Abstract
Propionibacterium acnes is a Gram-positive bacterium that is intimately associated with humans. The nature and consequences of this symbiosis are poorly understood; it might comprise both mutualistic and parasitic properties. Recent advances in distinguishing phylotypes of P. acnes have revealed that certain type I lineages are predominantly associated with acne vulgaris. Genome analyses revealed a highly conserved core genome and the existence of island-like genomic regions and possible mobile genetic elements as part of the flexible gene pool. The analysis of clustered regularly interspaced short palindromic repeats (CRISPR), found exclusively in type II P. acnes, recently revealed the presence of CRISPR spacers that derived from mobile genetic elements. These elements are present in a subset of P. acnes type I lineages. Their significance for type-specific host-interacting properties and their contribution to pathogenicity is currently under investigation.
Keywords: Propionibacterium acnes, acne vulgaris, clustered regularly interspaced short palindromic repeats (CRISPR), mobile genetic element, multilocus sequence typing, tight adherence
P. acnes is ubiquitously found on human skin, and has been mainly regarded as a commensal. It is also part of the human microbiota at other body sites, including the upper respiratory and gastrointestinal tracts. The organism is mostly known for its association with acne vulgaris, a skin disorder affecting around 80% of humans at some time in their life.1,2 In addition, P. acnes is isolated from a variety of other disease sites, including foreign body infections, sarcoidosis and prostate pathologies.3-5 However, so far clear-cut evidence of causal relationships is lacking. Even a role of P. acnes in acne vulgaris could not be unambiguously assigned, mainly due to the fact that the microorganism is isolated at similar frequencies from skin sites of healthy individuals.
Recent research has highlighted the population structure of P. acnes.6-8 It is a species with relatively little diversity with respect to the core genome. As judged from MLST analyses the species can be divided in three main lineages (termed types I, II and III). Type I strains are further divided into several subtypes (types I-1a, I-1b, I-26,8 or type IA1, IA2, IB, IC7), and each subtype consists of clonal complexes (CC). It has been suggested and partly confirmed that certain CCs of P. acnes are associated with acne vulgaris, e.g., CC18 strains (type I-1a), whereas others are more often isolated from healthy individuals.6 There is some circumstantial evidence indicating that strains of individual CCs have distinguishable inflammatory and host tissue interaction properties.6,7,9,10 However, despite extensive genome sequencing the genetic basis for these unique properties is unclear. One possible factor explaining phenotypic differences between strains is the absence and presence of genomic islands encoding CC-specific properties. Even within a given CC, strains could vary with respect to genomic island-encoding features, since the MLST-based schemes, according to principles of population genetics, relies on genes from the core genome, thus neglecting the flexible gene pool. The horizontal acquisition of genomic islands could be a relatively recent event in the evolutionary history of P. acnes, thus it might not be reflected in the phylogenetic tree of P. acnes.
We have identified a number of genomic regions that harbor signs of horizontal acquisition, such as an aberrant G+C content and the presence of flanking insertion sequences (Fig. 1).10-12 The gene content of such genomic regions is enriched with fitness functions such as antimicrobial defense, stress resistance, adhesion and metabolic functions (e.g., import/export and degradation of sugars and oligopeptides). For example, one island (designated ‘Thio’ in Fig. 1) harbors genes for the biosynthesis of a thiopeptide similar to berninamycin of Streptomyces bernensis13; thiopeptides often act as potent inhibitors of protein synthesis in Gram-positive bacteria. Another region (‘NRPS’ in Fig. 1) harbors a gene cluster for non-ribosomal peptide synthetases and genes involved in the synthesis of a cyclic lipopeptide.10 The genomic islands depicted in Figure 1 are only present in a subset of P. acnes strains; these strains often, but not exclusively, belong to the same CC.
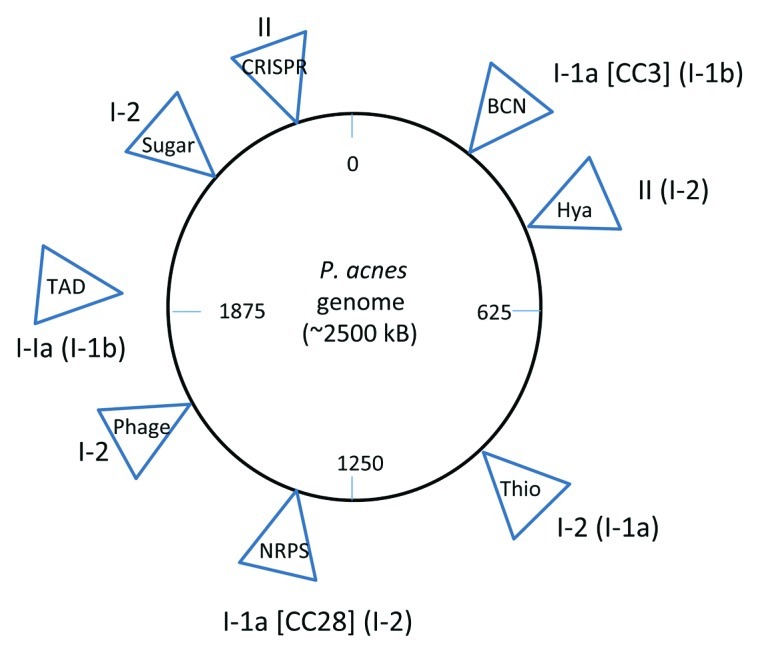
Figure 1. Island-like regions inserted into the core genome of P. acnes. Only islands larger than 10 kb are shown. The islands are named according to their gene content and/or most interesting function: BCN, bacteriocin synthesis cluster; Hya, hyaluronidase; Thio, thiopeptide synthesis cluster; NRPS, non-ribosomal peptide synthetases; phage, cryptic prophage; sugar, sugar-uptake and -degrading systems; CRISPR, clustered regularly interspaced short palindromic repeats; TAD, tight adherence system. The genomic insertion site of the TAD locus is not known, it might be an extrachromosomal element. For each island, the P. acnes type is given in which it most often occurs (e.g., the NRPS island is present mainly in type I-1a strains, in particular of clonal complex (CC) 28; it is occasionally present in I-2 strains).
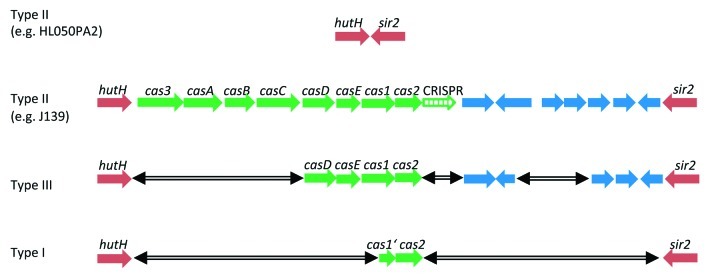
Another island-like region was recently investigated in more detail.12 It contains clustered regularly interspaced short palindromic repeats (CRISPRs) and CRISPR-associated genes (cas). The CRISPR /cas region is located on a 16 kb locus that is inserted into the core genome and exclusively present in type II P. acnes strains (Fig. 2). CRISPR/cas systems are present in a variety of bacteria and archaea and have been identified as a prokaryotic “adaptive immune” system; they provide acquired immunity against invasion of mobile genetic elements, phages and plasmids.14-18 The CRISPR regions of P. acnes type II strains thus provide a record of the genome parasites that these organisms have encountered. Like most CRISPR/cas systems, it contains sequence fragments (CRISPR spacers) of phages, more precisely of P. acnes-specific siphoviruses.19 Surprisingly, sequence analyses of the CRISPR spacers also revealed the presence of sequence fragments from two genomic islands that are found in other types of P. acnes, in particular in type I strains. Apparently, these islands are mobile genetic elements. They are designated BCN (bacteriocin) and TAD (tight adherence) loci, respectively (Fig. 1). The BCN island contains genes related to streptolysin biosynthesis (sagBCD) of streptococcal species. The TAD locus contains genes with homology to the tight adherence encoding genes of the Gram-negative Aggregatibacter actinomycetemcomitans; the TAD system in A. actinomycetemcomitans was shown to be important for host colonization and pathogenesis.20
Figure 2. The CRISPR/cas-containing locus in P. acnes. The 16 kb genomic region is present in most type II strains (such as in strain J139), inserted between hutH (encoding a histidine ammonia-lyase) and sir2 (encoding a Sir2 family protein). A few type II strains (e.g., HL050PA2) are CRISPR/cas locus-negative. Type III and type I strains possess fragments of this genomic island-like region inserted between hutH and sir2, indicative of deletion events (marked by the double-headed arrow).
It was further analyzed in which strains these islands occur: whereas the BCN locus is found in a phylogenetically distinct subtype of P. acnes (in CC3 strains), the TAD locus is present in a subset of diverse type I-1a strains (CC3, CC18, CC28 and CC31). The fact that all tested CC3 strains were positive for the BCN locus indicates that this locus was acquired by an ancestor strain of CC3. The TAD locus most likely spread more recently, since it is not restricted to strains of a specific phylogenetic clade. Moreover, the TAD locus was found also in other organisms, i.e., in the tentatively proposed species “Propionibacterium humerusii”21 and in Clostridium leptum, a bacterium isolated from the human gastrointestinal tract.22 The origin of this mobile genetic element is so far unclear. It has a G+C content (63%) that is closer to propionibacteria (ca. 60%) than to C. leptum (50.2%). Interesting open questions are if these mobile elements are still active and if their gene content contributes to strain-specific properties, e.g., if TAD locus-positive strains have special or stronger adherence properties and if BCN locus–positive strains are able to kill competitors (either BCN locus-negative P. acnes strains or other species present on the skin, e.g., Staphylococcus epidermidis).
The investigation of the CRISPR/cas containing genomic region in P. acnes also provides some hints about the evolution of the species. Analysis of all available genomes of P. acnes and additional PCR-based analysis of our strain collection revealed that there are type II strains that lack the entire CRISPR/cas harboring locus (Fig. 2). In contrast, all tested type I and type III strains have gene fragments of the locus inserted in their core genome. Type I strains harbor only a small locus fragment, whereas type III strains possess larger locus remnants. As a possible evolutionary scenario, type II strains have apparently acquired the locus by horizontal gene transfer. Subsequently, the locus was partially deleted, giving rise to type III strains; further deletion events occurred, resulting in type I strains. Alternatively, the partial CRISPR/cas locus deletion events in type I and type III strains occurred independently. Upon CRISPR/cas deletion, strains have become sensitive to the invasion of mobile genetic elements, which could contribute to speciation. Therefore, type I and type III strains may have the potential to evolve more rapidly than type II strains. Over time this could lead to increased genetic, thus phenotypic differences between the lineages. However, it should be noted that additional defense mechanisms exist in P. acnes such as restriction-modification systems, which can also prevent the acquisition of foreign DNA. Taken together, the acquisition and loss of mobile genetic elements and other island-like genomic regions, together with more subtle genomic alterations have contributed to the diversification of P. acnes. The understanding of the species evolution into lineages with distinct mutualistic or pathogenic potential is of high interest to unravel and distinguish the multiple roles of this bacterium in health and disease. Further studies on the identified mobile genetic elements and island-like regions are needed to comprehend their functional significance.
Acknowledgments
This work was supported by a grant of the European Skin Research Foundation (ESRF) to H.B.
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/21204
References
- 1.Kurokawa I, Danby FW, Ju Q, Wang X, Xiang LF, Xia L, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–32. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 2.Dessinioti C, Katsambas AD. The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clin Dermatol. 2010;28:2–7. doi: 10.1016/j.clindermatol.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Perry A, Lambert P. Propionibacterium acnes: infection beyond the skin. Expert Rev Anti Infect Ther. 2011;9:1149–56. doi: 10.1586/eri.11.137. [DOI] [PubMed] [Google Scholar]
- 4.Negi M, Takemura T, Guzman J, Uchida K, Furukawa A, Suzuki Y, et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod Pathol. 2012 doi: 10.1038/modpathol.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fassi Fehri L, Mak TN, Laube B, Brinkmann V, Ogilvie LA, Mollenkopf H, et al. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. Int J Med Microbiol. 2011;301:69–78. doi: 10.1016/j.ijmm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS One. 2010;5:e12277. doi: 10.1371/journal.pone.0012277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDowell A, Gao A, Barnard E, Fink C, Murray PI, Dowson CG, et al. A novel multilocus sequence typing scheme for the opportunistic pathogen Propionibacterium acnes and characterization of type I cell surface-associated antigens. Microbiology. 2011;157:1990–2003. doi: 10.1099/mic.0.049676-0. [DOI] [PubMed] [Google Scholar]
- 8.Kilian M, Scholz CF, Lomholt HB. Multilocus sequence typing and phylogenetic analysis of Propionibacterium acnes. J Clin Microbiol. 2012;50:1158–65. doi: 10.1128/JCM.r06129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy I, Pivarcsi A, Koreck A, Széll M, Urbán E, Kemény L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124:931–8. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
- 10.Brzuszkiewicz E, Weiner J, Wollherr A, Thürmer A, Hüpeden J, Lomholt HB, et al. Comparative genomics and transcriptomics of Propionibacterium acnes. PLoS One. 2011;6:e21581. doi: 10.1371/journal.pone.0021581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brüggemann H, Henne A, Hoster F, Liesegang H, Wiezer A, Strittmatter A, et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004;305:671–3. doi: 10.1126/science.1100330. [DOI] [PubMed] [Google Scholar]
- 12.Brüggemann H, Lomholt HB, Tettelin H, Kilian M. CRISPR/cas loci of type II Propionibacterium acnes confer immunity against acquisition of mobile elements present in type I P. acnes. PLoS One. 2012;7:e34171. doi: 10.1371/journal.pone.0034171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieland Brown LC, Acker MG, Clardy J, Walsh CT, Fischbach MA. Thirteen posttranslational modifications convert a 14-residue peptide into the antibiotic thiocillin. Proc Natl Acad Sci U S A. 2009;106:2549–53. doi: 10.1073/pnas.0900008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–70. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- 15.Deveau H, Garneau JE, Moineau S. CRISPR/Cas system and its role in phage-bacteria interactions. Annu Rev Microbiol. 2010;64:475–93. doi: 10.1146/annurev.micro.112408.134123. [DOI] [PubMed] [Google Scholar]
- 16.Marraffini LA, Sontheimer EJ. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat Rev Genet. 2010;11:181–90. doi: 10.1038/nrg2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–4. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–77. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lood R, Mörgelin M, Holmberg A, Rasmussen M, Collin M. Inducible Siphoviruses in superficial and deep tissue isolates of Propionibacterium acnes. BMC Microbiol. 2008;8:139. doi: 10.1186/1471-2180-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomich M, Planet PJ, Figurski DH. The tad locus: postcards from the widespread colonization island. Nat Rev Microbiol. 2007;5:363–75. doi: 10.1038/nrmicro1636. [DOI] [PubMed] [Google Scholar]
- 21.Butler-Wu SM, Sengupta DJ, Kittichotirat W, Matsen FA, 3rd, Bumgarner RE. Genome sequence of a novel species, Propionibacterium humerusii. J Bacteriol. 2011;193:3678. doi: 10.1128/JB.05036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD, et al. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799–807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]