Abstract
The role of human microbiota has been redefined during recent years and its physiological role is now much more important than earlier understood. Intestinal microbial colonization is essential for the maturation of immune system and for the developmental regulation of the intestinal physiology. Alterations in this process of colonization have been shown to predispose and increase the risk to disease later in life. The first contact of neonates with microbes is provided by the maternal microbiota. Moreover, mode of delivery, type of infant feeding and other perinatal factors can influence the establishment of the infant microbiota. Taken into consideration all the available information it could be concluded that the exposure to the adequate microbes early in gestation and neonatal period seems to have a relevant role in health. Maternal microbial environment affects maternal and fetal immune physiology and, of relevance, this interaction with microbes at the fetal-maternal interface could be modulated by specific microbes administered to the pregnant mother. Indeed, probiotic interventions aiming to reduce the risk of immune-mediated diseases may appear effective during early life.
Keywords: microbiota, early life, infant, health, probiotic
Human Microbiome
The human microbiome (HM) is a complex system of many microbial communities inhabiting a diversity of environmental niches throughout the human body. The human gastrointestinal tract (GIT) is inhabited by a complex and dynamic population of around 500–1000 different microbial species. HM exhibits large variation among individuals in relation to internal and external factors such as genetic factors, age, diet and health, and remains in a complex equilibrium. Although the exact composition of the microbiota is not known, advances in genomic technologies have recently begun to unravel our microbial partners. It is estimated that each individual houses at least 160 such species from a consortium of 1000 to 1150 prevalent bacterial species1 whose collective genome (“microbiome”) contains at least 100 times as many genes as the human genome. It is known that about 75% of the gut microbiome is covered by already known and dominant phyla (Actinobacteria, Firmicutes, and Bacteroidetes), while a fraction of about 25% is still unknown.
The microbiota has begun to receive growing attention in recent years since its remarkable relationship with health and disease has been identified. It plays an important role on human health by impacting nutrient utilization, by enhancement of gut barrier function and by stimulation of immune development. An adequate balance of the microbiota plays a critical role in maintaining the health status of the host. Intestinal colonization, a substantial antigen challenge for the newborn, is essential for the maturation of the gut-associated lymphoid tissue and for the developmental regulation of the intestinal physiology.2 Moreover, on molecular levels, members of the intestinal microbiota have been shown to possess a significant capacity to modulate the expression of a large number of host genes involved not only in mucosal barrier function but also in a variety of other intestinal functions, including nutrient absorption, metabolism, angiogenesis and intestinal maturation.3
Origin and Development of Microbiota
The development of the human microbiome is a complex process that has been traditionally assumed to start at birth when the infant is exposed to the mother’s microbiota and continues to develop and change during life. Furthermore, microbiome is known to play an essential role for the maturation of the immune system and the establishment of the gut barrier. Moreover, alteration in this process of colonization has been suggested to increase the risk of disease later in life.4
At birth, the neonate is going to be exposed to an avalanche of new microbes and the infant gut begins to be gradually colonised by a rapidly diversifying microbiota. The first microbes to colonise are facultative anaerobes such as enterobacteria, coliforms, lactobacilli and streptococci followed on the 2nd-3rd day by anaerobes such as bifidobacteria, bacteroides, clostridia and eubacteria as the main microbes in 1–2-week-old infant faeces.4 The most common bifidobacteria present in infant faeces are B. longum, B. infantis and B. breve.5,6 Molecular methods also demonstrate the presence of other types of bacteria such as Ruminococcus and Akkermansia-like bacteria already early in infant gut.5,7 Thereafter, colonization is further modulated not only by environmental bacteria but also by early breast milk, colostrum, which contains live microbes, especially bifidobacteria and lactic acid bacteria, and a spectrum of bioactive substances. The changes in microbiota composition during weaning are more drastic in breast-fed than in formula-fed infants.8 After weaning the gut microbiota continues to develop toward the adult-like pattern. Throughout the first year of life, the GIT microbiota changes continuously and increases in diversity until reaching an adult-like composition. Microbiome development in the GIT has been far more studied than in other body habitats. This process will be affected by feeding habits, health status, and sanitary conditions. The early GIT microbiota is often dominated by one or a few genera, namely Escherichia, Clostridium, Bacteroides or Bifidobacterium. After the first 6 mo of life when solid food is introduced to the infant, the gut microbiota becomes more diverse. The succession of Bacteroides, Clostridium and anaerobic bacteria increases rapidly while the proportion of bifidobacteria becomes more stable. However, the specific taxa present before and after this point may vary.9,10 Functional characterization of the infant microbiome lags behind studies of phylogenetic composition, but recent metagenomic analyses have initiated this task for gut microbiota development. In spite of taxonomical disparities, the infant gut rapidly acquires a functional gene repertoire dominated by carbohydrate metabolism genes and, as mentioned, broadly analogous to that of the adult. Specialized functional repertoires may exist at different stages, as the earliest microbiota can be enriched in genes facilitating lactate utilization whereas solid foods may promote enrichment in genes enabling utilization of a larger variety of carbohydrates, vitamin biosynthesis and xenobiotic degradation. However, genes involved in plant polysaccharide metabolism are also detected in the microbiota before the introduction of solid foods.9,10
Factors Influencing Microbiota Development
Microbiota development of the infant is rapid and depends on the first inoculum, the mother’s microbiota, mode of delivery, type of feeding and the environment, including weaning food practices and the use of antimicrobials.11
Maternal microbiota, diet and lifestyle
The maternal gut is the most important microbial source in the early colonisation of the infant’s gut. However, microbial colonization of the human body may begin earlier as bacteria have been found in umbilical cord, placenta, amniotic fluid and also in meconium.12-14 Microbiota differences have been reported during pregnancy and also, were associated with maternal health.15 Then, pregnant women may create a circle of unfavorable environment generated by inappropriate gut microbiota which would be transferred to the infant during pregnancy and birth.7 In addition, a clear association on bifidobacteria colonization between mother and infant has been found. Maternal colonization by B. bifidum had the most consistent effects on the infant's bifidobacterial microbiota.16 Another study17,18 suggested that early dietary and gut microbiological environments have a more complex effect on the metabolic programming of a child. Moreover, maternal nutritional habits during pregnancy appear to influence the type of microbes present in the meconium at the moment of birth. Infants whose mothers consumed an organic or biodynamic diet seemed to have slightly lower numbers of E. coli than did infants whose mothers consumed a regular diet.19,20
Mode of delivery
Differences in microbiota composition depending on delivery mode will affect the early stage of neonatal microbial colonisation.21 Of note, infants born by caesarean section have a different microbiota composition as compared with vaginally delivered newborn infants.22 This, unequivocally will have an impact on their health and indicates a mother–child transmission of microbiota during vaginal delivery. Indeed, an increased risk for atopic diseases such as allergic rhinitis, asthma and celiac disease among other was reported in children born by caesarean section.21,23,24
Vaginally delivered infants come in contact with the maternal vaginal and faecal microbiota which results in the gut colonisation by microbes from mother’s birth canal. In infants born by caesarean section (C-section) the establishment of gut microbiota is delayed. These infants are more often colonised with clostridia and bacteroides and less with bifidobacteria and lactobacilli.19,20,25,26 16S rRNA pyrosequencing has revealed that vaginally delivered infants acquire bacterial communities resembling their own mother’s vaginal microbiota, dominated by Lactobacillus, Prevotella or Sneathia, whereas C-section infants acquire bacterial communities similar to those found on the skin, dominated by Staphylococcus, Corynebacterium and Propionibacterium.22
Infant’s diet
Transfer of microbiota continues during breastfeeding which plays a key role during the infants first months of life. Hence, exclusively breastfed infants exhibit significant differences in gut microbiota as compared with formula fed.5,6,27 Breast milk has been shown to be an excellent and continuous source of bacteria including staphylococci, streptococci, lactic acid bacteria and bifidobacteria.28-30 In addition, components such as oligosaccharides support the growth of beneficial bacteria, which may critically contribute to the establishment of a healthy microbiota in breast-fed infants. Among breast-fed infants, Bifidobacterium-dominated microbiotae which can reach up to 60–90% of the total faecal microbiota are more frequent than among infants fed with formula, but other compositions are not uncommon.5,6,17 Other microbes present are Staphylococcus, Streptococcus, Corynebacterium, Lactobacillus, Micrococcus and Propionibacterium originating from nipple, milk ducts and the surrounding skin. Transmission of specific intestinal Bifidobacterium strains from mothers to infants have been recently reported31,32 suggesting that each mother-infant pair might have unique family-specific strains and breastfeeding could contribute to the microbial transfer from the mother to the infant and, therefore, impact on the infant gut colonization.33 Formula fed microbiota is more complex and similar to that of adults, and is characterized by the predominance of facultative anaerobes such as Bacteroides and Clostridium followed by Staphylococcus, Streptococcus and Enterobacteriaceae, and delayed colonisation by bifidobacteria.25,34, The target of infant formula research has been to develop product composition that closely resembles breast milk. There are, however, very little data on changes in the composition of the intestinal microbiota during early weaning and how the influence of pre-weaning feeding methods and perinatal events impact on the microbiota after the introduction of complementary foods.26
Lifestyle and geographical location
Single children tend to have lower counts of bifidobacteria in the gut at one month of age,19 non-E. coli enterobacteria and clostridia, and a lower ratio of anaerobic to facultative bacteria by one year of age compared with children with siblings.35 It has been also described, that infants living on a farm had a low diversity and a pronounced dominance of some Clostridium and Eubacterium species while other children had a more diverse microbiota.36
Based on numerous reports, it seems that the geographical differences in microbiota composition result from the different dietary habits in the region or the country. A recent study37 showed a “geographical gradient“ in the infant microbiota across Europe where northern infants showed higher levels of Bifidobacteria, Atopobium, C. perfringens, C. difficile while Southern infants presented higher proportion of Bacteroides, Eubacteria and Lactobacillus. Higher counts of lactobacilli, eubacteria and enterococci have been described in Estonian infants while Swedish infants showed high numbers of clostridia and bacteroides.38 Other studies also reported higher levels of bifidobacteria in Italian adult subjects as compared to Swedish, French and German adults.39 It has also been reported that Bifidobacteria genus, Bacteroides-Prevotella and Cl. histolyticum levels were higher in Malawi infants than Finnish infants at age of 6 months.40 A similar study demonstrated that children living in a rural village in Burkina Faso are characterised by an enrichment of Bacteroides while Italian children of Enterobacteriaceae.41
Antibiotics, probiotics and prebiotics
Antibiotics are very commonly used to treat specific infections but often without considering their impact on gut microbiota. Antimicrobials are not exclusively selective for pathogens and indiscriminately affect all members of the commensal gut microbial ecosystem, especially decreasing the levels of anaerobic bacteria, Bacteroides, E. coli and beneficial bifidobacteria and increasing the levels of potentially harmful clostridia and Klebsiella.19,42 Oral use of antibiotics (mainly amoxicillin) and antimycotics (miconazole) by the infant during the first month of life resulted in decreased numbers of bifidobacteria and B. fragilis-group species.19,20 The efficacy of probiotics reducing antibiotic-induced diarrhea has been extensively studied and their ability to efficaciously modulate the gut microbiota and restore the disturbed microbial ecosystem after antibiotic therapy proven.43 Meta-analyses conducted in this area44 concluded that available data provide sufficient evidence for the role of probiotics in the prevention of antibiotic-associated diarrhea (2874 participants; RR 0.52; 95% CI 0.38 to 0.72; I(2) = 56%).
Other factors
Gestational age has a profound role in determining the gut microbiota.19,45 In newborn infants, especially those prematurely born, who are hospitalised and therefore separated from their mothers for long periods immediately after birth, the environment becomes an important source of colonising bacteria.25,42
Microbiota and Human Health
In recent years, the increase in microbiota-related research has provided important advances toward establishing the identity of specific microbes and microbial groups or microbial molecules contributing to various aspects of host physiology and health. Studies on human microbiota should include microbial ecology and analysis of the complex metabolism of the microbial community, as well as various host–microbial interactions occurring at the interface between microbes and host intestinal epithelia. Such studies should lead to an understanding of the impact of the microbiota on human health and disease. Concurrently, host factors involved in various aspects of development and maturation targeted by the microbiota have been identified.
A balance among microbial groups present in the human gut is crucial for maintaining health. When this balance is disturbed, the host–microbe relationship can progress toward a disease state. The importance of the microbiome in relation to human disease has been stressed by a recent National Institutes of Health (NIH) initiative, the “Human Microbiome Project,” which aims to assess how changes in the microbiome correlate with human disease using genomic and metagenomic sequencing.46
Altered intestinal colonization by commensal microorganisms, increased occurrence of potential pathogens, as well as high interindividual variability and reduced microbial diversity have been reported in preterm infants45 increasing the risk to develop later disease. Several gastrointestinal pathologies such as IBD, IBS, NEC, obesity, various forms of colitis and even autism has been linked to disturbances in human-associated microbiota or alterations of the intimate cross-talk between these microbes and human cells.47 Numerous studies have linked early gut microbiota to the development of atopic diseases, but no specific microbes have yet been identified with consistently harmful or protective roles regarding atopy.48,49 Several studies indicate a link between bifidobacteria deficiency and increased incidence of atopy.50,51 However, large prospective case-control studies have failed to confirm this association20 and conflicting results have been obtained regarding the protective role of different Bifidobacterium species.51 The development of metabolic complications associated with obesity during childhood tracks into adulthood is related to risk factors such as diet, lifestyle, decreased physical activity and rapid infancy weight gain. However, some reports have suggested that the gut microbiota is an important factor affecting the regulation of host energy homeostasis and adiposity, as differences in microbial composition can explain an increased capacity of the obesity-associated microbiome to harvest energy from the diet.52,53 A subsequent metagenomic study54 with 154 individuals showed that obesity was associated with a markedly reduced bacterial diversity, a relative depletion of Bacteroidetes, and a higher proportion of Actinobacteria compared with lean subjects. However, lower ratios of Firmicutes to Bacteroidetes in overweight human adults compared with lean controls have recently been reported.55 In addition, diets based on a high intake of protein and/or low intake of carbohydrate or low fat consumption may alter microbial composition and activity in the large intestine and thus impact gut health.56 Other studies have examined gut microbiota composition in human obesity and type-2 diabetes and the impact of weight reduction on microbiota,57,58 some of which contest the link between the proportion of Bacteroidetes and Firmicutes and human obesity.59 In addition, it has been shown that infants with high numbers of Bifidobacterium and low numbers of Staphylococcus in early life may be protected from weight gain during later life.60 Hence, infants born to women with normal weight gain during pregnancy showed higher levels of bifidobacteria than those from women with excessive weight gain, suggesting a potential role for Bifidobacterium on infant microbiota and weight development.8,15 A recent study reported that high numbers of bifidobacteria may positively correlate with normalization of inflammatory status and improved glucose tolerance and glucose-induced insulin secretion.56 Diabetes mellitus is a group of metabolic diseases characterized by high blood sugar (glucose) levels that result from defects in insulin secretion, action, or both. A link between gut microbiota composition and activity and the management of glycemia associated with overweight and diabetes has been reported.61 In type-1 diabetes, a combination of multiple factors leading to the development of autoimmunity appears to include an aberrant intestinal microbiota, a leaky intestinal mucosal barrier and an altered intestinal immune responsiveness.62 Type 2 diabetes is a metabolic disease and the primary cause is obesity-linked insulin resistance. Recent research58 reported that type 2-diabetes is associated with compositional changes in the intestinal microbiota. The relative abundance of Firmicutes was significantly lower, while the proportion of Bacteroidetes and Proteobacteria was higher in diabetic persons compared with non-diabetic counterparts. Accordingly, the ratios of Bacteroidetes to Firmicutes positively correlated with reduced glucose tolerance. These results, assuming that diabetes and impaired glucose tolerance are linked to obesity, are in agreement with the recent evidence obtained for overweight persons by Schwiertz and colleagues.55
Early Microbial Exposure
As we outlined above, the gastrointestinal tract of the fetus has been generally considered microbiologically sterile but there are indications of microbial exposure already prior to delivery.12-14,63 Microbial colonization of the human body may begin earlier through transport of maternal bacteria via the bloodstream to the placenta from where they could reach the umbilical cord or the amniotic fluid, which is constantly being swallowed by the fetus.12,13,64 Different bacterial species in the amniotic fluid of preterm pregnancies were detected and further demonstrated that women whose amniotic fluid was PCR-positive for bacteria exhibited elevated levels of IL-6, histological chorioamnionitis and funisitis, which were strongly associated with the development of neonatal sepsis.65 In other study, Leptotrichia spp and other related bacterial species were detected using culture-independent molecular approaches in the amniotic fluid of women in preterm labor with a strong dose-dependent relationship between bacterial abundance in the amniotic fluid and gestational age at delivery.64 Furthermore, bacteria and/or their DNA have been also isolated from the meconium of healthy neonates.13,14,66,67 Of note, bacterial colonization of placenta in non-pathologic situations has been recently reported even in subjects delivered by elective caesarean section with a very low risk of contamination.66 Interestingly, translocation of bacteria from the oral cavity had been previously described.65 All these studies provide evidence for direct in utero microbial exposure and the possibility that introduction of the developing fetus to microbial products may play a role in shaping postnatal immune development.68 As contact with microbes takes place already in utero the impact of maternal intestinal microbiota may be more important than earlier understood.
Observational and interventional studies suggest that exposure to microbes during pregnancy may influence the metabolic and immunologic profiles of the pregnant uterus and, hence, the risk of disease developing in the offspring later in life.69,70 It has been reported microbiota differences during pregnancy associated with serum biochemical variables of relevance to the nutritional and health status during pregnancy (eg, cholesterol, folic acid, ferritin, and reduced transferrin) and therefore with possible consequences on fetal health programming.15,57 Moreover, in case of pregnant obese women, a vicious circle of unfavorable metabolic development may be generated if abnormal gut microbiota related to overweight, or excessive weight gain during pregnancy, is transferred to the infant.7,15 However, there is no direct evidence on the roles and mechanisms of action of each bacterial group in those instances. Under these circumstances, the possible role played by the composition of the gut microbiota in women’s health during pregnancy and its possible influence on the maternal-fetal interactions in utero have also been recently investigated.71-73 Of note is the fact that these studies led to the conclusion that maternal microbial exposure during pregnancy, and perinatal and postnatal periods may have a significant impact on infants’ health.
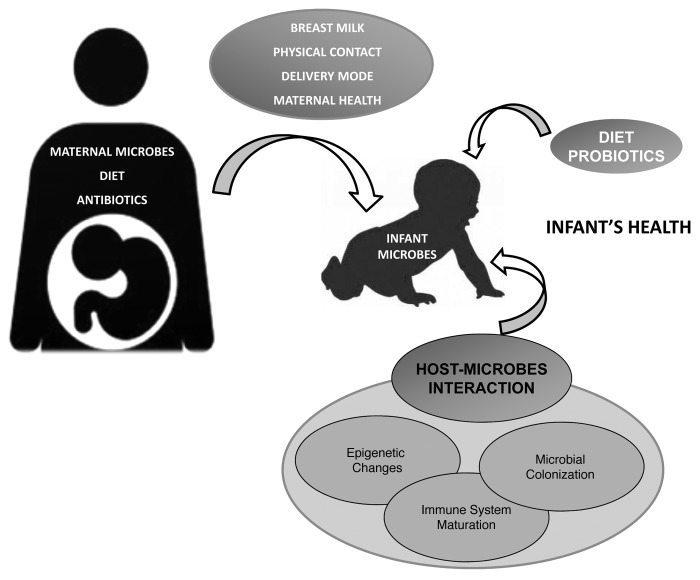
Altogether, the complex interactions between microbes and host combined with recent clinical observations and epidemiologic trends may point to the convergence of two hypotheses: the “hygiene hypothesis” and the “fetal programming hypothesis.” The hygiene hypothesis supports that the rapid increase of the incidence of allergy could be related to lower microbe exposure at early life and subsequent lower number of infections during infancy.74 Until recently it has been assumed that the human fetus develops in a sterile environment and is therefore born immunologically inexperienced and immature. However, it is established that maternal exposures during pregnancy may induce long-lasting or even permanent changes in fetal physiology and thereby have an impact on disease risk in later life (Fetal programming hypothesis).75 We are beginning to understand that exposure to microbes before conception, during gestation, and in the neonatal period have profound effects on health,76 since maternal microbial environment during pregnancy may remarkably influence the newborn immune development and, consequently, impact infant’s health early and later on in life. Recent evidence indicates the importance of prenatal and postnatal developmental processes in terms of maturation of balanced immune responses. It is well established that early microbial colonization events provide the neonate with vital stimuli that guide the immune maturation. Disturbances in this process may result in the development of immune disorders such as atopic disease, allergy and asthma, which are regarded as a failure in the balanced microbial composition and immune response.48,49,68 Experimental murine models demonstrate that maternal treatment with lipopolysaccharide or the commensal Acinetobacter lwoffii during gestation attenuates allergic sensitization and airway inflammation in the offspring.77 Also, epidemiological studies indicate that maternal farm environment exposure during pregnancy protects against allergic sensitization, asthma and disease, and have an upregulation of receptors of the innate immune system whereas exposures during infancy alone are under discussion.78,79 Furthermore, maternal infection with other organisms, such as parasites, viruses and exposure to viral antigens during gestation have also been recently shown to modulate the immune response in the offspring. These data, along with observations from probiotic intervention studies,80,81 show that maternal microbial exposure during gestation may lead to long-term health consequences for the offspring by influencing fetal immune development. However, continuous enhanced post-natal high microbial exposure may be required for optimal health protection78 and breastfeeding practice support the microbiota transfer as well as immune factors supporting equilibrated balance (Fig. 1). In this respect, the microbiota is quantitatively the most important environmental source of immune stimulation and may provide a primary signal for the maturation of a balanced post-natal innate and adaptive immune system51,82.
Figure 1. Factors influencing early microbiota colonization and its impact on infant health. Neonatal bacterial colonization is determined by environmental factors (e.g., maternal environment and diet) combined with host influences (genetics and gut characteristics). Maternal microbial environment affects maternal and fetal immune physiology and, of relevance, this interaction with microbes at the fetal-maternal interface could be modulated by specific microbes administered to the pregnant mother and/or neonates. The genetically-determined gut characteristics (structure, function, immunity), and the time and mode of birth (preterm, term, cesarean, vaginal), are crucial factors as well as maternal microbiota and nutritional status together diet (parenteral nutrition, formula, breast milk, and also, use of probiotics, prebiotics and/or antibiotics).
Due to the wide complexity of the microbiota, more detailed, basic microbial ecology studies, in clinically and immunologically well-characterized mother-infant pairs are needed, which are now made possible by advances in DNA sequencing technologies. Recent findings showed that adult gut microbiota can be classified according to 3 distinct microbial profiles or “enterotypes” that respond differently to diet and drug intake.83 These enterotypes are likely established during early life explaining why neonatal gut microbiota composition has a lasting effect on health and immunity84; however, the origin and factors affecting their development remain unclear. Moreover, how the maternal microbiota impacts on offspring’s microbiota development coupled to the effects on immune maturation during early life forms a key issue that deserves further investigation.
Breast Milk: Postnatal Link Between Mothers and their Offspring
Mother transfers the environmental information to her fetus through the placenta or to her infant through lactation (Fig. 1). Breast milk is recognized as the single most important postnatal element in metabolic and immunological programming of the child’s health.85 Breastfeeding emerged as a nutritional strategy following the divergence of mammals millions of years ago that was favored by natural selection.86 Despite the fact that milk production is very costly for mammalian females, it brings great benefits to the offspring. Today, breast milk is considered the gold standard for infant nutrition.87 Compilations of long-term studies have shown that breast-fed infants have lower risk of diabetes,88 hypercholesterolemia,89 cardiovascular disease90 and obesity91 in adulthood than formula-fed infants, although the causality is difficult to ascertain. It is likely that the beneficial impact achieved for the infant with breast-feeding is a combination of a balanced supply of nutrients, bioactive proteins and indigestible oligosaccharides, as well as bifidogenic bacteria in breast milk.87
Human milk contains large quantities of secretory immunoglobulins mainly IgA. These antibodies can bind to pathogens and prevent their attachment to an infant's cell. In addition to these immunologic components, breast milk contains several nonspecific factors, such as lysozyme, lactoferrin and oligosaccharides, which are able to block attachment of pathogens or virus to the infant's mucosa, preventing infections and supporting the growth of beneficial bacteria. Lysozyme inhibits the growth of many bacterial species by disrupting the bacterial cell wall. Lactoferrin, known as a multifunctional protein in human breast milk, also limits bacterial growth by removing essential iron and by stimulating cytokine production, and enhancing mucosal immunity, natural killer (NK) cell activity and macrophage cyto-toxicity. Nucleotides in human breast milk have been shown to enhance the immune function in infants. Breast milk is a continuous source of microbes and their growth factors and components are regulating host-microbe interactions.28,92-94 A delicate balance of such components is transferred from mother to infant via breastfeeding. Breast milk composition shows marked individual variation,34,95 and factors that determine human milk composition remain poorly understood, although maternal lifestyle, nutritional and immunological status, dietary habits and lactation time influence breast milk composition and quality.96,97
The origin and route of transfer of these bacteria have not yet been unveiled. The translocation of commensal bacteria has been demonstrated to occur in different parts of the body including the intestinal epithelium and skin.98-101 It has been suggested that bacteria could be transferred from the human gut to the mammary gland through an endogenous route (the so-called entero-mammary pathway) via the mucosa-associated lymphoid system.102 Another theory is endocytosis, which could be supported by our observed differences between elective and non-elective C-section, if the stress of labor and delivery affected gut permeability. This is further supported by reports sustaining that the enteric nerve system affects the sampling of bacteria in the gut and their transfer to Peyer’s patches.103 Another source of colonizing bacteria could be the skin and oral cavity of newborns exposed to the mother’s vaginal and intestinal microbiota during childbirth.22 In placenta, the penetration of bacteria has been observed in intrauterine infections, preterm birth and spontaneous abortion due to particular pathogens66 although bacterial colonization of placenta in non-pathologic situations was reported recently (see above).
Altogether this information leads to the speculation that it could be of relevance for the infant’s health to monitor bacterial population in pregnant women at different sites and to appropriately modify it when requested to adequately control microbiota transferred to the infant in the early vital inoculums.
Early Health Programming with Probiotics
There is a growing interest in beneficial microbes with specific functions in the human microbiota, which could be used in foods or supplements to improve human health and prevent and treat diseases. The latest evidence demonstrates that maternal lifetime nutrition influences the placental morphology, with a potential impact on the later health of their offspring.104 Pregnancy and early infancy are, to the current understanding, the most interesting critical stages and targets for dietary and food-based interventions aiming to reduce disease risk (Fig. 2). Hence, it is generally agreed that the faecal microbiota of the healthy, full-term, vaginally delivered, breast-fed infant constitutes the gold standard and therefore the target for any intervention in this regard.
Figure 2. Perinatal and postnatal probiotic interventions and their potential benefits for infant development and health. Early modulation driving healthy microbiota would be the aim of probiotic therapy and opens new prevention and treatment possibilities of diseases in which alteration of the microbiota plays relevant roles. Several studies have shown that administration of probiotics to pregnant women, nursing mothers, or newborns can influence the establishment and composition of infant gut microbiota and also, the development and maturation of immune system impacting early and later in life. NEC, necrotizing enterocolitis.
A probiotic has been defined as a “live microorganism which when administered in adequate amounts confers a health benefit on the host.”105 The most common probiotic bacteria are species belonging to Lactobacillus, Bifidobacterium, Enterococcus and also some Propionibacterium strains. During the last few decades, a large number of studies have been conducted to analyze the beneficial effects of probiotics, using different formula and focusing to the prevention or treatment of diseases. Therefore, modulation of an unbalanced indigenous microbiota would be the aim of probiotic therapy and opens new prevention and treatment possibilities of diseases in which alteration of the microbiota plays relevant roles. Studies have shown that administration of probiotics to pregnant women, nursing mothers, or newborns can influence the establishment and composition of infant gut microbiota16,71,72 impacting early and later in life (Fig. 2).
Prenatal interventions
Use of probiotics to promote infant microbiota development
Many clinical trials have been performed, in the perinatal period and during the first months of life aiming to test the influence of probiotic administration on the fecal microbial composition. It could be proved that prenatal maternal administration of L. rhamnosus LGG affected the transfer and initial establishment of bifidobacteria in neonates106 and was associated with modulation of the infant intestinal Bifidobacterium pattern toward that of a healthy breastfed infant.107 In particular, infants whose mothers received LGG during pregnancy showed higher prevalence of B. breve and a lower prevalence of B. adolescentis than placebo group.106 Another study showed that LGG temporarily colonized the infant’s gut after administration of the probiotic to the pregnant mother before delivery; this colonization was stable for up to 6 mo.108 Although, these results suggest that bacteria are transferred from mother to newborn maternal LGG consumption increased the bifidobacterial diversity in infants at 3 weeks and reduced the similarity of Bifidobacterium species pattern between mother and infant.106 Nevertheless, there are also studies where prenatal consumption of LGG failed to modulate the diversity of early infant gut microbiota despite promoting a beneficial bifidobacteria profile.109 Bifidobacteria also have a potential as prenatal probiotics as maternal colonization by B. bifidum had the most consistent effects on the infant's bifidobacterial microbiota.16
Use of probiotics in prevention of allergy diseases
The prevalence of allergic diseases, atopic eczema, allergic rhinitis and asthma, continues growing in the developed world and constitutes a common health problem among children.110 While the precise etiology is unclear, certain conditions or elements have a crucial role in the development of allergic diseases, such as environmental factors, aberrant gut microbiota composition and activity, impairment of intestinal mucosal barrier and shift of the Th1/Th2 –type cytokines balance toward a Th2 response.111
In human allergy intervention studies, perinatal maternal microbial supplementation with probiotics may have important preventive effects.73,81 Specific probiotics administered to allergic infants are effective in the prevention of atopic eczema, as well as modulating the immune system.112,113 The frequency of atopic eczema in the probiotic group was half that of the placebo group (15/64 [23%] vs 31/68 [46%]; relative risk 0.51 [95% CI 0.32–0.84]). LGG was administered to pregnant women for four weeks prior to delivery, then to newborns at high risk of allergy for six months, as result a significant reduction in early atopic disease was achieved.113 In prospective clinical studies ingestion of probiotic strains LGG and B. lactis Bb12 alleviated allergy-associated symptomatology in infants allergic to cow’s milk and atopic dermatitis.114 The effect of probiotics on the prevention of atopic dermatitis has been demonstrated in double-blinded randomized controlled studies where LGG was given to pregnant and lactating mothers, who had a strong family history of eczema, allergic rhinitis or asthma and to their infants for the first 6 mo after birth. The frequency of developing atopic dermatitis in the offspring was significantly reduced by 2, 4, and 7 y.50,80,112,113 Furthermore, several well-designed studies have provided evidence that specific strains of probiotics can be somewhat effective for the treatment of established atopic dermatitis.115 Pregnant women carrying high-risk children were given a mixture of 4 probiotic strains (Lactobacillus rhamnosus GG, L. rhamnosus LC705, Bifidobacterium breve Bb99, Propionibacterium freudenreichii ssp. shermanii JS) before delivery and later their infants received the same probiotics along with galacto-oligosaccharides for 6 mo after birth.116 Of note, prospective clinical studies showed that supplementation of probiotic bacteria failed to reduce the risk of atopic dermatitis and even resulted in increased sensitization to allergens in one of them.72,117,118 However, a meta-analysis report which included seven randomized, placebo-controlled trials comparing the development of atopic eczema in children whose mothers took probiotics vs placebo during pregnancy showed a significant reduction in the incidence of atopic eczema(reduction 5.7%; p = 0.022) in children aged 2 to 7 y whose mothers received probiotics during pregnancy.119 However, the beneficial effect was only significant for lactobacilli (reduction 10·6%; p = 0·045), but not for a probiotic combinationss (difference 3·06%, p = 0·204). Other recent reports indicate that probiotics had no effect on asthma development and related episodes,120,121 and airway inflammation.116 A major reason for these discrepancies may be the use of different probiotic strains, the food or vehicle used to deliver the probiotics and doses used. Furthermore, host factors such as genetic susceptibility, microbiota composition and environmental factors, such as geographic region and diet, may influence the results of these studies.
Use of probiotics in prevention of obesity
The prevalence of obesity is rapidly increasing in Western society constituting an important health issue. Genetic and environmental factors such as diet, socioeconomic status, maternal obesity, rapid infancy weight gain and reduced physical activity have unequivocal influence on the incidence of obesity and its related diseases. In addition, some reports have suggested that gut microbiota is an important factor affecting energy disposal and storage in adipocytes.53,54 Of note, gut microbiota composition may play a pivotal role in the microbial, metabolic and immunological programming of the infant although information in this regard is still scant.3 The association between gut microbiota composition and maternal nutritional status during pregnancy has been published by Collado et al.15 and by Santacruz et al.57 Interestingly, both studies are supportive to the view that a gut microbiota profile favoring a higher number of bifidobacteria and a lower number of Staphylococcus aureus may provide protection against maternal overweight development. Other studies provided clinical evidence of consistently improved plasma glucose concentrations and insulin sensitivity in healthy women during pregnancy and 12 mo postpartum when an adequate dietary intake was combined with probiotics; moreover those effects were extended to neonates and infants.122 Interestingly, nutrition counselling and probiotic intervention had a distinct effect on gestational diabetes; thus, while probiotics diminished the risk of gestational diabetes (13% (diet/probiotics) vs. 36% (diet/placebo) and 34% (control); p = 0.003), dietary counselling reduced the risk of associated fetal overgrowth [p = 0.035 for birth weight and p = 0.028 for birth length].17,18 In line with these observations, perinatal probiotic intervention with LGG moderated excessive weight gain especially among children who later became overweighed during the first years of life with a most pronounced impact at the age of 4 y.17,18 In another clinical placebo-controlled study a probiotic combination (LGG + Bifidobacterium lactis Bb12) administered during the perinatal period combined with an advantageous diet consistently improved plasma glucose concentrations and insulin sensitivity in healthy women during pregnancy and 12 mo postpartum.73,122 This intervention study also provided clinical evidence that probiotic consumption lowers the risk of central adiposity over the 6-mo postpartum period.123 The risk of central adiposity defined as waist circumference 80 cm or more was lowered in women in the diet/probiotics group compared with the control/placebo group (OR 0.30, 95%CI 0.11–0.85, p = 0.023 adjusted for baseline BMI), while the diet/placebo group did not differ from the controls (OR 1.00, 95% CI 0.38–2.68, p = 0.994) at 6 mo postpartum.
Use of probiotics in prevention of preterm birth
The effectiveness of probiotics in preventing preterm labor and birth has been the focus of recent studies since maternal infection may increase 30–50% the risk of preterm delivery.124 It has been suggested that specific probiotics could displace and inhibit pathogens responsible for triggering the inflammatory cascade that leads to preterm labor and delivery. Relevant clinical trials have suggested that oral administration of lactobacilli, could increase the number of vaginal Lactobacillus, restore the vaginal microbiota to normal, and treat women suffering bacterial vaginosis.125 Nevertheless, some trials found that intra-vaginal administration had no significant effect, and although most of the available studies and results concerning the effectiveness of the administration of lactobacilli for the treatment of bacterial vaginosis are mostly positive, further studies to conclude definitively that probiotics are useful are needed.125
Randomized controlled trials assessing the prevention of preterm birth by administration of probiotics in pregnant women and women planning pregnancy were recently reviewed.124 One of the studies enrolled women after 34 weeks’ gestation received fermented milk as a probiotic. In another study women with bacterial vaginosis in early pregnancy received commercially available yogurt vaginally. The results showed an 81% reduction in the risk of genital infection after the probiotics were administered (OR 0.19; 95% CI 0.08–0.48). However, there are insufficient data available to assess the actual effect on preterm birth and its complications126,127
Probiotics, cognitive function and mental development
A recent study surprisingly revealed that gut microbes which interact with the fetus before birth can modulate brain developmental pathways.128 This study shows that the commensal microbiota is necessary not only for the development of the immune system but also for brain development and that this regulation has explicit time constraints. It has been reported that gut microbes acquired early in life can impact brain development in mice and subsequent behavior such as decreasing physical activity and increasing anxiety.129 Furthermore, understanding the window of vulnerability when alterations in immune function can lead to brain impairment, and the therapeutic window when normal brain function can be restored is of clear importance. One study has shown that exposure to high levels of IL-6 in utero alters brain development and leads to permanent behavioral impairments.130 Consumption of certain probiotic strains may affect cytokine patterns and it has been shown that these molecules may affect cognitive functions131 and gut hormones and neuropeptides may affect the learning process.132
Postnatal interventions
Infants are one of the populations that may benefit more from the use of probiotics due the existence of a critical “window period” during the first months of life where there is an important opportunity for immune education when the intestinal microbiota and maturation of the immune system are not yet completed.19,48,49 Different newborn infants including preterm, formula-fed, C-section delivered, and those who required antibiotics or intensive care are prone to develop diseases including infections such as diarrhea, necrotizing enterocolitis, atopic eczema and other related allergic diseases.25 The main aim of probiotic intervention in these populations should be to avoid an aberrant microbiota development which may lead to impaired gut barrier function and abnormal immune responsiveness.133
Use of probiotics to promote infant microbiota development
It has been showed that the administration of L. rhamnosus GG to mothers during 3 weeks after delivery modifies the initial infant establishment of bifidobacteria than those receiving placebo.106 Infants whose mothers received probiotic had a higher prevalence of B. breve and a lower prevalence of B. adolescentis than did those in the placebo group at 5 d of age. It has been recently shown that prenatal administration to the mother of Lactobacillus rhamnosus GG (LGG) in late pregnancy modulated infant’s intestinal Bifidobacterium microbiota rendering it similar to that of a healthy breastfed infant.73 It has also been reported that specific probiotic combinations during early feeding via the mother or incorporated in early formula-feeding, shaped the intestinal microbiota composition in infants from Germany and Finland.134 Results showed that maternal intake of a probiotic mixture consisting of L. rhamnosus LPR and B .longum BL999, promoted the growth of lactic acid bacteria in the infant’s gut and that the impact was still evident 4 mo after discontinuation of probiotic administration. On the other hand, the specific probiotic combinations administered to formula-fed infants had only minor effects on microbiota composition toward lowering the bacterial counts of B. bifidum compared with the placebo group.
Use of probiotics for the prevention of allergic disease
An epidemic rise in allergic disease in “westernized” countries, especially food allergy and asthma, has occurred in parallel with many lifestyle changes. Studies have linked alterations in the intestinal microbiota in infants with the later development of allergic disease.48-50 These abnormalities particularly involved Bifidobacterium species51,135 and microbial diversity.136
A Cochrane Library meta-analysis of probiotic supplementation for the prevention of allergic disease in term infants found a significant reduction in eczema in infants who received probiotics compared with those that did not [typical RR 0.82, 95% CI 0.70, 0.95] and further trials are recommended to determine the reproducibility of the results.137 A recent meta-analysis71 including 12 randomized-controlled trials (781 participants) concluded that probiotics are not a clinically effective treatment for eczema (mean difference −0.90 points on a 20-point visual analog scale; 95% CI −2.84- 1.04), although when given during early infancy for the prevention rather than the treatment of eczema the results for some probiotic strains are promising. Another recent study138 evaluated the clinical evidence for the use of probiotics as a therapeutic modality for allergic rhinitis and asthma by reducing symptom severity and medication use, although, further good-quality studies are needed. Another study139 showed that beneficial effects of probiotic bacteria were obtained when treatment was given within the first 3 mo of life. Parental-reported eczema during the first 3 mo of life was significantly lower in the intervention group compared with placebo, 6/50 vs 15/52 (p = 0.035). It reported a relative risk reduction of 58% of parental reported eczema at 3 mo of age being the effect sustained until the age of 2 y, although relative risk reduction decreased with age. A more recent review and meta-analysis reported that a protective effect against allergic disease was only observed if probiotic supplementation were initiated in the prenatal period and continued postnatal, but not if probiotic supplementation was commenced postnatal.81 Some studies have indicated that probiotics during weaning may be effective in the prevention of eczema.140 The cumulative incidence of eczema at 13 mo was 11% (4–17%, 95% CI) and 22% (13–31%, 95% CI) in the probiotic and placebo groups, respectively (p < 0.05).Therefore, further studies are required to determine if postnatal administration of probiotics might be beneficial for prevention of allergic disease.
Use of probiotics in obesity
Of note, weight increments outweighing that of length during the first 6 mo of life were associated with increased risk of obesity at 3 y of age. Changes in weight status in infancy may influence risk of later obesity more than weight status at birth.141 It has been demonstrated that the infant fecal microbial composition is related to maternal weight and weight gain over pregnancy.7,15 In this study, Bacteroidetes and Staphylococcus levels were significantly higher in infants from overweighed mothers during the first six months of life and infants of women with normal weight gain over pregnancy showed higher levels of bifidobacteria than those born to women with excessive weight gain. Thus, higher infant weight correlates with lower numbers of bifidobacteria.7
Interestingly, differences in early fecal microbiota composition have been shown in one study to predict overweight in children early in life. Kalliomäki and colleagues60 have compared over time groups of children and observed that those who became overweight by 7 y of age had lower levels of bifidobacteria and higher levels of Staphylococcus aureus at 6 and 12 mo of age compared with those remaining normal-weight. Overall, these few studies support the hypothesis that bifidobacteria which comprises the predominant microbiota of healthy breast-fed infants142 might affect weight gain through mucosal host-microbe crosstalk, immune regulation, and control of inflammation.143 Clinical evidence was also provided that probiotic consumption lowers the risk of central adiposity over the 6-mo postpartum period.123
Use of probiotics in diarrhea-types
Diarrhea is an obvious target for probiotics and their role in prevention and treatment of infant’s diarrhea is the best studied probiotic effect. In fact, probiotics are considered among the alternatives for management of acute gastroenteritis in children. However, evidence of efficacy is limited to a few strains.144 In addition, specific probiotic strains have been shown to exert a protective effect against acute diarrhea, rotavirus diarrhea, antibiotic-associated diarrhea (AAD).44,145-147 The beneficial effects maybe a combination of different mechanisms as balancing the commensal gut microbiota and modulating of immune response as well as physical exclusion of pathogens by probiotics
Based on recent meta-analysis, most of the tested probiotics, such as Lactobacillus rhamnosus GG (LGG), B. lactis and S. thermophilus and S. boulardii can significantly reduce the incidence of antibiotic-associated diarrhea (AAD) in children.148 The combined risk ratio (RR) of developing AAD was significantly lower with probiotic compared with placebo (RR 0.35, 95% CI 0.19–0.67). In several RCTs it has been shown that probiotics mixed with milk or infant formula or given as an oral supplement are effective in the prevention of acute rotavirus diarrhea reducing and shortening the episodes in children144
Probiotics and prevention of sepsis and NEC
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in low birth weight infants. Delayed enteral feeding, frequent use of antibiotic therapy and altered acquisition of normal digestive microbiota are the most likely factors contributing to the development of NEC in preterm infants. Current evidence indicates that probiotic supplementation significantly reduces all-cause mortality and definite necrotising enterocolitis without significant adverse effects in preterm neonates.149,150 Further, certain probiotics can reduce both the severity and incidence of NEC in preterm neonates while no effect has been observed with other probiotic strains underlining the high strain-specificity of probiotics. Also, several RCTs have reported that enteral probiotic supplementation significantly reduced the incidence of NEC and mortality among premature infants.151 A meta-analysis study concluded that probiotics may be effective in reducing the risk of NEC in low-birth weight infants but more research is needed to clarify their safety, appropriate dose and type of probiotic to be used.150,152 It has also been hypothesized that very preterm infants, who have less microbial diversity in their GIT, may benefit from colonization with probiotics. Cultivation and molecular-based studies have demonstrated quantitative and qualitative changes in ffecalecal samples of infants suffering NEC; however, no causative microorganism has been identified. Depending on the strain(s) administered, these probiotics could potentially induce a similar microbial community to that of the term infant or adult gastrointestinal microbiome.153 These studies support the hypothesis that augmentation of the intestinal microbiome of premature infants with exogenous probiotics (healthy organisms) may favorably alter the microbiota resulting in an improvement in clinical outcomes. However, there is a lack of data regarding the optimal strain(s), dose, time to start and duration of probiotic therapy, as well as the taxonomy and quality of currently available probiotics formulations and products. Further large clinical trials are required to resolve these issues and to support the development of probiotic products.
Probiotics and autism spectrum disorders
On this area, autism is a biologically based disorder of brain development. The reported prevalence of autism spectrum disorders (ASDs) has increased by 5- to 10-fold over the past 20 y. Autism spectrum symptoms may be associated with the high prevalence of neurocognitive impairment in preterm children, particularly extremely preterm children, reflecting a different etiology and associated risk factors such as genetics and environmental factors. However, research is focused in early diagnostics and detection of environmental factors.
Further, as recently reviewed,46,154 alterations in the microbiome have been associated with autism. It has been shown that a significantly higher number of Clostridium spp is present in the fecal samples of autistic children when compared with healthy individuals. Children with autism have often been reported to have gastrointestinal problems that are more frequent and more severe than in children from the general population.155 Then, the potential use of probiotics would be beneficial for these infants. Whether the use of probiotics by children with autism can lead to improvements in behaviors needs to be established in well-controlled trials with sufficient group sizes. Important for these trials is the choice of the bacterial strains, as effects of probiotic bacteria can be highly strain specific.156
Probiotics and infant colic and crying
Infantile colic is a common problem within the first 3 mo of life and causes considerable distress for parents and pediatricians. Despite 40 y of research, its pathogenesis is incompletely understood and treatment remains an open issue.157 The etiology is complex and includes dietary protein hypersensitivity. The administration of probiotic L. reuteri ATCC 55730 has been shown to be effective in the treatment of colic in children.158,159
Conclusions
The first contact between fetus and microorganisms occurs in utero; however, the influence of maternal microbiota composition upon the offspring has not been consistently evaluated. What is known is that infant’s gut microbes’ colonization and establishment as well as its composition play a pivotal role in the metabolic and immunological development of the child. Moreover, infant’s microbiota will unequivocally influence its metabolic capacities and prompt future development of asthma and atopic disease among others. The first microbes are influenced also by other factors as the mode of delivery, environment during birth, prematurity, hygiene measures, maternal perinatal factors and the type of infant feeding. Taken into consideration all the available information it could be concluded that microbial exposition before birth and exposure to microbes during pregnancy and lactation may influence the metabolic and immunologic profiles of the pregnant uterus and the risk of disease development by the offspring later in life (Fig. 1). Therefore, exposure to the adequate microbes early in gestation and neonatal period seems to be highly relevant to the future well-being.
It has been demonstrated that an intimate interrelationship between diet, microbiome and immune system influences human health. Of note, those disorders associated with an aberrant microbiota or inappropriate immune responses are potential targets for probiotic intervention. Clinical efficacy of specific probiotic strains has been demonstrated in some diseases such as diarrhea, irritable bowel syndrome and food allergies. In addition, new evidences support the use of probiotics in the prevention and treatment of a number of diseases including atopic diseases, immune disorders, obesity, and diabetes. We are still far from specific clinical indications for the different conditions susceptible for being prevented or ameliorated with probiotics (Fig. 2). We need further studies adequately powered and comprising specific and uniform types of probiotics before we can reach protocols applicable in the clinical practice.
Acknowledgments
All authors participated in preparation of the manuscript. None of the authors have conflict of interests. This work was supported by and the grants 069/2010 and 071/2011 from Consellería de Sanidad, GVA, Spain and Fun-C-Food CSD2007–00063 from the Consolider-Ingenio program from the Spanish Ministry of Science and Innovation.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/21215
References
- 1.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. MetaHIT Consortium A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56:S5–18. doi: 10.1111/j.1753-4887.1998.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 3.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–4. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 4.Mshvildadze M, Neu J. The infant intestinal microbiome: friend or foe? Early Hum Dev. 2010;86(Suppl 1):67–71. doi: 10.1016/j.earlhumdev.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roger LC, McCartney AL. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology. 2010;156:3317–28. doi: 10.1099/mic.0.041913-0. a. [DOI] [PubMed] [Google Scholar]
- 6.Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology. 2010;156:3329–41. doi: 10.1099/mic.0.043224-0. b. [DOI] [PubMed] [Google Scholar]
- 7.Collado MC, Isolauri E, Laitinen K, Salminen S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr. 2010;92:1023–30. doi: 10.3945/ajcn.2010.29877. [DOI] [PubMed] [Google Scholar]
- 8.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaishampayan PA, Kuehl JV, Froula JL, Morgan JL, Ochman H, Francino MP. Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Biol Evol. 2010;2:53–66. doi: 10.1093/gbe/evp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98:229–38. doi: 10.1111/j.1651-2227.2008.01060.x. [Review] [DOI] [PubMed] [Google Scholar]
- 12.Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51:270–4. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, et al. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008;159:187–93. doi: 10.1016/j.resmic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Satokari R, Grönroos T, Laitinen K, Salminen S, Isolauri E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett Appl Microbiol. 2009;48:8–12. doi: 10.1111/j.1472-765X.2008.02475.x. [DOI] [PubMed] [Google Scholar]
- 15.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894–9. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 16.Grönlund MM, Grześkowiak Ł, Isolauri E, Salminen S. Influence of mother’s intestinal microbiota on gut colonization in the infant. Gut Microbes. 2011;2:227–33. doi: 10.4161/gmic.2.4.16799. [DOI] [PubMed] [Google Scholar]
- 17.Luoto R, Kalliomäki M, Laitinen K, Isolauri E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int J Obes (Lond) 2010;34:1531–7. doi: 10.1038/ijo.2010.50. [DOI] [PubMed] [Google Scholar]
- 18.Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103:1792–9. doi: 10.1017/S0007114509993898. b. [DOI] [PubMed] [Google Scholar]
- 19.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21. doi: 10.1542/peds.2005-2824. a. [DOI] [PubMed] [Google Scholar]
- 20.Penders J, Stobberingh EE, Thijs C, Adams H, Vink C, van Ree R, et al. Molecular fingerprinting of the intestinal microbiota of infants in whom atopic eczema was or was not developing. Clin Exp Allergy. 2006;36:1602–8. doi: 10.1111/j.1365-2222.2006.02599.x. b. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38:321–31. doi: 10.1016/j.clp.2011.03.008. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decker E, Engelmann G, Findeisen A, Gerner P, Laass M, Ney D, et al. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics. 2010;125:e1433–40. doi: 10.1542/peds.2009-2260. [DOI] [PubMed] [Google Scholar]
- 24.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38:629–33. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 25.Marques TM, Wall R, Ross RP, Fitzgerald GF, Ryan CA, Stanton C. Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin Biotechnol. 2010;21:149–56. doi: 10.1016/j.copbio.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, et al. INFABIO team Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology. 2011;157:1385–92. doi: 10.1099/mic.0.042143-0. [DOI] [PubMed] [Google Scholar]
- 27.Coppa GV, Zampini L, Galeazzi T, Gabrielli O. Prebiotics in human milk: a review. Dig Liver Dis. 2006;38(Suppl 2):S291–4. doi: 10.1016/S1590-8658(07)60013-9. [Review] [DOI] [PubMed] [Google Scholar]
- 28.Martín R, Heilig HG, Zoetendal EG, Jiménez E, Fernández L, Smidt H, et al. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res Microbiol. 2007;158:31–7. doi: 10.1016/j.resmic.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Martín R, Jiménez E, Heilig H, Fernández L, Marín ML, Zoetendal EG, et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl Environ Microbiol. 2009;75:965–9. doi: 10.1128/AEM.02063-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collado MC, Delgado S, Maldonado A, Rodríguez JM. Assessment of the bacterial diversity of breast milk of healthy women by quantitative real-time PCR. Lett Appl Microbiol. 2009;48:523–8. doi: 10.1111/j.1472-765X.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 31.Makino H, Kushiro A, Ishikawa E, Muylaert D, Kubota H, Sakai T, et al. Transmission of intestinal Bifidobacterium longum subsp. longum strains from mother to infant, determined by multilocus sequencing typing and amplified fragment length polymorphism. Appl Environ Microbiol. 2011;77:6788–93. doi: 10.1128/AEM.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi H, Mikami K, Nishino R, Matsuoka T, Kimura M, Koga Y. Comparative analysis of the properties of bifidobacterial isolates from fecal samples of mother-infant pairs. J Pediatr Gastroenterol Nutr. 2010;51:653–60. doi: 10.1097/MPG.0b013e3181f0e032. [DOI] [PubMed] [Google Scholar]
- 33.Martín V, Maldonado-Barragán A, Moles L, Rodriguez-Baños M, Campo RD, Fernández L, et al. Sharing of bacterial strains between breast milk and infant feces. J Hum Lact. 2012;28:36–44. doi: 10.1177/0890334411424729. [DOI] [PubMed] [Google Scholar]
- 34.Grönlund MM, Gueimonde M, Laitinen K, Kociubinski G, Grönroos T, Salminen S, et al. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin Exp Allergy. 2007;37:1764–72. doi: 10.1111/j.1365-2222.2007.02849.x. [DOI] [PubMed] [Google Scholar]
- 35.Adlerberth I, Strachan DP, Matricardi PM, Ahrné S, Orfei L, Aberg N, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007;120:343–50. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Dicksved J, Flöistrup H, Bergström A, Rosenquist M, Pershagen G, Scheynius A, et al. Molecular fingerprinting of the fecal microbiota of children raised according to different lifestyles. Appl Environ Microbiol. 2007;73:2284–9. doi: 10.1128/AEM.02223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. and Other Members of the INFABIO Team Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010;51:77–84. doi: 10.1097/MPG.0b013e3181d1b11e. [DOI] [PubMed] [Google Scholar]
- 38.Sepp E, Julge K, Vasar M, Naaber P, Björksten B, Mikelsaar M. Intestinal microflora of Estonian and Swedish infants. Acta Paediatr. 1997;86:956–61. doi: 10.1111/j.1651-2227.1997.tb15178.x. [DOI] [PubMed] [Google Scholar]
- 39.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027–33. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grześkowiak L, Collado MC, Mangani C, Maleta K, Laitinen K, Ashorn P, et al. Distinct gut microbiota in southeastern African and northern European infants. J Pediatr Gastroenterol Nutr. 2012;54:812–6. doi: 10.1097/MPG.0b013e318249039c. [DOI] [PubMed] [Google Scholar]
- 41.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savino F, Roana J, Mandras N, Tarasco V, Locatelli E, Tullio V. Faecal microbiota in breast-fed infants after antibiotic therapy. Acta Paediatr. 2011;100:75–8. doi: 10.1111/j.1651-2227.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- 43.Engelbrektson A, Korzenik JR, Pittler A, Sanders ME, Klaenhammer TR, Leyer G, et al. Probiotics to minimize the disruption of faecal microbiota in healthy subjects undergoing antibiotic therapy. J Med Microbiol. 2009;58:663–70. doi: 10.1099/jmm.0.47615-0. [DOI] [PubMed] [Google Scholar]
- 44.Johnston BC, Goldenberg JZ, Vandvik PO, Sun X, Guyatt GH. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;11:CD004827. doi: 10.1002/14651858.CD004827.pub3. [DOI] [PubMed] [Google Scholar]
- 45.Jacquot A, Neveu D, Aujoulat F, Mercier G, Marchandin H, Jumas-Bilak E, et al. Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J Pediatr. 2011;158:390–6. doi: 10.1016/j.jpeds.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 47.Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol. 2011;17:557–66. doi: 10.3748/wjg.v17.i5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–7. doi: 10.1136/gut.2006.100164. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–36. doi: 10.1111/j.1398-9995.2007.01462.x. b. [DOI] [PubMed] [Google Scholar]
- 50.Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–34. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 51.Sjögren YM, Jenmalm MC, Böttcher MF, Björkstén B, Sverremark-Ekström E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009;39:518–26. doi: 10.1111/j.1365-2222.2008.03156.x. [DOI] [PubMed] [Google Scholar]
- 52.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–8. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–5. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 56.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 57.Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 58.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008;32:1720–4. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 60.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 61.Delzenne NM, Cani PD. Gut microbiota and the pathogenesis of insulin resistance. Curr Diab Rep. 2011;11:154–9. doi: 10.1007/s11892-011-0191-1. [DOI] [PubMed] [Google Scholar]
- 62.Neu J, Lorca G, Kingma SD, Triplett EW. The intestinal microbiome: relationship to type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:563–71. doi: 10.1016/j.ecl.2010.05.008. [Review] [DOI] [PubMed] [Google Scholar]
- 63.Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A, Extremely Low Gestational Age Newborns (ELGAN) Study Investigators Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198:110–.e1-7. doi: 10.1016/j.ajog.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 64.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pettker CM, Buhimschi IA, Magloire LK, Sfakianaki AK, Hamar BD, Buhimschi CS. Value of placental microbial evaluation in diagnosing intra-amniotic infection. Obstet Gynecol. 2007;109:739–49. doi: 10.1097/01.AOG.0000255663.47512.23. [DOI] [PubMed] [Google Scholar]
- 66.Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156:20–5. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8:435–54. doi: 10.1586/eri.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–13. doi: 10.1016/S0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 70.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 71.Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for the treatment of eczema: a systematic review. Clin Exp Allergy. 2009;39:1117–27. doi: 10.1111/j.1365-2222.2009.03305.x. [DOI] [PubMed] [Google Scholar]
- 72.Boyle RJ, Ismail IH, Kivivuori S, Licciardi PV, Robins-Browne RM, Mah LJ, et al. Lactobacillus GG treatment during pregnancy for the prevention of eczema: a randomized controlled trial. Allergy. 2011;66:509–16. doi: 10.1111/j.1398-9995.2010.02507.x. [DOI] [PubMed] [Google Scholar]
- 73.Laitinen K, Poussa T, Isolauri E, Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr. 2009;101:1679–87. doi: 10.1017/S0007114508111461. [DOI] [PubMed] [Google Scholar]
- 74.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 75.Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res. 2011;69:465–72. doi: 10.1203/PDR.0b013e318217638a. [DOI] [PubMed] [Google Scholar]
- 76.Azad MB, Kozyrskyj AL. Perinatal programming of asthma: the role of gut microbiota. Clin Dev Immunol. 2012;2012:932072. doi: 10.1155/2012/932072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conrad ML, Ferstl R, Teich R, Brand S, Blümer N, Yildirim AO, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med. 2009;206:2869–77. doi: 10.1084/jem.20090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J, et al. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J. 2008;32:603–11. doi: 10.1183/09031936.00033707. [DOI] [PubMed] [Google Scholar]
- 79.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861–8. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 80.Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–71. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 81.Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr. 2010;22:626–34. doi: 10.1097/MOP.0b013e32833d9728. [DOI] [PubMed] [Google Scholar]
- 82.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 83.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. MetaHIT Consortium Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murgas Torrazza R, Neu J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J Perinatol. 2011;31(Suppl 1):S29–34. doi: 10.1038/jp.2010.172. [Review] [DOI] [PubMed] [Google Scholar]
- 85.Aaltonen J, Ojala T, Laitinen K, Poussa T, Ozanne S, Isolauri E. Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming: a prospective randomized controlled study. Eur J Clin Nutr. 2011;65:10–9. doi: 10.1038/ejcn.2010.225. [DOI] [PubMed] [Google Scholar]
- 86.Petherick A. Development: Mother’s milk: A rich opportunity. Nature. 2010;468:S5–7. doi: 10.1038/468S5a. [DOI] [PubMed] [Google Scholar]
- 87.Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr. 2010;156(Suppl):S3–7. doi: 10.1016/j.jpeds.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 88.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84:1043–54. doi: 10.1093/ajcn/84.5.1043. [Review] [DOI] [PubMed] [Google Scholar]
- 89.Owen CG, Whincup PH, Kaye SJ, Martin RM, Davey Smith G, Cook DG, et al. Does initial breastfeeding lead to lower blood cholesterol in adult life? A quantitative review of the evidence. Am J Clin Nutr. 2008;88:305–14. doi: 10.1093/ajcn/88.2.305. [DOI] [PubMed] [Google Scholar]
- 90.Owen CG, Whincup PH, Cook DG. Breast-feeding and cardiovascular risk factors and outcomes in later life: evidence from epidemiological studies. Proc Nutr Soc. 2011;70:478–84. doi: 10.1017/S0029665111000590. [DOI] [PubMed] [Google Scholar]
- 91.Owen CG, Martin RM, Whincup PH, Davey-Smith G, Gillman MW, Cook DG. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr. 2005;82:1298–307. doi: 10.1093/ajcn/82.6.1298. [Review] [DOI] [PubMed] [Google Scholar]
- 92.Brandtzaeg P. ‘ABC’ of mucosal immunology. Nestle Nutr Workshop Ser Pediatr Program. 2009;64(discussion 38-43, 251-7):23–38. doi: 10.1159/000235781. [DOI] [PubMed] [Google Scholar]
- 93.Snijders BE, Stelma FF, Reijmerink NE, Thijs C, van der Steege G, Damoiseaux JG, et al. CD14 polymorphisms in mother and infant, soluble CD14 in breast milk and atopy development in the infant (KOALA Study) Pediatr Allergy Immunol. 2010;21:541–9. doi: 10.1111/j.1399-3038.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 94.Hunt KM, Foster JA, Forney LJ, Schütte UM, Beck DL, Abdo Z, et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One. 2011;6:e21313. doi: 10.1371/journal.pone.0021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoppu U, Rinne M, Lampi AM, Isolauri E. Breast milk fatty acid composition is associated with development of atopic dermatitis in the infant. J Pediatr Gastroenterol Nutr. 2005;41:335–8. doi: 10.1097/01.mpg.0000168992.44428.fa. [DOI] [PubMed] [Google Scholar]
- 96.Hoppu U, Isolauri E, Laakso P, Matomäki J, Laitinen K. Probiotics and dietary counselling targeting maternal dietary fat intake modifies breast milk fatty acids and cytokines. Eur J Nutr. 2012;51:211–9. doi: 10.1007/s00394-011-0209-0. [DOI] [PubMed] [Google Scholar]
- 97.Le Huërou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23:23–36. doi: 10.1017/S0954422410000065. [Review] [DOI] [PubMed] [Google Scholar]
- 98.Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572–81. doi: 10.1078/0171-2985-00094. [DOI] [PubMed] [Google Scholar]
- 99.Pennisi E. Microbiology. Bacteria are picky about their homes on human skin. Science. 2008;320:1001. doi: 10.1126/science.320.5879.1001. [DOI] [PubMed] [Google Scholar]
- 100.Perez PF, Doré J, Leclerc M, Levenez F, Benyacoub J, Serrant P, et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119:e724–32. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- 101.Macpherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann N Y Acad Sci. 2004;1029:36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- 102.Donnet-Hughes A, Perez PF, Doré J, Leclerc M, Levenez F, Benyacoub J, et al. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc Nutr Soc. 2010;69:407–15. doi: 10.1017/S0029665110001898. [DOI] [PubMed] [Google Scholar]
- 103.Nickel T, Emslander I, Sisic Z, David R, Schmaderer C, Marx N, et al. Modulation of dendritic cells and toll-like receptors by marathon running. Eur J Appl Physiol. 2012;112:1699–708. doi: 10.1007/s00421-011-2140-8. [DOI] [PubMed] [Google Scholar]
- 104.Winder NR, Krishnaveni GV, Veena SR, Hill JC, Karat CL, Thornburg KL, et al. Mother’s lifetime nutrition and the size, shape and efficiency of the placenta. Placenta. 2011;32:806–10. doi: 10.1016/j.placenta.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.FAO/WHO. Guidelines for the evaluation of probiotics in food. Food and Agricultural Organization of the United Nations and World Health Organization. Working Group Report 2002.
- 106.Gueimonde M, Sakata S, Kalliomäki M, Isolauri E, Benno Y, Salminen S. Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J Pediatr Gastroenterol Nutr. 2006;42:166–70. doi: 10.1097/01.mpg.0000189346.25172.fd. [DOI] [PubMed] [Google Scholar]
- 107.Lahtinen SJ, Boyle RJ, Kivivuori S, Oppedisano F, Smith KR, Robins-Browne R, et al. Prenatal probiotic administration can influence Bifidobacterium microbiota development in infants at high risk of allergy. J Allergy Clin Immunol. 2009;123:499–501. doi: 10.1016/j.jaci.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 108.Schultz M, Göttl C, Young RJ, Iwen P, Vanderhoof JA. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38:293–7. doi: 10.1097/00005176-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 109.Ismail IH, Oppedisano F, Joseph SJ, Boyle RJ, Robins-Browne RM, Tang ML. Prenatal administration of Lactobacillus rhamnosus has no effect on the diversity of the early infant gut microbiota. Pediatr Allergy Immunol. 2012;23:255–8. doi: 10.1111/j.1399-3038.2011.01239.x. [DOI] [PubMed] [Google Scholar]
- 110.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. ISAAC Phase Three Study Group Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [Erratum in: Lancet 2007; 370] [9593] [DOI] [PubMed] [Google Scholar]
- 111.Isolauri E. Dietary modification of atopic disease: Use of probiotics in the prevention of atopic dermatitis. Curr Allergy Asthma Rep. 2004;4:270–5. doi: 10.1007/s11882-004-0070-9. [Review] [DOI] [PubMed] [Google Scholar]
- 112.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 113.Kalliomäki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1019–21. doi: 10.1016/j.jaci.2006.12.608. [DOI] [PubMed] [Google Scholar]
- 114.Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58:481–509, xii. doi: 10.1016/j.pcl.2011.02.002. [xii.] [DOI] [PubMed] [Google Scholar]
- 115.Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116–121.e11. doi: 10.1016/j.jaci.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 116.Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–8. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 117.Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–91. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 118.Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics. 2008;121:e850–6. doi: 10.1542/peds.2007-1492. [DOI] [PubMed] [Google Scholar]
- 119.Doege K, Grajecki D, Zyriax BC, Detinkina E, Zu Eulenburg C, Buhling KJ. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood--a meta-analysis. Br J Nutr. 2012;107:1–6. doi: 10.1017/S0007114511003400. [DOI] [PubMed] [Google Scholar]
- 120.Dotterud CK, Storrø O, Johnsen R, Oien T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol. 2010;163:616–23. doi: 10.1111/j.1365-2133.2010.09889.x. [DOI] [PubMed] [Google Scholar]
- 121.Rose MA, Stieglitz F, Köksal A, Schubert R, Schulze J, Zielen S. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin Exp Allergy. 2010;40:1398–405. doi: 10.1111/j.1365-2222.2010.03560.x. [DOI] [PubMed] [Google Scholar]
- 122.Laitinen K, Collado MC, Isolauri E. Early nutritional environment: focus on health effects of microbiota and probiotics. Benef Microbes. 2010;1:383–90. doi: 10.3920/BM2010.0045. [DOI] [PubMed] [Google Scholar]
- 123.Ilmonen J, Isolauri E, Poussa T, Laitinen K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clin Nutr. 2011;30:156–64. doi: 10.1016/j.clnu.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 124.Othman M, Neilson JP, Alfirevic Z. Probiotics for preventing preterm labour. Cochrane Database Syst Rev. 2007:CD005941. doi: 10.1002/14651858.CD005941.pub2. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Falagas ME, Betsi GI, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect. 2007;13:657–64. doi: 10.1111/j.1469-0691.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 126.Krauss-Silva L, Moreira ME, Alves MB, Braga A, Camacho KG, Batista MR, et al. A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: preliminary results. Trials. 2011;12:239. doi: 10.1186/1745-6215-12-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hantoushzadeh S, Golshahi F, Javadian P, Khazardoost S, Aram S, Hashemi S, et al. Comparative efficacy of probiotic yoghurt and clindamycin in treatment of bacterial vaginosis in pregnant women: A randomized clinical trial. J Matern Fetal Neonatal Med. 2012;25:1021–4. doi: 10.3109/14767058.2011.614654. [DOI] [PubMed] [Google Scholar]
- 128.Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Diamond B, Huerta PT, Tracey K, Volpe BT. It takes guts to grow a brain: Increasing evidence of the important role of the intestinal microflora in neuro- and immune-modulatory functions during development and adulthood. Bioessays. 2011;33:588–91. doi: 10.1002/bies.201100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–56. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 132.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 133.Rautava S, Ruuskanen O, Ouwehand A, Salminen S, Isolauri E. The hygiene hypothesis of atopic disease--an extended version. J Pediatr Gastroenterol Nutr. 2004;38:378–88. doi: 10.1097/00005176-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 134.Grześkowiak L, Grönlund MM, Beckmann C, Salminen S, von Berg A, Isolauri E. The impact of perinatal probiotic intervention on gut microbiota: double-blind placebo-controlled trials in Finland and Germany. Anaerobe. 2012;18:7–13. doi: 10.1016/j.anaerobe.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 135.Ouwehand AC, Isolauri E, He F, Hashimoto H, Benno Y, Salminen S. Differences in Bifidobacterium flora composition in allergic and healthy infants. J Allergy Clin Immunol. 2001;108:144–5. doi: 10.1067/mai.2001.115754. [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3:944–54. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007:CD006475. doi: 10.1002/14651858.CD006474.pub2. [DOI] [PubMed] [Google Scholar]
- 138.Vliagoftis H, Kouranos VD, Betsi GI, Falagas ME. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570–9. doi: 10.1016/S1081-1206(10)60219-0. [Review] [DOI] [PubMed] [Google Scholar]
- 139.Niers L, Martín R, Rijkers G, Sengers F, Timmerman H, van Uden N, et al. The effects of selected probiotic strains on the development of eczema (the PandA study) Allergy. 2009;64:1349–58. doi: 10.1111/j.1398-9995.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 140.West CE, Hammarström ML, Hernell O. Probiotics during weaning reduce the incidence of eczema. Pediatr Allergy Immunol. 2009;20:430–7. doi: 10.1111/j.1399-3038.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 141.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177–83. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 143.LeBouder E, Rey-Nores JE, Raby AC, Affolter M, Vidal K, Thornton CA, et al. Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk. J Immunol. 2006;176:3742–52. doi: 10.4049/jimmunol.176.6.3742. [DOI] [PubMed] [Google Scholar]
- 144.Guarino A, Lo Vecchio A, Canani RB. Probiotics as prevention and treatment for diarrhea. Curr Opin Gastroenterol. 2009;25:18–23. doi: 10.1097/MOG.0b013e32831b4455. [Review] [DOI] [PubMed] [Google Scholar]
- 145.Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010:CD003048. doi: 10.1002/14651858.CD003048.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thomas DW, Greer FR, American Academy of Pediatrics Committee on Nutrition. American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217–31. doi: 10.1542/peds.2010-2548. [Review] [DOI] [PubMed] [Google Scholar]
- 147.Preidis GA, Hill C, Guerrant RL, Ramakrishna BS, Tannock GW, Versalovic J. Probiotics, enteric and diarrheal diseases, and global health. Gastroenterology. 2011;140:8–14. doi: 10.1053/j.gastro.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kale-Pradhan PB, Jassal HK, Wilhelm SM. Role of Lactobacillus in the prevention of antibiotic-associated diarrhea: a meta-analysis. Pharmacotherapy. 2010;30:119–26. doi: 10.1592/phco.30.2.119. [DOI] [PubMed] [Google Scholar]
- 149.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011:CD005496. doi: 10.1002/14651858.CD005496.pub3. [DOI] [PubMed] [Google Scholar]
- 150.Deshpande GC, Rao SC, Keil AD, Patole SK. Evidence-based guidelines for use of probiotics in preterm neonates. BMC Med. 2011;9:92. doi: 10.1186/1741-7015-9-92. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ganguli K, Walker WA. Probiotics in the prevention of necrotizing enterocolitis. J Clin Gastroenterol. 2011;45(Suppl):S133–8. doi: 10.1097/MCG.0b013e318228b799. [DOI] [PubMed] [Google Scholar]
- 152.Mihatsch WA. What is the power of evidence recommending routine probiotics for necrotizing enterocolitis prevention in preterm infants? Curr Opin Clin Nutr Metab Care. 2011;14:302–6. doi: 10.1097/MCO.0b013e3283454e78. [Review] [DOI] [PubMed] [Google Scholar]
- 153.Neu J, Caicedo R. Probiotics: protecting the intestinal ecosystem? J Pediatr. 2005;147:143–6. doi: 10.1016/j.jpeds.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 154.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–53. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 155.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Critchfield JW, van Hemert S, Ash M, Mulder L, Ashwood P. The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract. 2011;2011:161358. doi: 10.1155/2011/161358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Critch J. Infantile colic: Is there a role for dietary interventions? Paediatr Child Health. 2011;16:47–9. [PMC free article] [PubMed] [Google Scholar]
- 158.Savino F, Cordisco L, Tarasco V, Palumeri E, Calabrese R, Oggero R, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526–33. doi: 10.1542/peds.2010-0433. [DOI] [PubMed] [Google Scholar]
- 159.Savino F, Tarasco V. New treatments for infant colic. Curr Opin Pediatr. 2010;22:791–7. doi: 10.1097/MOP.0b013e32833fac24. [Review] [DOI] [PubMed] [Google Scholar]