Abstract
The emergence of abundant-class mRNAs specific for contractile muscle proteins and their distribution between polysomal and free mRNP fractions were studied in skeletal muscle excised from chicken embryos during the transition from myoblasts (day 9) to myotubes (day 18). Muscle-specific cDNA was selectively prepared by hybridizing cDNA to template RNA (polysomal poly(A)+ mRNA) from day-14 embryos followed by isolation of the abundant class, which represents approximately 20% of total mRNA. The specificity of the cDNA probe for this class was confirmed by the differential degree of hybridization to cytoplasmic RNA from cultured myotube and myoblast cells and by its inability to hybridize with mRNA from nonmuscle cells such as liver. Except for muscle from day-9 embryos, the concentrations of the abundant-class muscle-specific mRNAs were higher in polysomes than in free mRNP fractions. Furthermore, the levels of these mRNAs in polysomes increased 12-fold from day 9 (myoblast) to day 14 (intermediate) with a further 3.6-fold increase from day 14 to day 18 (myotube). In contrast to this 45-fold net increase in the polysomal level of these mRNAs from day 9 to day 18, the levels in the free mRNP fraction showed only a 3-fold decrease during this period. Because the amount of mRNA lost from the mRNP fraction is much less than the net increase in the polysome fraction, mRNP does not serve as a reservoir of untranslated muscle-specific mRNA for transfer to polysomes. Consequently, the emergence of muscle-specific polysomal mRNA for contractile proteins during myogenesis in ovo appears to be regulated primarily by transcriptional control.
Full text
PDF

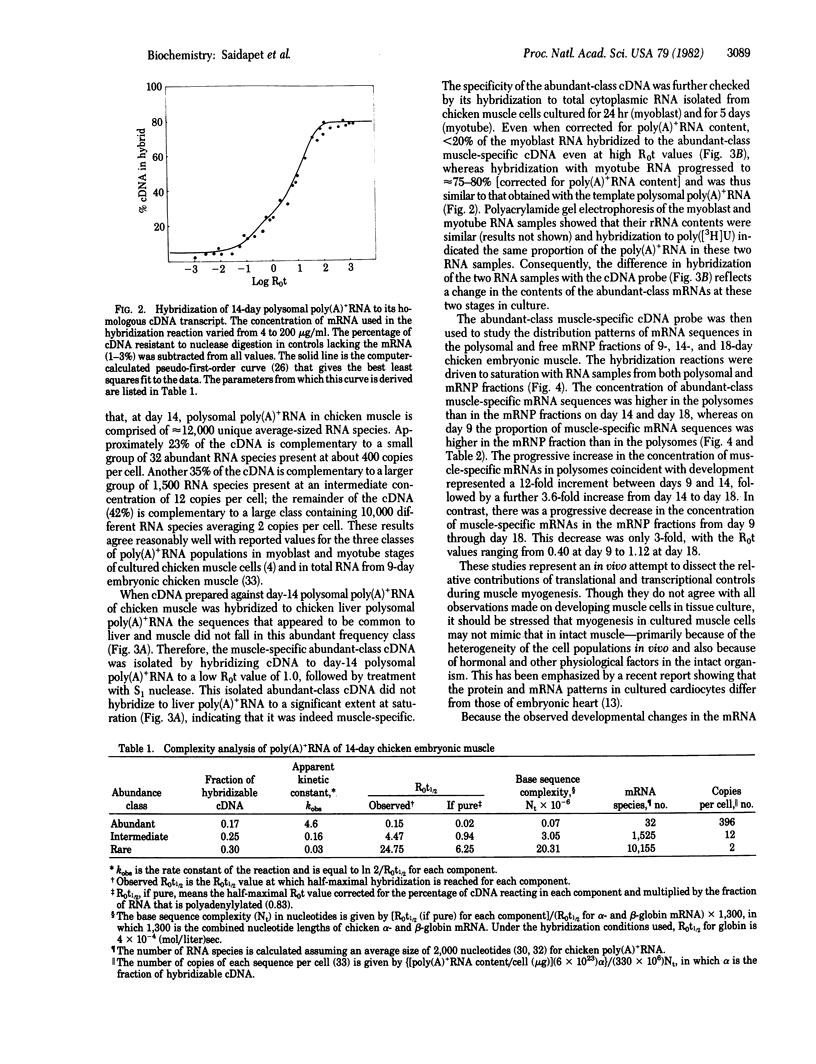
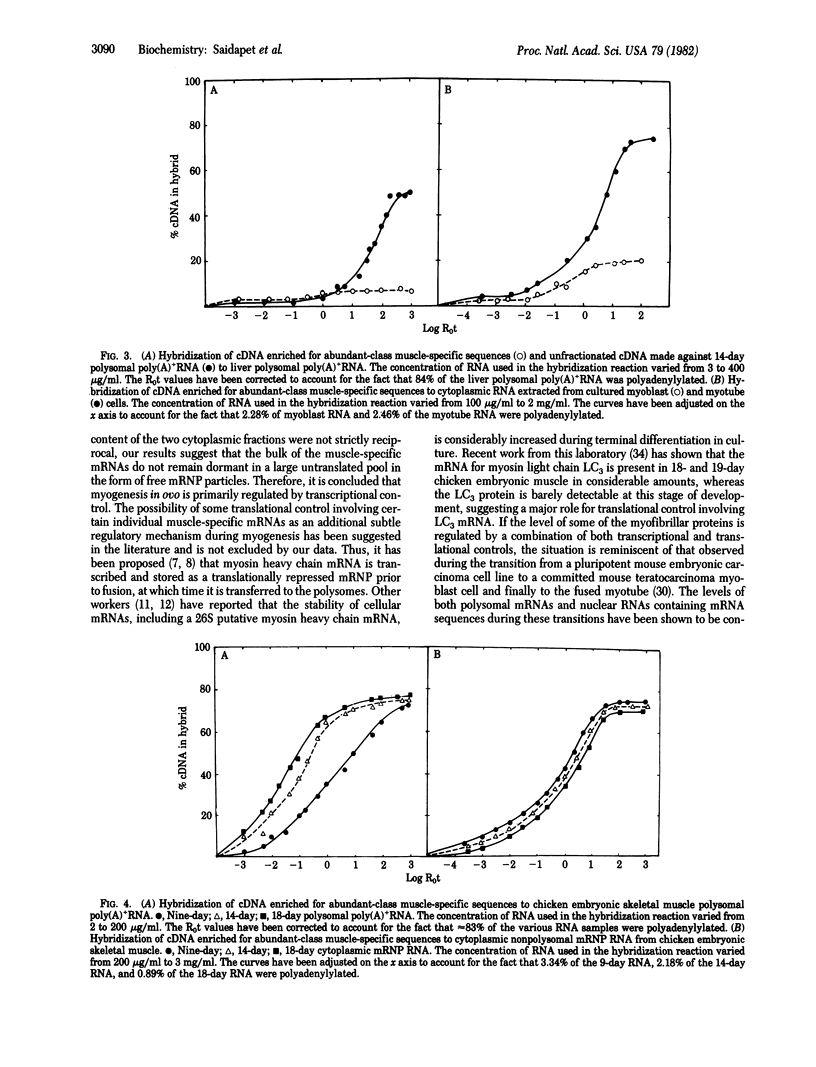

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N. A., Robert B., Jacquet M., Buckingham M. E., Gros F. Changes in gene expression during myogenic differentiation. I. Regulation of messenger RNA sequences expressed during myotube formation. J Mol Biol. 1980 Jul 15;140(4):441–458. doi: 10.1016/0022-2836(80)90264-8. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Bergmann I. E., Brawerman G. Loss of the polyadenylate segment from mammalian messenger RNA. Selective cleavage of this sequence from polyribosomes. J Mol Biol. 1980 May 25;139(3):439–454. doi: 10.1016/0022-2836(80)90140-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Breton R., Fiszman M., Gros F. Chick embryo cardiocytes in culture synthesize proteins not present in embryonic heart. Biochem Biophys Res Commun. 1980 Jul 16;95(1):281–288. doi: 10.1016/0006-291x(80)90736-6. [DOI] [PubMed] [Google Scholar]
- Buckingham M. E., Caput D., Cohen A., Whalen R. G., Gros F. The synthesis and stability of cytoplasmic messenger RNA during myoblast differentiation in culture. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1466–1470. doi: 10.1073/pnas.71.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M. E., Cohen A., Gros F. Cytoplasmic distribution of pulse-labelled poly(A)-containing RNA, particularly 26 S RNA, during myoblast growth and differentiation. J Mol Biol. 1976 May 25;103(3):611–626. doi: 10.1016/0022-2836(76)90220-5. [DOI] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Dym H. P., Kennedy D. S., Heywood S. M. Sub-cellular distribution of the cytoplasmic myosin heavy chain mRNA during myogenesis. Differentiation. 1979;12(3):145–155. doi: 10.1111/j.1432-0436.1979.tb01000.x. [DOI] [PubMed] [Google Scholar]
- Dym H., Turner D. C., Eppenberger H. M., Yaffe D. Creatine kinase isoenzyme transition in actinomycin D-treated differentiating muscle cultures. Exp Cell Res. 1978 Apr;113(1):15–21. doi: 10.1016/0014-4827(78)90082-4. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Herrmann H., Heywood S. M., Marchok A. C. Reconstruction of muscle development as a sequence of macromolecular synthesis. Curr Top Dev Biol. 1970;5:181–234. [PubMed] [Google Scholar]
- Heywood S. M., Kennedy D. S. Translational control in embryonic muscle. Prog Nucleic Acid Res Mol Biol. 1976;19:477–484. doi: 10.1016/s0079-6603(08)60939-3. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Sarkar S. Poly(riboadenylate)-containing messenger ribonucleoprotein particles of chick embryonic muscles. Biochemistry. 1979 Mar 6;18(5):745–753. doi: 10.1021/bi00572a002. [DOI] [PubMed] [Google Scholar]
- John H. A., Patrinou-Georgoulas M., Jones K. W. Detection of myosin heavy chain mRNA during myogenesis in tissue culture by in vitro and in situ hybridization. Cell. 1977 Oct;12(2):501–508. doi: 10.1016/0092-8674(77)90126-x. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Marchok A. C., Herrmann H. Studies of muscle development. I. Changes in cell proliferation. Dev Biol. 1967 Feb;15(2):129–155. doi: 10.1016/0012-1606(67)90010-3. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Schimke R. T. Ovalbumin messenger RNA: evidence that the initial product of transcription is the same size as polysomal ovalbumin messenger. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4327–4331. doi: 10.1073/pnas.71.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. Effect of estrogen on the sequence and population complexity of chick oviduct poly(A)-containing RNA. J Biol Chem. 1976 Jun 25;251(12):3738–3748. [PubMed] [Google Scholar]
- Mukherjee A. K., Guha C., Sarkar S. The translational inhibitory 10 S cytoplasmic ribonucleoprotein of chicken embryonic muscle is distinct from messenger ribonucleoproteins. FEBS Lett. 1981 May 5;127(1):133–138. doi: 10.1016/0014-5793(81)80359-6. [DOI] [PubMed] [Google Scholar]
- Ordahl C. P., Caplan A. I. High diversity in the polyadenylated RNA populations of embryonic myoblasts. J Biol Chem. 1978 Nov 10;253(21):7683–7691. [PubMed] [Google Scholar]
- Paterson B. M., Bishop J. O. Changes in the mRNA population of chick myoblasts during myogenesis in vitro. Cell. 1977 Nov;12(3):751–765. doi: 10.1016/0092-8674(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Davidson E. H., Britten R. J. A program for least squares analysis of reassociation and hybridization data. Nucleic Acids Res. 1977 Jun;4(6):1727–1737. doi: 10.1093/nar/4.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Robbins J., Heywood S. M. Quantification of myosin heavy-chain mRNA during myogenesis. Eur J Biochem. 1978 Jan 16;82(2):601–608. doi: 10.1111/j.1432-1033.1978.tb12056.x. [DOI] [PubMed] [Google Scholar]
- Roy R. K., Lau A. S., Munro H. N., Baliga B. S., Sarkar S. Release of in vitro-synthesized poly(A)-containing RNA from isolated rat liver nuclei: characterization of the ribonucleoprotein particles involved. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1751–1755. doi: 10.1073/pnas.76.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Mukherjee A. K., Guha C. A ribonuclease-resistant cytoplasmic 10 S ribonucleoprotein of chick embryonic muscle. A potent inhibitor of cell-free protein synthesis. J Biol Chem. 1981 May 25;256(10):5077–5086. [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Zevin-Sonkin D., Yaffe D. Accumulation of muscle-specific RNA sequences during myogenesis. Dev Biol. 1980 Feb;74(2):326–334. doi: 10.1016/0012-1606(80)90434-0. [DOI] [PubMed] [Google Scholar]