Abstract
There is growing interest in using Drosophila melanogaster to elucidate mechanisms that underlie the complex relationships between a host and its microbiota. In addition to the many genetic resources and tools Drosophila provides, its associated microbiota is relatively simple (1–30 taxa), in contrast to the complex diversity associated with vertebrates (> 500 taxa). These attributes highlight the potential of this system to dissect the complex cellular and molecular interactions that occur between a host and its microbiota. In this review, we summarize what is known regarding the composition of gut-associated microbes of Drosophila and their impact on host physiology. We also discuss these interactions in the context of their natural history and ecology and describe some recent insights into mechanisms by which Drosophila and its gut microbiota interact.
“Workers with Drosophila have been considered fortunate in that they deal with the first multicellular invertebrate to be cultured monoxenically (Delcourt and Guyenot, 1910); the first to be handled axenically on a semisynthetic diet (Guyenot, 1917); and the first to be grown on a defined diet (Schultz et al., 1946). This list of advantages is somewhat embarrassing, since it implies an interest in nutrition that, in reality, was only secondary. The very first studies were concerned with the reduction of variability in genetic experiments (Delcourt and Guyenot, 1910) and standardization of the nutritional environment.”
-James Sang, 1959 Ann NY Acad 1
Keywords: symbiosis, host-microbe interactions, immunity, gut, microbiota, yeast, bacteria
Introduction
A common condition of the metazoan gut is to be in association with a number of benign or beneficial microorganisms. Recent studies have shown that the influence of these resident microorganisms is profound, altering many aspects of host physiology, especially digestive and immune functions.1-3 Studies with gnotobiotic animals, coupled with genomic tools aimed to capture the full extent of microbial diversity and function within the gut, have greatly altered our vision of host-microbe interactions.4 To the extent that these interactions are now accepted as essential elements of host health and the conditioning of host immune defenses. Over the last two decades Drosophila melanogaster, the common fruit fly, has been largely used to decipher mechanisms of host-microbe interactions in the context of innate immunity and pathogenic associations.5 More recently, studies have suggested the utility of this model to elucidate mechanisms underlying more benign or beneficial host-gut microbiota interactions due to its amenability to genetic study, lower microbiota complexity, and the ease in raising axenic flies.
Ironically, studies of microbes associated with Drosophila are almost as old as the genetic model itself (TH Morgan 1909). Thus, the influence of microbes associated with Drosophila on the host was to some extent appreciated even before they were subject of direct study. As Sang’s quote above indicates, these initial studies were concerned with reducing variability in experiments6 by standardizing the impact of nutrition, as it was essential to distinguish environmental from genetic influences on phenotypic traits. This led to the common use of axenically raised flies and the development of a chemically defined fly medium,7-13 practices that were largely abandoned as the model’s use grew and as interests and efforts of the community shifted to studies in development. However, these early studies provided a wealth of detail on both biotic and abiotic factors that could influence Drosophila development and physiology. Studies into the 1960s by Sang and others established the precise chemical composition of fly medium required for normal growth of axenic flies.7,14,15 In the 1940s, studies led by Tatum and Beadle in both Neurospora crassa and Drosophila established the “one gene, one enzyme” hypothesis and as such, studies of mutations affecting Drosophila metabolism were at their apogee. Thus, in 1939, Tatum could report that the eye color of axenic vermillion (v) brown (bw) double mutant flies was altered and that re-inoculation of an unidentified Bacillus species to axenic v, bw cultures reverted the pigmentation phenotype.16 These results indicated the existence of rate limiting metabolic reactions in larvae grown in axenic conditions, pointing to a role of microbiota in optimizing host metabolism. Thus, the impacts of indigenous microbiota on host nutritional requirements and on the phenotypic expression of mutations were clearly appreciated. However, it was not until the late 1960s and the thesis work of Marion Bakula,17 University of New York, that we begin to understand the nature of bacteria associated with Drosophila. This pioneering work analyzed the composition, persistence, and transmission of gut-associated bacteria of laboratory wild-type flies. One of her key findings was the observation that microbes were transmitted to offspring by contamination of the eggshells, which are ingested by young instar larvae.18 Her experiments also supported the view that the persistence of bacteria during the Drosophila life cycle is non-fortuitous. This stand-alone study still provides many interesting observations for today’s scientists and lays a foundation for contemporary studies, which by broadening these concepts and integrating current technologies can begin to decipher the mechanistic basis of these interactions.
In this review, we present the ‘state of the art’ on the study of gut-associated microbial symbionts of Drosophila melanogaster, with an emphasis on bacteria. We summarize what is known about the diversity of microbial communities associated with laboratory and wild populations of D. melanogaster and discuss genetic and environmental factors that influence these interactions, including a consideration of their ecological context. We highlight recent studies demonstrating the varied impacts of these communities on host physiology and identification of some of their underlying genetic mechanisms. In addition, the advantages and limitations of the Drosophila model for studying host/microbiota interactions are discussed.
Composition of Drosophila Gut-associated Bacteria Populations
Laboratory-reared flies
Several independent studies analyzing Drosophila-associated microbiota have provided a good deal of insight into the diversity of bacteria in laboratory stocks of Drosophila melanogaster. These studies, summarized in Table 1, have analyzed samples from either whole flies19-22 or dissected guts of surface-sterilized flies.22-25 Bacteria were identified by either characterization of cultivable species or through culture-independent assessment of microbial diversity by direct PCR amplification and sequencing of 16S rRNA genes from extracted DNA. More recently, a study using high throughput sequencing of a large number of 16S rRNA amplicons allowed identification of almost all detectable bacterial species at unprecedented depth and across the different life stages.24 These studies indicate that laboratory stocks are associated with a relatively low number of taxa, corresponding to 1–13 OTUs (defined as 97–99% identity, depending on the study) and are most frequently associated with bacteria from two genera, Acetobacter and Lactobacillus. Some species are clearly laboratory specific, such as Enterococcus faecalis,19,23 Gluconobacter morbifer,25,26 and Enterobacteriaceae Group Orbus,22 while Lactobacillus plantarum and Acetobacter pomorum/pasteurianus have been found in most laboratory stocks.
Table 1. Summary of culture-based (A) and molecular-based (B) studies investigating the diversity of microbes associated with Drosophila melanogaster.
| (A) Culture-based analysis | ||||
|---|---|---|---|---|
|
Study |
Sample |
Sample source |
Culture conditions |
Isolate/clone analysis |
| Delcourt and Guyenot 19106 |
flies on medium |
nd |
potato medium |
Saccharoymyces mali, Bacillus acetia |
| Miller and Phaff 1962120 |
fig colonised by flies |
wild-caught |
malt agar |
Acetobacter melanogenus (Gluconobacter oxydans), various yeasts |
| Bakula 196717 | eggs, whole adults | OregonR | nutrient agar | Bacillus sp, Brevibacterium sp, Corynebacterium sp, Kurthia sp b |
| (B) 16S rRNA-based analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Study |
Sample |
Sample source |
#clones; OTU cut-off used |
Isolate/clone analysis |
||||
| Brummel et al. 200491 |
whole adults |
CantonS (n = 100) |
10 clones |
Lactobacillus, Gluconobacter, Enterobacter, Anaerococcusc |
||||
| Cox and Gilmore 200719 |
whole adults |
OregonR Bloomington OregonR-OK U MA-wild-caught (10 males/site) |
Or-Bloom- 238 Or-OK U- 237 MA-wild- 211 OTUs 97% |
49 OTUs across samples (9–25 per population) |
||||
|
Most common phyla α-Proteobacteria (236/686), 8 OTUs γ-Proteobacteria (183/686), 22 OTUs Firmicutes (256/686), 14 OTUs Actinobacteria (7/686), 3 OTUs Bacteroidetes (2/686), 2 OTUs |
Most common OTUs Wolbachia sp. (62/686) Acetobacter aceti (73/686)@ A. cerevisiae (72/686) A. pasteurianus (39/686)@ A. pomorum (13/686) Gluconobacter cerinus (33/686) Enterobacter cloacae (41/686) Klebsiella oxytoca (11/686) Lactobacillus plantarum (12/686) Leuconostoc mersenteroides (11/686) Enterococcus faecalis (186/686)@ |
|||||||
| Corby-Harris et al. 200728 |
whole adults |
11 wild-caught populations 5 males/site |
728 clones (31-86 per site) OTUs 97% |
74 OTUs across all sites (7–30 per site)d,e |
||||
|
Most common phyla α-Proteobacteria (125/728), 15 OTUs γ-Proteobacteria (59/728), 21 OTUs β-Proteobacteria, 5 OTUs ε-Proteobacteria, 1 OTUs Firmicutes, 17 OTUs Bacteroidetes, 5 OTUs unclassified (39/728) |
Most common Genera Acetobacter, Gluconobacter Wolbachia sp. (453/728)—10 OTUs Enterobacteriaceae, Pseudomonas Acidovorax Lactobacillus |
|||||||
| Ren et al. 200720 |
whole adults |
OregonR males (n = 10) |
Fly surface—97 clones Fly interior—100 clones OTUs 97% |
Fly surfacee Acetobacter tropicalis # Acetobacter pasteurianus# Acetobacter aceti (45/97)# Lactobacillus homohiochii (50/97) Lactobacillus plantarum # Lactobacillus fructivorous (1/97) unidentified (1/97) |
Fly interiore A. tropicalis (80/100) A. pasteurianus (1/100) # A. aceti (1/100) # L. brevis (15/100) L. plantarum (3/100) # Lactobacillus sp. MR-2 # Claudosporium sphaerosperum # |
|||
| Ryu et al. 200825 |
adult midgut |
c729-Gal4/+; 18 d-old adult flies (n = 100) (sex not indicated) |
CR wt- 251 clones* Cad-RNAi- 338 clones* OTUs 98% |
Wild-type Lactobacillus plantarum Lactobacillus brevis Acetobacter pomorum Gluconoacetobacter intermedius Gluconoacetobacter rhaeticus Acetobacter malorum Commensalibacter intestinalis |
Cad-RNAi L. plantarum L. brevis A. pomorum G. intermedius G. rhaeticus G. morbifer G. europaeus |
|||
| Sharon et al. 201023 |
whole flies |
OregonR (n = 5 males, 5 females) |
CMY- 64 clones Starch- 23 clones OTUs 99% |
CMY medium Acetobacter pomorum (13/64), 4 OTUs Bacillus firmus (8/64) Enterococcus faecalis (1/64) Lactobacillus plantarum (2/64) Low G+C Gram-positive bacterium T135 (4/64) Weisella paramesenteroides (4/64) - 2 OTUs |
Starch medium L. plantarum (6/23) |
|||
| Storelli et al. 201123 |
whole flies, adult midgut |
yw 20 d-old females (n = 20) |
whole flies—91 clones* adult midgut—93 clones* OTUs 98% |
whole flies Enterococcus faecalis Lactobacillus plantarum Aerococcus spp |
adult midgut E. faecalis L. plantarum Corynebacterium variabile |
|||
| Wong et al. 201124 |
eggs, whole larvae, larval gut pupae, adult gut |
Cantons S L1/L2 whole (n = 50) L3 guts (n = 50) Pupae (n = 30) Guts Y F (n = 50) Pupae (n = 30) Guts Y F (n = 50) Guts Y M (n = 50) Guts O F (n = 50) Guts O M (n = 50) |
HTS 16S rRNA V2 808,483 reads OTUs 97% |
122 OTUs across all stages (15–71 per stage) 5 OTUs represent 80=100% of OTUsf Lactobacillus brevis L. plantarum L. fructivorans Acetobacter pomorum A. tropicalis |
||||
| Chandler et al. 201122 | whole flies, adult gut, larval gut, pupae |
19 libraries (n) wild-caught flies (3)g CantonS (1) OregonR (2) Wild-derived (WO)/lab reared (11) (1 media, 1 external wash) (n = 7–20 flies) |
26–233 clones/library OTUs 97% |
21 OTUs total (1–16 per library) most common OTUs Lactobacillus 14/19 libraries (3 OTUs) Enterobacteriaceae Group Orbus 13/19 (5 OTUs) Commensalibacter 7/19 (1 OTU) Acetobacter 6/19 (2 OTUs) Serratia 6/19 (2 OTUs) 11 OTUs found in only one library 3 OTUs single clone in one library |
||||
Notes: aMost likely an Acetobacter sp, it was common practice to name any bacillus shaped bacteria “Bacillus sp.” bIsolates were classified based on morphological and physiological characteristics. Many of the species as listed are not recognized by current nomenclature standards. cNeither species nor number of clones per OTU were reported. dNo OTUs were common to all libraries (sites). eNumbers indicate (#clones of given OTU/total clones). fTwo additional laboratory wild-type stocks (OregonR and Ithaca) tested postive for top 5 OTUs by end point PCR. gWild-caught libraries: Flies from grapes: gut (4 OTUs), whole body (5 OTUs); Flies from oranges: gut (16 OTUs). @OTUs in common across the three populations. #identified by culture-dependent methods. *number of clones per OTU not reported. Remarks: The identification of bacterial species based on 16S rRNA sequence identity (OTUs 97%) should be interpreted with some caution since bacteria with identical or near-identical 16S rRNA genes could have distinct genomic or transcriptomic properties that could have important phenotypic consequences. Along this line, two novel cultivable Acetobacteraceae strains, Commensalibacter intestini and Gluconobacter morbifer, initially identified by the divergence of their 16S rRNA gene sequences (93.5% and 97.9% to type strains, respectively 25) were recently classified on the basis of phenotypic, genetic, and chemotaxonomic analyses.26 Interestingly, multiple studies have identified many novel OTUs (93–96% identity) most closely related to other commonly associated Lactobacillus and Acetobacter spp.21,22,24
Wild-caught flies
To date three studies have examined bacteria associated with Drosophila melanogaster collected in nature, all from sites within the United States19,22,27(Table 1). Two studies examined whole flies captured with banana baits19 or collected from a variety of natural food sources and environments.27 More recently, Chandler et al.22 analyzed the bacterial diversity of guts dissected from D. melanogaster adults collected at two geographical sites. Whereas the family Acetobacteraceae and the order Lactobacillales dominate the microbiota of laboratory-reared flies, a greater diversity of bacterial orders are associated with wild-caught flies, most notably among the Proteobacteria. It is not evident if this diversity merely reflects a higher number of transient bacteria in the gut due to the increased exposure to environmental bacteria, or whether they are forming stable associations with Drosophila in nature. Interestingly, a number of genera identified in these studies (e.g., Providencia, Serratia, Erwinia, Pantoea, and Pseudomonas) have been previously identified as pathogens of flies infected both in nature and the laboratory28-35. Yet, despite the increased diversity of bacteria associated with flies in nature, these studies confirm that Acetobacter/Gluconobacter and Lactobacillus are commonly associated members of the Drosophila melanogaster microbiota. Moreover, these studies indicate that, even when transiently ingested environmental bacteria are taken in account, natural Drosophila populations have a very restricted gut bacterial microbiome with low diversity.
General characteristics of the Drosophila microbiome
Altogether, these studies demonstrate that Drosophila, as has been reported for most insects, is associated with a much lower diversity of bacterial taxa than observed in mammals (tens to hundreds vs. thousands).4,36-38 Overall, this reduced diversity suggests that the niches provided by the Drosophila and mammalian gut are not equivalent. The reasons for this difference are not known, but a number of host factors have been suggested. It has been proposed that the adaptive immune system of higher metazoans has facilitated association with a greater diversity of microbes.39 Alternatively, the more frequent perturbation of the insect gut niche has been suggested as a limit to higher diversity.40 Insect guts tend to be transient, given the short life span of many insects, and encounter frequent episodes of disturbance. Regions of the gut (foregut and hindgut) are shed during molting and in holometabolous insects, including flies, the entire larval gut is replaced by a new adult gut during metamorphosis. Thus, the perturbed and transient nature of the guts of holometabolous insects such as Drosophila may be incompatible with the development of a highly diverse microbiome.
Inheritance and Persistence of Gut-associated Microbes along the Drosophila Life Cycle
Unlike intracellular symbionts, such as Wolbachia and Spiroplasma, which are transmitted within the embryo, gut-associated bacteria are acquired from the environment after birth.41-43 A major question in understanding the association of Drosophila with bacteria is to determine whether the association is fortuitous or controlled to some extent. Bakula addressed this question by monitoring the diversity and density of bacteria in flies across the life cycle.17,18 She concluded that Drosophila embryos are sterile, but that the eggshell is contaminated with bacteria most likely derived from feces of adults. In addition to the fact that axenic fly stocks can be obtained through removal of the embryonic chorion with bleach, she also demonstrated that bacteria could not be cultured from eggs aseptically removed from females. Furthermore, using methylene blue to externally stain embryos she elegantly demonstrated that, within an hour of hatching, blue dye was detected in the intestinal tract of first instar larvae.18 This suggested that larvae ingest the chorion of embryos, and thus acquire the bacteria coating them.
More recent studies using molecular techniques in addition to culturing have expanded Bakula’s findings that both the microbial composition and density change with developmental stages and as flies age.20,23,24,44 Eggshells carry a low number of bacteria of high diversity.24 Following ingestion from the egg or environment, bacterial density in the gut increases throughout the larval stage reaching a plateau in third-instar wandering larvae.18,23 The transition of insects, including Drosophila, into metamorphosis is associated with a sharp decrease in bacterial density 24 h after pupation, which increases again by 48 h. This reduction in bacterial numbers correlates with the increased expression of several antimicrobial peptide genes at the pupal stage45-48 and may be a host mechanism to limit the growth of bacteria before the adult tissues are formed.49 The larval midgut is one of the only larval structures that is not totally histolyzed during pupation, but is contained within a transient pupal epithelium around which the adult midgut develops.50 The larval midgut is the source of progenitor cells from which the adult gut is constructed51-53 and the larval/pupal gut, referred to as the yellow body,54 constitutes the first adult feces excreted as the meconium soon after emergence from the puparium. For these reasons, the meconium could serve as a reservoir for the microbiota and provide a mechanism of transmission to the adult, as has been shown for some Dipterans.55-57 In examining this possibility, Bakula was unable to detect culturable bacteria from homogenized whole flies aseptically removed from the pupal case prior to pupation, However, bacteria could be cultured from the inside of the pupal case, as had been previously reported in muscoid flies58,59. However, as these studies were limited to culture-based methods the transmission of microbiota by the meconium cannot be completely ruled out. What is certain is that bacterial counts in young adults are initially quite low, ranging from 40 to 1000 cells per gut.18,23,44 Interestingly, there is a marked increase in both external and internal bacterial density in old flies with counts increasing 10–1000 fold.20,44 In their study, Wong et al. also observed a shift in bacterial composition from dominance of 16S rRNA gene sequence of L. fructivorans in young flies to A. pomorum in old flies.24 Such shifts in aging have not been reported in other studies, but as Acetobacter, unlike Lactobacillus, grows rapidly under fully aerobic conditions, the authors hypothesize that the gut may become more oxic in old insects.
Host and Nutritional Factors Shaping the Community
Studies have shown that, though there are common players, gut-associated bacteria of Drosophila stocks can differ greatly between laboratories (Table 1) and even between stocks within the same laboratory.22 These differences between stocks are maintained even on an identical laboratory defined food source since fly stocks are essentially kept isolated as separate entities (Fig. 1B). Analyzing how different environmental and host factors shape the Drosophila microbiome is an important question in the field. Recently, a few studies have begun to provide some insight.
Figure 1. The natural and laboratory ecosystem of Drosophila. Drosophila melanogaster lives where it eats both in the wild (A, a fermenting kiwi fruit) and the lab (B, fly vial containing a cooked medium composed of dead yeast, cornmeal, sugar, and agar). Courting of adult female by males, fertilization of eggs, and oviposition all take place on the food source. The embryos hatch and larvae move and feed within their food source. At late stages larvae crawl out of the fruit/medium to pupate and after emerging as adults, begin the cycle anew.
Influence of diet
Diet has been shown to influence the bacterial composition of mammals and several insects and is proposed to be an important factor in shaping community composition. Similarly, Chandler et al.22 and Sharon et al.21 have demonstrated that host diet plays a substantial role in shaping bacterial microbiome composition in Drosophila as well. First, when comparing diverse, wild populations of Drosophila spp, Chandler et al. found that microbiota composition of species feeding on the same type of substrate were more similar to each other than to more closely related species that were feeding on different substrates. In addition, they found that switching a large pool of isogenic D. melanogaster onto different sterile diets led to changes in gut microbiota composition over time. Finally, raising three distantly related Drosophilids, which normally feed on different substrates in the wild, D. melanogaster (fruits), D. elegans (flowers), and D. virilis (sap fluxes and cambium), together on the same medium led to a convergence in microbiota composition across the three species. In another study, Sharon et al.21 found that shifting flies from a corn-molasses medium to a starch medium greatly reduced microbiota diversity. After 11 generations, starch-bred flies were mono-associated with L. plantarum, which represented a minor component (2.6%) of clones among the 10 taxa identified from molasses-bred flies.
Influence of host factors
To date, two studies have analyzed the impact of host factors on gut microbiome composition. Ryu et al.25 showed that flies with higher levels of antimicrobial peptides in the posterior midgut, due to the silencing of the homeobox gene Caudal, exhibit a shift in the community with an increase in the density of a minor member, Gluconobacter morbifer, and concomitant decrease in a dominant member, Commensalibacter intestini. This shift was explained by the increased resistance of G. morbifer to antimicrobial peptides and was also associated with increased gut defects due to the pathogenicity of G. morbifer. These results indicate that a specific genetic deficiency within the host can profoundly influence the gut microbial community and host physiology. Beyond the conceptual finding, the overall generalization of this finding remains to be determined since G. morbifer has not been identified in other laboratory stocks. Buchon et al.44 reported that Relish and PGRP-LC mutant flies with impaired Imd pathway activity have higher bacterial counts in their gut, suggesting that the basal level of antimicrobial peptides may regulate bacterial density. Nevertheless, this study, did not analyze the effect of Imd on flies recolonized with an identical microbiome and only examined two Imd-deficient backgrounds.
Taken together, these studies indicate that diet has a major effect on Drosophila gut microbiota and suggest a role of host immunity in controlling density and composition.
Environmental Factors Shaping the Microbiome: The Fly Substratum Niche
In the wild, Drosophila melanogaster is saprophytic and feeds on microbes growing on decaying substrate, most commonly rotting fruits. The substrate on which Drosophila develops and lives is an ephemeral ecosystem that can support a limited number of individuals before the population, or pioneering individuals, must leave and colonize a new substrate. Within the substrate, larvae are gregarious feeders and both larvae and adults often co-exist spatially and temporally. In this way, fermenting fruit (mixture of microbes and plant) provide both “gîte et couvert” (room and board; Figure 1A) to the fruit fly. Thus, the situation in Drosophila is rather distinct from mammals for which the gut microbiota largely differs from microbes associated with food. In this manner, separating the impacts of microbes as food or component of the substrate from their effect in the gut will be important.
A key question is whether there is a direct relationship between bacteria in the substrate and those in the gut? While the studies described above have provided a catalog of microbes that may associate with Drosophila, they cannot differentiate between transient or resident microbes, and in the case of culture-independent studies even between living or dead bacteria in the sample. Currently, our understanding of whether the gut microbiome differs from the external or substrate bacterial community is unclear, as two laboratory studies examining this question have conflicting results. While, Ren et al.20 observed a high degree of overlap in the external and internal bacterial communities of adult flies when they combined detection by culturing and PCR (Table 1), Chandler et al., found a significant difference in bacterial composition of external and internal samples from flies grown on non-sterile media, and between the guts of larvae vs. the medium.22 Studies have not examined the diversity of natural fly substrates, but it is assumed this environment would provide a greater diversity of microbes than flies encounter in the laboratory. At the same time, consideration on the fly-microbe-substratum niche is also important for the study of microbiota in laboratory stocks. In this case, adult flies are transferred into fresh and relatively sterile medium and serve as the inoculum source of bacteria for the next generation of larvae that will develop in the same vial (Fig. 1B).60 As a consequence, one could expect that fly medium generally contains the same bacteria that are associated with flies in the vial. In addition, preliminary studies indicate that the density of bacteria in the gut may reflect levels in the media and that bacterial loads can be reduced if flies are transferred at a high frequency onto fresh medium. For example, flies carrying multiple mutations in negative regulators of the Imd pathway survive longer if frequently transferred on sterile medium, rather than if left longer in vials, which is consistent with a lower load of gut bacteria.61 Of note, it has been reported that environmental contaminants can be eliminated from fly stocks by frequent transfer on sterile medium14 and this is a common practice in the laboratory when handling ‘sick’ stocks that have high levels of microbial growth in the medium.
Another important question is whether there is an active selection and retention of a specific consortium (‘core community’) or if the Drosophila microbiome assembles randomly based on available partners? One constraint is likely provided by the diet of Drosophila and the microbes that are able to colonize this environment. In addition, properties of the Drosophila intestinal environment, such as the immune and physiological state of the gut (Fig. 2), likely determine which of these bacteria persist. It is clear that the routine way of cultivating flies in the laboratory could induce a strong bias on both host choice and microbiome assembly. Of note, it is common practice to include broad-spectrum antimicrobials such as propionic acid and methyl paraben to fly medium to reduce spoilage. This selection has likely not only reduced the diversity of bacteria that can associate with Drosophila in laboratory conditions, but may also permit associations that would not normally occur in nature. For instance E. coli has been shown able to colonize axenic larvae and persist through pupation, but was completely displaced from the gut when these mono-associated flies were exposed to a bacterium normally associated with the gut.18
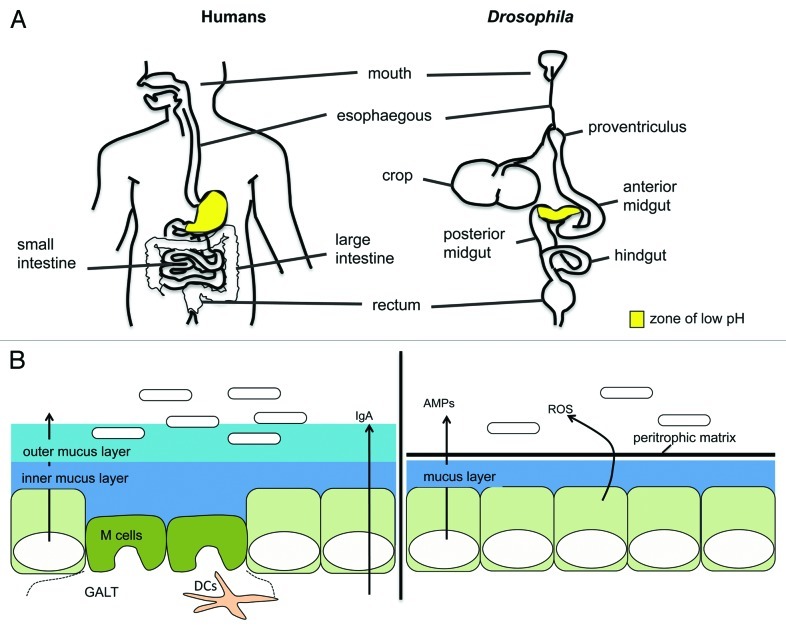
Figure 2. Comparison of the digestive tracts and intestinal epithelial-cell barriers of humans and Drosophila. (A) Diagrammatic representation of the human and adult Drosophila digestive tracts. The digestive tracts of mammals and Drosophila are similar in physiology and function. Both are divided into foregut, midgut, and hindgut segments, based on their embryonic origin, which give rise to specific gut structures and compartmentalized functions. (B) Mammals and Drosophila rely on several mechanisms to limit the contact between microbes in the lumen and intestinal epithelial cells. Mechanisms in common include an acidic zone (the stomach and copper cell region in mammals and Drosophila, respectively (A)), the secretion of mucins to form a protective mucus layer, and the secretion of antimicrobial peptides. In Drosophila, the peritrophic matrix, a layer of chitin and glycoproteins that lines the midgut epithelium, provides a physical barrier against ingested material, such as food particles, microbes, and pore-forming toxins. Reactive oxygen species (ROS) are also an important component of the Drosophila response to microbes, in controlling both levels of dietary microbes and pathogens. In mammals, specialized M cells overlie Peyer’s patches and lymphoid follicles (gut associated lymphoid tissue (GALT)) to facilitate the sampling of the lumen. IgA, produced by plasma cells and transcytosed across epithelial cells, is secreted into the lumen to limit microbes in the mucosa.
However, given the frequency of Drosophila’s association with specific bacteria, chance encounters with these bacteria in the environment cannot be the only driving force. This may be because certain combinations of yeast and bacteria occur frequently together in the wild (see Box 1 for discussion on yeasts), but host choice also likely plays a role. Importantly, adults can influence the quality of the larval food, not only through the initial choice of substrate for oviposition, but in the type and diversity of microbes they inoculate into the substrate. Since all gut bacteria must first be ingested, bacterial taxa that thrive on the feeding substrate will have the greatest chance of colonizing the guts of larvae and adults sharing the same substrate. While we know less about Drosophila behavior, it is known that C. elegans exhibits diverse behaviors in response to bacteria provided as a nutrient source, such as feeding, reproductive egg-laying, and changes in locomotion, including the ability to discriminate between nutritional, beneficial bacteria, and bacteria that represent poor nutritional sources or are pathogenic.62-66 Similarly, we can suppose that Drosophila in the wild have the choice for selecting rotting fruits that will be more beneficial for promoting growth, while avoiding pathogens, an over-populated substrate, or nutrient-deficient environment.
Finally, it has to be taken into account that, as in the wild, microbes in the fly medium strongly influence the nature of the substrate. They may alter its physico-chemical properties (texture/consistency, pH, oxygen levels, processing of nutrients) and supplement the medium with products of their metabolism. Therefore, results comparing axenic to conventionally raised flies does not only reflect the absence of gut microbiota, but also the absence of bacteria within the medium. The inability to distinguish between the effects of bacteria in the gut vs. bacteria in the medium is a limitation of gnotobiotic studies in Drosophila and important to consider in the design of experiments and interpretation of results (see below).
Impact of Drosophila Microbiota on the Host
The notion that intestinal bacteria communities directly affect host physiology and immunity is now largely acknowledged. Several recent studies have begun to investigate the role of Drosophila microbiota on the host through the use of axenic or gnotobiotic culture conditions. These studies have identified several important functions attributable to the microbiota associated with Drosophila and serve as launching points for future work.
Impact on Immune system
In contrast to the systemic immune response, which functions to ensure the sterility of the body cavity and hemolymph (insect blood), epithelial immune responses must tolerate the presence of benign/beneficial microbes while responding to and eliminating potential pathogens (Fig. 2). This implies a tight and specific regulation of the immune response in epithelia, balancing between immune activation and tolerance.5,67,68 In Drosophila, three lines of defense have been identified to limit microbial infection and pathogenesis in the gut: i) the production of microbicidal reactive oxygen species (ROS) by the NADPH-oxidase Duox,69 ii) the production of antimicrobial peptides by the Imd pathway,48,70 and iii) the protection against bacteria and pore-forming toxins by the chitinous peritrophic matrix.71
Under normal conditions, bacteria residing within the gut appear localized to the lumen within the endoperitrophic space, which is delimited by the peritrophic matrix, a chitinous barrier that lines the midgut72 (Fig. 2). This would indicate that gut microbiota are rarely in direct contact with intestinal epithelial cells. Several studies have shown that microbiota stimulate the Imd pathway at basal levels.20,25,44,61,73 Accordingly, axenic flies exhibit lower levels of expression of the antimicrobial peptide gene Diptericin in the gut. Moreover, the level of Diptericin, and other antimicrobial peptide genes increases as conventionally-reared flies age, consistent with an increase in bacterial load.20,44
The local antimicrobial response of the Drosophila gut is mediated by the Imd pathway upon detection of DAP-type peptidoglycan,70 a form of peptidoglycan found in Gram-negative bacteria and some Gram-positive bacilli (e.g., Bacillus, Lactobacillus, Listeria), through recognition by peptidoglycan recognition proteins (PGRPs). Recent studies in Drosophila have revealed that three levels of regulation are employed to reduce epithelial Imd pathway activity and thus to prevent excessive or prolonged immune activation by the microbiota.
Altering the immune reactivity of the host
Pirk (Poor Imd response upon knock-in), a protein that antagonizes PGRP-LC and PGRP-LE signaling capacity, has been shown to downregulate the Imd pathway receptor73-75 and participate in the precise control of Imd pathway induction in the gut.73 In particular, Lhocine et al.73 demonstrated that Pirk expression is Imd-dependent and basally induced by microbiota. Loss of Pirk activity results in constitutive activation of antimicrobial peptides in the gut in response to microbiota and a hyperactive immune response in response to pathogens. In this manner, Pirk is important in regulating the threshold of the immune response, and in conjunction with additional negative regulators (,61 see below) contributes to its precise control in the gut (Fig. 3). In addition, the ubiquitin-specific protease 36 (dUSP36) was shown to have a role in negative regulation of the Imd pathway by promoting degradation of the IMD protein.76 This deregulation was lost in axenic flies, indicating that dUSP36 prevents constitutive immune signal activation by microbiota. Finally, PGRP-LF, a membrane-bound PGRP with two PGRP domains was demonstrated to be a key negative regulator of Imd signaling by preventing PGRP-LC activation in the absence of infection.77 Developmental defects associated with constitutive activation of the Imd pathway, were reduced, but not completely abolished, when larvae with reduced PGRP-LF function were reared on antibiotics, suggesting that microbiota contribute to the regulation of the immune response by PGRP-LF.
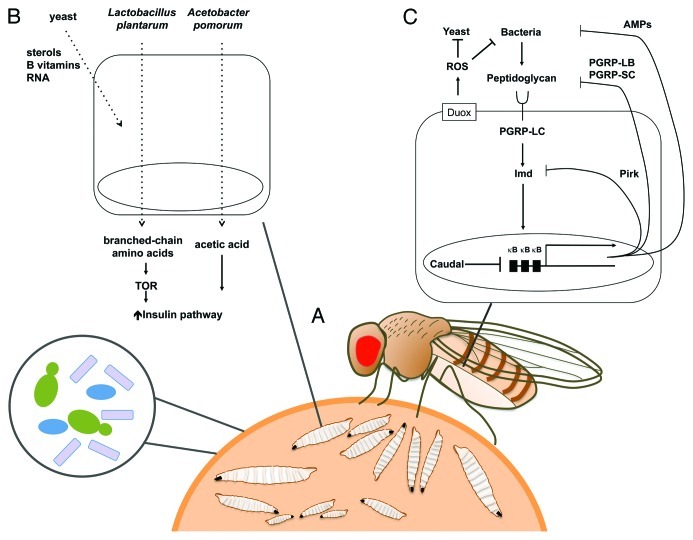
Figure 3. The fly-substratum niche and the impacts of gut associated microbes on Drosophila. Drosophila larvae and adults are saprophytic, feeding on microbes growing on decaying fruit (A) These microbes, or others acquired from the environment soon after birth, can also persist in the gut and form stable associations along the life cycle. These microbes have important impacts on host physiology, such as increasing larval growth rate by optimizing host metabolism and inducing the basal epithelial immune response. (B) Yeasts are essential to Drosophila development and nutrition by providing sterols, B vitamins, and RNA. Gut-associated bacteria increase larval growth. In nutrient-limited conditions this has been shown to be due to their impact on TOR-insulin signaling, either by production of acetic acid (Acetobacter pomorum) or increased amino acid metabolism (Lactobacillus plantarum). (C) The Imd pathway is activated upon the recognition of DAP-type peptidoglycan by PGRP-LC. The downstream nuclear translocation of the NF-κB factor Relish activates the transcription of genes encoding antibacterial peptides and negative regulators of the pathway, such as amidase PGRPs and Pirk. Amidase PGPRs reduce the immune response to microbiota by degrading peptidoglycan, while Pirk acts at the level of Imd pathway signaling. The transcription factor Caudal reduces antimicrobial peptide expression in the posterior midgut. This permits persistence of a beneficial microbiota in this segment. Dietary microbes induce the production of ROS by the NAPDH-oxidase Duox, which controls their density in the gut.
Altering the sensing of bacteria by peptidoglycan degrading PGRPs
Since peptidoglycan is mainly released by dividing bacteria, it is probable that only small amounts of peptidoglycan are released by the microbiota due to their reduced division rate. Nevertheless, even small amounts could trigger a persistent induction of the Imd pathway. Drosophila employs several mechanisms to limit peptidoglycan accumulation in the gut. In contrast to recognition PGRPs, proteins referred to as catalytic PGRPs have amidase activity that removes peptides from the glycan chains, thereby eliminating the immuno-stimulatory activity of peptidoglycans.78 Several of these enzymatic PGRPs (PGRP-LB and PGRP-SC1) are induced by both microbiota and ingested pathogens and participate in the downregulation of the Imd pathway in the gut by scavenging extracellular peptidoglycan, thereby preventing their binding to receptor PGRPs25,61,70,79 (Fig. 3). Differences in the expression pattern of amidase PGRPs in various gut regions, along with the superimposition of inducible and constitutive levels of expression, likely allow a precise control of the spatial and temporal activity of the Imd pathway in this tissue. In the absence of these negative regulators, conventionally, but not axenically-reared flies, express Diptericin at a higher level in absence of infection.61 Interestingly, flies lacking multiple negative regulators (e.g., PGRP-SC;LB double mutants or PGRP-SC:LB;pirk triple mutants) have reduced lifespans, which can be largely rescued by rearing flies in axenic conditions, suggesting that continuous stimulation of the immune system by indigenous microbiota is deleterious.61
Altering the immune response by compartmentalizing the host response
The gut is a compartmentalized organ with distinct immune-reactive domains.67,80,81 While the Imd pathway is activated all along the gut, antimicrobial peptide genes, such as Diptericin, are expressed with a complex and distinct pattern, indicating that additional levels of regulation restrict their expression to some regions. The Imd transactivator Relish is usually localized to the nucleus in the presence of microbiota in the posterior midgut,25 but antimicrobial peptides are not expressed in this region. This is because the homeobox gene caudal represses the expression of antimicrobial peptides in in this location.25 The suppression of antimicrobial peptides by caudal is proposed to be essential for the establishment of a beneficial microbiota in this segment of the gut (see above 25).
In conclusion, the level of basal immunity is largely dependent on microbiota density and multiple mechanisms are employed to reduce the level of immune activation by persistently associated microbes though both negative regulators that tightly tune Imd pathway activity and transcription factors that restrict antimicrobial peptide gene expression to specific regions of the gut.
Interference with pathogens
In mammals, the indigenous microbiota are also thought to reduce host susceptibility to microbial infection through either direct competition with pathogens or by indirectly stimulating host immune pathways. Direct antagonist interactions between gut microbiota and entomopathogens have been described in several insect species.82-86 However, to date little is known about how Drosophila microbiota impact host survival to pathogenic microbes. A recent study reported a protective role of Drosophila microbiota against larval ingestion of Candida albicans.87 Under conventional rearing, 90% of Drosophila larvae feeding on food contaminated with C. albicans survived to adulthood. However, survival of larvae dropped to 58% when the parental strain was grown in axenic conditions. Genetic experiments indicate that JNK-mediated cell death was the cause of death of wild-type axenic larvae infected with C. albicans. This protective effect was also observed in imd;Toll double mutant flies indicating that it was not mediated through classical immune pathways, but probably a result of direct interference. In another study, axenic PGRP-SC:LB;pirk flies lacking negative regulators of the Imd pathway (see above,61) tended to live longer than conventionally reared counterparts following ingestion of Erwinia carotovora carotovora 15. This protective effect is probably due to a reduction in the immune response that is deleterious in flies depleted for these negative regulators.
Impact on host growth and metabolism
In mammals, the link between gut microbiota and energy metabolism is well established and an area of intense research. Intestinal bacterial communities shape the nutrient environment of the host by contributing enzymatic activities that break down otherwise non-digestible carbohydrates. They also salvage energy through carbohydrate fermentation, leading to the production of short-chain fatty acids.88,89
The ability to raise fully healthy axenic flies indicates that under appropriate conditions, gut microbiota are not obligatory to any specific stage of the Drosophila life cycle. However, as stated above, the report that eye color of v, bw flies was affected by associated microbes already indicated the existence of rate limiting metabolic reactions in larvae that are grown axenically, pointing to a role of microbiota in the optimization of host metabolism.16 Furthermore, it was widely reported that the development of axenic flies was delayed, due to growth defects at the larval stage10,11,16-18,90 suggesting that Drosophila require symbiotic microbes to either make resources available or to provide supplemental nutrition. The latter seems most likely, given that providing dead yeast,12,91 or RNA and B vitamins14 in place of the yeast, is known to be sufficient to support the development of axenic flies.
However, even with an adequate diet (provided by sugar and dead yeast), bacterial symbionts are capable of further improving larval growth. Bakula reported that an isolated member of the microbiota could restore a normal development rate to axenic larvae, though conventionally-reared larvae (with complete microbiota, including live yeast) always exhibited the fastest development rate.18 Two recent studies have further analyzed the impact of bacteria on larval growth and have revealed some of the mechanisms important for their beneficial effect. First, Storelli et al.23 demonstrated that Drosophila microbiota promote larval growth in conditions of nutrient scarcity (reduction in yeast concentration). This effect is mediated by Lactobacillus plantarum, which is sufficient to recapitulate the natural microbiota growth-promoting effect. L. plantarum association correlates with increased systemic production of growth hormones (ecdysone and insulin) during larval growth. The observation that the host TOR kinase and the amino acid transporter Slimfast are essential for the effect of L. plantarum demonstrates that it acts upstream of the TOR-dependent host nutrient sensing system controlling hormonal growth signaling. This led the authors to suggest that L. plantarum exerts its beneficial effect through enhanced amino acid assimilation, as amino acids are known activators TOR kinase activity (Fig. 3). Using a similar approach, Shin et al.92 observed that axenic flies exhibit a small growth delay on normal media. Furthermore, while L. plantarum, C. intestini, or L. brevis could enhance larval growth, a single member of the community, Acetobacter pomorum, could fully restore optimal growth on poor protein diets (amount of dead yeast below 1% or yeast replaced entirely by casamino acids). This effect is mediated through the stimulation of the insulin pathway. A random mutagenesis screen of A. pomorum demonstrated that the insulin growth-promoting effect requires a functional PQQ-ADH–dependent oxidative respiratory chain. PQQ-ADH is the primary dehydrogenase in the ethanol oxidative respiratory chain of Acetobacter involved in acetic acid production. Along this line, growth defects in flies mono-associated with a PQQ-ADH-deficient A. pomorum were reversed by enhancing host insulin signaling or by supplementing the diet with acetic acid (Fig. 3). It is important to note that neither study excludes that the growth promoting effect could be mediated by the processing of food by bacteria in the medium rather than in the gut. The discrepancy between these studies concerning the microbe responsible for the growth promoting effect could reflect differences in fly medium composition or host genetic background. Future studies should address whether this effect is indeed mediated by microbiota residing in the gut, as well as identifying the mechanism of activation upstream of the insulin receptor.
Impact on epithelium renewal
To maintain homeostasis, the gut epithelium is constantly renewed throughout an organism’s life by the division and differentiation of intestinal stem cells. In Drosophila, gut stem cells are scattered along the basement membrane of the adult midgut.93,94 In normal conditions, the adult gut epithelium is renewed in approximately 1–2 weeks.93 Several groups have shown that increased epithelium renewal is observed upon some bacterial infections to repair associated damage.80,95-97 Interestingly, both the number of mitotic stem cells and the rate of epithelium renewal are reduced in axenic flies as compared with conventionally raised flies. This reduction in epithelium renewal is not due to an inability of axenic flies to activate renewal, as infection with pathogenic bacteria and reintroduction of culturable gut microbes increased mitotic activity in these flies.44 Moreover, in addition to the IMD pathway, the gut microbiota induce, to some extent, the basal levels of JAK/STAT and EGFR pathway activities, which in turn regulate stem cell activity. Almost no upd3 (the ligand activating the JAK/STAT pathway) expression was observed in the gut of axenic flies.44 Taken together, these data indicate that microbiota condition the basal level of epithelium renewal by stimulating stem cell division, probably through an increase in JAK/STAT, EGFR, and JNK activity. In this manner, Drosophila microbiota contribute to the development and architecture of the niche that it occupies. Interestingly, higher numbers of dividing stem cells were detected in the absence of infection in the guts of Relish flies, which are deficient for the Imd pathway, and associated with 10-fold higher bacterial counts than wild type, suggesting that an abnormally abundant microbiota can increase epithelium renewal. In an independent report, Shin et al. also reported a stimulation of stem activity by the microbiota that is insulin-dependent.92 Thus, stem cell activity could be linked to growth signaling by the insulin pathway, which then licenses them to divide. Future studies should decipher whether the microbiota effect on stem cells is local (restricted to the gut) or systemic.
Impact on longevity
Using axenic culture, Brummel et al.,98 reported that exposure to bacteria during the first week of adult life can increase lifespan. However, although Ren et al.20 observed that both the internal and external loads of bacteria associated with flies increased with age, there was no difference in longevity between axenic and conventionally raised flies. This suggests that the finding of Brummel cannot be generalized and may be specific to a fly strain or diet. Thus, additional studies are needed to determine if and how microbiota impacts longevity. Nonetheless, the observation that bacterial density in the gut increases with age has been confirmed by multiple studies.23,44,99 This suggests that any metabolic expenditure required by the fly to support the increased bacterial load and antimicrobial peptide expression that occurs with aging is endured without a significant cost to life span.20
Independent studies have also revealed that age-related deterioration of the intestinal epithelium is associated with excessive stem cell proliferation and aberrant differentiation.44,100-103 These age-dependent symptoms are exacerbated in Relish flies, whose guts are morphologically altered with regions devoid of enterocytes. Interestingly, guts from old axenic flies exhibit levels of epithelium renewal more similar to that of young axenic flies. Moreover, the guts of old axenic flies do not undergo alterations of intestinal integrity to the same extent as in conventionally raised flies. Since guts from both wild-type and Relish old flies contain higher counts of indigenous bacteria than their younger counterparts,20,44 age-related defects in the gut of older flies could be caused by the abundant microbiota.
Impact on host behavior
Assortative mating (nonrandom mating in which individuals mate preferentially according to phenotype) is considered to be an early event in speciation that is commonly attributed to genetic differences. In light of the hologenome theory of evolution, which proposes that the host and its associated microorganisms act as a unit of selection in evolutionary change,104 Sharon et al.21 have now provided evidence for a role of the microbiota of Drosophila in assortative mating. After rearing an isogenic population of flies on either a starch or molasses media, flies exhibited a significant mating preference for partners reared on the same medium. The switch in diet led to changes in gut microbiota composition and an antibiotic treatment abolished mating preference, suggesting that the fly microbiota was contributing to this effect. Furthermore, antibiotic-treated flies re-associated with a mix of Lactobacillus sp isolated from molasses-reared flies, or mono-associated with a Lactobacillus plantarum isolated from starch-reared flies exhibited mating preference.21 In comparing the cuticular hydrocarbons, differences were observed between the starch and molasses-reared flies, suggesting that microbiota were influencing mating preference by altering sex pheromones. This study points out how microbiota, through an impact on mating preference, could contribute to speciation and evolution in the wild. As fly populations living on different diets will be, at least to some extent, geographically separated, diet-induced mating preference would further reduce interbreeding of the populations, thus favoring speciation.
Perspectives
Although the major focus of research on Drosophila-microbe interactions has been in the context of the innate immune response to pathogens, there is now a growing emphasis to study beneficial interactions. Recent studies have provided a better understanding of the nature of the microbes residing within the Drosophila gut as well as identifying their influences on several host traits. The relative simplicity of the Drosophila bacterial microbiota, with many species being easily cultured, makes Drosophila a useful model to dissect host-microbiota interactions. The possibility to screen extensively for bacterial mutations that affect complex traits of microbiota such as the capacity to establish persistent associations over time, to interact with other associated microbes, or to impact on the host, is appealing. From this point of view, the genetic screen performed in Acetobacter pomorum that identified PQQ-ADH metabolism of acetic acid as a factor promoting larval growth92 is exemplary of the power of the Drosophila model. Additionally, genetic screens on the host side could complement these studies to identify and analyze host factors affected by microbiota and important for the association with microbes. Along these lines, there are ~200 fully sequenced isogenic lines to exploit for genome wide association studies.105 There are also a increasing number of Drosophila mutants proving useful as models of human disease,106,107 including many that have their origin in the gut or that have been associated with aberrant microbial communities in mammalian studies.108 In this context, Drosophila is an ideal system, providing the ability to carefully control environmental and experimental conditions coupled with a simplicity that will be important in deciphering the effects of microbiota on complex host traits.
However, our understanding of the molecular mechanisms underlying Drosophila-microbiota interactions is hampered to some extent by our lack of knowledge of how they interact in nature. It is not fully evident whether bacteria found in the Drosophila gut are residents in the strictest sense, or whether they are, like yeast, dietary microbes required for the saprophytic lifestyle of the flies. It is apparent that there are frequent and common associations with a narrow subset of bacteria, which Drosophila disseminates in nature, which persist throughout their life cycle, and are transmitted to progeny. In this sense it will be important to consider how natural habitat and ecology have shaped the nature of host-microbiota interactions. In some ways, the diversity of results observed in various laboratories, which may be attributable to diet, is already indicative of this complexity. At first glance this may seem a challenge that complicates inter-laboratory comparisons and experimental reproducibility that are required for scientific construction. Yet, these laboratory specificities are likely revealing the true nature of Drosophila-microbe interactions, as the nutritional quality of fruit also varies greatly. While this will require a more careful consideration of experimental design and reporting of laboratory conditions (rearing practices, medium composition, Drosophila genotypes, etc.), the expectation is that trends will emerge that will ultimately reflect the full spectrum of associations between Drosophila and their microbiota.
As stated by Felix and Braendle109, “Improbable as it may seem, rotting fruit/plant material thus unites three major lab model organisms in the same ecological context: Saccharomyces cerevisiae, Drosophila melanogaster and C. elegans. What the three species share is a rapid lifecycle, a likely legacy from their boom-and-bust lifestyle exploiting ephemeral resources.” This statement captures how natural conditions have shaped the lifestyles and genetics of animal models. Studies on Drosophila microbiota cannot ignore the ecological context, especially given the saprophagous lifestyle of Drosophila whose diet consists not only of dead organic matter, but also of associated microorganisms. This is exemplified by a recent study showing that food-derived odors (phenylacetic acid) promote male courtship in Drosophila110 or by the report indicating a role of microbiota in assortative mating.21 Future lines of research integrating behavior, neurobiology, and physiology with the natural ecology of Drosophila will be important.60 Thus, a better knowledge of the environment-Drosophila-microbiota interaction will likely reveal important facets of Drosophila biology and open routes for evolutionary studies since competition for rich and ephemeral resource patches is a key factor influencing animal-microbe interactions.
Box 1. Yeast: An Overlooked Constituent of the Drosophila Microbiome?
By and large, recent efforts to elucidate Drosophila-microbiota interactions have been focused solely on bacteria. However, an important, but largely overlooked, participant in this interaction undoubtedly includes fungi, especially yeast. A few factors have likely contributed to the undervaluing of yeast in the Drosophila microbiome. For one, the early identification of the importance of yeast to fly nutrition likely relegated it to being viewed merely as food, especially as dead yeast was a suitable substitute in laboratory medium.6,9,10,12,91,111 In addition, the recent boom in host-microbiota studies, and in particular the focus in mammals, has been almost exclusive to bacteria given their predominance in this environment. Yet, it is the normal state of Drosophila to be associated with both yeasts and bacteria112,113 and a wealth of studies point to the importance of yeast to the host outside of their contributions as food. Below, we highlight some aspects of Drosophila-yeast associations and show how they are of central importance in the ecology and evolutionary biology of Drosophila.
Essential role of yeast in nutrition
Drosophila, as a saprophytic animal, subsists on a diet of decomposing plant matter where it encounters a variety of microorganisms. Given that the substrate is relatively nutrient-poor, these associated microorganisms are often necessary to convert decaying matter into the dietary factors on which the insect depends. From this point of view, yeasts are considered to be a major food source for the majority of Drosophila species, in both larvae and adults.91,112 Early efforts to create a chemically defined medium demonstrated that yeasts provide many nutrients needed for successful metamorphosis and reproduction of Drosophila, such as amino acids, sterols, B vitamins, and fatty acids; compounds not generally present in decaying plant material. For example, diet composed of sterile banana, a carbohydrate-rich substrate, cannot support the growth of axenic larvae unless dietary yeasts are added114 or it is pre-fermented prior to sterilization.91 Additionally, yeasts can transform the substrate into concentrated nutrients and detoxify plant metabolites, thereby increasing the hosts’ ability to access and assimilate nutrients.115
Yeasts associated with Drosophila- are yeast symbionts?
Although they were not the focus of recent efforts in Drosophila microbiome research, considerable knowledge exists about yeasts that are associated with Drosophila. Several studies have shown that a relatively limited diversity of yeast genera are associated with Drosophila in the wild, the most common being Candida, Cryptococcus, Hanseniaspora, Hanusula, Kloeckera, Kluyveromyces, Pichia, Rhodotorula, Saccharomyces, Saccharomycopsis, and Torulopsis (for an extensive review see112,113). The species and their diversity most closely reflect the type of substrate the host utilizes.116,117 In the case of D. melanogaster, a cosmopolitan species that feeds essentially on fruit, Kloeckera, Pichia, and Saccharomycodes species have been most frequently recovered from wild-caught flies.113,118,119 Of note, although yeasts of the Saccharomyces genus are found associated with Drosophila,120 S. cerevisiae, the species most commonly used in laboratory medium, is usually not associated with D. melanogaster in the wild.121
Most studies characterizing the diversity of Drosophila-associated yeasts have isolated them from the adult crop by culturing on defined media, and identifying isolates by traditional morphological methods. The crop, a food storage organ off the foregut with little digestive capacity (Fig. 2), was chosen to optimize the recovery of ingested yeasts. After feeding on yeast, the crop can contain up to 105 yeast cells,122 yet the amount of living yeast found in the gut is quite low. That few yeast cells survive transit through the gut122 likely contributed significantly to the belief that they cannot be considered a bona fide member of the microbiota. Yet, it is known that Drosophila can transmit yeast to sterile media99 and that adults in the wild can transfer yeast to new substrates via their feces, by regurgitation while feeding, or attached to the bristled body surface.112,113,123 Furthermore, it has been shown that while vegetative cells are easily destroyed in the fly gut, spores are able to survive transit and can be cultured from feces.124 Interestingly, the passage of spores through the Drosophila gut increases yeast outbreeding,125 a strategy that would enhance genetic diversity and potentially the likelihood of success when deposited on a new substrate by the host. Thus, the interactions between Drosophila and yeast in the wild appear mutualistic as successful development of larvae depends on the availability of dietary yeasts, which then adult flies disseminate and even provide with an environment that promotes genetic mixing and increased diversity.
Impact of yeast on Drosophila ecology and behavior
Drosophila is known to be attracted to several fermentation products and to yeasts that carry out these functions.126-128 In Drosophila that feed on fruit, yeasts associated with the host are similar to those in the substrate, although the composition on the surface (where adults feed) may differ from that within the substrate (where larvae feed).119,129 This may generate an ecological separation that matches both preferences and partitions the resources between larvae and adults. Such different preferences are not completely understood, as studies have shown that some hosts choose yeasts beneficial for their growth, while others select yeast species previously associated with them even if not beneficial.114,130,131 However, several studies examining the association of Drosophila with microbes in the environment suggest that choice is indeed an important factor in the ecology and even speciation of Drosophila, as specific communities of microbes and flies appear to be associated with specific substrates. For example, while comparing preferences of five Drosophila species for various substrates in compost, Oakeshott117 found that both the Drosophila species and the microbes (bacteria, fermentative and non-fermentative yeasts, and filamentous molds) associated with different fruits were distributed non-randomly. In this study, D. melanogaster was positively associated with specific fruits (grapes and bananas) that were high in fermentative yeasts, and low in bacteria. Similarly, in a field study, D. melanogaster was found as a frequent successive colonizer of fig following inoculation of fermentative yeasts by fig wasps. Additionally, it was observed that sterile fig tissue inoculated with fermentative yeasts was more attractive to Drosophila than those inoculated with spoilage yeasts.132 Interestingly, the attraction of Drosophila to these fermentative yeasts was reduced by the addition of an Acetobacter sp More data are needed to understand how different substrate-microbe combinations attract Drosophila, but it is tempting to hypothesize that certain combinations may convey information about the nutritional quality or successional state of a substrate.
In addition to the role of yeasts in locating suitable habitats, the diversity of yeast species associated with Drosophila may be one factor that has contributed to speciation of Drosophilidae. In comparing a diversity of habitats representative of different feeding guilds of Drosophila, Starmer116 found that although the number of Drosophila-associated yeast species across diverse habitats was similar (mean of 10 species per habitat), distinct physiological characteristics of yeast were associated with each habitat. Yeast communities associated with poorer substrates, such as mushrooms or leaves, exhibited higher catabolic activity than the communities associated with richer substrates such as fruit. Differences in yeast communities linked to habitat are thought to have played a role in the radiation of related Drosophila species.
However, the effect of yeasts on Drosophila behavior extends beyond food choice. While it is well established that a significant density of Drosophila larvae are required for optimal growth (the Allee effect), recent studies point to a positive role of dietary yeasts in this process, since the introduction of live yeast increases fly survival at low density.133,134 It has even been suggested that competition between Drosophila larvae and filamentous fungi on decaying material could have selected for this density-dependent larval development.135 In the same context, dietary yeasts increase survival of axenic flies in the presence of Aspergillus nidulans.136 In addition, increased adult density (and likely higher introduction of microbes) prior to the introduction of larvae also enhances larval survival against filamentous fungi.133 Finally, dietary yeasts enhance the encapsulation ability of Drosophila toward parasitoids.137 The underlying mechanisms of these interactions are not known, but all these studies raise the intriguing hypothesis that Drosophila associated yeasts (and probably bacteria as well) could improve the nutritional value of the food substratum and protect against competing species.
While there is still a lot to learn about these interactions, these studies illustrate the complex and intimate relationships that occur within rotting fruit, between yeast, bacteria, and Drosophila melanogaster. With this in mind, it will be necessary in future studies to not only include yeast as a component of the microbiome, but also to investigate and compare interactions between yeasts and bacteria, both within the substrate and the Drosophila gut environment.
Acknowledgments
We are grateful to M. Rohlfs (University of Goettingen), M. Meister (University of Strasbourg), and N. Buchon (EPFL) for comments on an earlier version of this manuscript. . The Lemaitre lab is funded by the ERC Advanced Grant and the Swiss National Fund (3100A0–12079/1). N. A. Broderick is funded through a Human Frontiers Long-term Postdoctoral Fellowship.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/19896
References
- 1.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–23. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bäckhed F, Crawford PA. Coordinated regulation of the metabolome and lipidome at the host-microbial interface. Biochim Biophys Acta 2010; 1801:240-5. [DOI] [PMC free article] [PubMed]
- 3.Fraune S, Bosch TCG. Why bacteria matter in animal development and evolution. Bioessays. 2010;32:571–80. doi: 10.1002/bies.200900192. [DOI] [PubMed] [Google Scholar]
- 4.Robinson CJ, Bohannan BJM, Young VB. From structure to function: the ecology of host-associated microbial communities. Microbiol Mol Biol Rev. 2010;74:453–76. doi: 10.1128/MMBR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemaitre B, Hoffmann JA. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 6.Delcourt A, Guyenot E. The possibility of studying certain Diptera in a defined environment. CR Hebd Acad Sci. 1910;151:255–7. [Google Scholar]
- 7.Sang J. Circumstances affecting the nutritional requirements of Drosophila melanogaster. Ann N Y Acad Sci. 1959;77:352–65. doi: 10.1111/j.1749-6632.1959.tb36911.x. [DOI] [Google Scholar]
- 8.Guyenot E. Etudes biologiques sur une mouche, Drosophila ampelophila Low. III. Changements de milieu et adaptation. Compt Rend Acad Sci Paris. 1913;74:223–5. [Google Scholar]
- 9.Guyenot E. Etudes biologiques sur une mouche, Drosophila ampelophila Low. V. Nutrition des adultes et fecondite. Compt Rend Acad Sci Paris. 1913;74:332–4. [Google Scholar]
- 10.Guyenot E. Etudes biologiques sur une mouche, Drosophila ampelophila Low. IV. Nutrition des larves et fecondite. Compt Rend Acad Sci Paris. 1913;74:270–2. [Google Scholar]
- 11.Guyenot E. Etudes biologiques sur une mouche, Drosophila ampelophila Low. I. Possibilite de vie aseptique pour l'individu et la lignee Compt Rend Acad Sci Paris 1913; 74:97-9.
- 12.Guyenot E. Etudes biologiques sur une mouche, Drosophila ampelophila Low. II. Role des levures dans l'alimentation. Compt Rend Acad Sci Paris. 1913;74:178–80. [Google Scholar]
- 13.Begg M, Robertson FW. The nutritional requirements of Drosophila melanogaster. J Exp Biol. 1950;26:380. doi: 10.1038/161769a0. [DOI] [PubMed] [Google Scholar]
- 14.Sang JH. The quantitative nutritional requirements of Drosophila melanogaster. J Exp Biol. 1956;33:45–72. [Google Scholar]
- 15.Sang JH, King R. Nutritional requirements of axenically cultured Drosophila Melanogaster adults. J Exp Biol. 1961;38:793–809. [Google Scholar]
- 16.Tatum EL. Development of eye-colors in Drosophila: Bacterial synthesis of v hormone. Proc Natl Acad Sci U S A. 1939;25:486–90. doi: 10.1073/pnas.25.9.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakula M. The ecogenetics of a Drosophila-bacteria association Biology-Genetics: The City University of New York, 1967. [Google Scholar]
- 18.Bakula M. The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J Invertebr Pathol. 1969;14:365–74. doi: 10.1016/0022-2011(69)90163-3. [DOI] [PubMed] [Google Scholar]
- 19.Cox CR, Gilmore MS. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect Immun. 2007;75:1565–76. doi: 10.1128/IAI.01496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren C, Webster P, Finkel SE, Tower J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007;6:144–52. doi: 10.1016/j.cmet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2010;107:20051–6. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 2011;7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–14. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Wong CNA, Ng P, Douglas AE. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol. 2011;13:1889–900. doi: 10.1111/j.1462-2920.2011.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu JH, Kim S-H, Lee H-Y, Bai JY, Nam Y-D, Bae J-W, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–82. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 26.Roh SW, Nam YD, Chang HW, Kim KH, Kim MS, Ryu JH, et al. Phylogenetic characterization of two novel commensal bacteria involved with innate immune homeostasis in Drosophila melanogaster. Appl Environ Microbiol. 2008;74:6171–7. doi: 10.1128/AEM.00301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corby-Harris V, Pontaroli AC, Shimkets LJ, Bennetzen JL, Habel KE, Promislow DEL. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl Environ Microbiol. 2007;73:3470–9. doi: 10.1128/AEM.02120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewer J, Harrison M, Winston J. Survival of two varieties of Erwinia carotovora on Drosophila melanogaster Meigen and Drosophila busckii Coquillett, (Diptera: Drosophilidae) vectors of potato blackleg in Colorado. Am Potato J. 1981;15:439–49. doi: 10.1007/BF02874541. [DOI] [Google Scholar]
- 29.Kloepper J, Brewer J, Harrison M. Insect transmission of Erwinia carotovora var. carotovora and Erwinia carotovora var. atroseptica to potato plants in the field. Am Potato J. 1981;58:165–75. doi: 10.1007/BF02854416. [DOI] [Google Scholar]
- 30.Molina JJ, Harisson MD, Brewer JW. Transmission of Erwinia carotovora var. atropeptica by Drosophila melanogaster meig. I. Acquisition and transmission of the bacterium. Am Potato J. 1974;51:245–50. doi: 10.1007/BF02851435. [DOI] [Google Scholar]
- 31.Juneja P, Lazzaro BP. Providencia sneebia sp. nov. and Providencia burhodogranariea sp. nov., isolated from wild Drosophila melanogaster. Int J Syst Evol Microbiol. 2009;59:1108–11. doi: 10.1099/ijs.0.000117-0. [DOI] [PubMed] [Google Scholar]
- 32.Galac MR, Lazzaro BP. Comparative pathology of bacteria in the genus Providencia to a natural host, Drosophila melanogaster. Microbes Infect. 2011;13:673–83. doi: 10.1016/j.micinf.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flyg C, Kenne K, Boman HG. Insect pathogenic properties of Serratia marcescens: phage-resistant mutants with a decreased resistance to Cecropia immunity and a decreased virulence to Drosophila. J Gen Microbiol. 1980;120:173–81. doi: 10.1099/00221287-120-1-173. [DOI] [PubMed] [Google Scholar]
- 34.Nmorsi OP, Agbozele G, Ukwandu NC. Some aspects of epidemiology of filth flies: Musca domestica, Musca domestica vicina, Drosophilia melanogaster and associated bacteria pathogens in Ekpoma, Nigeria. Vector Borne Zoonotic Dis. 2007;7:107–17. doi: 10.1089/vbz.2006.0539. [DOI] [PubMed] [Google Scholar]
- 35.Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, Spellman P, et al. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci U S A. 2005;102:11414–9. doi: 10.1073/pnas.0502240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dethlefsen L, Huse SM, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 38.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6:776–88. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McFall-Ngai M. Adaptive immunity: care for the community. Nature. 2007;445:153. doi: 10.1038/445153a. [DOI] [PubMed] [Google Scholar]
- 40.Douglas AE. Lessons from studying insect symbioses. Cell Host Microbe. 2011;10:359–67. doi: 10.1016/j.chom.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris HL, Brennan LJ, Keddie BA, Braig HR. Bacterial symbionts in insects: balancing life and death. Symbiosis. 2010;51:37–53. doi: 10.1007/s13199-010-0065-3. [DOI] [Google Scholar]
- 42.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–51. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 43.Haselkorn TS. The Spiroplasma heritable bacterial endosymbiont of Drosophila. Fly (Austin) 2010;4:80–7. doi: 10.4161/fly.4.1.10883. [DOI] [PubMed] [Google Scholar]
- 44.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–44. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tryselius Y, Samakovlis C, Kimbrell DA, Hultmark D. CecC, a cecropin gene expressed during metamorphosis in Drosophila pupae. Eur J Biochem. 1992;204:395–9. doi: 10.1111/j.1432-1033.1992.tb16648.x. [DOI] [PubMed] [Google Scholar]
- 46.Russell V, Dunn P. Antibacterial proteins in the midgut of Manduca sexta during metamorphosis. J Insect Physiol. 1996;42:65–71. doi: 10.1016/0022-1910(95)00083-6. [DOI] [PubMed] [Google Scholar]
- 47.Samakovlis C, Kimbrell DA, Kylsten P, Engström A, Hultmark D. The immune response in Drosophila: pattern of cecropin expression and biological activity. EMBO J. 1990;9:2969–76. doi: 10.1002/j.1460-2075.1990.tb07489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, Lemaitre B, et al. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity. 2000;13:737–48. doi: 10.1016/S1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- 49.Russell VW, Dunn PE. Lysozyme in the midgut of Manduca sexta during metamorphosis. Arch Insect Biochem Physiol. 1991;17:67–80. doi: 10.1002/arch.940170202. [DOI] [PubMed] [Google Scholar]
- 50.Takashima S, Younossi-Hartenstein A, Ortiz PA, Hartenstein V. A novel tissue in an established model system: the Drosophila pupal midgut. Dev Genes Evol. 2011;221:69–81. doi: 10.1007/s00427-011-0360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–3. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–93. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Micchelli CA, Sudmeier L, Perrimon N, Tang S, Beehler-Evans R. Identification of adult midgut precursors in Drosophila. Gene Expr Patterns. 2011;11:12–21. doi: 10.1016/j.gep.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- 55.Moll RM, Romoser WS, Modrzakowski MC, Moncayo AC, Lerdthusnee K. Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J Med Entomol. 2001;38:29–32. doi: 10.1603/0022-2585-38.1.29. [DOI] [PubMed] [Google Scholar]
- 56.Leach J. Methods of survival of bacteria in puparia of the seed corn maggot (Hylemyia cilicrura Rond.) Z Agnew Entomol. 1934;20:150–61. doi: 10.1111/j.1439-0418.1934.tb00358.x. [DOI] [Google Scholar]
- 57.Buchner P. Endosymbiosis of animals with plant microorganisms. New York: Interscience Publishers, 1965. [Google Scholar]
- 58.Greenberg B, Klowden M. Enteric bacterial interactions in insects. Am J Clin Nutr. 1972;25:1459–66. doi: 10.1093/ajcn/25.12.1459. [DOI] [PubMed] [Google Scholar]
- 59.Radvan R. Persistence of bacteria during development in flies. Folia Microbiol (Praha) 1960;5:85–91. doi: 10.1007/BF02927472. [DOI] [Google Scholar]
- 60.Reaume CJ, Sokolowski MB. The nature of Drosophila melanogaster. Curr Biol. 2006;16:R623–8. doi: 10.1016/j.cub.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 61.Paredes JC, Welchman DP, Poidevin M, Lemaitre B. Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity. 2011;35:770–9. doi: 10.1016/j.immuni.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 62.Schulenburg H, Ewbank JJ. The genetics of pathogen avoidance in Caenorhabditis elegans. Mol Microbiol. 2007;66:563–70. doi: 10.1111/j.1365-2958.2007.05946.x. [DOI] [PubMed] [Google Scholar]
- 63.Pradel E, Zhang Y, Pujol N, Matsuyama T, Bargmann CI, Ewbank JJ. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:2295–300. doi: 10.1073/pnas.0610281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang HC, Paek J, Kim DH. Natural polymorphisms in C. elegans HECW-1 E3 ligase affect pathogen avoidance behaviour. Nature. 2011;480:525–9. doi: 10.1038/nature10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–84. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 66.Anyanful A, Easley KA, Benian GM, Kalman D. Conditioning protects C. elegans from lethal effects of enteropathogenic E. coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe. 2009;5:450–62. doi: 10.1016/j.chom.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis MM, Engström Y. Immune response in the barrier epithelia: Lessons from the fruit fly Drosophila melanogaster. J Innate Immun. 2012 doi: 10.1159/000332947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Royet J. Epithelial homeostasis and the underlying molecular mechanisms in the gut of the insect model Drosophila melanogaster. Cell Mol Life Sci. 2011;68:3651–60. doi: 10.1007/s00018-011-0828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ha E-M, Oh C-T, Bae Y-S, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–50. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 70.Zaidman-Rémy A, Hervé M, Poidevin M, Pili-Floury S, Kim M-S, Blanot D, et al. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity. 2006;24:463–73. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 71.Kuraishi T, Binggeli O, Opota O, Buchon N, Lemaitre B. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2011;108:15966–71. doi: 10.1073/pnas.1105994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol. 2009;54:285–302. doi: 10.1146/annurev.ento.54.110807.090559. [DOI] [PubMed] [Google Scholar]
- 73.Lhocine N, Ribeiro PS, Buchon N, Wepf A, Wilson R, Tenev T, et al. PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host Microbe. 2008;4:147–58. doi: 10.1016/j.chom.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Kleino A, Myllymäki H, Kallio J, Vanha-aho L-M, Oksanen K, Ulvila J, et al. Pirk is a negative regulator of the Drosophila Imd pathway. J Immunol. 2008;180:5413–22. doi: 10.4049/jimmunol.180.8.5413. [DOI] [PubMed] [Google Scholar]
- 75.Aggarwal K, Rus F, Vriesema-Magnuson C, Ertürk-Hasdemir D, Paquette N, Silverman N. Rudra interrupts receptor signaling complexes to negatively regulate the IMD pathway. PLoS Pathog. 2008;4:e1000120. doi: 10.1371/journal.ppat.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thevenon D, Engel E, Avet-Rochex A, Gottar M, Bergeret E, Tricoire H, et al. The Drosophila ubiquitin-specific protease dUSP36/Scny targets IMD to prevent constitutive immune signaling. Cell Host Microbe. 2009;6:309–20. doi: 10.1016/j.chom.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 77.Maillet F, Bischoff V, Vignal C, Hoffmann JA, Royet J. The Drosophila peptidoglycan recognition protein PGRP-LF blocks PGRP-LC and IMD/JNK pathway activation. Cell Host Microbe. 2008;3:293–303. doi: 10.1016/j.chom.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Royet J, Dziarski R. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat Rev Microbiol. 2007;5:264–77. doi: 10.1038/nrmicro1620. [DOI] [PubMed] [Google Scholar]
- 79.Ha E-M, Oh C-T, Ryu JH, Bae Y-S, Kang S-W, Jang I-H, et al. An antioxidant system required for host protection against gut infection in Drosophila. Dev Cell. 2005;8:125–32. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–11. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Ryu JH, Nam K-B, Oh C-T, Nam H-J, Kim S-H, Yoon J-H, et al. The homeobox gene Caudal regulates constitutive local expression of antimicrobial peptide genes in Drosophila epithelia. Mol Cell Biol. 2004;24:172–85. doi: 10.1128/MCB.24.1.172-185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dillon RJ, Vennard CT, Buckling A, Charnley AK. Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett. 2005;8:1291–8. doi: 10.1111/j.1461-0248.2005.00828.x. [DOI] [Google Scholar]
- 83.Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scott JJ, Oh D-C, Yuceer MC, Klepzig KD, Clardy J, Currie CR. Bacterial protection of beetle-fungus mutualism. Science. 2008;322:63. doi: 10.1126/science.1160423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cardoza YJ, Klepzig KD, Raffa KF. Bacteria in oral secretions of an endophytic insect inhibit antagonistic fungi. Ecol Entomol. 2006;31:636–45. doi: 10.1111/j.1365-2311.2006.00829.x. [DOI] [Google Scholar]
- 86.Evans JD, Armstrong TN. Antagonistic interactions between honey bee bacterial symbionts and implications for disease. BMC Ecol. 2006;6:4. doi: 10.1186/1472-6785-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Glittenberg MT, Kounatidis I, Christensen D, Kostov M, Kimber S, Roberts I, et al. Pathogen and host factors are needed to provoke a systemic host response to gastrointestinal infection of Drosophila larvae by Candida albicans. Dis Model Mech. 2011;4:515–25. doi: 10.1242/dmm.006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 89.Venema K. Role of gut microbiota in the control of energy and carbohydrate metabolism. Curr Opin Clin Nutr Metab Care. 2010;13:432–8. doi: 10.1097/MCO.0b013e32833a8b60. [DOI] [PubMed] [Google Scholar]
- 90.Sang J. The ecological determinants of population growth in a Drosophila culture. III. Larval and pupal survival. Physiol Zool. 1949 doi: 10.1086/physzool.22.3.30152044. [DOI] [PubMed] [Google Scholar]
- 91.Baumberger JP. The food of Drosophila melanogaster Meigen. Proc Natl Acad Sci U S A. 1917;3:122–6. doi: 10.1073/pnas.3.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, et al. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–4. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 93.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–9. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 94.Ohlstein B, Spradling AC. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–4. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 95.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–55. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–3. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brummel T, Ching A, Seroude L, Simon AF, Benzer S. Drosophila lifespan enhancement by exogenous bacteria. Proc Natl Acad Sci U S A. 2004;101:12974–9. doi: 10.1073/pnas.0405207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Begon M. Drosophila and “dead” laboratory medium. Drosoph Inf Serv. 1974;51:106. [Google Scholar]
- 100.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–55. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi NH, Kim JG, Yang DJ, Kim YS, Yoo M-A. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–34. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choi Y-J, Hwang M-S, Park J-S, Bae S-K, Kim YS, Yoo M-A. Age-related upregulation of Drosophila caudal gene via NF-kappaB in the adult posterior midgut. Biochim Biophys Acta 2008; 1780:1093-100. [DOI] [PubMed]
- 103.Park J-S, Kim Y-S, Yoo M-A. The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging (Albany NY) 2009;1:637–51. doi: 10.18632/aging.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 2008;32:723–35. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 105.Aitman TJ, Boone C, Churchill GA, Hengartner MO, Mackay TFC, Stemple DL. The future of model organisms in human disease research. Nat Rev Genet. 2011;12:575–82. doi: 10.1038/nrg3047. [DOI] [PubMed] [Google Scholar]
- 106.Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–20. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 107.O’Kane CJ. Modelling human diseases in Drosophila and Caenorhabditis. Semin Cell Dev Biol. 2003;14:3–10. doi: 10.1016/S1084-9521(02)00162-3. [DOI] [PubMed] [Google Scholar]
- 108.Apidianakis Y, Rahme LG. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis Model Mech. 2011;4:21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Félix M-A, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20:R965–9. doi: 10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 110.Grosjean Y, Rytz R, Farine J-P, Abuin L, Cortot J, Jefferis GSXE, et al. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–40. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- 111.Northrop J. The role of yeast in the nutrition of an insect (Drosophila) J Biol Chem. 1917;30:181–7. [Google Scholar]
- 112.Begon M. Yeasts and Drosophila In: Ashburner M, Carson H, Thompson Jr J, eds.: The genetics and biology of Drosophila, 1982:345-84. [Google Scholar]
- 113.Ganter P. Yeast and invertebrate associations. In: Peter G, Rosa C, eds. The Yeast Handbook: Springer Berlin Heidelberg, 2006:303-70. [Google Scholar]
- 114.Anagnostou C, Dorsch M, Rohlfs M. Influence of dietary yeasts on Drosophila melanogaster life‐history traits. Entomol Exp Appl. 2010;136:1–11. doi: 10.1111/j.1570-7458.2010.00997.x. [DOI] [Google Scholar]
- 115.Starmer W, Fogleman J. Coadaptation of Drosophila and yeasts in their natural habitat. J Chem Ecol. 1986;12:1037–55. doi: 10.1007/BF01638995. [DOI] [PubMed] [Google Scholar]
- 116.Starmer W. A comparison of Drosophila habitats according to the physiological attributes of the associated yeast communities. Evolution. 1981;35:38–52. doi: 10.2307/2407940. [DOI] [PubMed] [Google Scholar]
- 117.Oakeshott J, Vacek D, Anderson P. Effects of microbial floras on the distributions of five domestic Drosophila species across fruit resources. Oecologia. 1989;78:533–41. doi: 10.1007/BF00378745. [DOI] [PubMed] [Google Scholar]
- 118.Atkinson W, Shorrocks B. Breeding Site Specificity in Domestic Species of Drosophila. Oecologia. 1977;29:223–32. doi: 10.1007/BF00345697. [DOI] [PubMed] [Google Scholar]
- 119.Vacek D, Starmer W, Heed W. Relevance of the ecology of citrus yeasts to the diet of Drosophila. Microb Ecol. 1979;5:43–9. doi: 10.1007/BF02010577. [DOI] [PubMed] [Google Scholar]
- 120.Shehata A, Mrak E. Yeasts isolated from Drosophila and from their suspected feeding places in southern and central California. Mycologia. 1955 doi: 10.2307/3755504. [DOI] [Google Scholar]
- 121.Phaff H, Miller M, Recca J, Shifrine M. Yeasts found in the alimentary canal of Drosophila. Ecology. 1956 doi: 10.2307/1930176. [DOI] [Google Scholar]
- 122.Mrak E. The fate of yeast in the digestive tract of Drosophila. Am Nat. 1951 [Google Scholar]
- 123.Gilbert D. Dispersal of yeasts and bacteria by Drosophila in a temperate forest. Oecologia. 1980;46:135–7. doi: 10.1007/BF00346979. [DOI] [PubMed] [Google Scholar]
- 124.Coluccio AE, Rodriguez RK, Kernan MJ, Neiman AM. The yeast spore wall enables spores to survive passage through the digestive tract of Drosophila. PLoS One. 2008;3:e2873. doi: 10.1371/journal.pone.0002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reuter M, Bell G, Greig D. Increased outbreeding in yeast in response to dispersal by an insect vector. Curr Biol. 2007;17:R81–3. doi: 10.1016/j.cub.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 126.Hutner S, Kaplan H, Enzmann E. Chemicals attracting Drosophila. Am Nat. 1937;71:575–81. doi: 10.1086/280744. [DOI] [Google Scholar]
- 127.Lof ME, de Gee M, Hemerik L. Odor-mediated aggregation enhances the colonization ability of Drosophila melanogaster. J Theor Biol. 2009;258:363–70. doi: 10.1016/j.jtbi.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 128.Lebreton S, Becher PG, Hansson BS, Witzgall P. Attraction of Drosophila melanogaster males to food-related and fly odours. J Insect Physiol. 2012;58:125–9. doi: 10.1016/j.jinsphys.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 129.Morais PB, Martins MB, Klaczko LB, Mendonça-Hagler LC, Hagler AN. Yeast succession in the Amazon fruit Parahancornia amapa as resource partitioning among Drosophila spp. Appl Environ Microbiol. 1995;61:4251–7. doi: 10.1128/aem.61.12.4251-4257.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.da Cunha AB, Dobzhansky T, Sokoloff A. On food preferences of sympatric species of Drosophila. Evolution. 1951;5:97–101. doi: 10.2307/2405761. [DOI] [Google Scholar]
- 131.Dobzhansky T, Cooper D, Phaff H, Knapp E, Carson H. Studies on the ecology of Drosophila in the Yosemite region of California. 4. Differential attraction of species of Drosophila to different species of yeasts. Ecology. 1956;37:544–50. doi: 10.2307/1930178. [DOI] [Google Scholar]
- 132.Miller MW, Phaff HJ. Successive microbial populations in Calimyrna figs. Appl Microbiol. 1962;10:394–400. doi: 10.1128/am.10.5.394-400.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wertheim B, Marchais J, Vet L. Allee effect in larval resource exploitation in Drosophila: an interaction among density of adults, larvae, and micro‐organisms. Ecol Entomol. 2002;27:608–17. doi: 10.1046/j.1365-2311.2002.00449.x. [DOI] [Google Scholar]
- 134.Rohlfs M, Hoffmeister T. Maternal effects increase survival probability in Drosophila subobscura larvae. Entomol Exp Appl. 2005;117:51–8. doi: 10.1111/j.1570-7458.2005.00334.x. [DOI] [Google Scholar]
- 135.Rohlfs M, Obmann B, Petersen R. Competition with filamentous fungi and its implication for a gregarious life-style in insects living on ephemeral resources. Ecol Entomol. 2005;30:556–63. doi: 10.1111/j.0307-6946.2005.00722.x. [DOI] [Google Scholar]
- 136.Rohlfs M, Kürschner L. Saprophagous insect larvae, Drosophila melanogaster, profit from increased species richness in beneficial microbes. J Appl Entomol. 2010;134:667–71. [Google Scholar]
- 137.Anagnostou C, LeGrand EA, Rohlfs M. Friendly food for fitter flies? - Influence of dietary microbial species on food choice and parasitoid resistance in Drosophila. Oikos. 2010;119:533–41. doi: 10.1111/j.1600-0706.2009.18001.x. [DOI] [Google Scholar]