Abstract
Human cytomegalovirus (HCMV) infection alters the expression of many cellular genes, including IFN-stimulated genes (ISGs) [Zhu, H., Cong, J.-P., Mamtora, G., Gingeras, T. & Shenk, T. (1998) Proc. Natl. Acad. Sci. USA 95, 14470–14475]. By using high-density cDNA microarrays, we show that the HCMV-regulated gene expression profile in fibroblasts does not differ substantially from the response generated by IFN. Furthermore, we identified the specific viral component triggering this response as the envelope glycoprotein B (gB). Cells treated with gB, but not other herpesviral glycoproteins, exhibited the same transcriptional profile as HCMV-infected cells. Thus, the interaction of gB with its as yet unidentified cellular receptor is the principal mechanism by which HCMV alters cellular gene expression early during infection. These findings highlight a pioneering paradigm for the consequences of virus–receptor interactions.
As obligate intracellular parasites, viruses critically depend on cellular machinery and biosynthetic components for replication and propagation. During infection, viruses modulate host cell gene expression and influence cellular functions. High-density DNA microarrays, which allow the simultaneous monitoring of the expression of thousands of genes, represent a powerful approach to analyze the interplay between virus and cell (1, 2). Human cytomegalovirus (HCMV), a significant human pathogen that establishes a life-long relationship with its host, is known to alter cellular gene expression (3). Recently, DNA microarray analysis of human fibroblasts infected with HCMV revealed changes in the expression of hundreds of host genes (4). A major finding of this analysis was that HCMV strongly induced known IFN-stimulated genes (ISGs). Subsequently, the extent and specificity of the transcriptional response to IFN treatment also was elucidated by microarray analysis, with a greater number of genes being regulated by IFN than what was suspected previously (5). Thus it was possible that a much larger percentage of HCMV-induced genes also may be IFN responsive than was assumed previously. In an earlier study using differential-display analysis, it was shown that the HCMV-induced cellular changes in transcription did not depend on viral replication, suggesting that structural components of the virion triggered the response (3).
One of the potential candidates for this virion component is glycoprotein B (gB), the major envelope glycoprotein of HCMV. gB is a critical mediator of HCMV entry. As is true for other herpesvirus gB homologs, HCMV gB is an essential gene and strains lacking gB cannot be propagated (6). Anti-gB mAbs with neutralizing activity block HCMV infection at the level of virus entry (7). Lastly, as is true for the primary ligand of herpes simplex virus, gD, a soluble form of HCMV gB blocks virus entry and infection (8). Because ligand-receptor interactions frequently trigger signal-transduction cascades, we previously tested the ability of soluble gB to activate the expression of two IFN-inducible genes (9). In the present study, we defined the contribution of gB to HCMV-regulated changes in cellular gene expression by using a global microarray approach. Our results reveal that gB, most likely through an interaction with its cellular binding partner, is the principal mechanism by which HCMV alters cellular transcription early during infection.
Materials and Methods
RNase Protection Analysis.
PCR was used to amplify DNA fragments from genes for oligo adenylate synthetase (accession no. M87284, nucleotides 2060–2459) and IFN-stimulated gene of 54 kDa (accession no. M14659, nucleotides 1270–1609). The amplified products were cloned into the pDP19 vector (Ambion, Austin, TX). The actin probe was obtained (Ambion) commercially. RNase protection probes were generated from the cloned genes by using the MAXIscript T7 kit (Ambion) and 32P-labeled UTP (Amersham Pharmacia). Gel-purified probes and 4 μg of sample RNA were hybridized, digested, and separated by denaturing PAGE according to manufacturers instructions (RPA III kit; Ambion). The protected samples were visualized by using a phosphor imager (GS-525; Bio-Rad).
Cell Treatments and Viral Infections.
Neonatal human foreskin fibroblasts (HFFs; Clonetics, San Diego) were grown at 37°C/5% CO2 in DMEM (Life Technologies, Rockville, MD) supplemented with 10% FCS, 2 mM glutamine, and penicillin/streptomycin. Subconfluent HFF cells (5 × 106) were washed twice with PBS before the addition of HCMV (strain Ad169; multiplicity of infection = 2) in serum-free (SF) DMEM. Virus was present in the media for the entire length of the treatment. Similarly, IFN treatments were performed in SF DMEM using Type I (α and β) recombinant human IFNs (100 units/ml; BioSource International, Camarillo, CA). Recombinant HCMV gB was overexpressed and purified as described (9) and used at a final concentration of 13 μg/ml in SF DMEM. Mock cells were treated in an identical manner except no ligand was added to the SF DMEM. All cell treatments were repeated in at least two independent assays, with similar results obtained. Here we report a detailed analysis of one complete experiment.
RNA Preparation and Microarray Analysis.
Total RNA was extracted from cells by using the RNeasy kit (Qiagen, Chatsworth, CA), treated with DNase, and repurified. One round of T7 polymerase-based linear RNA amplification was performed by reverse transcription of RNA with a T7 promoter oligo(dT) primer and Cy3-dCTP-labeled fluorescent cDNA probes synthesized from the amplified RNA as described (10), except that to degrade the amplified RNA template, RNaseH (10 units) and RNaseA (10 units) were added and incubated at 37°C for 20 min. Then the probes were purified with a PCR purification kit (Qiagen), vacuum-dried, and resuspended in 50 μl of hybridization buffer [Version 2 hybridization buffer (Amersham Pharmacia Biotech, Piscataway, NJ) with 50% formamide] containing human Cot1 DNA (Life Technologies).
The cDNAs printed on the microarrays were from the IMAGE consortium (Integrated Molecular Analysis of Genome and their Expression) and Incyte libraries. All clones were sequence-verified before PCR amplification. The IMAGE clones were purchased from the Human UniGene Library (Research Genetics, Huntsville, AL). cDNAs were printed in duplicate on amino silane-coated slides (Corning) by using a Generation III Microarray Spotter (Molecular Dynamics). The cDNAs were PCR amplified and purified with a Qiagen 96 PCR purification kit, then were mixed 1:1 with a 10 M NaSCN printing buffer. The spots were ≈250 μm in diameter with a 280-μm center-to-center spacing. Each microarray included 30 plant genes for the determination of nonspecific hybridization (gift from Mark Schena, Stanford University). Printed microarrays were incubated in isopropanol at room temperature for 10 min. The probes were heated to 95°C for 2 min, to room temperature for 5 min, and applied to the slides. The slides were covered with glass coverslips, sealed with DPX (Fluka), and hybridized at 42°C overnight. Each probe was hybridized to two microarrays, each containing duplicate spots for each cDNA. Fluorescence intensity for each feature of the array was obtained by using autogene software (Biodiscovery, Los Angeles). A single raw-expression level for each gene was derived from the average of the spots representing each gene, and the coefficient of variation was calculated to control the data quality. The intensity level of each microarray was normalized so that the 75th percentile of the expression levels was equal across microarrays. Before calculating ratios, a threshold of 35 was assigned to any gene with an expression level below 35, because 35 represents the background intensity level. The raw data are presented in the supplemental data (which is published on the PNAS web site, www.pnas.org) without threshold adjustment.
Statistical Analysis of Coregulation.
Four hundred forty-one genes changed >2.3-fold in expression in Fig. 3 and constituted the sample for this probability calculation. The number of genes up-regulated by HCMV, IFN, and gB at 24 h were 52, 78, and 79, respectively. HCMV and IFN coregulated 32 genes, and HCMV and gB coregulated 35 genes. The probability (for the 32-gene example) was calculated as P = (441C52 ⋅ 52C32 ⋅ (441–52)C(78–32))/(441C52 ⋅ 441C78) = 1.696 × 10−14. nCk is the number of potential combinations of n items where k have been selected.
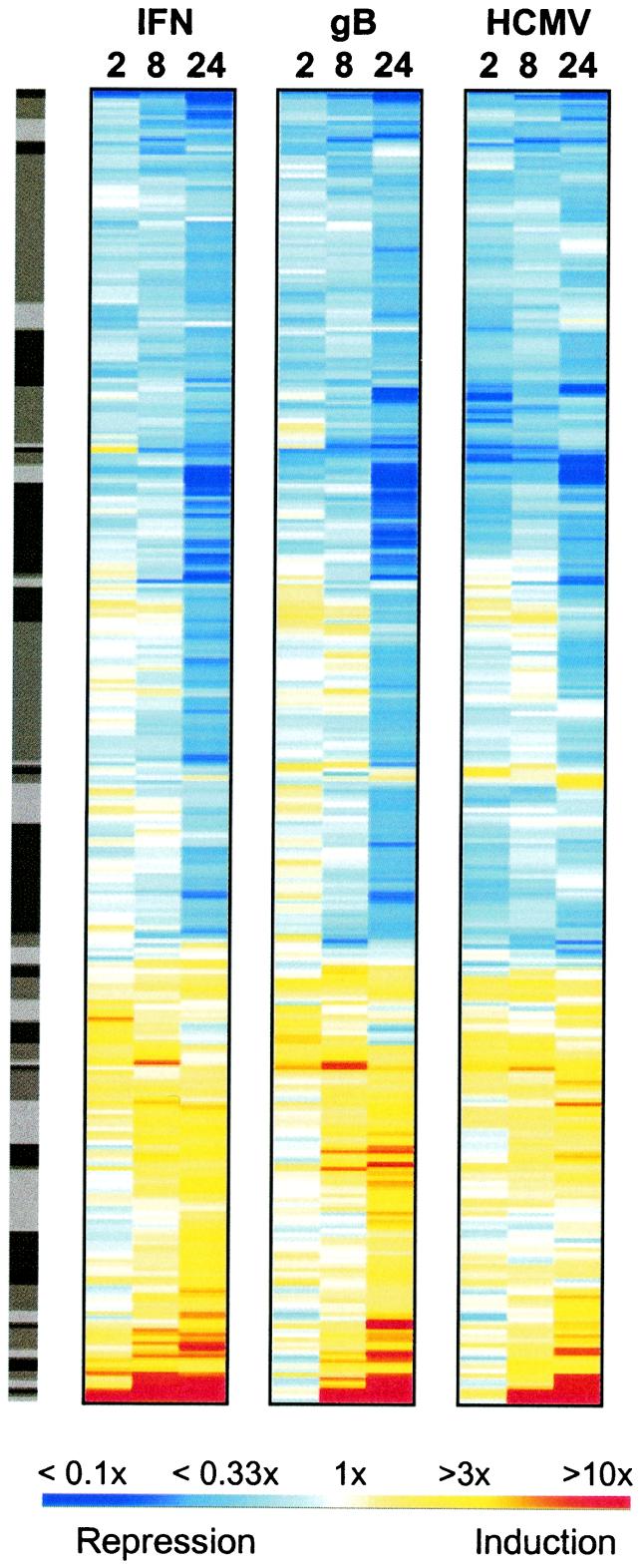
Figure 3.
Cluster analysis of genes changing in response to IFN, gB, or HCMV infection. Genes changing more than 2.3-fold in any one of the treatments were selected for hierarchical cluster analysis (using logarithm e transforms of the ratios) with the OMNIVIZ PRO software package (OmniViz, Maynard, MA), using the CorScape visualization. Depicted ratios were derived by comparing hybridization signals obtained from virus-, gB-, or IFN-treated cells to those of mock treatments. All ratios for which the mock intensity had a coefficient of variation of >50% were removed. This analysis yielded a set of 441 genes. Each gene is represented as a horizontal strip, with the color representing the relative linear induction or repression ratio as depicted by the color scale. The distribution of the genes into 50 clusters (shown in the gray sidebar) was generated with OMNIVIZ PRO, and the clusters are ordered similarly according to the hierarchical correlation algorithm used. The fluorescence intensities and ratios (relative to the mock) are provided in Table 2, which is published as supplemental data on the PNAS web site.
Results
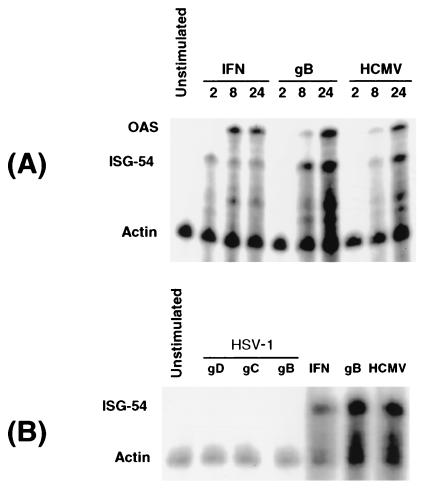
To investigate the specificity of ISG induction by gB further, expression of known ISGs [ISG-54 and oligo adenylate synthetase (OAS)] was examined in HFFs after incubation with HCMV gB and a panel of recombinant herpesviral glycoproteins by using RNase protection analysis. As shown in Fig. 1A, HCMV and purified gB elicited strong induction of ISG-54 and OAS by 8 h posttreatment. As reported, the kinetics of induction were delayed relative to IFN treatment (3, 9). By contrast, no ISG induction was observed after treatment with recombinant herpes simplex virus (HSV) glycoproteins gB, gC, and gD (Fig. 1B). The HSV glycoproteins were purified in a manner analogous to HCMV gB. Moreover, all three HSV glycoproteins have documented cell-binding activities: gB and gC bind to cell-surface heparin-sulfate proteoglycans, and gD binds to a family of HSV entry mediators (11). Thus, we conclude that the observed ISG induction by HCMV gB is specific and is not a function shared by all herpesviral glycoproteins, nor is it a trace contaminant inherent to production and purification from insect cells.
Figure 1.
Specificity of viral induction of ISGs. (A) HFFs were incubated with recombinant Type I IFNs (100 units/ml), recombinant HCMV gB (13 μg/ml), or HCMV [Ad169 strain, at a multiplicity of infection (MOI) of 2], and RNA samples were harvested at 2, 8, and 24 h posttreatment. The samples were analyzed by RNase protection with radiolabeled probes to oligo adenylate synthetase (OAS), ISG of 54 kDa (ISG-54), and actin. The protected samples were separated by denaturing gel electrophoresis and detected by using a phosphor imager (GS-525; Bio-Rad). (B) HFF cells were incubated with purified recombinant forms of HSV glycoproteins (gD, gC, and gB, at 13 μg/ml), recombinant Type I IFNs (100 units/ml), HCMV gB (13 μg/ml), and HCMV (MOI = 2) for 8 h. RNase protection analysis using probes to ISG-54 and actin was performed on RNA from treated samples, as described above. The apparent increase in actin signal seen after both HCMV and gB treatments was because of degradation of protected ISG-54 probes, which are more abundant in those samples with elevated ISG-54 message.
The IFN-stimulated genes ISG-54 and oligo adenylate synthetase were induced by HCMV with kinetics similar to gB (Fig. 1A). However, many other genes are known to be IFN-inducible (5), leaving the possibility that only a subset of ISGs were regulated by HCMV infection or gB treatment. Furthermore, the extent of overlap in gene expression between HCMV infection compared with gB treatment was not known. Thus, to investigate what fraction of the HCMV-mediated gene regulation is attributable to gB we applied global gene-transcriptional profiling by DNA microarrays.
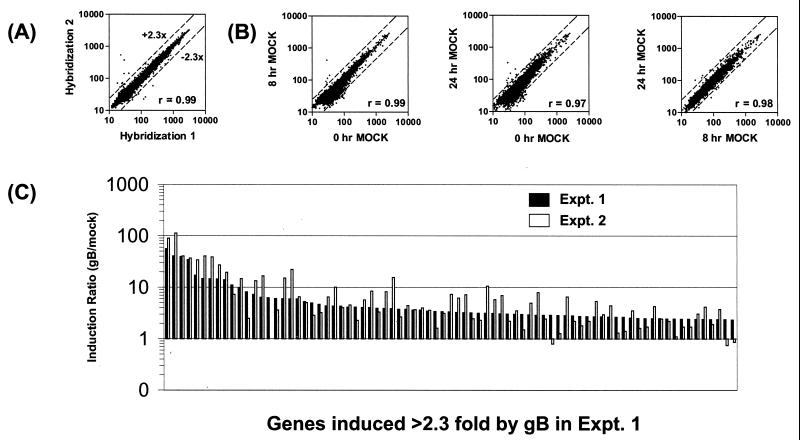
In preliminary experiments, we investigated the extent of microarray-to-microarray variation by using our single-dye labeling and detection method. Two independent hybridizations were performed by using a batch of fluorescent Cy-3-labeled cDNA probes prepared from fibroblast RNA. Each sample was hybridized to two identical arrays, each harboring two spots for each cDNA. Mean signal intensities obtained from each microarray hybridization were compared by scatter-plot analysis. A high correlation coefficient (Fig. 2A; r = 0.99) was obtained. Therefore, the single-dye method was used in subsequent experiments. This comparison also was used to set the threshold for changes regarded as significant. In Fig. 2A, 99.8% of the signals changed less than 2.3-fold because of nonspecific variation. Therefore, in this study, changes greater than 2.3-fold are regarded as significant. In addition, we addressed whether it would be necessary to use time-matched mock controls for the time course experiments. Fibroblasts were mock-treated and RNA was isolated after 0, 8, and 24 h. After labeling and microarray hybridization, comparison between the signals obtained for each time point revealed high correlation values and only minor changes in the low-intensity range (Fig. 2B). Because most of these signals changed below the 2.3-fold threshold, we conclude that the changes induced in fibroblasts as a result of mock treatment are negligible. Therefore, we decided to use a single mock-treatment sample in subsequent experiments.
Figure 2.
Microarray validation. (A) Cy3-labeled cDNA probes prepared from HFF total RNA were hybridized to two identical sets of microarrays harboring 8,942 genes. After scanning and normalization, the mean signal intensities (in arbitrary fluorescence units) from each gene were compared by using scatter-plot linear regression analysis. Greater than 99.8% of genes change less than 2.3-fold, as delineated by the dashed lines. The pair-wise similarity of gene-expression profiles was measured by the correlation coefficient (r value). (B) Gene expression of mock-treated fibroblasts at 0, 8, or 24 h was analyzed by scatter-plot analysis of signal intensities. Three comparisons, 0/8 h, 0/24 h, and 8/24 h, are shown. With few exceptions, changes were less than 2.3-fold or displayed a low-intensity signal. As a result, the r values are higher than 0.97 in the comparisons, indicating that the changes induced by mock treatment are not significant. (C) Reproducible induction of individual genes in two independent gB treatments. The induction ratios (relative to the mock) of genes induced >2.3-fold by a 24-h gB treatment in experiment (Expt.) 1 are plotted with their corresponding ratios from an independent 24-h gB treatment (Expt. 2). (Genes and ratios are provided in Table 1, which is published as supplemental data on the PNAS web site, www.pnas.org.) The genes are plotted along the x axis in order of decreasing induction in Expt. 1.
HFFs were infected with HCMV for 2, 8, or 24 h, and the cellular RNA was harvested. In parallel, RNAs were prepared from HFFs treated with recombinant HCMV gB. To further assess the overlap between HCMV-infected cells and IFN-treated cells, RNA also was isolated from fibroblasts treated with Type I IFNs for the same time periods. The time points were chosen to represent the early time period in the HCMV life cycle before viral DNA replication (which occurs ≈36 h postinfection). Fluorescent probes were generated from each RNA sample and hybridized to microarrays harboring a random collection of 8,942 human cDNAs. Expression changes for each cDNA were calculated from the fluorescence intensities relative to the values obtained from mock- treated fibroblasts. All of the infections and treatments were performed at least twice in independent assays. Although variation from experiment to experiment was observed with respect to signal intensities and ratios of induction or repression compared with mock-treated samples, most of the genes with a significantly changed ratio in one experiment were found to change above threshold level in the second experiment also. For example, comparison of data sets after two independent 24-h gB treatments of HFFs revealed that the majority of genes induced over 2.3-fold by gB treatment in one experiment were induced in the second experiment also (Fig. 2C and supplemental data). The median ratio for the induction ratios (relative to the mock) for these two experiments was 0.91 and the mean ratio was 1.1, showing the degree of reproducibility between independent gB treatments. Comparison between repeated HCMV infections and IFN treatment yielded similar results (data not shown). To minimize interexperimental variation, we performed all treatments and infections in parallel by using the same batch of cells. Moreover, the same print-batch of DNA arrays was used. This complete set of HFF treatments performed in parallel was used for detailed expression analysis.
To compare changes in gene expression between different stimuli, we used hierarchical cluster analysis of the ratios, compared with mock-treated cells, for all genes changing more than 2.3-fold in at least 1 of the 9 comparisons (12). In addition, we removed all genes (a total of 19) displaying a high coefficient of variation (>50% of the mean) in the control sample. This clustering method, using pair-wise Euclidean distances, reveals groups of genes with similar patterns of expression (13, 14). As a result, genes changing similarly on different treatments will be sorted to clusters distinct from those containing treatment-specific genes. Surprisingly, however, the cluster visualization of the 441 genes that met the selection criteria revealed a highly similar overall pattern between IFN treatment, HCMV infection, and gB treatment, suggesting that the vast majority of genes were regulated coordinately by all three stimuli (Fig. 3). By contrast, very few clusters contained genes that were up-regulated (red) strongly in one treatment and significantly down regulated (blue) in another. Moreover, such differences were mostly transient. For instance, at the 2-h time point, most genes induced strongly by IFN treatment were not induced, or only weakly induced, in the HCMV-infected or gB-treated samples with some of the genes being even slightly repressed. However, by 8 and 24 h, most of these strongly IFN-induced genes were induced in HCMV-infected or gB-treated cells also (Fig. 3). The reverse comparison also showed that genes strongly induced by HCMV (at the base of Fig. 3) were induced by IFN and gB. Of the 52 genes induced over 2.3-fold by HCMV at 24 h, 51 were induced by gB and IFN at 24 h also. Of these 51 genes, 35 (gB) or 32 (IFN) were induced >2.3-fold by gB and IFN, respectively, and 16 (gB) or 19 (IFN) were induced less than 2.3-fold. This correlation is highly significant, as shown by calculating the probability of random co-occurrence of a 2.3-fold increase in two treatments (given a sample of 441 genes, and assuming complete randomness as to each gene's behavior with regard to a >2.3-fold induction or not; see Materials and Methods). The probabilities for the random co-occurrence of 35 genes (HCMV and gB) and 32 genes (HCMV and IFN) were P = 1.1 × 10−17 and P = 1.7 × 10−14, respectively, lending strong support to the significance of the observed coregulations. The global view revealed by this analysis shows that most of the cellular genes induced or repressed by HCMV are induced or repressed, respectively, by IFN and gB treatment also.
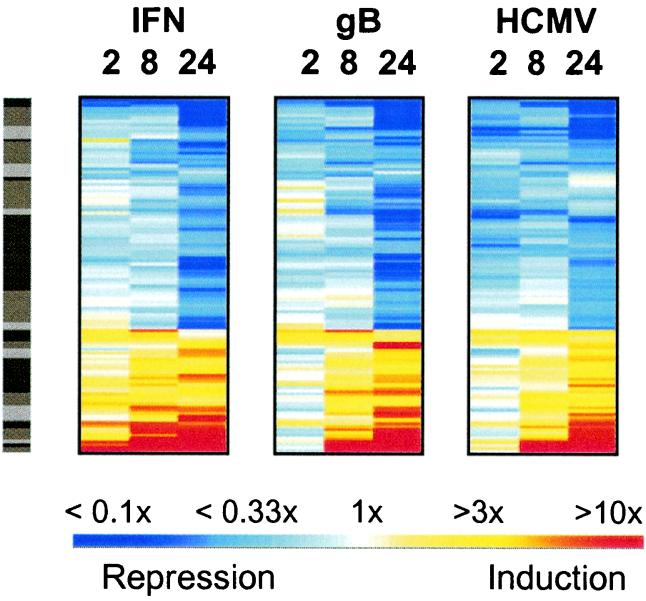
To examine whether the subset of genes showing the highest degree of coregulation by all three stimuli are known to be IFN-regulated, we focused on cellular genes that changed >2.3-fold in response to all three treatments (for at least one of the three time points). As shown in Fig. 4, a total of 112 genes fulfilled these criteria. Of these 112 (listed in Table 3, which is published as supplemental data on the PNAS web site), 14 were novel cDNAs or genes of unknown function, and 98 were known genes. Of those 98 genes, a significant proportion (20/98) consisted of known IFN-regulated genes, 11 of which were reported to be regulated by HCMV also (4, 5, 15). All other genes were not known previously to be regulated by either IFN or HCMV. This analysis clearly revealed the delayed onset of strongly induced genes in HCMV-infected and gB-treated cells compared with treatment by IFN as observed in the RNase protection experiments (Fig. 1).
Figure 4.
Stringent coregulation of genes early in viral infection. From the cluster view in Fig. 3, a second more stringent filter was applied to select only those genes changing >2.3-fold in response to all three treatments (in at least 1 of the 3 time points for each treatment). These criteria selected 112 of the 441 genes, and these were reclustered into 25 groups by using the logarithm e-transformed ratios as in Fig. 3 (using the OMNIVIZ PRO CorScape view).
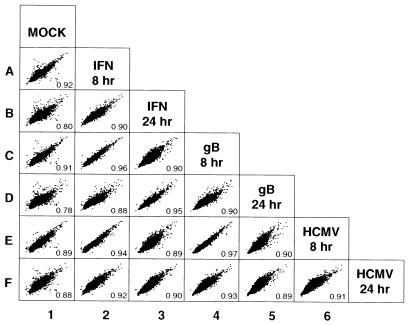
The previous analysis compared the ratios of intensities with respect to the intensities measured for mock-treated cells. To compare the cellular RNA profiles between the different stimuli directly, we used scatter-plot analysis of the log-transformed intensity values from all cDNAs on the microarrays obtained at the 8- and 24-h time points (Fig. 5). As expected, most data points lie on a diagonal regardless of the experimental comparison, because the vast majority of genes do not change after treatment or infection (Fig. 5). This fact is reflected also in observed correlation coefficients (r values) much closer to 1 than 0. The most divergent intensities, and the lowest correlation values, were obtained when IFN-treated, gB-treated, or HCMV-infected samples were compared with mock-treated cells (Fig. 5, column 1). By contrast, the comparison of IFN, HCMV, and gB treatments to each other yielded higher correlation coefficients, indicating highly similar RNA profiles. For example, all results obtained at 8 h showed a higher correlation between treated cells, with r values of 0.96 (IFN and gB; Fig. 5, plot C2), 0.94 (IFN and HCMV; Fig. 5, plot E2), and 0.97 (gB and HCMV; Fig. 5, plot E4), than any of the treated cells compared with mock-treated cells. Moreover, the tight distribution of all intensity values along the diagonal axis also indicates that the signals were very similar when treated or infected cells were compared with each other. Similarly, the 24-h IFN treatment was highly correlated to the 24-h gB treatment (Fig. 5, plot D3) as well as the 24-h HCMV infection (Fig. 5, plot F3), with r values of 0.95 and 0.90, respectively, whereas lower r values were obtained when treated cells were compared with mock-treated cells. These data strongly support and extend the above conclusion that the gene-expression patterns of cells infected with HCMV or treated with gB do not differ significantly from the expression profile of cells exposed to Type I IFN.
Figure 5.
Global analysis of fibroblast gene expression in response to IFN, gB, or HCMV infection. Intensity data from all of the cDNAs on the microarrays obtained at 8 and 24 h were log-transformed, and scatter plots were generated to assess differential gene expression between samples. Pair-wise similarity of gene-expression profiles was measured by the correlation coefficient; r values are indicated in the lower right corner of each scatter plot. Plots and calculations were made by using S-PLUS (http://www.mathsoft.com). The 2-h time points were omitted from this analysis because relatively few genes changed at this early time point.
Discussion
Because the arrays used in this study displayed a random collection of clones, it is highly unlikely that our results are biased toward IFN-regulated or otherwise related gene products. Moreover, this random collection also renders it unlikely that major pathways were missed in our analysis, although we cannot formally rule out the possibility that arrays covering the entire human genome will reveal differentially regulated genes.
The high degree of overlap between the gene-expression patterns of cells treated with gB or IFN is unexpected, because gB and IFN are known to signal through different receptors (9). It is further surprising that we failed to detect the regulation of additional genes by HCMV compared with gB because HCMV expresses high levels of the major immediate-early genes during the first 24 h of infection (16). These transcription factors were reported to regulate host cell gene expression (17, 18). However, during the course of infection, these or other viral factors may modulate the transcriptional events triggered by the initial gB contact with the cell surface. In fact, it seems that the magnitude of the regulation is lower in HCMV-infected cells than in gB-treated cells at 24 h (Tables 2 and 3).
Additionally, ISGs were induced upon HCMV infection or gB treatment of human aortic endothelial cells (J.S. and T.C., unpublished data). Thus, ISG induction also occurs in cell types reported to be a site of viral persistence (19). Interestingly, in both cell types, we failed to detect HCMV- or gB-induced activation of proinflammatory genes described in human monocytes after treatment with gB (20), suggesting that additional signaling pathways are triggered in the monocyte/macrophage lineage, the major reservoir for latent HCMV (21).
The molecular mechanism and signal cascade by which gB regulates ISGs is unknown. A variety of experimental evidence suggests the viral mechanism is distinct from the well characterized “traditional” IFN signaling (15, 22). As shown here and in earlier studies (3, 9), the onset of transcription modulation is delayed in HCMV-infected and gB-treated cells relative to IFN-treated cells. This difference may stem directly from the specific receptor used by HCMV or from the composition of virally activated signaling mediators. It is known that HCMV does not engage the Type I IFN receptor directly (9). Furthermore, tyk2 phosphorylation, which is diagnostic of Type I IFN receptor activation, does not take place in gB-treated cells (J.S. and T.C., unpublished data). Also compelling is the evidence that an indirect induction as a result of IFN production from HCMV-infected cells or gB-treated cells does not occur; neither experimental conditions require de novo synthesis for signaling to occur (3, 9, 23). Instead, gB seems to trigger IFN signaling through an unknown receptor and by incompletely characterized signaling mediators. Whether or not viral gB-mediated signaling can induce cytomegalovirus-induced factor, a complex which can bind to IFN-response elements within the promoters of HCMV-stimulated ISGs, remains to be addressed (23). Ultimately, identification of the receptor that confers HCMV entry and signal transduction will be required to dissect the initial events in infection.
The IFN response represents one of the major innate immune defenses of a cell against viral infection. Because HCMV is a highly host-adapted virus that has coevolved with humans, the question arises why HCMV actively induces this response during cell entry. Moreover, like many viruses (24), HCMV is known to interfere with IFN signaling by degrading the Jak-1 kinase, a critical component in IFN-mediated signal transduction (15, 22). However, this effect depends on viral replication (25, 26) and thus occurs after the time points measured here. It may be hypothesized that some of the virally induced ISGs have a beneficial effect for HCMV during the first hours of infection. The exact nature of such a putative advantageous effect and the specific genes involved remains to be investigated.
In conclusion, the preponderance of changes in cellular gene expression in the early phase of HCMV infection seems to be the direct consequence of the interaction between the viral ligand gB and its receptor. The ability of a single viral protein, gB, to elicit the same cellular transcriptional response as HCMV infection suggests that binding of gB to the cell surface during viral attachment is the key event in the initial stage of HCMV infection for altering cellular transcription. Our study reveals that this highly successful human pathogen programs cellular transcription from the earliest events in its life cycle, illuminating a previously unsuspected outcome of cellular contact.
Supplementary Material
Acknowledgments
We thank Peter Ghazal for helpful discussions throughout this study; Jose Galindo, Hong-Qing Guo, Amy Corrigan, and Kristine Flores for technical help with the microarray and scanning; Xiao Jun Ma and Xuejun Liu for bioinformatic support; Guang Chen for statistical consulting; and Gary Cohen and Roselyn Eisenberg (University of Pennsylvania) for the soluble HSV glycoproteins.
Abbreviations
- HCMV
human cytomegalovirus
- HFF
human foreskin fibroblasts
- gB
glycoprotein B
- ISG
IFN-stimulated gene
- HSV
herpes simplex virus
References
- 1.Lockhart D J, Dong H, Byrne M C, Follettie M T, Gallo M V, Chee M S, Mittmann M, Wang C, Kobayashi M, Horton H, Brown E L. Nat Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- 2.Chee M, Yang R, Hubbell E, Berno A, Huang X C, Stern D, Winkler J, Lockhart D J, Morris M S, Fodor S P A. Science. 1996;274:610–614. doi: 10.1126/science.274.5287.610. [DOI] [PubMed] [Google Scholar]
- 3.Zhu H, Cong J-P, Shenk T. Proc Natl Acad Sci USA. 1997;94:13985–13990. doi: 10.1073/pnas.94.25.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu H, Cong J-P, Mamtora G, Gingeras T, Shenk T. Proc Natl Acad Sci USA. 1998;95:14470–14475. doi: 10.1073/pnas.95.24.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Der S D, Zhou A, Williams B R G, Silverman R H. Proc Natl Acad Sci USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobom U, Brune W, Messerle M, Hahn G, Koszinowski U H. J Virol. 2000;74:7720–7729. doi: 10.1128/jvi.74.17.7720-7729.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro D, Paz P, Tugizov S, Topp K, La Vail J, Pereira L. Virology. 1993;197:143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 8.Boyle K A, Compton T. J Virol. 1998;72:1826–1833. doi: 10.1128/jvi.72.3.1826-1833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle K A, Pietropaolo R L, Compton T. Mol Cell Biol. 1999;19:3607–3613. doi: 10.1128/mcb.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salunga R C, Guo H, Luo L, Bittner A, Joy K C, Chambers J R, Wan J S, Jackson M R, Erlander M G. In: DNA Microarrays. Schema M, editor. Oxford: Oxford University Press; 1999. pp. 121–137. [Google Scholar]
- 11.Spear P G, Eisenberg R J, Cohen G H. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman L, Rousseeuw P. Finding Groups in Data–An Introduction to Cluster Analysis. New York: Wiley; 1990. [Google Scholar]
- 13.Iyer V R, Eisen M B, Ross D T, Schuler G, Moore T, Lee J C F, Trent J M, Staudt L M, Hudson J, Jr, Boguski M S, et al. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 14.White K P, Rifkin S A, Hurban P, Hogness D S. Science. 1999;286:2179–2184. doi: 10.1126/science.286.5447.2179. [DOI] [PubMed] [Google Scholar]
- 15.Boehm U, Klamp T, Groot M, Howard J C. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 16.Chambers J, Angulo A, Amaratunga D, Guo H, Jiang Y, Wan J S, Bittner A, Frueh K, Jackson M R, Peterson P A, et al. J Virol. 1999;73:5757–5766. doi: 10.1128/jvi.73.7.5757-5766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagemeier C, Walker S, Caswell R, Kouzarides T, Sinclair J. J Virol. 1992;66:4452–4456. doi: 10.1128/jvi.66.7.4452-4456.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryant L A, Mixon P, Davidson M, Bannister A J, Kouzarides T, Sinclair J H. J Virol. 2000;74:7230–7237. doi: 10.1128/jvi.74.16.7230-7237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fish K N, Soderberg-Naucler C, Mills L K, Stenglein S, Nelson J A. J Virol. 1998;72:5661–5668. doi: 10.1128/jvi.72.7.5661-5668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yurochko A D, Huang E-S. J Immunol. 1999;162:4806–4816. [PubMed] [Google Scholar]
- 21.Soderberg-Naucler C, Fish K N, Nelson J A. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 22.Stark G R, Kerr I M, Williams B R G, Silverman R H, Schreiber R D. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 23.Navarro L, Mowen K, Rodems S, Weaver B, Reich N, Spector D, David M. Mol Cell Biol. 1998;18:3796–3802. doi: 10.1128/mcb.18.7.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodbourn S, Didcock L, Randall R E. J Gen Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- 25.Miller D M, Zhang Y, Rahill B M, Waldman W J, Sedmak D D. J Immunol. 1999;162:6107–6113. [PubMed] [Google Scholar]
- 26.Miller D M, Rahill B M, Boss J M, Lairmore M D, Durbin J E, Waldman W J, Sedmak D D. J Exp Med. 1998;187:675–683. doi: 10.1084/jem.187.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.