Abstract
Rationale
Cardiac progenitor cells are important for maintenance of myocardial structure and function, but molecular mechanisms governing these progenitor cells remain obscure and require elucidation to enhance regenerative therapeutic approaches.
Objective
To understand consequences of stem cell antigen-1 (Sca-1) deletion upon functional properties of c-kit+ cardiac progenitor cells and myocardial performance using a Sca-1 knockout/Green Fluorescent Protein knock-in reporter mouse (ScaKI).
Methods and Results
Genetic deletion of Sca-1 results in early-onset cardiac contractile deficiency as determined by echocardiography and hemodynamics as well as age-associated hypertrophy. Resident cardiac progenitor cells in ScaKI mice do not respond to pathological damage in vivo, consistent with observations of impaired growth and survival of ScaKI cardiac progenitor cells in vitro. The molecular basis of the defect in ScaKI cardiac progenitor cells is associated with increased canonical Wnt signaling pathway activation consistent with molecular characteristics of lineage commitment.
Conclusions
Genetic deletion of Sca-1 causes primary cardiac defects in myocardial contractility and repair consistent with impairment of resident cardiac progenitor cell proliferative capacity associated with altered canonical Wnt signaling.
Keywords: Sca-1, c-kit, heart, cardiac progenitor cell, infarction, myocardium, Sca-1 knock-out, β–catenin, cardiac development
INTRODUCTION
Identification of multi-potent stem cells in adult tissues such as the myocardium rests with stem cell markers originally developed to define cells of hematopoietic lineage. The c-kit cell surface receptor is commonly used to mark stem/progenitor cells. c-kit has been identified on small non-myocytes within the myocardium referred to as cardiac progenitor cells (CPCs) that mediate myocardial repair following ischemic injury1, 2. CPCs also express the cell surface marker Stem cell antigen-1 (Sca-1) both in vitro3 and in vivo4. Sca-1 is an 18 kDa glycosyl phosphatidylinositol-anchored cell surface protein member of the Ly-6 antigen family with cell signaling and adhesion properties5 present on the surface of tissue-specific stem cells in the hematopoietic6, mammary gland7, and vascular niche8 in the mouse. Although typically associated with tissue stem or progenitor cells, Sca-1 is also expressed on a variety of fully differentiated cell types9.
Roles of Sca-1 protein in stem cell development and function primarily defined in the hematopoietic lineage initially described over 15 years ago in the mouse10 led to use for marking and enrichment of stem cells. Functions of Sca-1 include promotion of adhesion and proliferation that are critical for optimal hematopoietic activity6. Furthermore, Sca-1 is a part of a family of proteins that contain a urokinase plasminogen activator reporter (UPAR) domain involved in cellular adhesion, migration by modulating integrin function, and degradation of the extracellular matrix6. In addition, Sca-1 has been implicated in signaling for cellular differentiation11. Isolated mesenchymal stem cells positive for both Sca-1 and c-kit differentiate into cardiomyocytes, endothelial cells, and smooth muscle cells upon injection into the heart12. The number of Sca-1 positive cells increases in the left ventricle of the heart after myocardial infarction13 and progenitor cells from bone marrow also migrate to injured myocardium following infarction and contribute to repair14 suggesting that Sca-1+ cells contribute to regeneration and repair following a myocardial infarction (MI). Collectively, these characteristics make Sca-1 a critical protein for evaluating stem cells in the heart.
Consequences of Sca-1 deletion upon the functional properties of myocardial c-kit+ CPCs and myocardial performance were assessed in the present study using Sca-1 reporter mice with a single allele GFP knock-in, Sca-1GFP/+ that labels Sca-1+ cells in peripheral blood with GFP15. Findings were generated using this Sca-1 Knock-In (ScaKI) mouse model with both alleles of the Sca-1 protein coding sequence replaced with GFP downstream of the endogenous Sca-1 promoter, generating a mouse with a genetic deletion of ScaKI possessing the genotype of Sca-1GFP/GFP. Prior studies reveal the ScaKI mouse exhibits serial bone marrow stem cell repopulating defects but has not been previously studied for a cardiac phenotype6, although an unrelated murine model of Sca-1 deletion has been postulated to possess an impaired response to pathological challenge due to defective Nkx2.5 positive non-myocytes16. In comparison, our findings using the ScaKI line reveal deleterious effects of Sca-1 deletion upon c-kit+ CPCs and associated underlying signaling mechanisms.
METHODS
Transgenic animal models
Sca-1 knock-in transgenic mice were a generous gift from Dr. Timothy Graubert6, 15. A construct encoding GFP was inserted into the second exon of the Sca-1 gene at the start codon, functionally eliminating Sca-1 protein expression and creating a mouse in which GFP was expressed under the control of the endogenous Sca-1 promoter. These animals were then back-crossed into a C57/Bl6 background to create the ScaKI mice used in these experiments which are homologous for the recombined genes resulting in a transgenic line which is negative for Sca-1, but positive for GFP wherever Sca-1 promoter is expressed. ScaKI transgenic mice were created using the C57/Bl6 background. Hereafter, C57/Bl6 animals will be denoted as normal non-transgenic control animals (NTG). All experimental procedures were designed in accordance with NIH guidelines and approved by the San Diego State University and the Institutional Animal Care and Use Committees.
Surgical methods
Echocardiography was performed under minimal (1.25%) isoflurane anesthesia with 2.5% supplemented oxygen using an Acuson Sequoia C256 instrument. Myocardial infarctions were carried out under 1.5% isoflurane anesthesia by ligating the left descending coronary artery with 8-0 suture. To confirm adequate ligation, cyanosis and akinesia of the affected left ventricle were observed. Sham operations were performed by opening and closing the chest. Post-operative palliative care was administered immediately and at 12 hours. Hemodynamic measurements were accomplished with a closed chest by inserting a pressure/volume catheter through the aortic valve into the left ventricle under chloral hydrate sedation. Data was collected and analyzed using a Scisense microtip pressure transducer attached to an A/D converter. To generate paraffin embedded cardiac samples, mice were heavily sedated with chloral hydrate, and the hearts were arrested in diastole by catheterizing the abdominal aorta and flushing the heart with a high potassium chloride/cadmium chloride solution. Ten percent neutral buffered formalin (NBF) fixative was perfused into the coronary arteries at systolic pressure while the left ventricle was filled with NBF at diastolic pressure. Retroperfused hearts were then removed from the chest cavity and placed in NBF overnight, followed by processing for paraffin embedding using a Leica TP1050 automated tissue processor.
Immunofluorescence microscopy
Paraffin embedded tissue sections, cut at 5μm, were prepared from mouse hearts that had been processed as described above. Immunohistochemistry protocol is as previously described17 and is detailed in the method supplement. Antibody information is detailed in Online Table I. Confocal images and counts were acquired using a Leica TCS SP2 confocal microscope.
Parrafin embedded cell counts
Hearts were harvested at the specific developmental time points described in the text and paraffin embedded. At least three non-sequential sections were cut and stained per heart. The area included within the count was measured using the Leica LCS Lite (Leica AG) software.
Cardiac stem cell isolation and culture
CPCs were isolated and cultured as previously described1. Briefly, two mice per preparation were anesthetized using ketamine-xylazine solution and the heart was cannulated through the aortic arch and perfused at 37°C in oxygenated basic buffer (J-MEM, 0.7 g/L Hepes, 1.25g/L taurine, 20 units/L insulin, Pen/Strep/Glutamine, Amphotericin, Gentamicin, pH 7.3) on a Radnotti apparatus. The heart was then digested for 12 minutes at 37°C in 320units/Ml collagenase II in oxygenated basic buffer. Afterwards, the heart was minced in basic buffer containing 0.5% BSA and the cardiomyocytes pelleted for 1 min at 100g and discarded. Remaining cells in the supernatant were passed through a 25μm filter and pelleted. The cell pellet was resuspended and incubated with anti-c-kit (CD117) Miltenyi beads in PBS + 0.5% BSA; washed and isolated on a Miltenyi magnetic column to extract c-kit+ CPCs according to manufacturer’s instructions. CPCs were cultured according to standard tissue culture protocols in CPC media (DMEM/F12, 10% Embryonic Stem Cell-Grade FBS, PSG, Insulin-Transferrin-Selenium, 1000 units/mL LIF, 40ng/ml EGF, 20ng/mL bFGF).
Flow cytometry and apoptosis analysis
Cells were grown in complete media and analyzed by flow cytometry after non-enzymatic removal from the culture plates. The cells were resuspended at 100,000 cells in 100μL PBS+2% FBS, stained with directly conjugated antibodies and analyzed following two wash steps. For apoptosis treatment and analysis, CPCs were seeded into 6 well plates in CPC media for at least 12 hours before treatment. Treated cells received the indicated amount of hydrogen peroxide (H2O2) for 5 hours. After peroxide incubation, treated cells and untreated controls were labeled with Annexin V-APC Apoptosis Detection Kit (BD Pharmingen) according to manufacturer’s instructions. Briefly, cells were suspended at 100,000 cells per 100μL in Annexin V Binding Buffer. Each set of treated and untreated cells was incubated for 20 minutes at room temperature in the dark with 0.75μL Annexin V and 2μL 7-AAD per 100,000 cells. 200μL of Binding Buffer was added post incubation and the cells were analyzed by flow cytometry with a BD FACSAria instrument. Unstained and single stain controls were used to establish baseline fluorescence levels for the samples.
Trypan blue and cyquant assay
Cell cultures were counted after passage using 2% trypan blue solution by hemocytometer determination. For trypan blue assay, 15,000 cells per well were seeded at Day 0 in a 6 well plate and counted on a hemocytometer at the indicated days post plating. For Cyquant analysis, 1000 cells per well were seeded in triplicate Day 0 early in the morning in 100μl of full growth media. In the evening of Day 0 through Day 5, the growth media was gently removed from the appropriate wells and replaced with 1xHBSS containing Cyquant NF reagent diluted 1:500. Fluorescence was measured 30 minutes later on a Tecan Spectraflour Plus plate reader at an Excitation/Emmission of 485/535 with a standard gain of 70.
qPCR
mRNA was isolated from cells using Zymo Research quick RNA miniprep according to manufacturer’s instructions. Briefly, at least 100,000 cells per sample were collected in ZR RNA buffer, applied to a binding column and centrifuged at 12000 rpm for 1 minute. Flow through was discarded and washed once with RNA Wash Buffer. The RNA was eluted in DNAse/RNase free water and quantitated by spectrophotometer. RT-PCR was accomplished using the Bio-Rad iScript cDNA synthesis kit according to manufacture’s instructions. Briefly, 1μg of RNA per sample was added to 4μl of 5X Master Mix, 1μL of 20x reverse transcriptase and RNase free water up to 20μL. The rtPCR reaction conditions were 5 min at 25°C, 30 min at 42°C followed by 5 min 85°C. cDNA was diluted at least 1:60 in RNase/DNase free water. qPCR was accomplished using 20–30μg of cDNA, 3μmol/L forward and reverse primers and 2x Bio-Rad Sybr Green master mix at a final volume of 15μL. qPCR was performed on a Bio-rad/MJ research Opticon instrument with amplification protocol: 10 min at 95°C; then 15 sec at 95°C, 60 sec at 60°C, then plate read for 50 cycles followed by a melt curve analysis for product specificity. qPCR primer sequences are detailed in Online Table II. Fold difference analysis was calculated using the 2⊥ΔΔCt method using GAPDH as housekeeping gene control. qPCR arrays were accomplished according to manufacturer instructions by adding 25μL per well of 2x Bio-Rad Sybr Green master mix and 25μL per well of cDNA. SA Biosciences qPCR array primer sequences (RT2 Profiler™ PCR Array Mouse Signal Transduction PathwayFinder, PAMM-014A) are proprietary information.
siRNA
CPCs derived from FVB/N mice were plated in 10ml full growth media for 12 hours at 500,000 cells per 10cm plate. The media was replaced with Transfection Media consisting of CPC growth media containing 0% FBS. siRNA for mouse Sca-1 (Ly6A) was obtained from Bioneer (110454, proprietary sequence), and resuspended at 100μmol/l in DNAse/RNAse free water. Transfection mix containing 425μl of Transfection Media, 50μL of HiPerfect (Qiagen) transfection reagent and 15μl of 20μmol/L siRNA was added to the 10cm plate and incubated for 24 hours. The media was then changed to DMEM/F12 with 2.5% FBS and incubated for an additional 24 hours. Cells were then passaged and plated in full CPC media for the indicated assays.
SuperTopFlash luciferase assays
The SuperTopFlash 7x TCF/Lef-luciferase reporter in a lentiviral expression plasmid was a kind gift of Dr. Karl Willert at the University of California, San Diego. CPC lines were infected with an equal amount of SuperTopFlash lenti-virus and passaged several times to insure stable expression. Cells were plated overnight and then treated for 24 hours with GSK3β inhibitor (Santa Cruz 202636) at 1μmol/l or β–catenin inhibitor (EMD 219330) at 7.5μmol/L. Luciferase expression level was assessed using the Promega Bright-Glo luciferase assay according to manufacture’s instructions. Luminescence was quantitated on a Tecan Spectroflour Plus plate reader.
Statistical analysis
All p values were determined using a paired, two-tailed Student’s t-test with an N of at least three samples per group. Significance for qPCR analysis was determined by comparing ΔCt values of experimental versus control samples using a paired, two-tailed Student’s t-test with an N of at least three samples per group.
RESULTS
Depressed cardiac function in ScaKI mice and hypertrophy in older animals
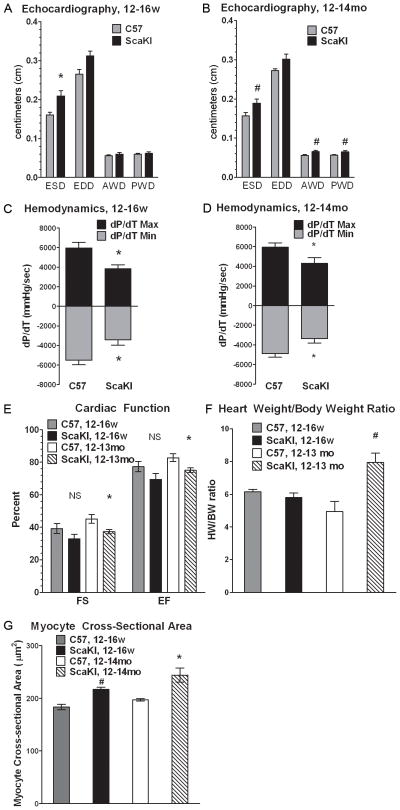
Phenotypic characterization was performed to assess defects in myocardial structure and function by echocardiography using gender matched ScaKI and nontransgenic control mice at 12–18 weeks (males) as well as 12–14 months (male and female groups). Both young and old ScaKI mice exhibit significantly larger End Systolic Diameter (ESD) measurements relative to nontransgenic controls (Figure 1A, B). In vivo hemodynamic measurements confirm poor cardiac function in ScaKI hearts as measured by change in pressure over change in time (dP/dT) where maximum pressure was significantly lower and minimum pressure was significantly higher in ScaKI hearts (Figure 1C, D). Fractional shortening (FS) and ejection fraction (EF) are both significantly lower in 1 year old ScaKI mice, which also exhibited significantly larger hearts as measured both by echocardiography of Anterior Wall Dimension (AWD), Posterior Wall Dimension (PWD) (Figure 1B) and heart weight/body weight ratio (Figure 1F). Increased weight of old ScaKI hearts and size can be attributed to cardiomyocyte hypertrophy due to an increase in average cardiomyocyte cross-sectional area as well as significantly increased AWD and PWD (Figure 1B and 1G).
Figure 1. ScaKI mice have depressed cardiac function in younger and older animals, and hypertrophy in older animals.
Echocardiographic measurements of NTG and ScaKI mice at (A) 12–16 weeks (w) old (n=3) and (B) 12–14 months (mo) old (n=6). End Systolic Diameter (ESD), End Diastolic Diameter (EDD), Anterior Wall Dimension (AWD), Posterior Wall Dimension (PWD). Hemodynamic measurements of change in pressure over change in time (dP/dT) in NTG and ScaKI mice at (C) 12–16w old (n=3) and (D) 12–14m old (n=3). (E) Calculated measurements of cardiac function, fractional shortening (FS) and ejection fraction (EF) in younger and older NTG and ScaKI mice. (F) Heart weight to body weight ratios of 12–16w (n=5) and 12–14mo old NTG and ScaKI mice (n=3). (G) Cardiomyocyte cross-sectional area in the left ventricle of 12–14mo old C57 and ScaKI animals. Error bars represent SEM. * p<0.05, # p<0.02, NS - not significant compared to age matched NTG controls. NTG – nontransgenic.
ScaKI mice also exhibit increased numbers of myocytes expressing atrial natriuretic peptide (ANP), a maker of hypertrophy, in both younger older animals (Online Figure I). Significantly reduced survival is observed in ScaKI mice relative to normal control subjects when challenged with a very large infarction (Online Figure II). Significantly fewer myocytes are labeled for BrdU in 28–30 week old ScaKI mice relative to normal controls (Online Figure III), consistent with loss of proliferative responsiveness in aged ScaKI hearts. Thus, hypertrophic remodeling in older animals may be accentuated in response to stress due in part to impaired cellular replacement.
Diminished number of CPCs in ScaKI mice
c-kit positive CPCs are present in normal or infarcted myocardium from both nontransgenic controls as well as ScaKI tissue samples (Figure 2A–C). However, significantly fewer total c-kit+ CPCs are present in ScaKI myocardial sections from normal tissue (1.5±0.6/mm2) compared with nontransgenic controls (2.6±0.8/mm2; Figure 2B). CPC migration to the zone of pathological damage increases in response to MI, peaking between 4–10 days post-MI18. As true for normal myocardium, significantly fewer CPCs are present within the infarct region of ScaKI hearts (2.4±1.0/mm2) relative to nontransgenic controls (8.7±1.2/mm2; Figure 2C), although infarct size is not significantly different between the groups as measured by percent of involved left ventricular free wall (Figure 2D). CPC proliferation, assessed using proliferating cell nuclear antigen19 (PCNA), revealed c-kit+/PCNA+ cells in the infarct and border region (Figure 2E) with significantly fewer c-kit+/PCNA+ cells in the ScaKI infarct region compared to nontransgenic controls (Figure 2F). Comparable proliferation data were obtained using Ki67 as a proliferation marker for c-kit+ cells in the infarct zone (Online Figure IV). Dual positive c-kit+/PCNA+ CPCs trended higher in nontransgenic control myocardium but increase was not statistically significant (Online Figure V). Vascular densities of infarct regions were not significantly different between normal nontransgenic versus ScaKI groups (Online Figure VII). CPCs are distinct from mast cells, which are positive for both tryptase and c-kit. Tryptase−/c-kit+ CPCs are embedded in the myocardium whereas tryptase+/c-kit+ mast cells are present just outside the myocardium, below the epicardium and are not included in this analysis (Online Figure VI).
Figure 2. ScaKI mice have fewer c-kit positive cells in normal myocardium and in the infarct and border zone of a 7 day myocardial infarction.
(A) Confocal images of c-kit+ cells in the normal myocardium of NTG (left) and ScaKI (right) mice. (B) Scans in the infarct zone of a 7 day MI in a NTG (left) heart and a ScaKI heart (right); c-kit (green), Tropomyosin (“Tmyo”, red) and Topro (blue). (C) Quantitation of c-kit positive cells/mm2 in normal and infarcted myocardium of NTG and ScaKI mice. (D) Infarct area as measured by percent of scarred left ventricular free wall (LVFW) at 7 days. (E) Confocal images of proliferating c-kit cells at 7 days post MI in NTG (left) and ScaKI mice (right); c-kit (green), Desmin (red) and proliferating cell nuclear antigen (PCNA, white). Scale bar is 40μm. (F) Quantitation of proliferating c-kit+ cells as percentage of PCNA+/c-kit+ cells over total c-kit+ cells. Arrowheads indicate c-kit+ cells. Error bars represent SEM. Infarct Zone (IZ), Border Zone (BZ). * p<0.05, # p<0.02, NS - not significant.
Consequences of Sca-1 knockout upon c-kit+ cells during postnatal development
The relationship between cardiac development, CPCs, and Sca-1 or Sca-1 driven GFP expression in the postnatal heart was determined in a developmental time course encompassing 2 days, 1 week, 2 weeks and 12 weeks of age. Presence of CPCs phenotyped as c-kit+ only as well as c-kit+/Sca-1+ were observed in normal nontransgenic control myocardial sections from two days up to adulthood (Figure 3A and B). Similarly, c-kit+ only and c-kit+/GFP+ CPCs were also observed in the ScaKI myocardium throughout all assessed time points (Figure 3C and D). Significantly fewer total c-kit+ CPCs were present in ScaKI hearts at 2 weeks and 12 weeks of age relative to nontransgenic controls (Figure 3E), but this difference was not present at 2 days and 1 week of age. Further characterization of the collective c-kit+ CPC population was performed by subdivision to determine the percentage of dual positive c-kit+/Sca-1+ CPCs in normal nontransgenic samples versus c-kit+/GFP+ CPCs in ScaKI sections (necessitated as GFP+ due to homozygous deletion of Sca-1 antigen in the ScaKI line). Significantly fewer c-kit+/GFP+ cells were present at postnatal day 2 in the ScaKI hearts relative to c-kit+/Sca-1+ cells in the normal control, with a possibly compensatory concurrent increase of c-kit+ only cells in ScaKI myocardium. Shortly thereafter at postnatal day 7 differences vanished between the controls and ScaKI for both c-kit+ alone and dual positive c-kit+/Sca-1+ cell categories. However by two weeks of age onward, ScaKI hearts had significantly fewer dual positive CPCs compared to nontransgenic controls. Since significant differences were not evident for the number c-kit+ only CPCs, decreases in the total c-kit+ CPC population can be attributed to fewer dual positive c-kit+/GFP+ CPCs in the ScaKI samples (Figure 3F) that are the counterparts to the dual positive c-kit+/Sca-1+ population in normal tissue. The number of Sca-1+ only or GFP+ only cells in the heart was comparable between the groups at all time points examined (Figure 3G). Vascular endothelium in the heart also expresses Sca-1, but knock-in of GFP in the ScaKI line does not overtly affect the number or gross morphology of vessels and capillaries observed in histologic sections (Online Figure VIII).
Figure 3. Sca-1 knockout consequences for c-kit+ cells during development.
Confocal images of adult heart sections of a NTG animal depicting a (A) c-kit+/Sca-1− cell and (B) c-kit+/Sca-1+ cell; c-kit (white), Sca-1 (green), Desmin (red), and Topro (blue). Confocal images of adult heart sections of a ScaKI animal depicting a (C) c-kit+/GFP− cell (D) c-kit+/GFP+ cell; c-kit (white), GFP (green), Tropomyosin (“Tmyo”, red), and Topro (blue). Arrowheads indicate c-kit+ cells. (E) Quantitation of total cells expressing c-kit at the indicated ages. (F) Quantitation of c-kit+ cells in the myocardium with respect to Sca-1 or GFP expression. (G) Quantiation of either Sca-1+ only or GFP+ only cells in the myocardium at indicated ages. Error Bars represent SEM. * p<0.05, # p<0.02, NS - not significant vs. NTG animals at the comparable age.
Stem cell marker expression, decreased proliferation and survival in adult ScaKI CPCs
CPCs were isolated using the c-kit marker from both ScaKI and nontransgenic control mice to determine phenotypic properties in vitro. Resulting cultures exhibit activation of the Sca1 promoter as evidenced by GFP expression in ScaKI (37.2%, background corrected) or Sca-1 protein in nontransgenic controls (51.1%, background corrected) by flow cytometry (Figure 4A). Proliferation of ScaKI CPCs is depressed relative to normal CPCs (Figure 4B). ScaKI CPCs also show increased sensitivity to oxidative stress induced by H2O2 exposure leading to apoptosis (Figure 4C).
Figure 4. Adult ScaKI CPCs express stem cell markers and demonstrate decreased proliferation and survival in vitro.
(A) Flow cytometric analysis of CPCs derived from NTG (left) and ScaKI (right) hearts and analysed for GFP expression (top) or stained for Sca-1 expression (bottom). (B) Growth curve of n=3 NTG and ScaKI CPCs using Trypan Blue Exclusion assay. (C) NTG and ScaKI CPCs treated with H2O2, stained with AnnexinV/7-AAD and analyzed with flow cytometry, n=4. (D) Flow cytometric analysis of Sca-1+ CPCs after siRNA of Sca-1 or Scrambled RNA at the indicated timepoints. (E) Cyquant proliferation assay of siSca-1 vs. siScramble CPCs at the indicated timepoints. (F) Growth curve of siSca-1 and siScramble CPCs using Trypan Blue Exclusion assay. Error bars represent SEM, * p<0.05.
Impaired proliferative phenotypes generated by genetic deletion of Sca-1 were confirmed using siRNA to Sca-1 to knockdown Sca-1 in normal CPCs generated from wild-type FVB/N mice. Number of Sca-1+ CPCs in the culture was reduced from an average of approximately 55% in siScramble transfected cells to 10% in siSca-1 transfected cells from Days 1–3 post plating (Figure 4D). Proliferative responses of siSca-1 cells are significantly reduced compared to siScramble cells by either Trypan Blue or Cyquant analyses (Figure 4E and F).
Altered β–catenin signaling in ScaKI CPCs
The mechanisms of Sca-1 signaling and specific genes affected by Sca-1 expression have yet to be described. However, phenotypic deficiencies in proliferation and survival in ScaKI CPCs (Fig. 2 – 4) suggest an underlying molecular basis. Screening assays by qPCR analysis using cDNA derived from each cell line under optimal growth conditions assessed activation of multiple signaling cascades (Figure 5A). The canonical Wnt Pathway exhibited the greatest number genes significantly changed between ScaKI CPCs versus nontransgenic controls. Several genes in the Wnt Pathway showed significant changes: WNT1 inducible signaling pathway protein 1 (Wisp1), Cadherin 1 (Cdh1), and Lymphoid enhancer binding factor 1 (Lef1) were decreased whereas Axin2 expression was significantly increased. Decreased expression of additional non-Wnt related genes for vascular cell adhesion molecule 1 (Vcam1) and NLR family, apoptosis inhibitory protein 1 (Naip1/Birc1a) were also statistically significant. Quantitative changes in gene expression were confirmed by qPCR of 3 additional cDNA samples from nontransgenic control and ScaKI CPCs performed in duplicate (Figure 5B).
Figure 5. Altered β–catenin signaling in ScaKI CPCs.
(A) qPCR gene expression array analysis for activated signaling pathways. Data from samples with greater than two-fold change is expressed as Fold Change of ScaKI CPC gene expression compared to NTG. (B) qPCR gene expression analysis of significantly depressed (left) and increased (right) genes involved in TCF/LEF signaling and survival signaling from screen depicted in A. (C) Relative luminescence of NTG and ScaKI CPCs transduced to express luciferase driven by a 7x TCF/Lef promoter (SuperTopFlash, STF) and treated with GSK3β inhibitor (GSK-I). (D) Gene expression analysis of differentiation markers in ScaKI CPCs compared to NTG CPCs. (E) Gene expression analysis of differentiation markers in ScaKI CPCs compared to NTG CPCs after treatment with β-catenin inhibitor (βcat-I). * p<0.05, B through E, n is at least 3 independent experiments.
Wnt/β–catenin gene expression alterations prompted further examination of pathway activation in ScaKI CPCs, revealing significantly elevated β–catenin mediated transcriptional activity increased by 2-fold in ScaKI CPCs compared to NTG control CPCs (Figure 5C). Inhibition of β–catenin phosphorylation and ubiquitination by the pharmaceutical inhibitor GSK3β increased β–catenin/TCF/Lef transcriptional activity in NTG control CPCs (1.5 fold) and ScaKI CPCs (2 fold), confirming functional activity of this pathway in the cultured cells (Figure 5C). β–catenin/TCF/Lef signaling is involved in switching from self-renewal to differentiated phenotypes in embryonic stem cells20 and, similarly, increased β–catenin signaling in ScaKI CPCs is associated with significant elevation of MEF2c, cardiac α–Actinin (Cα–Actinin),α–smooth muscle actin (αSMA) and SM22 relative to NTG control CPCs by qPCR analysis (Figure 5D). Participation of β–catenin in promoting commitment correlates with significant decreases in MEF2c and Cα–actinin were in ScaKI CPCs after three days of β–catenin inhibitor treatment (Figure 5E; 7.5μmol/l), calibrated to reduce SuperTopFlash luciferase activity but not impair proliferation (Online Figure IX). Similar to induction of cardiac specific transcripts in CPCs upon lineage commitment21, the impaired proliferation and increased cardiogenic commitment of ScaKI CPCs is consistent with enhancement of a lineage-committed phenotype that appears linked to aberrant canonical Wnt signaling involving β–catenin.
DISCUSSION
Maintenance and repair of myocardial structure and function depends upon CPCs, therefore understanding the molecular biology of CPCs will be essential for expanding our repertoire of methodological approaches for enhancing cellular therapeutic approaches. Although Sca-1 is a widely accepted marker of stem cells in the hematopoetic system, downstream molecular signaling and consequences of Sca-1 dysregulation are poorly understood in the hematopoietic context and essentially unknown in the myocardial environment. Therefore, this study was undertaken to characterize Sca-1+ cells and Sca-1+/c-kit+ CPCs in particular with respect to development, function, maintenance and repair of the heart. Sca-1 has been previously used by other groups to mark mouse3 and human22 CPCs. Lack of Sca-1 in the adult mouse heart results in minor developmental contractile defects in agreement with observations from prior studies16 as well as age-associated hypertrophy that has not been previously described (Figure 1).
A critical role for Sca-1 expression for optimal myocardial and CPC function is now clear from findings in this study, although prior publications examining the hematological system of the ScaKI mice failed to reveal an overt phenotype6. Since bone marrow of older (13 month old) ScaKI mice possess fewer c-kit+ cells (Online Figure X), we hypothesized that Sca-1 deletion would be consequential for the CPC population but only manifest in adult animals with exacerbation by aging or pathological challenge.
Myocardial function in ScaKI mice is characterized by reduced function in early adulthood and hypertrophy evolving with age. Enlarged myocytes are present in young adult ScaKI animals without a concomitant increase in HW/BW ratio (Figure 1). This age-associated increase in average myocyte size within the ScaKI heart is presumably due to decreased new myocyte formation by CPCs23 (Online Figure III). Fewer c-kit+ CPCs are present in ScaKI mice in normal or infarcted myocardium (Figure 2), consistent with the premise of a functionally compromised stem cell population in the ScaKI heart.
Cardiac impairment in young adult mice prompted a developmental timecourse analysis of CPCs spanning post-natal life (2 days) until adulthood (12 weeks). Deficiencies in c-kit+ CPCs of ScaKI hearts were significant at 2 weeks of age with lower c-kit+ CPC number due primarily to fewer c-kit+/GFP+ in ScaKI hearts compared to c-kit+/Sca-1+ CPCs in NTG controls (Figure 3). Based upon these observations, it is tempting to speculate that c-kit+/Sca-1+ and c-kit+/Sca-1−CPCs may act as distinct cell types, since if c-kit+ CPCs in the heart were derived from c-kit+/Sca-1+ CPCs then both groups of Sca-1+ and Sca-1− CPCs should be concomitantly reduced.
Cultured CPCs were successfully established from ScaKI myocardium despite lower numbers of CPCs exhibiting deficiencies in growth and survival (Figure 4) similar to prior reported findings in the non-cardiac context24. Poor growth potential of ScaKI CPCs observed in vitro (Figure 4) parallels the in vivo observation of impaired CPC response to infarction (Figure 2). Taken together, these results indicate that phenotypic characteristics of the ScaKI heart likely involve functional impairment of CPCs lacking Sca-1.
It is important to note here that two other types of cells which normally express Sca-1 may affect the overall cardiac phenotype of the ScaKI mice: the vasculature and a subpopulation of circulating hematopoietic cells. A structural quantification of the baseline and post-MI vasculature did not reveal any overt differences in the ScaKI heart in either baseline (Online Figure VIII) or post-MI (Online Figure VII). However, further studies are needed to assess if there is functional vascular phenotype in the ScaKI mouse. A detailed analysis of the post-MI inflammatory response is also necessary to rule out hematopoietic contributions to the ScaKI cardiac phenotype.
The molecular defect underpinning defective performance of Sca-1− CPCs appears to involve canonical Wnt signaling where β–catenin/TCF/Lef is the primary transcriptional effector for growth and survival (Figure 5). TCF/Lef also has non-Wnt associated functions25 and, in the absence of activated β–catenin, functions as a transcriptional repressor26. Wnt signaling is associated with adult stem/progenitor cell proliferation, survival and differentiation27. As well as cardiac development in embryogensis28, 29. ScaKI CPCs show higher β–catenin signaling associated with phenotypic characteristics of lineage commitment at the mRNA level (Figure 5). Indeed, increased β–catenin signaling promotes differentiation in several other types of progenitor cells, such as when epithelial progenitor cells exit cell cycle and initially differentiate into mature hair follicles30. Strength of β–catenin signaling differentially determines differentiation of mesenchymal progenitor cells to either a chondrocyte or osteoblast fate31 and β-catenin signaling inhibits proliferation together with promotion of neuronal differentiation32. A crucial role for Sca-1 in lineage commitment and c-kit expression has also been reported for hematopoietic stem cells6. A recent publication on cardiac side population cells (CSP), which are Sca-1+ and are a putative CPC, has revealed that an excess of β–catenin signaling results in fewer CSP cells post infarction, which is consistant with our findings33. Thus, overactive β-catenin signaling likely contributes, at least in part, to impairment of proliferation and up-regulation of cardiovascular lineage markers. Future studies will need to elaborate upon the role of Wnt pathway signaling downstream of Sca-1 in CPCs.
Supplementary Material
Novelty and Significance.
What Is Known?
Cardiac progenitor cells (CPCs) exist within the myocardium of multiple species, including humans and contribute to repair and regeneration of the heart after injury.
CPCs are small cycling cells in the myocardium, which may express the cell surface markers c-kit and Sca-1.
CPCs can be isolated from cardiac tissue, expanded and manipulated in culture, then reintroduced to provide therapeutic benefits after a heart attack.
What New Information Does This Article Contribute?
Sca-1 knock-out/GFP knock-in mice (ScaKI) exhibit a modest impairment of cardiac function as well as mild cardiac hypertrophic remodeling with age.
Fewer c-kit+ CPCs are present in the ScaKI heart: during development, after adulthood and after a heart attack.
Loss of Sca-1 expression in CPCs leads to decreased growth rate and increased differentiation marker expression.
Sca-1 in CPCs increases canonical Wnt/β–catenin signaling resulting in reduced cell cycling and premature differentiation of CPCs.
This rationale of this study is to describe the function of Sca-1 in the heart using the phenotypic consequences of Sca-1 deletion on cardiac structure and function. Sca-1 is a marker of stem cells and the vasculature in many tissues and its functions outside of the hematopoietic system have yet to be thoroughly investigated. Likewise, the intracellular signaling pathway for Sca-1 has yet to be elucidated. Our novel findings include that Sca-1 is important for the optimal cardiac function as well as the resident CPCs. The significance of the findings noteworthy because CPCs are an emerging clinical therapy for heart attacks and cardiomyopathy. This study is the first to indicate that canonical Wnt/β–catenin signaling is affected by deletion of Sca-1 in CPCs. Sca-1 is a surface protein and therefore a potential target for intervention to affect CPC function. Overall, our data confirm the importance of Sca-1 to maximal CPC function and the importance of CPC activity to optimal heart function.
Acknowledgments
The authors would like to thank Dr. Timothy Graubert for the kind gift of the Sca-1 Knock-In mouse line. We would also like to thank Dr. Karl Willert at the University of California, San Diego for gift of the SuperTopFlash Lentiviral reporter plasmid. In addition, we would like to acknowledge the vital assistance of the SDSU FACS Core Facility.
SOURCES OF FUNDING
This research is supported by NIH grants 2R01HL067245, 1R37HL091102-01, RC1HL100891-02, 1R21HL102714-01, P01HL085577-05, R01HL105759-01, 1R21HL104544-01 to M.A.S. B.B. is a recipient of a fellowship from the Rees-Stealy Research Foundation.
Non-standard Abbreviations
- αSMA
α–smooth muscle actin
- AWD
anterior wall dimension
- βcat-I
β–catenin Inhibitor
- BSA
bovine serum albumin
- BZ
border zone
- Cα–Actinin
cardiac α–actinin
- CPC
cardiac progenitor cell
- ESD
end systolic diameter
- EDD
end diastolic diameter
- FBS
fetal bovine serum
- GSK-I
GSK3β Inhibitor
- GFP/EGFP
green fluorescent protein/enhanced green fluorescent protein
- IZ
infarct zone
- MI
myocardial infarction
- NBF
neutral buffered formalin
- NTG
non-transgenic control mouse line, refers to C57/BL6 strain from which the Sca-1 knock-out/GFP knock in mouse was derived
- PBS
phosphate buffered saline
- PWD
posterior wall dimension
- RLU
relative luminescence units
- Sca-1
stem cell antigen -1
- ScaKI
Sca-1 knock-out/GFP knock-in mouse
- SEM
standard error of the mean
- Topro
To-pro-3-Iodide
- TN
tris-NaCl buffer
- TNB
tris-NaCl blocking buffer
Footnotes
DISCLOSURES
None.
References
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, Hoyt G, Yang P, Rosenberg J, Robbins RC, Wu JC. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53:1229–1240. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes C, Stanford WL. Concise review: Stem cell antigen-1: Expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 6.Bradfute SB, Graubert TA, Goodell MA. Roles of sca-1 in hematopoietic stem/progenitor cell function. Exp Hematol. 2005;33:836–843. doi: 10.1016/j.exphem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- 8.Pasquinelli G, Pacilli A, Alviano F, Foroni L, Ricci F, Valente S, Orrico C, Lanzoni G, Buzzi M, Luigi Tazzari P, Pagliaro P, Stella A, Paolo Bagnara G. Multidistrict human mesenchymal vascular cells: Pluripotency and stemness characteristics. Cytotherapy. doi: 10.3109/14653241003596679. [DOI] [PubMed] [Google Scholar]
- 9.van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL. Mouse hematopoietic stem-cell antigen sca-1 is a member of the ly-6 antigen family. Proc Natl Acad Sci U S A. 1989;86:4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gumley TP, McKenzie IF, Sandrin MS. Tissue expression, structure and function of the murine ly-6 family of molecules. Immunol Cell Biol. 1995;73:277–296. doi: 10.1038/icb.1995.45. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell PO, Mills T, O’Connor RS, Kline ER, Graubert T, Dzierzak E, Pavlath GK. Sca-1 negatively regulates proliferation and differentiation of muscle cells. Dev Biol. 2005;283:240–252. doi: 10.1016/j.ydbio.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Gojo S, Umezawa A. Plasticity of mesenchymal stem cells--regenerative medicine for diseased hearts. Hum Cell. 2003;16:23–30. doi: 10.1111/j.1749-0774.2003.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/cd31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 14.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 15.Hanson P, Mathews V, Marrus SH, Graubert TA. Enhanced green fluorescent protein targeted to the sca-1 (ly-6a) locus in transgenic mice results in efficient marking of hematopoietic stem cells in vivo. Exp Hematol. 2003;31:159–167. doi: 10.1016/s0301-472x(02)01021-4. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblatt-Velin N, Ogay S, Felley A, Stanford WL, Pedrazzini T. Cardiac dysfunction and impaired compensatory response to pressure overload in mice deficient in stem cell antigen-1. Faseb J. 26:229–239. doi: 10.1096/fj.11-189605. [DOI] [PubMed] [Google Scholar]
- 17.Bailey B, Izarra A, Alvarez R, Fischer KM, Cottage CT, Quijada P, Diez-Juan A, Sussman MA. Cardiac stem cell genetic engineering using the alphamhc promoter. Regen Med. 2009;4:823–833. doi: 10.2217/rme.09.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fransioli J, Bailey B, Gude NA, Cottage CT, Muraski JA, Emmanuel G, Wu W, Alvarez R, Rubio M, Ottolenghi S, Schaefer E, Sussman MA. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1324. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeymer U, Fishbein MC, Forrester JS, Cercek B. Proliferating cell nuclear antigen immunohistochemistry in rat aorta after balloon denudation. Comparison with thymidine and bromodeoxyuridine labeling. Am J Pathol. 1992;141:685–690. [PMC free article] [PubMed] [Google Scholar]
- 20.Miki T, Yasuda SY, Kahn M. Wnt/beta-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming. Stem Cell Rev. 7:836–846. doi: 10.1007/s12015-011-9275-1. [DOI] [PubMed] [Google Scholar]
- 21.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smits AM, van Vliet P, Metz CH, Korfage T, Sluijter JP, Doevendans PA, Goumans MJ. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: An in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc. 2009;4:232–243. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]
- 23.Rota M, Hosoda T, De Angelis A, Arcarese ML, Esposito G, Rizzi R, Tillmanns J, Tugal D, Musso E, Rimoldi O, Bearzi C, Urbanek K, Anversa P, Leri A, Kajstura J. The young mouse heart is composed of myocytes heterogeneous in age and function. Circ Res. 2007;101:387–399. doi: 10.1161/CIRCRESAHA.107.151449. [DOI] [PubMed] [Google Scholar]
- 24.Tateishi K, Ashihara E, Takehara N, Nomura T, Honsho S, Nakagami T, Morikawa S, Takahashi T, Ueyama T, Matsubara H, Oh H. Clonally amplified cardiac stem cells are regulated by sca-1 signaling for efficient cardiovascular regeneration. J Cell Sci. 2007;120:1791–1800. doi: 10.1242/jcs.006122. [DOI] [PubMed] [Google Scholar]
- 25.Carlsson P, Waterman ML, Jones KA. The hlef/tcf-1 alpha hmg protein contains a context-dependent transcriptional activation domain that induces the tcr alpha enhancer in t cells. Genes Dev. 1993;7:2418–2430. doi: 10.1101/gad.7.12a.2418. [DOI] [PubMed] [Google Scholar]
- 26.Dale TC. Signal transduction by the wnt family of ligands. Biochem J. 1998;329 ( Pt 2):209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 28.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: An essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- 29.Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 30.Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 33.Oikonomopoulos A, Sereti KI, Conyers F, Bauer M, Liao A, Guan J, Crapps D, Han JK, Dong H, Bayomy AF, Fine GC, Westerman K, Biechele TL, Moon RT, Force T, Liao R. Wnt signaling exerts an antiproliferative effect on adult cardiac progenitor cells through igfbp3. Circ Res. 109:1363–1374. doi: 10.1161/CIRCRESAHA.111.250282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.