis is significantly higher after prolonged (6 day) treatment. Thus the differentiating actions of BMP2 or -4 are distinct from those on survival, and prolonged exposure to high concentrations of BMPs compromises survival. This contrasts with other neurotrophic factors, such as neurotrophin-3 (NT-3), whereby differentiation and survival effects are linked. The data are consistent with previous findings that BMP2 promotes differentiation of neural crest-derived sympathetic and enteric progenitors into sympathetic or enteric neurons respectively and that it promotes the acquisition of dependence by these neurons on other gut-derived trophic factors (Pisano et al., 2000). Similarly cultured CNS progenitor cells exhibit strikingly different responses to BMP2 or -4 treatment depending on the embryonic stage and concentration of ligands. At E13, high concentrations of BMP2 or-4 inhibit cell proliferation and promote apoptosis that can be mitigated by FGF2 whereas at later stages (E16 and thereafter) BMP2 or -4 promote neuronal and later astrocytic differentiation (Mabie et al., 1999).
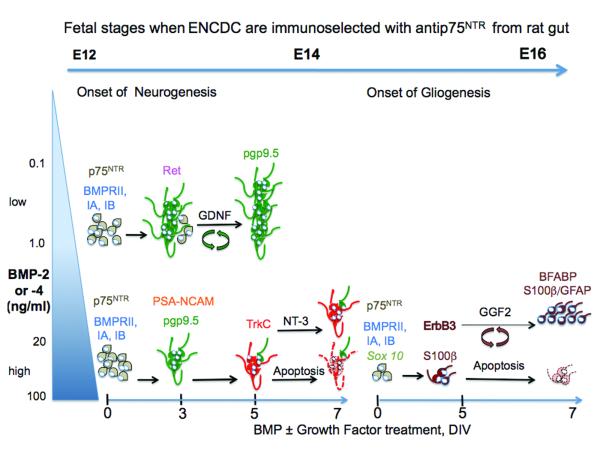
Figure 1. BMP2 and -4 promote enteric neuronal differentiation in early fetal development and glia at later stages and then dependence on other factors for their survival.
When ENCDC (in gray) are immunoselected at early fetal stages (E12, left) and grown for several days in vitro (DIV), BMP signaling promotes cell cycle exit and rapid neuronal differentiation, neuritic extension and clustering via PSA-NCAM of Ret and pgp9.5-expressing neurons (in green). In a subset of neurons (in red), BMP 2 or -4 promote precocious expression of TrkC, the specific transducing receptor for NT-3 (bottom left). However prolonged treatment with high BMP (20-100 ng/ml) does not sustain neuronal survival, and by E14 differentiated neurons become dependent on other neurotrophic factors (i.e. NT-3) for survival and undergo apoptosis in the absence of those factors (bottom center). High BMP concentrations interfere with the proliferative effects (green circular arrows) of GDNF. However with lower concentrations (0.1-1 ng/ml) and prolonged treatment, BMP2 or -4 increase neuronal differentiation, and this effect is enhanced by GDNF (center left). When ENCDC are immunoselected at later stages (E14 and thereafter) BMP 2 or -4 up-regulate expression of ErbB3, the specific binding receptor for GGF-2 in glial precursors that express BMP receptors, p75 NTR and Sox10 (gray cells, bottom right). As GGF-2 expression becomes maximal (by E15), ErbB3-expressing glial precursors proliferate with GGF-2 (brown circular arrows) and differentiate into mature glia (BFABP-S100β-GFAP-expressing) (right). The differentiated ErbB3-expressing glial cells (in brown) become dependent upon GGF-2 for their survival and undergo apoptosis in the absence of GGF-2 (bottom far right). High concentrations of BMP2 or -4 dampen the proliferative effects of GGF-2.
GDNF and BMP can cooperate to promote neurogenesis only if BMP signaling is limited
GDNF promotes proliferation of avian (Hearn et al., 1998), murine (Heuckeroth et al., 1998) and rat (Chalazonitis et al., 1998b) ENCDC in vitro. This effect is strong at E12 but wanes at later stages to be replaced with a differentiating/survival effect at E16 (Chalazonitis et al., 1998b). BMPs and TGFβ are potent promoters of cell cycle exit and can induce apoptosis in tumors via the Smad proteins (Ten Dijke et al., 2002). Further, as noted above, high concentrations of BMP2 or -4, or prolonged exposure to the ligands, reduces ENCDC survival. When ENCDC encounter both GDNF and BMP2 or -4, neurogenesis is enhanced by the survival effects of GDNF only if the BMP2 or -4 concentration is kept low or time of treatment is short. However if the BMP2 or -4 concentration is high or the treatment is prolonged, then GDNF–induced proliferation is inhibited and the degree of neurogenesis is diminished (Chalazonitis et al., 2004). A precise balance between the two factors is thus necessary to maintain and enhance neurogenesis in the ENS (diagram in Fig 1).
BMP2 or -4 promote acquisition of dependence of newly differentiated neurons on NT-3 for survival via up-regulation of TrkC
ENCDC immunoisolated at E14 but not at earlier stages (E12) respond to NT-3 by differentiating into neurons that grow extensive neuritic arbors and that become dependent on NT-3 for their survival (Chalazonitis et al., 1994); (Chalazonitis et al., 1998b) (Chalazonitis et al., 2001); (Chalazonitis, 2004). ENCDC immunoisolated from E12 gut express only low levels of the neurotrophin receptor, TrkC, but treatment with BMP2 or -4 (for 4-5d) promotes a 10-fold increase in the proportion of TrkC expressing-neurons. ENCDC isolated at E14 already express significant levels of TrkC, but BMP2 or -4 treatment increases levels of expression 5-6 fold. Concomitant with the induction of TrkC expression, a subset of enteric neurons become dependent upon NT-3 for their continued survival whereas they largely do not require NT-3 for survival if they have not encountered BMP signaling (Chalazonitis et al., 2004). Similarly fetal and neonatal sympathetic neurons isolated from the superior cervical ganglion express TrkC after treatment with BMP2 or -4, and they then acquire responsiveness to NT-3 with survival and extension and fasciculation of neuritic processes (Zhang et al., 1998) (diagram in Fig 1). BMP2 induced dependence for survival on other neurotrophic factors has also been demonstrated in immortalized sympatho-adrenal progenitor cells (MAH cells) which undergo apoptosis in a concentration-dependent manner when treated with BMP2 or -4 but which survive when co-treated with bFGF and NGF (Song et al., 1998). This type of complex interplay among different signaling molecules is particularly important for the development of enteric neuronal populations, which are much more diversified than PNS ganglia. For instance BMP2 and NT-3 promote the development of TH-expressing (dopaminergic) enteric neurons in vitro (Chalazonitis et al., 2008) and targeted deletion of NT-3 and TrkC promotes significant reduction in the CGRP-expressing intrinsic primary afferent neurons (IPANs) of the submucosal plexus (Chalazonitis et al., 2001). BMPs may also influence development of glial cells (see below) by regulating expression of other neurotrophin receptors such as TrkB, which is expressed by enteric glia (Levanti et al., 2009).
BMPs promote glial differentiation later in development than neuronal differentiation
ENCDC ultimately generate both neurons and glia. BMP2 or -4 induces neuronal but little glial differentiation of cells immunoisolated at early (E12) fetal stages (Chalazonitis et al., 2004); (Chalazonitis et al., 2011a). By E14 however, treatment with BMP4 significantly increases glial differentiation of ENCDC 7-fold. The gliogenic effects of BMP2 or -4 persist at E16 but at a lower efficacy than at E14. Differentiated glial cells respond in vitro directly and specifically to BMP4 exposure by exhibiting nuclear translocation of pSmad1/5/8 which can be inhibited upon co-exposure with noggin (Chalazonitis et al., 2011). The shift from pro-neuronal to pro-glial effects of BMP signaling in the ENS during development parallels what is observed in the CNS where a similar shift occurs (Mehler et al, 1997).
BMPs regulate expression of the GGF-2 receptor, ErbB3
Gliogenic factors such as GGF-2/NRG-1, type II (Marchionni et al., 1993) restrict pluripotent neural crest cells towards a glial fate (Shah et al., 1994). GGF-2 is effective in promoting glial differentiation of ENCDC immunoisolated at E16 but not at E12. Consistent with this in vitro observation, the abundance of transcripts for GGF2 in fetal gut increases by about 5-fold from E12 to E16 (Chalazonitis et al., 2011a) (diagram Fig1). Mice with loss of function of the GGF-2 receptor, ErbB3, lack enteric glia (Riethmacher et al., 1997) indicating that ErbB3 signaling is essential for enteric gliogenesis. ErbB3 is expressed by GFAP-expressing glial cells in E17 fetal gut and persists in the adult gut. Further, ENCDC immunoisolated at E15 express ErbB3 (Chalazonitis et al., 2011a). This suggested the possibility that BMP could regulate gliogenesis via promoting ErbB3 expression in glial precursors. In fact BMP4 treatment of ENCDC immunoisolated at E14 resulted in a 4-fold increase in GFAP-expressing cells co-expressing ErbB3.
BMP signaling attenuates GGF-2 proliferative effects but increases glial dependency on GGF2 for survival
GGF-2 promotes enteric gliogenesis by promoting proliferation of ENCDC glial progenitors (diagram Fig1). However BMP signaling interferes with the proliferative effects of GGF-2, consistent with its known effect in promoting exit from cell cycle of proliferating glia progenitor cells (Mabie et al., 1999); (Ten Dijke et al., 2002). Thus BMP signaling enhances glial differentiation but limits the ultimate number of glia by inhibiting proliferation of glial precursors. Cultured ENCDC exposed to GGF-2 begin to acquire a dependency for survival. Co-treatment with BMP4 greatly increases the number of cells that acquire such dependence on GGF-2 for survival (Chalazonitis et al., 2011a)
A major role for BMP signaling in the ENS is regulation of responses to other growth factors
An emerging theme is that BMP signaling exerts many of its pleiotropic effects on the ENS by regulating responses to other growth factors including GGF-2, GDNF, and NT-3. The effects of BMP2 or -4 on GDNF-induced neurogenesis remarkably parallel the effects on GGF-2-induced gliogenesis. In both cases BMP signaling enhances the effects of the factor on differentiation but limits effects on proliferation. Thus BMP signaling first regulates neurogenesis by modulating responses to GDNF and later regulates gliogenesis through its effects on GGF-2 responses (Chalazonitis et al., 2011a). Furthermore, BMPs promote growth factor dependency for survival of ENS neurons (on NT-3) and glia (on GGF-2) by inducing TrkC (neurons) and ErbB3 (glia). Taken together these observations in vitro point to the complicated effects of BMP signaling that are highly dependent upon the spatial and temporal window in which cells are exposed to BMP2 or -4 and the concentration of the factors that is encountered by the cells (see diagram in Fig1).
BMP4 REGULATES GANGLIOGENESIS AND NERVE PROCESSES FASCICULATION VIA POLYSIALYLATION OF THE NEURAL CELL ADHESION MOLECULE (N-CAM)
Once ENCDC have colonized the entire length of the gut and differentiated into neurons and glia, the cells coalesce into discrete ganglia that interconnect forming the chains that constitute the myenteric and submucosal plexuses (Epstein et al., 1991) (Newgreen and Hartley, 1995). Aggregation of neurons is promoted in vitro by high concentrations of BMP2 or -4, and the size of these clusters/ganglia is increased by co-treatment with GDNF (Chalazonitis et al., 2004). Although the precise molecular mechanisms underlying ganglion formation are unknown, several lines of evidence suggest that the neural cell adhesion molecule N-CAM and/or its polysialylated form, PSA-NCAM are involved. N-CAM is expressed in the developing ENS in chick, rat and human and PSA-NCAM plays a role in cell migration, axonal outgrowth and synapse formation during CNS development (Eckhardt et al., 2000), (Bruses, 2001). Expression of the sialyltransferases PST and STX is detected in fetal rat gut at E12, increases sharply by E14–E18, decreases at birth and is down-regulated in the adult (Faure et al., 2007). Neuronal clusters that form in vitro in response to BMP4 all express N-CAM, and BMP signaling promotes expression of PSA-NCAM, whereas GDNF signaling does not (Faure et al., 2007) (diagram in Fig1). Because polysialylation reduces the adhesive properties of N-CAM (Crossin and Krushel, 2000) (Weinhold et al., 2005), BMP signaling could alter the adhesion of neurons to one another or to the substrate via regulation of the expression of PSA-NCAM. To address this possibility, the polysialylation of N-CAM was inhibited by treating cultured ENCDC with two unnatural analogs of polysialic acids, both of which can be recognized by the sialyltransferases: N-butanoylmannosamine (ManBut) that blocks elongation of the sialic acid chain and N-propanoylmannosamine (ManProp) that does not (Mahal et al., 2001); (Charter et al., 2002). Clustering induced by BMP4 was completely inhibited with exposure to ManBut (2-5 mM) but not ManProp, indicating that PSA-NCAM is involved in the process. It is interesting to note that the highest levels of expression of both BMP4 and the sialyltransferase PST and STX, and the highest proportions of neurons expressing PSA-NCAM in both the myenteric plexus and the submucosal plexus (Faure et al., 2007), coincide at late embryonic stages of gut development. It is at these late fetal stages that reorganization of the chains of migrating neural crest cells takes place (Young et al., 2004), that the neurons become post-mitotic (Rothman et al., 1984) and that they cluster into ganglia (Epstein et al., 1991). Other studies of non-immunoisolated dissociated cultures and of explant cultures of fetal mouse gut also give evidence, in a more physiological, albeit less defined environment, of the importance of BMP induced polysialylation of N-CAM (Fu et al., 2006). BMP4 treatment restricted GDNF-induced migration of neural crest precursors in explants while noggin promoted it. Further, BMP4 treatment increased PSA-immunoreactivity on neural crest–cells and their neuritic processes and enhanced fasciculation of neuritic processes extending out of gut explants. Finally BMP4-induced aggregation of ganglion cells in murine bowel explants was confined to the outer wall of the bowel, while noggin treatment allowed the ganglion cells to remain dispersed through several layers (Fu et al., 2006).
BMP2 OR -4 LIMIT THE NUMBER OF ENTERIC NEURONS DEVELOPING IN VIVO
The studies using immunoisolated ENCDC provided quantitative analyses of the direct effects of BMP signaling on these cells and their progeny. To validate these culture findings in vivo where ENCDC interact with other cell layers and with multiple signaling molecules within an intact gut microenvironment, ENS development was examined in transgenic mice which overexpress either BMP4 (Gomes et al., 2003) or the BMP inhibitor noggin (Guha et al., 2004) under control of the neuron specific enolase (NSE) promoter. NSE expression becomes significant in mouse gut at E15-16 (Chalazonitis et al., 2008), so that transgenic changes in BMP signaling start at a time when colonization of the GI tract by the ENCDC is completed, neuronal progenitors are starting to differentiate, and glial progenitors are in a proliferative phase. The transgene continues to be expressed through the postnatal period; thus alterations in BMP signaling continue as subsets of neuronal precursors exit the cell cycle at distinct stages of late fetal and post-natal development and glia become differentiated. Quantitative analysis of the neuronal packing density was assessed using cuprolinic blue, a universal marker for enteric neurons (Karaosmanoglu et al., 1996) in transgenic mice aged P26-P34 (NSE-Nog) and P24-P25 (NSE-BMP4).
In the NSE-noggin mice there was a significant excess of neurons in both myenteric and submucosal plexuses in all regions of the GI tract (Chalazonitis et al., 2004) (see diagram Fig 2). In the myenteric plexus (MyP) the increase in homozygote transgenic mice ranged from 148% (ileum) to 168% (duodenum) of wild type (WT). There was a gene dosage effect, and the increase in density in heterozygote mice was significantly less in all regions of the gut ranging between 116% and 138% of WT. In contrast in the submucosal plexus (SmP) neurons numbers increased to similar degrees in heterozygotes and homozygotes mice from 123% (jejunum) to 156% (in duodenum) of WT. By contrast, in heterozygote mice over-expressing BMP4 there were no significant changes in neuronal density in either the myenteric or submucosal plexus (Chalazonitis et al., 2008). This suggests that the levels of BMP2 or -4 present in WT mice are already large relative to levels in the NSE-BMP4 transgenic mice. However in a different context where BMP signaling is constitutively active, the overall number of enteric neurons is significantly decreased from duodenum to colon in both plexuses (Chalazonitis et al., 2011b). This increased BMP signaling occurs in mice lacking the gene encoding the homeodomain interacting protein kinase-2 (HIPK-2) which is a transcriptional co-repressor that directly interacts with Smad1 and suppresses BMP-dependent reporter gene expression (Harada et al., 2003). The findings with these mice are consistent both with the observations in the noggin transgenic mice and with the inhibitory effects of BMP signaling observed in vitro (Chalazonitis et al., 2004) or in vivo (Sukegawa et al., 2000).
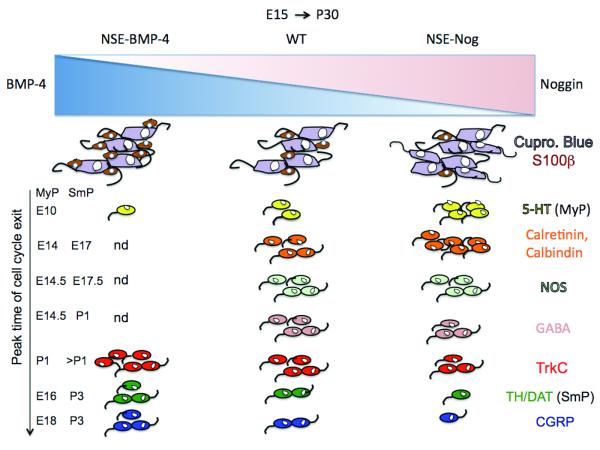
Figure 2. Altered BMP signaling in transgenic mice over-expressing noggin or BMP-4, changes the glia/neuron ratio and the proportions of neurons with defined phenotypes in relation to when they exit the cell cycle.
In mice over-expressing noggin under the control of the NSE promoter, BMP signaling is antagonized leading to an increased density of neurons (in mauve) in both plexuses, while glia density (in brown) is decreased (upper right). Conversely in mice over-expressing BMP-4 the density of neurons decreases while glial density increases (upper left). In the NSE-Nog mice the density and proportion of neurons exiting the cell cycle early, serotonin (in yellow), calbindin, calretinin (in orange) are increased (top of right column) whereas the proportions of those exiting the cell cycle late: GABA (in pink) , TrkC (in red) , TH/DAT (in green) , CGRP (in blue) are reduced (bottom of right column) in both plexuses. The proportion of the NOS-expressing neurons (light green) is not significantly altered since they exit the cell cycle neither too early nor too late compared to the other subtypes. In the NSE-BMP4 mice, the proportion of the earliest born serotonergic neurons is reduced (top left column) while the proportions of neurons that exit the cell cycle late (TrkC, TH/DAT, CGRP) are increased (bottom left column). nd: not determined.
BMP2 OR -4 PROMOTES GLIA DEVELOPMENT IN VIVO AND REGULATE THE GLIA/NEURON RATIO
In contrast to the effects on neuronal density, the glial packing density in NSE-noggin mice was significantly decreased (to 80% of WT) in both the MyP and SmP (Chalazonitis et al., 2011a). Conversely in heterozygote mice over–expressing BMP4 there was a significant increase (125% of WT) in the myenteric plexus of the duodenum. The size of the pool of ENCDC is finite; BMPs biological actions include promotion of cell cycle exit as well as differentiation on early and late developing populations of enteric precursors thus, depending on their concentration, the BMPs adjust the size of the pool destined for each neuronal and glial phenotypes. Thus increased BMP signaling enhances glial development at the expense of the number of neurons, and limiting BMP signaling conversely enhances neurogenesis at the expense of gliogenesis. The profound effects of BMP signaling in regulating the balance between neurogenesis and gliogenesis can be appreciated by comparing changes in the ratios of glia to neurons when BMP signaling is altered. For example, in NSE-noggin mice there was an extraordinary 2.5 fold decrease in the glia to neuron ratio in the myenteric plexus of the duodenum whereas there was a 50% to 75% increase in the ratio in NSE-BMP4 mice (Chalazonitis et al., 2011a). Taken together these data point out the critical role of BMP signaling in achieving a normal balance between neurogenesis and gliogenesis, thereby ensuring the integrity of ENS functions (see diagram in Fig. 2).
BMP SIGNALING REGULATES THE PHENOTYPIC DIVERSITY OF ENTERIC NEURONS
The diversity of neuronal phenotypes in the ENS is comparable to that of the CNS (Gershon, 2005; Furness, 2006). Defined enteric neuronal subsets are born at specific developmental times in the ENS i.e. serotonergic neurons are born first (from E10-E14) while those expressing the marker CGRP are among the last born neurons (from E17-late postnatal stages) (Pham et al., 1991). In general the subsets of neurons classified as being born early in the myenteric plexus (up to E14.5) are serotonergic, then calretinin- and calbindin- and then NOS-expressing-neurons. Because NOS neurons start to be born at E12.5 (Myp) and continue to be born beyond E17.5 (Myp) and P1 (SmP), this subset encompasses both early and late-born neurons. Subsets of neurons considered to be later born (from E14.5 until postnatal ages) include gabaergic, TrkC-CGRP- and dopaminergic/TH-expressing neurons. In the submucosal plexus cell cycle exit occurs for all phenotypes later than their myenteric plexus counterparts. Since BMP signaling regulates neuronal and glial differentiation in a time-dependent manner, the composition of neurons was examined in NSE-noggin and NSE-BMP4 mice to define a potential role for BMP signaling in regulating the composition of phenotypes (see diagram in Fig 2).
Decreased BMP signaling enhances early-born neuronal phenotypes and decreases later born ones
In the myenteric plexus of NSE-noggin mice, the density and proportions of serotonergic neurons (the earliest born population) increased 3-fold. The densities of calretinin- and calbindin-neurons also increased significantly but to a lesser extent than the serotonergic neurons. Similarly the density of NOS-expressing neurons increased slightly, although their proportion decreased (Chalazonitis et al., 2008) since the overall increase in neuron numbers was relatively larger. By contrast the densities and proportions of TrkC-neurons decreased significantly (2-3 fold compared to WT) in all gut regions (Chalazonitis et al., 2004). Similarly the densities and proportions of GABA- and CGRP-expressing neurons decreased significantly both in heterozygote and homozygote mice. In the submucosal plexus serotonergic neurons were not analyzed since they are expressed in the myenteric plexus only. However calretinin and calbindin neuronal densities both increased, particularly the calbindin-expressing neuronal population. By contrast the densities and proportions of TrkC-expressing neurons decreased greatly in all gut regions and by almost 4-fold in the ileum (Chalazonitis et al., 2004). In addition GABA-, CGRP-, TH- and DAT-expressing neurons all decreased significantly both in densities and proportions (Chalazonitis et al., 2008). Thus decreased BMP signaling enhanced early-born neuronal phenotypes and decreased later born ones whereas increased BMP signaling did the converse (see diagram in Fig 2).
Because most enteric neurons are cholinergic and co-express other neuropeptides (Rothman et al., 1984); (Sang and Young, 1998) it is likely that additional subsets of enteric neurons were altered that have not yet been analyzed. In fact the degree of overall neuronal hyperplasia that occurs in the NSE-noggin mice exceeds the added increases in proportions of serotonergic, calretinin, calbindin, and NOS neurons. However the data from both transgenic lines clearly indicate that BMP signaling acts to limit the size of the early born populations of neurons allowing the later born populations to increase in size, thus assuring the appropriate composition of the ENS. Since BMP signaling tends to promote exit from cell cycle, a change in the number of cell cycles that progenitors of each neuronal subset normally undergo prior to being born, may be the reason why 1) decreased BMP signaling exerts opposing effects on development of neurons that are born early (increase) from those that are born late (decrease) and why 2) increased BMP-signaling promotes the reverse. For example, in Drosophila early born neurons respond to a timer which depends on the number of cell cycles prior to differentiation while late-born neurons respond to a timer which is independent of cytokinesis (Harris, 2003; Pearson and Doe, 2003); (Grosskortenhaus et al., 2005). Thus it is plausible that decreased BMP signaling in the mouse gut may allow precursors of early-born neurons to prolong their cycling period thus generating more neurons, whereas increased BMP signaling may shorten their cycling period and reduce the number of progeny. As noted above, BMP signaling plays a critical role in the differentiation of later born populations (TrkC-TH-CGRP-expressing, etc) which helps to explain how it expands the pool of these cells and why loss of BMP signaling reduces the size of the later born populations.
BMP2 or -4 ALSO PROMOTE ENTERIC SMOOTH MUSCLE DIFFERENTIATION FROM NON-ENCDC DURING A LIMITED DEVELOPMENTAL WINDOW
Non-ENCDC (p75NTR−) cells include smooth muscle actin (SMA+)-expressing cells of mesodermal origin. Non-ENCDC isolated from E12 and E14 rat gut express BMPRII, -IA and –IB and respond to BMP in vitro with nuclear translocation of pSmad-1/5/8 (Chalazonitis et al., 2004). Treatment of SMA+-cells isolated from E12 with BMP2 or -4 increases the size of the population by more than 50%. By E14, however, SMA+-cells become less abundant, and neither BMP2 nor -4 treatment increases population size (Chalazonitis et al., 2004). These data are consistent with findings in early avian fetal gut that BMP4 enhances smooth muscle development (Roberts et al., 1995); (Roberts et al., 1998); (Roberts, 2000; Sukegawa et al., 2000). BMP2, BMPRII and –IA are expressed in the smooth muscle layer of E12-E13 mouse fetal gut. Moreover BMP2 treatment of gut-like structures that develop from mouse ES cells increased contractions and expression of SMA mRNA, and these effects were blocked by noggin (Torihashi et al., 2009).
To help define the role of BMP signaling on development of the smooth muscle layers in vivo, the thickness of the circular and longitudinal muscle layers was measured in E18 and 1 month post-natal NSE-noggin mice. In E18 fetal gut in NSE-noggin mice, development of the circular layer but not the longitudinal layer was enhanced. However, in 1 month-old NSE-noggin mice there was a significant increase in the number of layers in both the circular and longitudinal muscles (Chalazonitis et al., 2004). BMP2 or -4 treatment of immunoisolated non-ENCDCs promoted muscle cell differentiation at early embryonic stages and became ineffective at post-natal stages. Since noggin is not over expressed in the NSE-noggin mice until about E15, the predominant effect was blockade of the later inhibitory effects of BMP signaling thus leading to thicker smooth muscle layers. In toto these observations are consistent with a stimulatory effect of BMP signaling on differentiation of smooth muscle precursors during early fetal development (Roberts et al., 1995; Roberts D.J., 1998; Roberts, 2000) but an inhibitory effect later in development (Sukegawa et al., 2000).
GUT MOTILITY AND GI TRANSIT TIME ARE ABNORMAL IN NSE-NOGGIN MICE
Since the ENS of the NSE-noggin mice displayed significant neuronal hyperplasia, decreases in the glia/neuron ratios in both plexuses, changes in the relative proportions of many neuronal phenotypes, and increased thickness of the smooth muscle layers, anomalies in GI tract function might be expected. Stool frequency, wet weight and water content were all higher in the NSE-noggin compared to WT littermates, although body weights did not differ significantly. The gastrointestinal transit time (GTT) was determined through gavage of P-35-38 NSE-noggin and WT animals with spores of the heat resistant Bacillus stearothermophilus. Interestingly whereas the mean GTT was not significantly different between NSE-noggin (9.9 hr) and WT (10.2 hr) mice, the coefficient of variation of transit values was the double in NSE-noggin (24.2%) compared to WT (10.3%). Transit of spores in the GI tract of NSE-noggin mice was highly irregular; for example the percentage of spores recovered by 3 hr was more than 10% compared to less than 1% in WT, while the remaining 90% of the spores was retained for an abnormally long time compared to WT (Chalazonitis et al., 2008). While animals in protected environments may sustain such fluctuations in transit time, it is likely that in the wild (i.e. under stress) they would not adapt so well. The irregular periods of blockage of GTT in the NSE-noggin mice is reminiscent of the bowel obstruction that occurs in infants diagnosed with intestinal ganglioneuromatosis associated with the MEN2B mutations of the RET gene (Smith et al., 1999).
DEFECT IN THE GENE ENCODING SMAD-INTERACTING PROTEIN-1
(SIP-1/ZEB-2) CAUSES HIRSCHPRUNG’S DISEASE (HSCR) AS WELL AS OTHER NEURAL CREST DEFECTS
Identification of SIP-1 as a cause of HSCR provides additional genetic evidence of the essential and pleiotropic roles played by the BMPs in development of the ENS. SIP-1 is a family member of two-handed zinc finger/homeodomain proteins (Zinc finger-E box binding homeobox 2/ Zeb-2); it contains a homeodomain like sequence and 2 clusters of zinc fingers at the amino and carboxy terminals in addition to a Smad binding domain. SIP-1 can thus interfere with BMP signaling by interacting with receptors activated full length Smads (Wakamatsu et al., 2001). Human patients with de novo mutations identified in a deleted segment of chromosome 2q22, have HSCR associated with microcephaly, mental retardation, epilepsy and facial anomalies. SIP1/ZFHX1B/ZEB2 maps to this region, and its over-expression in Xenopus causes abnormal head phenotypes (Eisaki et al., 2000). There are 97% similarities in amino acids between mouse and human homologs of SIP1. Mice lacking the ZFHX1B gene, similarly exhibit neural crest-cells defects leading to HSCR because of suboptimal development of the vagal neural crest (Van de Putte et al., 2003). This phenotype demonstrates directly the essential role of SIP-1 in the migration and colonization of the distal gut by ENCDC, implicating suboptimal BMP signaling in this process.
CONCLUDING REMARKS AND OUTLOOK
The relative simplicity of peripheral nervous system ganglia is a major reason why Ira Black focused much of his attention on development of sympathetic and sensory ganglia (Black et al., 1971; Cochard et al., 1978). As knowledge of developmental neurobiology became more sophisticated, it also became necessary to understand how developing neural elements interact with other organ cells and structures. The gut is particularly suited for such analyses since it includes not only an extensive intrinsic nervous system, comprised of neuronal phenotypes as diverse as those in the CNS, but also a mucosal epithelium of variable morphology and secretory cells, an immune system, a lamina propria, a submucosa and layers of smooth muscle.
BMP signaling first regulates regionalization of the primordial gut with differentiation in the anterior to posterior direction into foregut, midgut and hindgut. The BMPs next establish the map of regionalization of the layers of tissues along the radial axis, from epithelium, lamina propria, to circular and longitudinal smooth muscle layers. When vagal ENCDC cells begin to enter the gut, BMP signaling regulates their migration to and colonization of their properly regionalized targets. These processes require BMP signaling to be exquisitely controlled for the right period of time, at the right concentration and at the right location so that: 1) adequate numbers of ENCDC migrate; 2) balanced numbers of neuronal and then glial precursors proliferate, exit the cell cycle and then differentiate; 3) diverse neuronal populations are established in the right proportions; 4) receptors for neurotrophic factors are up-regulated enabling neurons, or committed glia, to respond to specific survival factors; 5) neurons express polysialylated adhesion molecules to allow aggregation of neurons into ganglia. In contrast to ganglia of the PNS, it is remarkable that the nascent myenteric and submucosal ganglia are embedded within their target of innervation, the longitudinal and circular smooth muscle layers respectively. Since BMP signaling regulates smooth muscle layer differentiation as well as the proper axonal elongation and fasciculation of the neurons, it can help coordinate the establishment of the proper functional interactions between the pre-synaptic neurons and the smooth muscles. BMP signaling could also regulate interganglionic synaptic interactions within the plexuses of the ENS. In this regard, it is of interest to note that in mice deficient with the gene encoding the transcriptional co-repressor HIPK-2, an imbalance between TGFβ and BMP signaling occurs with excessive BMP signaling resulting in arrest of synaptic development and synaptic loss in the post-natal ENS (Chalazonitis et al., 2011b). There are many remaining challenges to understanding how BMP signaling regulates development of the ENS. However it is remarkable how the field of developmental neurobiology has expanded since Ira Black began his pioneering work just a few decades ago.
Acknowledgements
As long term collaborators with Dr. Michael D. Gershon, the authors are indebted to him for many reasons: most of the studies on the roles of BMPs in development of the ENS were performed in his laboratory; through the years of this collaborative effort, Dr. Gershon’s contributions and support have been essential to bring this body of work to fruition. Within Dr. Gershon’s group we thank Drs. Taube Rothman, Fabien D’Autréaux (Institut de Biologie, Université de Nice Sophia-Antipolis, Nice, France), Tuan D. Pham, Christophe Faure (Hospital St Justine, Montreal), Jason Chen, Zhishan Li and also the technical support of many, including Daniel Roman, Wanda Setlik and Valerie Boone. Within Dr. Kessler’s group thanks are due to Drs. Udayan Guha (National Cancer Institute, Bethesda MD), William Gomes (Montefiore Medical Center, N.Y.), and Lixin Kan; also to be acknowledged, is the recent collaboration with Dr. Eric Huang’s laboratory (UCSF, San Francisco, CA). We also wish to thank the many colleagues who have donated crucial antibodies to perform these studies, Drs. Moses Chao (Skirball Institute, N.Y.), Bob Rush (Flinders University, Adelaide), Aris Economides (Regeneron, Pharmaceuticals, N.Y.), Barbara Hempstead and Pouneh Kermani (Weill-Cornell Medical College, N.Y.), David Kaplan, (Sick Kids Hospital, Toronto, Canada), Peter ten-Dijke, (the Netherlands Cancer Institute, Amsterdam,), Marc Marchionni, (Alzcor, Pharmaceuticals, MA), Lena Hofer, (Acorda Therapeutics, N.Y.), David Anderson, (California Institute of Technology) and Thomas Müller, (Max Delbruck Center for Molecular Medicine, Berlin, Germany). This research has been supported by NIH grant numbers DK58056 (to AC), NS20778 and NS20013 (to JAK) and NS15547 (to MDG).
REFERENCES
- Attisano L, Wrana JL. Smads as transcriptional co-modulators. Current Opinion in Cell Biology. 2000;12:235–243. doi: 10.1016/s0955-0674(99)00081-2. [DOI] [PubMed] [Google Scholar]
- Baetge G, Pintar JE, Gershon MD. Transiently catecholaminergic (TC) cells in the bowel of fetal rats and mice: Precursors of non-catecholaminergic enteric neurons. Dev. Biol. 1990;141:353–380. doi: 10.1016/0012-1606(90)90391-u. [DOI] [PubMed] [Google Scholar]
- Black IB, Bloom FE, Hendry IA, Iversen LL. Growth and development of sympathetic ganglion: maturation of transmitter enzymes and synapse formation in the mouse superior cervical ganglion. J Physiol. 1971;215:24P–25P. [PubMed] [Google Scholar]
- Brewer KC, Mwizerva O, Goldstein AM. BMPRIA is a promising marker for evaluating ganglion cells in the enteric nervous system--a pilot study. Hum Pathol. 2005;36:1120–1126. doi: 10.1016/j.humpath.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bruses JL, Rutishauser U. Roles, regulation and mechanism of polysialic acid function during neural development. Biochimie. 2001;83:635–643. doi: 10.1016/s0300-9084(01)01293-7. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Thapar N. Advances in ontogeny of the enteric nervous system. Neurogastroenterol Motil. 2006;18:876–887. doi: 10.1111/j.1365-2982.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A. Neurotrophin-3 in the development of the enteric nervous system. Prog Brain Res. 2004;146:243–263. doi: 10.1016/S0079-6123(03)46016-0. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, D’Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA, Gershon MD. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24:4266–4282. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, D’Autreaux F, Pham TD, Kessler JA, Gershon MD. Bone morphogenetic proteins regulate enteric gliogenesis by modulating ErbB3 signaling. Dev Biol. 2011;350:64–79. doi: 10.1016/j.ydbio.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Pham TD, Li Z, Roman D, Guha U, Gomes W, Kan L, Kessler JA, Gershon MD. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J Comp Neurol. 2008;509:474–492. doi: 10.1002/cne.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Pham TD, Rothman TP, DiStefano PS, Bothwell M, Blair-Flynn J, Tessarollo L, Gershon MD. Neurotrophin-3 is required for the survival-differentiation of subsets of developing enteric neurons. J Neurosci. 2001;21:5620–5636. doi: 10.1523/JNEUROSCI.21-15-05620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Gershon MD. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: expression of GFRalpha-1 in vitro and in vivo. Dev Biol. 1998;204:385–406. doi: 10.1006/dbio.1998.9090. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Lamballe F, Barbacid M, Gershon MD. Neurotrophin-3 induces neural crest-derived cells from fetal rat gut to develop in vitro as neurons or glia. J Neurosci. 1994;14:6571–6584. doi: 10.1523/JNEUROSCI.14-11-06571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Vinson EN, MacLennan AJ, Gershon MD. Promotion of the development of enteric neurons and glia by neuropoietic cytokines: Interactions with neurotrophin-3. Dev. Biol. 1998;198:343–365. [PubMed] [Google Scholar]
- Chalazonitis A, Tang AA, Shang Y, Pham TD, Hsieh I, Setlik W, Gershon MD, Huang EJ. Homeodomain Interacting Protein Kinase 2 Regulates Postnatal Development of Enteric Dopaminergic Neurons and Glia via BMP Signaling. J Neurosci. 2011;31:13746–13757. doi: 10.1523/JNEUROSCI.1078-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Tennyson VM, Kibbey MC, Rothman TP, Gershon MD. The alpha1 subunit of laminin-1 promotes the development of neurons by interacting with LBP110 expressed by neural crest-derived cells immunoselected from the fetal mouse gut. J Neurobiol. 1997;33:118–138. doi: 10.1002/(sici)1097-4695(199708)33:2<118::aid-neu2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Charter NW, Mahal LK, Koshland DE, Jr., Bertozzi CR. Differential effects of unnatural sialic acids on the polysialylation of the neural cell adhesion molecule and neuronal behavior. J Biol Chem. 2002;277:9255–9261. doi: 10.1074/jbc.M111619200. [DOI] [PubMed] [Google Scholar]
- Cho KW, Blitz IL. BMPs, Smads and metalloproteases: extracellular and intracellular modes of negative regulation. Curr. Opin. in Gen. & Dev. 1998;8:443–449. doi: 10.1016/s0959-437x(98)80116-0. [DOI] [PubMed] [Google Scholar]
- Cochard P, Goldstein M, Black IB. Ontogenetic appearance and disappearance of tyrosine hydroxylase and catecholamines in the rat embryo. Proc Natl Acad Sci U S A. 1978;75:2986–2990. doi: 10.1073/pnas.75.6.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin KL, Krushel LA. Cellular signaling by neural cell adhesion molecules of the immunoglobulin superfamily. Dev Dyn. 2000;218:260–279. doi: 10.1002/(SICI)1097-0177(200006)218:2<260::AID-DVDY3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- De Santa Barbara P, Williams J, Goldstein AM, Doyle AM, Nielsen C, Winfield S, Faure S, Roberts DJ. Bone morphogenetic protein signaling pathway plays multiple roles during gastrointestinal tract development. Dev Dyn. 2005;234:312–322. doi: 10.1002/dvdy.20554. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Bukalo O, Chazal G, Wang L, Goridis C, Schachner M, Gerardy-Schahn R, Cremer H, Dityatev A. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci. 2000;20:5234–5244. doi: 10.1523/JNEUROSCI.20-14-05234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisaki A, Kuroda H, Fukui A, Asashima M. XSIP1, a member of two-handed zinc finger proteins, induced anterior neural markers in Xenopus laevis animal cap. Biochem Biophys Res Commun. 2000;271:151–157. doi: 10.1006/bbrc.2000.2545. [DOI] [PubMed] [Google Scholar]
- Epstein ML, Poulsen KT, Thiboldeaux R. Formation of ganglia in the gut of the chick embryo. J Comp Neurol. 1991;307:189–199. doi: 10.1002/cne.903070203. [DOI] [PubMed] [Google Scholar]
- Faure C, Chalazonitis A, Rheaume C, Bouchard G, Sampathkumar SG, Yarema KJ, Gershon MD. Gangliogenesis in the enteric nervous system: roles of the polysialylation of the neural cell adhesion molecule and its regulation by bone morphogenetic protein-4. Dev Dyn. 2007;236:44–59. doi: 10.1002/dvdy.20943. [DOI] [PubMed] [Google Scholar]
- Fu M, Lui VC, Sham MH, Pachnis V, Tam PK. Sonic hedgehog regulates the proliferation, differentiation, and migration of enteric neural crest cells in gut. J Cell Biol. 2004;166:673–684. doi: 10.1083/jcb.200401077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Vohra BP, Wind D, Heuckeroth RO. BMP signaling regulates murine enteric nervous system precursor migration, neurite fasciculation, and patterning via altered Ncam1 polysialic acid addition. Dev Biol. 2006;299:137–150. doi: 10.1016/j.ydbio.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB. The Enteric Nervous System. Blackwell Publishing Narayana Press; Odder, Denmark: 2006. [Google Scholar]
- Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39:S184–193. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosci. 2010;33:446–456. doi: 10.1016/j.tins.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Brewer KC, Doyle AM, Nagy N, Roberts DJ. BMP signaling is necessary for neural crest cell migration and ganglion formation in the enteric nervous system. Mech Dev. 2005;122:821–833. doi: 10.1016/j.mod.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Grosskortenhaus R, Pearson BJ, Marusich A, Doe CQ. Regulation of temporal identity transitions in Drosophila neuroblasts. Dev Cell. 2005;8:193–202. doi: 10.1016/j.devcel.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Guha U, Mecklenburg L, Cowin P, Kan L, O’Guin WM, D’Vizio D, Pestell RG, Paus R, Kessler JA. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729–740. doi: 10.1016/S0002-9440(10)63336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao MM, Anderson RB, Kobayashi K, Whitington PM, Young HM. The migratory behavior of immature enteric neurons. Dev Neurobiol. 2009;69:22–35. doi: 10.1002/dneu.20683. [DOI] [PubMed] [Google Scholar]
- Hao MM, Young HM. Development of enteric neuron diversity. J Cell Mol Med. 2009;13:1193–1210. doi: 10.1111/j.1582-4934.2009.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada J, Kokura K, Kanei-Ishii C, Nomura T, Khan MM, Kim Y, Ishii S. Requirement of the co-repressor homeodomain-interacting protein kinase 2 for ski-mediated inhibition of bone morphogenetic protein-induced transcriptional activation. J Biol Chem. 2003;278:38998–39005. doi: 10.1074/jbc.M307112200. [DOI] [PubMed] [Google Scholar]
- Harris WA. Developmental biology: clocking the birth of neurons. Nature. 2003;425:568–569. doi: 10.1038/425568a. [DOI] [PubMed] [Google Scholar]
- Hearn CJ, Murphy M, Newgreen D. GDNF and ET-3 differentially modulate the numbers of avian neural crest cells and enteric neurons in vitro. Dev.Biol. 1998;197:93–105. doi: 10.1006/dbio.1998.8876. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Lampe PA, Johnson EM, Jr., Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in Vitro. Dev. Biol. 1998;200:116–129. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Gen. Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Karaosmanoglu T, Aygun B, Wade PR, Gershon MD. Regional differences in the number of neurons in the myenteric plexus of the guinea pig small intestine and colon: an evaluation of markers used to count neurons. Anat. Rec. 1996;244:470–480. doi: 10.1002/(SICI)1097-0185(199604)244:4<470::AID-AR5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Levanti MB, Esteban I, Ciriaco E, Perez-Pinera P, Cabo R, Garcia-Suarez O, Pardo B, Silos-Santiago I, Cobo J, Vega JA. Enteric glial cells express full-length TrkB and depend on TrkB expression for normal development. Neurosci Lett. 2009;454:16–21. doi: 10.1016/j.neulet.2009.02.051. [DOI] [PubMed] [Google Scholar]
- Mabie PC, Mehler MF, Kessler JA. Multiple roles of Bone Morphogenetic Protein signaling in the regulation of cortical cell number and phenotype. J.Neurosci. 1999;19:7077–7088. doi: 10.1523/JNEUROSCI.19-16-07077.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahal LK, Charter NW, Angata K, Fukuda M, Koshland DE, Jr., Bertozzi CR. A small-molecule modulator of poly-alpha 2,8-sialic acid expression on cultured neurons and tumor cells. Science. 2001;294:380–381. doi: 10.1126/science.1062192. [DOI] [PubMed] [Google Scholar]
- Marchionni MA, Goodearl ADJ, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, Wroblewski D, Lynch C, Baldassare M, Hiles I, Davis JB, Hsuan JJ, Totty NF, Otsu M, McBurney RN, Waterfield MD, Stroobant P, Gwynne D. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic proteins in the nervous system. TINS. 1997;20:309–317. doi: 10.1016/s0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- Newgreen DF, Hartley L. Extracellular matrix and adhesive molecules in the early development of the gut and its innervation in normal and spotting lethal rat embryos. Acta Anat (Basel) 1995;154:243–260. doi: 10.1159/000147776. [DOI] [PubMed] [Google Scholar]
- Nielsen C, Murtaugh LC, Chyung JC, Lassar A, Roberts DJ. Gizzard formation and the role of Bapx1. Dev Biol. 2001;231:164–174. doi: 10.1006/dbio.2000.0151. [DOI] [PubMed] [Google Scholar]
- Pearson BJ, Doe CQ. Regulation of neuroblast competence in Drosophila. Nature. 2003;425:624–628. doi: 10.1038/nature01910. [DOI] [PubMed] [Google Scholar]
- Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J. Comp. Neurol. 1991;1991:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- Pisano JM, Colon-Hastings F, Birren SJ. Postmigratory enteric and sympathetic neural precursors share common, developmentally regulated, responses to BMP2. Dev Biol. 2000;227:1–11. doi: 10.1006/dbio.2000.9876. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Roberts DJ. Molecular Mechanisms of Development of the Gastrointestinal Tract. Developmental Dynamics. 2000;219:109–120. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1047>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Smith DM, Goff DJ, Tabin CJ. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–2801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- Rothman TP, Nilaver G, Gershon MD. Colonization of the developing murine enteric nervous system and subsequent phenotypic expression by the precursors of peptidergic neurons. J Comp Neurol. 1984;225:13–23. doi: 10.1002/cne.902250103. [DOI] [PubMed] [Google Scholar]
- Sang Q, Young HM. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat Rec. 1998;251:185–199. doi: 10.1002/(SICI)1097-0185(199806)251:2<185::AID-AR6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D, Kalcheim C. Regulation of the onset of neural crest migration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development. 1999;126:4749–4762. doi: 10.1242/dev.126.21.4749. [DOI] [PubMed] [Google Scholar]
- Selleck MA, Garcia-Castro MI, Artinger KB, Bronner-Fraser M. Effects of Shh and Noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm. Development. 1998;125:4919–4930. doi: 10.1242/dev.125.24.4919. [DOI] [PubMed] [Google Scholar]
- Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DA. Glial Growth Factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Smith DM, Nielsen C, Tabin CJ, Roberts DJ. Roles of BMP signaling and Nkx2.5 in patterning at the chick midgut-foregut boundary. Development. 2000;127:3671–3681. doi: 10.1242/dev.127.17.3671. [DOI] [PubMed] [Google Scholar]
- Smith VV, Eng C, Milla PJ. Intestinal ganglioneuromatosis and multiple endocrine neoplasia type 2B:implications for treatment. Gut. 1999;45:143–146. doi: 10.1136/gut.45.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Mehler M, Kessler JA. Bone morphogenetic proteins induce apoptosis and growth factor dependence of cultured sympathoadrenal progenitor cells. Dev.Biol. 1998;196:119–127. doi: 10.1006/dbio.1998.8847. [DOI] [PubMed] [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endoderm epithelium. Development. 2000;127:1971–1980. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Hattori T, Hasegawa H, Kurahashi M, Ogaeri T, Fujimoto T. The expression and crucial roles of BMP signaling in development of smooth muscle progenitor cells in the mouse embryonic gut. Differentiation. 2009;77:277–289. doi: 10.1016/j.diff.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Van de Putte T, Maruhashi M, Francis A, Nelles L, Kondoh H, Huylebroeck D, Higashi Y. Mice lacking ZFHX1B, the gene that codes for Smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the etiology of Hirschsprung disease-mental retardation syndrome. Am J Hum Genet. 2003;72:465–470. doi: 10.1086/346092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu N, Yamada Y, Yamada K, Ono T, Nomura N, Taniguchi H, Kitoh H, Mutoh N, Yamanaka T, Mushiake K, Kato K, Sonta S, Nagaya M. Mutations in SIP1, encoding Smad interacting protein-1, cause a form of Hirschsprung disease. Nat Genet. 2001;27:369–370. doi: 10.1038/86860. [DOI] [PubMed] [Google Scholar]
- Weinhold B, Seidenfaden R, Rockle I, Muhlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280:42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten-Dijke P, Heldin C-H, Miyazono K. Bone morphogenetic protein receptors. Bone. 1996;19:569–574. doi: 10.1016/s8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- Yntema C, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–542. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- Young HM, Bergner AJ, Anderson RB, Enomoto H, Milbrandt J, Newgreen DF, Whitington PM. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol. 2004;270:455–473. doi: 10.1016/j.ydbio.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Zhang D, Mehler MF, Song Q, Kessler JA. Development of bone morphogenetic protein receptors in the nervous system and possible roles in regulating trkC expression. J Neurosci. 1998;18:3314–3326. doi: 10.1523/JNEUROSCI.18-09-03314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]