Abstract
Carfilzomib is a second-generation proteasome inhibitor with well-documented clinical activity as a single agent in patients with relapsed/refractory multiple myeloma. Carfilzomib can partially overcome resistance in bortezomib-refractory patients and has significant efficacy in bortezomib-naïve patients. Responses generally occur rapidly and are durable in the majority of cases. Carfilzomib can be safely administered in patients with renal failure and adverse cytogenetics do not seem to interfere with its activity. Moreover, carfilzomib has the advantage of a favorable safety profile, especially a low incidence of peripheral neuropathy, which is often the dose-limiting factor in thalidomide and bortezomib-based regimens. The most frequently observed high-grade adverse event is cytopenia. However, long-term tolerability is good with no cumulative toxicity. The place of carfilzomib in the treatment of the advanced and the newly diagnosed myeloma patient is currently under examination in several ongoing phase 3 clinical trials.
Keywords: relapsed, refractory, myeloma, carfilzomib, proteasome inhibitor
Introduction
In the last decade, overall rates of survival of multiple myeloma have improved significantly due to the introduction of novel agents such as immunomodulatory drugs (thalidomide, lenalidomide) and proteasome inhibitors (bortezomib).1 Nonetheless, even with these drugs added to the anti-myeloma armamentarium, virtually all patients will eventually relapse and/or develop resistance. Furthermore, as overall survival rates increase, cumulating and irreversible toxicities may significantly jeopardize the patient’s quality of life. Thus, there remains an unmet need for innovative anti-myeloma drugs with favorable toxicity profiles. One of the most promising candidates, which is being introduced into clinical practice, is carfilzomib, a selective and irreversible proteasome inhibitor. Here, we discuss the mechanism of action of carfilzomib, review all the phase 1 and 2 studies on single-agent carfilzomib in relapsed/refractory (RR) myeloma and focus on specific issues such as adverse events, neuropathy, impact of cytogenetics on response, optimal dosing schedules, and renal failure. Finally, we discuss new combination regimens for RR patients with carfilzomib.
Mechanism of action
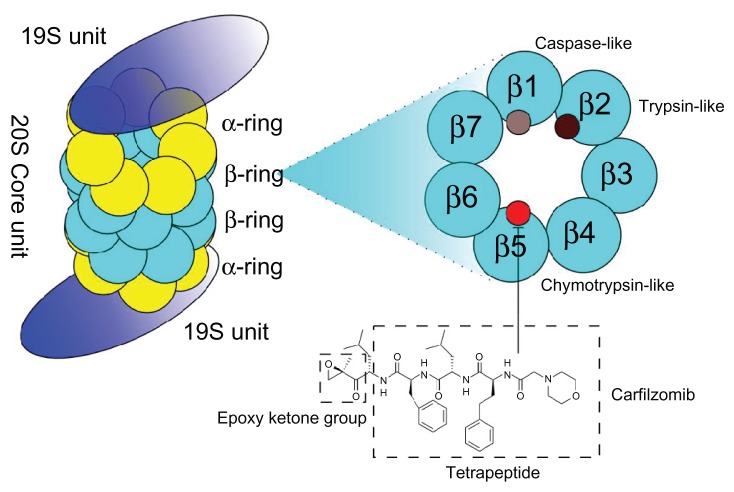
The proteasome, the target of carfilzomib, is a multi-catalytic intracellular protease complex that is responsible for the ubiquitin-dependent turnover of cellular proteins. It comprises a 20S core particle with one or two 19S caps at either end. Within the 20S core, two pairs of three major catalytic activities are located: a chymotrypsin-like activity found in the β5 subunit, a trypsin-like activity in subunit β2, and a postglutamyl peptide hydrolyzing (caspase-like activity) in the β1 subunit (Figure 1). Of these, the chymotrypsin- like domain has been shown to be the rate-limiting step of proteolysis in vitro and in vivo.2 Two main different isoforms of the proteasome exist: a constitutive form, which is present in most cells, and the immuno-proteasome, predominately expressed in cells of the lymphoid origin. Proteasome inhibitors have been shown to inhibit nuclear factor NF-κB activity by inhibiting the degradation of its inhibitor iκB;3 they deregulate the turnover of cyclins,4 stabilize the tumor suppressor p53,5 and shift the pro-apoptotic/anti-apoptotic balance in the BCL-2 family of proteins. Furthermore, it is believed that malignant plasma cells generate a large number of misfolded proteins and the inhibition of the proteasome leads to endoplasmatic stress and ultimately cell death.6
Figure 1.
The 26S proteasome is a large (2.5 MDa), multi-subunit, ATP-dependent proteolytic complex that degrades proteins into smaller peptides.
Notes: It comprises a hollow cylindrical 20S proteolytic core and one or two 19S regulatory particles. The 19S unit recognizes poly-ubiquitynated substrates, and prepares them for proteolysis, which occurs inside the 20S cores. The 20S core is a hollow cylindrical structure comprising 2 pairs of 14 different polypeptides arranged in 4 stacked rings. Six subunits carry catalytic residues for the proteolytic sites: two are chymotrypsin-like (β5), two trypsin-like (β2), and two caspase-like (β1). Carfilzomib, a peptide epoxyketone and derivative of the naturally occurring epoxomicin, irreversibly and specifically inhibits the chymotrypsin-like protease activity of the proteasome (β5) and immunoproteasome (β5i).
The first-in-class proteasome inhibitor bortezomib has provided adequate proof-of-principle of proteasome inhibition as a therapeutic approach in multiple myeloma. The development of “second-generation” proteasome inhibitors was undertaken mainly to mitigate bortezomib’s toxicity profile (especially neuropathy), overcome its drug-resistance, offer a more convenient way of administration (oral versus intravenous [IV] or subcutaneous [SC]), and try to obtain an irreversible binding to the proteasome. Five compounds have entered (mostly phase 1) clinical trials (Table 1). CEP-187707 and MLN-9708 are both peptide-boronate molecules (like bortezomib) but differ from the native compound by a different substrate specificity (both compounds) and being available orally (MLN-9708). Oprozomib (ONX-0912) is the orally available sister-compound to carfilzomib and both have an epoxyketone pharmacophore, which renders their binding to the proteasome irreversible.8 Marizomib is an irreversible β-lactone inhibitor, which has been shown to be the most potent proteasome inhibitor in clinical development, with the advantage of being orally available.9
Table 1.
Proteasome inhibitors used in clinical practice or in clinical trials for the treatment of multiple myeloma
| Compound | Chemical | Binding | Adm | Status | Proteasome inhibition |
|---|---|---|---|---|---|
| Bortezomib (PS-341) | Boronate | Reversible | IV/SC | FDA approved | β5/β5i > β1/β1i > β2i |
| CEP-18770 | Boronate | Reversible | IV/oral | Phase 1–2 | β5/β5i > β1/β1i |
| MLN-9708 | Boronate | Reversible | Oral/IV | Phase 1–2 | β5/β5i > β1/β1i |
| Carfilzomib (PR-171) | Epoxyketone | Irreversible | IV | FDA approved | β5/β5i > > β2i~β1i |
| Oprozomib (ONX-0912) | Epoxyketone | Irreversible | Oral/IV | Phase 1 | β5/β5i |
| Marizomib (NPI-0052) | β-lactone | Irreversible | IV/oral | Phase 1 | β5/β5i > β2/β2i > β1/β1i |
Note: © 2012, Elsevier. Adapted with permission from Kisselev AF, van der Linden WA, Overkleeft HS. Proteasome inhibitors: an expanding army attacking a unique target. Chem Biol. 2012;19(1):99–115.34
Abbreviations: Adm, administration; IV, intravenous; SC, subcutaneous.
The high selectivity of carfilzomib for proteasomes, as well as its weak activity on other protease classes, may contribute to greater tolerability in vivo (see the section on neuropathy). Another notable difference of carfilzomib from bortezomib is its ability to irreversibly inhibit proteasomes. Carfilzomib has demonstrated activity against bortezomib-resistant cell lines and primary multiple myeloma (MM) cells.10 The mechanisms underlying this resistance remain largely obscure. In vitro, prolonged exposure to increasing sublethal concentrations of bortezomib can render neoplastic cells resistant (Table 2). Recent work shows that apoptotic sensitivity to bortezomib in myeloma cells depends on the balance between proteasomal workload and the proteasomal degradative capacity.11 In other words, plasma cells with lower intrinsic proteasomal expression/activity12,13 and/or higher workload seem to be more prone to the cytotoxic effects of bortezomib. This might explain why carfilzomib, an irreversible proteasome inhibitor, has a prolonged effect on this equilibrium compared to bortezomib.14
Table 2.
Mutations described in vitro rendering different tumor cell lines resistant to bortezomib
| Cell line | Target | Mutation | Mechanism | Carfilzomib sensitivity |
|---|---|---|---|---|
| HT-29 adenocarcinoma | β5 | Cys63Phe35 | Near α-ring, inhibits bortezomib binding? | Yes35 |
| HT-29 adenocarcinoma | Pro-peptide (β5) | Arg24Cys35 | Altered recovery of proteasome activity following bortezomib exposure | Yes35 |
| HT-29 adenocarcinoma | Pro-peptide (β5i) | Phe50Ile14 | Altered recovery of proteasome activity following bortezomib exposure | Yes14 |
| Jurkat cells | β5 | Ala108Thr36 | Reduces affinity between bortezomib and the chymotrypsin-like active site | Unknown |
| Jurkat cells | β5 | Ala49Val37 | Reduces affinity between bortezomib and the chymotrypsin-like active site | Unknown |
| Jurkat cells | β5 | Ala49Thr and Ala50Val37 | Reduces affinity between bortezomib and the chymotrypsin-like active site | Unknown |
| THP-1 KMS-11/OPM-2 |
β5 | Ala49Thr12,38 | Reduces affinity between bortezomib and the chymotrypsin-like active site | Unknown |
Activity in relapsed and refractory myeloma
Carfilzomib was initially explored in two phase 1 studies in patients with RR hematological malignancies using two different administration schedules.
In the first study, PX-171-001, patients received a carfilzomib IV push at doses varying from 1.2 to 20 mg/m2 on days 1–5 of 14-day cycles.15 Due to patients’ inconvenience of attending the clinic for 5 consecutive days, an alternative dosing schedule was pursued in the PX-171-002 trial, with carfilzomib being administered as an IV push on a 28-day cycle (day 1, 2, 8, 9, 15, and 16 with 12 days rest) at doses from 1.2 mg/m2 to 27 mg/m2.16 A total of 37 patients with various RR hematological malignancies were treated, including 16 at or above the minimal effective dose of 15 mg/m2. Five responses were observed, all in myeloma patients: four partial and one minimal response. This 48-hour proteasome suppression regimen was further used in the subsequent phase 2 studies.
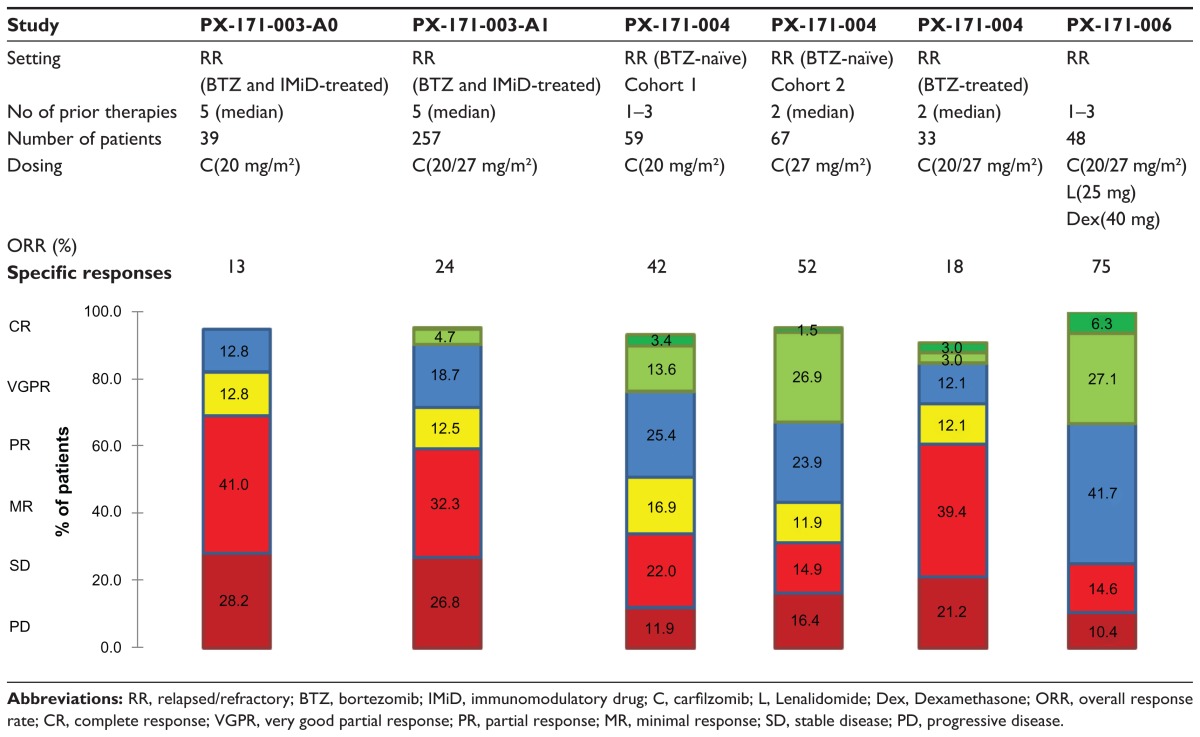
The pilot phase 2 study evaluating single-agent carfilzomib in the RR myeloma setting was the PX-171-003-A017 (Table 3). Patients were eligible if they had relapsed from more than two prior therapies, failed bortezomib and at least one immunomodulatory agent (thalidomide or lenalidomide), and were refractory to last treatment (defined as progressing on or within 60 days of last therapy or had less than a 25% response to the last treatment regimen). Carfilzomib 20 mg/m2 was given as an IV infusion on day 1, 2, 8, 9, 15, and 16 every 28 days for up to 12 cycles. Of the 39 patients that completed at least 1 cycle of carfilzomib, the overall response rate (ORR, ie, partial response or better) was 13% and an additional 13% of patients had a minimal response (MR). The median time to progression (TTP) was 6.2 months and the median duration of response (DOR) was 7.4 months.
Table 3.
Best responses (according to the IMWG criteria) of carfilzomib in different PX-171 trials in relapsed/refractory multiple myeloma patients
Abbreviations: RR, relapsed/refractory; BTZ, bortezomib; IMiD, immunomodulatory drug; C, carfilzomib; L, Lenalidomide; Dex, Dexamethasone; ORR, overall response rate; CR, complete response; VGPR, very good partial response; PR, partial response; MR, minimal response; SD, stable disease; PD, progressive disease.
Based on these results, an additional 257 patients were included in the extended second arm of the study (PX-171- 003-A1)18 (Table 3). The dose of carfilzomib was escalated (20 mg/m2 during the first cycle and 27 mg/m2 beyond) to a maximum of 12 cycles and patients were allowed to be more heavily pretreated after a median of 5 lines of treatment and including 83% having progressed on or within 60 days of last therapy. The ORR was 24% and a clinical benefit response (CBR, defined as ORR + minimal response) was seen in 36% of patients. Responses were durable with a DOR of 7.4 months. The results of the 003-A1 trial were submitted to the Food and Drug Administration (FDA) and this led on July 20 2012 to the approval of carfilzomib (Kyprolis®) for myeloma patients, who have received at least two prior therapies, including bortezomib and an immunomodulatory agent, and have demonstrated disease progression on or within 60 days of the completion of the last therapy. The European Medicines Agency (EMA), however, requested a supplemental randomized study designed to demonstrate that patients with relapsed and refractory myeloma derive a clinical benefit from carfilzomib. This led to the initiation of FOCUS, a randomized open-label phase 3 study of single-agent carfilzomib versus best supportive care in myeloma patients who have no available, approved, or alternative therapies and would otherwise be offered supportive and/or palliative care. The estimated study completion date is January 2015.
A parallel study, PX-171-004 (Table 3), evaluated the efficacy of single-agent carfilzomib in less advanced RR MM patients (1 to 3 prior lines of therapy, responsive to at least one).19 Bortezomib-naïve patients were either scheduled for a fixed-dose regimen of 20 mg/m2 carfilzomib (cohort 1; n = 59) or an escalated-dose regimen (starting with 20 mg/m2 carfilzomib during cycle 1 followed by 27 mg/m2 in all subsequent cycles) (cohort 2; n = 70). Cohort 1 and 2 were well balanced in terms of cytogenetics, but the International Staging System (ISS) III stage was more than double in cohort 2 (23% vs 10%). Although exposure to an immunomodulatory agent (lenalidomide or thalidomide) was similar, lenalidomide had been given to only 46% of patients in cohort 1 versus 70% in cohort 2. In cohort 1, 29% of patients completed 12 cycles of carfilzomib, with 41% withdrawals due to progressive disease and 22% due to adverse events. Although the dose escalated, 41% of patients in cohort 2 completed 12 cycles, with 34% dropouts due to progression and only 10% due to adverse events. ORR was 42.4% in cohort 1 vs 52.2% in cohort 2. Responses seemed durable with a median TTP of at least 8.3 months and a median DOR of at least 13.1 months in cohort 1. Cohort 2 did not yet reach median TTP or DOR.
Among PX-171-004, bortezomib-treated patients comprised a smaller cohort, who were treated with a fixed-dose carfilzomib regimen (20 mg/m2). Thirty-five patients were included, of whom 14 were refractory to their most recent treatment. The ORR in this cohort was 18%. Median DOR and TTP were 9.0 and 5.3 months, respectively.20
One would be tempted to compare these results to the use of single-agent bortezomib in RR myeloma in the APEX trial, where ORR was 38%, with a median TTP of 6.2 months.21 However, these studies are difficult to compare because of differences in response definition, prior treatment regimens, the lack of ISS reporting, and/or paucity of available cytogenetics. For example, in the APEX trial, prior treatment regimens included mostly alkylating agents (91%) and thalidomide (48%) since lenalidomide was at that time not readily available. In another older study, Orlowski et al reported an ORR (EMBT criteria) of 41% and a median TTP of 6.5 months of single-agent bortezomib in RR myeloma.22
Time to response
The time to response to treatment with carfilzomib in relapsed/refractory patients was evaluated in patients enrolled in the PX-171-003-A1 and PX-171-004 trials.23 In the 003-A1 trial, the median time of achieving a partial response or better in the 61/257 (23.7%) evaluable patients was 1.9 months (range 0.3–5.6 months). In the 004 trial, the bortezomib-naïve patients (60/127) and bortezomib-pretreated patients (6/35) had a partial response or better after a median of 1.7 months (range 0.5–3.7 months) vs 1.4 months (range 0.5–1.9 months), respectively. These data illustrate that carfilzomib as a salvage agent has a fast response.
Optimal dosing
In preclinical studies,10,24 a dose-dependent proteasome inhibition was thought to be correlated to better efficacy. Accumulating clinical data is adding credence to this hypothesis. For example, side-by-side comparison of the ORR of patients enrolled in the PX-171-003-A0/PX-171- 003-A1 study and both cohorts of the PX-171-004 study suggest superior outcomes of patients receiving carfilzomib 27 mg/m2 vs those that received 20 mg/m2. This dose-response relationship was evaluated using a statistically rigorous multivariate analysis (adjusting for study protocol, carfilzomib dose, cytogenetics, and ISS stage).25 The odds of achieving a partial response or better for a given patient on carfilzomib 27 mg/m2 was 4.1-fold higher (95% CI: 2.3–7.2, P < 0.001) than those treated with 20 mg/m2. This probability of ORR, DOR, PFS, and OS increased stepwise for each 1 mg/m2 increase in average carfilzomib dose. The Phase 1b/2 PX-171-007 evaluated a 30-minute stepwise incremental infusion of carfilzomib, stratifying patients starting at 20 mg/m2 at day 1 and 2 for the first cycle to 36, 45, 56, or 70 mg/m2 onwards.26 Low-dose dexamethasone (4 mg for ≤45 mg/m2; 8 mg for > 45 mg/m2) was given to mitigate the infusion-related reaction. In the highest dose cohort (20/70 mg/m2), both patients had dose-limiting toxicity and 20/56 mg/m2 was considered the maximal tolerated dose. This cohort was expanded to 24 patients. Of the 20 evaluable patients, an ORR of 60% was observed with thrombocytopenia (38%), anemia (21%), and hypertension as main ≥ grade 3 adverse events. It should be noted that at this dosing regimen, carfilzomib inhibited all three subunits of the proteasome, resulting in a 78% inhibition in total activity.
The impact of adverse cytogenetic characteristics
The impact of adverse cytogenetics (defined as the presence of deletion of chromosome 13, hypodiploidy, and/or deletion of 17p13, t[4;14] and t[14;16]) in terms of efficacy and treatment outcomes of single-agent carfilzomib in relapsed/refractory myeloma patients was studied in a subanalysis of the PX-171- -003-A1 trial.27 A total of 234 patients were included, of which 76% had both metaphase and fluorescence in situ hybridization data available for analysis. Seventy-five (32%) had more than one adverse cytogenetic abnormality and an advanced ISS stage was more frequently observed in this group. In this study, there was no clear impact of adverse cytogenetics observed in terms of response rate or response duration, with even a trend toward higher response rates in patients with t[4;14]. The impact of cytogenetics on the outcome in myeloma after treatment with carfilzomib requires further study in larger patient cohorts.
Use in case of impaired renal function
The PX-171-005 study evaluated single-agent carfilzomib in RR myeloma patients with a varying degree of renal dysfunction.28 Fifty patients of whom 96% received bortezomib during a prior treatment were enrolled in this phase 2 study. Patients were stratified according to their renal function (Group 1 [CrCl > 80 mL/min], n = 14; group 2 [CrCl 50–80 mL/min], n = 10; group 3 [CrCl 30–49 mL/min], n = 8; group 4 [CrCl < 30 mL/min], n = 10; group 5 [dialysis], n = 8). Treatment consisted of carfilzomib on day 1, 2, 8, 9, 15, and 16 of 28-day cycles with dose escalations (15 mg/m2 at cycle 1, 20 mg/m2 at cycle 2, and 27 mg/m2 at cycle 3 and beyond). If after the first cycle a partial response was not obtained, 40 mg dexamethasone/week was added. Among groups 1 to 4, no differences in adverse and serious adverse events were observed. Thirty-five patients discontinued the study (22 due to progressive disease and 6 due to adverse events [sepsis, arthralgia, fatigue, veno-occlusive disease, congestive heart failure, and bacteremia]). Pharmacokinetics revealed a half-life of carfilzomib from 30 to 60 minutes, with undetectable plasma levels within 3 hours irrespective of renal function. Proteasome recovery was complete in peripheral blood mononuclear cells by the next measurement at day 8 in all groups. These results demonstrate that there is no need for dose adjustment based on renal function, mirroring the experience with bortezomib. Moreover, an ORR of 21.7% could be observed in this heavily pretreated patient group.
Toxicity
An updated safety report of single-agent carfilzomib in the relapsed/refractory setting was recently presented.29 All patients (n = 526) who participated in the three phase 2 studies (PX-171-003 [A017 and A118], 004,19 and 00528) were analyzed. The most-frequent adverse events ( occurring in at least 30% of patients) and ≥grade 3 events (occurring in at least 10% of patients) are summarized in Table 4. The most common treatment-emergent and treatment-related adverse events were cytopenia (including grade 3 or higher) and fatigue, nausea, and dyspnea, respectively. Carfilzomib treatment was halted in 51% of patients due to progressive disease while 15% stopped because of adverse events. There were 37 (7%) deaths on the study of which 22 (4.2%) were due to disease progression. However, adverse events contributed to 14 of these deaths, including in order of frequency, cardiac events, hepatic failure, and infection.
Table 4.
Adverse events and frequency (occurring in ≥30%) (left panel) and ≥grade 3 adverse events and frequency (occurring in ≥10%) (right panel) of 526 patients enrolled in PX-171-003- A0, PX-171-003-A1, PX-171-004, and PX-171-005 trials
| Adverse event | Frequency (%) | ≥grade 3 adverse event | Frequency (%) |
|---|---|---|---|
| Fatigue | 55 | Thrombocytopenia | 23 |
| Anemia | 47 | Anemia | 22 |
| Nausea | 42 | Lymphopenia | 18 |
| Thrombocytopenia | 36 | Pneumonia | 11 |
| Dyspnea | 35 | Neutropenia | 10 |
| Diarrhea | 33 | ||
| Pyrexia | 30 |
Long-term treatment and tolerability (>12 cycles) of single- agent carfilzomib was evaluated in the PX-171-010 study.30 Of the 575 patients enrolled in the induction studies, 59 (10%) received > 12 cycles of carfilzomib and 42 were available for analysis. The median duration of carfilzomib treatment was 14 months, and the longest duration was 28 months. Most patients (76%) had received carfilzomib in dosages of 27 mg/m2 (with or without dexamethasone dosages of 4 mg) and 46% had a reduced dosing frequency. Of the 17 patients who discontinued carfilzomib maintenance therapy, 16 did so due to progressive disease. Overall adverse events were similar to those reported in other studies with single-agent carfilzomib without relevant neuropathy or renal dysfunction. Serious adverse events were rare (n = 3) and all patients were able to restart carfilzomib upon recovery. Cumulative toxicities were not observed. These data suggest that carfilzomib is well tolerated, even at an escalated dose, when administered for a prolonged period of time.
Patients with RR myeloma often suffer from disabling polyneuropathy (PNP), be it causatively related to their disorder or due to the use of bortezomib or thalidomide in preceding therapies. In an in vitro model of differentiating neuroblastoma cells (SH-SY5Y), bortezomib but not carfilzomib showed a significant reduction in average and total neurite length. This effect was independent of proteasome inhibition but seems to be mediated by off-target effects of bortezomib but not carfilzomib on serine proteases such as HtrA2/Omi, which is implicated in neuronal survival.31
These in vitro findings are mirrored by clinical data. In a cross-trial study of the PX-171-003-A0, 003-A1, 004, and 005 trials, a majority of 85% of 526 patients had a medical history of PNP in prior treatments (42.6% due to bortezomib, 43.3% due to thalidomide), which resulted in discontinuation of treatment in 25.9% and 21.1% of patients, respectively. A total of 71.9% suffered from active PNP at baseline (all grade 1 or 2). During carfilzomib treatment, in a minority of patients (13.9%), PNP occurred (all grades) with only seven cases of grade 3 and none with ≥grade 4 PNP. One patient stopped carfilzomib treatment and four needed dose modifications due to PNP.32
Combination regimens
Carfilzomib may be particularly suitable for combination strategies because of the encouraging results as a single agent and its limited toxicity profile.
The combination of carfilzomib/lenalidomide/low-dose dexamethasone (CRd) was studied in relapsed/refractory myeloma in a phase 1b multi-center dose-escalation study (PX-171-006 trial).32 Six cohorts combining various concentrations of carfilzomib (15–20–27 mg) and lenalidomide (10–15–20–25 mg) were tested. Maximal tolerated dose was not reached, so the highest dosing cohort (combining carfilzomib (20–27 mg/m2 on day 1, 2, 8, 9, 15, and 16), lenalidomide 25 mg (day 1 to day 21) and dexamethasone 40 mg (day 1, 8, 15, and 22), was expanded in four-week cycles. Adverse events were generally mild and manageable. At least one serious adverse event occurred in 28/84 patients over all dosing cohorts (33%), of which 9/84 were considered possibly or probably related to carfilzomib, lenalidomide, and/or dexamethasone. The ORR in the highest dosage cohort (n = 48) was 75% (see Table 3), irrespective of cytogenetics, ISS stage, or prior therapies. At 12 months follow-up, median duration of response had not yet been reached. This work paved the way for the ASPIRE trial, a randomized, multi-centric phase 3 trial (n = 780), which will compare CRd versus lenalidomide/low-dose dexamethasone in relapsed MM (1–3 prior therapies): lenalidomide (25 mg/day), low-dose dexamethasone (40 mg/week) with or without carfilzomib (20–27 mg/m2).33 Enrollment of patients was completed in February 2012 and interim results could be available as early as the first half of 2013.
Conclusion
Carfilzomib, used as a single agent, exerts a clinically significant effect in relapsed/refractory myeloma patients. Adverse events are manageable and long-term tolerability is good. It lacks relevant neuropathy and is a highly interesting treatment option for patients with this prior treatment-related or myeloma-related affliction. Carfilzomib can also be safely used in patients with compromised renal function and end-stage renal disease. Because of the encouraging results as a single agent and its limited toxicity profile, combination regimens of carfilzomib and other anti-myeloma drugs are being pursued in the relapsed/refractory setting.
Footnotes
Disclosure
The authors have nothing to disclose and indicate no potential conflict of interest.
References
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock KL, Gramm C, Rothstein L, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78(5):761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 3.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4(5):349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 4.Koepp DM, Harper JW, Elledge SJ. How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell. 1999;97(4):431–434. doi: 10.1016/s0092-8674(00)80753-9. [DOI] [PubMed] [Google Scholar]
- 5.Pandit B, Gartel AL. Proteasome inhibitors induce p53-independent apoptosis in human cancer cells. Am J Pathol. 2011;178(1):355–360. doi: 10.1016/j.ajpath.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obeng EA, Carlson LM, Gutman DM, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piva R, Ruggeri B, Williams M, et al. CEP-18770: A novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood. 2008;111(5):2765–2775. doi: 10.1182/blood-2007-07-100651. [DOI] [PubMed] [Google Scholar]
- 8.Zhou HJ, Aujay MA, Bennett MK, et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047) J Med Chem. 2009;52(9):3028–3038. doi: 10.1021/jm801329v. [DOI] [PubMed] [Google Scholar]
- 9.Potts BC, Albitar MX, Anderson KC, et al. Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011;11(3):254–284. doi: 10.2174/156800911794519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn DJ, Chen Q, Voorhees PM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110(9):3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi G, Oliva L, Cascio P, et al. The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood. 2009;113(13):3040–3049. doi: 10.1182/blood-2008-08-172734. [DOI] [PubMed] [Google Scholar]
- 12.Oerlemans R, Franke NE, Assaraf YG, et al. Molecular basis of bortezomib resistance: proteasome subunit beta5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood. 2008;112(6):2489–2499. doi: 10.1182/blood-2007-08-104950. [DOI] [PubMed] [Google Scholar]
- 13.Ruckrich T, Kraus M, Gogel J, et al. Characterization of the ubiquitin-proteasome system in bortezomib-adapted cells. Leukemia. 2009;23(6):1098–1105. doi: 10.1038/leu.2009.8. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki E, Demo S, Arastu-Kapur S, et al. Bortezomib-resistant cell lines have increased proteasome levels but remain sensitive to carfilzomib. ASH Annual Meeting Abstracts. 2009;114(22):Abstr: 2852. [Google Scholar]
- 15.O’Connor OA, Stewart AK, Vallone M, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15(22):7085–7091. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alsina M, Trudel S, Vallone M, et al. Phase 1 single agent antitumor activity of twice weekly consecutive day dosing of the proteasome inhibitor carfilzomib (PR-171) in hematologic malignancies. ASH Annual Meeting Abstracts. 2007;110(11):Abstr: 411. [Google Scholar]
- 17.Jagannath S, Vij R, Stewart K, et al. Final results of PX-171-003-A0, part 1 of an open-label, single-arm, phase II study of carfilzomib (CFZ) in patients (pts) with relapsed and refractory multiple myeloma (MM) J Clin Oncol. 2009;27(Suppl 15s):Abstr 8504. [Google Scholar]
- 18.diCapua Siegel DS, Martin T, Wang M, et al. Results of PX-171-003-A1, an open-label, single-arm, phase 2 (Ph 2) study of carfilzomib (CFZ) in patients (pts) with relapsed and refractory multiple myeloma (MM) ASH Annual Meeting Abstracts. 2010;116(21):Abstr: 985. [Google Scholar]
- 19.Vij R, Wang M, Kaufman JL, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012 doi: 10.1182/blood-2012-03-414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vij R, Siegel DS, Kaufman JL, et al. Results of an ongoing open-label, phase II study of carfilzomib in patients with relapsed and/or refractory multiple myeloma (R/R MM) J Clin Oncol. 2010;28(Suppl 15s):Abstr 8000. [Google Scholar]
- 21.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 22.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Siegel DS, Jakubowiak AJ, et al. The speed of response to single- agent carfilzomib in patients with relapsed and/or refractory multiple myeloma: an exploratory analysis of results from 2 multicenter phase 2 clinical trials. ASH Annual Meeting Abstracts. 2011;118(21):Abstr: 3969. [Google Scholar]
- 24.Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67(13):6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 25.Squifflet P, Michiels S, Siegel DS, et al. Multivariate modelling reveals evidence of a dose-response relationship in phase 2 studies of single-agent carfilzomib. ASH Annual Meeting Abstracts. 2011;118(21):Abstr: 1877. [Google Scholar]
- 26.Papadopoulos KP, Lee P, Singhal S, et al. A Phase 1b/2 study of prolonged infusion carfilzomib in patients with relapsed and/or refractory (R/R) multiple myeloma: updated efficacy and tolerability from the completed 20/56 mg/m2 expansion cohort of PX-171-007. ASH Annual Meeting Abstracts. 2011;118(21):Abstr: 2930. [Google Scholar]
- 27.Jakubowiak AJ, Siegel DS, Singhal S, et al. Unfavorable cytogenetic characteristics do not adversely impact response rates in patients with relapsed and/or refractory multiple myeloma treated with single-agent carfilzomib on the 003 (A1) study. ASH Annual Meeting Abstracts. 2011;118(21):Abstr: 1875. [Google Scholar]
- 28.Badros AZ, Vij R, Martin T, et al. Phase II study of carfilzomib in patients with relapsed/refractory multiple myeloma and renal insufficiency. J Clin Oncol. 2010;28(Suppl 15s):Abstr: 8128. [Google Scholar]
- 29.Singhal S, Siegel DS, Martin T, et al. Integrated safety from phase 2 studies of monotherapy carfilzomib in patients with relapsed and refractory multiple myeloma (MM): an updated analysis. ASH Annual Meeting Abstracts. 2011;118(21):Abstr: 1876. [Google Scholar]
- 30.Jagannath S, Vij R, Kaufman JL, et al. Long-term treatment and tolerability of the novel proteasome inhibitor carfilzomib (CFZ) in patients with relapsed and/or refractory multiple myeloma (R/R MM) ASH Annual Meeting Abstracts. 2010;116(21):Abstr: 1953. [Google Scholar]
- 31.Arastu-Kapur S, Anderl JL, Kraus M, et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011;17(9):2734–2743. doi: 10.1158/1078-0432.CCR-10-1950. [DOI] [PubMed] [Google Scholar]
- 32.Jagannath SJ, Vij RV, Martin TM, Badros AB, Patel PP, McCulloch LM. Carfilzomib is associated with a low rate of typically mild to moderate, non-dose limiting treatment-emergent peripheral neuropathy. European Hematological Association Annual Meeting; 2012; Amsterdam. p. Abstr: 0857. [Google Scholar]
- 33.Bensinger W, Wang M, Orlowski RZ, et al. Dose-escalation study of carf ilzomib (CFZ) plus lenalidomide (LEN) plus low-dose dexamethasone (Dex) (CRd) in relapsed/refractory multiple myeloma (R/R MM) J Clin Oncol. 2010;28(15):8029. [Google Scholar]
- 34.Moreau P, Palumbo A, Stewart AK, et al. A randomized, multicenter, phase (Ph) III study comparing carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (Dex) to LEN and Dex in patients (Pts) with relapsed multiple myeloma (MM) J Clin Oncol. 2011;29(15):TPS225. [Google Scholar]
- 35.Kisselev AF, van der Linden WA, Overkleeft HS. Proteasome inhibitors: an expanding army attacking a unique target. Chem Biol. 2012;19(1):99–115. doi: 10.1016/j.chembiol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki E, Demo S, Deu E, et al. Molecular mechanisms of bortezomib-resistant adenocarcinoma cells. PLoS One. 2011;6(12):e27996. doi: 10.1371/journal.pone.0027996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu S, Yang J, Song X, et al. Point mutation of the proteasome beta5 subunit gene is an important mechanism of bortezomib resistance in bortezomib-selected variants of Jurkat T cell lymphoblastic lymphoma/ leukemia line. J Pharmacol Exp Ther. 2008;326(2):423–431. doi: 10.1124/jpet.108.138131. [DOI] [PubMed] [Google Scholar]
- 38.Lu S, Yang J, Chen Z, et al. Different mutants of PSMB5 confer varying bortezomib resistance in T lymphoblastic lymphoma/leukemia cells derived from the Jurkat cell line. Exp Hematol. 2009;37(7):831–837. doi: 10.1016/j.exphem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Ri M, Iida S, Nakashima T, et al. Bortezomib-resistant myeloma cell lines: a role for mutated PSMB5 in preventing the accumulation of unfolded proteins and fatal ER stress. Leukemia. 2010;24(8):1506–1512. doi: 10.1038/leu.2010.137. [DOI] [PubMed] [Google Scholar]