Abstract
Background
To assess the relationship between surgical delay and mortality in elderly patients with hip fracture. Systematic review and meta-analysis of retrospective and prospective studies published from 1948 to 2011. Medline (from 1948), Embase (from 1974) and CINAHL (from 1982), and the Cochrane Library. Odds ratios (OR) and 95% confidence intervals for each study were extracted and pooled with a random effects model. Heterogeneity, publication bias, Bayesian analysis, and meta-regression analyses were done. Criteria for inclusion were retro- and prospective elderly population studies, patients with operated hip fractures, indication of timing of surgery and survival status.
Methodology/Principal Findings
There were 35 independent studies, with 191,873 participants and 34,448 deaths. The majority considered a cut-off between 24 and 48 hours. Early hip surgery was associated with a lower risk of death (pooled odds ratio (OR) 0.74, 95% confidence interval (CI) 0.67 to 0.81; P<0.000) and pressure sores (0.48, 95% CI 0.38 to 0.60; P<0.000). Meta-analysis of the adjusted prospective studies gave similar results. The Bayesian probability predicted that about 20% of future studies might find that early surgery is not beneficial for decreasing mortality. None of the confounders (e.g. age, sex, data source, baseline risk, cut-off points, study location, quality and year) explained the differences between studies.
Conclusions/Significance
Surgical delay is associated with a significant increase in the risk of death and pressure sores. Conservative timing strategies should be avoided. Orthopaedic surgery services should ensure the majority of patients are operated within one or two days.
Introduction
Hip fractures are common and serious: they always cause short-term pain, disability and can lead to longer-term pain, disability and even deformity. Mortality rate is estimated to be 5–10% at one month and 12–27% at one year from surgery [1]. Recent data from the UK, USA and Canada show that patients are mostly older than 75 years and female [2], [3], [4]. Mortality in the elderly may reach 10% at one month, 20% at four months and 30% at one year [5]. These patients are the frailest among those who are admitted to hospital, and their outcomes are likely to depend closely on how their care is managed.
In the last decade efforts have been made to boost our knowledge of the prognostic factors influencing the course and management of hip fracture. As the majority are treated surgically, time to surgery may be decisive. Some studies report that pre-operative delay might lead to an increase in mortality and adversely influence other clinical outcomes such as infection and pressure sores [6], [7], [8], [9]. Clinical guidelines recommend immediate reparative surgery, within 24–48 hours from hospital admission [10], [11]. However, several observational studies found no association between time and mortality and concluded that further research is needed on whether functional outcomes are worsened by delaying surgery [12], [13], [14]. This approach is supported by geriatricians who offer an explicit rationale for postponing surgery: delay may be necessary and beneficial for stabilizing patients with co-morbidities [12].
A large number of observational studies have explored prognostic factors influencing hip fracture surgery, but with discordant results. Reasons might depend on the variability in adjusting for different confounders among studies, the lack of a consistent choice of the reference group and use of a common surgical delay cut-off.
A meta-analysis published in 2010 investigating the effect of surgical delay on mortality at different follow -up times, found significantly higher all-cause mortality for patients treated surgically more than 24, 48 and 72 hours from admission [15]. We moved from this high-quality meta-analysis to conduct a comprehensive meta-analysis which considered all prospective and retrospective evidence. The aims of this systematic review were: (i) to identify and describe all the studies which assessed whether surgical delay increases the mortality of elderly patients treated for hip fracture; (ii) to see whether delay is associated with increased mortality; (iii) to examine the influence of a wide range of a priori selected variables (age, sex, co-morbidities, etc.) using meta-regression; and (iv) to test the robustness of results using a Bayesian approach.
Methods
Eligibility
Studies were included if they met the following criteria: i) randomized, quasi-randomized (e.g. allocation based on date of admission), prospective and retrospective cohort and case-controlled studies; ii) patients with operated hip fractures; iii) patients aged 65 years or older (median or mean age per study); iv) reporting of timing of hip surgery; v) survival status adequately reported for meta-analysis; vi) published in English, French, Italian or Spanish after 1980. Evidence from controlled observational studies was included: it is unlikely that patients were randomized to receive immediate or postponed surgery to obtain evidence of the mortality-delay association because of ethical concerns. We did not define the optimal surgical delay from hospital admission to reparative surgery a priori but accepted what the authors claimed at face value. When authors did not give a cut-off, we arbitrarily selected 24 hours as optimal. Different time cut-offs were used as strata in our analyses.
Search strategy
Studies were identified by searching electronic databases and scanning reference lists of articles. This search was applied to Medline (1948 – /September 2011), and adapted for Embase (1974 – September 2011) and CINAHL (1982 – December 2011). The strategy was developed using the following key items: hip fracture, arthroplasty, and timing surgery (see Search strategy – Table S1) [16], [17]. Manual searches of the reference lists of included studies, reviews, meta-analyses and guidelines on hip fracture surgery and prognostic factors were also done.
Study selection
The literature search was conducted independently and two reviewers (AP and LG) independently searched the literature who then selected potentially eligible studies for inclusion. Disagreements between them were resolved by consensus; if no agreement could be reached, a third author (LM) was called in to decide. The fulltext of all eligible citations was examined in more detail.
Data Extraction
We developed a data extraction sheet, pilot-tested it on five randomly-selected studies, and refined it accordingly. One review author (AP) extracted the following data from studies included and entered in the data extraction form: study design, study year, participants (age, sex, case-mix and co-morbidities), country of origin and setting. A second author (CR) checked the extracted data to ensure quality. Disagreements were solved by discussion between the two review authors; if no agreement was reached, a third author (LM) could decide.
The primary outcome was unambiguous overall mortality. If applicable short- (<30 days) and long-term mortality (>30 days) were combined. Secondary outcomes were post-operative complications, i.e. infections, pressure sores, post-operative chronic pain, hospital length of stay, and readmission. If necessary, percentages of mortality or other outcomes were converted into frequencies.
For all studies that addressed mortality, we recorded the unadjusted matched odds ratios (OR) for: i) the comparison of early and delayed surgery; ii) whether any adjustment was made for covariates (e.g. age); iii) the adjusted estimate of the ORs and 95% confidence intervals (CIs). When univariate and multivariate adjusted models were available, both were abstracted. Since some studies reported only adjusted hazard ratios, we converted them to ORs using a formula to compute risk ratio from OR [18]. We used as control mortality risk the median control risk based on our primary meta-analysis.
If a study considered both young patients (<65 years or high-velocity injuries) and old patients, the data were extracted separately if possible, and only the elderly group was considered. If sorting was not possible and at least 90% of the patients in a study were classifiable as elderly, the study was included; if not it was excluded.
Methodological quality
Methodological quality was independently assessed by two review authors (AP and LG). For observational studies the Newcastle-Ottawa (NOS) scale for cohort and case-control studies was used [19], [20]. This scale has three groups of items: selection, exposure/outcome and comparability. A study can be awarded a maximum of one star for each numbered item in ‘patient selection’ (four items) and ‘exposure’ (three items) and a maximum of two stars in the ‘comparability of study groups’ (two items), for a total of nine stars. Since we were interested in mortality of operated patients, we expected three items would be scored positively across all studies, specifically Ascertainment of exposure (secure surgical record), Demonstration that outcome of interest was not present at start of study, Assessment of outcome (record linkage). In fact in our meta-analysis the NOS scale could have ranged between three and nine. For randomized controlled trials (RCTs) we summarized the risk of bias for mortality across the following specific domains within study: sequence generation, allocation concealment, and incomplete outcome data [21]. We decided a priori that only prospective observational studies that met eight or nine of the Newcastle-Ottawa Scale criteria were to be considered of high quality, whereas RCTs were considered of high quality if they satisfied two or more.
Data analysis
We did an overall quantitative synthesis using all ORs on mortality across all studies, with unadjusted data from each study. The results were pooled using the inverse variance method and ordered by study year. The random effects model described by DerSimonian and Laird [22] was used to synthesize data rather than the fixed effect model because it incorporates intra- and inter-study variability. This model was selected a priori as the meta-analysis was expected to include primarily observational studies with inherently more variability than RCTs. A Mantel-Haenszel OR was also computed using fixed effect and compared to the DerSimonian and Laird estimate to investigate any influence of small study effects on the pooled OR, since the DerSimonian and Laird methods tend to attribute greater weight to small studies with increasing heterogeneity [23]. The degree of heterogeneity between trials was assessed by the I-squared (I2) statistic, with its 95% CI for each outcome. We accounted for heterogeneity in the results of our primary meta-analysis, using the prediction interval (estimated in a Bayesian setting) for the true effect in a new study, which describes the full distribution of effects in a random-effects meta-analysis [21].
Since meta-analyses of observational studies often give spuriously precise results, we tested the robustness of results with two approaches. First, we calculated the probability of the OR being less than 1 from the predictive Bayesian interval. Second, we used credibility ceilings, a technique which assumes that methodological limitations implicit in included observational studies cannot give us more than a maximum certainty that an effect is in a particular direction and not null or in the other direction [24].
Sensitivity to prior assumptions was checked in the Bayesian estimation of the pooled OR as well as the predictive interval. We assumed different priors for the shape of the random effects distribution: Gamma (0.001, 0.001) on precision; uniform (0, 50) on inter-study variance tau2; uniform(0, 50) on inter-study standard deviation tau; and three functions of mean intra-study variance: uniform shrinkage on tau2, DuMouchel on tau, and half-normal on tau2.
Further sensitivity analysis was done to account for methodological differences between the studies. Data were synthesized for studies of high and low quality, using adjusted study-specific estimates, preferring the ones with the most extensive adjustment [25].
The extent to which study-level variables explained heterogeneity in predicting mortality was explored by fitting random effects meta-regression models to account for the design (prospective or retrospective), nature of data (administrative or clinical), methodological quality (8–9 stars or 1–7), health status or co-morbidities (to identify patients at high risk of mortality), location of study (United States or other), different optimal time cut-points, age, prevalence of females, study year, and optimal time treated as a continuous variable. The effect of mortality baseline risk was investigated in a Bayesian linear regression model.
We checked for potential publication and small study effects by the contour enhanced funnel plot [26], [27] integrating visual inspection of the plot with the test proposed by Harbord [28].
Statistical analyses were done using Stata v.11 [29] and WinBUGS v.1.3 software [30] P-values less than 0.05 were considered statistically significant.
Results
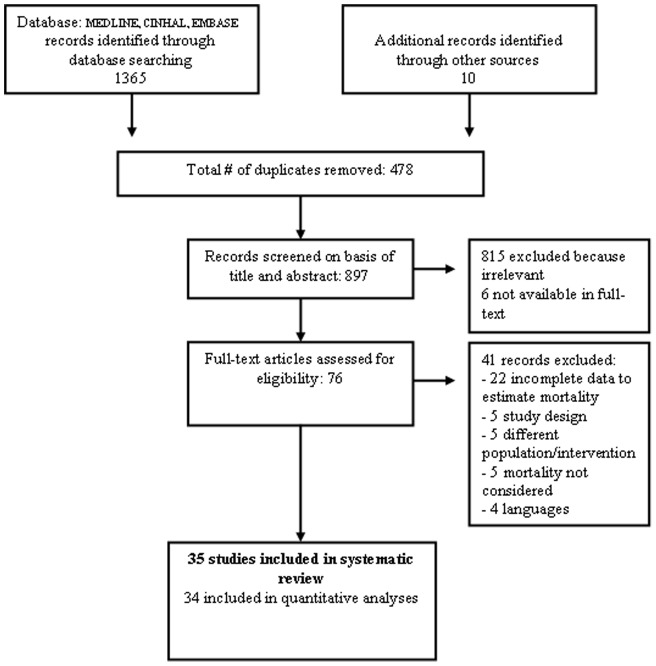
Database searches yielded 1375 references. Hand-searching produced 10 more. Exclusion of duplicates and irrelevant references left 76 descriptive studies. We excluded 41 studies because of: incomplete data (n = 22), study design (n = 5), different population/intervention (n = 5), mortality not considered as an outcome (n = 5), language (n = 4). We included 35 studies fulfilling our inclusion criteria, all except one [31] providing data for our analyses ( Figure 1 ) [6], [7], [13], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]. This corresponds to 34 cohort studies, 14 using a prospective design and 20 a retrospective one, and one RCT. summarizes the main features of the 35 articles included, which included 191,873 participants. The number of participants in each trial ranged from 65 to 57,315 ( Table 1 ). The mean age was 80 years (range 76–83 years). The mean proportion of women was 74% (0–83%) in the 32 studies reporting sex. Three studies were published in the 80’s, six in the 90’s and 26 (71.8%) after 2000. Twelve were from the USA, four from UK, three each from Canada and Italy, two each from Israel and Netherlands; Australia, Austria, Denmark, Finland, German, Ireland, New Zealand, Turkey and Sweden provided one study each.
Figure 1. Selection for studies exploring the association between mortality and optimal time to surgery in patients with hip fractures.
Table 1. Characteristics of included studies.
| Source | Design | Source of data | Data source for pre-operative time and confounding factor | Country | No of participants | Average age | % Female | Optimal Time (hours) | Methodology score |
| Al Ani 2008 | Prospective | Two centres | Clinical records | Sweden | 744 | 81 | 73 | 24 | 7 |
| Bergeron 2006 | Retrospective | One centre | Unclear | Canada | 977 | 81 | 74 | 24 | 7 |
| Bredahl 1992 | Retrospective | One centre | Clinical records | Denmark | 778 | 79 | 73 | 12 | 5 |
| Carretta 2011 | Retrospective | One centre | Administrative data | Italy | 1320 | 83 | 77 | 48 | 7 |
| Davis 1988 | Prospective | Two centres | Clinical records | USA | 230 | 81 | 82 | 48 | 6 |
| Dorotka 2003 | Prospective | One centre | Clinical records | Austria | 182 | 78 | 76 | 24 | 7 |
| Doruk 2003 | Prospective | One centre | Clinical records | Turkey | 65 | 76 | 64 | 120 | 7 |
| Elliott 2003 | Prospective | Two centres | Clinical Records | Ireland | 1780 | NR | 77 | 24 | 9 |
| Franzo 2005 | Retrospective | 18 centres | Administrative data | Italy | 6629 | 82 | 81 | 48 | 8 |
| Gdalevich 2004 | Retrospective | One centre | Clinical records | Israel | 651 | NR | 75 | 48 | 8 |
| Grimes 2002 | Retrospective | 20 centres | Clinical records** | USA | 8383 | 80 | 79 | 24 | 8 |
| Hamlet 1997 | Retrospective | One centre | Clinical records | USA | 171 | 77 | 80 | 24 | 6 |
| Holt 2008 | Prospective | 22 centres | Clinical records | UK | 18692 | NR | 79 | 24 | 9 |
| Kenzora 1986 | Retrospective | One centre | Clinical records | USA | 406 | 76 | 76 | 24 | 6 |
| Maggi 2009 | Prospective | Nine centres | Clinical records | Italy | 2473 | 82 | 79 | 24 | 8 |
| Majumdar 2006 | Retrospective | Multicentre* | Administrative data | Canada | 3864 | 82 | 71 | 24 | 8 |
| Mc Guire 2004 | Retrospective | Multicentre* | Administrative data | USA | 18208 | 82 | 79 | 48 | 8 |
| Moran 2005 | Prospective | One centre | Clinical records | UK | 2148 | 80 | 76 | 96 | 9 |
| Mullen 1989 | Prospective | One centre | Clinical records | USA | 400 | 79 | NR | 24 | 6 |
| Novack 2007 | Retrospective | Seven centres | Administrative data | USA | 3815 | 82 | 73 | 48 | 9 |
| Orosz 2004 | Prospective | Four centres | Clinical records | USA | 1576 | 82 | 81 | 24 | 9 |
| Parker 1992 | Prospective | One centre | Clinical records | UK | 468 | 81 | 83 | 48 | 7 |
| Peleg 2011 | Retrospective | Seven centres | Administrative data | Israel | 6442 | NR | NR | 48 | 6 |
| Radcliff 2008 | Retrospective | 181 centres | Administrative data | USA | 5682 | 77 | 0 | 96 | 6 |
| Rademakers 2007 | Retrospective | One centre | Clinical records | Netherlands | 722 | 82 | 76 | 12 | 5 |
| Rae 2007 | Prospective | One centre | Clinical records | Australia | 222 | 79 | 72 | 48 | 8 |
| Roos 1996 | Retrospective | Multicentre* | Administrative data | USA | 26213 | 81 | 79 | 48 | 5 |
| Sexson 1988 | Retrospective | One centre | Clinical records | USA | 300 | NR | 77 | 24 | 8 |
| Smektala 2008 | Prospective | 268 centres | Clinical records | German | 2916 | NR | 80 | 48 | 8 |
| Siegmeth 2005 | Prospective | One centre | Clinical records | UK | 3628 | 81 | NR | 48 | 8 |
| Stoddart 2002 | Retrospective | One centre | Clinical records | New Zealand | 138 | 83 | 75 | 24 | 7 |
| Sund 2005 | Retrospective | Multicentre | Administrative data | Finland | 16881 | 82 | 75 | 48 | 9 |
| Swanson 1998 | RCT | One centre | Clinical records | USA | 71 | 79 | 78 | 48 | NA |
| Verbeek 2007 | Retrospective | One centre | Clinical records | Netherlands | 192 | 80 | 77 | 24 | 9 |
| Weller 2005 | Retrospective | One centre | Administrative data | Canada | 57315 | 78 | 75 | 24 | 8 |
Number of participating centres not reported. **Data already collected for other purpose. NR Not Reported. NA Not applicable.
Patient data were collected from clinical records in 24 studies, in ten from national or regional administrative databases, and in one the source was unclear. The optimal surgical delay was 12 hours in two studies, 24 hours in 16, 36 hours in one study, 48 in thirteen, and >72 in three. The quality measured through the NOS scale ranged from five to nine points, with a median of seven ( Table 2 ).
Table 2. Methodological Quality Assessment of Observational Studies Based on the Newcastle-Ottawa (NOS) scale.
| Study ID | Selection | Comparability | Outcome | Total score* | |||
| Representativeness of early cohort | Selection of delay cohort | Controlled for age | Controlled for co-morbidities | Follow- up length | Adequacy follow-up | ||
| Al Ani 2008 | * | * | * | * | 7 | ||
| Bergeon 2006 | * | * | * | * | 7 | ||
| Bredahl 1992 | * | * | 5 | ||||
| Carretta 2011 | * | * | * | * | 7 | ||
| Davis 1988 | * | * | * | 6 | |||
| Dorotka 2003 | * | * | * | * | 7 | ||
| Doruk 2003 | * | * | * | * | 7 | ||
| Elliott 2003 | * | * | * | * | * | * | 9 |
| Franzo 2005 | * | * | * | * | * | 8 | |
| Gdalevich 2004 | * | * | * | * | * | 8 | |
| Grimes 2002 | * | * | * | * | * | 8 | |
| Kenzora 1986 | * | * | * | 6 | |||
| Hamlet 1997 | * | * | * | 6 | |||
| Holt 2008 | * | * | * | * | * | * | 9 |
| Maggi 2009 | * | * | * | * | * | 8 | |
| Majumdar 2006 | * | * | * | * | * | 8 | |
| Mc Guire 2004 | * | * | * | * | * | 8 | |
| Moran 2005 | * | * | * | * | * | * | 9 |
| Mullen 1989 | * | * | * | * | 6 | ||
| Novack 2007 | * | * | * | * | * | * | 9 |
| Orosz 2004 | * | * | * | * | * | * | 9 |
| Parker 1992 | * | * | * | * | 7 | ||
| Peleg 2011 | * | * | * | 6 | |||
| Radcliff 2008 | * | * | * | 6 | |||
| Rademakers 2007 | * | * | 5 | ||||
| Rae 2007 | * | * | * | * | * | 8 | |
| Roos 1996 | * | * | 5 | ||||
| Sexson 1988 | * | * | * | * | * | 8 | |
| Siegmeth 2005 | * | * | * | * | * | 8 | |
| Smektala 2011 | * | * | * | * | * | 8 | |
| Stoddart 2002 | * | * | * | * | 7 | ||
| Sund 2005 | * | * | * | * | * | * | 9 |
| Verbeek 2007 | * | * | * | * | * | * | 9 |
| Weller 2005 | * | * | * | * | * | 8 | |
Total score: sum of row totals plus 3 points scored positively across all studies (see methods section for details).
The only RCT was at high risk of bias [57]. Swanson randomized a limited number of patients (71) to a multifaceted intervention named ‘early intervention’. Early surgery, early mobilization, and intensive support by health professionals were the main components, although it is difficult to evaluate their relative roles. The groups did not truly differ in terms of time to surgery: 90% of patients in the intervention group and 80% in the control group were operated within 48 hours. The generation of the randomization sequence and allocation concealment were likely to be inappropriate, possibly being affected by the time of hospital admission.
Time to surgery and mortality
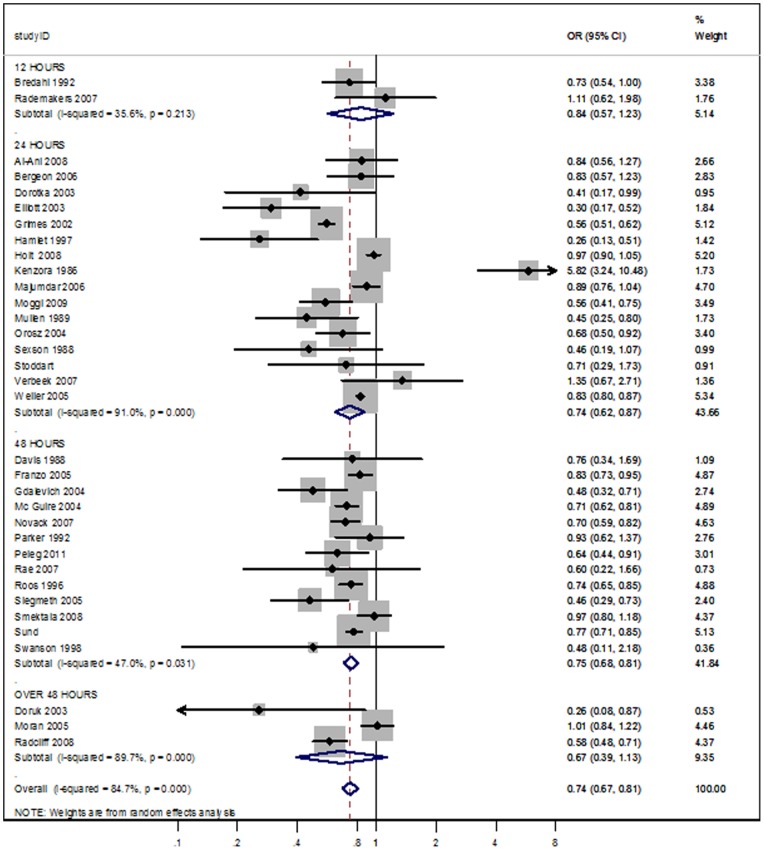
Patients who underwent early surgery had significantly lower odds of death than those whose surgery was delayed (OR 0.74; 95% CI 0.67 to 0.81; p<0,0001.). Substantial heterogeneity (I2 = 84.7%) was detected as shown in the forest plots ( Figure 2 ). The OR predictive interval was 0.48 to 1.13, meaning that no effect or an adverse effect of early surgery might be a plausible finding in a new study. The Mantel-Haenszel OR was 0.79 (95% CI 0.77 to 0.81; p<0.0001), suggesting a modest impact of small studies on the random effects estimate towards more beneficial values.
Figure 2. Meta-analysis of Early versus Delayed surgery time according to cut-off points (12, 24, 48, and over 48 hours). Outcome: overall mortality.
Bayesian meta-analysis yielded an OR of 0.72 and a 95% credible interval (0.61 to 0.84) the upper bound of which was relatively insensitive to assumptions about prior distribution for random effects. The likelihood of early surgery being found to be beneficial varied between 78% and 82% according to assumptions on priors, suggesting that no or an adverse effect of early surgery could be predicted in about 20% of future studies.
The benefit was resistant to conservative interpretation with sceptical credibility ceilings: only when we considered that there was no chance of any single study convincing us more than about 22% that the effect of early surgery was beneficial did the pooled estimate predict that early and delayed surgery were equivalent.
Meta-regression analyses
Risk of bias
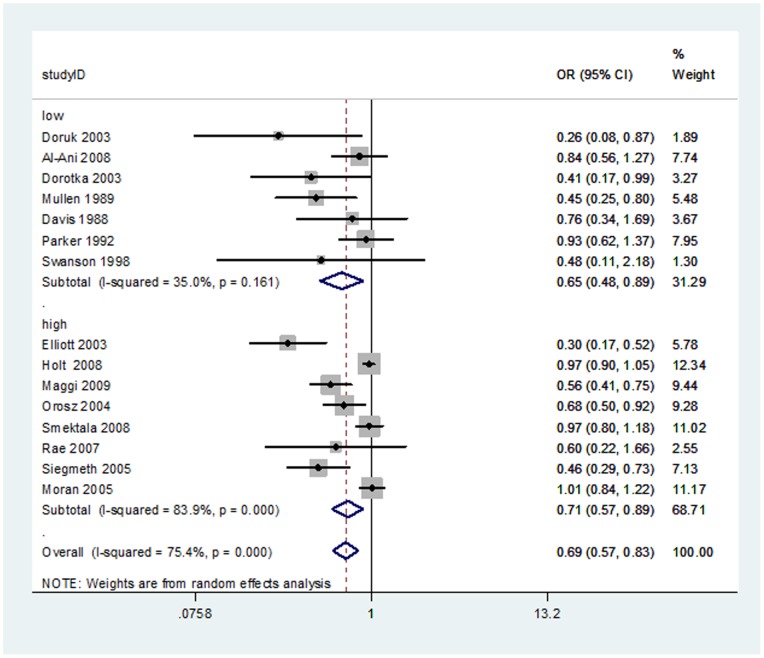
Fourteen studies, seven of low quality [32], [34], [35], [36], [44], [46], [57] and eight of high quality [37], [40], [43], [45], [50], [53], [54], reported prospective adjusted measures of effect. The overall random effects meta-analysis of ORs yielded a pooled estimate favouring early surgery (OR: 0.69; 95% CI: 0.57–0.83; I2 = 75.4%). The test for differences between study quality subgroups was not statistically significant (random effects meta-regression P = 0.42). The results are reported in Figure 3 .
Figure 3. Meta-analysis of high and low-quality adjusted prospective studies comparing early versus delayed surgery time. Outcome: overall mortality.
Other confounders
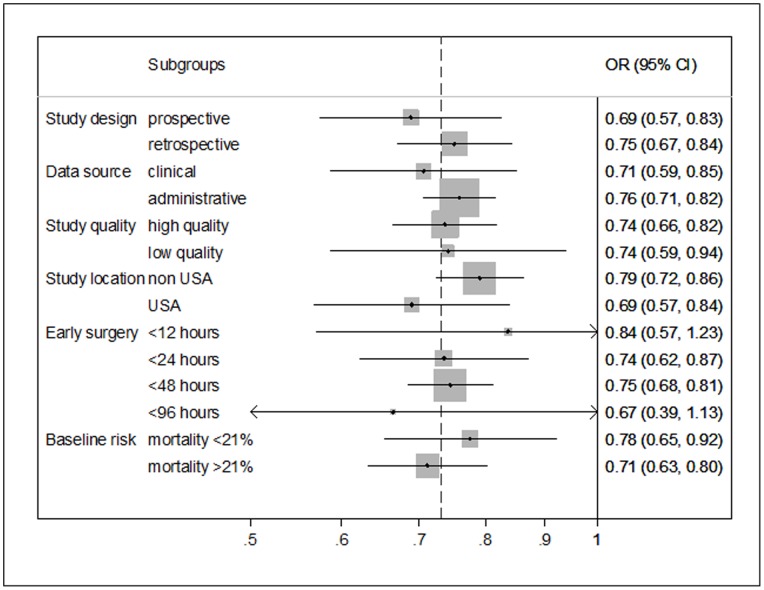
Table 3 summarizes meta-regression and Figure 4 subgroup results on unadjusted data. In all studies the reporting of mortality stratified for comorbidity was incomplete and we were therefore unable to do a reliable meta-regression analysis of this predictor. None of the covariates studied with meta-regression or subgroup analyses yielded any significant effects on mortality (p≥0.05). However, the power of these analyses is typically low and exploration of confounders may be possibly subject to aggregation bias.
Table 3. Random effects meta-regression analyses of Early and Delayed surgery time for overall mortality.
| Characteristic | N. studies | N. participants | OR (95% CI) | p-value |
| Continuous variables | ||||
| Age (per 10 years) | 28 | 159772 | 0.97 (0.36 to 2.63) | 0.96 |
| Female prevalence (per 10% more) | 32 | 187885 | 1.03 (0.92 to 1.16) | 0.55 |
| Study year (per 10 years) | 34 | 191873 | 0.90 (0.7 to 1.18) | 0.46 |
| Continuous time (per 24 hours) | 34 | 191873 | 0.22 (0.00077 to 63.62) | 0.59 |
| Categorical variables | ||||
| Study design | ||||
| Prospective (reference) | 15 | 35112 | 1 | |
| Retrospective | 20 | 156761 | 1.17 (0.82 to 1.66) | 0.36 |
| Data source category | ||||
| Clinical data (reference) | 24 | 46843 | 1 | |
| Administrative data | 10 | 144053 | 1.05 (0.72 to 1.53) | 0.78 |
| Study quality | ||||
| 8–9 stars (reference) | 18 | 149228 | 1 | |
| 1–7 stars | 17 | 42645 | 1.05 (0.73 to 1.5) | 0.79 |
| Study location | ||||
| Non-US study (reference) | 23 | 129179 | 1 | |
| US study | 12 | 62694 | 0.94 (0.65 to 1.35) | 0.74 |
| Early surgery time cut-off definition | ||||
| <12 hours | 2 | 1500 | 1.2 (0.55 to 2.65) | 0.62 |
| <24 hours (reference) | 16 | 97100 | 1 | |
| <48 hours | 14 | 85378 | 0.97 (0.65 to 1.43) | 0.87 |
| <96–120 hours | 3 | 7895 | 0.90 (0.46 to 1.78) | 0.77 |
| Baseline risk | ||||
| Risk<21% | 17 | 85826 | 1 | |
| Risk>21% | 18 | 106047 | 0.86 (0.61 to 1.22) | 0.40 |
Figure 4. Subgroups analyses of Early and Delayed surgery time for overall mortality.
The association between the OR for being operated earlier or later and study baseline risk approached statistical significance, but did not cross it (regression coefficient: −0.14; 95% Bayesian credible interval from −0.40 to 0.09) i.e., the decrease in mortality baseline risk corresponds to a decrease in the difference between early or late surgery (OR close to 1). The probability of the regression coefficient being different from nil was 0.88%.
Time to surgery and secondary outcomes
We were able to do additional meta-analyses for pressure sores (six studies, 4590 patients, random effects pooled OR 0.48, 95% CI, 0.38–0.60; I2 = 0%). The studies were extremely heterogeneous in terms of post-operative complications (i.e. different complications were grouped) and hospital length of stay. Considering the subsample of studies published since 2000, based on an arbitrary period focused on the last decade, mean length of stay varied between seven and 46 days, raising concern that studies differed in their postoperative pathways and hospital discharge policies. These health service differences prevented any meta-analysis of these outcomes. We found data from one study each for post-operative chronic pain and readmission.
Small study effects
Visual inspection of the contour-enhanced funnel plot ( Figure 5 ) indicated that pooled data did not appear to be heavily influenced by publication bias. This means that slight asymmetry of the plot is possible, with relatively few studies existing midway in the area of non-significance. It is also possible that others are ‘missing’ from this area. Nevertheless Harbord’s test was not statistically significant (P = 0.173).
Figure 5. Contour enhanced funnel plot of studies comparing Early and Delayed surgery time for overall mortality.
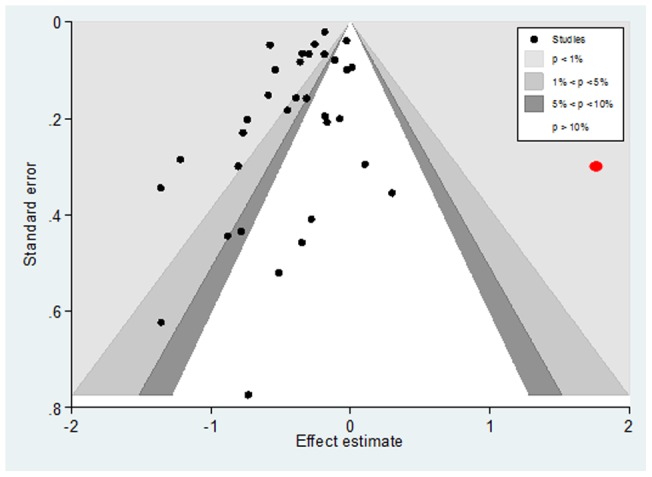
Caption: Kenzora 1986 (in red), while laying in the area of statistical significance favouring late surgery, may have interfered with the effect of small studies in the funnel plot.
Discussion
This meta-analysis of 35 studies showed that elderly patients operated for hip fracture sooner – i.e. within one or two days from hospital admission – have significantly less mortality than patients scheduled for surgery after the second day. After adjustment for age, female prevalence, location, and year, or after omitting low-quality and retrospective studies, this association remained consistently significant. This result was resistant to conservative approaches that increase the confidence attributed to significant effects based on nominal statistical significance. Observational study designs have limitations, but our results do indicate that there probably are differences in mortality outcomes between early and delayed hip fracture surgery, and may even be large in terms of patient benefits. Given the wide diversity of these studies and the frequent lack of control for clinically relevant confounders such as comorbidities, the quantitative results must be seen as merely strongly suggestive, not conclusive.
We observed substantial intra-study heterogeneity that was not explained by any of the study-level variables. Given the absolute majority of non-randomized studies and the variability in settings, health status and comorbidities, adjusting factors, and databases used, some heterogeneity is to be expected. Relying on quantitative inferences generated by observational studies, however, can lead to misleading claims when the overall data suffers from substantial heterogeneity that remains unexplained. On the other hand we explored and found no major differences between studies with adjustments for covariates or unadjusted studies and between studies using clinical or administrative data sources: This might limit the effects of confounders.
In patients immobilized for hip fractures, mortality is influenced by multiple risk factors. Less than half the studies considered comorbidity. Other covariates, such as the centre in multi-centre studies or cognitive impairment, were often ignored. Inadequacies in adjusting for important confounders is an important threat to this systematic review and it is very hard to make inferences or probe their exact depth ex post. This might reflect the excessive use of administrative databases rather than medical records to obtain a critical mass of data. Administrative databases are clearly a poor source of information on even major confounders and the rudimentary quality of this type of data has been questioned [25]. Another caveat is that large-scale studies based on administrative databases may have far more weight in a meta-analysis than smaller studies based on meticulous examination of full health records, even using methods to down-weight the spurious precision (i.e. random effects model).
Other threats to internal validity are plausible and relevant to this systematic review. Selection bias is the confounding of treatment effects with population differences: clinicians may without hesitation refer healthier patients for surgery, so differences in mortality tend to be confounded by the indication to surgery. This bias could be combined with another threat: in some studies patients were considered ineligible on the basis of acute medical conditions and surgical delays due to admission and pre-operative management. This builds in the plausible likelihood of group differences that can masquerade as treatment effect. If treatment improves the outcome and patients operated earlier are on average healthier, they could gain more from the treatment, returning to health faster than the controls, thus boosting the differences between groups.
There are other systematic reviews published in a short time on the relation between mortality and hip surgery [59], [60]. Our results confirm the previous findings by Simunovic et al [15]. Although the large number of patients and studies increases the power of our meta-analysis, the precision of effect sizes should not be overinterpreted. Our meta-analysis reports the prediction interval that addresses the actual dispersion of effect sizes across studies and shows little change with more studies. The Bayesian meta-analysis predicts values of OR larger than 1 in about 20% of future studies. Thus, the mean benefit is unlikely to be found across all patients and clinical settings. Early surgery may not save lives, or can even cause more deaths. The interplay of clinical and organizational determinants of quality of care and the potential selection of patients at higher or lower risk of mortality at each hospital makes surgery and admission for hip fracture a complex procedure. In Table 4 methods and results of the review by Simunovic are compared to this review to highlight the similar conclusions made by two independent groups.
Table 4. Similarities and differences between this systematic review and an independent one by Simunovic et al. 2010 [15].
| Systematic review | Simunovic 2010 | Moja 2012 |
| Objectives | We conducted a systematic review and meta-analysis to determine the effect of early surgery on the risk of death and common postoperative complications among elderly patients with hip fracture. | The aims of this systematic review are: (i) to identify and describe all the studies which assessed whether operative delay increases the mortality of elderly patients treated surgically for hip fracture; (ii) to see whether delay is associated with increased mortality; and (iii) to examine the influence of a priori selected variables (age, sex, co-morbidities, etc.). |
| Results on mortality | ||
| Overall | 16 studies, 13 478 patients, RR 0.55, 95% CI 0.40–0.75, p<0.001, I2 = 71%. | 34 studies, 191 873 patients, OR 0.74, 95% CI 0.67 to 0.81, P<0.0001, I2 = 85%. |
| High-quality studies | 5 studies, 4 208 patients, RR 0.81, 95% CI 0.68–0.96, p = 0.01, I2 = 0%. | 8 studies, 33435 patients OR: 0.81; 95%CI: 0.71 to 0.59, p = 0.0001, I2 = 86%. |
| Authors’ conclusions on mortality | Earlier surgery was associated with a lower risk of death and lower rates of postoperative pneumonia and pressure sores among elderly patients with hip fracture. These results suggest that reducing delays may reduce mortality and complications. | Surgical delay is associated with a significant increase in the risk of death and pressure sores. Conservative timing strategies should be limited to patients who may benefit most. Orthopaedic surgery services should ensure the majority of patients are operated between one and two days. |
| Methods | ||
| Outcomes | Mortality, pressure sores, pneumonia, deep vein thrombosis, pulmonary embolism | Mortality, pressure sores |
| Eligibility criteria | i) Patients 60 years of age or older; ii) who underwent surgery for a low-energy hip fracture; iii) evaluation of preoperative surgical delay; iv) consideration of all-cause mortality as an outcome; and v) prospective design. | i) Randomized, quasi-randomized (i.e. allocation based on date of admission), prospective and retrospective cohort and case-controlled studies; ii) inclusion of patients with operated hip fractures; iii) inclusion of timing of hip surgery; iv) inclusion of patients older than 65 years (median or mean age per study); v) survival status adequately reported for meta-analysis. |
| Study identification | No language and year restrictions. | Studies published in English, French, Italian or Spanish after 1980. |
| Risk of bias | Newcastle-Ottawa Scale criteria | Newcastle-Ottawa Scale criteria |
| Summary statistics | Relative Risk | Odds Ratio |
| Statistical approaches | Random-effects model of DerSimonian and Laird. | Primary: Random-effects model of DerSimonian and Laird, prediction interval. Secondary: fixed-effect model of Mantel-Haenszel and Bayesian. |
| Stratification for | Time according to follow-up mortality (short, medium and long-term). | Time according to cut-off points for surgery (12, 24, 48, and over 48 hours). |
| Strengths | Extensive study search Postoperative complications. | Meta-regression and Bayesian meta-analysis. |
Many different cut-off times have been used to distinguish early and late surgery: most studies used 24 and 48 hours while a few others used shorter (up to 6 hours) or longer (up to 72 hours) times. Although we grouped studies based on the cut-off time selected, it is difficult to draw a precise line between early and delayed surgery, and it is clear that all these observational comparison groups could still be unbalanced and give rise to significant biases. The optimal timing might be between 12 and 48 hours, identifying two time windows: an immediate timing strategy so that surgery is scheduled on the same or next calendar day after hospital admission and a rapid early timing strategy so that surgery is scheduled within two days of admission.
Although a randomized trial in this context has been opposed because it could be unethical [7], a stronger design such as a randomized trial evaluating the timing of surgery (immediate or rapid) is an attractive idea to reduce the threats supporting causal inferences. This approach is generally accepted in cardiology where there are several examples of randomized trials evaluating the optimal timing of invasive interventions [61], [62]. In our systematic review we found only one RCT examining the effect of delayed hip surgery in the context of a multifaceted intervention [57]. Although this trial suffered by several shortcomings including poor intervention integrity, it can still be considered a key-milestone study because it offered patients a potential genuine equipoise. If observational designs are preferred, we invite investigators to use more quasi-experimental design elements: propensity scores based on medical records have been successfully used in some studies [41], [45] and merit more attention.
In Italy, only one third of patients are operated within three days after admission (region ranges from 11% to 60%) [63]. The feasibility of operating sooner after the injury depends on the efficient use of hospital resources: key contextual elements are the number of surgeons, anaesthetists and operating rooms available and the absence of prolonged clinical assessments due to administrative delays or low-clinical-value investigations. The results of this study encourage hospitals to shorten the time from admission to hip fracture surgery: operating rooms available by night and day 33, 48, application of risk scores at admission [37], multi-disciplinary management 51, and timing as a quality indicator [56], have all been proposed to improve quality. Clearly, further economic analyses are needed to assess the cost-effectiveness of these strategies in various health care systems.
Early hip fracture surgery does appear to provide a survival benefit in comparison with later intervention; it was also associated with a significant reduction in pressure sores. Conservative timing strategies should be limited to patients who will benefit most (i.e. those requiring stabilization) because, besides consuming considerable resources, and physician and nursing time, they may severely affect a patient’s health. Cardiac or renal failure is a compelling reason for delay: cardiologists or nephrologists are consulted to set the timing for surgery, and patients often require additional treatments and tests that take time. This unavoidable delay keeps the patient in bed, increasing the risk of pulmonary, skin and urinary infections and may erode the benefit brought by the specialist approach. Whenever possible the consultation should be completed in 24–48 hours. Administrative delays are unjustifiable. This strategy should be pursued in high- and low-volume hip fracture centres. The early surgery strategy is not intended as a race against time to operate patients in a few hours but everything possible should be done to ensure the majority of patients are operated within one to two days.
Supporting Information
Electronic search strategies.
(DOC)
Acknowledgments
The authors wish to thank Judith Baggott, Haris Vasiliadis and Gustavo Zanoli who helped with translation or provided comments and editorial input to this review; Vanna Pistotti who revised the search strategy; Michela Cinquini and Rita Banzi who helped with data extraction from studies.
Funding Statement
This review was funded by the Istituto di Ricovero e Cura a Carattere Scientifico Galeazzi. Another source of institutional support was the Department of Public Health of the University of Milan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Parker M, Johansen A (2006) Hip fracture. Bmj 333: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Improvement IfIa (2006) Delivering quality and value: focus on fractured neck of femur. NHS.
- 3. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB (2009) Incidence and mortality of hip fractures in the United States. JAMA 302: 1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leslie WD, O'Donnell S, Jean S, Lagace C, Walsh P, et al. (2009) Trends in hip fracture rates in Canada. JAMA 302: 883–889. [DOI] [PubMed] [Google Scholar]
- 5. Roberts SE, Goldacre MJ (2003) Time trends and demography of mortality after fractured neck of femur in an English population, 1968–98: database study. Bmj 327: 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weller I, Wai EK, Jaglal S, Kreder HJ (2005) The effect of hospital type and surgical delay on mortality after surgery for hip fracture. Journal of Bone & Joint Surgery – British Volume 87: 361–366. [DOI] [PubMed] [Google Scholar]
- 7. Novack V, Jotkowitz A, Etzion O, Porath A (2007) Does delay in surgery after hip fracture lead to worse outcomes? A multicenter survey. International Journal for Quality in Health Care 19: 170–176. [DOI] [PubMed] [Google Scholar]
- 8. Bottle A, Aylin P (2006) Mortality associated with delay in operation after hip fracture: observational study. Bmj 332: 947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gdalevich M, Cohen D, Yosef D, Tauber C (2004) Morbidity and mortality after hip fracture: the impact of operative delay. Archives of Orthopaedic & Trauma Surgery 124: 334–340. [DOI] [PubMed] [Google Scholar]
- 10. Mak JC, Cameron ID, March LM (2010) Evidence-based guidelines for the management of hip fractures in older persons: an update. Med J Aust 192: 37–41. [DOI] [PubMed] [Google Scholar]
- 11.Scottish Intercollegiate Guideline Network (SIGN) (2009) SIGN 111: Management of hip fracture in older people. Edinburgh: Scottish Intercollegiate Guideline Network.
- 12. Orosz GM, Hannan EL, Magaziner J, Koval K, Gilbert M, et al. (2002) Hip fracture in the older patient: reasons for delay in hospitalization and timing of surgical repair. Journal of the American Geriatrics Society 50: 1336–1340. [DOI] [PubMed] [Google Scholar]
- 13. Bergeron E, Lavoie A, Moore L, Bamvita J-M, Ratte S, et al. (2006) Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? [see comment]. Journal of Trauma-Injury Infection & Critical Care 60: 753–757. [DOI] [PubMed] [Google Scholar]
- 14. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL (2002) The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. American Journal of Medicine 112: 702–709. [DOI] [PubMed] [Google Scholar]
- 15. Simunovic N, Devereaux PJ, Sprague S, Guyatt GH, Schemitsch E, et al. (2010) Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ 182: 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handoll HH, Sherrington C (2007) Mobilisation strategies after hip fracture surgery in adults. Cochrane Database Syst Rev: CD001704. [DOI] [PubMed]
- 17.Parker MJ, Handoll HH, Griffiths R (2004) Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev: CD000521. [DOI] [PubMed]
- 18.Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, et al. (2008) Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008] The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org. Accessed 2010 Dec 15.
- 19.Higgins JPT, Altman DG (2008) Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008] The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org. Accessed 2010 Dec 15.
- 20.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, et al. (2007) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed: August 2012.
- 21. Higgins JP, Thompson SG, Spiegelhalter DJ (2009) A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 172: 137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 23.Sterne JAC, Egger M, Moher D (2008) Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions: Version 5.0.1 [updated September 2008] The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org. Accessed: 2010 Dec 15.
- 24. Salanti G, Ioannidis JP (2009) Synthesis of observational studies should consider credibility ceilings. J Clin Epidemiol 62: 115–122. [DOI] [PubMed] [Google Scholar]
- 25. Papanikolaou PN, Christidi GD, Ioannidis JP (2006) Patient outcomes with teaching versus nonteaching healthcare: a systematic review. PLoS Med 3: e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palmer TM, Peters JL, Sutton AJ, Moreno SG (2008) Contour-enhanced funnel plots for meta-analysis. Stata J 8: 242–254. [Google Scholar]
- 27. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2008) Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 61: 991–996. [DOI] [PubMed] [Google Scholar]
- 28. Harbord RM, Egger M, Sterne JA (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25: 3443–3457. [DOI] [PubMed] [Google Scholar]
- 29.StataCorp (2009) Stata Statistical Software: Release 11. College Station, TX: StataCorp LP.
- 30.Spiegelhalter DJ, Thomas A, Best NG (2000) WinBUGS Version 1.3 User Manual. Cambridge: MRC Biostatistics Unit. Available at: www.mrc-bsu.cam.ac.uk/bugs. Accessed: 2010 Dec 15.
- 31. Carretta E, Bochicchio V, Rucci P, Fabbri G, Laus M, et al. (2011) Hip fracture: effectiveness of early surgery to prevent 30-day mortality. Int Orthop 35: 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Ani AN, Samuelsson B, Tidermark J, Norling A, Ekstrom W, et al. (2008) Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. Journal of Bone & Joint Surgery – American Volume 90: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 33. Bredahl C, Nyholm B, Hindsholm KB, Mortensen JS, Olesen AS (1992) Mortality after hip fracture: results of operation within 12 h of admission. Injury 23: 83–86. [DOI] [PubMed] [Google Scholar]
- 34. Davis TR, Sher JL, Porter BB, Checketts RG (1988) The timing of surgery for intertrochanteric femoral fractures. Injury 19: 244–246. [DOI] [PubMed] [Google Scholar]
- 35. Dorotka R, Schoechtner H, Buchinger W (2003) The influence of immediate surgical treatment of proximal femoral fractures on mortality and quality of life. Operation within six hours of the fracture versus later than six hours [see comment]. Journal of Bone & Joint Surgery – British Volume 85: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 36. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V (2004) The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr 39: 179–185. [DOI] [PubMed] [Google Scholar]
- 37. Elliott J, Beringer T, Kee F, Marsh D, Willis C, et al. (2003) Predicting survival after treatment for fracture of the proximal femur and the effect of delays to surgery. J Clin Epidemiol 56: 788–795. [DOI] [PubMed] [Google Scholar]
- 38. Holt G, Smith R, Duncan K, Finlayson DF, Gregori A (2008) Early mortality after surgical fixation of hip fractures in the elderly: an analysis of data from the scottish hip fracture audit. J Bone Joint Surg Br 90: 1357–1363. [DOI] [PubMed] [Google Scholar]
- 39.Kenzora JE, McCarthy RE, Lowell JD, Sledge CB (1984) Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res: 45–56. [PubMed]
- 40. Maggi S, Siviero P, Wetle T, Besdine RW, Saugo M, et al. (2009) A multicenter survey on profile of care for hip fracture: predictors of mortality and disability. Osteoporos Int 21: 223–231. [DOI] [PubMed] [Google Scholar]
- 41. Majumdar SR, Beaupre LA, Johnston DWC, Dick DA, Cinats JG, et al. (2006) Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study [see comment]. Medical Care 44: 552–559. [DOI] [PubMed] [Google Scholar]
- 42.McGuire KJ, Bernstein J, Polsky D, Silber JH (2004) The 2004 Marshall Urist award: delays until surgery after hip fracture increases mortality. Clinical Orthopaedics & Related Research: 294–301. [DOI] [PubMed]
- 43. Moran CG, Wenn RT, Sikand M, Taylor AM (2005) Early mortality after hip fracture: is delay before surgery important? Journal of Bone & Joint Surgery – American Volume 87: 483–489. [DOI] [PubMed] [Google Scholar]
- 44.Mullen JO, Mullen NL (1992) Hip fracture mortality. A prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res: 214–222. [PubMed]
- 45. Orosz GM, Magaziner J, Hannan EL, Morrison RS, Koval K, et al. (2004) Association of timing of surgery for hip fracture and patient outcomes. JAMA 291: 1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parker MJ, Pryor GA (1992) The timing of surgery for proximal femoral fractures. Journal of Bone & Joint Surgery – British Volume 74: 203–205. [DOI] [PubMed] [Google Scholar]
- 47. Peleg K, Savitsky B, Yitzhak B, Avi I (2011) Different reimbursement influences surviving of hip fracture in elderly patients. Injury 42: 128–132. [DOI] [PubMed] [Google Scholar]
- 48. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, et al. (2008) Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am 90: 34–42. [DOI] [PubMed] [Google Scholar]
- 49. Rademakers LMF, Vainas T, van Zutphen 2 SWAM, Brink PRG, van Helden SH (2007) Pressure Ulcers and Prolonged Hospital Stay in Hip Fracture Patients Affected by Time-to-Surgery. European Journal of Trauma and Emergency Surgery 33: 238–244. [DOI] [PubMed] [Google Scholar]
- 50. Rae HC, Harris IA, McEvoy L, Todorova T (2007) Delay to surgery and mortality after hip fracture. ANZ Journal of Surgery 77: 889–891. [DOI] [PubMed] [Google Scholar]
- 51. Roos LL, Walld RK, Romano PS, Roberecki S (1996) Short-term mortality after repair of hip fracture. Do Manitoba elderly do worse? Medical Care 34: 310–326. [DOI] [PubMed] [Google Scholar]
- 52. Sexson SB, Lehner JT (1987) Factors affecting hip fracture mortality. Journal of Orthopaedic Trauma 1: 298–305. [DOI] [PubMed] [Google Scholar]
- 53. Siegmeth AW, Gurusamy K, Parker MJ (2005) Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. Journal of Bone & Joint Surgery – British Volume 87: 1123–1126. [DOI] [PubMed] [Google Scholar]
- 54. Smektala R, Endres HG, Dasch B, Maier C, Trampisch HJ, et al. (2008) The effect of time-to-surgery on outcome in elderly patients with proximal femoral fractures. BMC Musculoskelet Disord 9: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stoddart J, Horne G, Devane P (2002) Influence of preoperative medical status and delay to surgery on death following a hip fracture. ANZ J Surg 72: 405–407. [DOI] [PubMed] [Google Scholar]
- 56. Sund R, Liski A (2005) Quality effects of operative delay on mortality in hip fracture treatment. Quality & Safety in Health Care 14: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Swanson CE, Day GA, Yelland CE, Broome JR, Massey L, et al. (1998) The management of elderly patients with femoral fractures. A randomised controlled trial of early intervention versus standard care. Medical Journal of Australia 169: 515–518. [PubMed] [Google Scholar]
- 58. Verbeek DOF, Ponsen KJ, Goslings JC, Heetveld MJ (2008) Effect of surgical delay on outcome in hip fracture patients: a retrospective multivariate analysis of 192 patients. International Orthopaedics 32: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shiga T, Wajima Z, Ohe Y (2008) Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anaesth 55: 146–154. [DOI] [PubMed] [Google Scholar]
- 60. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ (2009) Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury 40: 692–697. [DOI] [PubMed] [Google Scholar]
- 61. Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, et al. (2009) Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 360: 2165–2175. [DOI] [PubMed] [Google Scholar]
- 62. Montalescot G, Cayla G, Collet JP, Elhadad S, Beygui F, et al. (2009) Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA 302: 947–954. [DOI] [PubMed] [Google Scholar]
- 63.Seccareccia F, D’Errigo P, Rosato S, Maraschini A, Badoni G, et al.. (2010) Programma “Valutazione degli esiti per promuovere il miglioramento dell’efficacia nell’erogazione delle prestazioni ricomprese nei livelli essenziali di assistenza (LEA)”. Istituto Superiore di Sanità; Rapporti ISTISAN 10/43.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic search strategies.
(DOC)