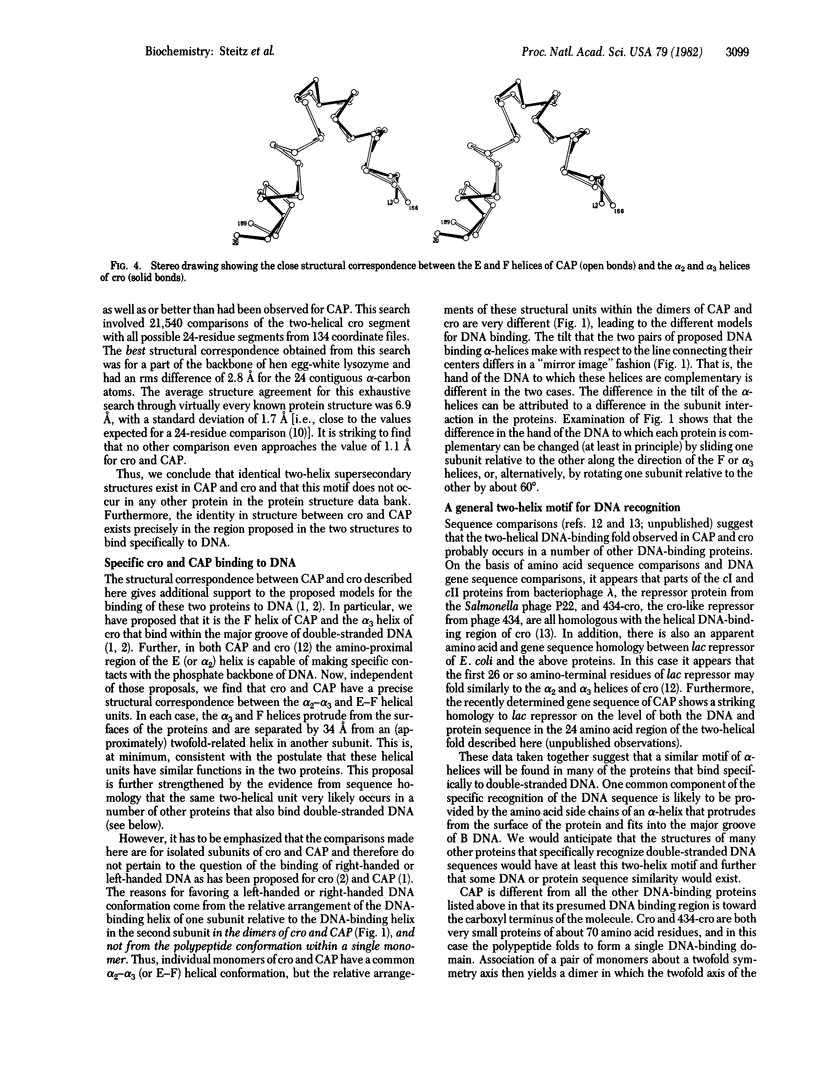
Abstract
It is shown that there is a structural similarity between the presumed DNA-binding regions of the Escherichia coli catabolite gene activator protein ("CAP") and the cro repressor protein ("cro") from bacteriophage lambda. The correspondence between the two proteins is particularly striking for a structural unit consisting of two consecutive alpha-helices. The 24 alpha-carbon atoms that constitute the two-helical structural units in the two proteins can be superimposed with a root-mean-square disagreement of 1.1 A. It is shown that this agreement is very unlikely to be due to a chance correspondence. For both CAP activator and cro repressor proteins it is the second alpha-helix of the two-helical unit that has been proposed to bind within the major groove of left-handed or right-handed B DNA, respectively [McKay, D. B. & Steitz, T. A. (1981) Nature (London) 290, 744-749; Anderson, W. F., Ohlendorf, D. H., Takeda, Y. & Matthews, B. W. (1981) Nature (London) 290, 754-758]. The structural correspondence between CAP and cro seen here, together with other recent evidence of sequence homologies between cro, CAP, and other proteins that bind double-stranded DNA, suggests that the two-helical unit is likely to be a common feature of many DNA-binding proteins. The results also suggest that some principles of specific protein-double-stranded DNA interaction may be general and include recognition via alpha-helices fitting into the major groove of the DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Ohlendorf D. H., Takeda Y., Matthews B. W. Structure of the cro repressor from bacteriophage lambda and its interaction with DNA. Nature. 1981 Apr 30;290(5809):754–758. doi: 10.1038/290754a0. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Echols H. Developmental pathways for the temperate phage: lysis vs lysogeny,. Annu Rev Genet. 1972;6(0):157–190. doi: 10.1146/annurev.ge.06.120172.001105. [DOI] [PubMed] [Google Scholar]
- Hsiang M. W., Cole R. D., Takeda Y., Echols H. Amino acid sequence of Cro regulatory protein of bacteriophage lambda. Nature. 1977 Nov 17;270(5634):275–277. doi: 10.1038/270275a0. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Ohlendorf D. H., Anderson W. F., Takeda Y. Structure of the DNA-binding region of lac repressor inferred from its homology with cro repressor. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1428–1432. doi: 10.1073/pnas.79.5.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D. B., Steitz T. A. Structure of catabolite gene activator protein at 2.9 A resolution suggests binding to left-handed B-DNA. Nature. 1981 Apr 30;290(5809):744–749. doi: 10.1038/290744a0. [DOI] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Jeffrey A., Johnson A. D., Maurer R., Meyer B. J., Pabo C. O., Roberts T. M., Sauer R. T. How the lambda repressor and cro work. Cell. 1980 Jan;19(1):1–11. doi: 10.1016/0092-8674(80)90383-9. [DOI] [PubMed] [Google Scholar]
- Remington S. J., Matthews B. W. A systematic approach to the comparison of protein structures. J Mol Biol. 1980 Jun 15;140(1):77–99. doi: 10.1016/0022-2836(80)90357-5. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Argos P. Exploring structural homology of proteins. J Mol Biol. 1976 Jul 25;105(1):75–95. doi: 10.1016/0022-2836(76)90195-9. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Argos P. The taxonomy of protein structure. J Mol Biol. 1977 Jan 5;109(1):99–129. doi: 10.1016/s0022-2836(77)80048-x. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Pan J., Hopper P., Hehir K., Brown J., Poteete A. R. Primary structure of the phage P22 repressor and its gene c2. Biochemistry. 1981 Jun 9;20(12):3591–3598. doi: 10.1021/bi00515a044. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]



