Abstract
Multiple endocrine neoplasia type 1 (MEN1) is characterized by the combined occurrence of pituitary, pancreatic and parathyroid tumors showing loss of heterozygosity in the putative tumor suppressor gene MEN1. This gene encodes the protein menin, the overexpression of which inhibits cell proliferation in vitro. In this study, we conducted a preclinical evaluation of MEN1 gene therapy in pituitary tumors of Men1+/− mice, using a recombinant non-replicating adenoviral serotype 5 vector that contained the murine Men1 cDNA under control of a cytomegalovirus promoter (Men1.rAd5). Pituitary tumours in 55 Men1+/− female mice received a transauricular, intratumoral injection of Men1.rAd5 or control treatments, followed by 5-bromo-2-deoxyuridine (BrdU) in drinking water for four weeks before magnetic resonance imaging (MRI) and immunohistochemical analysis. Immediate procedure-related and four-week mortalities were similar in all groups, indicating that the adenoviral gene therapy was not associated with a higher mortality. Menin expression was higher in the Men1.rAd5-treated mice when compared to other groups. Daily proliferation rates assessed by BrdU incorporation were reduced significantly in Men1.rAd5-injected tumors relative to control treated tumors. In contrast, apoptotic rates, immune T cell response and tumor volumes remained similar in all groups. Our findings establish that MEN1 gene replacement therapy can generate menin expression in pituitary tumors, and significantly reduce tumor cell proliferation.
Keywords: Neuroendocrine tumours (NETs), adenoviral vector, tumour suppressor, apoptosis, immune reaction, Men1
Introduction
Multiple endocrine neoplasia type 1 (MEN1), which is an autosomal dominant disorder, is characterised by the combined occurrence of tumours of the parathyroids, pancreatic islets (e.g. gastrinomas and insulinomas) and anterior pituitary (e.g. prolactinomas and somatotrophinomas) (1). In addition, some patients with MEN1 develop adrenocortical tumours, foregut carcinoids, lipomas, meningiomas, facial angiofibromas and collagenomas (1). Treatment for pancreatic islet cell tumours, anterior pituitary adenomas and foregut carcinoids, which comprise the neuroendocrine tumours (NETs) in MEN1 patients, is more difficult than for those same tumours in non-MEN1 patients, for several reasons (1-3). First, the MEN1 tumours, with the exception of anterior pituitary adenomas, are multiple. For example, in MEN1 patients, unlike non-MEN1 patients, multiple submucosal duodenal and pancreatic gastrinomas develop, thereby reducing the success rates for surgery, such that only ~15% of MEN1 patients, compared to ~45% of non-MEN1 patients, are free of disease immediately after surgery, and at 5 years this number has decreased to ~5% in MEN1 patients, compared to ~40% in non-MEN1 patients (1, 4, 5). Second, NET metastatic disease, which may be occult, is more prevalent in MEN1 patients than in patients with sporadic endocrine tumours; for example metastases may be present at the time of presentation in up to 50% of patients with MEN1-associated insulinomas, whereas the lifetime risk of metastatic insulinoma in non-MEN1 patients is <10% (1, 5). Third, the majority (~80%) of NETs have low proliferation rates, with a Ki-67 index <2% (6, 7), and as such are not responsive to chemotherapy or radiotherapy. Fourth, NETs in MEN1 are larger, more aggressive, and resistant to treatment (2, 3). For example, ~85% of anterior pituitary adenomas in MEN1 patients, as opposed to 64% in non-MEN1 patients (8), are macroadenomas; ~30% of anterior pituitary adenomas in MEN1 patients have invaded surrounding tissue (Hardy classification grades III and IV), compared to 10% in non-MEN1 patients (9); and >45% of anterior pituitary adenomas in MEN1 patients had persistent hormonal over-secretion following appropriate medical, surgical and radiotherapy treatments compared to between 10% and 40% in non-MEN1 patients (10-12). Thus, there is a clinical need for better and alternative treatments for NETs in MEN1 patients.
One possible new treatment could be MEN1 gene replacement therapy as the majority (>90%) of MEN1 NETs have loss of heterozygosity (LOH) for the MEN1 allele located on chromosome 11q13, consistent with a tumour suppressor role for the encoded 610 amino acid protein, menin (13, 14), which is predominantly a nuclear protein that interacts with proteins that are involved in transcription regulation, genome stability and cell division (15). For example, menin interacts with JunD to inhibit its transcriptional activity and thereby cell proliferation; and menin interaction with the mixed lineage leukaemia protein 1 (MLL1), which is a histone H3 lysine 4 methyl transferase that functions as an oncogenic cofactor to up-regulate gene transcription, promotes MLL1-fusion-protein-induced leukaemogenesis (16). MEN1 tumours have been shown to have germline and somatic mutations, consistent with the Knudson two-hit hypothesis, and to lack menin expression (15). Furthermore, in vitro studies in which menin was expressed by the use of recombinant plasmid, adenoviral or retroviral vectors, in either menin-null mouse embryonic fibroblasts (MEFs), RAS-transformed NIH3T3 MEFs, or rat insulinoma cell lines, have demonstrated that menin-overexpression can result in decreased cell proliferation and increased apoptosis (17-21). One study, in which RAS-transformed NIH3T3 MEFs that over-expressed menin were injected into athymic nude mice, demonstrated that menin reduced tumour growth (17). We, therefore, explored the feasibility of in vivo Men1 gene replacement therapy in a mouse model for MEN1, using a recombinant replication-deficient adenovirus serotype 5 (rAd5). We chose rAd5 because of its ability to infect dividing and non-dividing cells and its high efficiency of transduction and transient gene expression (22, 23); these qualities are well suited for cancer gene therapy where the goal is to achieve short-term expression that destroys abnormal tissue, that consists of dividing and non-dividing cells, which is particularly important for endocrine tumours which are slow growing and have a low rate of cell division (24). Moreover, adenoviral vectors: remain epichromosomal and therefore do not cause insertional mutagenesis; can accommodate large quantities of foreign DNA through deletions of the viral genome; replicate efficiently in vitro and can be produced in high titres; are the most commonly used vectors in cancer gene therapy; continue to show increasing promise as gene therapy delivery vehicles; and have been approved for clinical use in Phase I and II trials of various cancers including malignant glioma, and those of lung, prostate and the head and neck (23, 25, 26). These phase I and II trials have established the safety of adenoviral gene therapy, and some trials have also demonstrated efficacy, which may be limited, in the treatment of prostate, lung and other solid tumours (25-28).
We used an established mouse model for MEN1, generated by deletion of exons 1 and 2 of the mouse Men1 gene, and in which Men1+/− mice aged 12-21 months developed tumours of the parathyroids, pancreatic islets, anterior pituitary, adrenal cortex, ovaries and testes with hypercalcaemia, hypophosphataemia, and hypercorticosteronaemia. (29). Moreover, these tumours had loss of menin expression, consistent with a tumour suppressor role for the Men1 gene, and the model, with the exception of the occurrence of the gonadal tumours and the absence of gastrinomas, is representative for the disorder in man (29). To explore the feasibility of in vivo Men1 gene replacement in this MEN1 mouse model, we initially treated the anterior pituitary adenomas, as opposed to the pancreatic NETs, as they are well localised; usually occur as a single tumour mass as opposed to multiple tumours in the pancreas; and are accessible to direct injection of a therapeutic vector that has a good chance of reaching the majority of cells, and even partial efficacy can be of benefit and assessed.
Materials and Methods
Recombinant adenoviral vectors
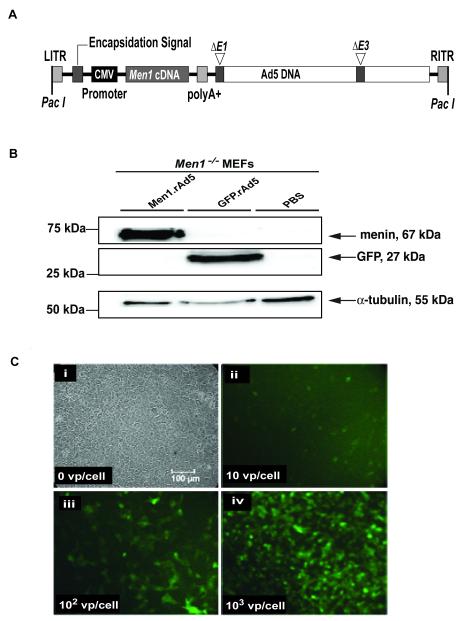
The replication-deficient adenovirus serotype, which has the E1 and E3 regions deleted (Ad5ΔE1/ΔE3) was used to generate the construct expressing the murine Men1 cDNA (Men1.rAd5), as follows (AdEasy Vector System, QBiogene, USA). An expression cassette, containing the murine Men1 cDNA under control of the cytomegalovirus (CMV) promoter, was assembled into a transfer plasmid through multiple cloning steps. Transient expression of this plasmid in human embryonic kidney (HEK293) cells showed that it was able to express menin (data not shown). Therefore, the expression cassette was transferred to an E1/E3-deleted Ad5 genome, by homologous recombination in E. coli (Figure 1A). HEK293 cells were transfected with the recombinant adenoviral DNA (Men1.rAd5) to produce viral plaques. Viral particles were further amplified and purified by caesium chloride, producing 8.6×1011 vp/ml. A commercially available recombinant E1/E3-deleted green fluorescent protein (GFP)-expressing adenovirus (GFP.rAd5), under the control of the CMV promoter, was used as a control (AdenoExpress, Qbiogene, USA).
Figure 1. Men1 recombinant adenoviral vector.
A, Genomic organization of the Men1 recombinant vector generated by using the replication-deficient adenovirus serotype 5 (Men1.rAd5). The adenovirus has the E1 and E3 regions deleted, thereby making it replication deficient (Ad5ΔE1/ΔE3). The expression cassette consisting of 1833bp Men1 cDNA, CMV promoter and polyA+ sequence, was inserted into the E1 region of the adenoviral genome. The locations of the left and right inverted terminal repeats (LITR and RITR, respectively) together with the PacI restriction endonuclease sites are shown. A recombinant adenoviral vector expressing green fluorescent protein (GFP.rAd5) (not shown) was used as a control. B, Detection of menin and GFP expression by Western blot analysis of lysates from Men1−/− MEFs, following 48-hours of infection with either the Men1.rAd5 or GFP.rAd5 vectors or treatment with control PBS. Menin (67kDa) and GFP (27kDa) protein expression were detected only in lysates from Men1.rAd5 or GFP.rAd5-treated MEFs, respectively. Detection of α-tubulin (55kDa) expression was used as a positive control. C, Adenovirus infectivity test. Men1+/− pituitary tumour cells in culture were infected with GFP.rAd5. (i) Uninfected cells viewed by phase contrast microscopy; (ii-iv) Cells infected with 10, 102 and 103 viral particles (vp) per cultured cell, respectively, and observed by fluorescence microscopy, 24-hours after infection.
Animals
Mice were kept in accordance with UK Home Office welfare guidelines and project license restrictions. Heterozygous mice deleted for exons 1 and 2 of the Men1 gene develop MEN1-associated tumours (29), and Men1+/− mice on C57BL/6 and 129S6/SvEv backgrounds have been found to develop similar MEN1-associated tumours (29). However, pituitary adenomas develop predominantly in female Men1+/− mice (29), and to facilitate a reduction in the number of mice required for the study, only congenic female Men1+/− mice aged between 18 and 21 months, on C57BL/6 (n=97) or 129S6/SvEv (n=53) backgrounds, were used. Mice were fed a standard diet (RMI expanded diet, Special Diet Services, Ltd, UK) and provided with water ad libitum, which for four-weeks, prior to sacrifice, contained 5-bromo-2-deoxyuridine (BrdU), 1mg/ml, to allow for an assessment of cell proliferation (30).
Cell cultures
HEK293 cells were obtained from the American Type Culture Collection (ATCC), that had characterized the cell lines by short tandem repeat (STR) profiling, cell morphology, karyotyping and cytochrome C oxidase I (COI) assay (http://www.lgcstandards-atcc.org/ATCCCulturesandProducts/CellBiology/tabid/980/Default.aspx). HEK293 cells, MEFs derived from Men1−/− embryos aged 10.5 days post-coitum (dpc) and primary cell cultures of pituitary tumours from Men1+/− mice were maintained in culture, in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 5-10% foetal calf serum (FCS) (Invitrogen) and incubated at 37°C in a humidified 5% CO2 atmosphere, as previously described (31-34). Men1−/− MEFs were infected with 8.5 × 109 viral particles of Men1.rAd5, or GFP.rAd5 (35) or 1ml of phosphate buffered saline (PBS) and 48-hours later, DNA, RNA and protein extracted (32, 36). Pituitary tumour primary cell cultures were infected with different known amounts of GFP.rAd5 to obtain multiplicities of infection (MOI) of 0, 1 (not shown), 10, 102, 103 and 104 (not shown) viral particles (VP) per cultured cell. GFP expression was assessed using fluorescent microscopy using an Eclipse E400 microscope (Nikon) as described (37).
Analysis of DNA and proteins
Men1 genotypes were determined using DNA and PCR primers (29, 38), and the vector construct was sequenced using methods previously described (39). Western blot analysis to assess GFP and menin expression was performed using rabbit antibodies (anti-menin (AbCam, UK), anti-GFP (Santa Cruz Biotechnology, Inc., USA) and anti-α-tubulin (AbCam)), and a secondary antibody (horseradish peroxidase (HRP)-conjugated goat anti-rabbit (Bio-Rad Laboratories, USA), as previously described (29).
Delivery of adenoviral vectors to pituitary tumours
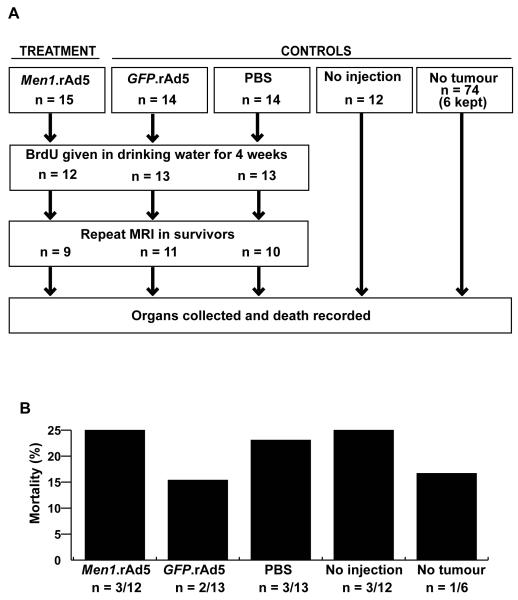
Anaesthetised female Men1+/− mice with pituitary tumours, identified by cranial magnetic resonance imaging (MRI, see below), were randomised to receive a 20μl transauricular, intra-tumoural injection of Men1.rAd5 (5 × 1010 viral particles), GFP.rAd5 (5 × 1010 viral particles), or control PBS (Figure 2). Accurate targeting of tumours was confirmed, in a subset of treated female Men1+/− mice, by MRI immediately after the transauricular injection (Figure 3A-C), which was delivered using a Hamilton syringe and 30-gauge needle (40). To eliminate any bias in the analysis and interpretation of the results, the investigators were blinded to the contents of the vials containing the solutions for injections. A control non-injected group of female Men1+/− mice was included in the study (Figure 2). Four-weeks following administration of treatment, the mice had another MRI scan, were sacrificed, a necropsy undertaken and tumours and tissues harvested.
Figure 2. Details of the treatment and control arms of the trial.
A, Men1+/− female mice aged ~18 months with pituitary adenomas were treated with a transauricular, intra-tumoural injection of either Men1.rAd5, GFP.rAd5 (control for adenovirus), or PBS (control for injection). Additional control arms included an uninjected group of Men1+/− mice with pituitary adenomas (control for disease progression) and a group of Men1+/− mice who had no tumours, on MRI or subsequent necropsy, and were not injected (control for age-related mortality). B, Mortality at four-weeks post-injection was similar in the treatment and control groups. The weights of the Men1+/− female mice in the different groups were not significantly different (data not shown) and none of the Men1+/− mice had dysmorphic features.
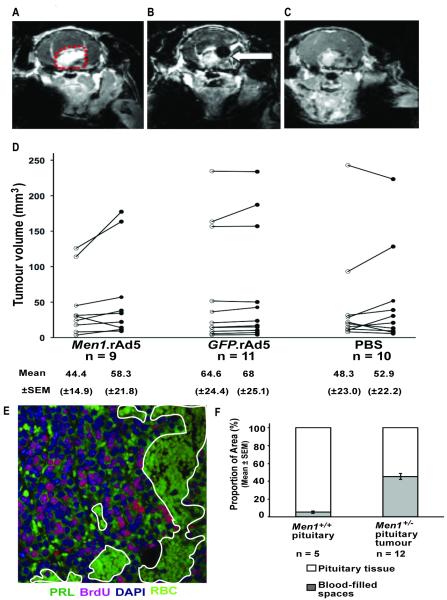
Figure 3. Pituitary tumour imaging and volumetric measurements.
A, Coronal MRI with gadodiamide enhancement, demonstrating a pituitary tumour (outlined in red dots). B, MRI section of the same tumour, immediately after delivery of an intra-tumoural injection; the black area in the brain, shown by the arrow, corresponds to the injected bolus. C, MRI section of the same tumour 24-hours after injection showing complete absorption of the bolus. D, MRI was used to assess tumour volumes, pre-treatment and four-weeks after intra-tumoural injection. Data are shown for each tumour; pre-treatment (open circles), and post-treatment (filled circles). E, Assessment of blood-filled spaces in Men1+/− pituitary tumours. A large area of a pituitary tumour is made up of spaces which are filled with blood (outlined in white, magnification x40). BrdU, 5-bromo-2′-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; PRL, prolactin; RBC, red blood cells. F, Quantification of pituitary sections from wild type mice (n=5) and pituitary tumours from Men1+/− mice (n=12) indicating the proportion of tissue area that is filled by blood-filled spaces (grey bars) compared to pituitary parenchyma cells (white bars).
Magnetic resonance imaging
Mice were anaesthetised with isoflurane and MRI of the cranium performed using a non-ionic MRI contrast medium, gadodiamide (Omniscan, Amersham Health AS, Norway) which was injected intra-peritoneally at 0.1mmol/kg. An 11.7 Tesla (500MHz) MR system was used, image reconstruction performed using purpose-written software in Matlab (Mathworks, USA), and image data exported into TIFF-format and loaded into Scion Image (Scion Corporation, USA). The MRI scans were coded so that investigators undertaking the analysis did not know whether the images were pre- or post-treatment. Pituitary tumour volumes were quantified as the sum of the area of all 1mm sections containing the tumour.
Histology, immunohistochemistry and analysis
Histology and immunostaining of tumours and tissues was performed using previously described methods (29) and appropriate primary and secondary antibodies. Sections were counterstained with either haematoxylin or 4,6-diamino-2-phenylindole (DAPI) (ProLong Gold Antifade reagent with DAPI, Molecular Probes, USA). Apoptotic cells were labelled using ApopTag® Fluorescein In Situ Apoptosis Detection Kit (Chemicon, USA) according to the manufacturer’s instructions. Sections were viewed by light or fluorescent microscopy using an Eclipse E400 microscope (Nikon), and images captured using a DXM1200C digital camera and NIS-Elements BR 2.30 software (both Nikon). Images were acquired at x20 magnification. Nuclear menin staining was quantified using a three-point grading system, with no staining=0; staining in fewer than half the cells=1; and staining in over half the cells=2. BrdU-labelled pituitary cell proliferation rates were ascertained from an average of 6 x20-fields, from each animal.
Statistics
All results were reported as the mean±SEM for equivalent groups. The mean values of tumour volume variation over the four-week study and 95% Confidence Intervals (CI) were calculated for each experimental group. Results were compared with independent t-tests (unpaired and two tailed) or Chi-squared (χ2) tests.
Results
Recombinant adenoviral vector construction, infectivity, and expression of menin
DNA sequence analysis (data not shown) confirmed the presence of the murine Men1 1833bp cDNA in the recombinant Men1.rAd5 vector (Figure 1A). Expression of the 67kDa menin by the Men1.rAd5 was demonstrated by Western blot analysis in Men1−/− MEFs (Figure 1B) and in HEK293 cells (data not shown). Furthermore, expression of GFP was shown in Men1−/− MEFs that were infected with GFP.rAd5 (Figure 1B). The ability of the recombinant adenoviral vector to infect Men1+/− pituitary tumour cells was assessed by an infectivity test in which increasing amounts of GFP.rAd5 viral particles were used (Figure 1C). This revealed that the number of pituitary tumour cells which were infected with GFP.rAd5 increased commensurately with the increased dose of viral particles. Thus, these in vitro results establish that Men1.rAd5 expressed menin and that rAd5 vectors can infect Men−/− MEFs and primary Men1+/− pituitary tumour cells.
In vivo effects of Men1.rAd5 vector
We embarked on in vivo studies aimed at investigating the potential anti-proliferative effects of Men1 gene replacement therapy, using Men1.rAd5, as our in vitro studies demonstrated menin expression by Men1.rAd5 and the ability of rAd5 vectors to infect Men1+/− pituitary tumour cells (Figure 1). To assess such an effectiveness of the Men1.rAd5 vector, we designed a randomised controlled trial (Figure 2) in which investigators were blinded to the treatment received by the Men1+/− female mice. The presence of pituitary tumours was assessed by MRI using gadodiamide enhancement (Figure 3A). MRI was performed in a total of 150 Men1+/− female mice, aged 18.5±0.2 months, and anterior pituitary adenomas, ranging in diameter from >2mm to <5mm, were identified in ~37% of mice (n=55) consistent with the previously observed frequency of pituitary tumours at this age in Men1+/− mice (29). Twenty-one of the 95 Men1+/− mice in which pituitary adenomas were not identified by MRI, had pituitary adenomas identified at necropsy; however these tumours were <1mm in diameter. Overall, these findings indicate that the use of MRI for the detection of anterior pituitary adenomas in mice has a sensitivity of 72% and a specificity of 100%. Histological examination of the pituitary adenomas revealed that the majority immunostained for prolactin and the remainder immunostained for growth-hormone, which is consistent with previous findings (29) and a pars distalis origin for these tumours (29).
The 55 Men1+/− mice with pituitary adenomas were randomised to one of the following four treatment groups which received a transauricular intra-tumoural injection of either Men1.rAd5 (n=15), GFP.rAd5 (n=14) or PBS (n=14), or no injection (n=12) (Figure 2A). Accurate targeting of injections to the tumours was confirmed by MRI immediately post-injection (Figure 3B) in a subset of mice and MRI 24-hours later revealed absorption of the injected solution (Figure 3C). In addition, six of 74 Men1+/− mice that did not have MRI-identifiable anterior pituitary adenomas and did not receive any treatment, were retained as age-matched controls (Figure 2). Five of the 43 Men1+/− anaesthetised mice (12%) that received the transauricular intra-tumoural injection died within 10 minutes. Three of these mice had received Men1.rAd5, one had received GFP.rAd5 and one had received PBS. However, this immediate post-injection mortality was not significantly different (p>0.14, χ2 test) in the three groups that received an injection. This finding indicates that the immediate mortality was likely due to the trauma of the procedure and anaesthesia, rather than a consequence of the rAd5 vectors. A further 8 Men1+/− mice died over the next four-weeks post-injection, distributed amongst the three treatment groups, giving an overall mortality of 21% over the study period. However, the mortality was not significantly different (p>0.62, χ2 test) in any of the treated or non-treated groups; indeed the highest mortality of 25% (n=3 out of 12 mice) was observed both in the Men1.rAd5 treated group and the age-matched non-injected and non-anaesthetised control group (Figure 2B). This mortality figure is consistent with that which we previously reported in Men1+/− mice of this age (29). These results indicate that mortality in these 18-20 month old Men1+/− mice was likely due to old age, rather than a consequence of Men1 gene replacement therapy with the Men1.rAd5 vector.
Successful treatment of tumours may result in a reduction of tumour volume and/or decreased tumour cell proliferation or increased apoptosis, and we assessed for these effects by measuring tumour volume by MRI, BrdU incorporation into the DNA of dividing cells, and TUNEL assay by immunohistochemistry, respectively (Figures 3 and 4). MRI assessment of pre- and post-treatment pituitary tumour volumes in the three groups receiving transauricular intra-tumoural injections did not reveal any significant differences (Figure 3D). The absence of a significant reduction in pituitary tumour volume in the Men1.rAd5 group may be due to several reasons. First, the Men1 gene may not be stably expressed for the four-weeks duration between injection and culling. However, immunohistochemical studies of pituitary tumour sections revealed an increased and stable expression of menin, as assessed by a three-point grading system, at four-weeks in the mice treated with Men1.rAd5 (Figure 4A) when compared to those treated with GFP.rAd5 or PBS (1.5±0.13 in Men1.rAd5; 0.6±0.32 in GFP.rAd5; and 0.5±0.16 in PBS-only, p<0.04). GFP expression occurred only in the pituitary tumours of Men1+/− mice treated with GFP.rAd5 (Figure 4B). Second, it is possible that the four-week time interval post-treatment is too short to detect any significant changes in tumour volume. However, it was not possible to continue observing the mice for substantially longer periods because of deaths due to old age. Third, pituitary tumours of 4 different Men1+/− mouse models are known to be highly vascular and contain spaces that are filled with blood (29, 41-43), and the Men1.rAd5 will not have an effect on these non-tumourous cells. Analysis of Men1+/− pituitary tumours revealed that 46±3% of tumours were formed by blood-filled spaces, compared to 5±1% in Men1+/+ normal pituitaries (p<0.00001) (Figure 3E-F). Therefore, the pituitary tumour cells formed ~55% of the total tumour volume indicating that approximately half of the tumour would be unaffected by gene replacement therapy. The Men1.rAd5 vector may have an anti-proliferative effect on pituitary tumour cells, which form half of the tumour volume, but this effect may be too small to be detected by MRI.
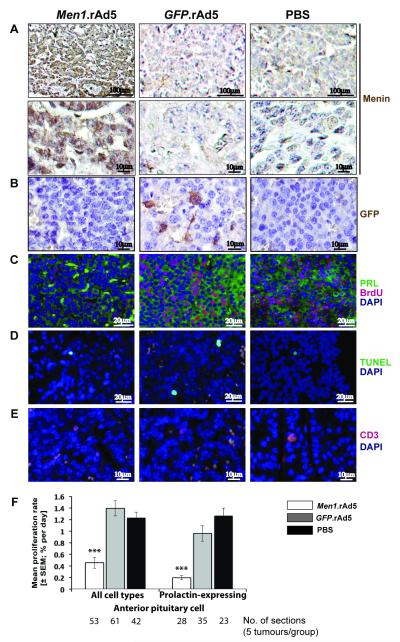
Figure 4. Assessment of proliferation, apoptosis and immune response in Men1+/− pituitary tumours at four-weeks after intra-tumoural injection of either Men1.rAd5, GFP.rAd5, or PBS (control).
A, Menin immunostaining, using DAB, and nuclei counterstaining with haematoxylin, at four-weeks. Menin expression was absent in Men1+/− pituitary tumours (PBS control and GFP.rAd5), but specific nuclear expression of menin (brown) was observed in Men1+/− pituitary tumours injected with Men1.rAd5 (p<0.04). Top and bottom panels represent views at x20 and x40 magnification respectively. B, GFP immunostaining, using DAB, and nuclei counterstaining with haematoxylin. Cytoplasmic GFP expression (brown, middle panel) was observed only in Men1+/− pituitary tumours that were injected with GFP.rAd5. C, Proliferation in prolactin expressing cells. Immunofluorescent labelling for prolactin (PRL, green) and BrdU (red) with nuclear DAPI counterstain (blue). BrdU labelling is reduced in Men1+/− pituitary tumours injected with Men1.rAd5. D, Apoptosis. Apoptotic cells (green) labelled using the TUNEL assay with DAPI counterstain (blue). Apoptosis was similar in the three groups. E, Immune response. Immunofluorescent labelling for CD3 (red) with DAPI counterstain (blue). Numbers of CD3 positive cells were similar in all three groups. F, Proliferation rates in Men1+/− pituitary tumours. Intra-tumoural injection of Men1.rAd5 resulted in a significant decrease in proliferation rates of prolactin-expressing cells and all pituitary tumour cells. Data shown as mean±SEM, ***p<0.000001 (Men1.rAd5 v GFP.rAd5 and Men1.rAd5 v PBS).
To assess for alterations in pituitary tumour cell proliferation that may occur in response to the expression of menin by the Men1.rAd5 vector, we used immunohistochemistry to identify cells labelled with BrdU, which is a thymidine analogue that is incorporated into DNA during the synthesis (S) phase of dividing cells (Figure 4C). In addition, we assessed for apoptosis (Figure 4D), as menin has been reported to promote apoptosis (44), and for an immune response (Figure 4E), as the injection of an adenovirus may induce an immune reaction. Treatment with Men1.rAd5 resulted in a significant decrease in BrdU incorporation by pituitary tumour cells such that there was a three-fold reduction in the daily proliferation rate when compared to treatment with GFP.rAd5 and PBS-only (0.45±0.09% in Men1.rAd5 treated; 1.39±0.13% in GFP.rAd5 treated; and 1.23±0.10% in PBS treated, p<0.0001) (Figure 4F). We further examined the proliferation rates of prolactin-expressing cells (Figure 4C) in pituitary tumours, as the majority (>65%) of Men1+/− anterior pituitary tumour cells immunostain for prolactin (29). This revealed that treatment with Men1.rAd5 resulted in a six-fold reduction in daily proliferation rates of prolactin-containing cells (Figure 4F) when compared to treatment with GFP.rAd5 and PBS (0.19±0.03% in Men1.rAd5 treated; 0.96±0.13% in GFP.rAd5 treated; and 1.3±0.13% in PBS-only treated, p<0.00001). Menin expression by the Men1.rAd5 vector in anterior pituitary tumours was not associated with an increase in apoptosis, which was assessed using the TUNEL assay (Figure 4D). Thus, the mean apoptotic rates in anterior pituitary tumours treated with Men1.rAd5, GFP.rAd5 or PBS were similar at 0.17±0.06%, 0.19±0.21%, and 0.20±0.09%, respectively (p>0.2).
Gene therapy using adenoviral vectors has been reported to cause cell-mediated immune responses, and we therefore assessed for a T-cell response by immunostaining for CD3, which is a membrane protein in T-cell receptor complexes (Figure 4E). This revealed that intra-tumoural injection of rAd5 vectors was not associated with a cell-mediated immune response as the mean percentage of cells immunostaining with CD3 in anterior pituitary tumours treated with Men1.rAd5, GFP.rAd5 and PBS were similar at 0.17±0.09%, 0.08±0.02%, and 0.13±0.08%, respectively (p>0.3). Localised delivery of gene therapy using adenoviral vectors may potentially be associated with systemic spread, and to assess this we immunostained for GFP expression in the cerebrum, liver, lymph nodes, and adrenals of Men1+/− mice treated with GFP.rAd5. This revealed an absence of GFP expression in these tissues (data not shown), indicating that GFP.rAd5 had remained localised to the pituitary tumour (Figure 4B) and not spread systemically.
Discussion
Our studies demonstrate that in vivo expression of menin by use of a recombinant adenoviral vector in pituitary adenomas of Men1+/− immunocompetent mice is effective in reducing tumour cell proliferation (Figure 4) and is not associated with significant adverse effects or increased mortality (Figure 2). These results are comparable to those of adenovirus-mediated retinoblastoma (Rb) gene therapy in pituitary melanotroph tumours in Rb-deficient mice, where intra-tumoural Rb cDNA delivery inhibited tumour growth (40). Thus, our results represent an important pre-clinical advance for the development of MEN1 gene therapy which is likely to be of use: in MEN1 patients for the treatment of anterior pituitary adenomas, foregut NETs and pancreatic NETs; in non-MEN1 patients with foregut and pancreatic NETs as >40% of these have MEN1 somatic mutations and loss of menin expression (15); and in non-MEN1 patients with pancreatic ductal adenocarcinomas as ~45% of these have decreased expression of the MEN1 gene (45). In addition, MEN1 gene replacement therapy may have a possible wider use in other tumours that have other genetic abnormalities, as in vitro studies have reported that menin over-expression inhibits cell proliferation of non-MEN1 human endocrine pancreatic tumour cells (BON1 cell line) (46), and of mouse RAS-transformed NIH3T3 MEFs (17). However, improvements in the delivery of the recombinant adenoviral vector, e.g. by a systemic route as opposed to direct intra-tumoural injection which may need to be repeated, will be required to achieve better targeting of multiple NETs that occur in MEN1 patients, and to avoid the immune responses that are associated with multiple exposures to the adenovirus (47, 48). Such advances would likely represent important steps in providing effective treatments for NETs, particularly as metastatic gastroenteropancreatic NETs are a major determinant of mortality in MEN1 patients (1-5).
Our studies, which involved only one intra-tumoural injection, did not detect an immune response to the recombinant adenoviral vector in immunocompetent Men1+/− mice, and this contrasts with the results of other studies that have injected recombinant adenoviral vectors into the pituitaries of rats and sheep. The injection of recombinant adenoviral vectors to rat pituitaries was associated with an immune response, e.g. an increase in CD8 positive T-cells, that peaked at 14 days post-injection and was resolved by 2 months (49), and the injection of recombinant adenoviral vector to ovine pituitaries resulted, within seven days, in a significant hypophysitis with lymphocytic infiltration (50). These differences in the immune reactions may be partly attributable to differences in: the immune responses of the three species studied; the recombinant adenoviral vectors; and the time points at which the analyses were undertaken. It is important to note that our study only examined for an immune response at one time point, and further studies at additional time points will be required to determine the occurrence, if any, of an immune response. Indeed, more detailed studies of the innate and adaptive immune responses which are known to limit vector transduction efficiency and duration of transgene expression are required to facilitate improvements in MEN1 gene replacement therapy.
Menin expression, following intra-tumoural injection of the recombinant Men1 adenovirus, was not associated with increased apoptosis (Figure 4). This is surprising, as in vitro studies in HEK293 cells and menin-null MEFs, have reported that menin expression induced apoptosis; thus, menin expression in menin-deficient pituitary tumour cells, in our study, would have been predicted to increase tumour cell apoptosis. However, the lack of an observed increase in apoptosis following in vivo menin expression in anterior pituitary tumour cells may in part be attributable to the differences in intrinsic oncogenic and apoptotic pathways of the cell types studied, and to the role of the surrounding cells of the matrix of the tumour in vivo, as well as the possibility that our analysis at four-weeks may have missed any increase in apoptosis at earlier time points. Nevertheless, overall our results suggest that the predominant in vivo effect of menin-loss is to increase cell proliferation rather than reduce apoptosis and, hence, MEN1 gene replacement is likely to lead to a decrease in tumour cell proliferation and growth.
In summary, our study has established the proof of concept for in vivo Men1 gene replacement in reducing cell proliferation of anterior pituitary adenomas which develop in Men1+/− mice.
Precis: Findings offer a preclinical proof of concept for gene replacement therapy of pituitary adenomas, using a relevant tumor suppressor gene often mutated in this setting.
Acknowledgments
Financial support: This work was supported by the UK Medical Research Council (MRC) grants G9825289 and G1000467 to GVW, JJ, AACR, BH, SP, PTC and RVT; a MRC Clinical Research Training Fellowship grant G0501780/76451 to GVW; a Portuguese Foundation for Science and Technology grant BD/12415/2003 to ML; and a Wellcome Trust Clinical Research Training Fellowship grant 087332/Z/08/Z to MJ.
Footnotes
Disclosure of Potential Conflicts of Interest: No authors have any conflict of interest to declare
References
- 1.Thakker RV. Multiple Endocrine Neoplasia Type 1. In: De Groot JL, Jameson LJ, editors. Endocrinology. Saunders Elsevier; 2010. pp. 2719–2741. [Google Scholar]
- 2.Beckers A, Betea D, Valdes Socin H, Stevenaert A. The treatment of sporadic versus MEN1-related pituitary adenomas. J Intern Med. 2003;253:599–605. doi: 10.1046/j.1365-2796.2003.01164.x. [DOI] [PubMed] [Google Scholar]
- 3.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, et al. Clinical Practice Guidelines for Multiple Endocrine Neoplasia Type 1 (MEN1) J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 4.Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243:477–88. doi: 10.1046/j.1365-2796.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 5.Akerstrom G, Hellman P. Surgery on neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:87–109. doi: 10.1016/j.beem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Scherubl H, Jensen RT, Cadiot G, Stolzel U, Kloppel G. Management of early gastrointestinal neuroendocrine neoplasms. World J Gastrointest Endosc. 2011;3:133–9. doi: 10.4253/wjge.v3.i7.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trouillas J, Labat-Moleur F, Sturm N, Kujas M, Heymann MF, Figarella-Branger D, et al. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients. The American journal of surgical pathology. 2008;32:534–43. doi: 10.1097/PAS.0b013e31815ade45. [DOI] [PubMed] [Google Scholar]
- 8.Gsponer J, De Tribolet N, Deruaz JP, Janzer R, Uske A, Mirimanoff RO, et al. Diagnosis, treatment, and outcome of pituitary tumors and other abnormal intrasellar masses. Retrospective analysis of 353 patients. Medicine (Baltimore) 1999;78:236–69. doi: 10.1097/00005792-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Wu ZB, Su ZP, Wu JS, Zheng WM, Zhuge QC, Zhong M. Five years follow-up of invasive prolactinomas with special reference to the control of cavernous sinus invasion. Pituitary. 2008;11:63–70. doi: 10.1007/s11102-007-0072-4. [DOI] [PubMed] [Google Scholar]
- 10.Arafah BM, Nasrallah MP. Pituitary tumors: pathophysiology, clinical manifestations and management. Endocr Relat Cancer. 2001;8:287–305. doi: 10.1677/erc.0.0080287. [DOI] [PubMed] [Google Scholar]
- 11.Qu X, Wang M, Wang G, Han T, Mou C, Han L, et al. Surgical outcomes and prognostic factors of transsphenoidal surgery for prolactinoma in men: a single-center experience with 87 consecutive cases. Eur J Endocrinol. 2011;164:499–504. doi: 10.1530/EJE-10-0961. [DOI] [PubMed] [Google Scholar]
- 12.Kreutzer J, Buslei R, Wallaschofski H, Hofmann B, Nimsky C, Fahlbusch R, et al. Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur J Endocrinol. 2008;158:11–8. doi: 10.1530/EJE-07-0248. [DOI] [PubMed] [Google Scholar]
- 13.Larsson C, Skogseid B, Oberg K, Nakamura Y, Nordenskjold M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988;332:85–7. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- 14.Thakker RV, Bouloux P, Wooding C, Chotai K, Broad PM, Spurr NK, et al. Association of parathyroid tumors in multiple endocrine neoplasia type 1 with loss of alleles on chromosome 11. N Engl J Med. 1989;321:218–24. doi: 10.1056/NEJM198907273210403. [DOI] [PubMed] [Google Scholar]
- 15.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Gurung B, Wan B, Matkar S, Veniaminova NA, Wan K, et al. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature. 2012;482:542–6. doi: 10.1038/nature10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YS, Burns AL, Goldsmith PK, Heppner C, Park SY, Chandrasekharappa SC, et al. Stable overexpression of MEN1 suppresses tumorigenicity of RAS. Oncogene. 1999;18:5936–42. doi: 10.1038/sj.onc.1203005. [DOI] [PubMed] [Google Scholar]
- 18.Sayo Y, Murao K, Imachi H, Cao WM, Sato M, Dobashi H, et al. The multiple endocrine neoplasia type 1 gene product, menin, inhibits insulin production in rat insulinoma cells. Endocrinology. 2002;143:2437–40. doi: 10.1210/endo.143.6.8950. [DOI] [PubMed] [Google Scholar]
- 19.Schnepp RW, Mao H, Sykes SM, Zong WX, Silva A, La P, et al. Menin induces apoptosis in murine embryonic fibroblasts. J Biol Chem. 2004;279:10685–91. doi: 10.1074/jbc.M308073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La P, Yang Y, Karnik SK, Silva AC, Schnepp RW, Kim SK, et al. Menin-mediated caspase 8 expression in suppressing multiple endocrine neoplasia type 1. J Biol Chem. 2007;282:31332–40. doi: 10.1074/jbc.M609555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Ozawa A, Zaman S, Prasad NB, Chandrasekharappa SC, Agarwal SK, et al. The tumor suppressor protein menin inhibits AKT activation by regulating its cellular localization. Cancer Res. 2011;71:371–82. doi: 10.1158/0008-5472.CAN-10-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khare R, Chen CY, Weaver EA, Barry MA. Advances and future challenges in adenoviral vector pharmacology and targeting. Curr Gene Ther. 2011;11:241–58. doi: 10.2174/156652311796150363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMenamin MM. Translational Benefits of Gene Therapy to Date. Clin Oncol Cancer Res. 2011;8:10–15. [Google Scholar]
- 24.Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs) Gut. 2011 doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonpavde G, Thompson TC, Jain RK, Ayala GE, Kurosaka S, Edamura K, et al. GLIPR1 tumor suppressor gene expressed by adenoviral vector as neoadjuvant intraprostatic injection for localized intermediate or high-risk prostate cancer preceding radical prostatectomy. Clin Cancer Res. 2011;17:7174–82. doi: 10.1158/1078-0432.CCR-11-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vachani A, Moon E, Wakeam E, Haas AR, Sterman DH, Albelda SM. Gene therapy for lung neoplasms. Clin Chest Med. 2011;32:865–85. doi: 10.1016/j.ccm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu W, Zheng S, Li XF, Huang JJ, Zheng X, Li Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: a pilot phase II clinical trial. World J Gastroenterol. 2004;10:3634–8. doi: 10.3748/wjg.v10.i24.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas SM, Grandis JR. The current state of head and neck cancer gene therapy. Hum Gene Ther. 2009;20:1565–75. doi: 10.1089/hum.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harding B, Lemos MC, Reed AA, Walls GV, Jeyabalan J, Bowl MR, et al. Multiple endocrine neoplasia type 1 knockout mice develop parathyroid, pancreatic, pituitary and adrenal tumours with hypercalcaemia, hypophosphataemia and hypercorticosteronaemia. Endocr Relat Cancer. 2009;16:1313–27. doi: 10.1677/ERC-09-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–67. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 31.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook JRD. Molecular Cloning: A laboratory manual. 3rd ed Cold Spring Harbour Laboratory Press; 2001. [Google Scholar]
- 33.Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J. Preparation, Culture and Immortalization of Mouse Embryonice Fibroblasts. Current Protocols in Molecular Biology. 2005 doi: 10.1002/0471142727.mb2801s70. ed. J.W. Sons. [DOI] [PubMed] [Google Scholar]
- 35.Qi X, Chang Z, Song J, Gao G, Shen Z. Adenovirus-mediated p53 gene therapy reverses resistance of breast cancer cells to adriamycin. Anticancer Drugs. 2011;22:556–62. doi: 10.1097/CAD.0b013e328345b4e7. [DOI] [PubMed] [Google Scholar]
- 36.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 37.Gunkel M, Erdel F, Rippe K, Lemmer P, Kaufmann R, Hormann C, et al. Dual color localization microscopy of cellular nanostructures. Biotechnol J. 2009;4:927–38. doi: 10.1002/biot.200900005. [DOI] [PubMed] [Google Scholar]
- 38.Bassett JH, Forbes SA, Pannett AA, Lloyd SE, Christie PT, Wooding C, et al. Characterization of mutations in patients with multiple endocrine neoplasia type 1. Am J Hum Genet. 1998;62:232–44. doi: 10.1086/301729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemmens I, Van de Ven WJ, Kas K, Zhang CX, Giraud S, Wautot V, et al. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum Mol Genet. 1997;6:1177–83. doi: 10.1093/hmg/6.7.1177. [DOI] [PubMed] [Google Scholar]
- 40.Riley DJ, Nikitin AY, Lee WH. Adenovirus-mediated retinoblastoma gene therapy suppresses spontaneous pituitary melanotroph tumors in Rb+/− mice. Nat Med. 1996;2:1316–21. doi: 10.1038/nm1296-1316. [DOI] [PubMed] [Google Scholar]
- 41.Bertolino P, Tong WM, Galendo D, Wang ZQ, Zhang CX. Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol Endocrinol. 2003;17:1880–92. doi: 10.1210/me.2003-0154. [DOI] [PubMed] [Google Scholar]
- 42.Crabtree JS, Scacheri PC, Ward JM, Garrett-Beal L, Emmert-Buck MR, Edgemon KA, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A. 2001;98:1118–23. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loffler KA, Biondi CA, Gartside M, Waring P, Stark M, Serewko-Auret MM, et al. Broad tumor spectrum in a mouse model of multiple endocrine neoplasia type 1. Int J Cancer. 2007;120:259–67. doi: 10.1002/ijc.22288. [DOI] [PubMed] [Google Scholar]
- 44.Hussein N, Casse H, Fontaniere S, Morera AM, Asensio MJ, Bakeli S, et al. Reconstituted expression of menin in Men1-deficient mouse Leydig tumour cells induces cell cycle arrest and apoptosis. Eur J Cancer. 2007;43:402–14. doi: 10.1016/j.ejca.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 45.Cavallari I, Silic-Benussi M, Rende F, Martines A, Fogar P, Basso D, et al. Decreased expression and promoter methylation of the menin tumor suppressor in pancreatic ductal adenocarcinoma. Genes Chromosomes Cancer. 2009;48:383–96. doi: 10.1002/gcc.20650. [DOI] [PubMed] [Google Scholar]
- 46.Stalberg P, Grimfjard P, Santesson M, Zhou Y, Lindberg D, Gobl A, et al. Transfection of the multiple endocrine neoplasia type 1 gene to a human endocrine pancreatic tumor cell line inhibits cell growth and affects expression of JunD, delta-like protein 1/preadipocyte factor-1, proliferating cell nuclear antigen, and QM/Jif-1. J Clin Endocrinol Metab. 2004;89:2326–37. doi: 10.1210/jc.2003-031228. [DOI] [PubMed] [Google Scholar]
- 47.Wu TL, Ertl HC. Immune barriers to successful gene therapy. Trends Mol Med. 2009;15:32–9. doi: 10.1016/j.molmed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6:215–26. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci U S A. 2000;97:7482–7. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis JR, McMahon RF, Lowenstein PR, Castro MG, Lincoln GA, McNeilly AS. Adenovirus-mediated gene transfer in the ovine pituitary gland is associated with hypophysitis. J Endocrinol. 2002;173:265–71. doi: 10.1677/joe.0.1730265. [DOI] [PubMed] [Google Scholar]