Abstract
Obesity is associated with metabolic alterations related to glucose homeostasis and cardiovascular risk factors. These metabolic alterations are associated with low-grade inflammation that contributes to the onset of these diseases. We and others have provided evidence that gut microbiota participates in whole-body metabolism by affecting energy balance, glucose metabolism, and low-grade inflammation associated with obesity and related metabolic disorders. Recently, we defined gut microbiota-derived lipopolysaccharide (LPS) (and metabolic endotoxemia) as a factor involved in the onset and progression of inflammation and metabolic diseases. In this review, we discuss mechanisms involved in the development of metabolic endotoxemia such as the gut permeability. We also discuss our latest discoveries demonstrating a link between the gut microbiota, endocannabinoid system tone, leptin resistance, gut peptides (glucagon-like peptide-1 and -2), and metabolic features. Finally, we will introduce the role of the gut microbiota in specific dietary treatments (prebiotics and probiotics) and surgical interventions (gastric bypass).
Keywords: gut microbiota, LPS, metabolic endotoxemia, gut permeability, GLP-1, GLP-2, endocannabinoid, adipose tissue, liver, RYGB
Introduction
A growing body evidence suggests that obesity is influenced by both genetic and lifestyle factors. Although an imbalance between energy intake and energy expenditure could explain the growing incidence of obesity, the metabolic alterations associated with obesity are not solely explained by genetic factors. Obesity is classically associated with metabolic alterations related to glucose homeostasis (e.g., glucose intolerance, type 2 diabetes, and insulin resistance) and cardiovascular risk factors (e.g., hypertension and dyslipidemia).1 These metabolic alterations are associated with low-grade inflammation that contributes to the onset of these diseases.2 Given the existing link between inflammation and metabolism in the context of the metabolic syndrome, identifying the origin of inflammation is of the utmost importance. Although numerous investigations have demonstrated a relationship between inflammation and macrophage infiltration into organs (e.g., adipose tissue, muscles, and the liver), the exact role of macrophages and the source and type of triggering factors activating the immune system remain a matter of debate.2-8 Hence, the major pathogenic mechanism linking inflammation with changes in liver and adipose tissue metabolism remain to be determined. Given the plethora of inflammatory markers causally associated with the development of impaired insulin action (or insulin resistance) and the numerous molecular interactions between the immunity and insulin signaling, we have researched potential integrating factors that might provide a mechanism. Our studies focused on the gut microbiota as a putative candidate.
Gut Microbiota and Metabolic Endotoxemia
Among the environmental factors that are hypothesized to interfere with energy homeostasis, growing evidence demonstrates that the gut microbiota plays a critical role.9-16
The human microbiota consists of as many as 10 to 100 trillion microorganisms, a number that is at least 10-fold more than cells that make up the human body,17 meaning that the cells that compose our body are 10% human and 90% microbes.18 Therefore, it is now widely accepted that this consortium of cells provides important metabolic and biological functions that cannot be performed by our human metabolism.19
We and others have provided evidence that gut microbiota participates in whole-body metabolism by affecting energy balance,11,13,14 glucose metabolism,9,14,20-22 and low-grade inflammation9,10,14,23-25 associated with obesity and related metabolic disorders.
Recently, we defined gut microbiota-derived lipopolysaccharide (LPS) as factors involved in the onset and progression of inflammation and metabolic diseases.14 We found that in pathological situations (obesity and type 2 diabetes) specific microbes-associated molecular patterns (MAMPs) such as LPS play a major role in the onset the diseases associated with obesity.9,10,14,22,25-27 LPS is a component of Gram-negative bacteria cell walls and are among the most potent and well-studied inducers of inflammation. We demonstrated that dietary fat facilitates the development of metabolic endotoxemia (e.g., increased plasma LPS levels).14,28 This process can be initiated by physiological mechanisms, such as the transport of LPS from the gut lumen toward target tissues by newly synthesized chylomicrons from epithelial intestinal cells in response to fat feeding.29,30 We and others have confirmed these data in human subjects; therefore, this phenomenon could contribute to higher plasma LPS levels and low-grade inflammation, as is observed with a high-fat diet.14,31-35 Given that genetic models of obesity fed with a normal chow diet still develop metabolic endotoxemia, we cannot exclude the involvement of factors other than fat absorption.10,25,36,37 Therefore, we hypothesized that metabolic endotoxemia could be linked to the gut microbiota.
Gut Microbiota and High-Fat Diet
We first demonstrated that high-fat diet profoundly affects gut microbiota composition in 2007.14,27 More precisely, we found, that diet-induced obesity strongly altered gut microbiota composition by reducing Bifidobacterium spp and Bacteroides-related bacteria, Eubacterium rectale-Blautia coccoides group content.14,27 These data have been confirmed and extended by the discovery that fat feeding also decreases Lactobacillus spp and Roseburia spp.38 Several other studies have characterized the gut microbiota composition of diet-induced obese mice with different metagenomic approaches.39-43 Importantly, all of these studies are relatively consistent regarding gut microbiota modulation in mice fed a high-fat diet; Firmicutes become more abundant, and Bacteroidetes decrease in number. More specifically, Hildebrandt et al. showed decreased Bacteriodetes and increased Firmicutes and Proteobacteria in mice fed a high-fat diet.41 Murphy et al. found an increased proportion of Firmicutes and a reduction in Bacteroidetes in similar dietary conditions.42 More recently, Ravussin et al. confirmed that mice on a high-fat diet had increased levels of Firmicutes.43 Although most of the results exploring the impact of high-fat diet on gut microbiota composition are convergent, no study has identified a specific link with metabolic endotoxemia onset.
Gut Microbiota and the Innate Immune System Link Metabolic Endotoxemia to Insulin Resistance
Toll-like receptors (TLRs) are a family of pattern-recognition receptors that play a critical role in innate immunity by integrating signals from microbiota-host interactions (e.g., proinflammatory signals). The innate immune system detects LPS via its interaction with specific proteins that complex with TLR4 (CD14/TLR4 complex).44 Several experimental arguments support metabolic endotoxemia as a factor involved in the development of low-grade inflammation associated with insulin resistance and type 2 diabetes (Fig. 1). We found that a high-fat diet increased fat mass, body weight and low-grade inflammatory state (in the liver, adipose tissues and muscle) through an LPS-dependent mechanism. We also discovered that mice that lack functional LPS receptors (CD14 knockout mice) are resistant to diet-induced obesity and related disorders, including hepatic insulin resistance.14 We demonstrated that the chronic subcutaneous infusion of LPS (mimicking metabolic endotoxemia) induces significant inflammation and insulin resistance to a similar extent as observed following high-fat diet feeding. The origin of gut microbiota in this phenotype has been further demonstrated in mice chronically treated with broad-spectrum antibiotics. In these conditions, mice fed a high-fat diet that received antibiotics exhibited reduced metabolic endotoxemia, inflammation, insulin resistance and fat mass development.9 These effects were confirmed in genetically obese (ob/ob) mice.9,45 More recently, it was shown that germ-free mice did not develop inflammation following a high-fat diet, further supporting the role of gut microbiota in low-grade inflammation onset.23 Different studies have proposed that saturated fatty acids promote low-grade inflammation and insulin resistance through a TLR-4 dependent mechanism46-48; however, this effect is still a matter of debate.49 It can be proposed with more certainty that fatty acids stimulate the innate immune system, but probably in conjunction with initial stimulation by LPS of the TLR-4/CD14 complex and subsequent TLR-2 stimulation. For instance, both axenic mice and antibiotic treated mice are resistant to the development of high-fat diet–induced inflammation and insulin resistance. Given that these mice fully digest and absorb the ingested fat, and display functional TLR-4/2 receptors9,23,50 suggest that a signaling cascade initiated by an LPS/TLR-4/CD14-dependent mechanism in turn activates TLR-2 expression to support innate immune system inflammatory responses.
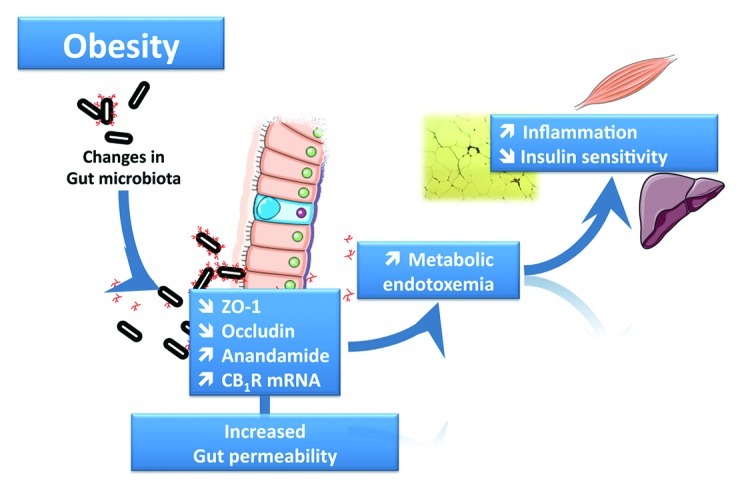
Figure 1. The gut microbiota controls gut barrier function and the onset of metabolic endotoxemia. Diet-induced obesity and genetic (ob/ob or db/db mice) obesity are associated with changes in gut microbiota composition. This leads to gut barrier function alteration through several mechanisms, including an altered distribution of the tight junction proteins ZO-1 and Occludin and an increased eCB system tone with a higher expression of anandamide and CB1R. These phenomena promote metabolic endotoxemia and initiate the development of low-grade inflammation and insulin resistance in the liver, muscles and adipose tissue.
Because of these studies, it is now increasingly recognized that the innate immune system and metabolic pathways are functionally intertwined,2 making them attractive obesity and diabetes targets. Recent data indicate that low-grade inflammation and insulin resistance are also controlled by TLR2.51-53 It has been proposed that both TLR516 and TLR254 knock out mice exhibited altered gut microbiota composition and these receptors play a central role in the development of obesity and associated disorders. These data further support the hypothesis that microorganisms and/or derived compounds play a crucial role in the onset of metabolic disorders associated with obesity. However, there is no clear evidence of a unique coupling system between gut microbial-host signals and the onset or progression of metabolic alterations associated with obesity.
Metabolic Endotoxemia and Gut Barrier Function
One of the mechanisms explaining the development of obesity-related metabolic endotoxemia is that gut microbiota links gut permeability to low-grade inflammation and insulin resistance.9,14,25,37 More specifically, we have shown that a high-fat diet contributes to the disruption of the tight-junction proteins (Zonula Occludens-1 and Occludin) involved in the gut barrier function (Fig. 1). This effect is directly dependent on the gut microbiota because antibiotic treatment abolished diet-induced gut permeability.9,10 In accordance with this hypothesis, we also found that the specific modulation of gut microbiota composition with non-digestible carbohydrates (e.g., prebiotics) improves gut barrier integrity, reduces metabolic endotoxemia, and lowers inflammation and glucose intolerance (Fig. 1).10,25,27,37
Among the putative mechanisms linking the gut microbiota with the development of obesity and related disorders, we have proposed a role for the endocannabinoid system (eCB). The eCB system is composed of endogenous lipids that activate specific G protein-coupled receptors termed cannabinoid receptors 1 and 2 (CB1R and CB2R). Among these lipids, N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) are the most studied.55 AEA and 2-AG are both widely present throughout the body, and their tissue levels are regulated by the balance between synthesis and inactivation.56
Importantly, our latest discoveries demonstrate a link between the gut microbiota, eCB system tone and metabolic features associated with obesity.24,37 For instance, we have demonstrated that the gut microbiota controls the eCB tone in intestine and adipose tissue.24,37 We also found that the gut microbiota regulates the CB1R expression, AEA content and its degrading enzyme fatty acid amide hydrolase (FAAH), not only in the intestine but also in mouse adipose tissue (Fig. 1).37 However, the mechanisms behind this constitutive crosstalk in obese and type 2 diabetes are largely unknown. Interestingly, several studies have suggested a relationship between LPS and the eCB system. Indeed, LPS controls eCB synthesis in macrophages, and the macrophage infiltration of adipose tissue and the liver during obesity is a key factor in metabolic disorder development.5,57 We demonstrated that macrophage infiltration is dependent on LPS activation and its interaction with its co-receptor CD14, as well as gut microbiota composition.9,14 Finally, we used pharmacological interventions to demonstrate that the eCB system contributes to gut barrier function (in vivo and in vitro) and metabolic endotoxemia via putative CB1 receptor-dependent mechanisms. More precisely, the eCB system controls gut barrier function through the distribution and localization of tight junction proteins (ZO-1 and occludin), independently of food intake behavior. Furthermore, we identified the eCB system in the gut and adipose tissue as a prominent pathway for adipogenesis regulation.37 Altogether, these data support interplay between these three partners, namely, the gut microbiota, the innate immune system and the endocannabinoid system, in the development of obesity and related disorders.
Communication Between Gut Microbes and Adipose Tissue Metabolism
Adipose tissue is a key organ with a central role in metabolism regulation.58 Although its role in energy storage has been recognized for centuries, its function as an endocrine organ affecting energy homeostasis, innate immunity and inflammation has only been known for a few decades.58 Insight into the mechanisms underlying adipose tissue biology is important for a better understanding of altered metabolism in obesity and type 2 diabetes. As mentioned already, the gut microbiota can be considered as an “exteriorized organ” that contributes to host metabolism and homeostasis via different functions and mechanisms.18 Growing evidence suggests that the gut microbiota contributes to host metabolism through communication with adipose tissue, which influences the development of metabolic alterations associated with obesity.
Several studies support such a communication axis. First, mice with gut microbiota (conventionally raised) have over 40% more fat mass than germ-free mice (without gut microbiota).11 Second, the transplantation of cecal content (gut microbiota) isolated from obese mice to germ-free mice resulted in a greater increase in total body fat mass compared with colonization with gut microbiota isolated from lean donors.13 A recent study demonstrated an increased lipolysis and decreased lipogenesis in the brown adipose tissue of germ-free vs. conventionally raised mice, suggesting that gut microbiota stimulates brown adipose tissue lipid metabolism.59 Ob/ob mice fed prebiotics have a different gut microbiota composition and decreased adiposity index compared with obese mice fed a normal chow diet.25 Conventionalization of germ-free mice has also highlighted a general increase in activity of the enzyme lipoprotein lipase (LPL), which catalyzes the release of fatty acids from circulating triglycerides and lipoproteins in muscle and adipose tissue. This mechanism may be related to suppression of the fasting-induced adipose factor (FIAF) in the gut. FIAF is an inhibitor of LPL activity, and its blunted activity in conventionalized mice may participate in the accumulation of fatty acids in adipose tissue.11 Finally the demonstration that germ-free mice are resistant to high-fat diet-induced body weight gain and fat mass accumulation suggests that gut microbiota promotes fat storage.50 Different mechanisms leading to the resistance of diet-induced obesity have been proposed and are extensively discussed in recent reviews.18,60
We have previously suggested a link between gut microbiota composition, metabolic endotoxemia, and adipose tissue development during high-fat diet feeding.9,14,27 In addition, we showed that gut microbiota modulation by prebiotics may control adipogenesis by acting on lipogenic enzymes and markers of adipocyte differentiation.37 We proposed that selective changes in the gut microbiota of obese mice impact LPS levels, a process that may participate in adipogenesis regulation.37
Based on a recent study from our lab,24 we suggest that the impact of gut microbiota modulation on adipose tissue metabolism can be mediated by the eCB system. Different studies suggest a role for eCBs in adipose tissue metabolism in both rodents and humans,61-66 but the exact molecular mechanisms underlying this regulation are still under investigation. Recently, we discovered that modulating gut microbiota with prebiotics changes the eCB system tone, not only in the gut (as discussed earlier) but also in adipose tissue.37 eCB activation stimulates adipogenesis, whereas LPS stimulates eCB system tone.37,67 More recently, we observed that genetically obese and diabetic mice (db/db) exhibited a different gut microbiota composition compared with their lean littermates.24 With high-throughput culture-independent technologies (pyrosequencing and MITchip analysis), we found that gut microbiota composition varied between the two groups. In addition, we have shown that db/db mice present an altered adipose eCB system tone, characterized by elevated AEA levels and increased expression of CB1 receptor and synthesizing enzyme N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD), in comparison to their lean littermates (Fig. 1).24 We also examined adipose tissue metabolism, represented here by apelin, a newly identified adipokine. This key peptide is involved in several physiological functions in both the central nervous system and peripheral tissues. Apelin plays a role in cardiovascular functions (e.g., heart contractility, fluid homeostasis, and blood pressure) and seems to be a potential regulator of metabolism; it has been demonstrated that apelin affects glucose homeostasis by stimulating glucose uptake in skeletal muscle and adipose tissue.68-70 Interestingly, we found that higher expression of apelin and its receptor (APJ) in the adipose tissue of obese and type 2 diabetic mice was significantly correlated with several taxa.24 These genetic models are known to have inflammation, altered gut barrier function and endotoxemia.36 We confirmed this by measuring inflammatory markers (cytokine IL-1β, chemokine MCP-1, macrophage markers F4/80, CD11c, CD68), which are all increased in obese vs. lean mice; there were positive correlations between these inflammatory markers and the apelinergic system.24 To ascertain the mechanistic link between altered adipose tissue metabolism and inflammation (i.e., LPS and cytokines) or the eCB system, we stimulated adipose tissue explants from lean wild-type mice with an eCB system agonist, with or without concomitant LPS administration. We found that eCB activation downregulates expression of apelin and its receptor, and low-dose LPS significantly increases these two markers. Surprisingly, concomitant treatment of adipose tissue with eCB agonist and LPS increases apelin and APJ; in this scenario the eCB agonist is unable to neutralize LPS-mediated effects on the apelinergic system.24 These results indicate that eCBs impact adipose tissue metabolism in a physiological state and that these effects can be modified in pathological situations (e.g., obesity with endotoxemia, inflammation and eCB activation), which strongly suggests that LPS and inflammation regulate adipose tissue metabolism.24 Taken together, these data suggest a potential relationship between gut microbiota and adipose tissue that may be mediated by the eCB system.
Gut Peptides as Communication Agents Between Gut Microbiota and Host Metabolism in a Context of Obesity
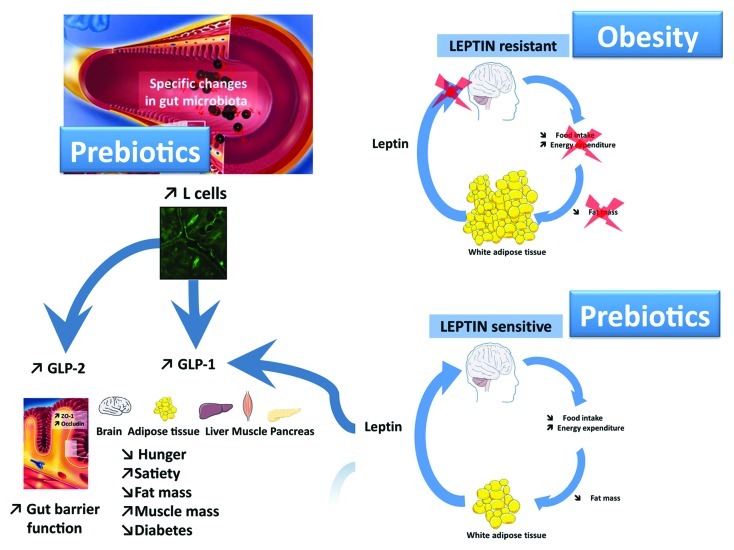
Gut microbiota composition is influenced by several factors, including host-dependent factors (e.g., genetic background, age, sex, immune system and gut motility), treatment (e.g., antibiotics and gastric bypass) and diet (e.g., nondigestible carbohydrates, fat, prebiotics or probiotics).60 Prebiotics are selectively fermented dietary ingredients that cause specific changes in the composition and/or activity of the gut microbiota (e.g., bifidobacteria and lactobacilli) that confer benefit(s) to host health.71,72 Hence, prebiotics are often used to modulate gut microbiota and to promote health. We have previously shown that prebiotics improve gut barrier function and reduce the metabolic inflammation and insulin resistance associated with obesity by increasing release of gut peptides, such as glucagon-like peptide-1 and -2 (GLP-1 and -2).10,18,20-22,26,73,74 We found that prebiotic-induced changes in the gut microbiota promote GLP-1 and GLP-2 synthesis (proglucagon mRNA, GLP-1 and GLP-2 peptides) in the proximal colon (Fig. 2).25,74 Recently, we demonstrated that prebiotics, such as oligofructose, modulate 102 different taxa in obese and type 2 diabetic mice.25 These modulations were associated with lower fat mass accumulation, increased muscle mass and improved glucose and lipid metabolism.25 In addition, we confirmed that these effects were associated with decreased gut permeability, metabolic endotoxemia and whole body inflammation.25 In the same study, we identified a novel mechanism explaining higher endogenous GLP-1 and GLP-2 production; prebiotic treatment increases the number of enteroendocrine cells producing GLP-1 and GLP-2 (L-cells) in the jejunum and colon (Fig. 2).25 Therefore, these findings suggest that targeting enteroendocrine function could be a novel therapeutic approach to treat the inflammatory phenotype associated with obesity and type 2 diabetes.
Figure 2. Prebiotic-induced changes in the gut microbiota affect enteroendocrine function and leptin sensitivity. Prebiotics profoundly affect gut microbiota composition in a complex way in response to a high-fat diet or genetic obesity (e.g., increased Bifidobacterium spp. and Akkermansia muciniphila). Prebiotic treatment decreases gut permeability and metabolic endotoxemia and improves insulin sensitivity, steatosis and low-grade inflammation via several mechanisms, including the following: 1) an increased L-cell number and endogenous GLP-1 and GLP-2 production and 2) an increased leptin sensitivity, which controls energy homeostasis and GLP-1 production.
Leptin is produced by adipocytes in proportion to the amount of adipose tissue to inform whole the body of nutritional status.75,76 Leptin is involved in food intake and energy homeostasis regulation and is also linked to glucose homeostasis regulation and numerous gastrointestinal functions such as induction of GLP-1 secretion77 (Fig. 2). Leptin resistance is a hallmark of obesity78 and we were the first to demonstrate that gut microbiota control leptin action.25 More precisely, we found that altering gut microbiota composition with prebiotics improves leptin sensitivity in diet-induced obese and type 2 diabetic mice (Fig. 2).25 We cannot exclude that improved leptin sensitivity could be responsible for increased plasma GLP-1. Therefore, we propose that targeted gut microbiota modulations could be a novel therapeutic target to reset leptin sensitivity during obesity.
In addition to data obtained in pre-clinical studies, several effects of prebiotics have been partially confirmed in humans. For instance, it has been shown that prebiotic consumption (5 to 20 g per day) changes the gut microbiota composition and increases plasma GLP-1 levels.79-82 These effects have been associated with several interesting effects, including the following: 1) lower postprandial glycemia,81,83 2) an increased satiety and a decreased hunger and energy intake,80-83 and 3) a reduced visceral fat mass.80-83 Altogether, these results strongly suggest that gut peptides and their “regulator elements” contribute to communication between gut microbiota and the host, with benefits in terms of obesity pathology. Deciphering the mechanisms involved in this communication will allow the design of novel therapies for the treatment of obesity and associated metabolic disorders.
Gut Microbiota and Non-Alcoholic Fatty Liver Disease
The relationship between changes in gut microbiota and the development and progression of liver diseases has been known for over 50 y. Endotoxemia and gut-derived toxins are suggested to have causative roles in the onset and progression of liver inflammation and damage in chronic liver diseases.84,85 Non-alcoholic fatty liver disease (NAFLD) is the most typical chronic liver complication observed in obesity and metabolic syndrome. This hepatic component of metabolic syndrome involves a complex spectrum of pathological changes, including steatosis, nonalcoholic steatohepatitis (NASH), fibrosis and cirrhosis.86
Similar to the mechanisms underlying endotoxemia and inflammation described in the previous sections, diet-induced intestinal bacterial overgrowth, gut leakiness and increased endotoxin absorption have all been associated with NAFLD/NASH in both rodents and human patients.10,36,87,88 Changes in tight junction protein expression and distribution are suggested as critical factors in the impairment of gut barrier function and subsequent alterations in gut permeability observed in NAFLD patients.89 As a consequence, hepatic exposure to gut-derived endotoxins is thought to increase TLR activation, especially on Kupffer cell membranes, and to activate nuclear transcription factors resulting in the release of numerous proinflammatory cytokines that ultimately lead to hepatic injury and fibrosis.90-92
Both high-fat and high-fructose diets induce important changes in gut microbiota and trigger inflammatory reactions associated with the development of systemic and hepatic insulin resistance and NAFLD/NASH.14,27,93,94 Recently, several studies have attempted to show that proinflammatory pathway activation in Kupffer cells and hepatic resident macrophages is involved in the development of diet-induced hepatic insulin resistance.57,95-97 Although the inhibition of Kupffer cell functioning prevents adiposity, adipose tissue inflammation, and exerted antidiabetic and antiobesity effects in response to a short exposure to HFD,57 the role of Kupffer cells in chronic HFD-induced insulin resistance remains unclear.95,96
However, treatment with antibiotics or loss of TLR-4 significantly attenuate the development of hepatic steatosis in fructose-fed mice.98 It was recently demonstrated that TLRs other than TLR4 may be involved in fructose-induced NAFLD onset.99
The hypothesis is that diet-induced NAFLD may be mediated by a MyD88-dependent pathway,94 whereas the development of other forms of steatohepatitis (e.g., alcohol-related) seems to involve the MyD88-independent pathway.100
Additional confirmation of the close relationship between gut and liver comes from work in mice with non-alcoholic liver steatosis, in which probiotics ameliorate NAFLD/NASH and metabolic syndrome. Li et al. demonstrated that probiotic treatment improves insulin resistance, hepatic histology and total fatty acid content in mice with NASH.101 Furthermore, the use of VSL#3, a mix of probiotic strains, decreases levels of TNF-α and reduces hepatic inflammatory signaling in liver steatosis.102,103 Similar data were reproduced in humans, suggesting that prebiotic administration could ameliorate oxidative and inflammatory liver damage associated with NAFLD.102
However, the lack of randomized clinical trials prevented a recent Cochrane Library review to assess the clinical effects of probiotic therapy in NAFLD/NASH patients. Therefore, additional studies are required to further prove the beneficial effects of probiotic therapy for NASH.104
Gut Microbiota and Gastric Bypass Surgery
Currently, strategies involving diet and increased physical activity as well as the use of anti-obesity medications offer only limited efficacy in terms of significant weight loss and long-term effectiveness. In fact, body weight loss is usually rapidly regained once dieting or drug therapy is discontinued.105
Presently, the most effective weight loss strategy is bariatric surgery.106 Several reports and clinical studies analyses reported that bariatric surgery significantly reduces body weight and fat mass in obese patients over time; it also ameliorates or even cures type 2 diabetes and metabolic syndrome and has profound effects on the cardiovascular system.107-110 Among the different bypass surgeries, including biliopancreatic diversion, ileal transposition, and duodenal-jejunal bypass, the Roux-en-Y gastric bypass (RYGB) surgery is the most effective treatment for morbid obesity.111
The modification of food intake behavior, altered gut hormone secretion,112 gastric emptying, bile acid metabolism and changes in intestinal gluconeogenesis113 have all been postulated as possible explanations for improvements in body composition and nutritional and inflammatory metabolism associated with RYGB. However, mechanisms for weight loss and metabolic changes in RYGB are not fully understood.
RYGB induces eating behavior changes, with decreased appetite and meal frequency114 and, paradoxically, increased energy expenditure.115 Gut hormone signaling is also modified by the surgery. Postprandial levels of PYY and GLP-1 rise soon after surgery and remain elevated for many months.107,116 GLP-2 levels and mucosal crypt cell proliferation are also increased after surgery.117 Higher GLP-2 production may account for gut absorptive surface area restoration, which would limit malabsorption after RYGB.117 Interestingly, these metabolic and hormonal changes can be detected before weight loss occurs. Furthermore, diabetes remission is estimated to be approximately 80–85% just days after surgery.106 For these reasons, RYGB is now considered a potential treatment strategy for diabetes, even in the absence of obesity. In addition to the immediate and long-term adaptations associated with gastric bypass surgery, recent studies reported that microbial ecology also changes in obese subjects after RGYB.118 In fact, post-surgery changes in gut microbiota have been suggested to play a role in the improvement of several features that characterize the pathological statuses of human obesity and type 2 diabetes.119,120 Zhang et al. used pyrosequencing technology to show that gastric bypass surgery significantly decreased Firmicutes and increased Gammaproteobacteria, a member of the Enterobacteriaceae family, in three obese patients.
Additionally, the proportion of Bacteroides and Prevotella (a subgroup of Bacteroidetes) were shown to rapidly increase in the distal gut microbiota of 30 morbidly obese subjects after surgery.120 Escherichia coli species were inversely associated with body fat mass and metabolic changes in the same patients 3 months after surgery. Interestingly, other specific bacterial groups (e.g., Faecalibacterium prausnitzii) were better correlated with changes in inflammatory markers and inflammation than with changes in calorie intake.120
However, in the same study, Lactobacillus/Leuconostoc/Pediococcus and Bifidobacterium levels were decreased after surgery.120 This is in contrast with previous studies that reported positive correlations between Bifidobacterium levels and improvements in metabolic and inflammatory states.121-123 However, another study in morbidly obese patients undergoing RYGB revealed that probiotic treatment with Lactobacillus significantly increased the percentage of weight loss compared with control patients up to three months after surgery.124 Li et al. reported higher concentrations of Proteobacteria, specifically Enterobacter hormaechei, after RYGB in a non-obese rat model. Gut microbiota analysis of fecal samples from RYGB rats showed lower concentrations of Firmicutes and Bacteroidetes in comparison with sham-operated rats.125 Although all of these results support a new role of gut microbiota in adaptations associated with RYGB, a definitive explanation for the beneficial improvements observed following bypass surgery has not been fully elucidated.118
Future studies are needed to explore the molecular and physiological mechanisms observed post-RYGB, with particular attention given to the relationships between changes in eating behavior, gut hormone levels and the gut microbial community.
In addition to the specific dietary treatments (prebiotics and probiotics) and surgical interventions (gastric bypass) showing the interaction between the gut microbiota and host metabolism, recent preliminary experiments have shown the interest of fecal transplantation in humans. Vrieze et al. have shown that fecal transplantation from lean donors to 18 obese patients significantly improved peripheral insulin sensitivity compared with those who received an autologous transplant. Although the final characterization of the gut microbiota composition was not known, and more information are required to strengthen the results, this original approach challenge the hypothesis that fecal transplantation might be view as a “new way to treat the metabolic syndrome” (Vrieze A, et al. EASD 2010; Abstract 90).
Conclusion
Compelling evidence supports the concept that the gut microbiota participates in the development of fat mass, insulin resistance and low-grade inflammation associated with obesity. Over the last five years, numerous emerging concepts have helped elucidate this “small world within”126 in the context of the “MicrObesity”.18 The gut microbiota seems to play a crucial role in numerous conditions associated with obesity and type 2 diabetes, from metabolic endotoxemia to gut barrier function and liver diseases. It also seems to be involved in specific dietary treatments (prebiotics and probiotics) and surgical interventions (gastric bypass).
Nevertheless, the numerous mechanisms by which gut bacteria interact with the host, including the direct involvement of specific gut microbes and/or of microbial metabolites, remains to be elucidated.
Acknowledgments
PDC is a Research Associate from the FRS-FNRS (Fonds de la Recherche Scientifique, Belgique) and the recipient of subsidies from FSR (fonds spéciaux de recherche), UCL (Université catholique de Louvain) and the FRSM (Fonds de la Recherche Scientifique Medicale: n°3.4579.11).
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/19625
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 3.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–26. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–79. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tordjman J, Guerre-Millo M, Clément K. Adipose tissue inflammation and liver pathology in human obesity. Diabetes Metab. 2008;34:658–63. doi: 10.1016/S1262-3636(08)74601-9. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Messina JL. Acute insulin resistance following injury. Trends Endocrinol Metab. 2009;20:429–35. doi: 10.1016/j.tem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 10.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 14.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 15.Martínez I, Wallace G, Zhang C, Legge R, Benson AK, Carr TP, et al. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl Environ Microbiol. 2009;75:4175–84. doi: 10.1128/AEM.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–33. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 18.Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther. 2011;130:202–12. doi: 10.1016/j.pharmthera.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Jia W, Li H, Zhao L, Nicholson JK. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov. 2008;7:123–9. doi: 10.1038/nrd2505. [DOI] [PubMed] [Google Scholar]
- 20.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes Res. 2005;13:1000–7. doi: 10.1038/oby.2005.117. [DOI] [PubMed] [Google Scholar]
- 21.Cani PD, Daubioul CA, Reusens B, Remacle C, Catillon G, Delzenne NM. Involvement of endogenous glucagon-like peptide-1(7-36) amide on glycaemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol. 2005;185:457–65. doi: 10.1677/joe.1.06100. [DOI] [PubMed] [Google Scholar]
- 22.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92:521–6. doi: 10.1079/BJN20041225. [DOI] [PubMed] [Google Scholar]
- 23.Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948–59. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- 24.Geurts L, Lazarevic V, Derrien M, Everard A, Van Roye M, Knauf C, et al. Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: impact on apelin regulation in adipose tissue. Front Microbiol. 2011;2:149. doi: 10.3389/fmicb.2011.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–86. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes. 2006;55:1484–90. doi: 10.2337/db05-1360. [DOI] [PubMed] [Google Scholar]
- 27.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 28.Cani PD, Delzenne NM. Gut microflora as a target for energy and metabolic homeostasis. Curr Opin Clin Nutr Metab Care. 2007;10:729–34. doi: 10.1097/MCO.0b013e3282efdebb. [DOI] [PubMed] [Google Scholar]
- 29.Vreugdenhil AC, Rousseau CH, Hartung T, Greve JW, van ’t Veer C, Buurman WA. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J Immunol. 2003;170:1399–405. doi: 10.4049/jimmunol.170.3.1399. [DOI] [PubMed] [Google Scholar]
- 30.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–7. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219–23. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 32.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–92. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 33.Laugerette F, Vors C, Géloën A, Chauvin MA, Soulage C, Lambert-Porcheron S, et al. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem. 2011;22:53–9. doi: 10.1016/j.jnutbio.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Al-Attas OS, Al-Daghri NM, Al-Rubeaan K, da Silva NF, Sabico SL, Kumar S, et al. Changes in endotoxin levels in T2DM subjects on anti-diabetic therapies. Cardiovasc Diabetol. 2009;8:20. doi: 10.1186/1475-2840-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34:392–7. doi: 10.2337/dc10-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–25. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 37.Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Van Holle A, Muccioli GG, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem. 2011;22:712–22. doi: 10.1016/j.jnutbio.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–24, e1-2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy EF, Cotter PD, Healy S, Marques TM, O’Sullivan O, Fouhy F, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59:1635–42. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 43.Ravussin Y, Koren O, Spor A, Leduc C, Gutman R, Stombaugh J, et al. Responses of Gut Microbiota to Diet Composition and Weight Loss in Lean and Obese Mice. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bäckhed F, Normark S, Schweda EK, Oscarson S, Richter-Dahlfors A. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes Infect. 2003;5:1057–63. doi: 10.1016/S1286-4579(03)00207-7. [DOI] [PubMed] [Google Scholar]
- 45.Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416–26. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 46.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–9. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- 48.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 49.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29:1944–9. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 50.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–84. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, et al. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia. 2010;53:1795–806. doi: 10.1007/s00125-010-1747-3. [DOI] [PubMed] [Google Scholar]
- 52.Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010;24:731–9. doi: 10.1096/fj.09-141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis JE, Braucher DR, Walker-Daniels J, Spurlock ME. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J Nutr Biochem. 2011;22:136–41. doi: 10.1016/j.jnutbio.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Caricilli AM, Picardi PK, de Abreu LL, Ueno M, Prada PO, Ropelle ER, et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 2011;9:e1001212. doi: 10.1371/journal.pbio.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Lambert DM, Muccioli GG. Endocannabinoids and related N-acylethanolamines in the control of appetite and energy metabolism: emergence of new molecular players. Curr Opin Clin Nutr Metab Care. 2007;10:735–44. doi: 10.1097/MCO.0b013e3282f00061. [DOI] [PubMed] [Google Scholar]
- 56.Muccioli GG. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov Today. 2010;15:474–83. doi: 10.1016/j.drudis.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Lanthier N, Molendi-Coste O, Horsmans Y, van Rooijen N, Cani PD, Leclercq IA. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2010;298:G107–16. doi: 10.1152/ajpgi.00391.2009. [DOI] [PubMed] [Google Scholar]
- 58.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–39. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 59.Mestdagh R, Dumas ME, Rezzi S, Kochhar S, Holmes E, Claus SP, et al. Gut microbiota modulate the metabolism of brown adipose tissue in mice. J Proteome Res. 2012;11:620–30. doi: 10.1021/pr200938v. [DOI] [PubMed] [Google Scholar]
- 60.Delzenne NM, Neyrinck AM, Backhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol (2011) [DOI] [PubMed] [Google Scholar]
- 61.D’Eon TM, Pierce KA, Roix JJ, Tyler A, Chen H, Teixeira SR. The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids. Diabetes. 2008;57:1262–8. doi: 10.2337/db07-1186. [DOI] [PubMed] [Google Scholar]
- 62.Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A, Izzo AA, et al. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring) 2008;16:553–65. doi: 10.1038/oby.2007.106. [DOI] [PubMed] [Google Scholar]
- 63.Sarzani R, Bordicchia M, Marcucci P, Bedetta S, Santini S, Giovagnoli A, et al. Altered pattern of cannabinoid type 1 receptor expression in adipose tissue of dysmetabolic and overweight patients. Metabolism. 2009;58:361–7. doi: 10.1016/j.metabol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–80. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 65.Gasperi V, Fezza F, Pasquariello N, Bari M, Oddi S, Agrò AF, et al. Endocannabinoids in adipocytes during differentiation and their role in glucose uptake. Cell Mol Life Sci. 2007;64:219–29. doi: 10.1007/s00018-006-6445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartelt A, Orlando P, Mele C, Ligresti A, Toedter K, Scheja L, et al. Altered endocannabinoid signalling after a high-fat diet in Apoe (-/-) mice: relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia. 2011;54:2900–10. doi: 10.1007/s00125-011-2274-6. [DOI] [PubMed] [Google Scholar]
- 67.Liu J, Batkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, et al. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem. 2003;278:45034–9. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- 68.Duparc T, Naslain D, Colom A, Muccioli GG, Massaly N, Delzenne NM, et al. Jejunum inflammation in obese and diabetic mice impairs enteric glucose detection and modifies nitric oxide release in the hypothalamus. Antioxid Redox Signal. 2011;14:415–23. doi: 10.1089/ars.2010.3330. [DOI] [PubMed] [Google Scholar]
- 69.Duparc T, Colom A, Cani PD, Massaly N, Rastrelli S, Drougard A, et al. Central apelin controls glucose homeostasis via a nitric oxide-dependent pathway in mice. Antioxid Redox Signal. 2011;15:1477–96. doi: 10.1089/ars.2010.3454. [DOI] [PubMed] [Google Scholar]
- 70.Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buléon M, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008;8:437–45. doi: 10.1016/j.cmet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 72.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(Suppl 2):S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 73.Delzenne NM, Cani PD, Neyrinck AM. Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: experimental data. J Nutr. 2007;137(Suppl):2547S–51S. doi: 10.1093/jn/137.11.2547S. [DOI] [PubMed] [Google Scholar]
- 74.Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr. 2007;98:32–7. doi: 10.1017/S0007114507691648. [DOI] [PubMed] [Google Scholar]
- 75.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. doi: 10.1016/S0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 76.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 77.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–9. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 78.Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–4. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 79.Piche T, des Varannes SB, Sacher-Huvelin S, Holst JJ, Cuber JC, Galmiche JP. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology. 2003;124:894–902. doi: 10.1053/gast.2003.50159. [DOI] [PubMed] [Google Scholar]
- 80.Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr. 2006;60:567–72. doi: 10.1038/sj.ejcn.1602350. [DOI] [PubMed] [Google Scholar]
- 81.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236–43. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- 82.Verhoef SP, Meyer D, Westerterp KR. Effects of oligofructose on appetite profile, glucagon-like peptide 1 and peptide YY3-36 concentrations and energy intake. Br J Nutr. 2011;106:1757–62. doi: 10.1017/S0007114511002194. [DOI] [PubMed] [Google Scholar]
- 83.Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89:1751–9. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nolan JP, Leibowitz AI. Endotoxins in liver disease. Gastroenterology. 1978;75:765–6. [PubMed] [Google Scholar]
- 85.Nolan JP. The role of endotoxin in liver injury. Gastroenterology. 1975;69:1346–56. [PubMed] [Google Scholar]
- 86.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 87.Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026–33. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–11. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–87. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 90.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 91.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–35. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 92.Miura K, Seki E, Ohnishi H, Brenner DA. Role of toll-like receptors and their downstream molecules in the development of nonalcoholic Fatty liver disease. Gastroenterol Res Pract. 2010;2010:362847. doi: 10.1155/2010/362847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Königsrainer A, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138:1452–5. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 94.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 95.Neyrinck AM, Cani PD, Dewulf EM, De Backer F, Bindels LB, Delzenne NM. Critical role of Kupffer cells in the management of diet-induced diabetes and obesity. Biochem Biophys Res Commun. 2009;385:351–6. doi: 10.1016/j.bbrc.2009.05.070. [DOI] [PubMed] [Google Scholar]
- 96.Lanthier N, Molendi-Coste O, Cani PD, van Rooijen N, Horsmans Y, Leclercq IA. Kupffer cell depletion prevents but has no therapeutic effect on metabolic and inflammatory changes induced by a high-fat diet. FASEB J. 2011;25:4301–11. doi: 10.1096/fj.11-189472. [DOI] [PubMed] [Google Scholar]
- 97.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–57. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983–92. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 99.Wagnerberger S, Spruss A, Kanuri G, Volynets V, Stahl C, Bischoff SC, et al. Toll-like receptors 1-9 are elevated in livers with fructose-induced hepatic steatosis. Br J Nutr. 2011:1–12. doi: 10.1017/S0007114511004983. [DOI] [PubMed] [Google Scholar]
- 100.Nolan JP. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology. 2010;52:1829–35. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- 101.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–50. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 102.Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–3. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 103.Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–97. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lirussi F, Mastropasqua E, Orando S, Orlando R. Probiotics for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. 2007:CD005165. doi: 10.1002/14651858.CD005165.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bray GA. Lifestyle and pharmacological approaches to weight loss: efficacy and safety. J Clin Endocrinol Metab. 2008;93(Suppl 1):S81–8. doi: 10.1210/jc.2008-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19:1605–11. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 107.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sjöström CD. Surgery as an intervention for obesity. Results from the Swedish obese subjects study. Growth Horm IGF Res. 2003;13(Suppl A):S22–6. doi: 10.1016/S1096-6374(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 109.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 110.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 111.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 112.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–42. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1273–82. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bueter M, Löwenstein C, Olbers T, Wang M, Cluny NL, Bloom SR, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845–53. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 116.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–97. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252:50–6. doi: 10.1097/SLA.0b013e3181d3d21f. [DOI] [PubMed] [Google Scholar]
- 118.Cani PD, Delzenne NM. Benefits of bariatric surgery: an issue of microbial-host metabolism interactions? Gut. 2011;60:1166–7. doi: 10.1136/gut.2011.242503. [DOI] [PubMed] [Google Scholar]
- 119.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 122.Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 123.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894–9. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 124.Woodard GA, Encarnacion B, Downey JR, Peraza J, Chong K, Hernandez-Boussard T, et al. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: a prospective randomized trial. J Gastrointest Surg. 2009;13:1198–204. doi: 10.1007/s11605-009-0891-x. [DOI] [PubMed] [Google Scholar]
- 125.Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60:1214–23. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zoetendal EG, Vaughan EE, de Vos WM. A microbial world within us. Mol Microbiol. 2006;59:1639–50. doi: 10.1111/j.1365-2958.2006.05056.x. [DOI] [PubMed] [Google Scholar]